Synthesis of Thiazolo[5,4-d]thiazoles in an Eco-Friendly L-Proline–Ethylene Glycol Mixture
Abstract
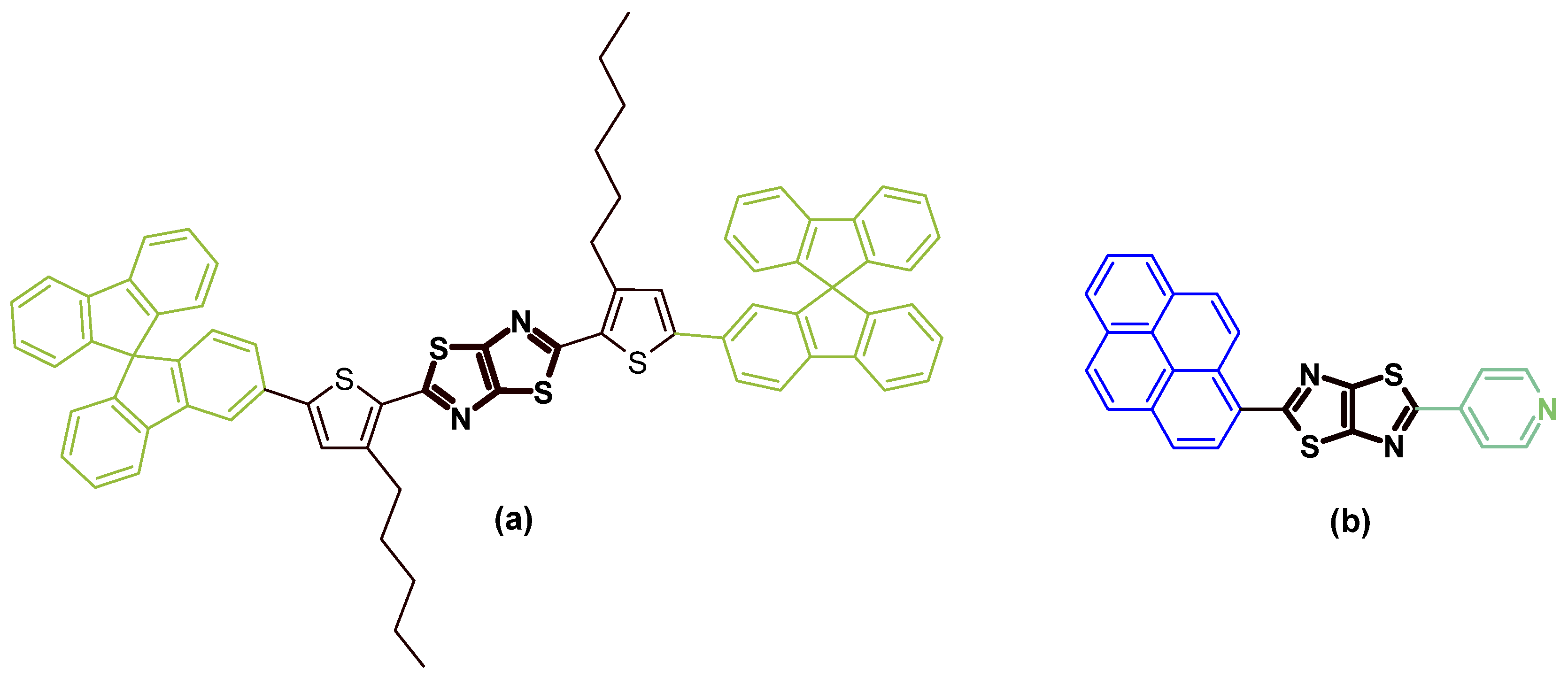
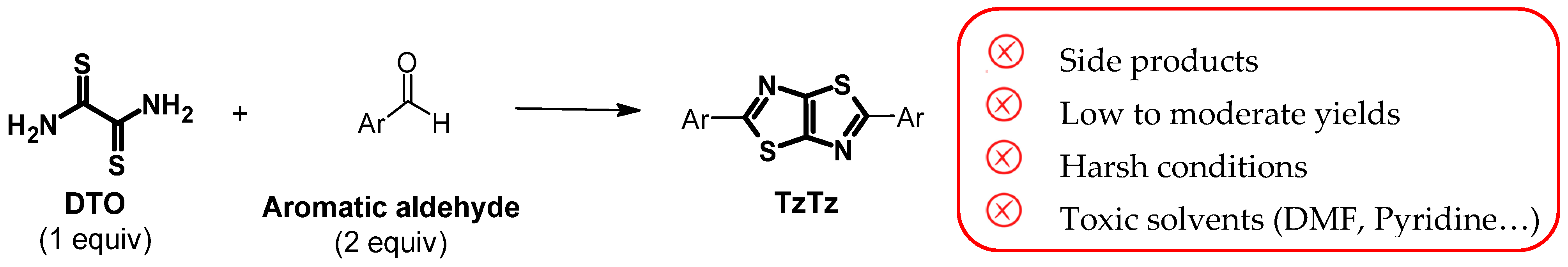
1. Introduction
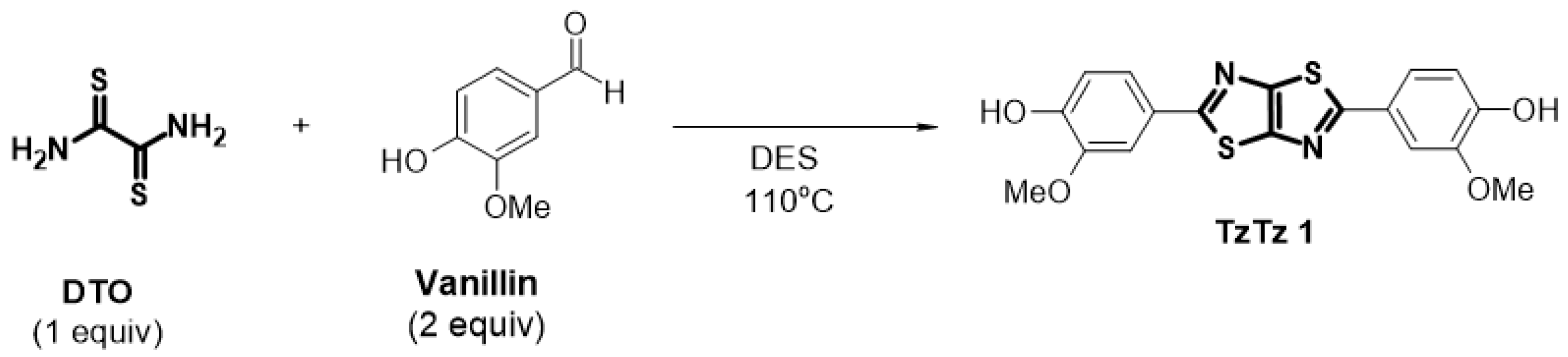
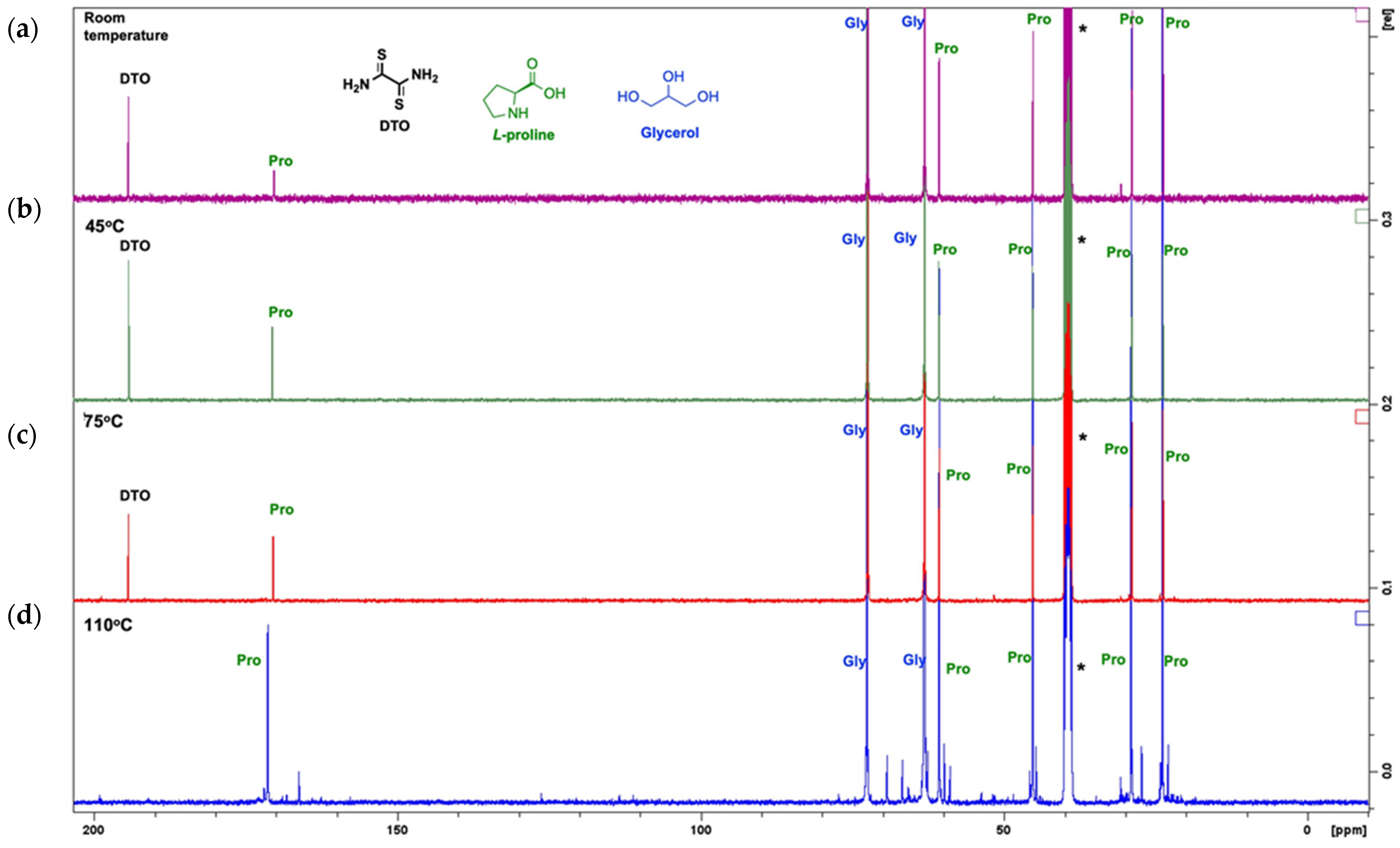
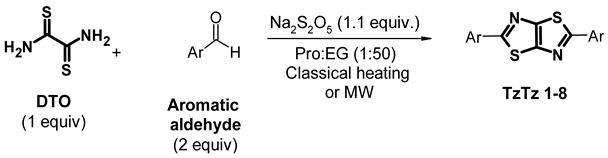
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, M.Y.; Zysman-Colman, E. Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef]
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S. A brief history of OLEDs—Emitter development and industry milestones. Adv. Mater. 2021, 33, 2005630. [Google Scholar] [CrossRef]
- Chaudhry, M.U.; Muhieddine, K.; Wawrzinek, R.; Sobus, J.; Tandy, K.; Lo, S.; Namdas, E.B. Organic light-emitting transistors: Advances and perspectives. Adv. Funct. Mater. 2020, 30, 1905282. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Bevk, D.; Marin, L.; Lutsen, L.; Vanderzande, D.; Maes, W. Thiazolo[5,4-d]thiazoles—Promising building blocks in the synthesis of semiconductors for plastic electronics. RSC Adv. 2013, 3, 11418–11431. [Google Scholar] [CrossRef]
- Tokárová, Z.; Eckstein-Andicsová, A.; Balogh, R.; Tokár, K. Survey of the Ketcham reaction for series of furan-substituted thiazolo[5,4-d]thiazoles. Tetrahedron 2021, 89, 132155. [Google Scholar] [CrossRef]
- Eckstein-Andicsová, A.; Tokárová, Z.; Kozma, E.; Balogh, R.; Vykydalová, A.; Mróz, W.; Tokár, K. Thiazolo[5,4-d]thiazoles with a spirobifluorene moiety as novel D-π-A type organic hosts: Design, synthesis, structure-property relationship and applications in electroluminescent devices. New J. Chem. 2023, 47, 11165–11175. [Google Scholar] [CrossRef]
- Kumar, V.; Sony, S.; Kaur, N.; Mobin, S.M.; Kaur, P.; Singh, K. Thiazolothiazole based donor-π-acceptor fluorophore: Protonation/deprotonation triggered molecular switch, sensing and bio-imaging applications. Anal. Chim. Acta 2022, 1206, 339776. [Google Scholar] [CrossRef]
