Triacylglycerol Composition of Seed Oil from Corema album Berries
Abstract
1. Introduction
2. Results and Discussion
2.1. Oil Content
2.2. Composition of Fatty Acids
2.3. TAG Composition
2.4. Fatty Acid Distribution Between sn-2 and sn-1,3 Positions of Triacylglycerols
2.5. Composition of Tocochromanols
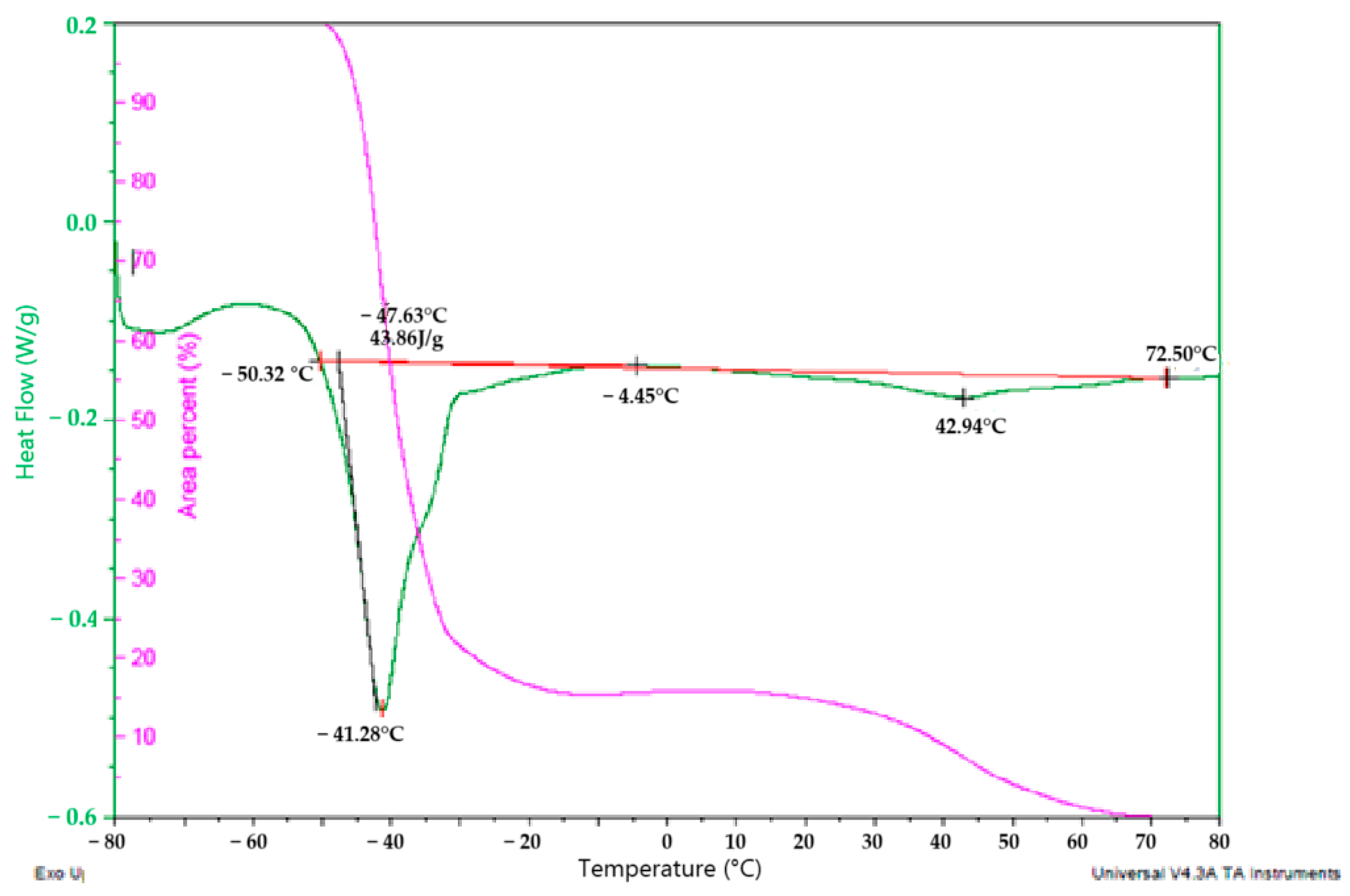
2.6. Differential Scanning Calorimetry (DSC) of Corema Seed Oil
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Oil Extraction
3.4. Fatty Acid Composition
3.5. TAG Analysis
3.6. TAG Lipolysis
3.7. Analysis of Tocochromanols
3.8. Calorimetric Analysis by DSC
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IA | atherogenicity index |
| IT | thrombogenicity index |
| H | Soxhlet extraction with hexane |
| HR | Hara and Radin method |
| BD | Bligh and Dyer method |
| DW | dry weight |
| TAGs | triacylglycerols |
| PUFA | polyunsaturated fatty acid |
| MUFA | monounsaturated fatty acid |
| SFA | saturated fatty acid |
| n-3 | omega-3 |
| n-6 | omega-6 |
| ALA | α-linolenic acid |
| LA | linoleic acid |
| EPA | eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| POP | dipalmitoyl–oleoyl–glycerol |
| PLP | dipalmitoyl–linoleoyl–glycerol |
| PLnP | dipalmitoyl–linolenoyl–glycerol |
| POS | palmitoyl–oleoyl–stearoyl–glycerol |
| POO | dioleoyl–palmitoyl–glycerol |
| PSL | palmitoyl–stearoyl–linoleoyl–glycerol |
| POL | palmitoyl–oleoyl–linoleoyl–glycerol |
| PLL | dilinoleoyl–palmitoyl–glycerol |
| POLn | palmitoyl–oleoyl–linolenoyl–glycerol |
| PLLn | palmitoyl–linoleoyl–linolenoyl–glycerol |
| PLnLn | dilinolenoyl–palmitoyl–glycerol |
| SOO | dioleoyl–stearoyl–glycerol |
| SLS | distearoyl–linoleoyl–glycerol |
| OOO | triolein |
| SOL | stearoyl–oleoyl–linoleoyl–glycerol |
| OOL | dioleoyl–linoleoyl–glycerol |
| SOLn | stearoyl–oleoyl–linolenoyl–glycerol |
| SLL | dilinoleoyl–stearoyl–glycerol |
| OLL | dilinoleoyl–oleoyl–glycerol |
| OOLn | dioleoyl–linolenoyl–glycerol |
| SLLn | stearoyl–linoleoyl–linolenoyl–glycerol |
| SLnLn, | dilinolenoyl–stearoyl–glycerol |
| LLL | trilinolein |
| OLLn | oleoyl–linoleoyl–linolenoyl–glycerol |
| LLLn | dilinoleoyl–linolenoyl–glycerol |
| OLnLn | dilinolenoyl–oleoyl–glycerol |
| LLnLn | dilinolenoyl–linoleoyl–glycerol |
References
- Álvarez-Cansino, L.; Zunzunegui, M.; Díaz Barradas, M.C.; Esquivias, M.P. Gender-specific costs of reproduction on vegetative growth and physiological performance in the dioecious shrub Corema album. Ann. Bot. 2010, 106, 989–998. [Google Scholar] [CrossRef] [PubMed]
- López-Dóriga, I.L. The Archaeobotany and Ethnobotany of Portuguese or White Crowberry (Corema album (L.) D. Don). Ethnobiol. Lett. 2018, 9, 19–32. [Google Scholar] [CrossRef]
