Reaction Mechanism Study of LiNH2BH3 and (LiH)n (n = 1–5) Clusters Based on Density Functional Theory
Abstract
1. Introduction
2. Results
2.1. Reaction Between LiNH2BH3 and LiH Clusters
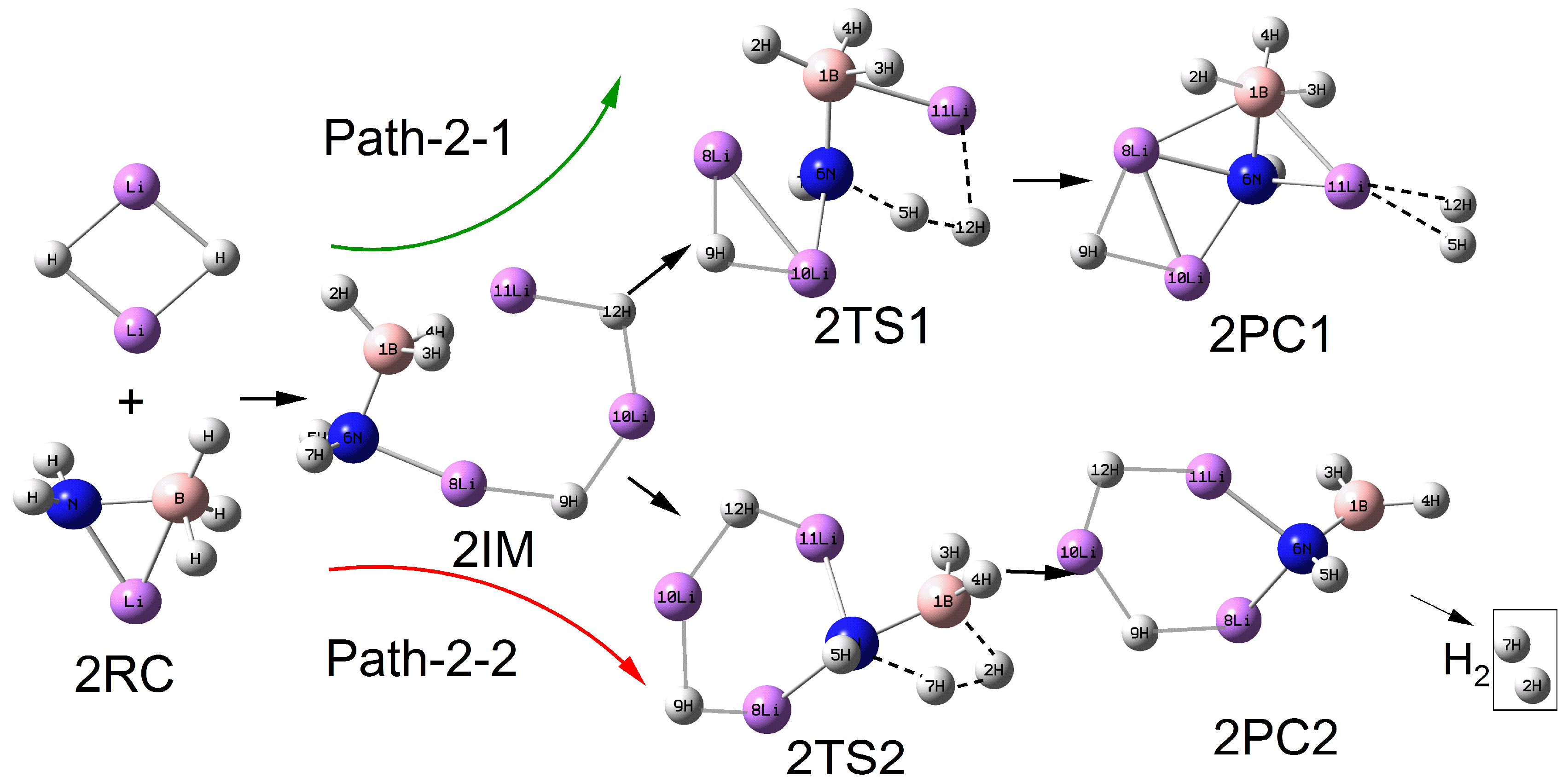
2.2. Reaction Between LiNH2BH3 and (LiH)n (n = 2–5) Clusters
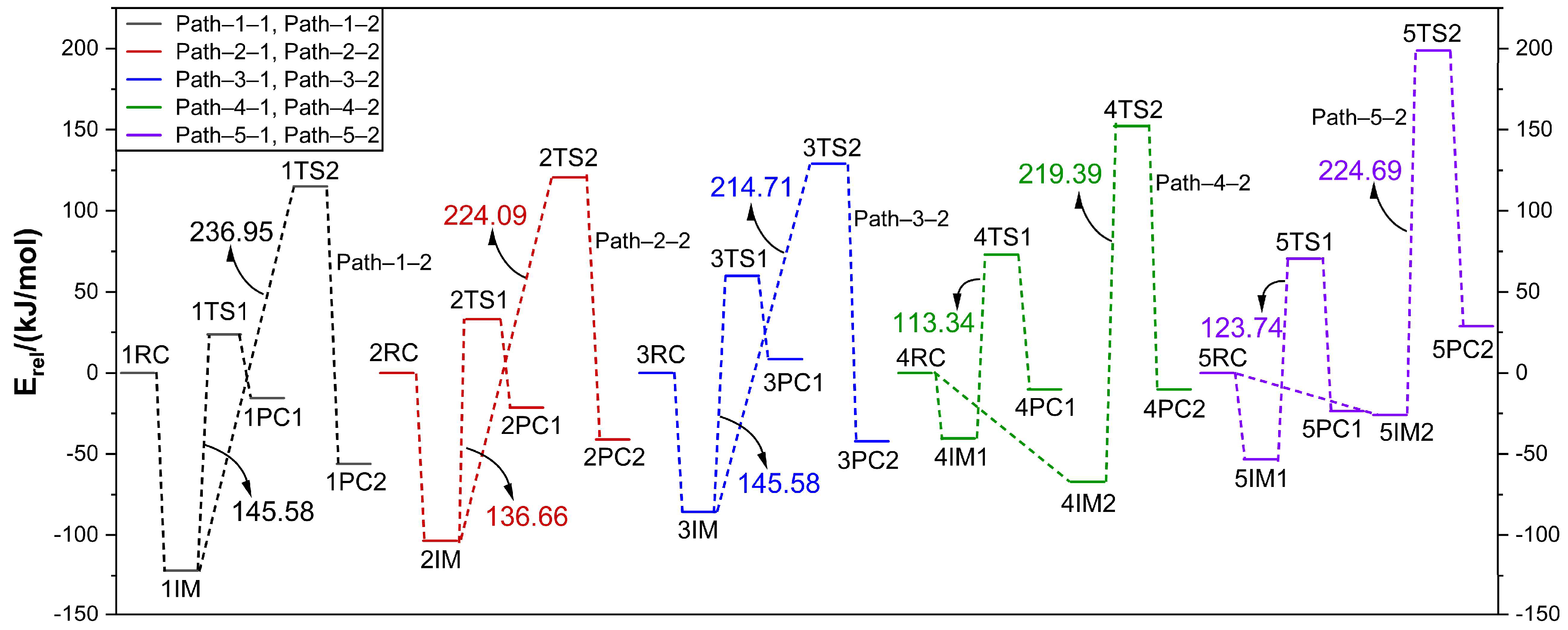
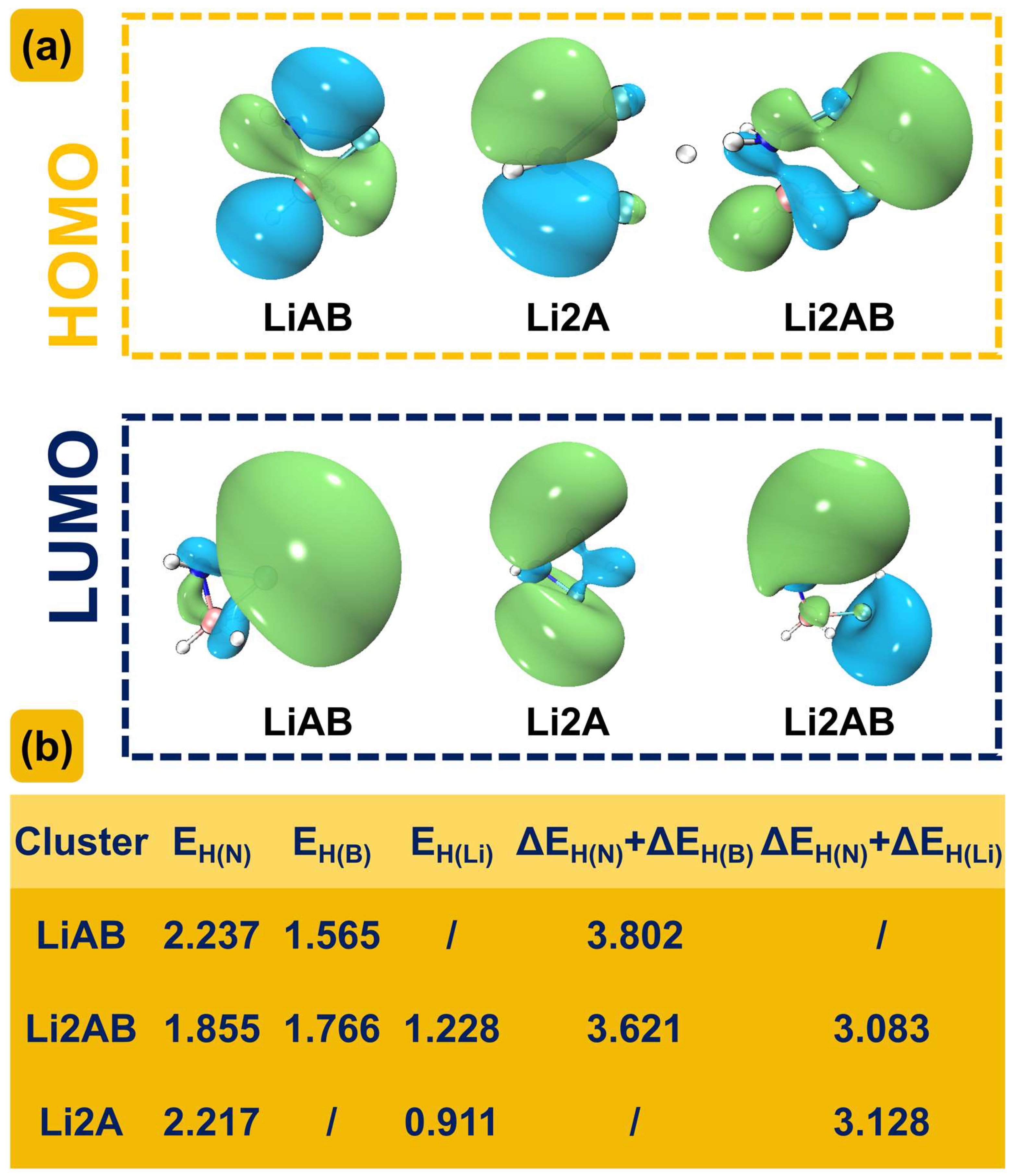
2.3. Analysis of Reaction Energy and Mechanism
3. Discussion
4. Calculation Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, Q. Chemistry of Materials for Energy and Environmental Sustainability. Molecules 2024, 29, 5929. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Sun, Y.; Zhang, Y.Q.; Liu, J.Z.; Bo, X.; Wang, Z.L. Tungsten carbide/tungsten oxide catalysts for efficient electrocatalytic hydrogen evolution. Molecules 2024, 30, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; Zhou, T.; Liao, J.F.; Li, X.F.; Ma, W.H.; Lv, G.Q.; Zhao, S.M. Hydrogen generation from the hydrolysis of diamond–wire sawing silicon waste powder vibration–ground with KCl. Molecules 2025, 30, 223. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Jiang, L.J.; Jiang, W.Q.; Wu, Y.F.; Guo, X.M.; Li, Z.N.; Yuan, H.P.; Luo, M. Recent advances of boron nitride nanosheets in hydrogen storage application. J. Mater. Res. Technol. 2023, 26, 2028–2042. [Google Scholar] [CrossRef]
- Li, C.; Liu, B.J.; Li, Y.F.; Yuan, Z.M.; Zhou, D.S.; Wang, H.Y. A significantly improved hydrogen storage performance of nanocrystalline Ti–Fe–Mn–Pr alloy. J. Mater. Res. Technol. 2023, 23, 4566–4575. [Google Scholar] [CrossRef]
