Sensory Evaluation of Effervescent Nutritional Supplements: Identification and Characterisation of Off-Tastes
Abstract
1. Introduction
2. Results
2.1. The Sensory Characterisation of the Nutritional Supplements
2.1.1. Orthonasal Olfactory Properties (Smell)
2.1.2. Retronasal Olfaction
2.1.3. Aftertaste and Retronasal Odour Persistence
2.2. Effect of Retronasal Odour Perception on Taste Perceptions
3. Discussion
3.1. The Relationship Between the Composition of Nutritional Supplements and Their Sensory Characteristics
3.2. Perceptual Interactions
3.3. The Outcomes of the Study
4. Materials and Methods
4.1. Experimental Conditions
4.2. Products
4.3. Sensory Profile Procedure
4.3.1. The Recruitment and Selection of the Panel
4.3.2. Presentation of Sensory Analysis to Panellists
4.3.3. Establishment of Descriptor List
4.3.4. Training of Sensory Panel and Monitoring of Individual Performances
4.3.5. Measurement Sessions
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolton, J.; Shannon, L.; Smith, V.; Abbott, R.; Bell, S.J.; Stubbs, L.; Slevin, M.L. Comparison of short-term and long-term palatability of six commercially available oral supplements. J. Hum. Nutr. Diet. 1990, 3, 317–321. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cancer cachexia: Metabolic alterations and clinical manifestations. Nutrition 1997, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Delompré, T.; Guichard, E.; Briand, L.; Salles, C. Taste perception of nutrients found in nutritional supplements: A review. Nutrients 2019, 11, 2050. [Google Scholar] [CrossRef]
- Delompré, T.; Lenoir, L.; Martin, C.; Briand, L.; Salles, C. Characterizing the Dynamic Taste and Retro-Nasal Aroma Properties of Oral Nutritional Supplements Using Temporal Dominance of Sensation and Temporal Check-All-That-Apply Methods. Foods 2020, 9, 1456. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Redel, U.; Blank, K.; Meyerhof, W. The human bitter taste receptor TAS2R7 facilitates the detection of bitter salts. Biochem. Biophys. Res. Com. 2019, 512, 877–881. [Google Scholar] [CrossRef]
- Laffitte, A.; Neiers, F.; Briand, L. Characterization of taste compounds: Chemical structures and sensory properties. In Flavour: From Food to Perception; Guichard, E., Le Bon, A.M., Morzel, M., Salles, C., Eds.; Wiley-Blackwell: Oxford, UK, 2017; pp. 154–191. [Google Scholar]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Delompré, T.; Belloir, C.; Martin, C.; Salles, C.; Briand, L. Detection of Bitterness in Vitamins Is Mediated by the Activation of Bitter Taste Receptors. Nutrients 2022, 14, 4141. [Google Scholar] [CrossRef] [PubMed]
- Sinding, C.; Saint-Eve, A.; Thomas-Danguin, T. Chapter 7—Multimodal sensory interactions. In Flavor: From Food to Behaviors, Wellbeing and Health, 2nd ed.; Guichard, E., Salles, C., Eds.; Elsevier Ltd.: Cambridge, MA, USA, 2023; pp. 205–231. [Google Scholar]
- Ipci, K.; Öktemer, T.; Birdane, L.; Altintoprak, N.; Bayar Muluk, N.; Passali, D.; Lopatin, L.; Bellussi, L.; Mladina, R.; Pawankar, R.; et al. Effervescent tablets: A safe and practical delivery system for drug administration. ENT Updates 2016, 6, 46–50. [Google Scholar] [CrossRef]
- Ozcagli, T.G.; Stelling, J.; Stanford, J. A study in four European countries to examine the importance of sensory attributes of oral nutritional supplements on preference and likelihood of compliance. Turk. J. Gastroenterol. 2013, 24, 266–272. [Google Scholar] [CrossRef]
- Andrea, R. Arma release during in-mouth process. In Flavour: From Food to Perception; John Wiley & Sons, Inc.: Chichester, UK, 2017; pp. 235–265. [Google Scholar]
- Lawless, H.T.; Schlake, S.; Smythe, J.; Lim, J.Y.; Yang, H.D.; Chapman, K.; Bolton, B. Metallic taste and retronasal smell. Chem. Senses 2004, 29, 25–33. [Google Scholar] [CrossRef]
- Sowalsky, R.A.; Noble, A.C. Comparison of the effects of concentration, pH and anion species on astringency and sourness of organic acids. Chem. Senses 1998, 23, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Pulkkinen, E.; Fischer, I.; Laska, M. Sour, but acceptable: Taste responsiveness to five food-associated acids in zoo-housed white-faced sakis. Physiol Behav. 2024, 286, 114679. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Opekun, A.R.; Abudayyeh, S.; Graham, D.Y. Effect of different organic acids (citric, malic and ascorbic) on intragastric urease activity. Alim. Pharm. Ther. 2005, 21, 1145–1148. [Google Scholar] [CrossRef]
