Rapid Fluorescent Probe Detection of Magnesium Impurities in High-Purity Lithium Carbonate Brine Systems
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Assay Reagents
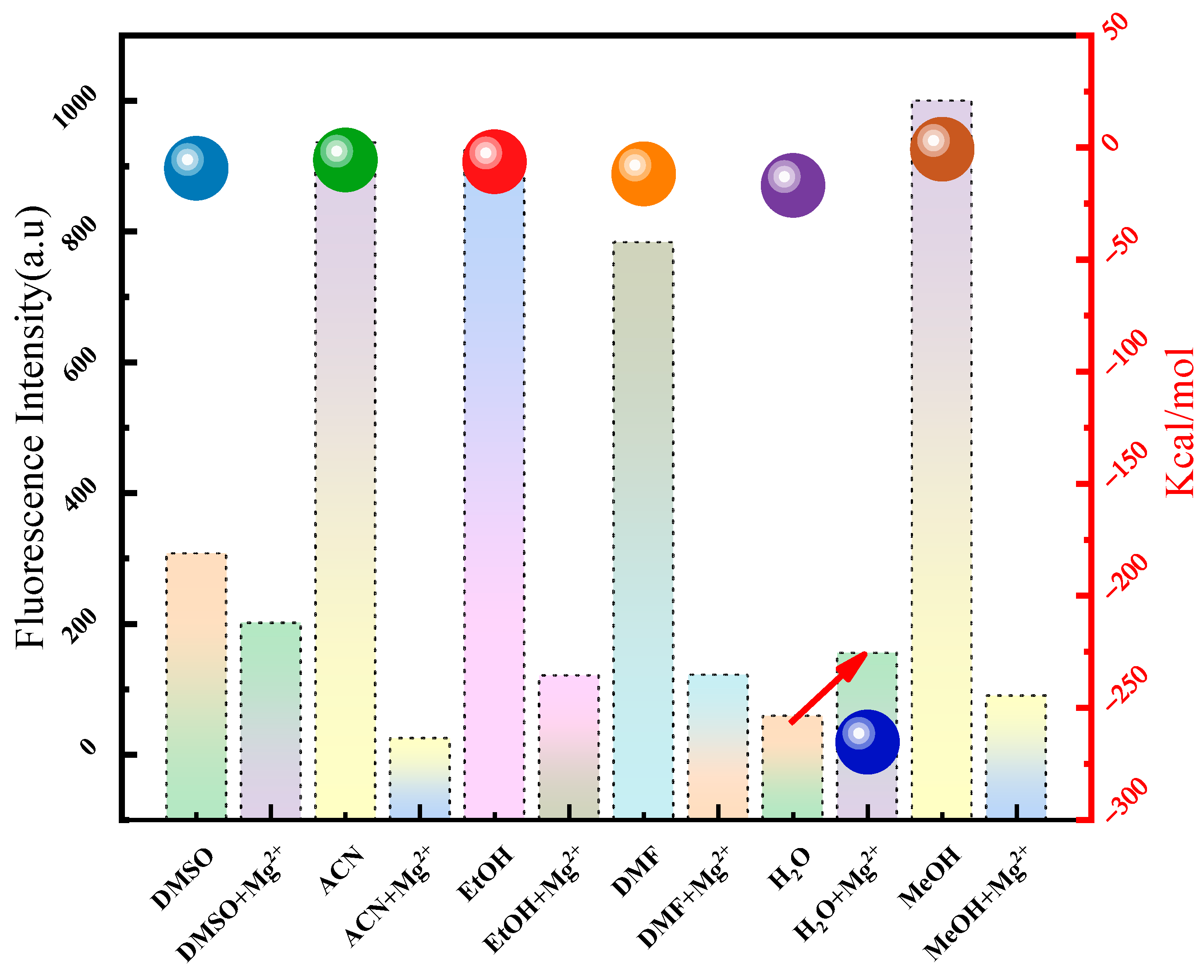
2.2. Optical Properties of Detection Reagents in Brine System
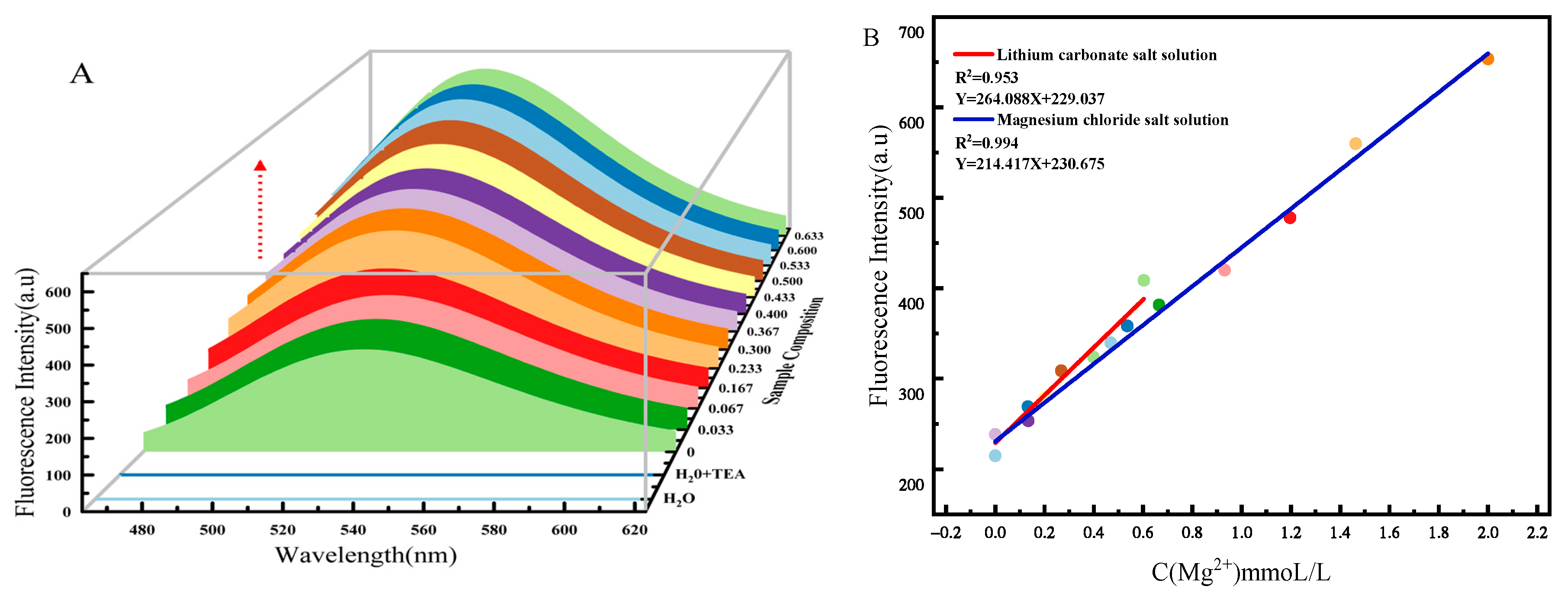
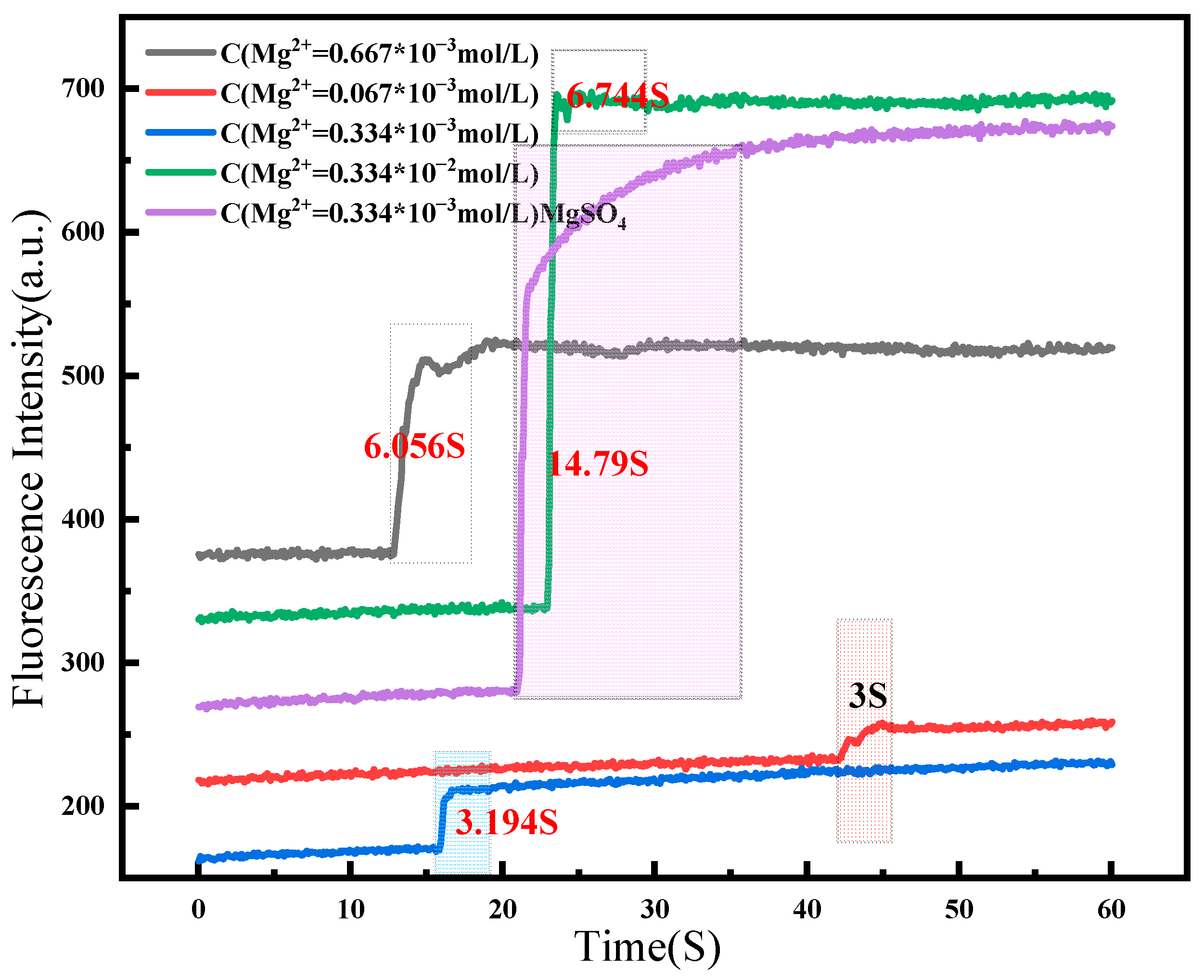
2.3. Detection Sensitivity for Mg2+ in Brine System
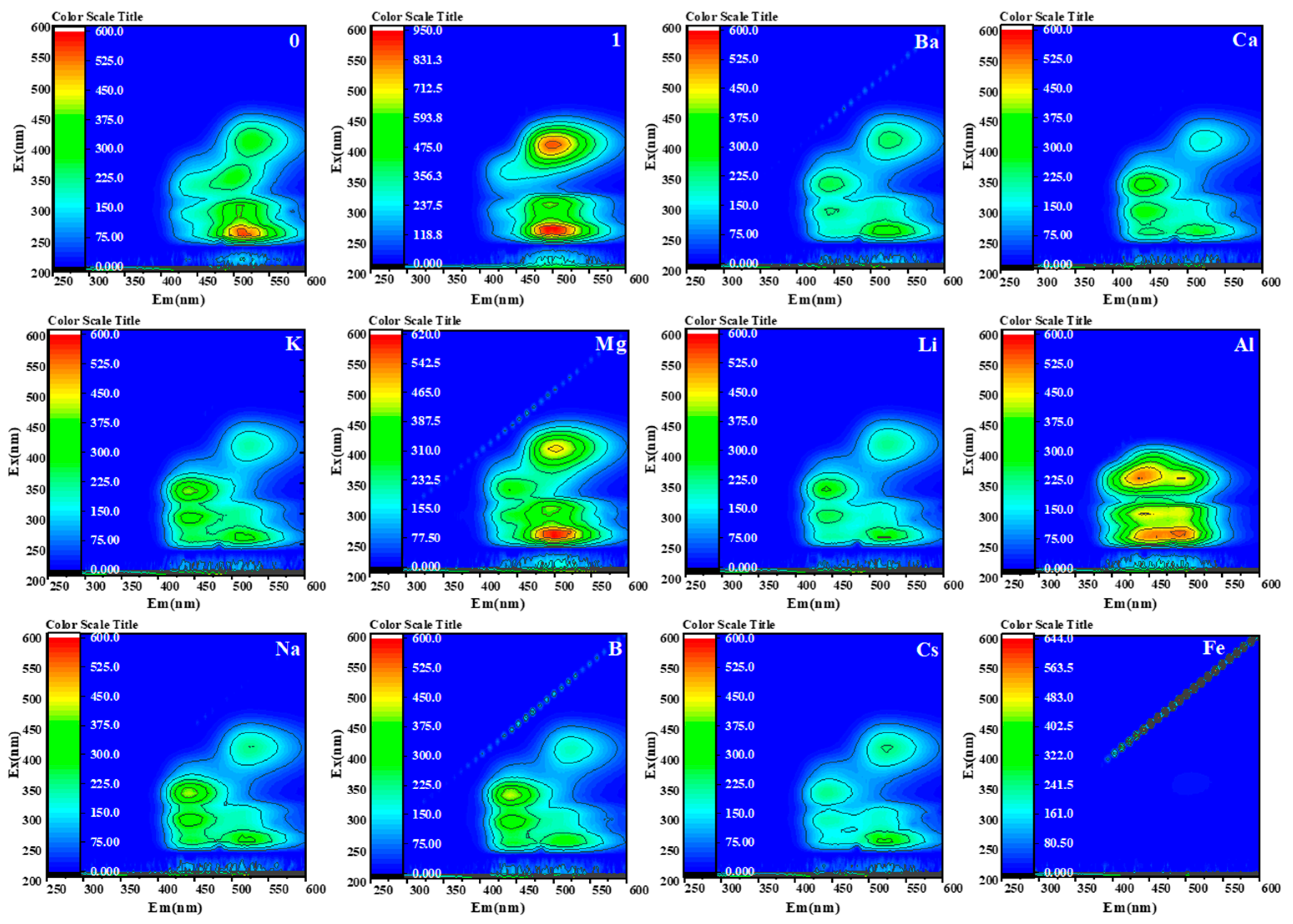
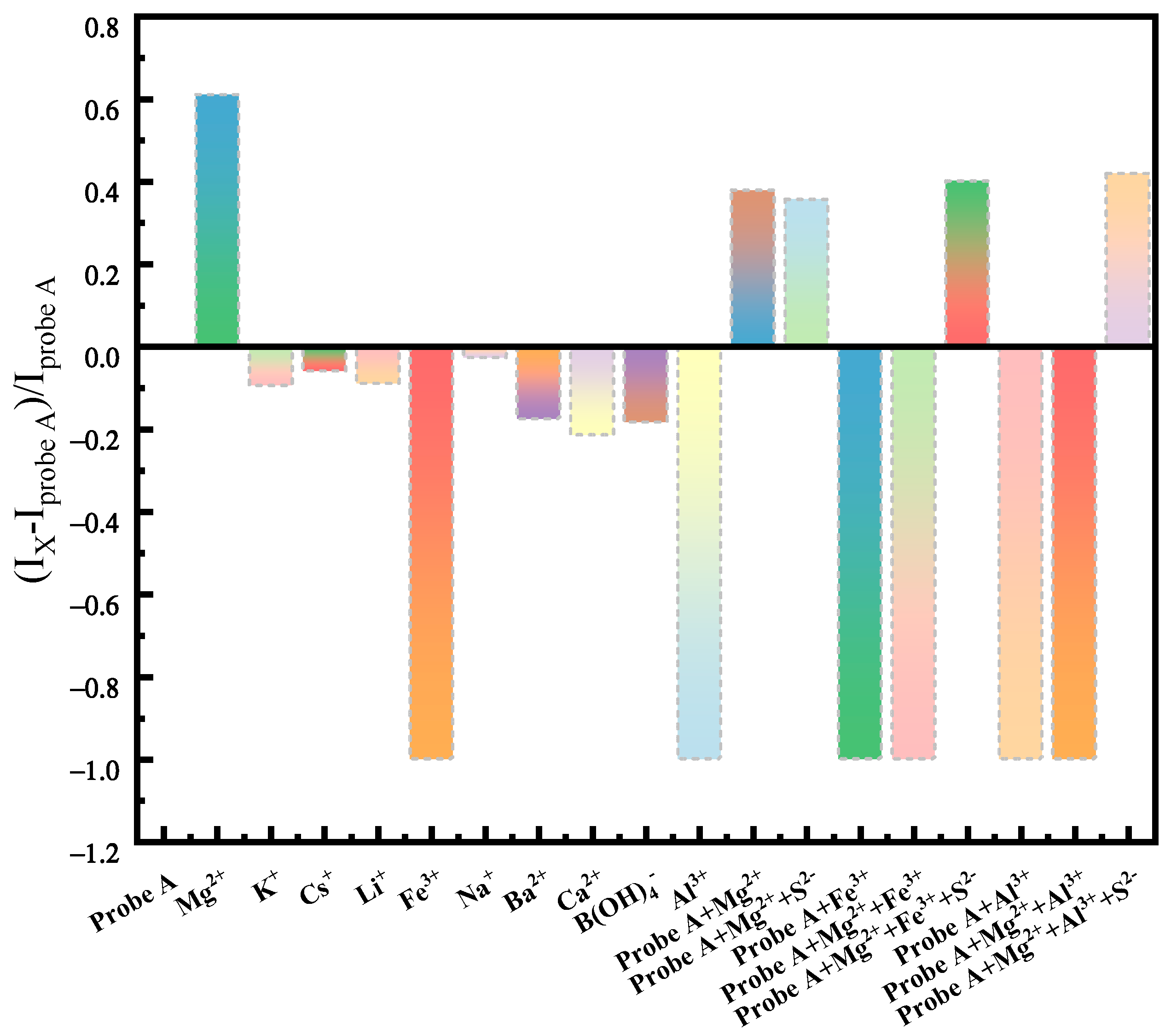
2.4. Specific Detection of Mg2+ in Saline Solution
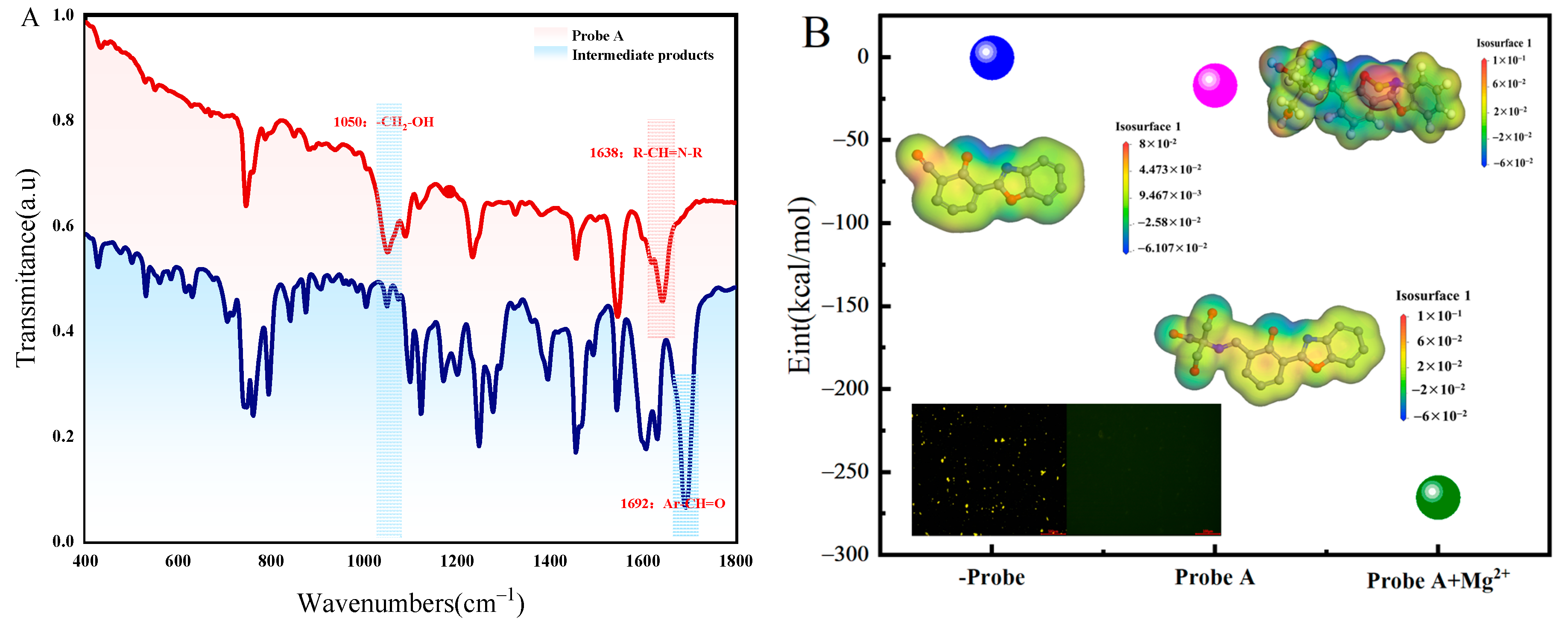
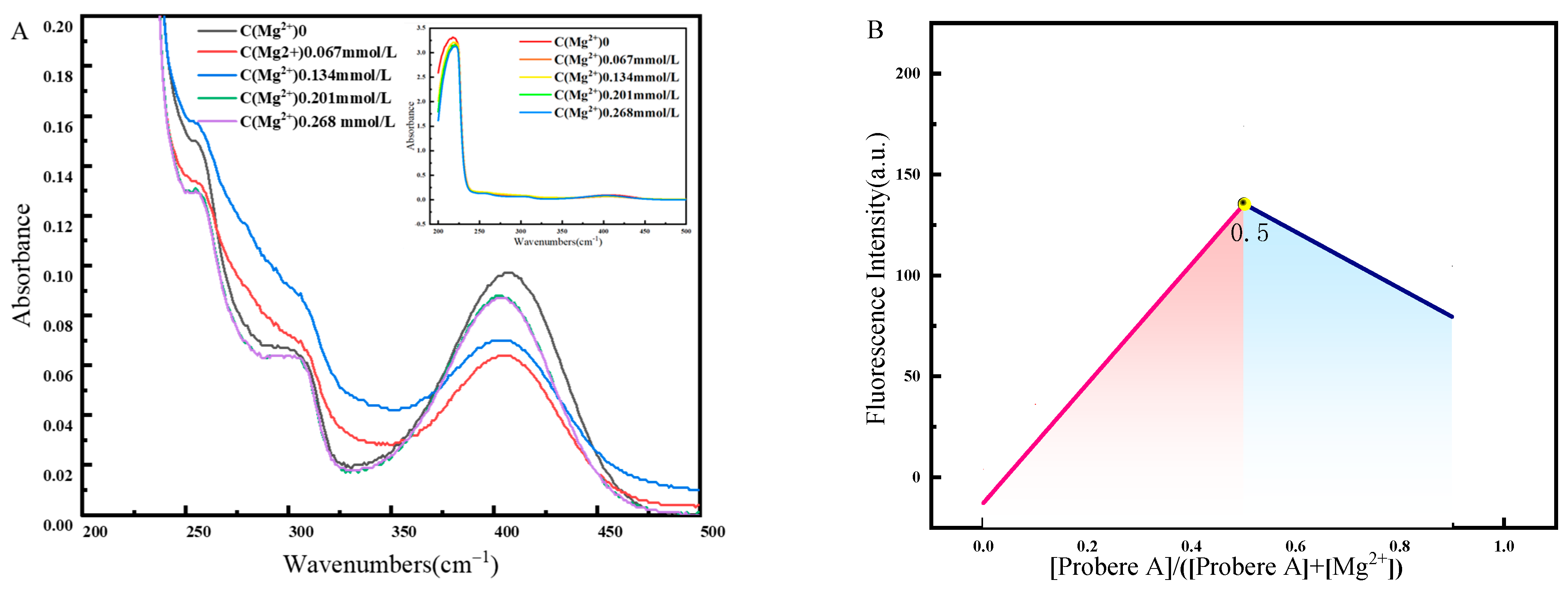
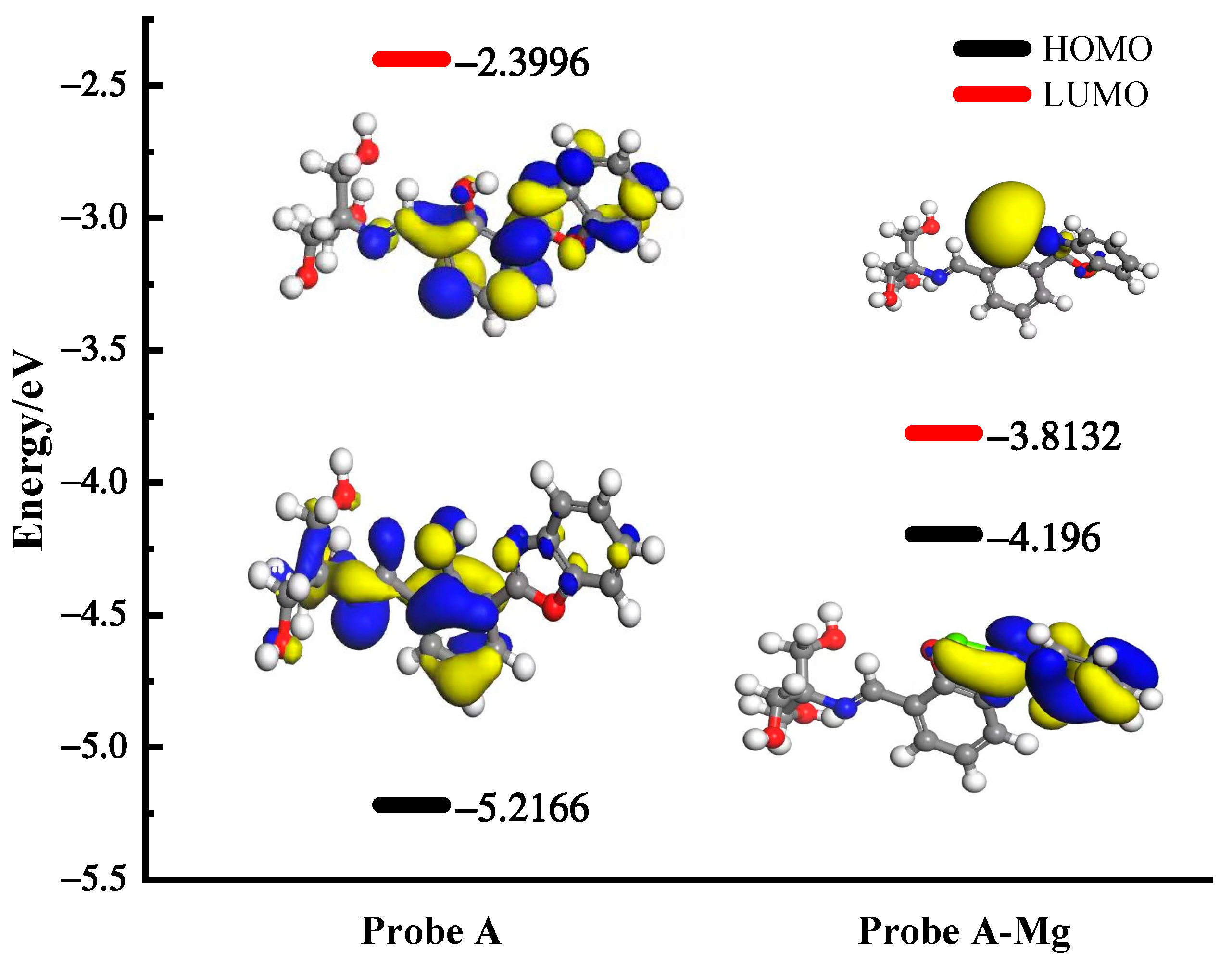
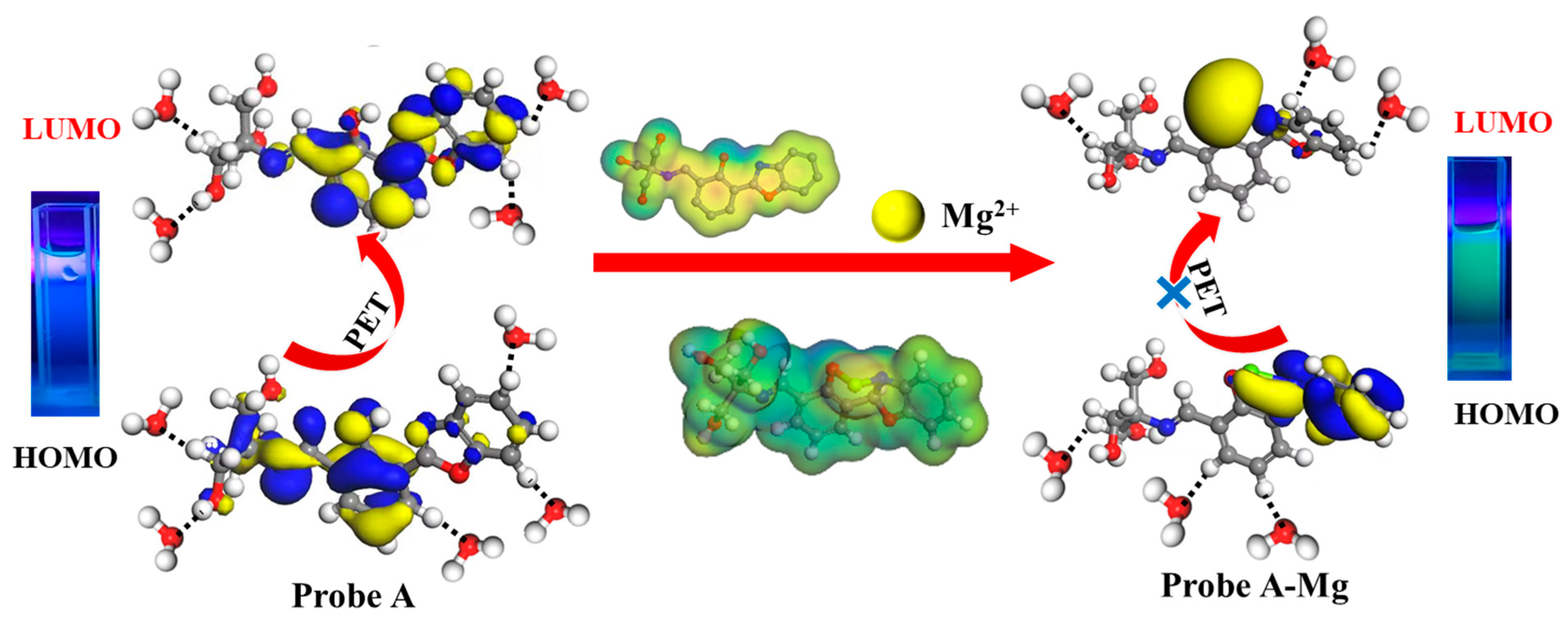
2.5. Detection Mechanisms
2.6. Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khakmardan, S.; Li, W.; Giurco, D.; Crawford, R. Life cycle assessment of lithium carbonate production: Comparing sedimentary deposits. J. Clean. Prod. 2024, 467, 142955. [Google Scholar] [CrossRef]
- Xu, W.; Liu, D.; He, L.; Zhao, Z. A Comprehensive Membrane Process for Preparing Lithium Carbonate from High Mg/Li Brine. Membranes 2020, 10, 371. [Google Scholar] [CrossRef]
- Choe, G.; Kim, H.; Kwon, J.; Jung, W.; Park, K.-Y.; Kim, Y.-T. Re-evaluation of battery-grade lithium purity toward sustainable batteries. Nat. Commun. 2024, 15, 1185. [Google Scholar] [CrossRef]
- Yi, W.-t.; Yan, C.-y.; Ma, P.-h. Removal of calcium and magnesium from LiHCO3 solutions for preparation of high-purity Li2CO3 by ion-exchange resin. Desalination 2009, 249, 729–735. [Google Scholar] [CrossRef]
- Linneen, N.; Bhave, R.; Woerner, D. Purification of industrial grade lithium chloride for the recovery of high purity battery grade lithium carbonate. Sep. Purif. Technol. 2019, 214, 168–173. [Google Scholar] [CrossRef]