- Xu, N.L.; Chen, Y.; Chen, S.J.; Zhang, W.B.; Li, S.; Song, R.J.; Zhang, J.Y. First–principles investigations for the hydrogen storage properties of XVH3 (X = Na, K, Rb, Cs) perovskite type hydrides. J. Mater. Res. Technol. 2023, 26, 4825–4834. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Li, Z.; Wang, X.B.; Xu, Y.F.; Chen, S.S.; Wang, Z. Optimized synthesis of Zr(IV) metal organic frameworks (MOFs–808) for efficient hydrogen storage. N. J. Chem. 2019, 43, 4092–4099. [Google Scholar] [CrossRef]
- Al Obeidli, A.; Ben Salah, H.; Al Murisi, M.; Sabouni, R. Recent advancements in MOFs synthesis and their green applications. Int. J. Hydrogen Energy 2022, 47, 2561–2593. [Google Scholar] [CrossRef]
- Ariharan, A.; Viswanathan, B.; Nandhakumar, V. Nitrogen–incorporated carbon nanotube derived from polystyrene and polypyrrole as hydrogen storage material. Int. J. Hydrogen Energy 2018, 43, 5077–5088. [Google Scholar] [CrossRef]
- Pedicini, R.; Maisano, S.; Chiodo, V.; Conte, G.; Policicchio, A.; Agostino, R.G. Posidonia Oceanica and Wood chips activated carbon as interesting materials for hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 14038–14047. [Google Scholar] [CrossRef]
- Cao, W.C.; Ding, X.; Chen, R.R.; Zhang, J.X.; Zhang, Y.; Shen, H.X.; Fu, H.Z. In–situ precipitation of ultrafine Mg2Ni particles in Mg–Ni–Ag metal fibers and their hydrogen storage properties. Chem. Eng. J. 2023, 475, 146252. [Google Scholar] [CrossRef]
- Zang, J.H.; Wang, S.F.; Hu, R.R.; Man, H.; Zhang, J.C.; Wang, F.; Sun, D.L.; Song, Y.; Fang, F. Ni, beyond thermodynamic tuning, maintains the catalytic activity of V species in Ni3(VO4)2 doped MgH2. J. Mater. Chem. A 2021, 9, 8341–8349. [Google Scholar] [CrossRef]
- Wang, L.X.; Hu, Y.W.T.; Lin, J.Y.; Leng, H.Y.; Sun, C.H.; Wu, C.Z.; Li, Q.; Pan, F.S. The hydrogen storage performance and catalytic mechanism of the MgH2–MoS2 composite. J. Magnes. Alloys 2023, 11, 2530–2540. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.T.; Luo, J.Z.; Lin, J.Y.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef]
