Novel Quaternary Ammonium Urethane-Dimethacrylates for Copolymers with Low Water Sorption and Solubility
Abstract
1. Introduction
2. Results
2.1. Synthesis
2.2. Spectroscopic Analysis of Monomers
2.2.1. 1H NMR
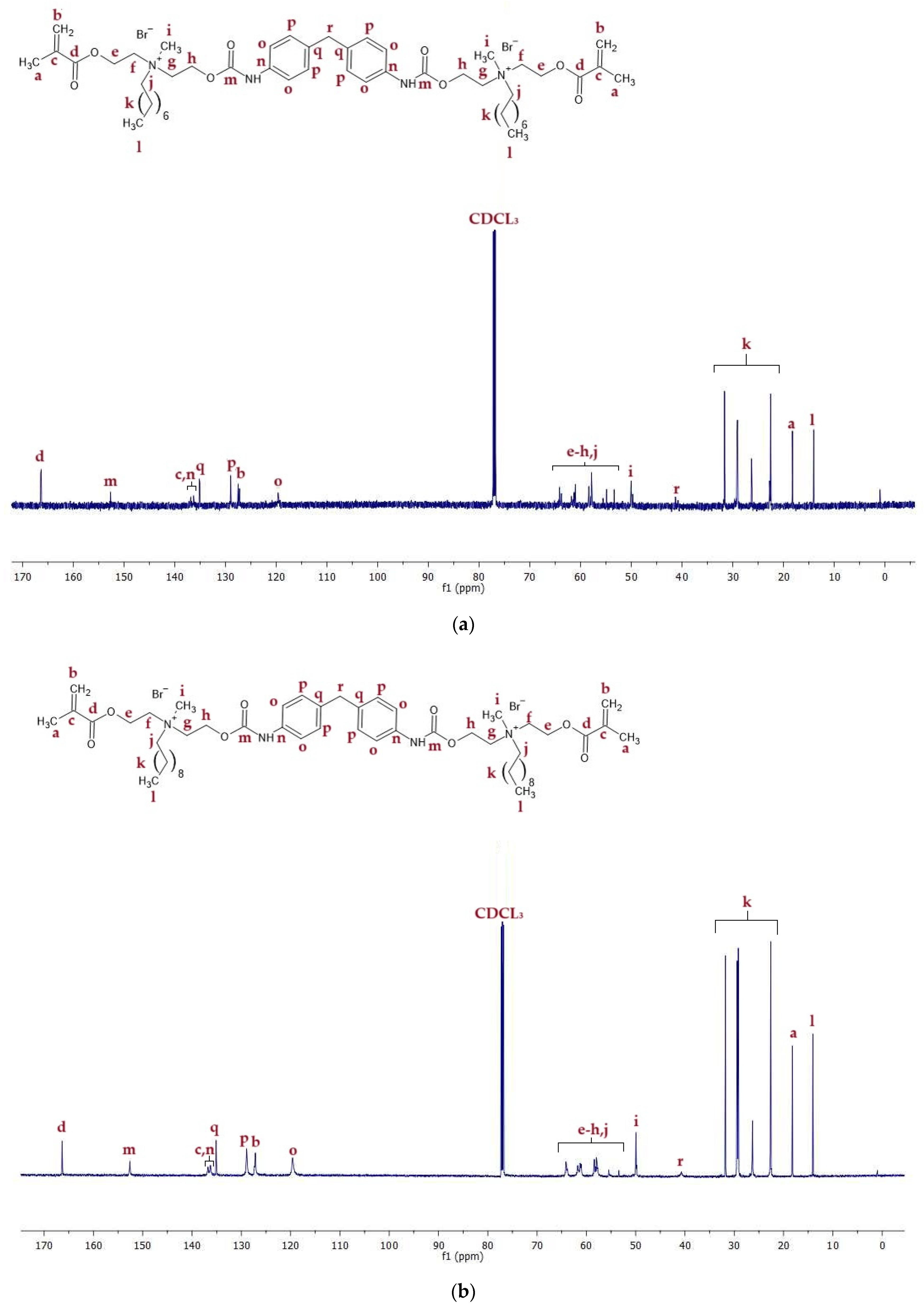
2.2.2. 13C NMR
2.2.3. FTIR
2.3. Monomers
2.4. Copolymers
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemical Syntheses
4.2.1. N,N-(2-Hydroxyethyl)methylaminoethyl Methacrylate (HAMA)
4.2.2. 2-(Methacryloyloxy)ethyl-2-Hydroxyethylmethylalkylammonium Bromides (QAHAMA-n)
4.2.3. Quaternary Ammonium Urethane-Dimethacrylates (QAUDMA)
4.3. Curing Procedure and Sample Preparation
- 40(QA8+IPDI)p: QA8+IPDI 40 wt.%, Bis-GMA 40 wt.%, and TEGDMA 20 wt.%
- 40(QA10+IPDI)p:QA10+IPDI 40 wt.%, Bis-GMA 40 wt.%, and TEGDMA 20 wt.%
- 40(QA8+CHMDI)p: QA8+CHMDI 40 wt.%, Bis-GMA 40 wt.%, and TEGDMA 20 wt.%
- 40(QA10+CHMDI)p: QA10+CHMDI 40 wt.%, Bis-GMA 40 wt.%, and TEGDMA 20 wt.%
- 40(QA8+MDI)p: QA8+MDI 40 wt.%, Bis-GMA 40 wt.%, and TEGDMA 20 wt.%
- 40(QA10+MDI)p: QA10+MDI 40 wt.%, Bis-GMA 40 wt.%, and TEGDMA 20 wt.%.