- Fu, L.; Huang, G.; Hu, Y.; Chen, X.; Wang, J.; Pan, F. Development of a novel strategy for the quantification of ultra-trace impurity elements in high-purity magnesium using inductively coupled plasma tandem mass spectrometry. J. Magnes. Alloys 2023, in press. [Google Scholar] [CrossRef]
- Figueres, L.; Prot-Bertoye, C.; Morla, L.; Ferriere, E.; Griveau, C.; Brideau, G.; Baron, S.; Houillier, P. Performance of ion chromatography to measure picomole amounts of magnesium in nanolitre samples. J. Physiol. 2020, 598, 5613–5625. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, J.; Zhao, X.; Zhu, C. Determination of calcium and magnesium contents in alkaline degreaser for cold rolling by flame atomic absorption spectrometry with microwave digestion. Electroplat. Coat. 2023, 42, 71–76. [Google Scholar]
- Valek, R.A.; Tanner, K.B.; Taggart, J.B.; Ryan, R.L.; Cardall, A.C.; Woodland, L.M.; Oxborrow, M.J.; Williams, G.P.; Miller, A.W.; Sowby, R.B. Regulated Inductively Coupled Plasma-Optical Emission Spectrometry Detectible Elements in Utah Lake: Characterization and Discussion. Water 2024, 16, 2170. [Google Scholar] [CrossRef]
- Hussein, I.A.; Khathum, L.H.; Mohammed, M.K. Study Analytical of The Effect Magnesium ion Concentration to Determination the Total Hardness And Comparison Spectrophotometry. Baghdad Sci. J. 2013, 10, 892–897. [Google Scholar] [CrossRef]
- Majidi, B.; Amiri, A.; Badiei, A.; Shayesteh, A. Dual mode colorimetric-fluorescent sensor for highly sensitive and selective detection of Mg2+ ion in aqueous media. J. Mol. Struct. 2020, 1213, 128156. [Google Scholar] [CrossRef]
- Paisuwan, W.; Lertpiriyasakulkit, T.; Ruangpornvisuti, V.; Sukwattanasinitt, M.; Ajavakom, A. 8-Hydroxyjulolidine aldimine as a fluorescent sensor for the dual detection of Al3+ and Mg2+. Sens. Bio-Sens. Res. 2020, 29, 100358. [Google Scholar] [CrossRef]