- Wei, S.; Liu, J.X.; Xia, Y.P.; Zhang, H.Z.; Cheng, R.G.; Sun, L.X.; Xu, F.; Bu, Y.T.; Liu, Z.Y.; Huang, P.R.; et al. Enhanced hydrogen storage properties of LiAlH4 by excellent catalytic activity of XTiO3@h–BN (X = Co, Ni). Adv. Funct. Mater. 2021, 32, 2110180. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.X.; Zhang, L.C.; Huang, Z.G.; Hu, J.J.; Gao, M.X.; Pan, H.G.; Liu, Y.F. Single–pot solvothermal strategy toward support–free nanostructured LiBH4 featuring 12 wt% reversible hydrogen storage at 400 °C. Chem. Eng. J. 2022, 428, 132566. [Google Scholar] [CrossRef]
- Wei, J.; Leng, H.Y.; Li, Q.; Chou, K.C. Improved hydrogen storage properties of LiBH4 doped Li–N–H system. Int. J. Hydrogen Energy 2014, 39, 13609–13615. [Google Scholar] [CrossRef]
- Shore, S.G.; Parry, R.W. The crystalline compound ammonia–borane,1 H3NBH3. J. Am. Chem. Soc. 1955, 77, 6084–6085. [Google Scholar] [CrossRef]
- Yuan, B.; Shin, J.W.; Bernstein, E.R. Dynamics and fragmentation of van der Waals and hydrogen bonded cluster cations: (NH3)n and (NH3BH3)n ionized at 10.51 eV. J. Chem. Phys. 2016, 144, 144315. [Google Scholar] [CrossRef]
- Benedetto, S.D.; Carewska, M.; Cento, C.; Gislon, P.; Pasquali, M.; Scaccia, S.; Prosini, P.P. Effect of milling and doping on decomposition of NH3BH3 complex. Thermochim. Acta 2006, 441, 184–190. [Google Scholar] [CrossRef]
- Liu, X.R.; Wang, S.M.; Wu, Y.F.; Li, Z.N.; Jiang, L.J.; Guo, X.M.; Ye, J.H. Dehydrogenation properties of two phases of LiNH2BH3. Int. J. Hydrogen Energy 2020, 45, 2127–2134. [Google Scholar] [CrossRef]