4.4. Nuclear Magnetic Resonance Spectroscopy (NMR)
4.5. Fourier Transform Infrared Spectroscopy (FTIR)
4.6. Refractive Index
4.7. Density
4.8. Water Sorption and Solubility
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Rezaei, T.; Mehramouz, B.; Gholizadeh, P.; Yousefi, L.; Ganbarov, K.; Ghotaslou, R.; Taghizadeh, S.; Kafil, H.S. Factors Associated with Streptococcus mutans Pathogenicity in the Oral Cavity. Biointerface Res. App. Chem. 2023, 13, 368–387. [Google Scholar]
- Ghazal, T.S.; Levy, S.M.; Childers, N.K.; Carter, K.D.; Caplan, D.J.; Warren, J.J.; Cavanaugh, J.E.; Kolker, J. Mutans Streptococci and Dental Caries: A New Statistical Modeling Approach. Caries Res. 2018, 52, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Ren, B.; He, J.; Peng, X.; Guo, Q.; Zheng, L.; Li, J.; Dai, H.; Chen, V.; Zhang, L.; et al. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J. 2021, 15, 894–908. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Wakade, R.S.; Ribeiro, A.A.; Hu, W.; Bittinger, K.; Simon-Soro, A.; Kim, D.; Li, J.; Krysan, D.J.; Liu, Y.; et al. Human Tooth as a Fungal Niche: Candida albicans Traits in Dental Plaque Isolates. mBio 2023, 14, e02769-22. [Google Scholar] [CrossRef]
- Hernández, P.; Sánchez, M.C.; Llama-Palacios, A.; Ciudad, M.J.; Collado, L. Strategies to Combat Caries by Maintaining the Integrity of Biofilm and Homeostasis during the Rapid Phase of Supragingival Plaque Formation. Antibiotics 2022, 11, 880. [Google Scholar] [CrossRef]
- Ray, R.R. Dental biofilm: Risks, diagnostics and management. Biocatal. Agric. Biotechnol. 2022, 43, 102381. [Google Scholar] [CrossRef]
- Brookes, Z.; McGrath, C.; McCullough, M. Antimicrobial Mouthwashes: An Overview of Mechanisms-What Do We Still Need to Know? Int. Dent. J. 2023, 73, S64–S68. [Google Scholar] [CrossRef]
- Arif, W.; Rana, N.F.; Saleem, I.; Tanweer, T.; Khan, M.J.; Alshareef, S.A.; Sheikh, H.M.; Alaryani, F.S.; AL-Kattan, M.O.; Alatawi, H.A.; et al. Antibacterial Activity of Dental Composite with Ciprofloxacin Loaded Silver Nanoparticles. Molecules 2022, 27, 7182. [Google Scholar] [CrossRef]
- Zhang, R.; Jones, M.M.; Moussa, H.; Keskar, M.; Huo, N.; Zhang, Z.; Visser, M.B.; Sabatini, C.; Swihart, M.T.; Cheng, C. Polymer–antibiotic conjugates as antibacterial additives in dental resins. Biomater. Sci. 2019, 7, 287. [Google Scholar] [CrossRef]
- Chen, L.; Suh, B.; Yang, J. Antibacterial dental restorative materials: A review. Am. J. Dent. 2018, 31, 6B–12B. [Google Scholar]
- Beyth, N.; Farah, S.; Domb, A.; Weiss, E. Antibacterial dental resin composites. Reac. Funct. Polym. 2014, 75, 81–88. [Google Scholar] [CrossRef]
- Ferrando-Magraner, E.; Bellot-Arcís, C.; Paredes-Gallardo, V.; Almerich-Silla, J.M.; García-Sanz, V.; Fernández-Alonso, M.; Montiel-Company, J.M. Antibacterial Properties of Nanoparticles in Dental Restorative Materials. A Systematic Review and Meta-Analysis. Medicina 2020, 56, 55. [Google Scholar] [CrossRef] [PubMed]
- Domb, A.J.; Weiss, E.; Beyth, N.; Farber, I.; Perez-Davidi, M. Antimicrobial Nanoparticulate Additives Forming Non-Leachable Sustained Antimicrobial Polymeric Compositions. U.S. Patent 20080226728A1, 18 September 2008. [Google Scholar]
- Fernandes, J.; Menezes, V.; Albuquerque, A.; Oliveira, M.; Meira, K.; Menezes, R., Jr.; Sampaio, F.C. Improving Antimicrobial Activity of Dental Restorative Materials. In Emerging Trends in Oral Health Sciences and Dentistry; InTech: London, UK, 2015. [Google Scholar]
- Badr, S.; Abdulrahman, A.; Abdullah, A.; Essam, A.; Mohammad, Y.; Shahzeb, H. Effect of various antibacterial materials in dental composites: A systematic review. Ann. Dent. Spec. 2021, 9, 39–44. [Google Scholar]
- Mehdawi, I.; Young, A. Antibacterial composite restorative materials for dental applications. In Non-Metallic Biomaterials for Tooth Repair and Replacement; Elsevier: Amsterdam, The Netherlands, 2013; pp. 270–293. [Google Scholar]
- Zhou, X.; Huang, X.; Li, M.; Peng, X.; Wang, S.; Zhou, X.; Cheng, L. Development and status of resin composite as dental restorative materials. J. Appl. Polym. Sci. 2019, 136, 48180. [Google Scholar] [CrossRef]
- Chan, D.; Hu, W.; Chung, K.; Larsen, R.; Jensen, S.; Cao, D.; Gaviria, L.; Ong, J.; Whang, K.; Eiampongpaiboon, T. Reactions: Antibacterial and bioactive dental restorative materials: Do they really work? Am. J. Dent. 2018, 15, 32B–36B. [Google Scholar]