- Johnson, J.R.; Ketcham, R. Thiazolothiazoles. I. The Reaction of Aromatic Aldehydes with Dithioöxamide. J. Am. Chem. Soc. 1960, 82, 2719–2724. [Google Scholar] [CrossRef]
- Jouaiti, A.; Giuso, V.; Cebrián, C.; Mercandelli, P.; Mauro, M. Linear, two- and four-armed pyridine-decorated thiazolo[5,4-d]thiazole fluorophores: Synthesis, photophysical study and computational investigation. Dye. Pigment. 2022, 208, 110780. [Google Scholar] [CrossRef]
- Zampese, J.A.; Keene, F.R.; Steel, P.J. Diastereoisomeric dinuclear ruthenium complexes of 2,5-di(2-pyridyl)thiazolo[5,4-d]thiazole. Dalton Trans. 2004, 4124–4129. [Google Scholar] [CrossRef]
- Thomas, D.A. Derivatives of thiazolo[5,4-d]thiazole. J. Heterocycl. Chem. 1970, 7, 457–462. [Google Scholar] [CrossRef]
- Knighton, R.C.; Hallett, A.J.; Kariuki, B.M.; Pope, S.J.A. A one-step synthesis towards new ligands based on aryl-functionalised thiazolo[5,4-d]thiazole chromophores. Tetrahedron Lett. 2010, 51, 5419–5422. [Google Scholar] [CrossRef]
- Sayresmith, N.A.; Saminathan, A.; Sailer, J.K.; Patberg, S.M.; Sandor, K.; Krishnan, Y.; Walter, M.G. Photostable voltage-sensitive dyes based on simple, solvatofluorochromic, asymmetric thiazolothiazoles. J. Am. Chem. Soc. 2019, 141, 18780–18790. [Google Scholar] [CrossRef]
- Turhan, O.; Dikmen, Z.; Bütün, V. Novel approach to increase chemosensing ability of fluorophore via cross-linked hydrogel film formation. J. Photochem. Photobiol. A 2023, 443, 114828. [Google Scholar] [CrossRef]
- Dessi, A.; Calamante, M.; Mordini, A.; Zani, L.; Taddei, M.; Reginato, G. Microwave-activated synthesis of thiazolo[5,4-d]thiazoles by a condensation/oxidation sequence. RSC Adv. 2014, 4, 1322–1328. [Google Scholar] [CrossRef]
