Synthesis, Photo-Physical Properties, and Electroluminescence Characteristics of Iridium Phosphorescent Materials Based on Different β-Diketonate Ancillary Ligands
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Thermal Properties
2.2. Crystalline Structures
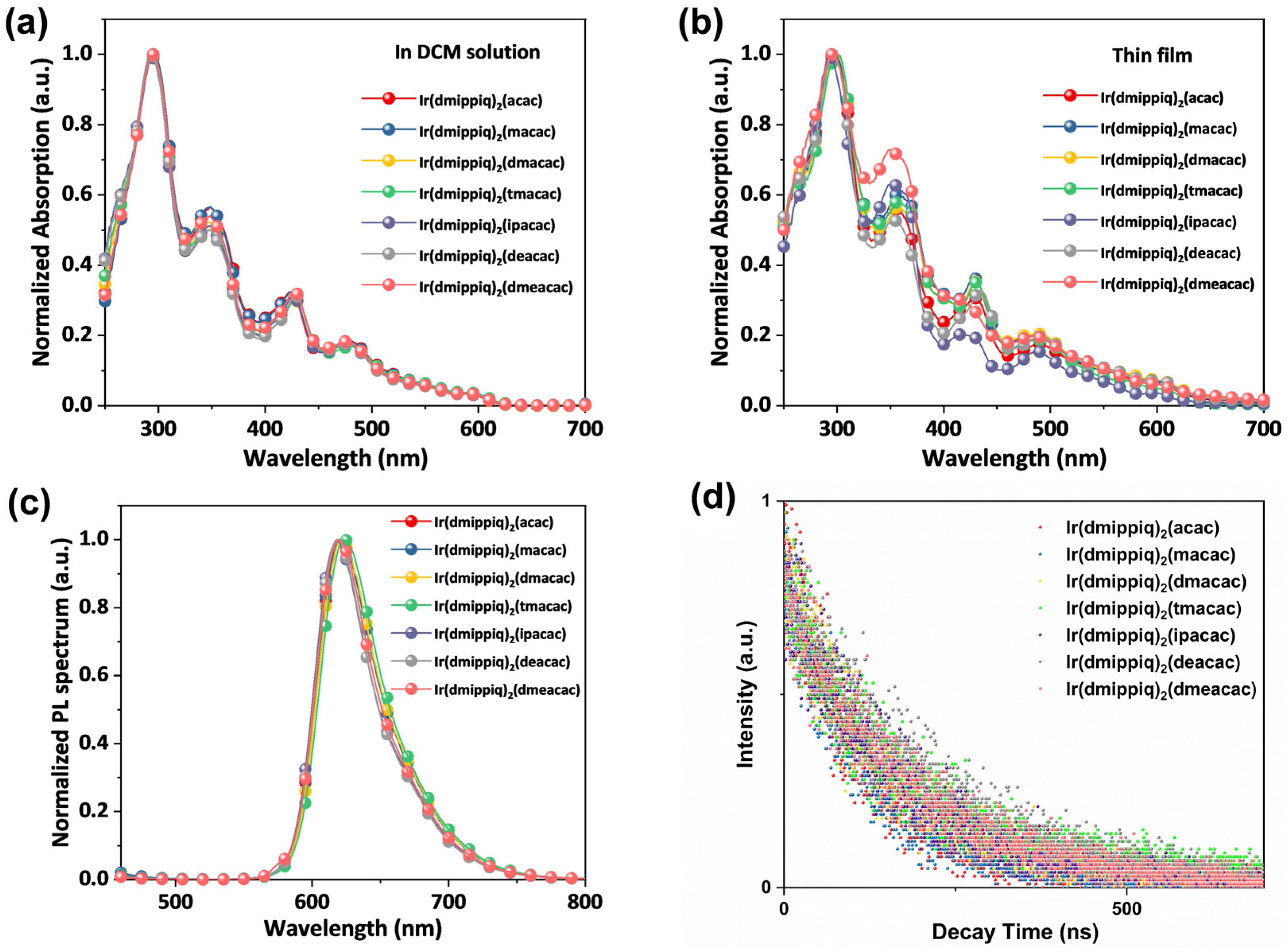
2.3. Optical and Electrochemical Properties
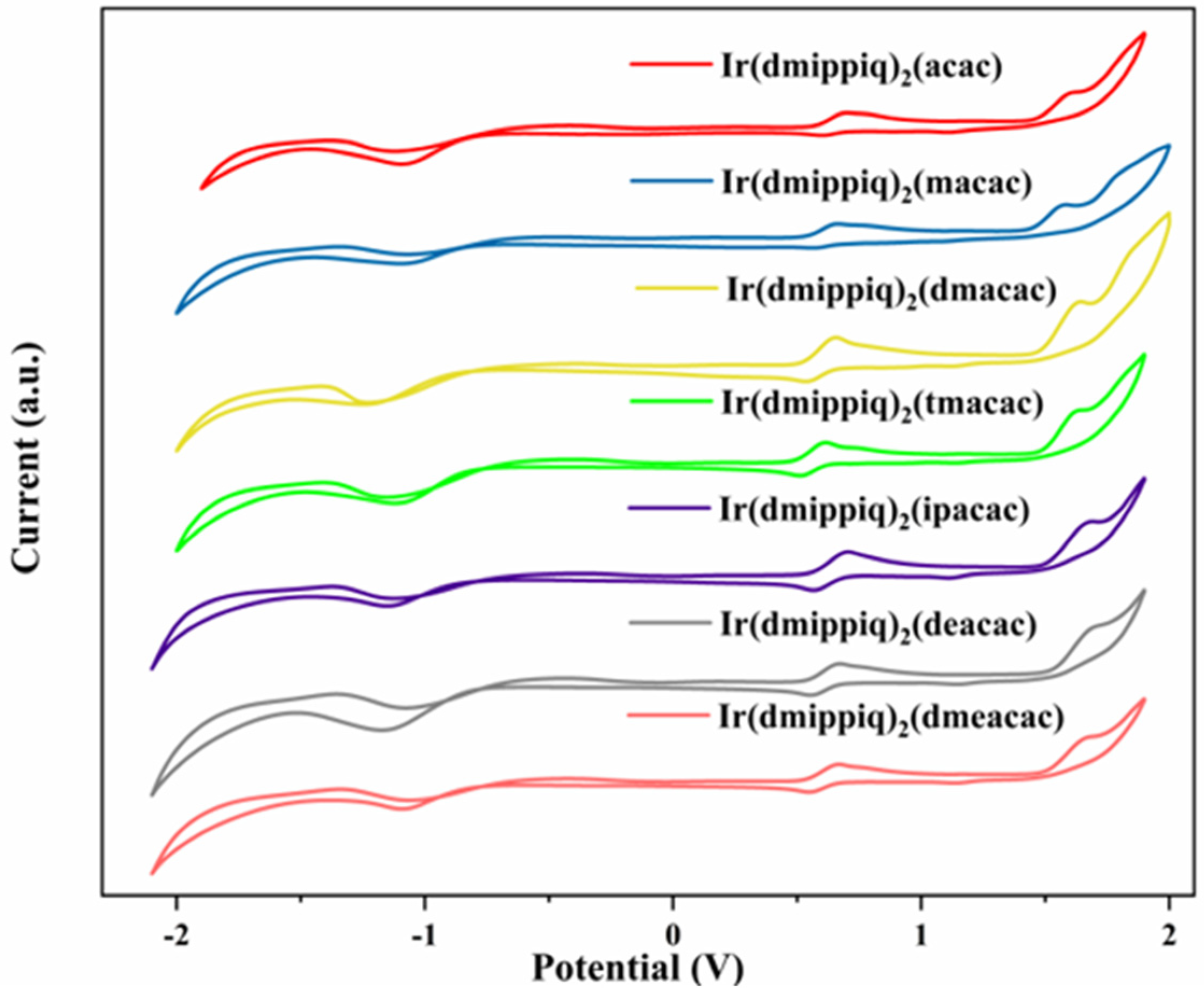
2.4. Electrochemical Properties
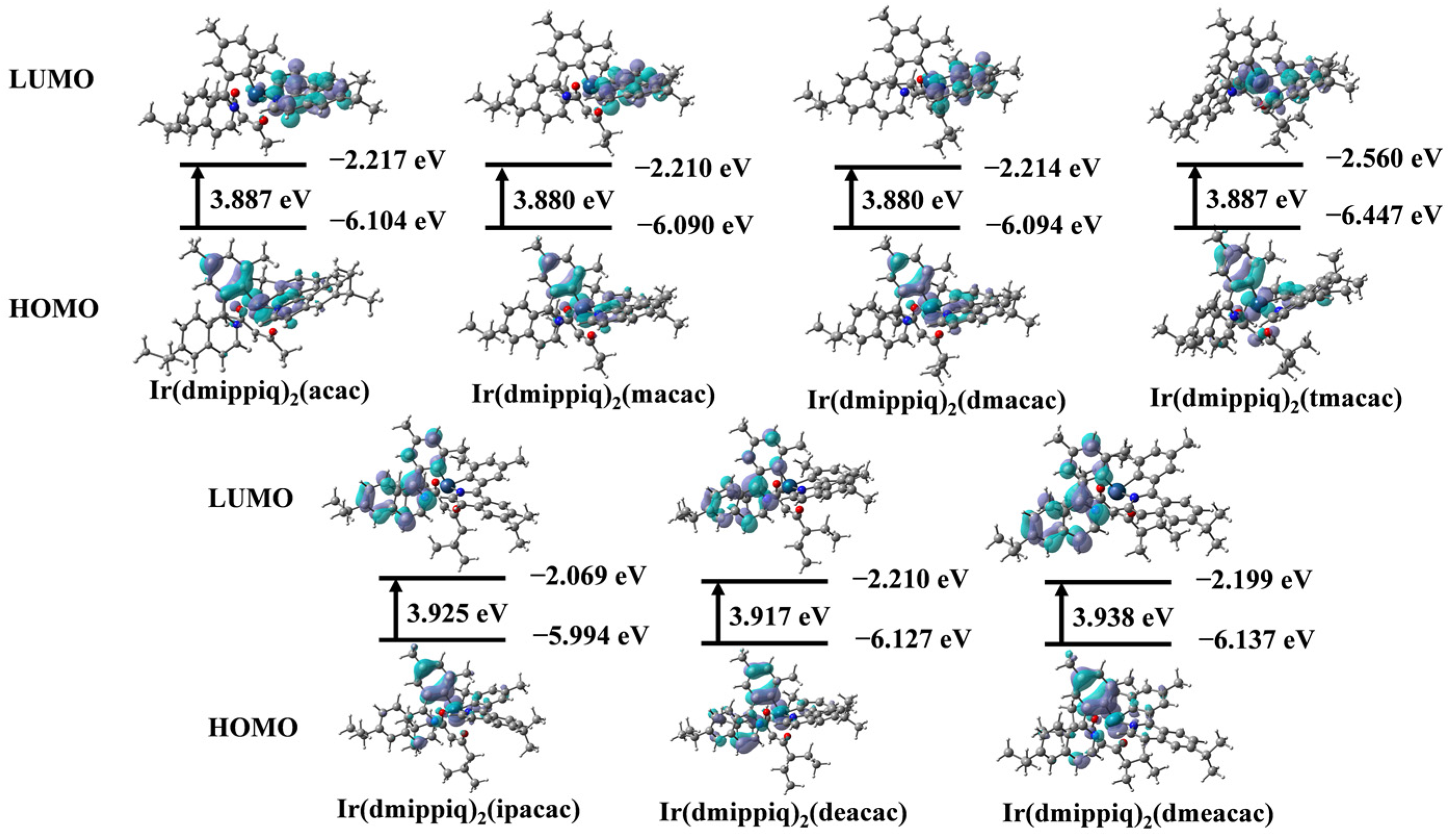
2.5. Theorectical Calculations
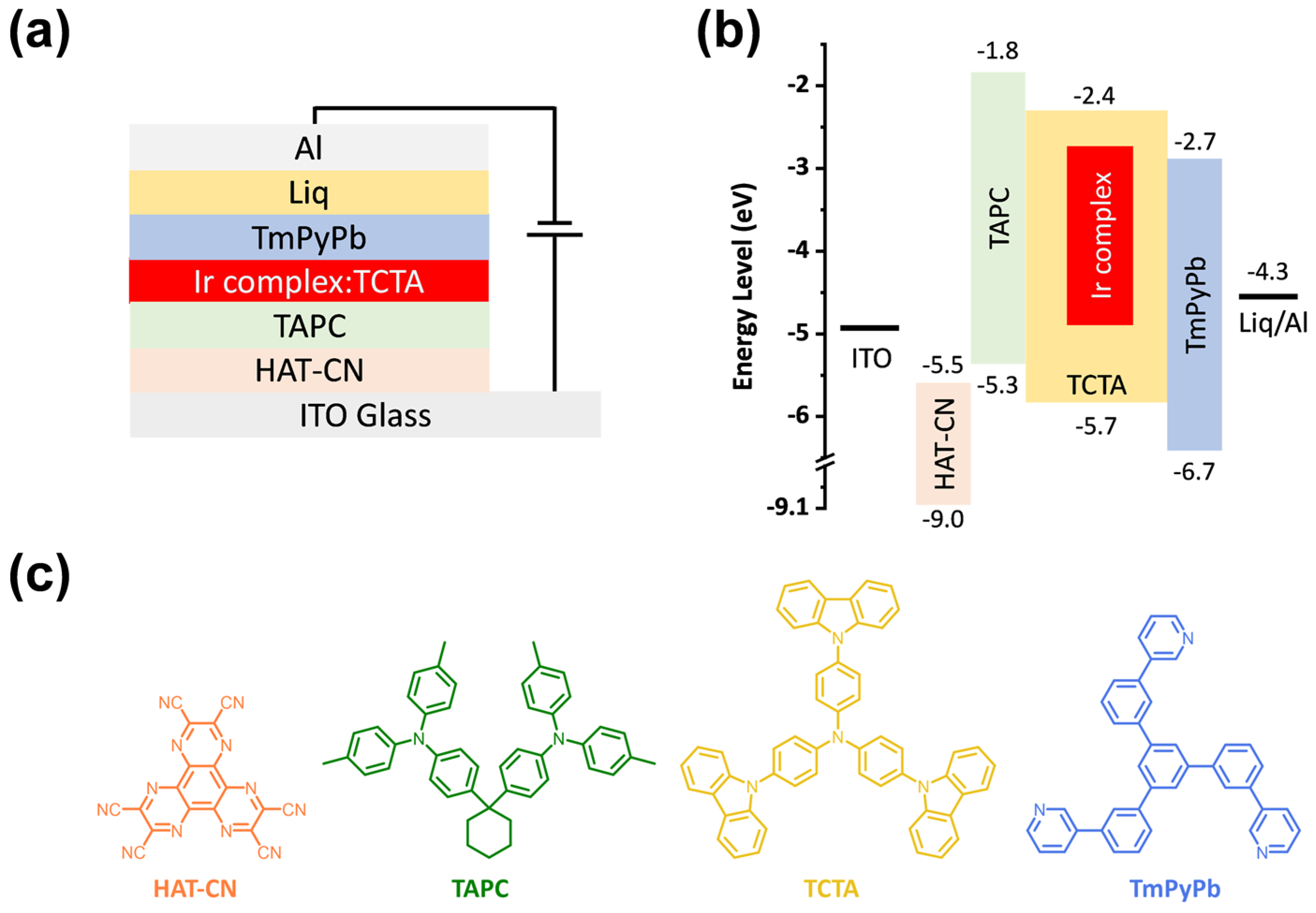
2.6. EL Device Performance
3. Materials and Methods
3.1. General Information
3.2. Device Fabrication and Measurement
3.3. Compound Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Brase, S. A Brief History of OLEDs-Emitter Development and Industry Milestones. Adv. Mater. 2021, 33, e2005630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, T.; Duan, L. Emerging Self-Emissive Technologies for Flexible Displays. Adv. Mater. 2020, 32, e1902391. [Google Scholar] [CrossRef] [PubMed]
- Kamtekar, K.T.; Monkman, A.P.; Bryce, M.R. Recent Advances in White Organic Light-Emitting Materials and Devices (WOLEDs). Adv. Mater. 2010, 22, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Yang, C. Yellow/Orange Emissive Heavy-Metal Complexes as Phosphors in Monochromatic and White Organic Light-Emitting Devices. Chem. Soc. Rev. 2014, 43, 6439–6469. [Google Scholar] [CrossRef] [PubMed]
- Sasabe, H.; Kido, J. Development of High Performance OLEDs for General Lighting. J. Mater. Chem. C. 2013, 1, 1699–1707. [Google Scholar] [CrossRef]