- Breslin, P.; Beauchamp, G. Suppression of bitterness by sodium: Variation among bitter taste stimuli. Chem. Senses 1995, 20, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lawless, H.T. Detection thresholds and taste qualities of iron salts. Food Qual. Prefer. 2006, 17, 513–521. [Google Scholar] [CrossRef]
- Yang, H.H.L.; Lawless, H.T. Descriptive analysis of divalent salts. J. Sens. Stud. 2005, 20, 97–113. [Google Scholar] [CrossRef]
- Clark, R.A.; Hewson, L.; Bealin-Kelly, F.; Hort, J. The interactions of CO2, ethanol, hop acids and sweetener on flavour perception in a model beer. Chem. Percept. 2011, 4, 42–54. [Google Scholar] [CrossRef]
- Hewson, L.; Hollowood, T.; Chandra, S.; Hort, J. Taste–aroma interactions in a citrus flavoured model beverage system: Similarities and differences between acid and sugar type. Food Qual. Prefer. 2008, 19, 323–334. [Google Scholar] [CrossRef]
- Symoneaux, R.; Le Quéré, J.-M.; Baron, A.; Bauduin, R.; Chollet, S. Impact of CO2 and its interaction with the matrix components on sensory perception in model cider. LWT-Food Sci. Technol. 2015, 63, 886–891. [Google Scholar] [CrossRef]
- Le Calvé, B.; Goichon, H.; Cayeux, I. CO2 perception and its influence on flavour. In Expression of Multidisciplinary Flavour Science; Blank, I., Würst, M., Yeretzian, C., Eds.; ZHAW: Winterhur, Switzerland, 2010; pp. 55–58. [Google Scholar]
- Saint-Eve, A.; Déléris, I.; Feron, G.; Ibarra, D.; Guichard, E.; Souchon, I. How trigeminal, taste and aroma perceptions are affected in mint-flavored carbonated beverages. Food Qual. Prefer. 2010, 21, 1026–1033. [Google Scholar] [CrossRef]
- Voorpostel, C.R.; Dutra, M.B.d.L.; Bolini, H.M.A. Sensory profile and drivers of liking for grape nectar among smoker and nonsmoker consumers. Food Sci. Technol. 2014, 34, 164–173. [Google Scholar] [CrossRef]
- Methven, L.; Rahelu, K.; Economou, N.; Kinneavy, L.; Ladbrooke-Davis, L.; Kennedy, O.B.; Mottram, D.S.; Gosney, M.A. The effect of consumption volume on profile and liking of oral nutritional supplements of varied sweetness: Sequential profiling and boredom tests. Food Qual. Prefer. 2010, 21, 948–955. [Google Scholar] [CrossRef]
- Dalmasso, J.P.; Wiese, K.L. Sensory and chemical effects of tomato juice acidified with malic and citric acids. J. Food Qual. 1991, 14, 145–152. [Google Scholar] [CrossRef]
- Shi, Y.G.; Pu, D.N.; Zhou, X.W.; Zhang, Y.Y. Recent Progress in the Study of Taste Characteristics and the Nutrition and Health Properties of Organic Acids in Foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef] [PubMed]
- Keast, R.S.J.; Breslin, P.A.S. An overview of binary taste–taste interactions. Food Qual. Prefer. 2003, 14, 111–124. [Google Scholar] [CrossRef]
- Lubran, M.B.; Lawless, H.T.; Lavin, E.; Acree, T.E. Identification of metallic-smelling 1-octen-3-one and 1-nonen-3-one from solutions of ferrous sulfate. J. Agric. Food Chem. 2005, 53, 8325–8327. [Google Scholar] [CrossRef] [PubMed]
- Ömür-Özbek, P.; Dietrich, A.M.; Duncan, S.E.; Lee, Y. Role of Lipid Oxidation, Chelating Agents, and Antioxidants in Metallic Flavor Development in the Oral Cavity. J. Agric. Food Chem. 2012, 60, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Guichard, E. Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Rev. Int. 2002, 18, 49–70. [Google Scholar] [CrossRef]
- Engel, E.; Nicklaus, S.; Salles, C.; Le Quéré, J.L. Relevance of omission tests to determine flavour-active compounds in food: Application to cheese taste. Food Qual. Prefer. 2002, 13, 505–513. [Google Scholar] [CrossRef]
- Salles, C. Odour-taste interactions in flavour perception. In Flavour in Food, CRC Press ed.; Voilley, A., Etiévant, P., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2006; Volume 3, pp. 345–368. [Google Scholar]
- Lawless, H.T.; Heymann, H. Physiological and Psychological Foundations of Sensory Function. In Sensory Evaluation of Food: Principles and Practices; Lawless, H.T., Heymann, H., Eds.; Food Science Text Series; Springer: New York, NY, USA, 2010; pp. 19–56. [Google Scholar]
- AFNOR V 09-105; Analyse Sensorielle—Directives Générales pour L'implantation de Locaux Destinés à L'analyse Sensorielle. AFNOR: La Plaine Saint-Denis, France, 1987.
