Exploring the Effects of Sweet Potato Leaves on Skin Health—From Antimicrobial to Immunomodulator
Abstract
1. Introduction
2. Results
2.1. Phenolic Compounds Fingerprint by uHPLC-DAD
2.2. Phenolic Compounds Identification by LC–MS
2.3. MIC and MBC Determination
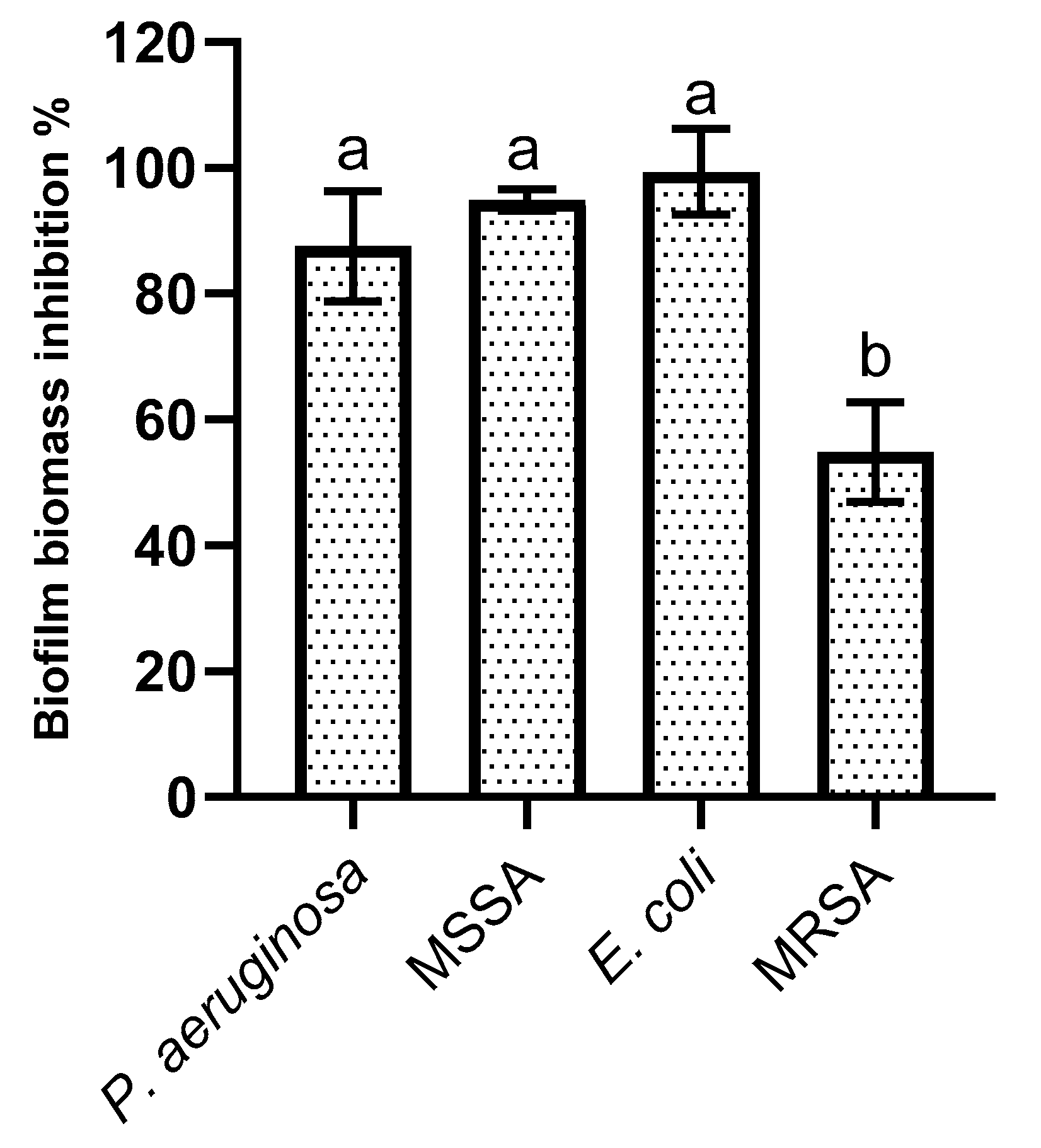
2.4. Biofilm Formation Inhibition
2.5. SPL Extract Impact on HaCat Cells
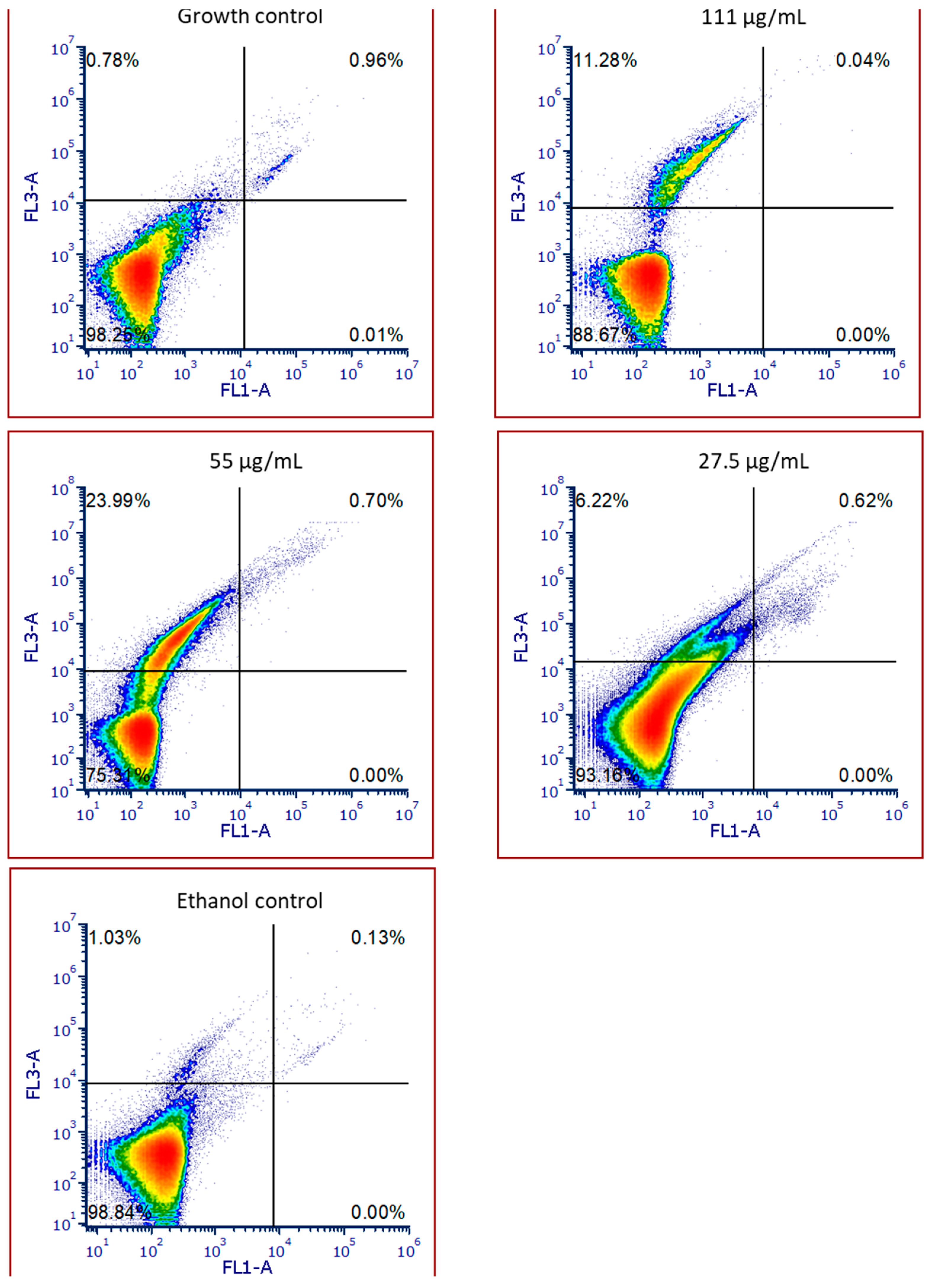
2.6. SPL Extract Impact on HaCat Cell Viability
2.7. IL-6 Production by HaCat Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Raw Materials
4.2. Compound Extraction and Characterization
4.2.1. Extraction
4.2.2. Phenolic Compounds Fingerprint by uHPLC-DAD
4.2.3. High Resolution LC–MS
4.2.4. Data Analysis
4.3. Bacterial Strains and Cell Lines
4.4. MIC and MBC Determination
4.5. Antibiofilm Activity
4.6. Cytotoxicity Evaluation
4.6.1. Cellular Metabolism
4.6.2. Cellular Proliferation
4.6.3. Cell Viability by Flow Cytometry
4.7. Inflammatory Potential
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| AUC | Area Under the Curve |
| CAE | Chlorogenic Acid Equivalents |
| E. coli | Escherichia coli |
| EtOH | Ethanol |
| GNPS | Global Natural Products Social Molecular Networking |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| LC–MS | Liquid Chromatography–Mass Spectrometry |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| LPS | Lipopolysaccharide |
| MBC | Minimal Bactericidal Concentration |
| MCF-10A | Human Mammary Epithelial Cells |
| MHB | Muller Hinton Broth |
| MIC | Minimal Inhibitory Concentration |
| MRSA | Methicillin Resistant Staphylococcus aureus |
| MSSA | Methicillin Sensitive Staphylococcus aureus |
| NCTC | National Collection of Type Cultures |
| P. aeruginosa | Pseudomonas aeruginosa |
| PCA | Plate Count Agar |
| RT | Retention Time |
| RP-uHPLC-DAD | Reverse-Phase Ultra-High-Performance Liquid Chromatography with Diode Array Detection |