- Farrugia, C.; Camilleri, J. Antimicrobial properties of conventional restorative filling materials and advances in antimicrobial properties of composite resins and glass ionomer cements—A literature review. Dent. Mater. 2015, 31, e89–e99. [Google Scholar] [CrossRef]
- Ibrahim, M.; Garcia, I.; Kensara, A.; Balhaddad, A.; Collares, F.; Williams, M.; Ibrahim, A.; Lin, N.; Weir, M.; Xu, H.; et al. How we are assessing the developing antibacterial resin-based dental materials? A scoping review. J. Dent. 2020, 99, 103369. [Google Scholar] [CrossRef]
- Chen, Z.; Chu, Z.; Jiang, Y.; Xu, L.; Qian, H.; Wang, Y.; Wang, W. Recent advances on nanomaterials for antibacterial treatment of oral diseases. Mater. Today Bio. 2023, 20, 100635. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Hu, Y.; Huang, F.; Xiao, Y. Quaternary ammonium compounds in dental restorative materials. Dent. Mater. J. 2018, 37, 183–191. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, L.; Bai, R.; Zhuang, Z.; Zhang, Y.; Yu, T.; Peng, L.; Xin, T.; Chen, S.; Han, B. Recent Progress in Antimicrobial Strategies for Resin-Based Restoratives. Polymers 2021, 13, 1590. [Google Scholar] [CrossRef]
- Ramburrun, P.; Pringle, N.; Dube, A.; Adam, R.; D’Souza, S.; Aucamp, M. Recent Advances in the Development of Antimicrobial and Antifouling Biocompatible Materials for Dental Applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial Quaternary Ammonium Compounds in Dental Materials: A Systematic Review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, S.; Zhou, X.; Wang, H.; Xu, H.H.K.; Cheng, L. The Use of Quaternary Ammonium to Combat Dental Caries. Materials 2015, 8, 3532–3549. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Chen, J.H.; Ma, S.; Izutani, N.; Li, F. Antibacterial Resin Monomers Based on Quaternary Ammonium and Their Benefits in Restorative Dentistry. Jap. Dent. Sci. Rev. 2012, 48, 115–125. [Google Scholar] [CrossRef]
- Featherstone, J. Dental restorative materials containing quaternary ammonium compounds have sustained antibacterial action. J. Am. Dent. Assoc. 2022, 153, 1114–1120. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Guo, X.; Wang, H.; Cheng, L. Applications of quaternary ammonium compounds in the prevention and treatment of oral diseases: State-of-the-art and future directions. J. Dent. 2023, 137, 104678. [Google Scholar] [CrossRef]
- Jiao, Y.; Niua, L.; Maa, S.; Li, J.; Tayd, F.; Chena, J. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Mousavinasab, S. Biocompatibility of composite resins. Dent. Res. J. 2011, 8, S21–S29. [Google Scholar]
- Huang, L.; Xiao, Y.H.; Xing, X.D.; Li, F.; Ma, S.; Qi, L.L.; Chen, J.H. Antibacterial Activity and Cytotoxicity of Two Novel Cross-Linking Antibacterial Monomers on Oral Pathogens. Arch. Oral. Biol. 2011, 56, 367–373. [Google Scholar] [CrossRef]
- Huang, L.; Yu, F.; Sun, X.; Dong, Y.; Lin, P.T.; Yu, H.H.; Xiao, Y.H.; Chai, Z.G.; Xing, X.D.; Chen, J.H. Antibacterial Activity of a Modified Unfilled Resin Containing a Novel Polymerizable Quaternary Ammonium Salt MAE-HB. Sci. Rep. 2016, 6, 33858. [Google Scholar] [CrossRef]
- Liang, X.; Söderling, E.; Liu, F.; He, J.; Lassila, L.V.J.; Vallittu, P.K. Optimizing the Concentration of Quaternary Ammonium Dimethacrylate Monomer in Bis-GMA/TEGDMA Dental Resin System for Antibacterial Activity and Mechanical Properties. J. Mater. Sci. Mater. Med. 2014, 25, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.T.; He, J.W.; Lin, Z.M.; Liu, F.; Lassila, L.V.J.; Vallittu, P.K. Physical and Chemical Properties of an Antimicrobial Bis-GMA Free Dental Resin with Quaternary Ammonium Dimethacrylate Monomer. J. Mech. Behav. Biomed. Mater. 2016, 56, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Huang, Q.; Liu, F.; He, J.; Lin, Z. Synthesis of Novel Antibacterial Monomers (UDMQA) and Their Potential Application in Dental Resin. J. Appl. Polym. Sci. 2013, 129, 3373–3381. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, Z.; Liang, X.; Liu, F.; He, J. Preparation and Characterization of Antibacterial Dental Resin with UDMQA-12. Adv. Polym. Technol. 2014, 33, 21395. [Google Scholar] [CrossRef]