- Meilgaard, M.C.; Carr, B.T.; Civille, G.V. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Moskowitz, H.R. Product Testing and Sensory Evaluation of Foods: Marketing and R&D Approaches; Food & Nutrition Press, Inc.: Trumbull, CT, USA, 1983. [Google Scholar]
- AFNOR-ISO 8586; Sensory analysis. General Guidance for the Selection, Training and Monitoring of Assessors. Part 1: Selected Assessors. AFNOR: La Plaine Saint-Denis, France, 2012.
- Stone, H.; Sidel, J.L. Introduction to sensory evaluation. In Sensory Evaluation Practices, 3rd ed.; Academic Press: San Diego, CA, USA, 2004; pp. 1–19. [Google Scholar]
- Peltier, C. Statistical Analysis of Sensory Profiling Data Revisited by a Database Approach. Ph.D. Thesis, University of Burgundy, Dijon, France, 2015. [Google Scholar]
- O’Mahony, M. Sensory Evaluation of Food: Statistical Methods and Procedures; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
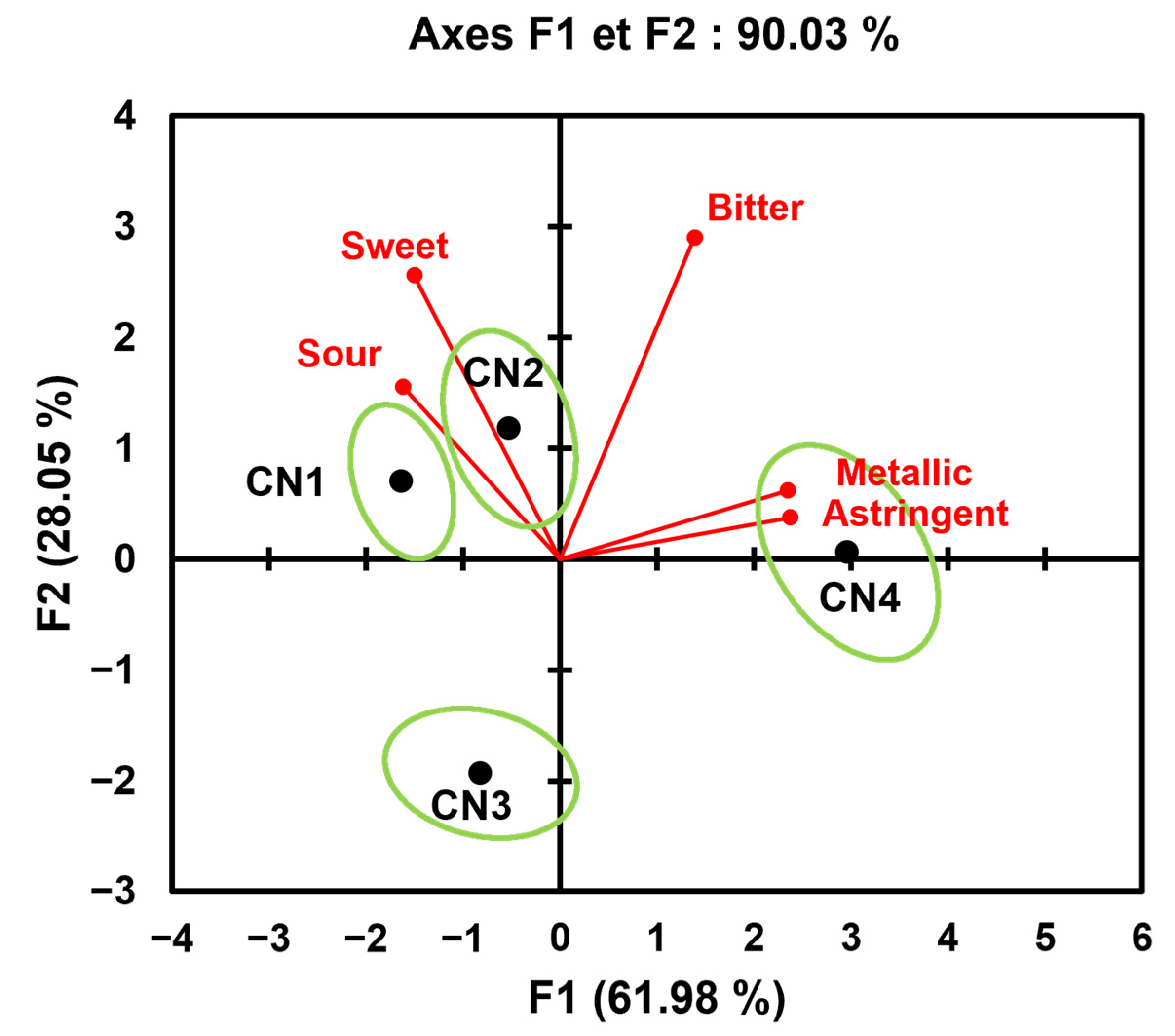
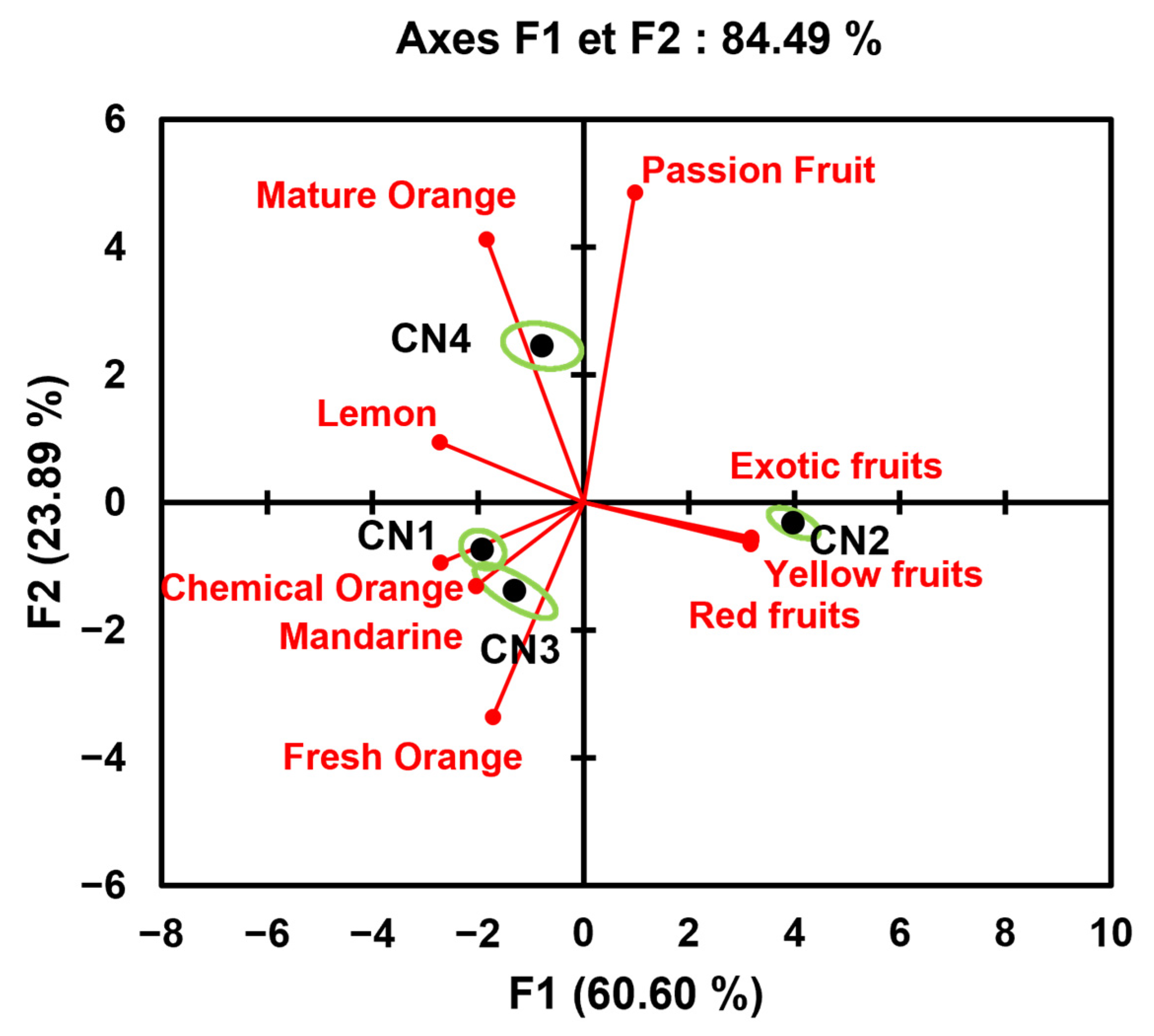
| Attribute | CN1 | CN2 | CN3 | CN4 |
|---|---|---|---|---|
| Orthonasal odour | ||||
| Chemical orange | 5.60 ± 0.078 a | 0.56 ± 0.078 d | 3.46 ± 0.078 b | 2.40 ± 0.078 c |
| Fresh orange | 1.10 ± 0.070 b | 0.35 ± 0.070 c | 3.13 ± 0.070 a | 0.43 ± 0.070 c |
| Mature orange | 1.29 ± 0.073 b | 0.42 ± 0.073 c | 0.35 ± 0.073 c | 2.65 ± 0.073 a |
| Lemon | 0.16 ± 0.050 a | 0.22 ± 0.050 a | 0.33 ± 0.050 a | 0.25 ± 0.050 a |
| Mandarin | 0.37 ± 0.066 b | 0.18 ± 0.066 b | 1.21 ± 0.066 a | 0.33 ± 0.066 b |
| Passion fruit | 0.33 ± 0.059 c | 2.19 ± 0.059 b | 0.29 ± 0.059 c | 3.90 ± 0.059 a |
| Exotic fruits | 0.18 ± 0.064 c | 2.34 ± 0.064 a | 0.29 ± 0.064 c | 0.55 ± 0.064 b |
| Yellow fruits | 0.19 ± 0.112 b | 1.39 ± 0.112 a | 0.14 ± 0.112 b | 0.36 ± 0.112 b |
| Red fruits | 0.11 ± 0.039 b | 0.13 ± 0.039 a | 0.21 ± 0.039 b | 0.15 ± 0.039 b |
| Attributes | CN1 | CN2 | CN3 | CN4 |
|---|---|---|---|---|
| Retronasal odour | ||||
| Chemical orange | 5.69 ± 0.097 a | 0.55 ± 0.097 c | 2.88 ± 0.097 b | 2.60 ± 0.097 b |
| Fresh orange | 1.15 ± 0.060 b | 0.26 ± 0.060 c | 2.83 ± 0.060 a | 0.37 ± 0.060 c |
| Mature orange | 1.48 ± 0.041 b | 0.25 ± 0.041 d | 0.62 ± 0.041 c | 2.49 ± 0.041 a |