- Reineke, S.; Lindner, F.; Schwartz, G.; Seidler, N.; Walzer, K.; Lussem, B.; Leo, K. White Organic Light-Emitting Diodes with Fluorescent Tube Efficiency. Nature 2009, 459, 234–238. [Google Scholar] [CrossRef]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly Efficient Phosphorescent Emission from Organic Electroluminescent Devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Baldo, M.A.; Thompson, M.E.; Forrest, S.R. High-Efficiency Fluorescent Organic Light-Emitting Devices Using a Phosphorescent Sensitizer. Nature 2000, 403, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Adachi, C.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Nearly 100% Internal Phosphorescence Efficiency in an Organic Light-Emitting Device. J. Appl. Phys. 2001, 90, 5048–5051. [Google Scholar] [CrossRef]
- Chou, P.-T.; Chi, Y.; Chung, M.-W.; Lin, C.-C. Harvesting Luminescence via Harnessing the Photophysical Properties of Transition Metal Complexes. Coord. Chem. Rev. 2011, 255, 2653–2665. [Google Scholar] [CrossRef]
- Xiang, H.; Cheng, J.; Ma, X.; Zhou, X.; Chruma, J.J. Near-Infrared Phosphorescence: Materials and Applications. Chem. Soc. Rev. 2013, 42, 6128–6185. [Google Scholar] [CrossRef]
- You, Y.; Park, S.Y. Phosphorescent Iridium(III) Complexes: Toward High Phosphorescence Quantum Efficiency Through Ligand Control. Dalton. Trans. 2009, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.-H.; Kumar, S.; Agrawal, A.; Li, T.-H.; Sahoo, S. Approaches for Fabricating High Efficiency Organic Light Emitting Diodes. J. Mater. Chem. C 2015, 3, 2974–3002. [Google Scholar] [CrossRef]
- Minaev, B.; Baryshnikov, G.; Agren, H. Principles of Phosphorescent Organic Light Emitting Devices. Phys. Chem. Chem. Phys. 2014, 16, 1719–1758. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Wu, J.; Wu, Z.G.; Zheng, Y.X.; Zuo, J.L.; Pan, Y. Rational Design of Phosphorescent Iridium(III) Complexes for Emission Color Tunability and Their Applications in OLEDs. Coord. Chem. Rev. 2018, 374, 55–92. [Google Scholar] [CrossRef]