- Manouchehri, F.; Sadeghi, B.; Najafi, F.; Mosslemin, M.H.; Niakan, M. Synthesis and Characterization of Novel Polymerizable Bis-Quaternary Ammonium Dimethacrylate Monomers with Antibacterial Activity as an Efficient Adhesive System for Dental Restoration. Polym. Bull. 2019, 76, 1295–1315. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghaemy, M.; Mohseni, M. Synthesis and Characterization of Photo-Curable Bis-Quaternary Ammonium Dimethacrylate with Antimicrobial Activity for Dental Restoration Materials. Eur. Polym. J. 2016, 74, 81–90. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Z.; Wang, R.; Zuo, W.; Zhu, M. Bis-quaternary ammonium betulin-based dimethacrylate: Synthesis, characterization, and application in dental restorative resins. Mater. Adv. 2023, 4, 2127–2137. [Google Scholar] [CrossRef]
- Chrószcz, M.W.; Barszczewska-Rybarek, I.M. Synthesis and Characterization of Novel Quaternary Ammonium Urethane-Dimethacrylate Monomers—A Pilot Study. Int. J. Mol. Sci. 2021, 22, 8842. [Google Scholar] [CrossRef]
- Chrószcz-Porębska, M.; Kazek-Kęsik, A.; Chladek, G.; Barszczewska-Rybareka, I. Novel mechanically strong and antibacterial dimethacrylate copolymers based on quaternary ammonium urethane-dimethacrylate analogues. Dent. Mater. 2023, 39, 659–664. [Google Scholar] [CrossRef]
- Drejka, P.; Chrószcz-Porębska, M.; Kazek-Kęsik, A.; Chladek, G.; Barszczewska-Rybarek, I. Chemical Modification of Dental Dimethacrylate Copolymer with Tetramethylxylylene Diisocyanate-Based Quaternary Ammonium Urethane-Dimethacrylates—Physicochemical, Mechanical, and Antibacterial Properties. Materials 2024, 17, 298. [Google Scholar] [CrossRef]
- Drejka, P.; Chrószcz-Porębska, M.; Kazek-Kęsik, A.; Barszczewska-Rybarek, I. Antibacterial Properties of Dental Copolymer Modified with Monomers Possessing Quaternary Ammonium Groups. Biol. Life Sci. Forum 2024, 35, 10. [Google Scholar] [CrossRef]
- Chrószcz-Porębska, M.W.; Barszczewska-Rybarek, I.M.; Chladek, G. Physicochemical Properties of Novel Copolymers of Quaternary Ammonium UDMA Analogues, Bis-GMA, and TEGDMA. Int. J. Mol. Sci. 2023, 24, 1400. [Google Scholar] [CrossRef] [PubMed]
- ISO 4049:2019; Dentistry—Polymer-Based Restorative Materials. International Organization for Standardization: Geneva, Switzerland, 2019.
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Oivanen, M.; Keulemans, F.; Garoushi, S.; Vallittu, P.K.; Lassila, L. The effect of refractive index of fillers and polymer matrix on translucency and color matching of dental resin composite. Biomater. Investig. Dent. 2021, 8, 48–53. [Google Scholar] [CrossRef]
- Bauer, J.; Gutke, M.; Heinrich, F.; Edling, M.; Stoycheva, V.; Kaltenbach, A.; Burkhardt, M.; Gruenefeld, M.; Gamp, M.; Gerhard, C.; et al. Novel UV-transparent 2-component polyurethane resin for chip-on-board LED micro lenses. Opt. Mater. Express 2020, 10, 2085–2099. [Google Scholar] [CrossRef]
- Zec, N.; Vraneš, M.; Bešter-Rogač, M.; Trtić-Petrović, T.; Dimitrijević, A.; Čobanov, I.; Gadžurić, S. Influence of the alkyl chain length on densities and vol-umetric properties of 1,3-dialkylimidazolium bromide ionic liquids and their aqueous solutions. J. Chem. Thermodyn. 2018, 121, 72–78. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wang, J.; Pan, Z.; Liu, J. Molecular Simulation Study on the Density Behavior of n-Alkane/CO2 Systems. ACS Omega 2021, 6, 29618–29628. [Google Scholar] [CrossRef]
- Bastioli, C.; Romano, G.; Migliaresi, C. Water sorption and mechanical properties of dental composites. Biomaterials 1990, 11, 219–223. [Google Scholar] [CrossRef]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; You, C.K.; Carrilho, M.R. Water sorption/solubility of dental adhesive resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef]
- Bociong, K.; Szczesio, A.; Sokolowski, K.; Domarecka, M.; Sokolowski, J.; Krasowski, M.; Lukomska-Szymanska, M. The Influence of Water Sorption of Dental Light-Cured Composites on Shrinkage Stress. Materials 2017, 10, 1142. [Google Scholar] [CrossRef]
- Hopkins Hatzopoulos, M.; Eastoe, J.; Dowding, P.J.; Grillo, I.; Demé, B.; Rogers, S.E.; Heenan, R.; Dyer, R. Effects of structure variation on solution properties of hydrotropes: Phenyl versus cyclohexyl chain tips. Langmuir 2012, 28, 9332–9340. [Google Scholar] [CrossRef]
- ISO 489:2022; Plastics—Determination of Refractive Index. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 1675:2022; Plastics—Liquid Resins—Determination of Density by the Pycnometer Method. International Organization for Standardization: Geneva, Switzerland, 2022.