| SPL | Sweet Potato Leaves |
| TFC | Total Flavonoid Content |
| TPC | Total Phenolic Content |
| TNF-α | Tumor Necrosis Factor Alpha |
| uHPLC-DAD | Ultra-High Performance Liquid Chromatography with Diode Array Detection |
| UHR−QqTOF | Ultra-High-Resolution Quadrupole−Quadrupole Time of Flight |
| TLA | Three Letter Acronym |
| LD | Linear Dichroism |
References
- Machado, M.; Silva, S.; Costa, E.M. Are Antimicrobial Peptides a 21st-Century Solution for Atopic Dermatitis? Int. J. Mol. Sci. 2023, 24, 13460. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W. Air pollution and skin disorders. Int. J. Women’s Dermatol. 2021, 7, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Silva, S.; Costa, E.M. Byproducts as a Sustainable Source of Cosmetic Ingredients. Appl. Sci. 2024, 14, 10241. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Chen, C.-C.; Lin, K.-H.; Chao, P.-Y.; Lin, H.-H.; Huang, M.-Y. Bioactive Compounds, Antioxidants, and Health Benefits of Sweet Potato Leaves. Molecules 2021, 26, 1820. [Google Scholar] [CrossRef]
- Yi, J.; Li, L.; Li, X.; Duan, X.; Wang, J.; Han, Y.; Gao, Y. Valorisation of sweet potato leaves as a potential agri-food resource: Assessing nutritional and nutraceutical values altered by food processing—A review. Int. J. Food Sci. Technol. 2024, 59, 8825–8836. [Google Scholar] [CrossRef]
- Zhang, Z.-c.; Zhou, Q.; Yang, Y.; Wang, Y.; Zhang, J.-l. Highly acylated anthocyanins from purple sweet potato (Ipomoea batatas L.) alleviate hyperuricemia and kidney inflammation in hyperuricemic mice: Possible attenuation effects on allopurinol. J. Agric. Food Chem. 2019, 67, 6202–6211. [Google Scholar] [CrossRef] [PubMed]
- Solihah, I.; Herlina, H.; Munirah, E.; Haryanti, H.; Amalia, M.; Rasyid, R.S.P.; Suciati, T.; Fatma, F. The hypoglycemic effect of purple sweet potato leaf fractions in diabetic rats. J. Adv. Pharm. Educ. Res. 2023, 13, 64–72. [Google Scholar] [CrossRef]