- Mei, X.; Luo, W.; Li, H.; Pu, S. Synthesis and photophysical investigation of Schiff base as a Mg2+ and Zn2+ fluorescent chemosensor and its application. Luminescence 2023, 38, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Wang, R.; Dong, W.; Li, X.; Li, M.; Zhao, L.; Yang, S.; Sun, F. Laser induced fluorescence (LIF) technique visualizes and characterizes concentration polarization and fouling layer in the cross-flow nanofiltration. Sep. Purif. Technol. 2019, 212, 520–527. [Google Scholar] [CrossRef]
- Parker, H.E.; Sengupta, S.; Harish, A.V.; Soares, R.R.G.; Joensson, H.N.; Margulis, W.; Russom, A.; Laurell, F. A Lab-in-a-Fiber optofluidic device using droplet microfluidics and laser-induced fluorescence for virus detection. Sci. Rep. 2022, 12, 3539, Erratum in Sci. Rep. 2022, 12, 5104. [Google Scholar] [CrossRef]
- Feng, W.; Xiao, Q.; Wang, L.; Yang, Y. A New Fluorescent Probe for Hydrogen Sulfide Detection in Solution and Living Cells. Molecules 2023, 28, 6195. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Rivera-Fuentes, P. A general strategy to develop fluorogenic polymethine dyes for bioimaging. Nat. Chem. 2024, 16, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xue, S.; Xiao, G.; Du, C.; Liu, J.; Xie, Z. Study of the Synthesis and Application of a Fluorescence-Enhanced Mg2+ Probe. Chemistryselect 2021, 6, 13809–13816. [Google Scholar] [CrossRef]
- Sadhanala, H.K.; Aryal, S.; Sharma, K.; Orpaz, Z.; Michaeli, S.; Gedanken, A. Nitrogen-doped carbon dots as a highly selective and sensitive fluorescent probe for sensing Mg2+ ions in aqueous solution, and their application in the detection and imaging of intracellular Mg2+ ions. Sens. Actuators B-Chem. 2022, 366, 131958. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, S.; Pang, X.; Ren, X.; Zhang, B.; Kong, J.; Li, L. Solvent-directed multiple correspondence fluorescent probe for highly selective and sensitive detection of Cu2+ and Mg2+. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2023, 303, 123205. [Google Scholar] [CrossRef]
