Sustainable MXene Synthesis via Molten Salt Method and Nano-Silicon Coating for Enhanced Lithium-Ion Battery Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
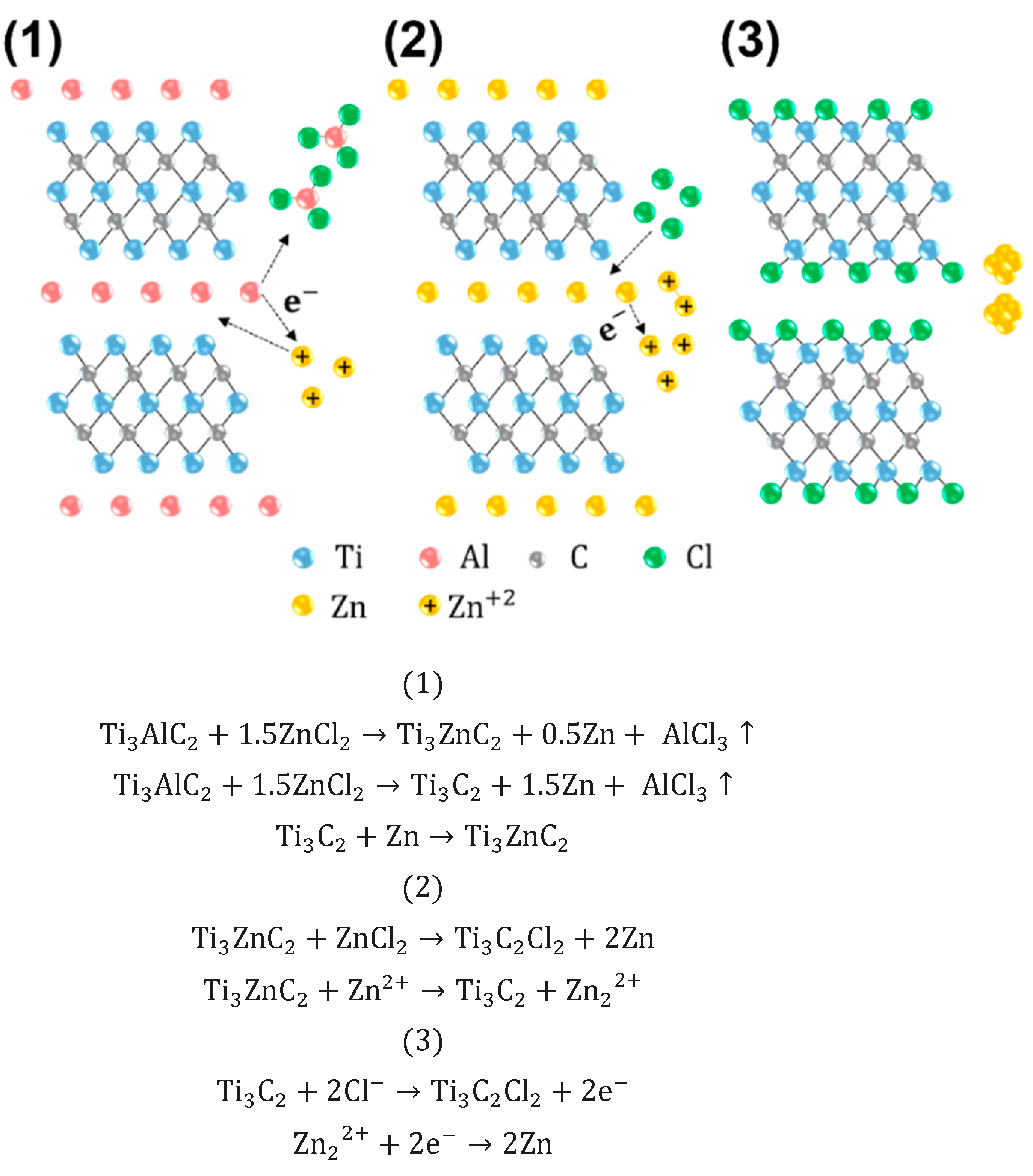
2.2. MAX Etching for Multi-Layer MXene
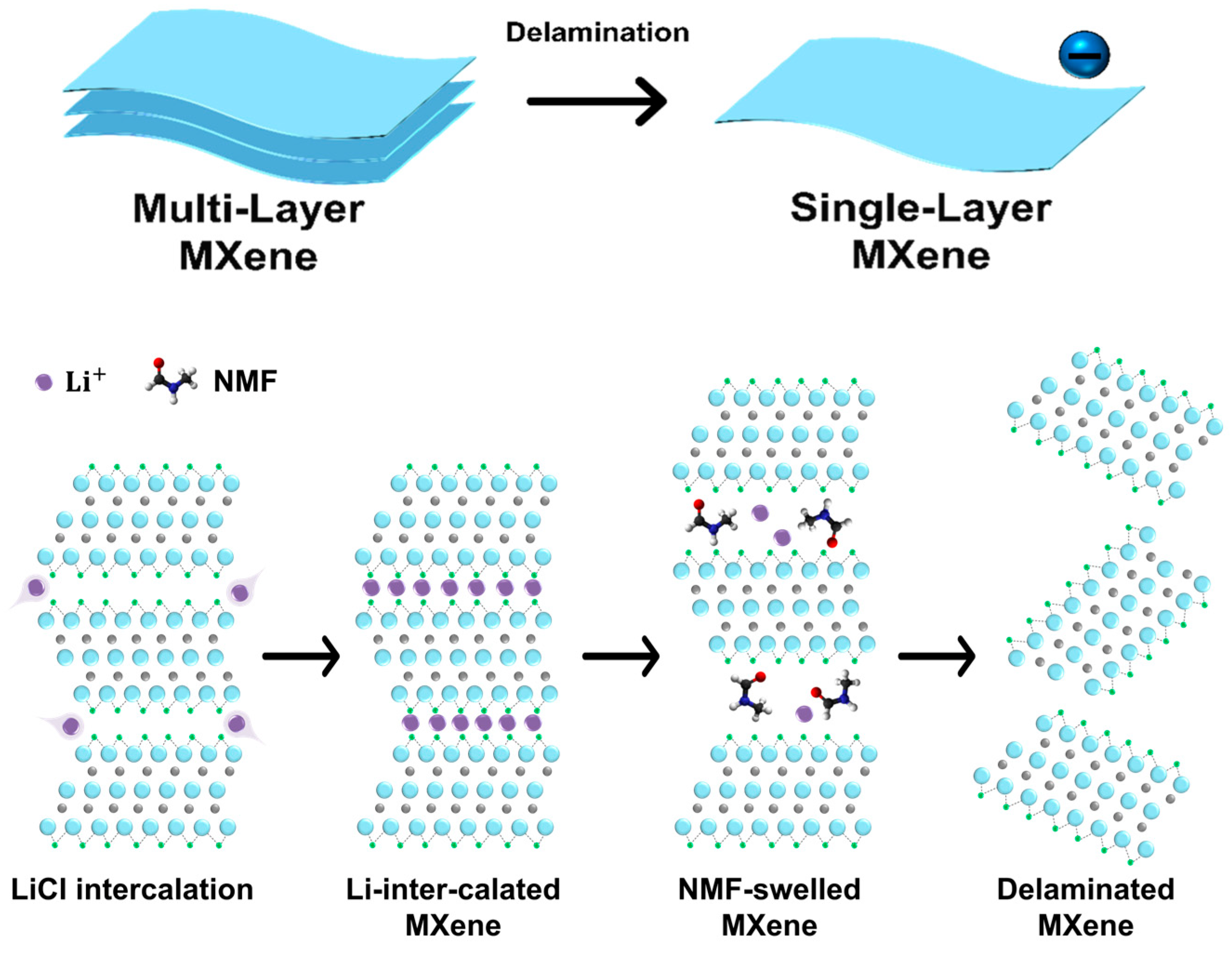
2.3. Delamination for Single-Layer MXene
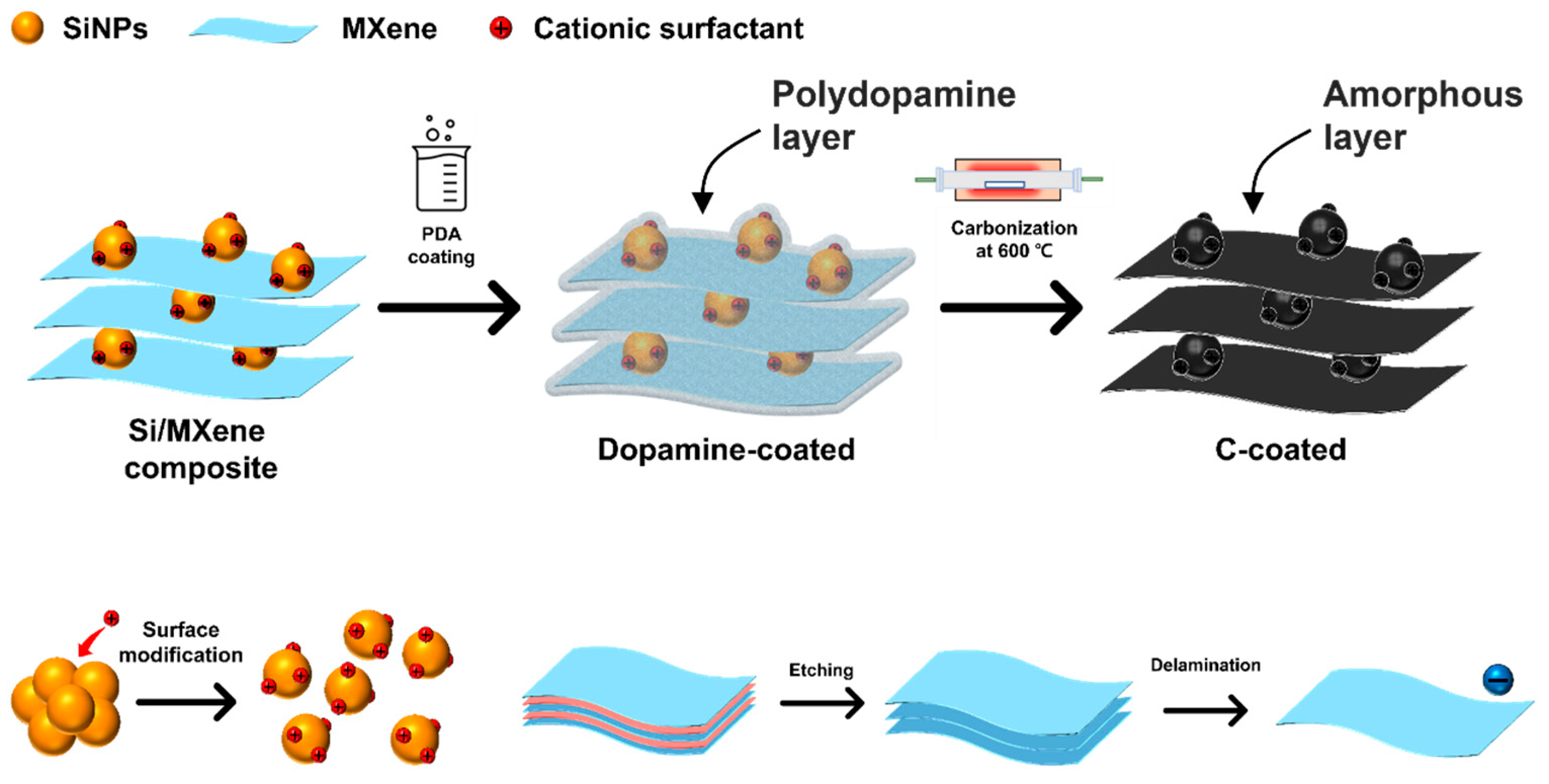
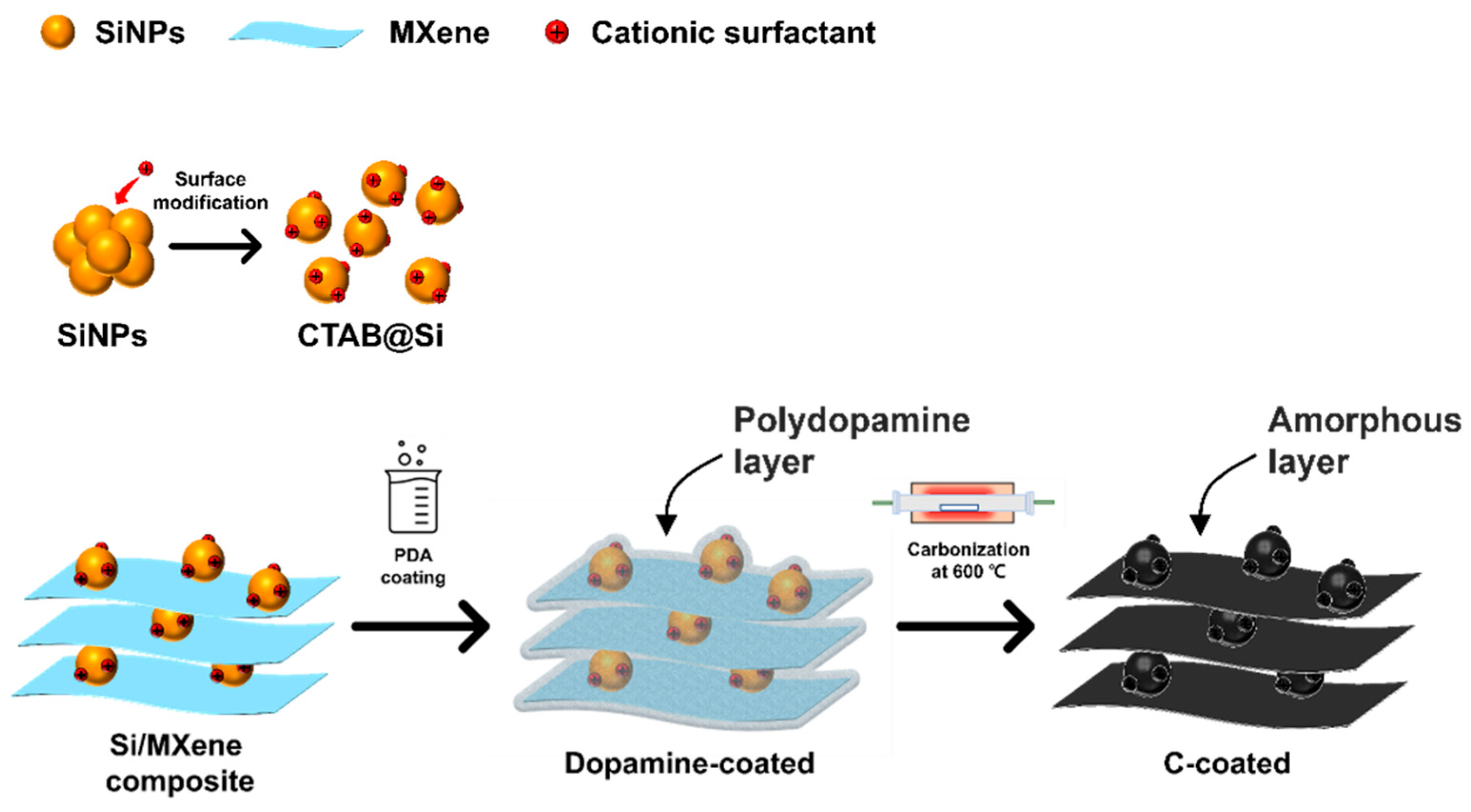
2.4. Self-Assembly of Si-MXene Composite and Polydopamine Coating
2.5. Characterizations
2.6. Fabrication of Si-MXene Composite Andoe for Half Cell
3. Results and Discussion
3.1. Ecofriendly Synthesis of MXene
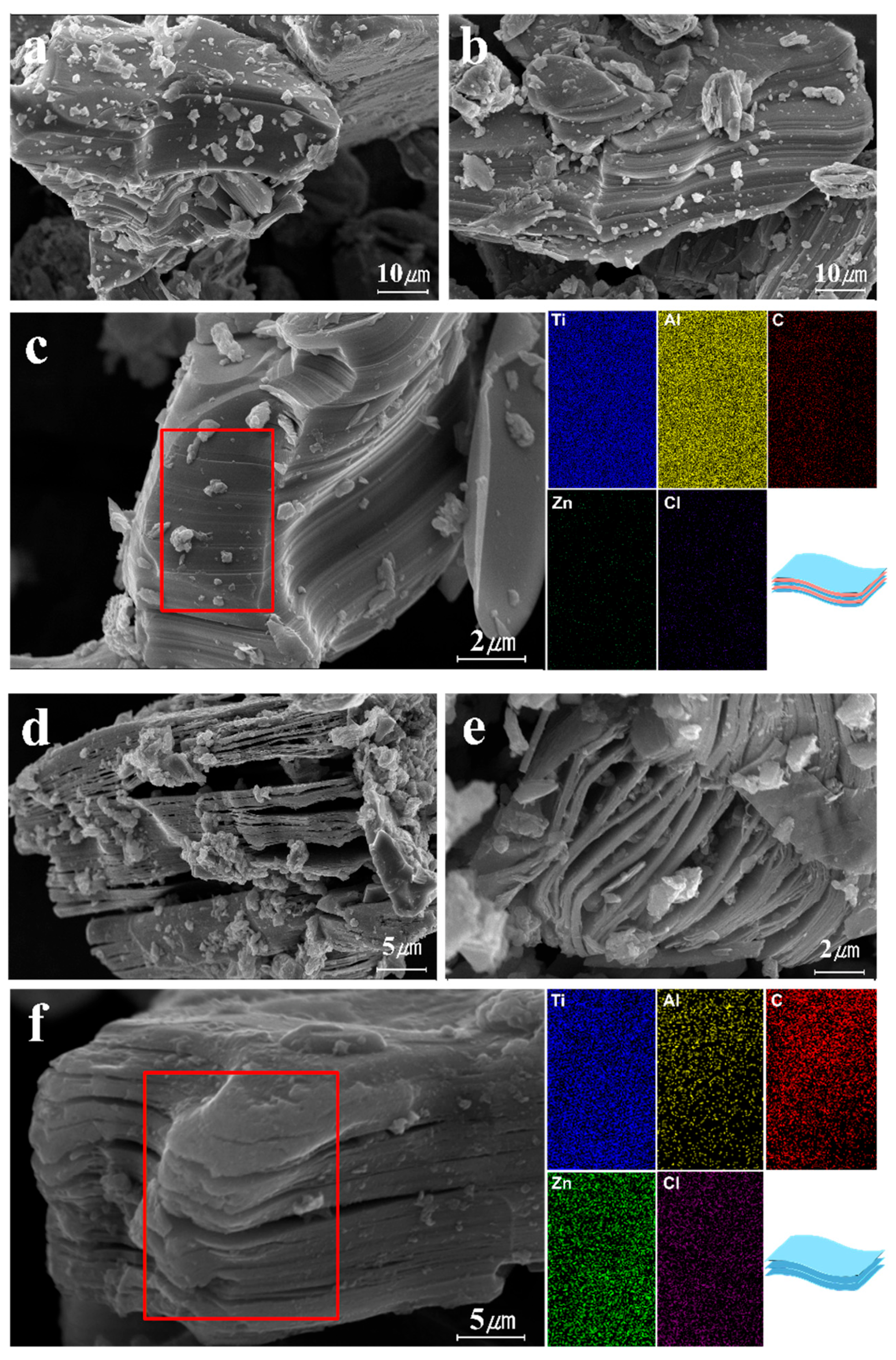
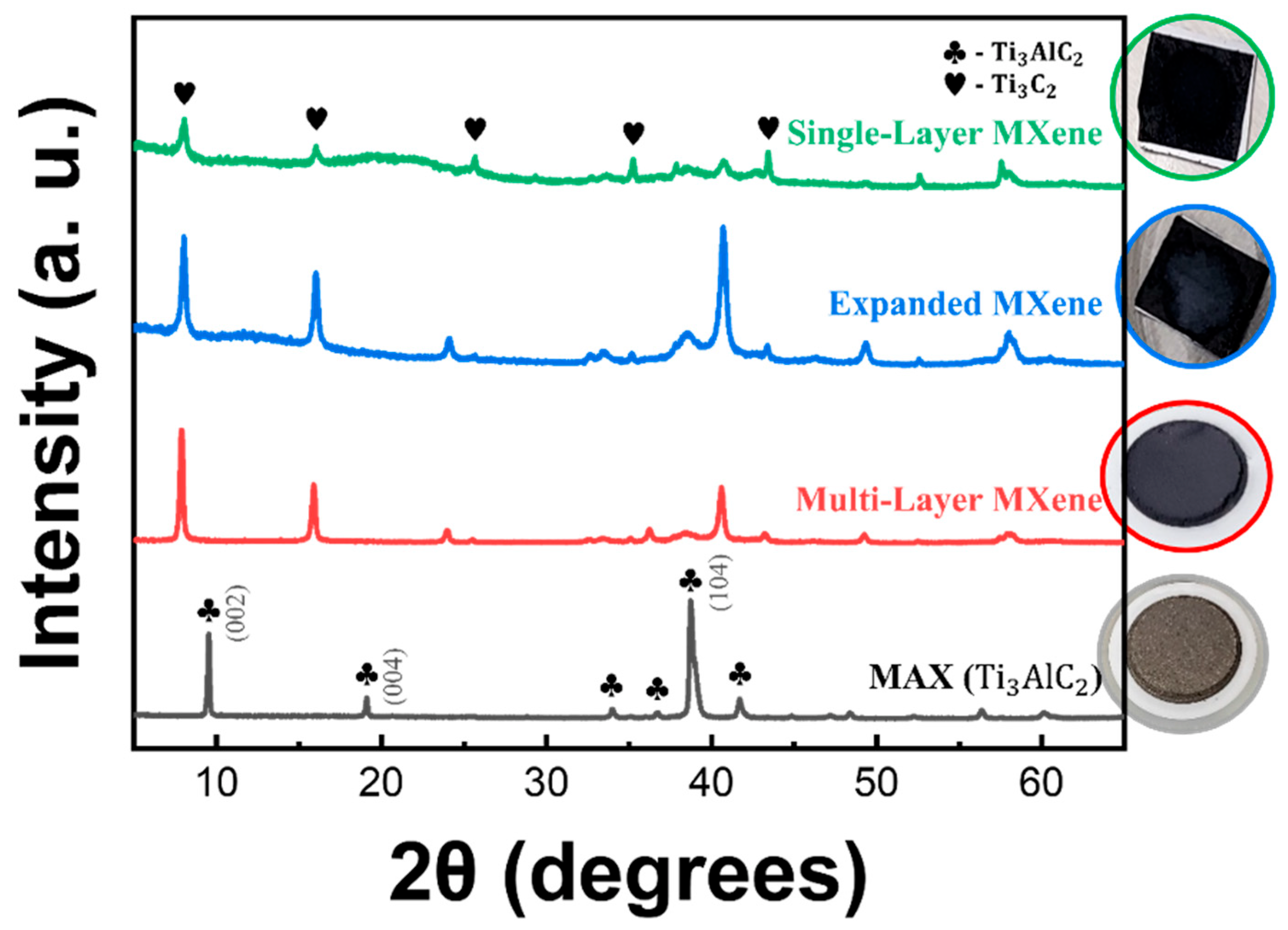
3.1.1. Synthesis of Multi-Layer MXene
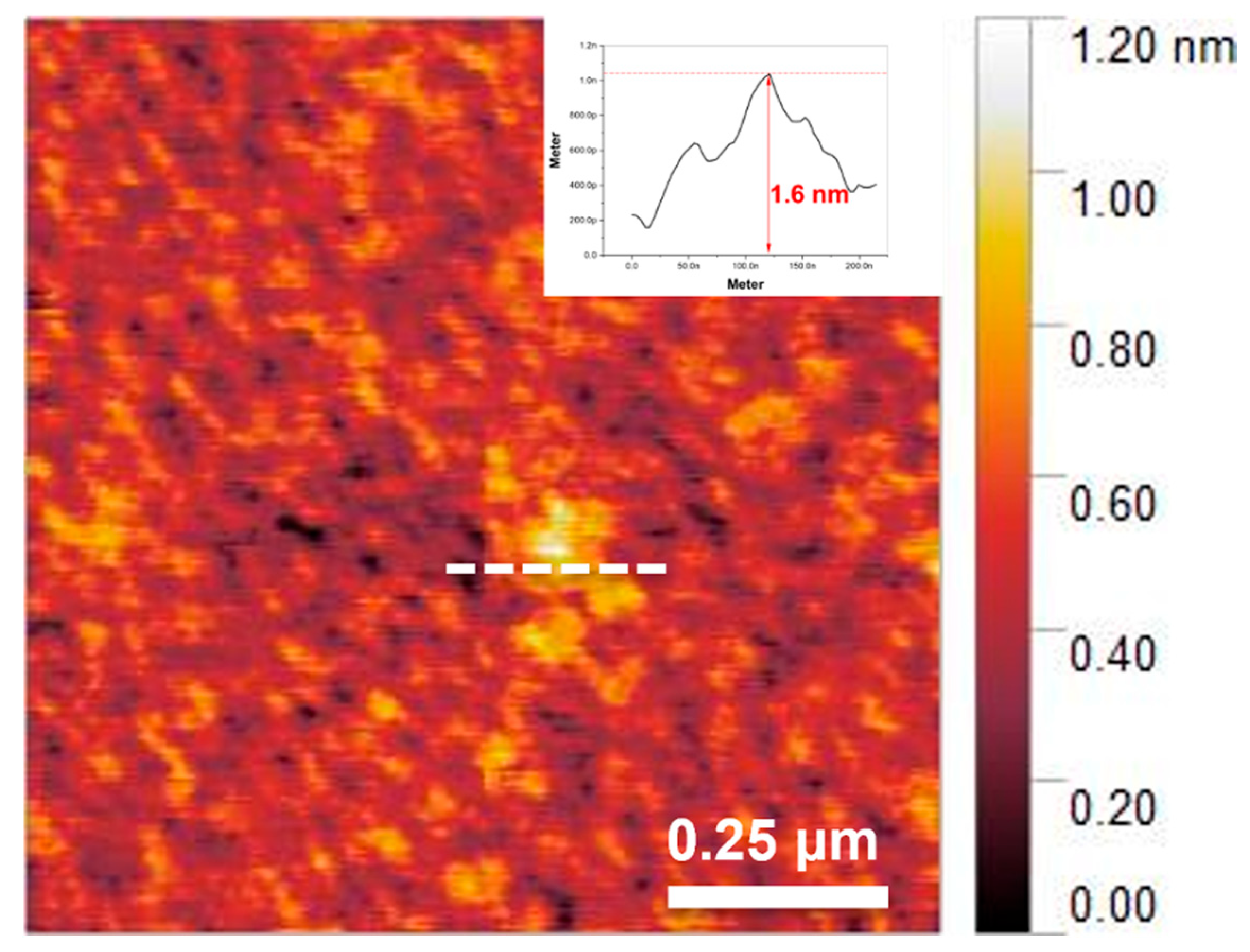
3.1.2. Synthesis of Single-Layer MXene
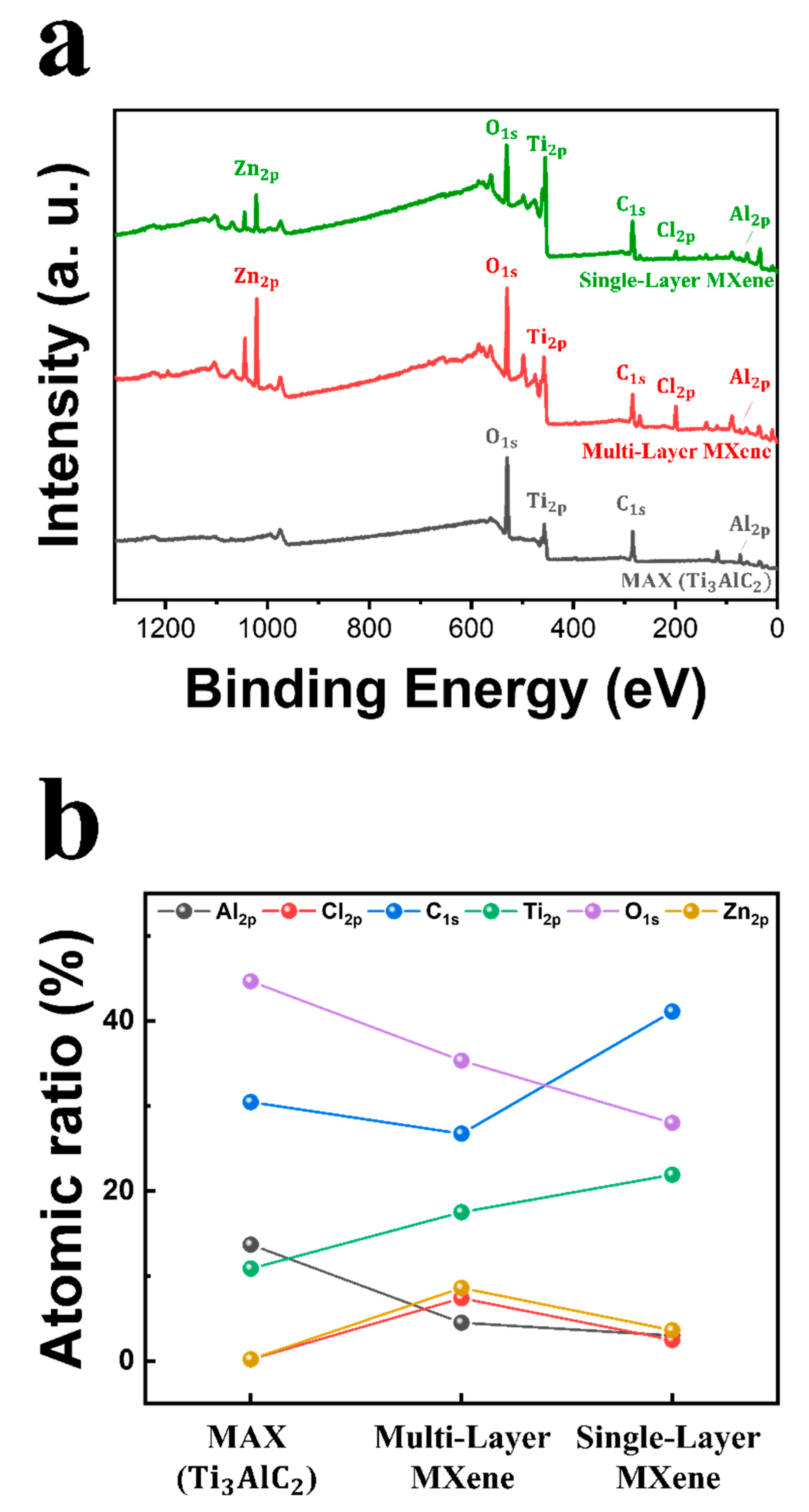
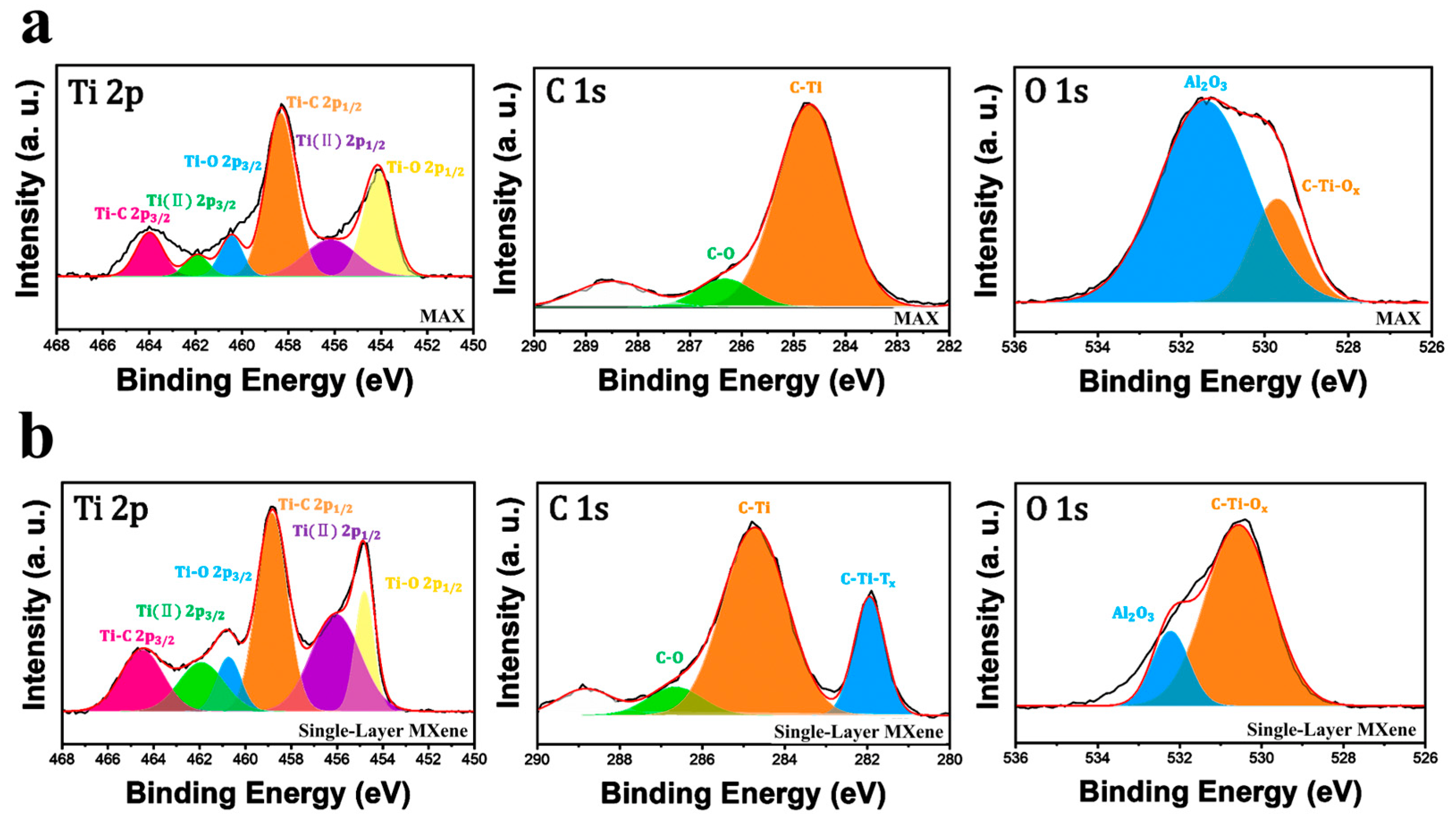
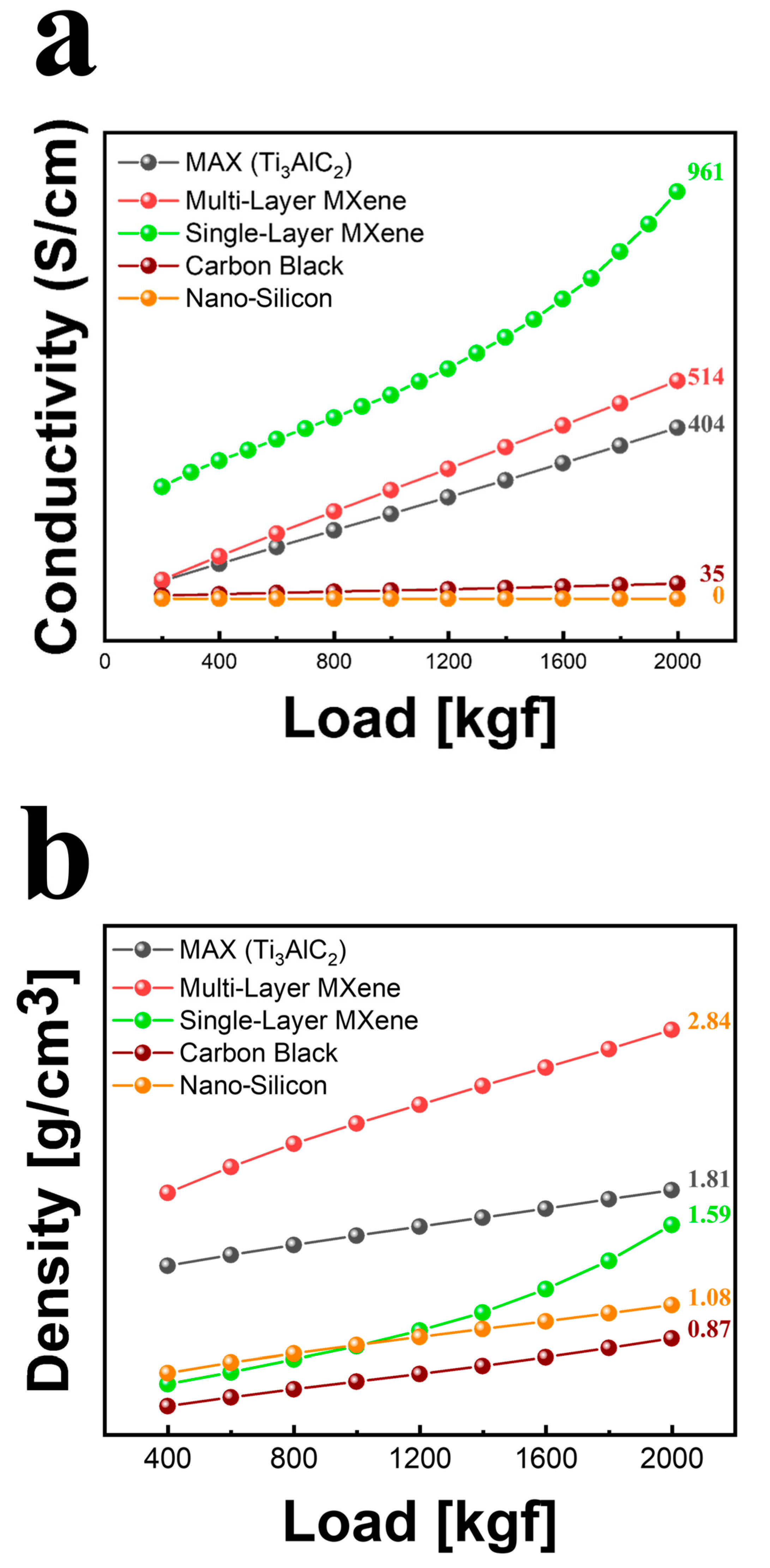
3.1.3. Analysis of Ecofriendly Synthesized MXenes
3.2. Preparation and Battery Applications of Si-MXene/PDA Composite
3.2.1. Preparation of Si-MXene/PDA Composite
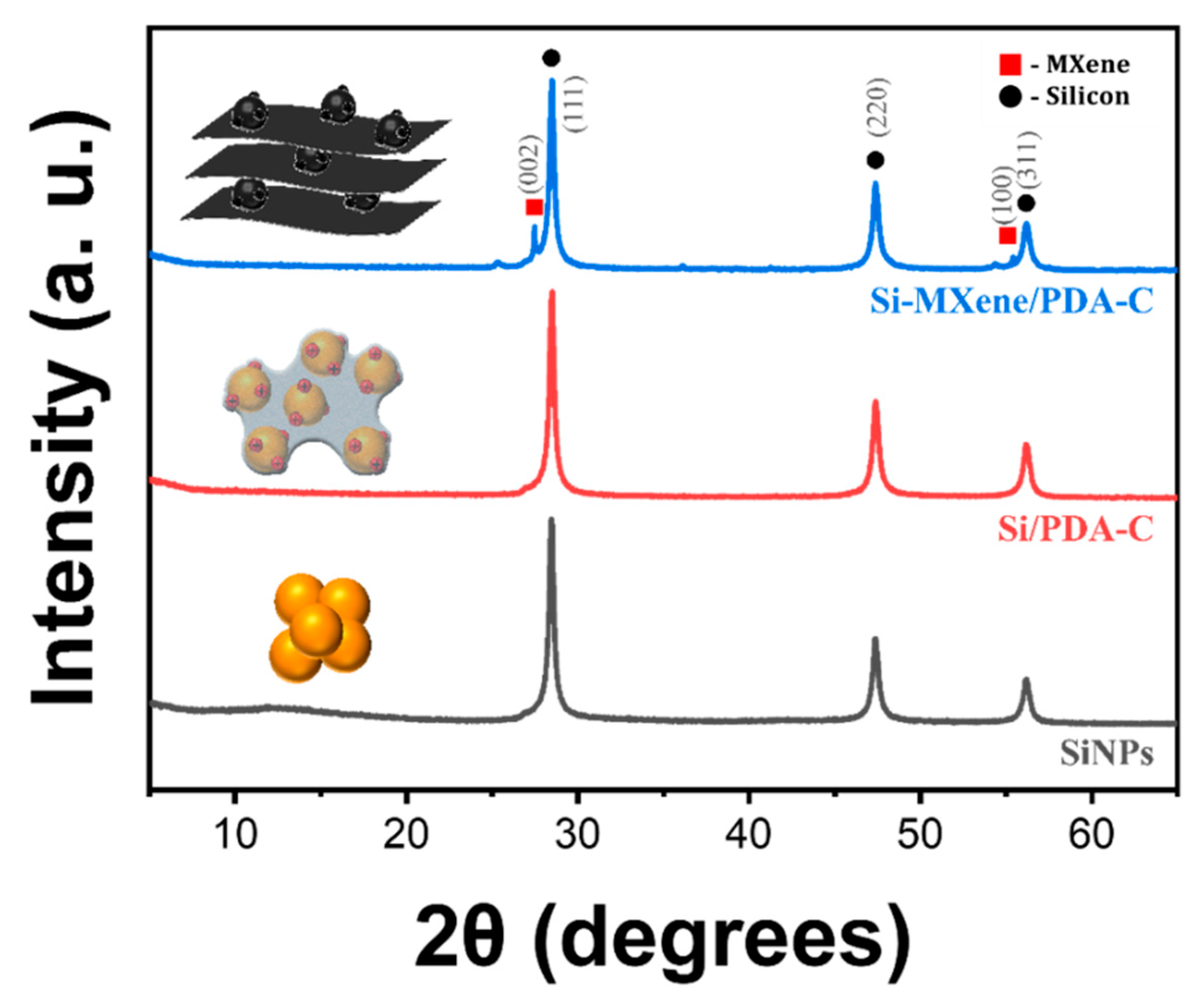
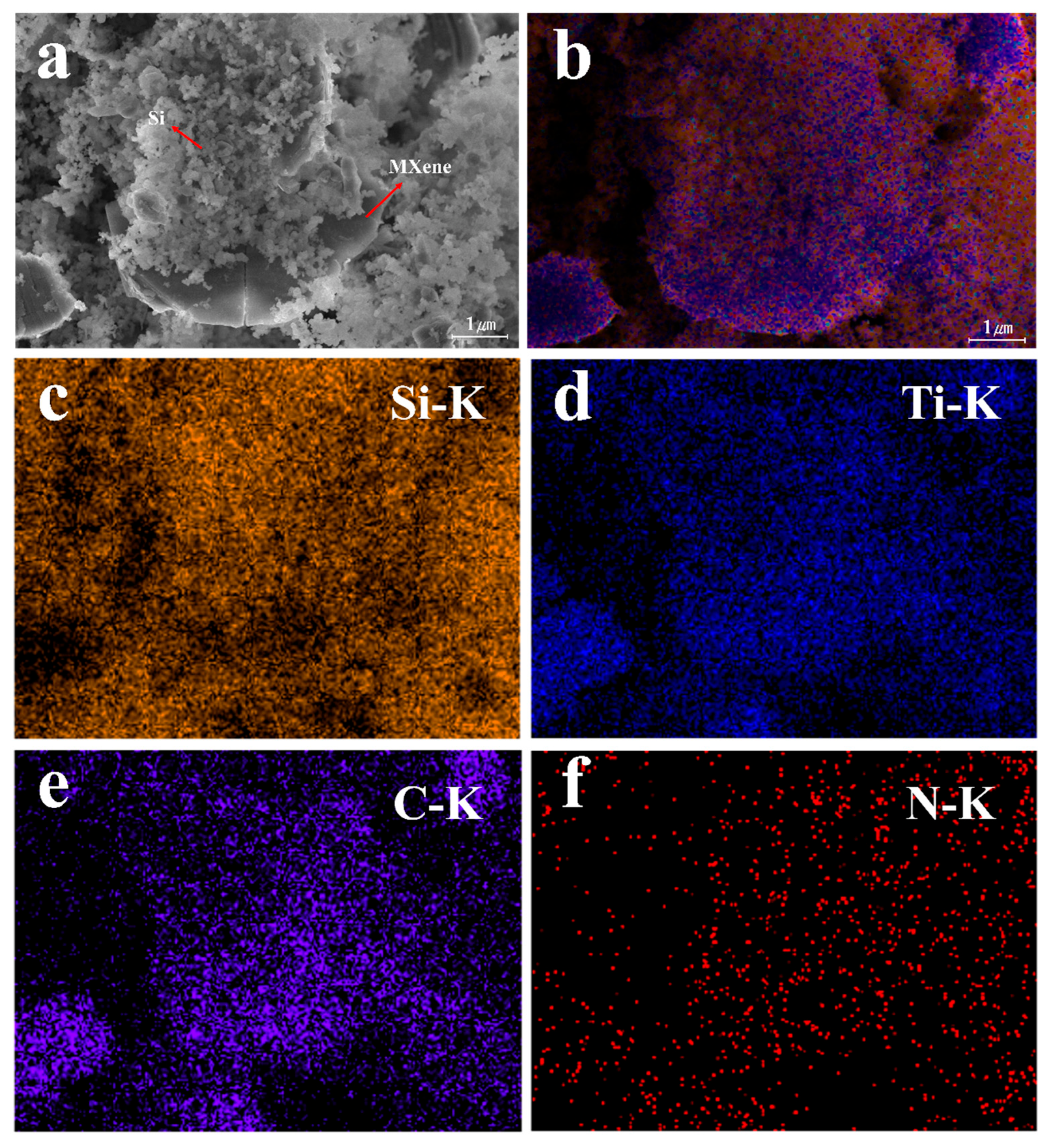
3.2.2. Morphology Analysis of Si-MXene/PDA Composite
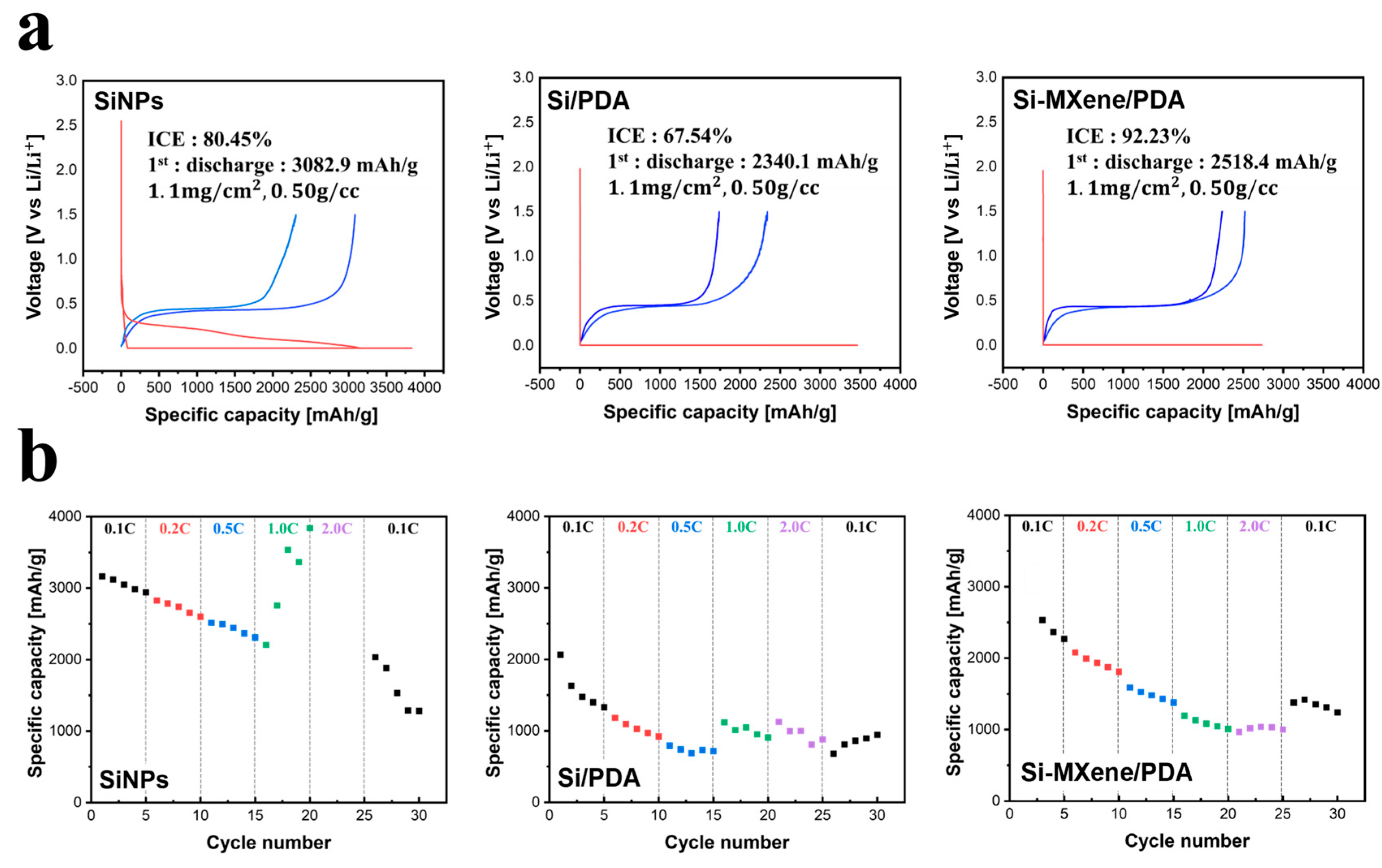
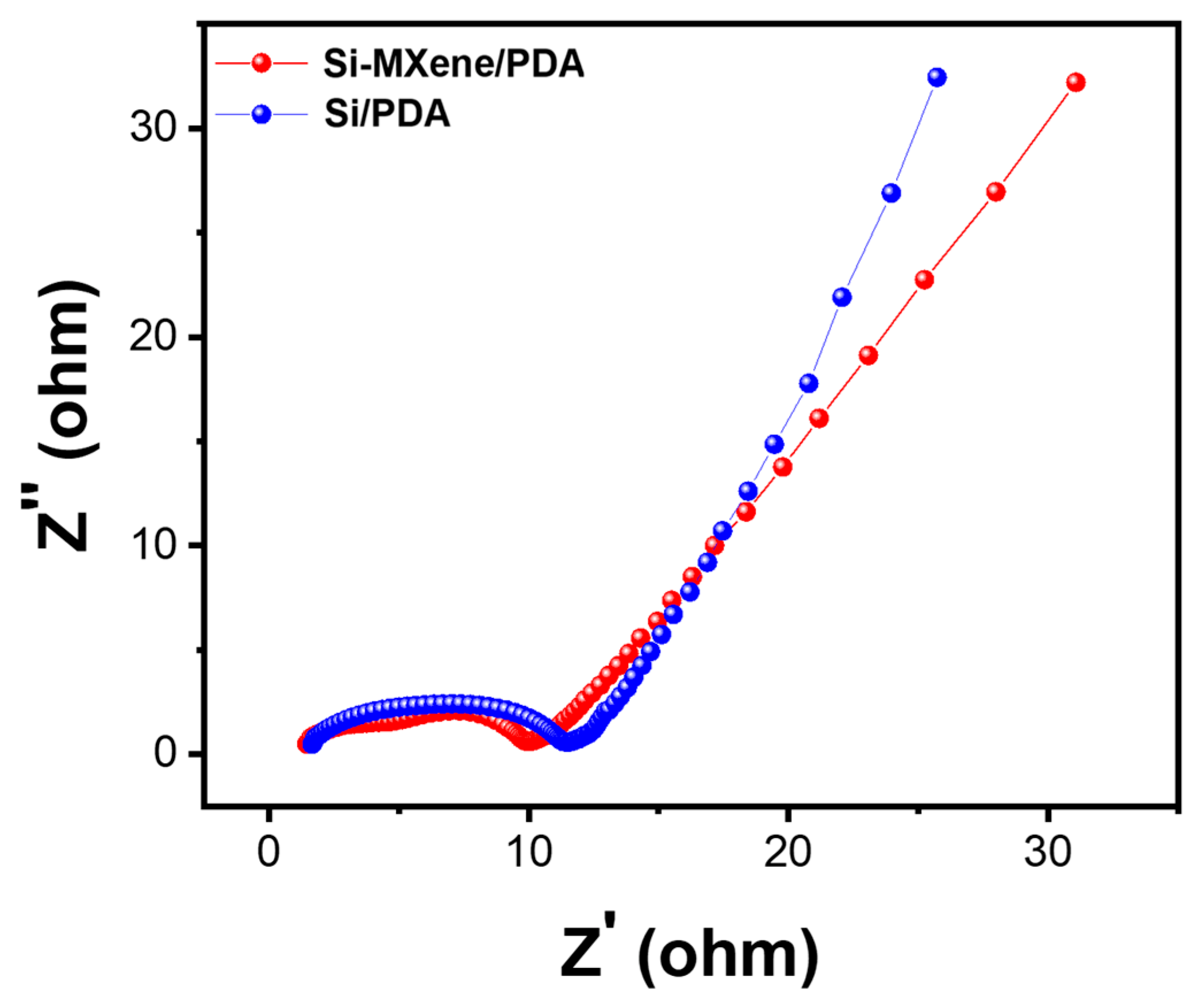
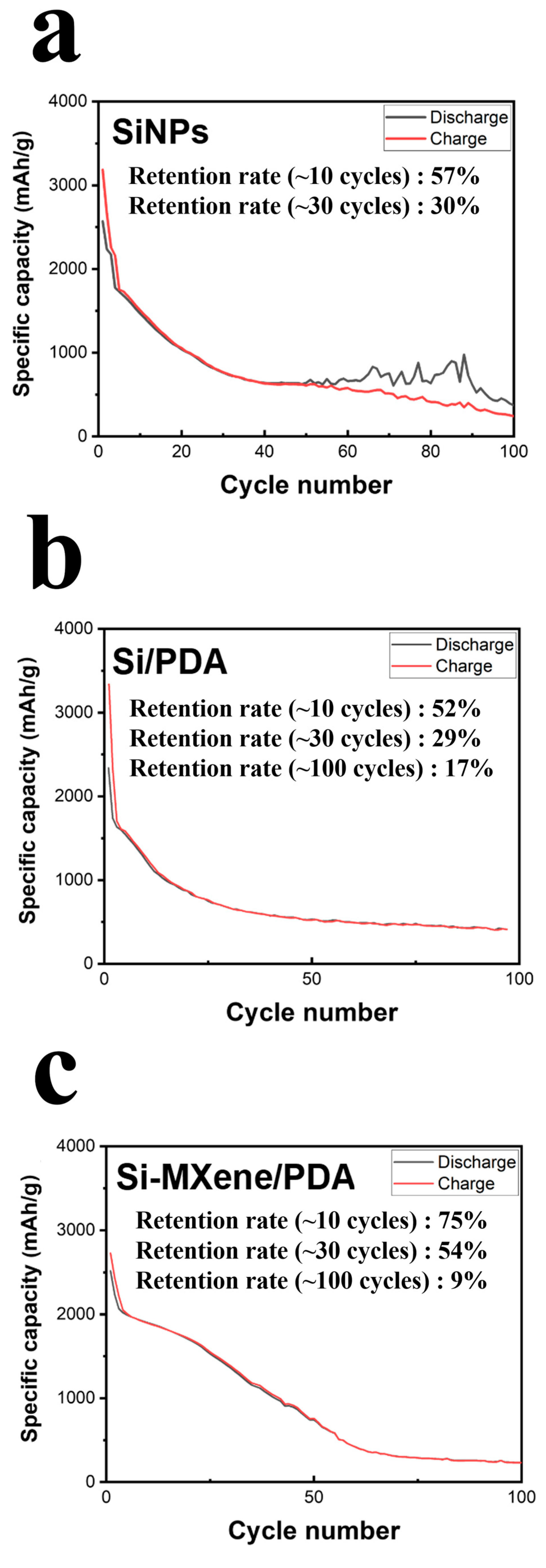
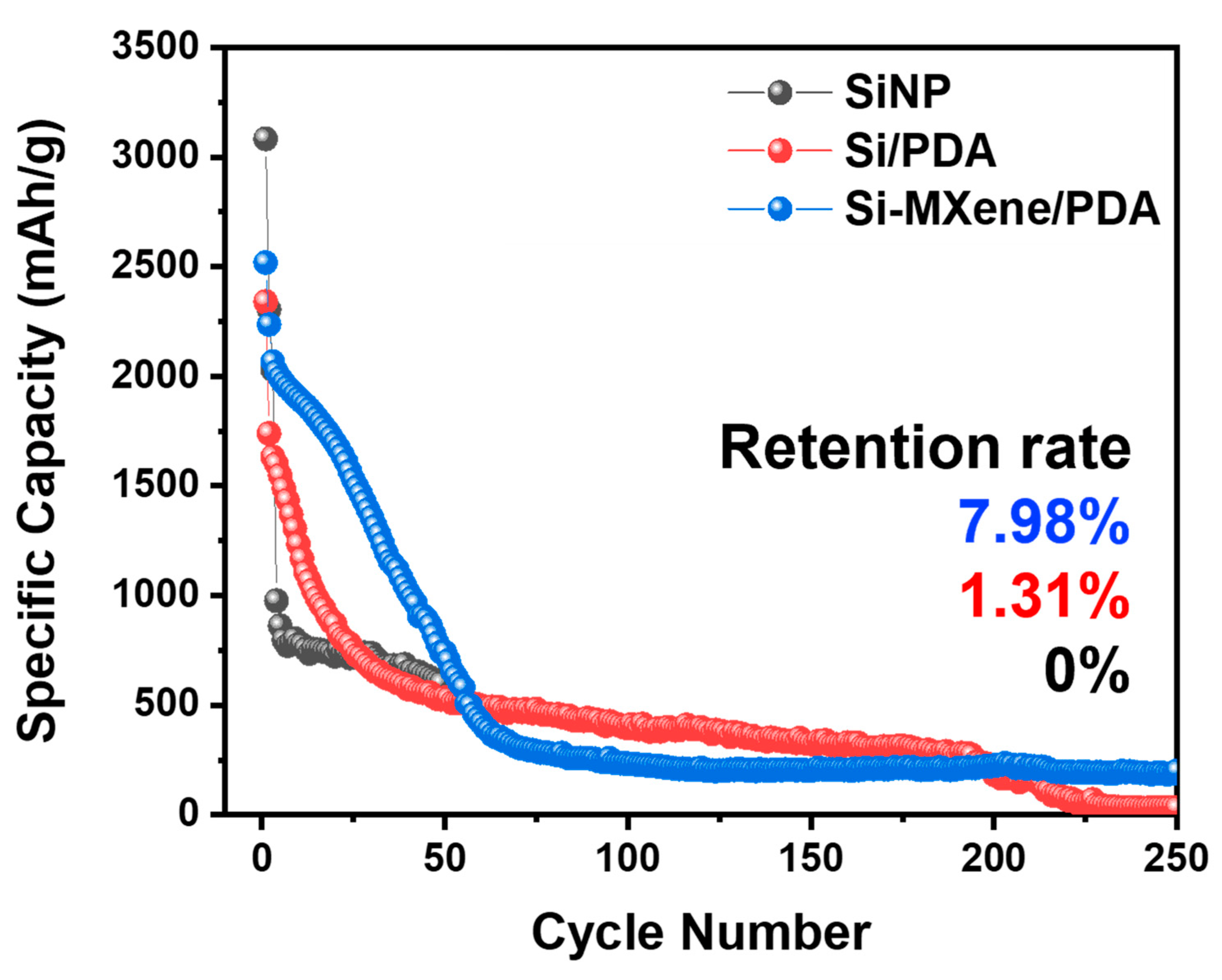
3.3. Electrochemical Performance of Si-MXene Composite Anode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. In MXenes; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2023; pp. 15–29. [Google Scholar]
- Kajiyama, S.; Szabova, L.; Sodeyama, K.; Iinuma, H.; Morita, R.; Gotoh, K.; Tateyama, Y.; Okubo, M.; Yamada, A. Sodium-ion intercalation mechanism in MXene nanosheets. ACS Nano 2016, 10, 3334–3341. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Anasori, B. The rise of Mxenes. In MXenes; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2023; pp. 3–11. [Google Scholar]
- Lee, J.T.; Wyatt, B.C.; Davis Jr, G.A.; Masterson, A.N.; Pagan, A.L.; Shah, A.; Anasori, A.; Sardar, R. Covalent surface modification of Ti3C2Tx MXene with chemically active polymeric ligands producing highly conductive and ordered microstructure films. ACS Nano 2021, 15, 19600–19612. [Google Scholar] [CrossRef] [PubMed]