- Flores, E.; Munoz-Osses, M.; Torrent, C.; Vásquez-Martinez, Y.; Gómez, A.; Cortez-San Martin, M.; Vega, A.; Martí, A.A.; Godoy, F.; Mascayano, C. Design, synthesis and biological evaluation of ferrocenyl thiazole and thiazolo[5,4-d]thiazole catechols as inhibitors of 5-hLOX and as antibacterials against Staphylococcus aureus. Structural relationship and computational studies. Organometallics 2020, 39, 2672–2681. [Google Scholar] [CrossRef]
- Costa, L.D.; Guieu, S.; Faustino, M.A.F.; Tomé, A.C. Staightforward synthesis of thiazolo[5,4-c]isoquinolines from dithiooxamide and 2-halobenzaldehydes. New J. Chem. 2022, 46, 3602–3615. [Google Scholar] [CrossRef]
- Al-Dujaili, A.H.; Atto, A.T.; Al-Kurde, A.M. Synthesis and liquid crystalline properties of models and polymers containing thiazolo[5,4-d]thiazole and siloxane flexible spacers. Eur. Polym. J. 2001, 37, 927–932. [Google Scholar] [CrossRef]
- Li, D.; Yuan, Y.; Bi, H.; Yao, D.; Zhao, X.; Tian, W.; Wang, Y.; Zhang, H. Boron-bridged π-conjugated ladders as efficient electron-transporting emitters. Inorg. Chem. 2011, 50, 4825–4831. [Google Scholar] [CrossRef]
- Ando, S.; Kumaki, D.; Nishida, J.; Tada, H.; Inoue, Y.; Tokito, S.; Yamashita, Y. Synthesis, physical properties and field-effect transistors of novel thiazolothiazole-phenylene co-oligomers. J. Mater. Chem. 2007, 17, 553–558. [Google Scholar] [CrossRef]
- Olgun, U.; Dikmen, Z.; Çetin, H.; Arican, F.; Gülfen, M. Synthesis, optical dyes properties and band gap energies of silver hydroxy-aryl thiazolo[5,4-d]thiazole complexes. J. Mol. Struct. 2022, 1250, 131816. [Google Scholar] [CrossRef]
- Dikmen, Z.; Isik, M.; Turhan, O.; Akbari, M.; Tuncer, C.; Javanifar, R.; Bütün, V. Thiazolo[5,4-d]thiazole based dye modified microspheres as metal nanoparticle reactor template and hybrid catalyst. Eur. Polym. J. 2022, 175, 111391. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Zaib, Q.; Eckelman, M.J.; Yang, Y.; Kyung, D. Are deep eutectic solvents really green?: A life-cycle perspective. Green Chem. 2022, 24, 7924–7930. [Google Scholar] [CrossRef]