- Neri, J.M.; Latocheski, E.; de Araujo, J.G.L.; de Lima, R.P.; Cavalcanti, L.N.; Neves, A.C.O.; Gasparotto, L.H.S.; Domingos, J.B.; Menezes, F.G. Quinoxaline-functionalized silver nanoparticles as chromogenic probe for the multiple selective detection of cysteine, Mg2+ and Sn2+ in aqueous solution. Sens. Actuators B-Chem. 2021, 349, 130743. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, J.; Xie, Z.; Chang, G. Synthesis of a? Turn-On? Mg2+ fluorescent probe and its application in hydrogel adsorption. J. Mol. Struct. 2023, 1281, 135085. [Google Scholar] [CrossRef]
- Purkait, R.; Sinha, C. Solvent-tuned discriminant sensing of Al3+, Mg2+ and HF2−by vanilinyl-picolinyl hydrazide Schiff base. New J. Chem. 2019, 43, 9815–9823. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, C.; Liu, T.; Chao, J.; Zhang, Y.; Huo, F. Selective ”off-on“ detection of magnesium (II) ions using a naphthalimide-derived fluorescent probe. Dye. Pigment. 2017, 146, 344–351. [Google Scholar] [CrossRef]
- Abou-Hatab, S.; Carnevale, V.; Matsika, S. Modeling solvation effects on absorption and fluorescence spectra of indole in aqueous solution. J. Chem. Phys. 2021, 154, 064104. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xing, H.; Zhou, Z.; Liu, J.; Sung HH, Y.; Williams, I.D.; Halpert, J.E.; Zhao, Z. How do molecular interactions affect fluorescence behavior of AIEgens in solution and aggregate states? Sci. China Chem. 2022, 65, 135–144. [Google Scholar] [CrossRef]
- Hou, X.; Feng, G.; Chen, Z.; Wu, H.; Zhao, H.; Cao, S.; Hallett, J.P. Tailoring a suitable partner system for cholinium cation to build effective solvents for biomass deconstruction. Green Chem. 2024, 26, 5977–5987. [Google Scholar] [CrossRef]