- Hosayn, C. Étude des Matériaux Bidimensionnels par des Calculs Ab-Initio. Ph.D. Thesis, Université 8 mai 1945 Guelma, Guelma, Algeria, 2023. [Google Scholar]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D titanium carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Sang, X.; Xie, Y.; Lin, M.W.; Alhabeb, M.; Van Aken, K.L.; Gogotsi, Y.; Kent, P.R.C.; Xiao, K.; Unocic, R.R. Atomic defects in monolayer titanium carbide (Ti3C2Tx) MXene. ACS Nano 2016, 10, 9193–9200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, G.; Liu, K.; Cui, Y. Design of complex nanomaterials for energy storage: Past success and future opportunity. Acc. Chem. Res. 2017, 50, 2895–2905. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, S.; Mahesh, N.; Kamaraj, M.; Saranya, T.; Babu, P.S.; Aravind, J.; Kim, W.; Govarthanan, M. Customized carbon composite nanomaterials for the mitigation of emerging contaminants: A review of recent trends. Carbon Lett. 2024, 34, 1091–1114. [Google Scholar] [CrossRef]
- Gohar, O.; Khan, M.Z.; Bibi, I.; Bashir, N.; Tariq, U.; Bakhtiar, M.; Karim, M.R.A.; Ali, F.; Hanif, M.B.; Motola, M. Nanomaterials for advanced energy applications: Recent advancements and future trends. Mater. Des. 2024, 241, 112930. [Google Scholar] [CrossRef]
- Come, J.; Xie, Y.; Naguib, M.; Jesse, S.; Kalinin, S.V.; Gogotsi, Y.; Kent, P.R.C.; Balke, N. Nanoscale elastic changes in 2D Ti3C2Tx (MXene) pseudocapacitive electrodes. Adv. Energy Mater. 2016, 6, 1502290. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Xu, S.; Wei, G.; Li, J.; Han, W.; Gogotsi, Y. Flexible MXene–graphene electrodes with high volumetric capacitance for integrated co-cathode energy conversion/storage devices. J. Mater. Chem. A 2017, 5, 17442–17451. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides. In MXenes: From Discovery to Applications of Two-Dimensional Metal Carbides and Nitrides; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2023; pp. 933–947. [Google Scholar]
- Lyu, B.; Kim, M.; Jing, H.; Kang, J.; Qian, C.; Lee, S.; Cho, J.H. Large-area MXene electrode array for flexible electronics. ACS Nano 2019, 13, 11392–11400. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene gas sensors with ultrahigh signal-to-noise ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef]