- Tatarin, S.T.; Krasnov, L.V.; Nykhrikova, E.V.; Minin, M.M.; Smirnov, D.E.; Churakov, V.C.; Bezzubov, S.I. Predicting Emission Wavelengths and Quantum Yields of Diverse Bis-cyclometalated Iridium(III) Complexes Using Machine Learning. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Chang, Q.W.; Chen, Z.A.; Wang, Z.A.; Jiang, J.; Yu, J.; Liu, W.P.; Yan, C.X.; Chen, L. Iridium Phosphorescent Complexes Based on Modified Phenylquinoline Ligand and Their High-efficiency Pure Red Organic Electroluminescent Device. Chin. J. Lumin. 2022, 43, 1583–1591. [Google Scholar] [CrossRef]
- Chang, Q.W.; Chen, Z.A.; Feng, L.; Jiang, W.; Yan, C.X.; Liu, W.P.; Bai, F.Q. Synthesis and photophysical properties of phenylquinoline iridium complexes controlled by electron-withdrawing groups. Chin. J. Inorg. Chem. 2023, 39, 255–262. [Google Scholar] [CrossRef]
- Jiang, W.; Hou, W.M.; Yan, C.X.; Nie, Z.F.; Chang, Q.W.; Li, X.G.; Liu, W.P. Synthesis and Performance of Deep-Red Phosphorescent Iridium Complexes with Pyrone as an Auxiliary Ligand. Molecules 2024, 29, 3183. [Google Scholar] [CrossRef]
- Ulbricht, C.; Beyer, B.; Friebe, C.; Winter, A.; Schubert, U.S. Recent Developments in the Application of Phosphorescent Iridium(III) Complex Systems. Adv. Mater. 2009, 21, 4418–4441. [Google Scholar] [CrossRef]
- Chi, Y.; Chou, P.T. Transition-Metal Phosphors with Cyclometalating Ligands: Fundamentals and Applications. Chem. Soc. Rev. 2010, 39, 638–655. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Nam, W. Photofunctional Triplet Excited States of Cyclometalated Ir(III) Complexes: Beyond Electroluminescence. Chem. Soc. Rev. 2012, 41, 7061–7084. [Google Scholar] [CrossRef]