- Shi, K.; Ma, F.; Fu, Z.; Liu, J.; Song, R.; Yuan, D.; Tang, Y. Investigation of the cohesive behavior of water-intervened rejuvenated SBS-modified bitumen. J. Clean. Prod. 2024, 467, 142986. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Li, Y.; Liu, M.; Chang, G. Selection and Simulation of Ketone Extraction Dephenol Solvent. J. Qingdao Univ. Sci. Technol. (Nat. Sci. Ed.) 2021, 42, 47–53. [Google Scholar] [CrossRef]
- Shabashini, A.; Panja, S.K.; Biswas, A.; Bera, S.; Nandi, G.C. ICT based photoacid probe for microsolvation and H-bonding assisted proton transfer process from solute to solvents. J. Photochem. Photobiol. a-Chem. 2022, 432, 114087. [Google Scholar] [CrossRef]
- Anderson, B.; Bryant, D.L.; Gozem, S.; Brambley, C.; Handy, S.T.; Farone, A.; Miller, J.M. Solvent-Dependent Emissions Properties of a Model Aurone Enable Use in Biological Applications. J. Fluoresc. 2024, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Sarangi, M.K.; Ray, A.; Bhattacharyya, D.; Maity, D.K. Excited state hydrogen bonding fluorescent probe: Role of structure and environment. J. Lumin. 2016, 173, 105–112. [Google Scholar] [CrossRef]
- Danilkina, N.A.; Andrievskaya, E.V.; Vasileva, A.V.; Lyapunova, A.G.; Rumyantsev, A.M.; Kuzmin, A.A.; Bessonova, E.A.; Balova, I.A. 4-Azidocinnoline-Cinnoline-4-amine Pair as a New Fluorogenic and Fluorochromic Environment-Sensitive Probe. Molecules 2021, 26, 7460. [Google Scholar] [CrossRef] [PubMed]
- Janakipriya, S.; Tamilmani, S.; Thennarasu, S. A novel 2-(2′-aminophenyl)benzothiazole derivative displays ESIPT and permits selective detection of Zn2+ ions: Experimental and theoretical studies. RSC Adv. 2016, 6, 71496–71500. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, A.; Li, C.; Yu, Q.; Li, Z.; Wang, L.; Wu, X.; Zhang, Z. Synthesis of aggregation-induced emission fluorescent probe and the application in recognition of Al3+. Chin. J. Anal. Lab. 2024, 42, 1721–1727. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Kandel, H.M.; Al-Shamiri, H.A.; Negm, N.A.; Elwahy, A.H.; Abou Kana, M.T. Spectroscopic Study of Solvent Polarity on the Optical and Photo-Physical Properties of Novel 9,10-bis(coumarinyl)anthracene. J. Fluoresc. 2018, 28, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Juan, O.-Y.; Li, Y.-F.; Wu, P.; Jiang, W.-L.; Liu, H.-W.; Li, C.-Y. Detecting and Imaging of γ-Glutamytranspeptidase Activity in Serum, Live Cells, and Pathological Tissues with a High Signal-Stability Probe by Releasing a Precipitating Fluorochrome. ACS Sens. 2018, 3, 1354–1361. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, S.; Wang, G.; Cui, J.; Lu, Y.; Rong, X.; Luo, Y.; Zhang, L.; Cheng, Z.; Gao, C. Nitrogen and sulfur co-doped carbon quantum dots as ”on-off-on“ fluorescence probes to detect Hg2+and MnO4− and improving the photostability of Rhodamine B. Anal. Chim. Acta 2023, 1277, 341683. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Liang, Z.-Q.; Yan, X.; Li, M.-M.; Ye, C.-Q.; Wang, X.-M.; Tao, X.-T. The effect of triethylamine on dye-sensitized upconversion luminescence and its application in nanoprobes and photostability. Phys. Chem. Chem. Phys. 2023, 25, 12401–12408. [Google Scholar] [CrossRef]
- Kamacı, M.; Kaya, İ. A highly selective, sensitive and stable fluorescent chemosensor based on Schiff base and poly(azomethine-urethane) for Fe3+ ions. J. Ind. Eng. Chem. 2017, 46, 234–243. [Google Scholar] [CrossRef]
- Li, L.-S.; Xu, L. Highly fluorescent silicon quantum dots decorated silica microspheres for selective detection and removal of Au3+ and subsequent catalytic application. J. Ind. Eng. Chem. 2020, 84, 375–383. [Google Scholar] [CrossRef]