- Luo, J.H.; Wu, H.; Zhou, W.; Kang, X.D.; Wang, P. Li2(NH2BH3)(BH4)/LiNH2BH3: The first metal amidoborane borohydride complex with inseparable amidoborane precursor for hydrogen storage. Int. J. Hydrogen Energy 2013, 38, 197–204. [Google Scholar] [CrossRef]
- Liu, X.R.; Wu, Y.F.; Wang, S.M.; Li, Z.N.; Guo, X.M.; Ye, J.H.; Jiang, L.J. Current progress and research trends on lithium amidoborane for hydrogen storage. J. Mater. Sci. 2019, 55, 2645–2660. [Google Scholar] [CrossRef]
- Shimoda, K.; Doi, K.; Nakagawa, T.; Zhang, Y.; Miyaoka, H.; Ichikawa, T.; Tansho, M.; Shimizu, T.; Burrell, A.K.; Kojima, Y. Comparative study of structural changes in NH3BH3, LiNH2BH3, and KNH2BH3 during dehydrogenation process. J. Phys. Chem. C 2012, 116, 5957–5964. [Google Scholar] [CrossRef]
- Ramzan, M.; Silvearv, F.; Blomqvist, A.; Scheicher, R.H.; Lebègue, S.; Ahuja, R. Structural and energetic analysis of the hydrogen storage materials LiNH2BH3 and NaNH2BH3 from ab initio calculations. Phys. Rev. B 2009, 79, 132102. [Google Scholar] [CrossRef]
- Wu, C.Z.; Wu, G.T.; Xiong, Z.T.; Han, X.W.; Chu, H.L.; He, T.; Chen, P. LiNH2BH3·NH3BH3: Structure and hydrogen storage properties. Chem. Mater. 2009, 22, 3–5. [Google Scholar] [CrossRef]
- Li, W.; Scheicher, R.H.; Araújo, C.M.; Wu, G.T.; Blomqvist, A.; Wu, C.Z.; Ahuja, R.; Feng, Y.P.; Chen, P. Understanding from first–principles why LiNH2BH3·NH3BH3 shows improved dehydrogenation over LiNH2BH3 and NH3BH3. J. Phys. Chem. C 2010, 114, 19089–19095. [Google Scholar] [CrossRef]
- Lee, T.B.; McKee, M.L. Mechanistic study of LiNH2BH3 formation from (LiH)4+NH3BH3 and subsequent dehydrogenation. Inorg. Chem. 2009, 48, 7564–7575. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Chem. Phys. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- El–Azhary, A.A.; Suter, H.U. Comparison between optimized geometries and vibrational frequencies calculated by the DFT methods. J. Phys. Chem. 1996, 100, 15056–15063. [Google Scholar] [CrossRef]