- Liao, Z.J.; Zhu, T.J.; Mi, B.X.; Gao, Z.Q.; Fan, Q.L.; Huang, W. Molecular Iridium (III) Complexes and Their Corresponding Electrophosphorescent Devices. Prog. Chem. 2011, 23, 1627–1643. [Google Scholar]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Lee, H.E.; Adachi, C.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. Highly Phosphorescent Bis-Cyclometalated Iridium Complexes: Synthesis, Photophysical Characterization, and Use in Organic Light Emitting Diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Park, S.Y. Inter-Ligand Energy Transfer and Related Emission Change in the Cyclometalated Heteroleptic Iridium Complex: Facile and Efficient Color Tuning over the Whole Visible Range by the Ancillary Ligand Structure. J. Am. Chem. Soc. 2005, 127, 12438–12439. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, Q.; Yang, C.; Zhang, K.; Wang, Q.; Zou, T.; Qin, J.; Ma, D. Solution-Processable Highly Efficient Yellow- and Red-Emitting Phosphorescent Organic Light Emitting Devices from a Small Molecule Bipolar Host and Iridium Complexes. J. Mater. Chem. 2008, 18, 4091–4096. [Google Scholar] [CrossRef]
- Kwon, T.H.; Cho, H.S.; Kim, M.K.; Kim, J.W.; Kim, J.J.; Lee, K.H.; Park, S.J.; Shin, I.S.; Kim, H.; Shin, D.M.; et al. Color Tuning of Cyclometalated Iridium Complexes through Modification of Phenylpyrazole Derivatives and Ancillary Ligand Based on ab Initio Calculations. Organometallics 2005, 24, 1578–1585. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, N.S.; Oh, H.Y.; Yang, J.H.; Jeon, W.S.; Park, J.S.; Suh, M.C.; Kwon, J.H. Highly Efficient Red Phosphorescent Dopants in Organic Light-Emitting Devices. Adv. Mater. 2011, 23, 2721–2726. [Google Scholar] [CrossRef]
- Duan, J.P.; Sun, P.P.; Cheng, C.H. New Iridium Complexes as Highly Efficient Orange–Red Emitters in Organic Light-Emitting Diodes. Adv. Mater. 2003, 15, 224–228. [Google Scholar] [CrossRef]