| Lemon | 0.35 ± 0.050 ab | 0.18 ± 0.050 b | 0.47 ± 0.050 a | 0.44 ± 0.050 a |
| Mandarin | 1.05 ± 0.070 a | 0.21 ± 0.070 b | 0.37 ± 0.070 b | 0.35 ± 0.070 b |
| Passion fruit | 0.29 ± 0.080 c | 1.37 ± 0.080 b | 0.20 ± 0.080 c | 2.53 ± 0.080 a |
| Exotic fruits | 0.43 ± 0.079 b | 2.56 ± 0.079 a | 0.41 ± 0.079 b | 0.47 ± 0.079 b |
| Yellow fruits | 0.23 ± 0.063 b | 1.45 ± 0.063 a | 0.28 ± 0.063 b | 0.28 ± 0.063 b |
| Red fruits | 0.15 ± 0.051 b | 2.20 ± 0.051 a | 0.13 ± 0.051 b | 0.14 ± 0.051 b |
| Metallic | 0.32 ± 0.063 b | 0.50 ± 0.063 b | 0.33 ± 0.063 b | 1.70 ± 0.063 b |
| Taste | ||||
| Sour | 4.89 ± 0.087 a | 3.48 ± 0.087 b | 3.17 ± 0.087 bc | 2.97 ± 0.065 c |
| Bitter | 1.20 ± 0.059 a | 1.70 ± 0.059 a | 0.47 ± 0.059 c | 1.82 ± 0.059 a |
| Salty | 1.74 ± 0.066 ab | 1.58 ± 0.066 b | 1.86 ± 0.066 a | 1.84 ± 0.066 a |
| Sweet | 4.12 ± 0.080 ab | 4.26 ± 0.080 a | 3.87 ± 0.80 bc | 3.80 ± 0.80 c |
| Astringent | 0.41 ± 0.073 b | 0.49 ± 0.073 b | 0.37 ± 0.073 b | 0.98 ± 0.073 a |
| Attributes | CN1 | CN2 | CN3 | CN4 |
|---|---|---|---|---|
| Astringent | 0.516 ± 0.065 b | 0.418 ± 0.065 b | 0.462 ± 0.065 b | 1.113 ± 0.065 a |
| Metallic | 0.261 ± 0.045 b | 0.282 ± 0.045 b | 0.179 ± 0.045 b | 1.671 ± 0.045 a |
| Salty | 1.500 ± 0.068 a | 1.018 ± 0.068 b | 0.953 ± 0.068 b | 1.112 ± 0.068 b |
| Sour | 2.005 ± 0.082 a | 1.766 ± 0.082 a | 1.992 ± 0.082 a | 1.857 ± 0.082 a |
| Bitter | 1.406 ± 0.072 ab | 1.647 ± 0.072 a | 0.369 ± 0.072 c | 1.274 ± 0.072 b |
| Sweet | 2.751 ± 0.078 b | 3.313 ± 0.078 a | 2.841 ± 0.78 b | 2.694 ± 0.78 b |
| CN1 | CN2 | CN3 | CN4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attributes | With nose-clip | Without nose-clip | p-value | With nose-clip | Without nose-clip | p-value | With nose-clip | Without nose-clip | p-value | With nose-clip | Without nose-clip | p-value |
| During tasting | ||||||||||||
| Sour | 5.03 | 4.91 | 0.168 | 3.68 | 3.49 | 0.258 | 3.28 | 3.15 | 0.309 | 3.45 | 2.96 | 0.001 |
| Bitter | 1.44 | 1.23 | 0.035 | 2.10 | 1.68 | <0.0001 | 1.17 | 0.44 | <0.0001 | 1.37 | 1.72 | 0.005 |
| Astringent | 0.97 | 0.44 | <0.0001 | 0.79 | 0.49 | 0.064 | 0.48 | 0.37 | 0.396 | 1.39 | 0.96 | <0.0001 |