- Hussain, I.; Arifeen, W.U.; Khan, S.A.; Aftab, S.; Javed, M.S.; Hussain, S.; Ahmad, M.; Chen, X.; Zhao, J.; Rosaiah, P.; et al. M4X3 MXenes: Application in Energy Storage Devices. Nano-Micro Lett. 2024, 16, 215. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Gogotsi, Y. 2D materials with nanoconfined fluids for electrochemical energy storage. Joule 2017, 1, 443–452. [Google Scholar] [CrossRef]
- Wozniak, J.; Jastrzębska, A.; Olszyna, A. Challenges and opportunities in tailoring MAX phases as a starting materials for MXenes development. Mater. Technol. 2022, 37, 1639–1650. [Google Scholar] [CrossRef]
- Meng, F.; Seredych, M.; Chen, C.; Gura, V.; Mikhalovsky, S.; Sandeman, S.; Ingavle, G.; Ozulumba, T.; Miao, L.; Anasori, B.; et al. MXene sorbents for removal of urea from dialysate: A step toward the wearable artificial kidney. ACS Nano 2018, 12, 10518–10528. [Google Scholar] [CrossRef] [PubMed]
- Shuck, C.E.; Gogotsi, Y. Taking MXenes from the lab to commercial products. Chem. Eng. J. 2020, 401, 125786. [Google Scholar] [CrossRef]
- Zha, X.H.; Luo, K.; Li, Q.; Huang, Q.; He, J.; Wen, X.; Du, S. Role of the surface effect on the structural, electronic and mechanical properties of the carbide MXenes. Europhys. Lett. 2015, 111, 26007. [Google Scholar] [CrossRef]
- Natu, V.; Sokol, M.; Verger, L.; Barsoum, M.W. Effect of edge charges on stability and aggregation of Ti3C2Tz MXene colloidal suspensions. J. Phys. Chem. C 2018, 122, 27745–27753. [Google Scholar] [CrossRef]
- Xiu, L.-Y.; Wang, Z.-Y.; Qiu, J.-S. General synthesis of MXene by green etching chemistry of fluoride-free Lewis acidic melts. Rare Met. 2020, 39, 1237–1238. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Dyatkin, B.; Gogotsi, Y.; Barsoum, M.W. Kinetics of aluminum extraction from Ti3AlC2 in hydrofluoric acid. Mater. Chem. Phys. 2013, 139, 147–152. [Google Scholar] [CrossRef]
- Zhou, J.; Zha, X.; Zhou, X.; Chen, F.; Gao, G.; Wang, S.; Shen, C.; Chen, T.; Zhi, C.; Eklund, P.; et al. Synthesis and electrochemical properties of two-dimensional hafnium carbide. ACS Nano 2017, 11, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhou, Z.; Shen, P. Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’with high volumetric capacitance. In MXenes; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2023; pp. 379–399. [Google Scholar]