- Popović, B.M.; Gligorijević, N.; Arandelović, S.; Macedo, A.C.; Jurić, T.; Uka, D.; Mocko-Blažek, K.; Serra, A.T. Cytotoxicity profiling of choline-based natural deep eutectic solvents. RSC Adv. 2023, 13, 3520–3527. [Google Scholar] [CrossRef]
- Bystrzanowska, M.; Tobiszewski, M. Assessment and design of greener deep eutectic solvents—A multicriteria decision analysis. J. Mol. Liq. 2021, 321, 114878. [Google Scholar] [CrossRef]
- Meredith, L.; Elbourne, A.; Greaves, T.L.; Bryant, G.; Bryant, S.J. Physico-chemical characterization of glycerol-and ethyleneglycol-based deep eutectic solvents. J. Mol. Liq. 2024, 394, 123777. [Google Scholar] [CrossRef]
- Fonseca, D.P.; Amorim, A.C.; Carreiro, E.P.; Ramalho, J.P.P.; Hermann, G.J.; Federsel, H.-J.; Duarte, A.R.C.; Burke, A.J. Sustainable organocatalyzed enantioselective catalytic Michael additions in betaine-derived deep eutectic solvents. SynOpen 2023, 7, 374–380. [Google Scholar] [CrossRef]
- Zárate-Roldán, S.; Trujillo-Rodríguez, M.; Gimeno, M.C.; Herrera, R.P. L-proline-based deep eutectic solvents as green and enantioselective organocatalyst/media for aldol reaction. J. Mol. Liq. 2024, 396, 123971. [Google Scholar] [CrossRef]
- Juaristi, E. Recent developments in next generation (S)-proline-derives chiral organocatalysts. Tetrahedron 2021, 88, 132143. [Google Scholar] [CrossRef]
- Tiecco, M.; Alonso, D.A.; Niguez, D.R.; Ciancaleoni, G.; Guillena, G.; Ramon, D.J.; Bonillo, A.A.; Germani, R. Assessment of the organocatalytic activity of chiral L-proline-based deep eutectic solvents based on their structural features. J. Mol. Liq. 2020, 313, 113573. [Google Scholar] [CrossRef]
