Identification and Therapeutic Potential of Polymethoxylated Flavones in Citri Reticulatae Pericarpium for Alzheimer’s Disease: Targeting Neuroinflammation
Abstract
1. Introduction
2. Results
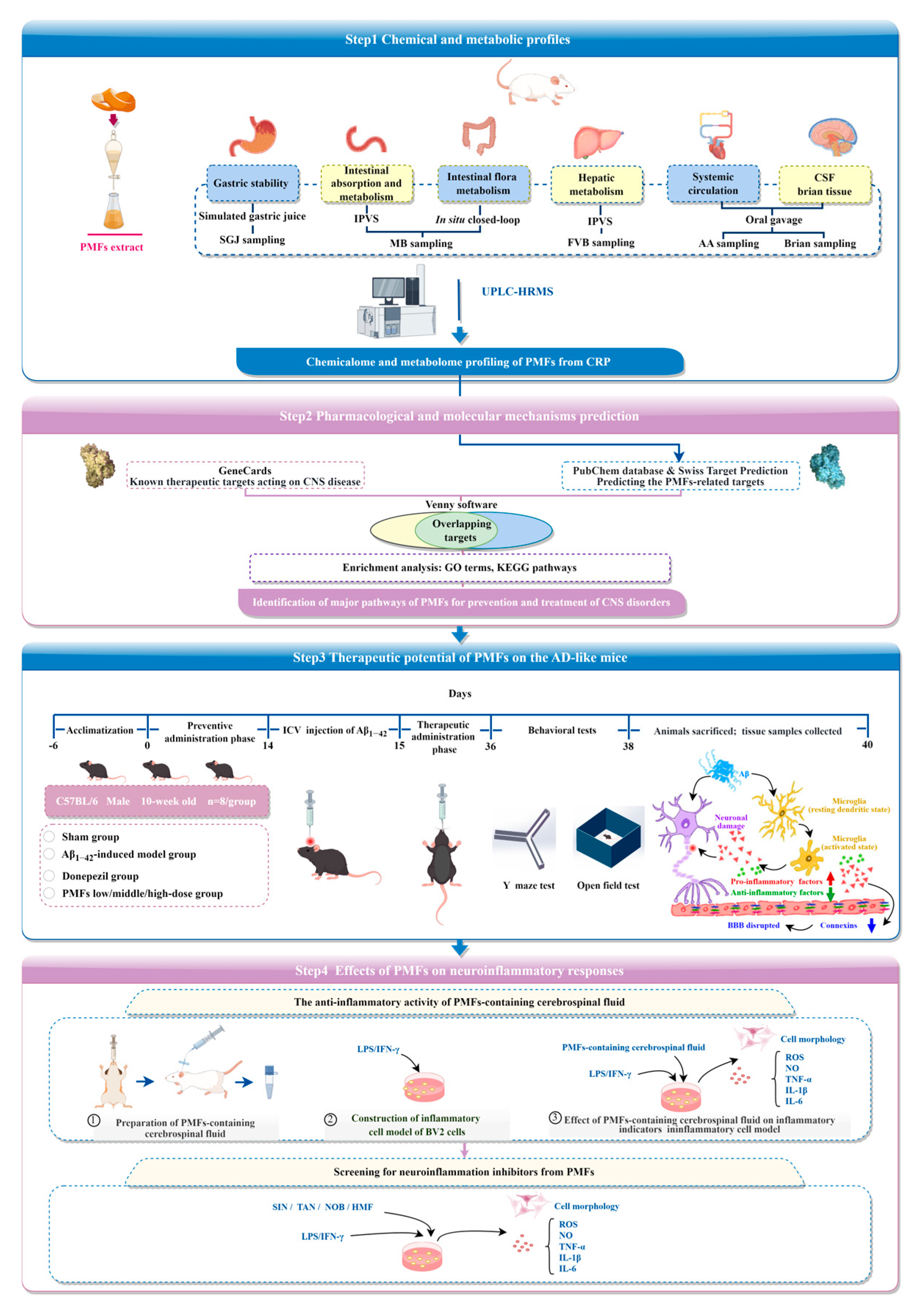
2.1. Chemical and Metabolic Profiles of PMFs in CRP