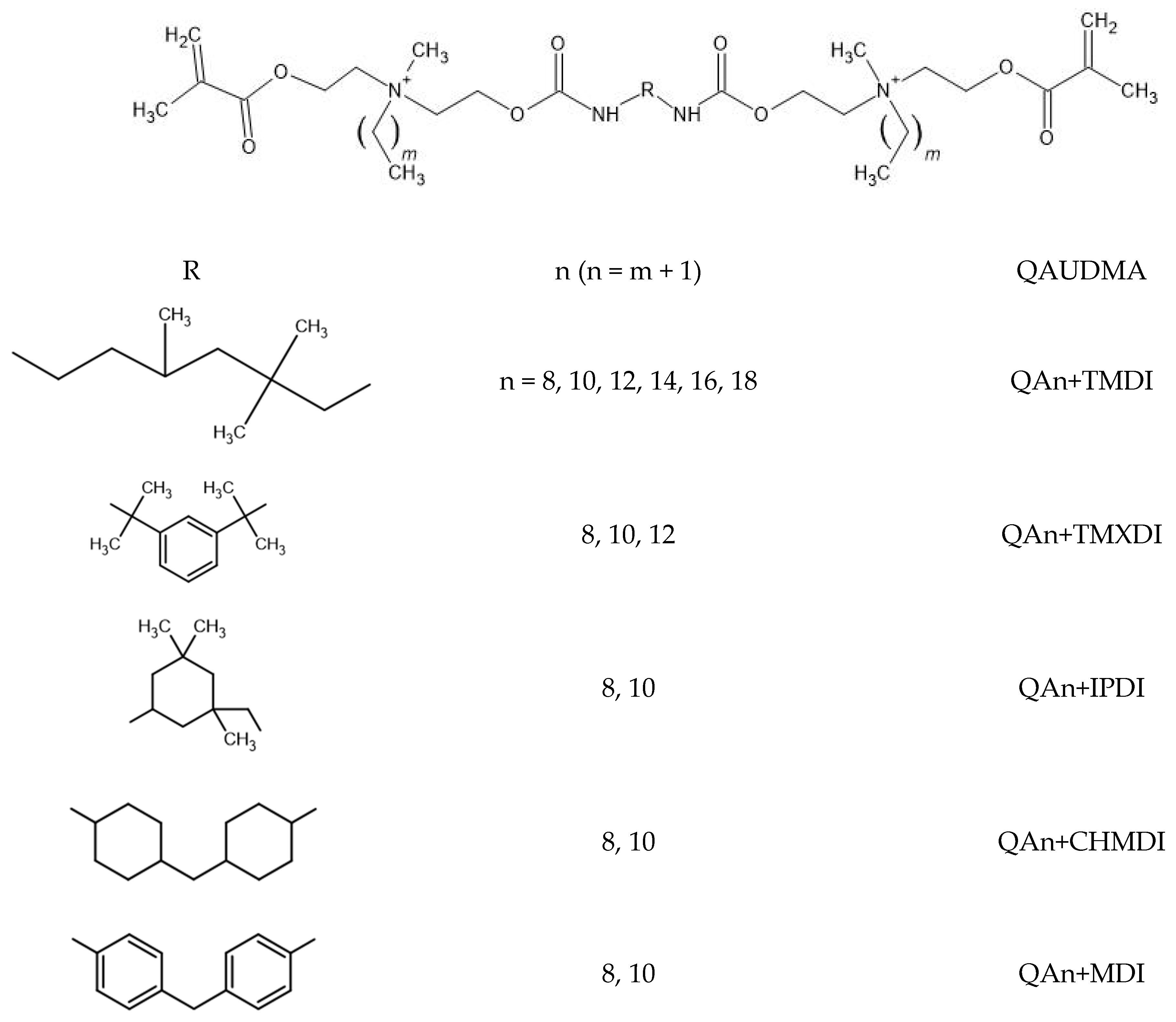

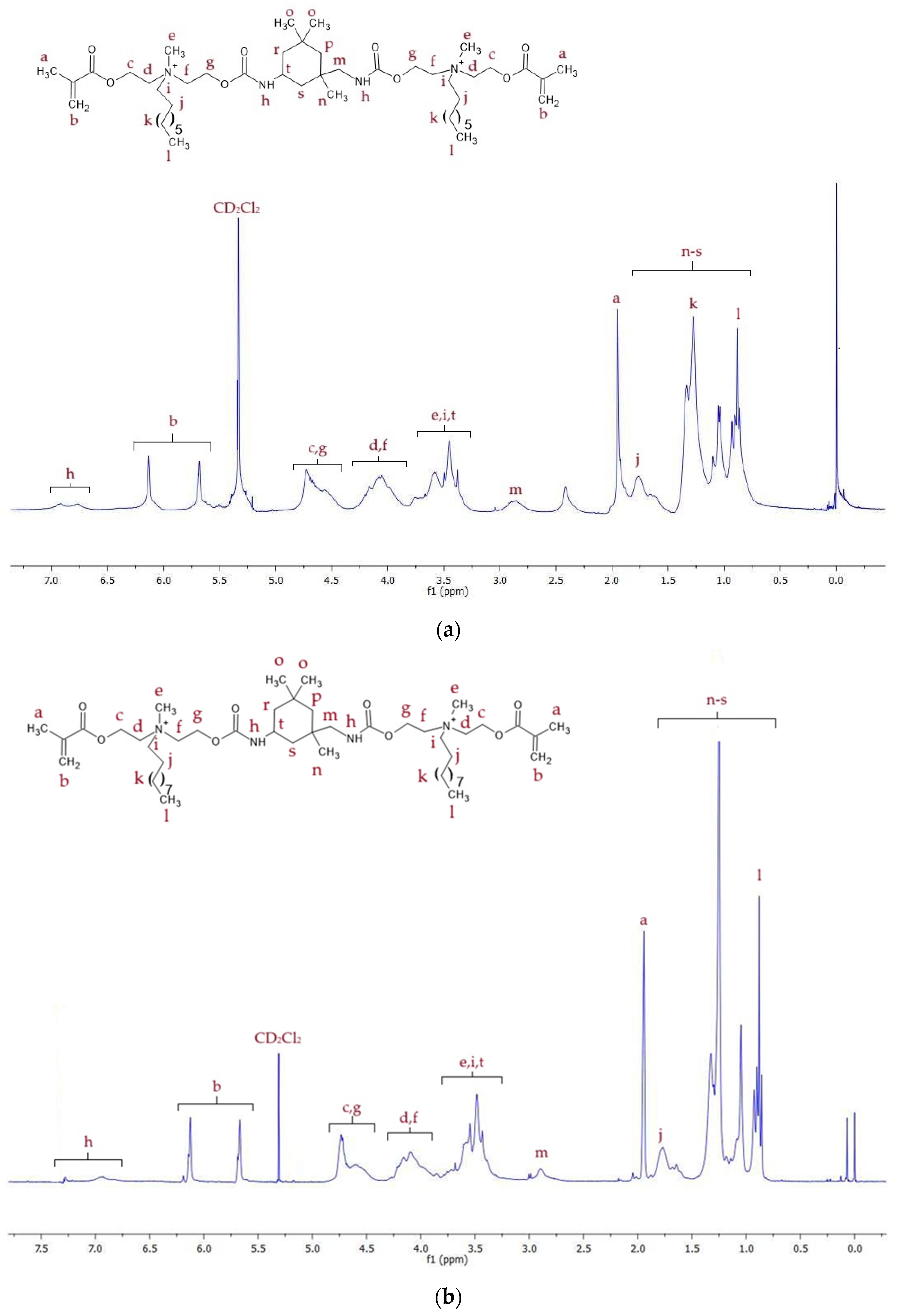
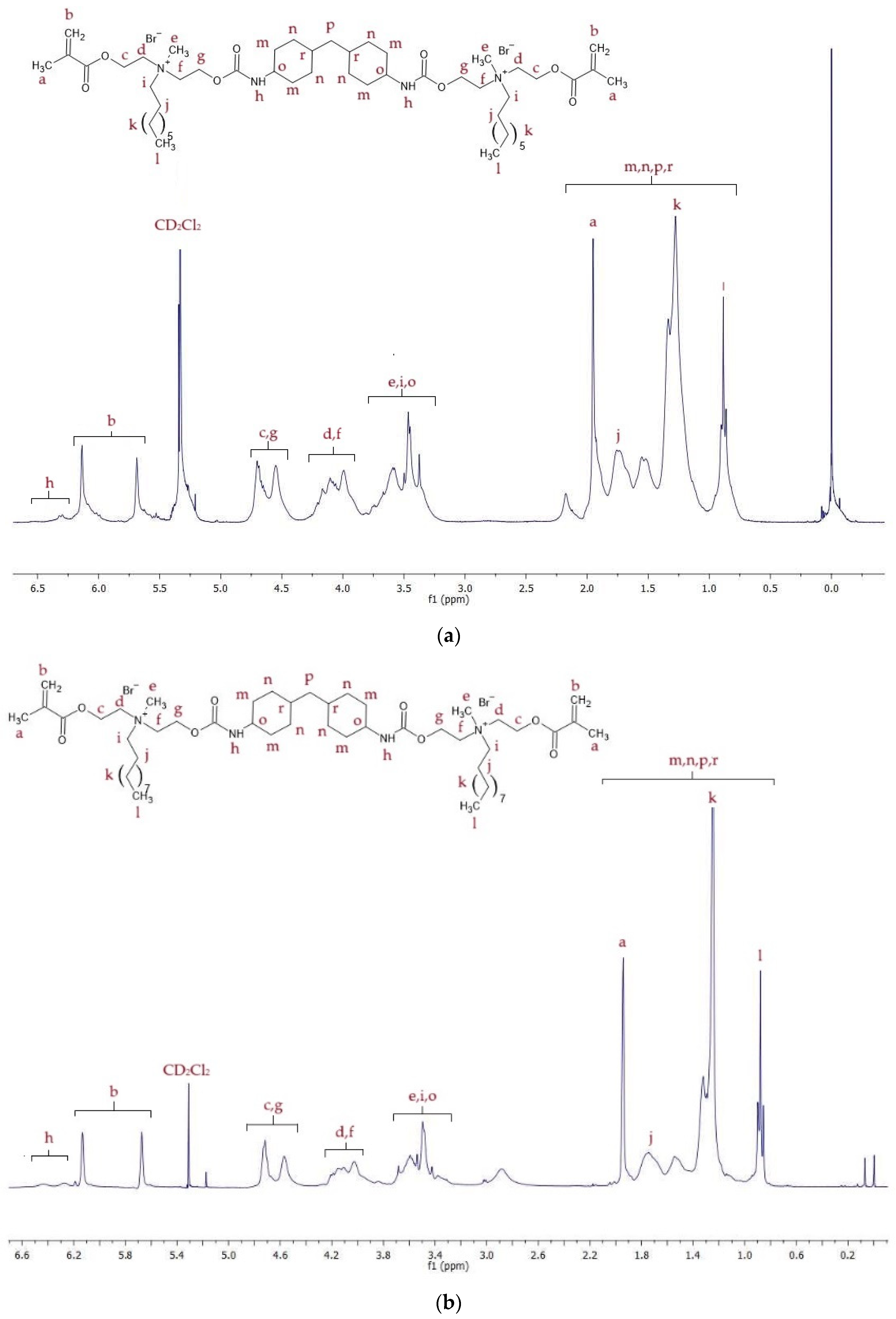

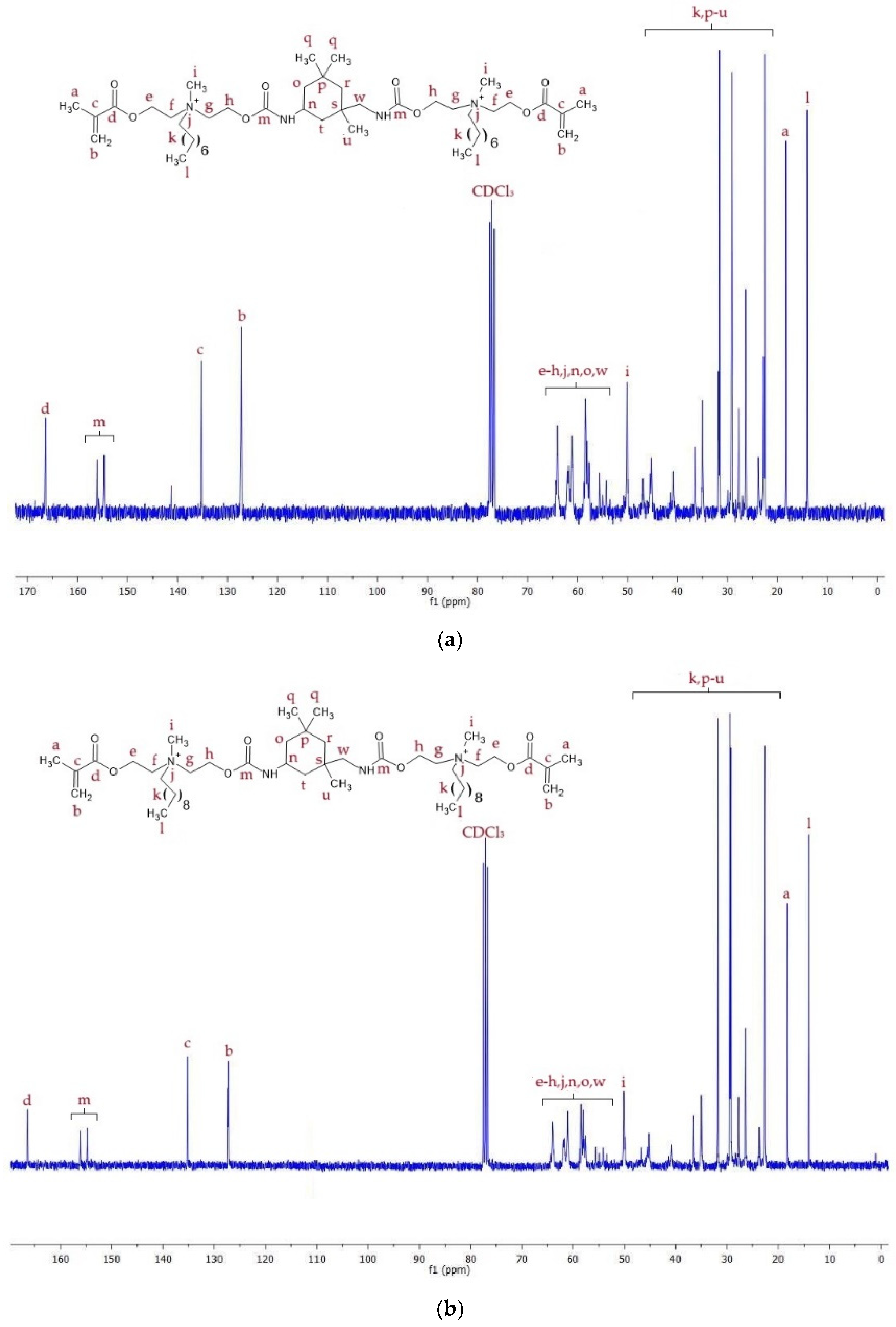


| Monomer | MW (g/mol) | xDB = xUB (mol/kg) | RI | dm (g/cm3) | |
|---|---|---|---|---|---|
| AV 1 | AV | SD | |||
| Experimental monomers | |||||
| QA8+IPDI | 983 | 2.03 | 1.5211 | 1.24 a | 0.04 |
| QA10+IPDI | 1039 | 1.92 | 1.5130 | 1.18 b | 0.02 |
| QA8+CHMDI | 1023 | 1.96 | 1.5234 | 1.25 a | 0.01 |
| QA10+CHMDI | 1079 | 1.85 | 1.5205 | 1.19 b | 0.02 |
| QA8+MDI | 1011 | 1.98 | 1.5595 | 1.32 | 0.02 |
| QA10+MDI | 1067 | 1.87 | 1.5535 | 1.52 | 0.01 |
| Reference monomer | |||||
| UDMA | 470 | 4.25 | 1.4614 | 1.09 | 0.01 |
| Copolymer | WS (μg/mm3) | SL (μg/mm3) | ||
|---|---|---|---|---|
| AV | SD | AV | SD | |