- Hesse, S. Synthesis of 5-arylidenerhodanines in L-proline-based deep eutectic solvent. Beilstein J. Org. Chem. 2023, 19, 1537–1544. [Google Scholar] [CrossRef]
- Hesse, S.; Hertzog, J.; Rup-Jacques, S. L-proline-based DES in Knoevenagel synthesis of arylidene rhodanines, thiazolidine-2,4-diones and barbituric derivatives. J. Heterocycl. Chem. 2024, 61, 1015–1023. [Google Scholar] [CrossRef]
- Gendron, D. Vanillin: A promising biosourced building block for the preparation of various heterocycles. Front. Chem. 2022, 10, 949355. [Google Scholar] [CrossRef]
- Biswal, B.P.; Becker, D.; Chandrasekhar, N.; Seenath, J.S.; Paasch, S.; Machill, S.; Hennersdorf, F.; Brunner, E.; Weigand, J.J.; Berger, R.; et al. Exploration of thiazolo[5,4-d]thiazole linkages in conjugated porous organic polymers for chemoselective molecular sieving. Chem. Eur. J. 2018, 24, 10868–10875. [Google Scholar] [CrossRef]
- Papernaya, L.K.; Shatrova, A.A.; Albanov, A.I.; Levkovskaya, G.G.; Rozentsveig, I.B. One-pot microwave-assisted synthesis of 2,5-bis(pyrazol-4-yl)[1,3]thiazolo[5,4-d]thiazoles from pyrazole-4-carbaldehydes and dithiooxamide. Arkivoc 2016, v, 142–150. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.; Wang, S.; Liu, J.; Xie, K.; Wang, A.; Tan, Z. Synthesis of 2-aryl benzothiazoles via K2S2O8-mediated oxidative condensation of benzothiazoles with aryl aldehydes. J. Org. Chem. 2012, 77, 7086–7091. [Google Scholar] [CrossRef]
- Wang, J.; Zong, Y.; Zhang, X.; Gao, Y.; Li, Z.; Yue, G.; Quan, Z.; Wang, X. Synthesis of N-benzothiazol-2-yl-amides by iron-catalyzed oxidative C(sp2)-H functionalization. Synlett 2014, 25, 2143–2148. [Google Scholar] [CrossRef]
- Kumar, M.; Pandey, S.K.; Chaudhary, N.; Mishra, A.; Gupta, D. Highly efficient method for the synthesis of substituted benzimidazoles using sodium metabisulfite adsorbed on silica gel. Results Chem. 2022, 4, 100403. [Google Scholar] [CrossRef]