- Xia, R.; Zhang, Z.; Guo, J.; Dou, D.; Wang, K.; Cao, J.; Wang, Z. Tuning the Position of Methyl Groups to Realize Highly Efficient Deep-Red PhOLEDs. Dyes Pigment. 2021, 195, 109738. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.; Moon, C.K.; Kim, S.Y.; Park, Y.S.; Lee, J.H.; Woo Lee, J.; Huh, J.; You, Y.; Kim, J.J. Phosphorescent Dye-Based Supramolecules for High-Efficiency Organic Light-Emitting Diodes. Nat. Commun. 2014, 5, 4769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Ho, C.-L.; Wong, W.-Y.; Wang, Q.; Ma, D.; Wang, L.; Lin, Z.; Marder, T.B.; Beeby, A. Manipulating Charge-Transfer Character with Electron-Withdrawing Main-Group Moieties for the Color Tuning of Iridium Electrophosphors. Adv. Funct. Mater. 2008, 18, 499–511. [Google Scholar] [CrossRef]
- Tao, R.; Qiao, J.; Zhang, G.; Duan, L.; Chen, C.; Wang, L.; Qiu, Y. High-Efficiency near-Infrared Organic Light-Emitting Devices Based on an Iridium Complex with Negligible Efficiency Roll-off. J. Mater. Chem. C 2013, 1, 6446–6454. [Google Scholar] [CrossRef]
- Yang, X.; Guo, H.; Liu, B.; Zhao, J.; Zhou, G.; Wu, Z.; Wong, W.Y. Diarylboron-Based Asymmetric Red-Emitting Ir(III) Complex for Solution-Processed Phosphorescent Organic Light-Emitting Diode with External Quantum Efficiency above 28%. Adv. Sci. 2018, 5, 1701067. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.N.; Teets, T.S. Ancillary Ligand Effects on Red-Emitting Cyclometalated Iridium Complexes. Chem. Eur. J. 2019, 25, 6026–6037. [Google Scholar] [CrossRef] [PubMed]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Kwong, R.; Tsyba, I.; Bortz, M.; Mui, B.; Bau, R.; Thompson, M.E. Synthesis and Characterization of Phosphorescent Cyclometalated Iridium Complexes. Inorg. Chem. 2001, 40, 1704–1711. [Google Scholar] [CrossRef]