- Talreja, N.; Chauhan, D.; Ashfaq, M. Two-Dimensional Hybrid Composites: Synthesis, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.; Feng, X. Fluoride-free synthesis of two-dimensional titanium carbide (MXene) using a binary aqueous system. Angew. Chem. 2018, 130, 15717–15721. [Google Scholar] [CrossRef]
- Frisenda, R.; Molina-Mendoza, A.J.; Mueller, T.; Castellanos-Gomez, A.; Van Der Zant, H.S. Atomically thin p–n junctions based on two-dimensional materials. Chem. Soc. Rev. 2018, 47, 3339–3358. [Google Scholar] [CrossRef]
- Urbankowski, P.; Anasori, B.; Makaryan, T.; Er, D.; Kota, S.; Walsh, P.L.; Zhao, M.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene). Nanoscale 2016, 8, 11385–11391. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, B.; Ouyang, C.; Yang, S.A.; Yao, Y. Investigations on V2C and V2CX2 (X = F, OH) monolayer as a promising anode material for Li ion batteries from first-principles calculations. J. Phys. Chem. C 2014, 118, 24274–24281. [Google Scholar] [CrossRef]
- Kruger, D.D.; García, H.; Primo, A. Molten Salt Derived MXenes: Synthesis and Applications. Adv. Sci. 2024, 11, 2307106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, F.; Han, J.; Bai, S.; Tan, J.; Liu, J.; Li, F. Challenges and recent progress on silicon-based anode materials for next-generation lithium-ion batteries. Small Struct. 2021, 2, 2100009. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Lin, D.; Lu, Z.; Hsu, P.C.; Lee, H.R.; Liu, N.; Zhao, J.; Wang, H.; Liu, C.; Cui, Y. A high tap density secondary silicon particle anode fabricated by scalable mechanical pressing for lithium-ion batteries. Energy Environ. Sci. 2015, 8, 2371–2376. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.; Yu, G. Material and structural design of novel binder systems for high-energy, high-power lithium-ion batteries. Acc. Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef]
- Li, P.; Zhao, G.; Zheng, X.; Xu, X.; Yao, C.; Sun, W.; Dou, S.X. Recent progress on silicon-based anode materials for practical lithium-ion battery applications. Energy Storage Mater. 2018, 15, 422–446. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Li, F.; Xu, J.; Hou, Z.; Li, M.; Yang, R. Silicon anodes for high-performance storage devices: Structural design, material compounding, advances in electrolytes and binders. ChemNanoMat 2020, 6, 720–738. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, Z.; Liu, Q.; Tian, J.; Liu, M.; Dong, K.; Lei, Z. Research progress of silicon suboxide-based anodes for lithium-ion batteries. Front. Mater. 2021, 7, 628233. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Improving cycle stability of Si anode through partially carbonized polydopamine coating. J. Electroanal. Chem. 2020, 876, 114738. [Google Scholar] [CrossRef]
- Tamakloe, W.; Agyeman, D.A.; Park, M.; Yang, J.; Kang, Y.M. Polydopamine-induced surface functionalization of carbon nanofibers for Pd deposition enabling enhanced catalytic activity for the oxygen reduction and evolution reactions. J. Mater. Chem. A 2019, 7, 7396–7405. [Google Scholar] [CrossRef]
- Zanoletti, A.; Carena, E.; Ferrara, C.; Bontempi, E. A Review of Lithium-Ion Battery Recycling: Technologies, Sustainability, and Open Issues. Batteries 2024, 10, 38. [Google Scholar] [CrossRef]
- Lei, C.; Han, F.; Li, D.; Li, W.C.; Sun, Q.; Zhang, X.Q.; Lu, A.H. Dopamine as the coating agent and carbon precursor for the fabrication of N-doped carbon coated Fe3O4 composites as superior lithium ion anodes. Nanoscale 2013, 5, 1168–1175. [Google Scholar] [CrossRef]
- Zhang, T.; Shevchuk, K.; Wang, R.J.; Kim, H.; Hourani, J.; Gogotsi, Y. Delamination of Chlorine-Terminated MXene Produced Using Molten Salt Etching. Chem. Mater. 2024, 36, 1998–2006. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Zhang, J.; Li, G.; Huang, H.; Zhang, X.; Jiang, Q. Enhancement of the electrical properties of MXene Ti3C2 nanosheets by post-treatments of alkalization and calcination. Mater. Lett. 2015, 160, 537–540. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Lim, G.P.; Soon, C.F.; Morsin, M.; Ahmad, M.K.; Nayan, N.; Tee, K.S. Synthesis, characterization and antifungal property of Ti3C2Tx MXene nanosheets. Ceram. Int. 2020, 46, 20306–20312. [Google Scholar] [CrossRef]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at every step, from their precursors to single flakes and assembled films. Prog. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Syamsai, R.; Kollu, P.; Jeong, S.K.; Grace, A.N. Synthesis and properties of 2D-titanium carbide MXene sheets towards electrochemical energy storage applications. Ceram. Int. 2017, 43, 13119–13126. [Google Scholar] [CrossRef]