- Villar, L.; Castroviejo, S.; Aedom, C.; Gómez Campo, C.; Laínz, M.; Monserrat, P.; Morales, R.; Muñoz Garmendia, F.; Nieto Feliner, G.; Rico, E.; et al. Corema D. Don. In Flora Iberica; Real Jardín Botánico, CSIC: Madrid, Spain, 1993; Volume 4, pp. 524–536. [Google Scholar]
- Larrinaga, A.R.; Guitián, P. Intraspecific Variation in Fruit Size and Shape in Corema album (Ericaceae) Along a Latitudinal Gradient: From Fruits to Populations. Biol. J. Linn. Soc. 2016, 118, 940–950. [Google Scholar] [CrossRef]
- Barroca, M.J.; da Silva, A.M. From folklore to the nutraceutical world: The Corema album potential. In Gastronomy and Food Science; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 119–135. [Google Scholar]
- Quer, P. Plantas Medicinales: El Dioscórides Renovado; Ed. Labor: Barcelona, Spain, 1976; p. 1031. [Google Scholar]
- León-González, A.J.; Navarro, I.; Acero, N.; Muñoz-Mingarro, D.; Martín-Cordero, C. The Fruit of Corema album (L.) D. Don, a Singular White Berry with Potential Benefits in Nutrition and Health. Phytochem. Rev. 2022, 21, 525–536. [Google Scholar] [CrossRef]
- León-González, A.J.; Truchado, P.; Tomás-Barberán, F.A.; López-Lázaro, M.; Barradas, M.C.D.; Martín-Cordero, C. Phenolic acids, flavonols and anthocyanins in Corema album (L.) D. Don berries. J. Food Compos. Anal. 2013, 29, 58–63. [Google Scholar] [CrossRef]
- Canoyra, A.; Martín-Cordero, C.; Muñoz-Mingarro, D.; León-González, A.J.; Parsons, R.B.; Acero, N. Corema album Berry Juice as a Protective Agent Against Neurodegeneration. Pharmaceuticals 2024, 17, 1535. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Mateos, R.; Ramos, S.; Martín, M.Á.; Sarriá, B.; Martín-Cordero, C.; López-Lázaro, M.; Bravo, L.; Goya, L. Chemo-Protective Activity and Characterization of Phenolic Extracts from Corema album. Food Res. Int. 2012, 49, 728–738. [Google Scholar] [CrossRef]
- Sławinska, N.; Prochon, K.; Olas, B. A Review on Berry Seeds—A Special Emphasis on Their Chemical Content and Health-Promoting Properties. Nutrients 2023, 15, 1422. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Laakso, P.I.; Kallio, H. Characterization of seed oils of wild, edible Finnish berries. Zeitschrift Lebensmitteluntersuchung-Forschung A 1997, 204, 300–307. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Sampels, S. Nutritional value of fish: Lipids, proteins, vitamins, and minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- Paszczyk, B.; Luczynska, J. The Comparison of Fatty Acid Composition and Lipid Quality Indices in Hard Cow, Sheep, and Goat Cheeses. Foods 2020, 9, 1667. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, P. The Effect of Omega-3 Fatty Acids on Rheumatoid Arthritis. Mediterr. J. Rheumatol. 2020, 31, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.A.; Alfaia, C.M.; Pestana, J.M.; Prates, J.A.M. Structured Lipids Engineering for Health: Novel Formulations Enriched in n-3 Long-Chain Polyunsaturated Fatty Acids with Potential Nutritional Benefits. Metabolites 2023, 13, 1060. [Google Scholar] [CrossRef] [PubMed]