- Dikmen, Z.; Turhan, O.; Yaman, M.; Bütün, V. An effective fluorescent optical sensor: Thiazolo-thiazole based dye exhibiting anion/cation sensitivities and acidochromism. J. Photochem. Photobiol. A 2021, 419, 113456. [Google Scholar] [CrossRef]
- Dikmen, Z.; Dikmen, G.; Bütün, V. Fluorophore-assisted green fabrication of flexible and cost-effective Ag nanoparticles decorated PVA nanofibers for SERS based trace detection. J. Photochem. Photobiol. A 2023, 445, 115074. [Google Scholar] [CrossRef]
- Mari, S.; Ando, S.; Yamaguchi, T. Novel aromatic proton exchange membranes based on thiazolothiazole units. Polym. J. 2017, 49, 745–749. [Google Scholar] [CrossRef]
- Saber, A.F.; Elewa, A.M.; Chou, H.-H.; El-Mahdy, A.F.M. Donor-acceptor carbazole-based conjugated microporous polymers as photocatalysts for visible-light-driven H2 and O2 evolution from water splitting. Appl. Catal. B 2022, 316, 121624. [Google Scholar] [CrossRef]
- Hua, C.; Rizzuto, F.J.; Zhang, X.; Tuna, F.; Collison, D.; D’Alessandro, D.M. Spectroelectrochemical properties of a Ru(II) complex with a thiazolo[5,4-d]thiazole triarylamine ligand. New J. Chem. 2017, 41, 108–114. [Google Scholar] [CrossRef]
| Entry | DES | Conversion 1 |
|---|---|---|
| 1 | CC:Gly (1:2) | Slow (24 h) |
| 2 | CC:urea (1:2) | Slow (24 h) |
| 3 | Pro:Gly (1:2) | Fast (1 h) |
| Entry | Temperature | Consumption of Vanillin 1 | TzTz 1 Formation |
|---|---|---|---|
| 1 | 110 °C | 1 h | Yes |
| 2 | 45 °C | 24 h | No |
| 3 | 75 °C | 5 h | Yes |
| Entry | Compound | Classical Heating | Yield (%) | Microwave Heating | Yield (%) | Literature |
|---|---|---|---|---|---|---|
| 1 | 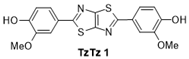 | 130 °C—1 h | 75 | 130 °C—25 min | 92 | Pyridine, reflux, 24 h 79% [15] |
| 2 | 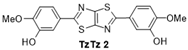 | 130 °C—1 h | 70 | 130 °C—25 min | 75 | |