| Metallic | 0.20 | 0.35 | 0.061 | 0.20 | 0.50 | 0.007 | 0.12 | 0.32 | 0.022 | 0.24 | 1.66 | <0.0001 |
| Salty | 1.98 | 1.75 | 0.017 | 1.39 | 1.59 | 0.128 | 1.79 | 1.83 | 0.744 | 1.90 | 1.83 | 0.617 |
| Sweet | 3.61 | 4.13 | <0.0001 | 3.52 | 4.25 | <0.0001 | 3.35 | 3.83 | <0.0001 | 3.62 | 3.78 | 0.266 |
| After tasting | ||||||||||||
| Sour | 3.68 | 1.99 | <0.0001 | 1.46 | 1.77 | 0.040 | 1.34 | 2.02 | <0.0001 | 1.89 | 1.82 | 0.63 |
| Bitter | 1.29 | 1.42 | 0.093 | 2.77 | 1.57 | <0.0001 | 0.69 | 0.37 | 0.004 | 0.97 | 1.21 | 0.06 |
| Astringent | 1.27 | 0.56 | <0.0001 | 0.53 | 0.43 | 0.503 | 0.62 | 0.46 | 0.149 | 1.44 | 1.04 | 0.009 |
| Metallic | 0.18 | 0.28 | 0.180 | 0.25 | 0.28 | 0.718 | 0.19 | 0.20 | 0.852 | 0.16 | 1.60 | <0.0001 |
| Salty | 1.14 | 1.46 | 0.015 | 0.93 | 0.97 | 0.791 | 0.84 | 0.95 | 0.400 | 0.85 | 1.14 | 0.061 |
| Sweet | 1.96 | 2.69 | <0.0001 | 2.19 | 3.28 | <0.0001 | 2.16 | 2.83 | <0.0001 | 2.28 | 2.62 | 0.005 |
| CN1 | CN2 | CN3 | CN4 | |
|---|---|---|---|---|
| Vitamins | ||||
| B1 (Thiamine phosphate; Thiamine hydrochloride) | 15 mg | 1.4 mg | 3.1 mg | |
| B2 (Riboflavin phosphate) | 15 mg | 1.6 mg | 4 mg | |
| B3 (Nicotinamide) | 50 mg | 18 mg | 45 mg | |
| B5 (Calcium pantothenate) | 23 mg | 6 mg | 17 mg | |
| B6 (Pyridoxine hydrochloride) | 10 mg | 2 mg | 4 mg | |
| B8 (D-biotin) | 0.15 mg | 0.15 mg | 145 µg | |
| B9 (Folic acid) | 0.4 mg | 200 µg | 430 µg | |
| B12 (Cobalamin (0.1%)) | 10 µg | 1 µg | 3 µg | |
| C (Ascorbic acid) | 500 mg | 60 mg | 1000 mg | 180 mg |
| A (Palmitate (100%)) | 0.86 mg | |||
| D (Cholecalciferol (100%)) | 12 mg | |||
| E (Tocopherol acetate (50%)) | 5 µg | |||
| K (Phylloquinone (5%)) | 20 µg | |||
| Minerals | ||||
| Calcium (Calcium carbonate; Calcium phosphate) | 100 mg | 100 mg | 120 mg | |
| Magnesium (Magnesium carbonate; Magnesium sulphate; Magnesium oxide) | 100 mg | 100 mg | 80 mg | |
| Iron (Iron pyrophosphate) | 8 mg | |||
| Iodide | 150 µg | |||
| Zinc (Zinc citrate trihydrate) | 10 mg | 5 mg | 4.4 mg | |
| Manganese (Manganese sulphate) | 500 µg | |||
| Copper (Copper citrate hemipentahydrate) | 1 mg | |||
| Molybdenum (Sodium molybdenum) | 50 µg | |||
| Selenium (Sodium selenite) | 55 µg | |||
| Caffeine | 40 mg |