- Kang, H.; Lee, S.-G. Protective effect of purple sweet potato leaf (Ipomoea batatas Linn Convolvulaceae) against alcohol-induced liver damage in mice. Trop. J. Pharm. Res. 2021, 20, 301–308. [Google Scholar] [CrossRef]
- Xi, L.; Mu, T.; Sun, H. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Krochmal-Marczak, B.; Cebulak, T.; Kapusta, I.; Oszmiański, J.; Kaszuba, J.; Żurek, N. The content of phenolic acids and flavonols in the leaves of nine varieties of sweet potatoes (Ipomoea batatas L.) depending on their development, grown in Central Europe. Molecules 2020, 25, 3473. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef]
- Suárez, S.; Mu, T.; Sun, H.; Añón, M.C. Antioxidant activity, nutritional, and phenolic composition of sweet potato leaves as affected by harvesting period. Int. J. Food Prop. 2020, 23, 178–188. [Google Scholar] [CrossRef]
- Padda, M.S.; Picha, D. Antioxidant activity and phenolic composition in ‘Beauregard’sweetpotato are affected by root size and leaf age. J. Am. Soc. Hortic. Sci. 2007, 132, 447–451. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, D.; Wu, L.; Zhang, J.; Li, X.; Wu, W. Chemical Characterization and Antioxidant Properties of Ethanolic Extract and Its Fractions from Sweet Potato (Ipomoea batatas L.) Leaves. Foods 2020, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-f.; Tu, Z.-c.; Zhang, L.; Wang, H.; Wen, Q.-h.; Huang, T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Luo, D.; Mu, T.; Sun, H. Profiling of phenolic acids and flavonoids in sweet potato (Ipomoea batatas L.) leaves and evaluation of their anti-oxidant and hypoglycemic activities. Food Biosci. 2021, 39, 100801. [Google Scholar] [CrossRef]