- Seppanen, C.M.; Song, Q.; Csallany, A.S. The antioxidant functions of tocopherol and tocotrienol homologues in oils, fats, and food systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Kourimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Van Hoed, V.; De Clercq, N.; Echim, C.; Andjelkovic, M.; Leber, E.; Dewettinck, K.; Verhé, R. Berry seeds: A source of specialty oils with high content of bioactives and nutritional value. J. Food Lipids 2009, 16, 33–49. [Google Scholar] [CrossRef]
- Takic, M.; Pokimica, B.; Petrovic-Oggiano, G.; Popovic, T. Effects of Dietary Linolenic Acid Treatment and the Efficiency of Its Conversion to Eicosapentaenoic and Docosahexaenoic Acids in Obesity and Related Diseases. Molecules 2022, 27, 4471. [Google Scholar] [CrossRef] [PubMed]
- Schunck, W.H.; Konkel, A.; Fischer, R.; Weylandt, K.H. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 2018, 183, 177–204. [Google Scholar] [CrossRef] [PubMed]
- Górska, A.; Piasecka, I.; Wirkowska-Wojdyła, M.; Brys, J.; Kienc, K.; Brzezinska, R.; Ostrowska-Ligeza, E. Berry Seeds. A By-Product of the Fruit Industry as a Source of Oils with Beneficial Nutritional Characteristics. Appl. Sci. 2023, 13, 5114. [Google Scholar] [CrossRef]
- Piasecka, I.; Górska, A.; Obranovic, M.; Kalisz, S.; Dobrincic, A.; Dobroslavic, E.; Ostrowska-Ligeza, E.; Brzezinska, R.; Dragović’s-Uzelac, V. The Quality Assessment of Oils Obtained from Berry Fruit Seeds Using Pressurized Liquid Extraction. Biol. Life Sci. Forum 2023, 26, 84. [Google Scholar] [CrossRef]
- Taborska, N.; Martyka, A.; Kubicka-Figiel, M.; Ujma, P. Essential fatty acid and analysis of their impact on the human body based on latest research. J. Educ. Health Sport 2024, 51, 166–178. [Google Scholar] [CrossRef]
- Serra, A.; Conte, G.; Ciucci, F.; Bulleri, E.; Corrales-Retana, L.; Cappucci, A.; Buccioni, A.; Mele, M. Dietary linseed supplementation affects the fatty acid composition of the sn-2 position of triglycerides in sheep milk. J. Dairy Sci. 2018, 101, 6742–6751. [Google Scholar] [CrossRef]
- Deng, M.; Chen, H.; Zhang, W.; Cahoon, E.B.; Zhou, Y.; Zhang, C. Genetic improvement of tocotrienol content enhances the oxidative stability of canola oil. Front. Plant Sci. 2023, 14, 1247781. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Alexander Iii, J.; Ward, T.; Del Tredici, P.; Nicholson, R. Phylogenetic relationships of Empetraceae inferred from sequences of chloroplast gene matK and nuclear ribosomal DNA ITS region. Mol. Phylogenet. Evol. 2002, 25, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, I.; Górska, A.; Ostrowska-Ligeza, E.; Kalisz, S. The Study of Thermal Properties of Blackberry, Chokeberry and Raspberry Seeds and Oils. Appl. Sci. 2021, 11, 7704. [Google Scholar] [CrossRef]