| Consumption Phases and Assessment Guidelines | Sensory Modalities Assessed | Descriptors | Definitions | Sensory References |
|---|---|---|---|---|
| Before tasting «Place the tablet in the provided cup. Wait for the tablet to completely dissolve in water, indicated by the visual disappearance of the tablet in the cup. Bring the sample to your nose and record the olfactory intensity of the specified attributes.» | Olfactory (9) | Fresh orange | Characteristic odour of freshly squeezed orange | NI |
| Mature orange | Characteristic odour of an orange in an advanced state of decomposition (presence of mould) | NI | ||
| Chemical orange | Characteristic odour of certain confectionery products or carbonated orange-flavoured drinks. | NI | ||
| Mandarin | Characteristic odour of the fruit and/or its juice | NI | ||
| Passion fruit | Characteristic odour of the fruit and/or its juice | NI | ||
| Lemon | Characteristic odour of the fruit and/or its juice | NI | ||
| Exotic fruits | Characteristic odour of a blend of different exotic fruits (mango, pineapple, banana) squeezed together | NI | ||
| Red fruits | Characteristic odour of a blend of different pressed red fruits (strawberry, raspberry) | NI | ||
| Yellow fruits | Characteristic odour of a blend of different pressed yellow fruits (peach, apricot, nectarine) | NI | ||
| During tasting «After evaluating the olfactory descriptors, proceed with the assessment of the gustatory descriptors. To do so, bring the sample to your mouth and promptly record the gustatory intensity of the specified attributes.» | Gustatory (5) | Sour | Elemental flavour produced by dilute aqueous solutions of various substances such as citric acid or tartaric acid | Negative terminal (0): Evian water Positive terminal (10): Citric acid (1.8 g/L) + Evian water |
| Sweet | A basic flavour produced by dilute solutions of various substances such as sucrose or aspartame | Negative terminal (0): Evian water Positive terminal (10): Sucrose (80 g/L) + Evian water | ||
| Salty | Elemental flavour caused by diluted substances such as sodium chloride | Negative terminal (0): Evian water Positive terminal (10): Sodium chloride (6 g/L) + Evian water | ||