- Sultana, T.; Islam, S.; Azad, M.A.K.; Akanda, M.J.H.; Rahman, A.; Rahman, M.S. Phytochemical Profiling and Antimicrobial Properties of Various Sweet Potato (Ipomoea batatas L.) Leaves Assessed by RP-HPLC-DAD. Foods 2024, 13, 2787. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. Aqueous-Methanol Extracts of Orange-Fleshed Sweet Potato (Ipomoea batatas) Ameliorate Oxidative Stress and Modulate Type 2 Diabetes Associated Genes in Insulin Resistant C2C12 Cells. Molecules 2018, 23, 2058. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yue, Z.; Guo, M.; Fang, L.; Bai, L.; Li, X.; Tao, Y.; Wang, S.; Liu, Q.; Zhi, D. Dietary flavonoid hyperoside induces apoptosis of activated human LX-2 hepatic stellate cell by suppressing canonical NF-κB signaling. BioMed Res. Int. 2016, 2016, 1068528. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kaur, J.; Goyal, J.; Vaid, L.; Singh, T.G.; Singh, R.; Devi, S. Multifaceted Role of Tiliroside in Inflammatory Pathways: Mechanisms and Prospects. Recent Adv. Inflamm. Allergy Drug Discov. 2024. [Google Scholar] [CrossRef]
- Pochapski, M.T.; Fosquiera, E.C.; Esmerino, L.A.; Dos Santos, E.B.; Farago, P.V.; Santos, F.A.; Groppo, F.C. Phytochemical screening, antioxidant, and antimicrobial activities of the crude leaves’ extract from Ipomoea batatas (L.) Lam. Pharmacogn. Mag. 2011, 7, 165. [Google Scholar]
- Mbaeyi-Nwaoha, I.; Emejulu, V. Evaluation of phytochemical composition and antimicrobial activity of sweet potato (Ipomoea batatas) leaf. Pak. J. Nutr. 2013, 12, 575–586. [Google Scholar] [CrossRef]
- Sheikhy, M.; Karbasizade, V.; Ghanadian, M.; Fazeli, H. Evaluation of chlorogenic acid and carnosol for anti-efflux pump and anti-biofilm activities against extensively drug-resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa. Microbiol. Spectr. 2024, 12, e03934-23. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, Y.; Fu, S.; Li, Z.; Zhang, J.; Xu, Y.; Han, X.; Miao, J. Application of Chlorogenic acid as a substitute for antibiotics in Multidrug-resistant Escherichia coli-induced mastitis. Int. Immunopharmacol. 2023, 114, 109536. [Google Scholar] [CrossRef]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Horta, B.; Calhau, C.; Morais, R.M.; Pintado, M.M. Anti-biofilm potential of phenolic acids: The influence of environmental pH and intrinsic physico-chemical properties. Biofouling 2016, 32, 853–860. [Google Scholar] [CrossRef]