- Becke, A.D. Density–functional thermochemistry. III. the role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle–Salvetti correlation–energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, N.; Kose, A.; Fellah, M.F. A DFT investigation of hydrogen adsorption and storage properties of Mg decorated IRMOF–16 structure. Colloid. Surface. A 2022, 641, 128510. [Google Scholar] [CrossRef]
- Gopalsamy, K.; Subramanian, V. Hydrogen storage capacity of alkali and alkaline earth metal ions doped carbon based materials: A DFT study. Int. J. Hydrogen Energy 2014, 39, 2549–2559. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Yuan, R.; Li, G.; Du, A. Reaction Mechanism Study of LiNH2BH3 and (LiH)n (n = 1–5) Clusters Based on Density Functional Theory. Molecules 2025, 30, 929. https://doi.org/10.3390/molecules30040929
Dong X, Yuan R, Li G, Du A. Reaction Mechanism Study of LiNH2BH3 and (LiH)n (n = 1–5) Clusters Based on Density Functional Theory. Molecules. 2025; 30(4):929. https://doi.org/10.3390/molecules30040929
Chicago/Turabian StyleDong, Xiao, Rong Yuan, Genzhuang Li, and Aochen Du. 2025. "Reaction Mechanism Study of LiNH2BH3 and (LiH)n (n = 1–5) Clusters Based on Density Functional Theory" Molecules 30, no. 4: 929. https://doi.org/10.3390/molecules30040929
APA StyleDong, X., Yuan, R., Li, G., & Du, A. (2025). Reaction Mechanism Study of LiNH2BH3 and (LiH)n (n = 1–5) Clusters Based on Density Functional Theory. Molecules, 30(4), 929. https://doi.org/10.3390/molecules30040929