| Bitter | Elemental flavour caused by dilute solutions of various substances such as caffeine or quinine hydrochloride | Negative terminal (0): Evian water Positive terminal (10): Caffeine (1.5 g/L) + Evian water | ||
| Astringent | A complex sensation, accompanied by a contraction, stretching or wrinkling of the oral mucosa, produced by substances such as the tannins in wine | Negative terminal (0): Evian water Positive terminal (10): Tannic acid (1 g/L) + Evian water |
| Consumption Phases and Assessment Guidelines | Sensory Modalities Assessed | Descriptors | Definitions | Sensory References |
|---|---|---|---|---|
| During tasting «After evaluating the olfactory descriptors, proceed with the assessment of the aromatic descriptors. To do so, bring the sample to your mouth and promptly record the aromatic intensity of the specified attributes.» | Aromatic (10) | Fresh orange | Characteristic aroma of freshly squeezed orange | NI |
| Mature orange | Characteristic of an orange in an advanced state of decomposition (presence of mould) | NI | ||
| Chemical orange | Characteristic aroma of certain confectionery products or carbonated orange-flavoured drinks | NI | ||
| Mandarin | Characteristic aroma of the fruit and/or its juice | NI | ||
| Passion fruit | Characteristic aroma of the fruit and/or its juice | NI | ||
| Lemon | Characteristic aroma of the fruit and/or its juice | NI | ||
| Exotic fruits | Characteristic aroma of a blend of different exotic fruits (mango, pineapple, banana) squeezed together | NI | ||
| Red fruits | Characteristic aroma of a blend of different pressed red fruits (strawberry, raspberry) | NI | ||
| Yellow fruits | Characteristic aroma of a blend of different pressed yellow fruits (peach, apricot, nectarine) | NI | ||
| Metallic | Sensation caused by substances such as ferrous sulphate, similar to that felt when the tongue or oral mucosa is cut | Negative terminal (0): Evian water Positive terminal (10): Ferrous sulphate (0.1 g/L) + Evian water | ||