- Carlson, G.A.; Djurovich, P.I.; Watts, R.J. Widely Varying Photophysical Properties of Ligand-Nitrated Bis(μ-Chloro)Tetrakis(2-Phenylpyridinato)Diiridium(III). Inorg. Chem. 2002, 32, 4483–4484. [Google Scholar] [CrossRef]
- Colombo, M.G.; Brunold, T.C.; Riedener, T.; Guedel, H.U.; Fortsch, M.; Buergi, H.-B. Facial Tris Cyclometalated Rhodium(3+) And Iridium(3+) Complexes: Their Synthesis, Structure, and Optical Spectroscopic Properties. Inorg. Chem. 2002, 33, 545–550. [Google Scholar] [CrossRef]
- Garces, F.O.; King, K.A.; Watts, R.J. Synthesis, Structure, Electrochemistry, and Photophysics of Methyl-Substituted Phenylpyridine Ortho-Metalated Iridium(III) Complexes. Inorg. Chem. 2002, 27, 3464–3471. [Google Scholar] [CrossRef]


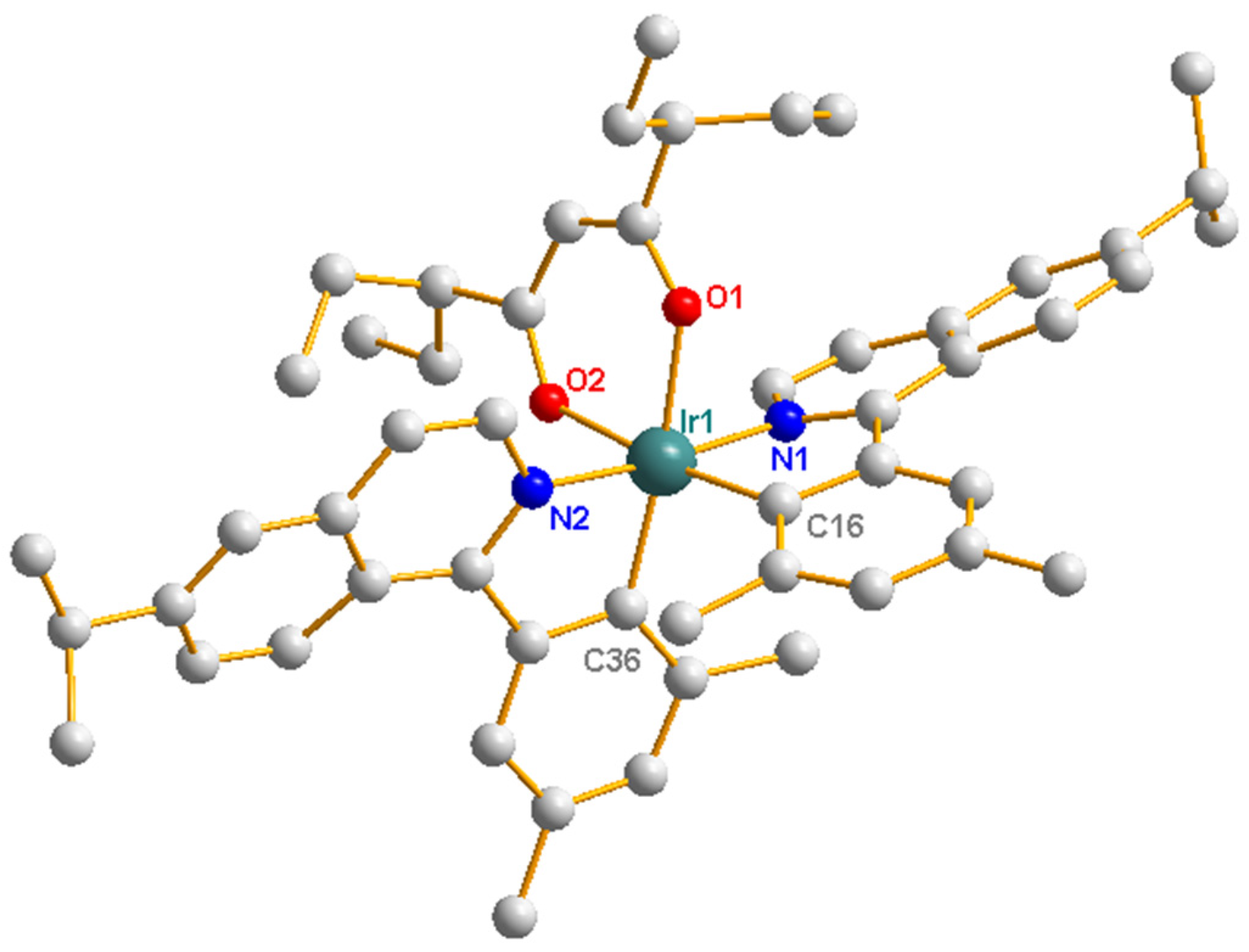

| Complexes | Ir(dmppiq)2(dpacac) |
|---|---|
| Formula | C53H63IrN2O2 |
| Formula weight | 952.25 |
| Crystal system | monoclinic |
| Space group | P121/c1 |
| a [Å] | 15.9566(13) |
| b [Å] | 14.4918(12) |
| c [Å] | 40.128(3) |
| α [°] | 90° |
| β [°] | 90.850(2)° |
| γ [°] | 90° |
| V [Å3] | 9278.2(13) |
| Z | 8 |
| ρcalc [g cm−3] | 1.363 |
| Reflections collected | 90,736 |
| Independent reflections | 15,975 |
| R, WR2(all) | 0.0943, 0.1711 |
| R, WR2 [I > 2σ(I)] | 0.0599, 0.1546 |
| GOF | 1.078 |
| Compound | Ir(dmppiq)2(dpacac) | ||
|---|---|---|---|
| Ir(1)-O(1) | 2.128(5) | Ir(1)-O(2) | 2.130(5) |
| Ir(1)-N(1) | 2.032(6) | Ir(1)-N(2) | 2.044(6) |
| Ir(1)-C(16) | 2.007(7) | Ir(1)-C(36) | 2.006(8) |
| O(1)-Ir(1)-O(2) | 88.6(2) | C(16)-Ir(1)-N(1) | 80.3(3) |
| C(36)-Ir(1)-N(2) | 80.1(3) | N(1)-Ir(1)-N(2) | 174.1(2) |
| C(16)-Ir(1)-O(1) | 174.5(3) | C(36)-Ir(1)-O(2) | 175.2(3) |
| Compound | λabs a (nm) | λex a (nm) | λPL a (nm) | ФPL a | τ b (ns) | kr b (×106 s−1) | knr b (×106 s−1) | Td (°C) |