- Sarycheva, A.; Gogotsi, Y. Raman spectroscopy analysis of the structure and surface chemistry of Ti3C2Tx Mxene. In MXenes; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2023; pp. 333–355. [Google Scholar]
- Yang, Q.; Eder, S.J.; Martini, A.; Grützmacher, P.G. Effect of surface termination on the balance between friction and failure of Ti3C2Tx MXenes. npj Mater. Degrad. 2023, 7, 6. [Google Scholar] [CrossRef]
- Melchior, S.A.; Raju, K.; Ike, I.S.; Erasmus, R.M.; Kabongo, G.; Sigalas, I.; Iyuke, S.E.; Ozoemena, K.I. High-voltage symmetric supercapacitor based on 2D titanium carbide (MXene, Ti2CTx)/carbon nanosphere composites in a neutral aqueous electrolyte. J. Electrochem. Soc. 2018, 165, A501–A511. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Q.; Li, J.; Liu, H. Boosting the photocatalytic activity of CdLa2S4 for hydrogen production using Ti3C2 MXene as a co-catalyst. Appl. Catal. B Environ. 2020, 267, 118379. [Google Scholar] [CrossRef]
- Bao, Z.; Lu, C.; Cao, X.; Zhang, P.; Yang, L.; Zhang, H.; Sha, D.; He, W.; Zhang, W.; Pan, L.; et al. Role of MXene surface terminations in electrochemical energy storage: A review. Chin. Chem. Lett. 2021, 32, 2648–2658. [Google Scholar] [CrossRef]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef]
- Maity, S.; Chatterjee, A. Conductive polymer based electro-conductive textiles for novel applications. Tech. Text. 2015, 1, E16–E18. [Google Scholar]
- Li, R.; Hu, X.; Shan, S.; Li, Y.; Cui, W.; Liu, L. Study on the controlled release properties of modified multi-walled carbon nanotubes on sulforaphane. Carbon Lett. 2024, 34, 757–765. [Google Scholar] [CrossRef]
- Suresh, S.; Wu, Z.P.; Bartolucci, S.F.; Basu, S.; Mukherjee, R.; Gupta, T.; Hundekar, P.; Shi, Y.; Lu, T.-M.; Koratkar, N. Protecting silicon film anodes in lithium-ion batteries using an atomically thin graphene drape. ACS Nano 2017, 11, 5051–5061. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, P.; Ramachandran, K.; Maheshvaran, K.; Senthil, T.S.; Manivel, P. Simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid using Au decorated carbon nanofibers modified screen printed electrode. Carbon Lett. 2024, 34, 2325–2341. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, Z.Y.; Wang, X.Y.; Hirano, A.; Imanishi, N.; Takeda, Y. Electrochemical behaviors of Si/C composite synthesized from F-containing precursors. J. Power Sources 2009, 189, 733–737. [Google Scholar] [CrossRef]