- Liao, W.C.; Lai, Y.-C.; Yuan, M.-C.; Hsu, Y.-L.; Chan, C.-F. Antioxidative activity of water extract of sweet potato leaves in Taiwan. Food Chem. 2011, 127, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, V.R.; Renjith, R.S.; Mukherjee, A.; Anil, S.R.; Sreekumar, J.; Jyothi, A.N. Comparative Study on the Chemical Structure and In Vitro Antiproliferative Activity of Anthocyanins in Purple Root Tubers and Leaves of Sweet Potato (Ipomoea batatas). J. Agric. Food Chem. 2019, 67, 2467–2475. [Google Scholar] [CrossRef]
- Moura, I.O.; Santana, C.C.; Lourenço, Y.R.F.; Souza, M.F.; Silva, A.R.S.T.; Dolabella, S.S.; de Oliveira e Silva, A.M.; Oliveira, T.B.; Duarte, M.C.; Faraoni, A.S. Chemical Characterization, Antioxidant Activity and Cytotoxicity of the Unconventional Food Plants: Sweet Potato (Ipomoea batatas (L.) Lam.) Leaf, Major Gomes (Talinum paniculatum (Jacq.) Gaertn.) and Caruru (Amaranthus deflexus L.). Waste Biomass Valoriz. 2021, 12, 2407–2431. [Google Scholar] [CrossRef]
- Lee, S.-L.; Chin, T.-Y.; Tu, S.-C.; Wang, Y.-J.; Hsu, Y.-T.; Kao, M.-C.; Wu, Y.-C. Purple Sweet Potato Leaf Extract Induces Apoptosis and Reduces Inflammatory Adipokine Expression in 3T3-L1 Differentiated Adipocytes. Evid.-Based Complement. Altern. Med. 2015, 2015, 126302. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.-Y.; Huang, Y.-P.; Hsieh, W.-B. Inhibitive effect of purple sweet potato leaf extract and its components on cell adhesion and inflammatory response in human aortic endothelial cells. Cell Adhes. Migr. 2013, 7, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-D.; Brownmiller, C.; Sorker, H.; Islam, S.; Lee, S.-O. Sweetpotato Leaves Inhibit Lipopolysaccharide-Induced Inflammation in RAW 264.7 Macrophages via Suppression of NF-κB Signaling Pathway. Foods 2021, 10, 2051. [Google Scholar] [CrossRef]