| 3 | 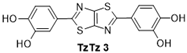 | 130 °C—1 h | 99 | 130 °C—25 min | 61 | Nitrobenzene, 130 °C, 24 h 79% [13] |
| 4 | 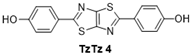 | 130 °C—1 h | 72 | 130 °C—25 min | 36 | Pyridine, reflux, 5 h 74% [43,44] EtOH, NaOH, 80 °C, 2 h [22] Phenol, reflux, 2 h—38% [19] |
| 5 | 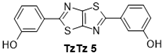 | 130 °C—1 h | 85 | 130 °C—25 min | 30 | Excess aldehyde, phenol reflux 15 min—38% [9] |
| 6 | 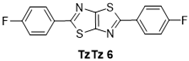 | 140 °C—1 h | 58 | 130 °C—25 min | / | DMF, 150 °C, 6 h—50% [45] |
| 7 | 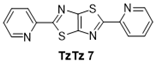 | 140 °C—1 h | 66 | 130 °C—25 min | / | EtOH, Et3N, reflux, 48 h—58% [38] (i) Nitrobenzene, 150 °C, mw 30 min (ii) chloranil, THF, reflux, 10 min—76% [16] Nitrobenzene, 130 °C, 24 h—60% [13] Excess aldehyde, reflux, 45 min—19% [11] |
| 8 | 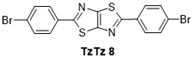 | 130 °C—1 h | 36 | 130 °C—25 min | / | DMF, reflux, 3 h—27% [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyễn, T.T.T.; Longevial, J.-F.; Hesse, S. Synthesis of Thiazolo[5,4-d]thiazoles in an Eco-Friendly L-Proline–Ethylene Glycol Mixture. Molecules 2025, 30, 938. https://doi.org/10.3390/molecules30040938
Nguyễn TTT, Longevial J-F, Hesse S. Synthesis of Thiazolo[5,4-d]thiazoles in an Eco-Friendly L-Proline–Ethylene Glycol Mixture. Molecules. 2025; 30(4):938. https://doi.org/10.3390/molecules30040938
Chicago/Turabian StyleNguyễn, Thiên Thuý Trang, Jean-François Longevial, and Stéphanie Hesse. 2025. "Synthesis of Thiazolo[5,4-d]thiazoles in an Eco-Friendly L-Proline–Ethylene Glycol Mixture" Molecules 30, no. 4: 938. https://doi.org/10.3390/molecules30040938
APA StyleNguyễn, T. T. T., Longevial, J.-F., & Hesse, S. (2025). Synthesis of Thiazolo[5,4-d]thiazoles in an Eco-Friendly L-Proline–Ethylene Glycol Mixture. Molecules, 30(4), 938. https://doi.org/10.3390/molecules30040938