- Zuo, X.; Wang, X.; Xia, Y.; Yin, S.; Ji, Q.; Yang, Z.; Wang, M.; Zheng, X.; Qiu, B.; Liu, Z.; et al. Silicon/carbon lithium-ion battery anode with 3D hierarchical macro-/mesoporous silicon network: Self-templating synthesis via magnesiothermic reduction of silica/carbon composite. J. Power Sources 2019, 412, 93–104. [Google Scholar] [CrossRef]
- Wu, D.; Xing, Y.; Zhang, D.; Hao, Z.; Dong, Q.; Han, Y.; Liu, L.; Wang, M.; Zhang, R. Optimized interfacial compatibility of carbon fiber and epoxy resin via controllable thickness and activated ingredients of polydopamine layer. Carbon Lett. 2024, 34, 351–359. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, J.; Chen, X.; Li, Y.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Enhanced cycling performance of Si-MXene nanohybrids as anode for high performance lithium ion batteries. Chem. Eng. J. 2019, 378, 122212. [Google Scholar] [CrossRef]
- Han, X.; Zhou, W.; Chen, M.; Chen, J.; Wang, G.; Liu, B.; Luo, L.; Chen, S.; Zhang, Q.; Shi, S.; et al. Interfacial nitrogen engineering of robust silicon/MXene anode toward high energy solid-state lithium-ion batteries. J. Energy Chem. 2022, 67, 727–735. [Google Scholar] [CrossRef]
- Xia, M.; Chen, B.; Gu, F.; Zu, L.; Xu, M.; Feng, Y.; Zhang, Z.; Zhang, H.; Zhang, C.; Yang, J. Ti3C2Tx MXene nanosheets as a robust and conductive tight on Si anodes significantly enhance electrochemical lithium storage performance. ACS Nano 2020, 14, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Sahiner, M.; Suner, S.S.; Sahiner, N. Improved biomedical properties of polydopamine-coated carbon nanotubes. Micromachines 2021, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Park, J.H.; Seo, M.K.; Kim, S. Synthesis and electrochemical performances of platinum decorated polydopamine-coated carbon nanotubes/graphene composites as fuel cell catalysts. J. Alloys Compd. 2020, 822, 153586. [Google Scholar] [CrossRef]
- Zhou, G.; Kim, N.R.; Chun, S.E.; Lee, W.; Um, M.K.; Chou, T.W.; Islam, M.F.; Byun, J.-H.; Oh, Y. Highly porous and easy shapeable poly-dopamine derived graphene-coated single walled carbon nanotube aerogels for stretchable wire-type supercapacitors. Carbon 2018, 130, 137–144. [Google Scholar] [CrossRef]