- Pastore, S.; Lulli, D.; Potapovich, A.I.; Fidanza, P.; Kostyuk, V.A.; Dellambra, E.; De Luca, C.; Maurelli, R.; Korkina, L.G. Differential modulation of stress-inflammation responses by plant polyphenols in cultured normal human keratinocytes and immortalized HaCaT cells. J. Dermatol. Sci. 2011, 63, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Monforte, A.R.; Martins, S.I.F.S.; Silva Ferreira, A.C. Impact of Phenolic Compounds in Strecker Aldehyde Formation in Wine Model Systems: Target and Untargeted Analysis. J. Agric. Food Chem. 2020, 68, 10281–10286. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Tavaria, F.K.; Pintado, M. Insights into the Biocompatibility and Biological Potential of a Chitosan Nanoencapsulated Textile Dye. Int. J. Mol. Sci. 2022, 23, 14234. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Veiga, M.; Baptista, P.; Tavaria, F.K.; Pintado, M.E. Textile dyes loaded chitosan nanoparticles: Characterization, biocompatibility and staining capacity. Carbohydr. Polym. 2021, 251, 117120. [Google Scholar] [CrossRef]
- Teixeira, F.S.; Pimentel, L.L.; Vidigal, S.S.M.P.; Azevedo-Silva, J.; Pintado, M.E.; Rodríguez-Alcalá, L.M. Differential Lipid Accumulation on HepG2 Cells Triggered by Palmitic and Linoleic Fatty Acids Exposure. Molecules 2023, 28, 2367. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Pereira, C.F.; Ribeiro, A.A.; Casanova, F.; Freixo, R.; Pintado, M.; Ramos, O.L. Characterization and Evaluation of Commercial Carboxymethyl Cellulose Potential as an Active Ingredient for Cosmetics. Appl. Sci. 2022, 12, 6560. [Google Scholar] [CrossRef]

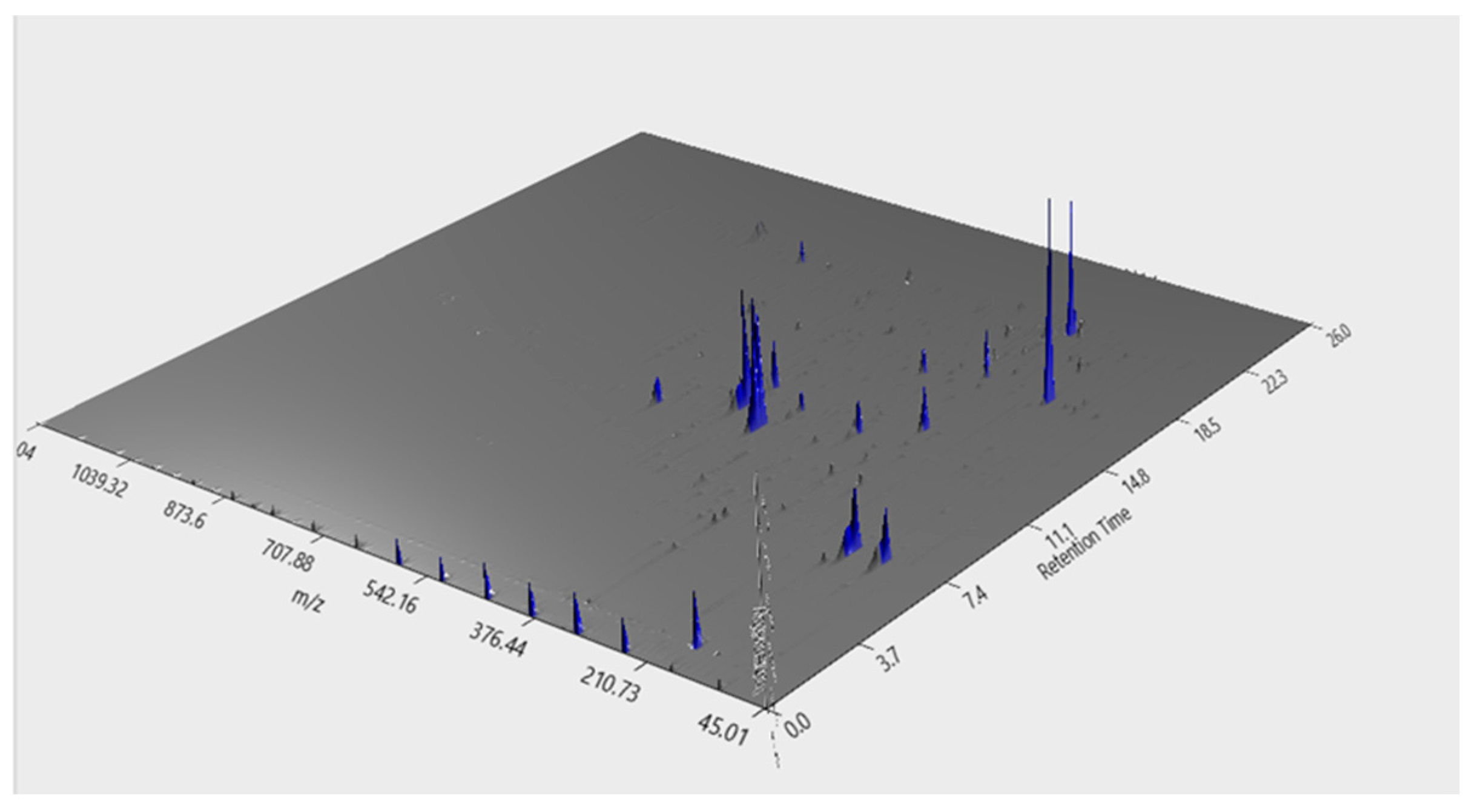


| N° | RT (min) | Area (a.u) | m/z | Molecular Formula | Metabolite |
|---|---|---|---|---|---|
| 1 | 4.81 | 8.48 × 105 | 707.18/707.19 | C16H18O9 | Chlorogenic acid |