- Micić, D.M.; Ostojić, S.B.; Simonović, M.B.; Pezo, L.L.; Simonović, B.R. Thermal behavior of raspberry and blackberry seed flours and oils. Thermochim. Acta 2015, 617, 21–27. [Google Scholar] [CrossRef]
- Silva, T.J.; Barrera-Arellano, D.; Ribeiro, A.P.B. Margarines: Historical approach, technological aspects, nutritional profile, and global trends. Food Res. Int. 2021, 147, 110486. [Google Scholar] [CrossRef]
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.M. A rapid method of lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Nash, A.M.; Frankel, E.N. Limited extraction of soybean with hexane. J. Am. Oil Chem. Soc. 1986, 63, 244–246. [Google Scholar] [CrossRef]
- Garcés, R.; Mancha, M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 1993, 211, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 38, 985–992. [Google Scholar] [CrossRef]
- Martínez-Force, E.; Ruiz-López, N.; Garcés, R. Influence of specific fatty acids on the asymmetric distribution of saturated fatty acids in sunflower (Helianthus annuus L.) triacylglycerols. J. Agric. Food Chem. 2009, 57, 1595–1599. [Google Scholar] [CrossRef]
- Verdonck, E.; Schaap, K.; Thomas, L.C. A discussion of the principles and applications of Modulated Temperature DSC (MTDSC). Int. J. Pharm. 1999, 192, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Samyn, P.; Schoukens, G.; Vonck, L.; Stanssens, D.; Van den Abbeele, H. Quality of Brazilian vegetable oils evaluated by (modulated) differential scanning calorimetry. J. Therm. Anal. Calorim. 2012, 110, 1353–1365. [Google Scholar] [CrossRef]
- Ping Tan, C.; Nehdi, I.A. DSC Analysis of Vegetable Oils—Relationship Between Thermal Profiles and Chemical Composition. In Differential Scanning Calorimetry—Applications in Fats and Oils Technology; Chiavaro, E., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 3–26. [Google Scholar]

| H | HR | BD | |
|---|---|---|---|
| % Oil | 2.77 ± 0.40 | 2.06 ± 0.19 | 2.17 ± 0.19 |
| FA | H | HR | BD | H–HR–BD |
|---|---|---|---|---|
| 16:0 | 5.00 | 4.77 | 5.07 | 4.95 ± 0.16 |
| 18:0 | 1.80 | 2.15 | 2.22 | 2.06 ± 0.23 |
| 18:1 (9) | 13.04 | 12.08 | 11.66 | 12.26 ± 0.71 |
| 18:1 (11) | 0.48 | 0.36 | 0.32 | 0.39 ± 0.08 |
| 18:2 (n-6) | 32.54 | 35.64 | 34.51 | 34.23 ± 1.57 |
| 18:3 (n-3) | 47.12 | 44.99 | 45.54 | 45.88 ± 1.11 |
| 18:2 (n-6)/18:3 (n-3) | 0.69 | 0.79 | 0.76 | 0.75 ± 0.05 |
| SFA | 6.80 | 6.93 | 7.29 | 7.01 ± 0.25 |
| MUFA | 13.52 | 12.44 | 11.98 | 12.65 ± 0.79 |
| PUFA | 79.65 | 80.63 | 80.05 | 80.11 ± 0.49 |