| After tasting «You will proceed with the evaluation of the persistence of gustatory descriptors. To do so, bring the sample to your mouth and record the gustatory intensity of the specified attributes 10 s after complete ingestion of the sample.» | Aftertaste (5) | Sour | Elemental flavour produced by dilute aqueous solutions of various substances such as citric acid or tartaric acid | Negative terminal (0): Evian water Positive terminal (10): Citric acid (1.8 g/L) + Evian water |
| Sweet | A basic flavour produced by dilute solutions of various substances such as sucrose or aspartame | Negative terminal (0): Evian water Positive terminal (10): Sucrose (80 g/L) + Evian water | ||
| Salty | Elemental flavour caused by diluted substances such as sodium chloride | Negative terminal (0): Evian water Positive terminal (10): Sodium chloride (6 g/L) + Evian water | ||
| Bitter | Elemental flavour caused by dilute solutions of various substances such as caffeine or quinine hydrochloride | Negative terminal (0): Evian water Positive terminal (10): Caffeine (1.5 g/L) + Evian water | ||
| Astringent | A complex sensation, accompanied by a contraction, stretching or wrinkling of the oral mucosa, produced by substances such as the tannins in wine | Negative terminal (0): Evian water Positive terminal (10): Tannic acid (1 g/L) + Evian water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delompré, T.; Martin, C.; Briand, L.; Salles, C. Sensory Evaluation of Effervescent Nutritional Supplements: Identification and Characterisation of Off-Tastes. Molecules 2025, 30, 854. https://doi.org/10.3390/molecules30040854
Delompré T, Martin C, Briand L, Salles C. Sensory Evaluation of Effervescent Nutritional Supplements: Identification and Characterisation of Off-Tastes. Molecules. 2025; 30(4):854. https://doi.org/10.3390/molecules30040854
Chicago/Turabian StyleDelompré, Thomas, Christophe Martin, Loïc Briand, and Christian Salles. 2025. "Sensory Evaluation of Effervescent Nutritional Supplements: Identification and Characterisation of Off-Tastes" Molecules 30, no. 4: 854. https://doi.org/10.3390/molecules30040854
APA StyleDelompré, T., Martin, C., Briand, L., & Salles, C. (2025). Sensory Evaluation of Effervescent Nutritional Supplements: Identification and Characterisation of Off-Tastes. Molecules, 30(4), 854. https://doi.org/10.3390/molecules30040854