| Experimental copolymers | ||||
| 40(QA8+IPDI)p | 14.00 a,g,h,i, | 1.61 | 2.70 a,g,h | 0.29 |
| 40(QA10+IPDI)p | 13.18 b,j,k,l | 1.02 | 2.62 b,i,j,k,l | 0.26 |
| 40(QA8+CHMDI)p | 13.75 c,m,n,o | 1.32 | 2.94 c,i,m,n | 0.21 |
| 40(QA10+CHMDI)p | 11.79 d,g,j,m | 0.14 | 3.08 d,j,o,p | 0.20 |
| 40(QA8+MDI)p | 11.67 e,h,k,n | 0.32 | 7.68 e,g,k,m,o,r | 0.48 |
| 40(QA10+MDI)p | 11.63 f,i,l,o | 0.47 | 6.61 f,h,l,n,p,r | 0.53 |
| Reference copolymer | ||||
| 40(UDMA)p | 5.85 a,b,c,d,e,f | 0.49 | 1.13 a,b,c,d,e,f | 0.04 |
| Substance | Mass (g) | Moles (mol) |
|---|---|---|
| MMA | 100.12 | 1.00 |
| MDEA | 79.85 | 0.67 |
| K2CO3 | 14.40 | 1 |
| PTZ | 0.05 | 1 |
| Toluene | 346 | 1 |
| Substance | Mass (g) | Moles (mol) |
|---|---|---|
| HAMA | 20.00 | 0.107 |
| OB | 20.66 | 0.107 |
| DB | 23.67 | 0.107 |
| PTZ | 0.01 | 1 |