| PUFA/SFA | 11.71 | 11.63 | 10.98 | 11.43 ± 1.94 |
| AI | 0.05 | 0.05 | 0.05 | 0.05 |
| TI | 0.04 | 0.04 | 0.04 | 0.04 |
| TAG Type | H | HR | BD | H–HR–BD |
|---|---|---|---|---|
| POP | 0.22 | 0.22 | 0.30 | 0.25 ± 0.05 |
| PLP | 0.30 | 0.22 | 0.30 | 0.62 ± 0.05 |
| PLnP | 0.27 | 0.22 | 0.30 | 0.26 ± 0.04 |
| POS | 0.18 | 0.22 | 0.29 | 0.23 ± 0.06 |
| POO | 0.57 | 0.22 | 0.29 | 0.36 ± 0.19 |
| PSL | 0.26 | 0.48 | 0.66 | 0.47 ± 0.20 |
| POL | 1.98 | 2.36 | 2.16 | 2.17 ± 0.19 |
| PLL + POLn | 4.78 | 5.75 | 4.84 | 5.12 ± 0.54 |
| PLLn + PLnLn | 6.00 | 6.63 | 5.22 | 5.95 ± 0.71 |
| SOO + SLS + SLnL | 2.12 | 3.61 | 3.48 | 3.07 ± 0.83 |
| OOO | 1.28 | 1.72 | 1.76 | 1.59 ± 0.27 |
| SOL | 0.84 | 1.49 | 0.00 | 0.78 ± 0.75 |
| OOL + SOLn | 2.48 | 2.92 | 2.74 | 2.71 ± 0.22 |
| SLL | 1.30 | 1.91 | 1.15 | 1.45 ± 0.40 |
| OLL + OOLn | 7.66 | 8.19 | 6.97 | 7.61 ± 0.61 |
| SLLn + SLnLn | 1.91 | 2.05 | 1.74 | 1.9 ± 0.16 |
| LLL + OLLn | 17.18 | 16.24 | 14.15 | 15.86 ± 1.55 |
| LLLn + OLnLn | 23.15 | 21.52 | 20.55 | 21.74 ± 1.31 |
| LLnLn | 18.7 | 15.96 | 16.98 | 17.21 ± 1.38 |
| LnLnLn | 8.82 | 8.1 | 9.78 | 8.9 ± 0.84 |
| FA | FA(Sn2) MAG % | FA (Sn1, 2, 3) TAG % | FA (Sn1, 3) DAG % |
|---|---|---|---|
| 16:0 | 3.31 | 9.49 | 12.58 |
| 18:0 | 5.76 | 4.99 | 4.61 |
| 18:1 (9) | 16.64 | 11.11 | 8.35 |
| 18:1 (11) | 0 | 0.6 | 0.9 |
| 18:2 | 35.18 | 30.13 | 27.61 |
| 18:3 | 39.10 | 43.67 | 45.96 |
| Tocopherols | Tocotrienols | ||||||
|---|---|---|---|---|---|---|---|
| 26.3% | 72.47% | ||||||
| γ + δ | α + β | γ + δ | α + β | ||||
| 13.59% | 12.71% | 69.73% | 2.74% | ||||
| α | β | γ | δ | α | β | γ | δ |
| 12.19% | 0.52% | 11.93% | 1.66% | 2.74% | - | 57.51% | 12.22% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Cordero, C.; Martinez-Force, E.; Acero de Mesa, N.; Muñoz-Mingarro, D.; León-González, A.J. Triacylglycerol Composition of Seed Oil from Corema album Berries. Molecules 2025, 30, 914. https://doi.org/10.3390/molecules30040914
Martín-Cordero C, Martinez-Force E, Acero de Mesa N, Muñoz-Mingarro D, León-González AJ. Triacylglycerol Composition of Seed Oil from Corema album Berries. Molecules. 2025; 30(4):914. https://doi.org/10.3390/molecules30040914
Chicago/Turabian StyleMartín-Cordero, Carmen, Enrique Martinez-Force, Nuria Acero de Mesa, Dolores Muñoz-Mingarro, and Antonio J. León-González. 2025. "Triacylglycerol Composition of Seed Oil from Corema album Berries" Molecules 30, no. 4: 914. https://doi.org/10.3390/molecules30040914
APA StyleMartín-Cordero, C., Martinez-Force, E., Acero de Mesa, N., Muñoz-Mingarro, D., & León-González, A. J. (2025). Triacylglycerol Composition of Seed Oil from Corema album Berries. Molecules, 30(4), 914. https://doi.org/10.3390/molecules30040914