- Fu, Z.-H.; Qin, J.-C.; Wang, Y.-W.; Peng, Y.; Zhang, Y.-M.; Zhao, D.-M.; Zhang, Z.-H. A quinoline-based chromogenic and ratiometric fluorescent probe for selective detection of Mg2+ ion: Design, synthesis and its application in salt lake brines and bioimaging. Dye. Pigment. 2021, 185, 108896. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, B.; Yu, H.; Zhu, L.; Feng, Y.; Zhu, M.; Guo, Q.; Meng, X. Two-Photon Fluorescent Probes for Biological Mg2+ Detection Based on 7-Substituted Coumarin. J. Org. Chem. 2015, 80, 4306–4312. [Google Scholar] [CrossRef]
- Qiu, S.; Yu, J.; Zhou, T.; Zhang, K.; Duan, Y.; Ban, X.; Zhu, Q.; Shi, L.; Zhang, D. Thermally activated delayed fluorescence fluorescent probe based on triazine as emission core for metal ions detection. Opt. Mater. 2021, 119, 111303. [Google Scholar] [CrossRef]
- Marimuthu, P.; Ramu, A. A ratiometric fluorescence chemosensor for Mg2+ ion and its live cell imaging. Sens. Actuators B-Chem. 2018, 266, 384–391. [Google Scholar] [CrossRef]
- Li, X.; Tao, Y.; Zhu, L.; Ma, S.; Luo, S.; Zhao, Z.; Sun, N.; Ge, X.; Ye, Z. Optical and chemical properties and oxidative potential of aqueous-phase products from OH and 3C∗-initiated photooxidation of eugenol. Atmos. Chem. Phys. 2022, 22, 7793–7814. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Yang, M.; Li, Z. The Fabrication and Mechanism of a Crystalline Organic Fluorescent Probe Based on Photoinduced Electron Transfer. Molecules 2023, 28, 6774. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, X.; Li, M.; Liao, N.; Bi, A.; Jiang, Y.; Liu, S.; Gong, Z.; Zeng, W. Fluorescent probes for the detection of magnesium ions (Mg2+): From design to application. RSC Adv. 2018, 8, 12573–12587. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Liu, J.; O’Connor, H.M.; Gunnlaugsson, T.; James, T.D.; Zhang, H. Photoinduced electron transfer (PeT) based fluorescent probes for cellular imaging and disease therapy. Chem. Soc. Rev. 2023, 52, 2322–2357. [Google Scholar] [CrossRef] [PubMed]