| 2 | 4.9 | 7.29 × 106 | 353.08/353.09 | C16H18O9 | Neochlorogenic Acid |
| 3 | 8.3531 | 8.97 × 105 | 179.03/179.04 | C9H8O4 | Caffeic acid |
| 4 | 4.9072 | 1.05 × 106 | 431.19/413.19 | C19H30O8 | 3,5,5-Trimethyl-4beta-hydroxy-4-[3-(beta-D-glucopyranosyloxy)-1-butenyl]-2-cyclohexene-1-one |
| 5 | 9.3774 | 8.38 × 105 | 625.14/625.14 | C27H30O17 | Quercetin-3-O-sophoroside |
| 6 | 10.9606 | 5.19 × 106 | 609.14/609.15 | C27H30O16 | Rutin |
| 7 | 11.3332 | 6.62 × 106 | 463.09/463.12 | C21H20O12 | Spiraeoside |
| 8 | 11.3332 | 2.50 × 106 | 927.13/927.18 | C21H20O12 | Hyperoside |
| 9 | 12.3578 | 1.88 × 107 | 512.12/512.11 | C25H24O12 | Cynarin |
| 10 | 16.9211 | 3.46 × 105 | 593.13/593.15 | C30H26O13 | tiliroside |
| 11 | 17.0142 | 3.99 × 106 | 677.15/677.15 | C34H30O15 | 3,4,5-Tricaffeoylquinic acid |
| MIC | MBC | |
|---|---|---|
| P. aeruginosa | 5 | 5 |
| MRSA | 2.5 | n.d. |
| MSSA | 5 | 5 |
| E. coli | 5 | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, M.; Silva, S.; Pintado, M.; Costa, E.M. Exploring the Effects of Sweet Potato Leaves on Skin Health—From Antimicrobial to Immunomodulator. Molecules 2025, 30, 855. https://doi.org/10.3390/molecules30040855
Machado M, Silva S, Pintado M, Costa EM. Exploring the Effects of Sweet Potato Leaves on Skin Health—From Antimicrobial to Immunomodulator. Molecules. 2025; 30(4):855. https://doi.org/10.3390/molecules30040855
Chicago/Turabian StyleMachado, Manuela, Sara Silva, Manuela Pintado, and Eduardo M. Costa. 2025. "Exploring the Effects of Sweet Potato Leaves on Skin Health—From Antimicrobial to Immunomodulator" Molecules 30, no. 4: 855. https://doi.org/10.3390/molecules30040855
APA StyleMachado, M., Silva, S., Pintado, M., & Costa, E. M. (2025). Exploring the Effects of Sweet Potato Leaves on Skin Health—From Antimicrobial to Immunomodulator. Molecules, 30(4), 855. https://doi.org/10.3390/molecules30040855