|---|---|---|---|---|---|---|---|---|
| Ir(dmippiq)2(acac) | 295(6.86), 348(3.56), 424(2.16), 479(1.32) | 281 | 620 | 0.74 | 111.32 | 6.65 | 2.34 | 399 |
| Ir(dmippiq)2(macac) | 296(7.41), 349(3.67), 425(1.96), 477(1.21) | 285 | 620 | 0.75 | 117.48 | 6.38 | 2.13 | 357 |
| Ir(dmipiq)2(dmacac) | 295(8.23), 347(3.96), 426(2.40), 478(1.35) | 273 | 621 | 0.68 | 144.97 | 4.69 | 2.21 | 364 |
| Ir(dmippiq)2(tmacac) | 294(6.58), 348(3.14), 429(1.87), 480(1.04) | 281 | 623 | 0.72 | 209.05 | 3.44 | 1.34 | 355 |
| Ir(dmippiq)2(ipacac) | 294(7.36), 348(3.05), 426(2.20), 478(1.25) | 277 | 619 | 0.70 | 157.04 | 4.46 | 1.91 | 377 |
| Ir(dmippiq)2(deacac) | 294(7.23), 348(3.45), 428(2.16), 478(1.24) | 277 | 618 | 0.66 | 198.55 | 3.32 | 1.71 | 343 |
| Ir(dmipiq)2(dmeacac) | 295(7.63), 348(3.79), 427(2.41), 478(1.44) | 287 | 619 | 0.72 | 164.53 | 4.38 | 1.70 | 333 |
| Compound | Eox(Ir3+/Ir4+) (V) | Eg a (eV) | HOMO b (eV) | LUMO b (eV) |
|---|---|---|---|---|
| Ir(dmippiq)2(acac) | 0.55 | 1.99 | −4.95 | −2.96 |
| Ir(dmippiq)2(macac) | 0.52 | 2.01 | −4.92 | −2.91 |
| Ir(dmippiq)2(dmacac) | 0.51 | 1.98 | −4.91 | −2.93 |
| Ir(dmippiq)2(tmacac) | 0.48 | 1.99 | −4.88 | −2.89 |
| Ir(dmippiq)2(ipacac) | 0.55 | 1.99 | −4.95 | −2.96 |
| Ir(dmippiq)2(deacac) | 0.52 | 1.99 | −4.92 | −2.93 |
| Ir(dmippiq)2(dmeacac) | 0.51 | 1.98 | −4.91 | −2.93 |
| HIC | Doping Concentration | Lmax a (cd/m2) | CEmax b (cd/A) | PEmax c (lm/W) | EQE d (%) | VD (V) @ 1, 100, 1000 cd/m2 | EL Peak e (nm) | CIE (x, y) |
|---|---|---|---|---|---|---|---|---|
| Ir(dmippiq)2(acac) | 12% | 4651 | 7.49 | 5.30 | 14.80 | 4.3, 7.7, 10.2 | 624 | (0.68, 0.32) |
| Ir(dmippiq)2(macac) | 10% | 7026 | 7.55 | 3.95 | 13.42 | 5.9, 7.9, 9.6 | 624 | (0.68, 0.32) |