- Kawski, A.; Ostrowska, B.; Ston, M. Influence of Environment on the Electronic Spectra of Donor-Acceptor-Substituted trans-Stilbenes in Solvent Mixtures. Z. Naturforschung A 1981, 36, 999–1001. [Google Scholar] [CrossRef]
- Viard, M.; Gallay, J.; Vincent, M.; Meyer, O.; Robert, B.; Paternostre, M. Laurdan solvatochromism: Solvent dielectric relaxation and intramolecular excited-state reaction. Biophys. J. 1997, 73, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xin, C.; Zhu, L.; Sun, C. Influence of intermolecular hydrogen bond interaction on fluorescence mechanism for ESIPT characteristic o-Hydroxybenzaldehyde. Chem. Phys. 2022, 561, 111622. [Google Scholar] [CrossRef]
- Mohammadzadeh, F.; Golshan, M.; Haddadi-Asl, V.; Salami-Kalajahi, M. Dual-mode fluorescent probe for rapid lead detection in water and gasoline based on a perylene/β-cyclodextrin hybrid sensor. J. Ind. Eng. Chem. 2024, in press. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cheng, H.; He, Y.; Zhao, J. Rapid Fluorescent Probe Detection of Magnesium Impurities in High-Purity Lithium Carbonate Brine Systems. Molecules 2025, 30, 776. https://doi.org/10.3390/molecules30040776
Li Y, Cheng H, He Y, Zhao J. Rapid Fluorescent Probe Detection of Magnesium Impurities in High-Purity Lithium Carbonate Brine Systems. Molecules. 2025; 30(4):776. https://doi.org/10.3390/molecules30040776
Chicago/Turabian StyleLi, Yan, Huaigang Cheng, Yueyue He, and Jing Zhao. 2025. "Rapid Fluorescent Probe Detection of Magnesium Impurities in High-Purity Lithium Carbonate Brine Systems" Molecules 30, no. 4: 776. https://doi.org/10.3390/molecules30040776
APA StyleLi, Y., Cheng, H., He, Y., & Zhao, J. (2025). Rapid Fluorescent Probe Detection of Magnesium Impurities in High-Purity Lithium Carbonate Brine Systems. Molecules, 30(4), 776. https://doi.org/10.3390/molecules30040776