- Zou, R.; Liu, F.; Hu, N.; Ning, H.; Gong, Y.; Wang, S.; Huang, K.; Jiang, X.; Xu, C.; Fu, S.; et al. Graphene/graphitized polydopamine/carbon nanotube all-carbon ternary composite films with improved mechanical properties and through-plane thermal conductivity. ACS Appl. Mater. Interfaces 2020, 12, 57391–57400. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. EIS study on the formation of solid electrolyte interface in Li-ion battery. Electrochim. Acta 2006, 51, 1636–1640. [Google Scholar] [CrossRef]
- Xia, W.; Cheng, M.; Hu, J.; Liu, Q.; Wei, T.; Wang, R.; Li, W.; Liu, B. Facile and controllable synthesis of nitrogen self-doped chitosan-derived carbon for high-performance Li-ion batteries. Carbon Lett. 2024, 34, 85–94. [Google Scholar] [CrossRef]
- Xu, Z.L.; Liu, X.; Luo, Y.; Zhou, L.; Kim, J.K. Nanosilicon anodes for high performance rechargeable batteries. Prog. Mater. Sci. 2017, 90, 1–44. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Chen, L.; Wu, Z.; Liang, Y. A high capacity nano Si composite anode material for lithium rechargeable batteries. Electrochem. Solid-State Lett. 1999, 2, 547. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jung, Y.; Lee, W.; Jeon, Y.-P.; Hong, J.-Y.; Lee, J.U. Sustainable MXene Synthesis via Molten Salt Method and Nano-Silicon Coating for Enhanced Lithium-Ion Battery Performance. Molecules 2025, 30, 812. https://doi.org/10.3390/molecules30040812
Kim H, Jung Y, Lee W, Jeon Y-P, Hong J-Y, Lee JU. Sustainable MXene Synthesis via Molten Salt Method and Nano-Silicon Coating for Enhanced Lithium-Ion Battery Performance. Molecules. 2025; 30(4):812. https://doi.org/10.3390/molecules30040812
Chicago/Turabian StyleKim, Hansu, Yunki Jung, Wonhwa Lee, Young-Pyo Jeon, Jin-Yong Hong, and Jea Uk Lee. 2025. "Sustainable MXene Synthesis via Molten Salt Method and Nano-Silicon Coating for Enhanced Lithium-Ion Battery Performance" Molecules 30, no. 4: 812. https://doi.org/10.3390/molecules30040812
APA StyleKim, H., Jung, Y., Lee, W., Jeon, Y.-P., Hong, J.-Y., & Lee, J. U. (2025). Sustainable MXene Synthesis via Molten Salt Method and Nano-Silicon Coating for Enhanced Lithium-Ion Battery Performance. Molecules, 30(4), 812. https://doi.org/10.3390/molecules30040812