| Ir(dmippiq)2(dmacac) | 10% | 8355 | 7.71 | 4.03 | 13.31 | 5.8, 7.8, 9.5 | 624 | (0.68, 0.32) |
| Ir(dmippiq)2(tmacac) | 12% | 9973 | 10.89 | 8.77 | 13.45 | 2.7, 3.9, 5.7 | 625 | (0.68, 0.32) |
| Ir(dmippiq)2(ipacac) | 10% | 9288 | 11.03 | 8.89 | 13.29 | 2.7, 3.8, 5.7 | 626 | (0.68, 0.32) |
| Ir(dmippiq)2(deacac) | 10% | 5024 | 11.77 | 9.48 | 13.49 | 2.9, 5.3, 8.4 | 624 | (0.68, 0.32) |
| Ir(dmippiq)2(dmeacac) | 16% | 13,960 | 15.63 | 12.60 | 18.26 | 2.6, 3.6, 5.4 | 625 | (0.68, 0.32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Q.; Zhang, K.; Yan, C.; Xie, L.; Yi, Y.; Su, W.; Liu, W. Synthesis, Photo-Physical Properties, and Electroluminescence Characteristics of Iridium Phosphorescent Materials Based on Different β-Diketonate Ancillary Ligands. Molecules 2025, 30, 861. https://doi.org/10.3390/molecules30040861
Chang Q, Zhang K, Yan C, Xie L, Yi Y, Su W, Liu W. Synthesis, Photo-Physical Properties, and Electroluminescence Characteristics of Iridium Phosphorescent Materials Based on Different β-Diketonate Ancillary Ligands. Molecules. 2025; 30(4):861. https://doi.org/10.3390/molecules30040861
Chicago/Turabian StyleChang, Qiaowen, Ke Zhang, Caixian Yan, Liming Xie, Yuanqiuqiang Yi, Wenming Su, and Weiping Liu. 2025. "Synthesis, Photo-Physical Properties, and Electroluminescence Characteristics of Iridium Phosphorescent Materials Based on Different β-Diketonate Ancillary Ligands" Molecules 30, no. 4: 861. https://doi.org/10.3390/molecules30040861
APA StyleChang, Q., Zhang, K., Yan, C., Xie, L., Yi, Y., Su, W., & Liu, W. (2025). Synthesis, Photo-Physical Properties, and Electroluminescence Characteristics of Iridium Phosphorescent Materials Based on Different β-Diketonate Ancillary Ligands. Molecules, 30(4), 861. https://doi.org/10.3390/molecules30040861