| QAUDMA | Substance | Mass (g) | Moles (mol) |
|---|---|---|---|
| QA8+IPDI | QAHAMA-8 | 20.34 | 0.053 |
| IPDI | 5.94 | 0.027 | |
| DBTDL | 0.008 | 1 | |
| QA10+IPDI | QAHAMA-10 | 20.34 | 0.050 |
| IPDI | 5.54 | 0.025 | |
| DBTDL | 0.01 | 1 | |
| QA8+CHMDI | QAHAMA-8 | 22.82 | 0.060 |
| CHMDI | 7.97 | 0.030 | |
| DBTDL | 0.01 | 1 | |
| QA10+CHMDI | QAHAMA-10 | 22.82 | 0.056 |
| CHMDI | 7.33 | 0.028 | |
| DBTDL | 0.01 | 1 | |
| QA8+MDI | QAHAMA-8 | 23.50 | 0.062 |
| MDI | 7.73 | 0.031 | |
| DBTDL | 0.01 | 1 | |
| QA10+MDI | QAHAMA-10 | 23.50 | 0.058 |
| MDI | 7.20 | 0.029 | |
| DBTDL | 0.01 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drejka, P.; Kula, P.; Barszczewska-Rybarek, I. Novel Quaternary Ammonium Urethane-Dimethacrylates for Copolymers with Low Water Sorption and Solubility. Molecules 2025, 30, 769. https://doi.org/10.3390/molecules30040769
Drejka P, Kula P, Barszczewska-Rybarek I. Novel Quaternary Ammonium Urethane-Dimethacrylates for Copolymers with Low Water Sorption and Solubility. Molecules. 2025; 30(4):769. https://doi.org/10.3390/molecules30040769
Chicago/Turabian StyleDrejka, Patryk, Patrycja Kula, and Izabela Barszczewska-Rybarek. 2025. "Novel Quaternary Ammonium Urethane-Dimethacrylates for Copolymers with Low Water Sorption and Solubility" Molecules 30, no. 4: 769. https://doi.org/10.3390/molecules30040769
APA StyleDrejka, P., Kula, P., & Barszczewska-Rybarek, I. (2025). Novel Quaternary Ammonium Urethane-Dimethacrylates for Copolymers with Low Water Sorption and Solubility. Molecules, 30(4), 769. https://doi.org/10.3390/molecules30040769