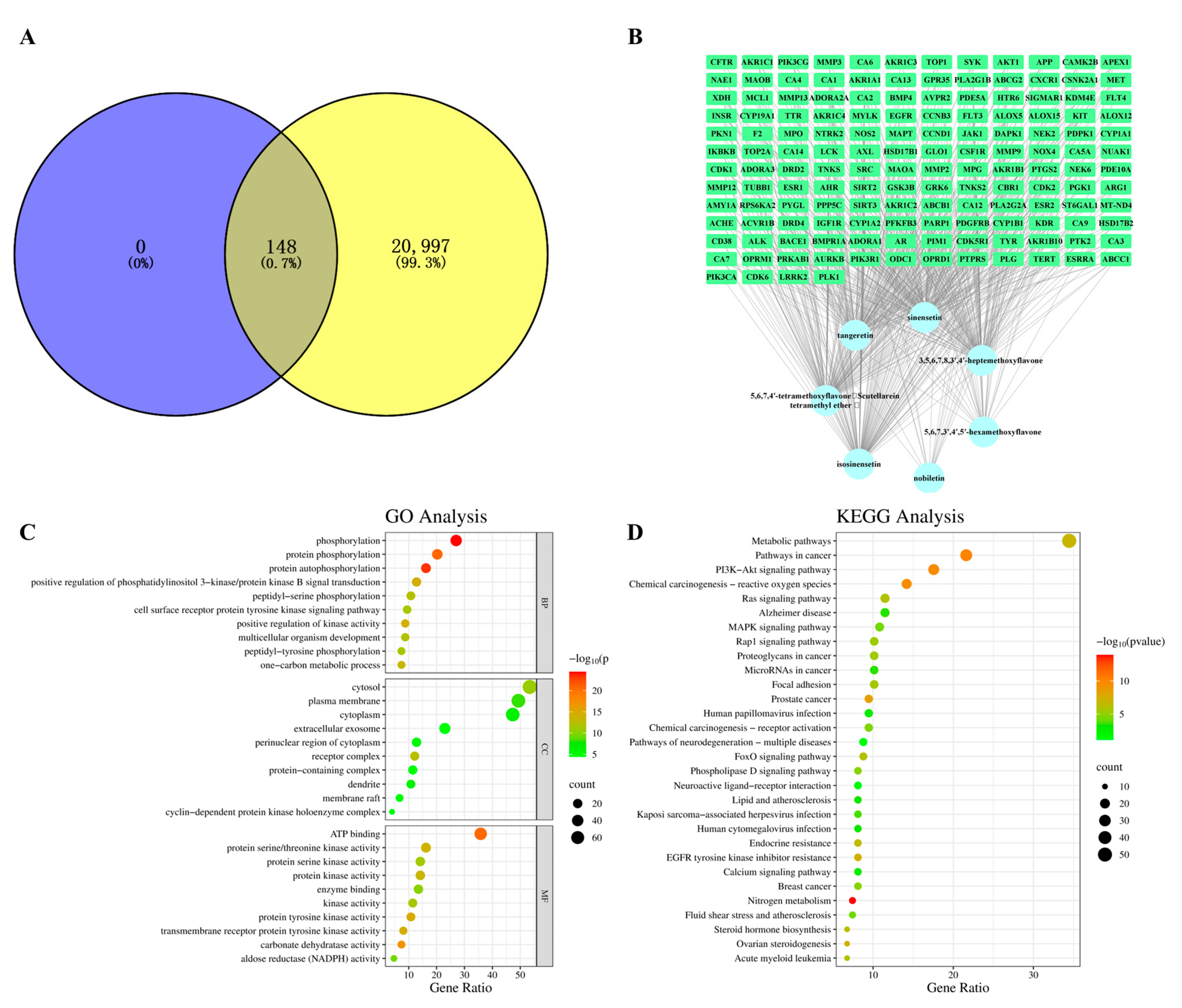
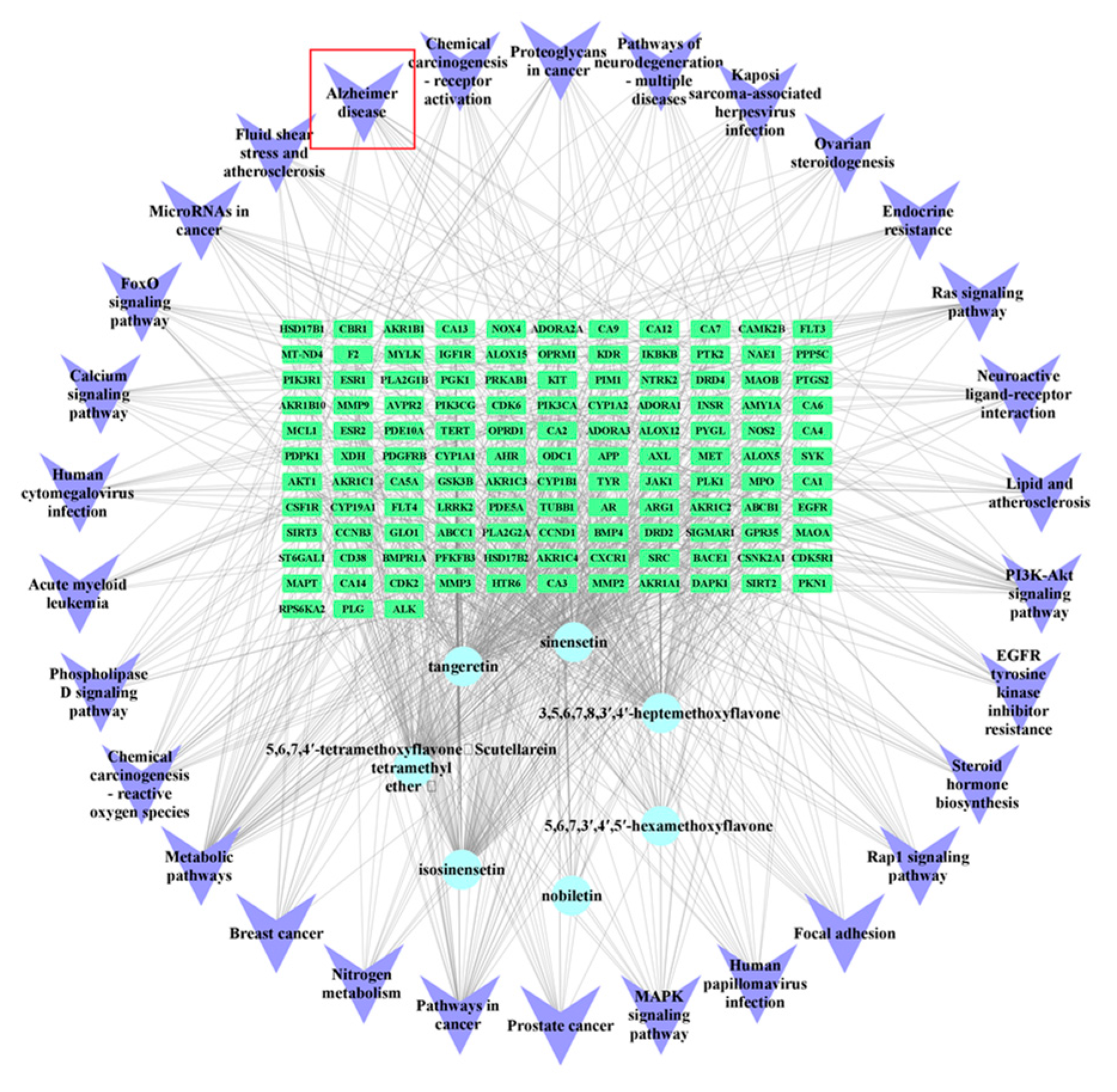
2.2. Network Pharmacology Analysis
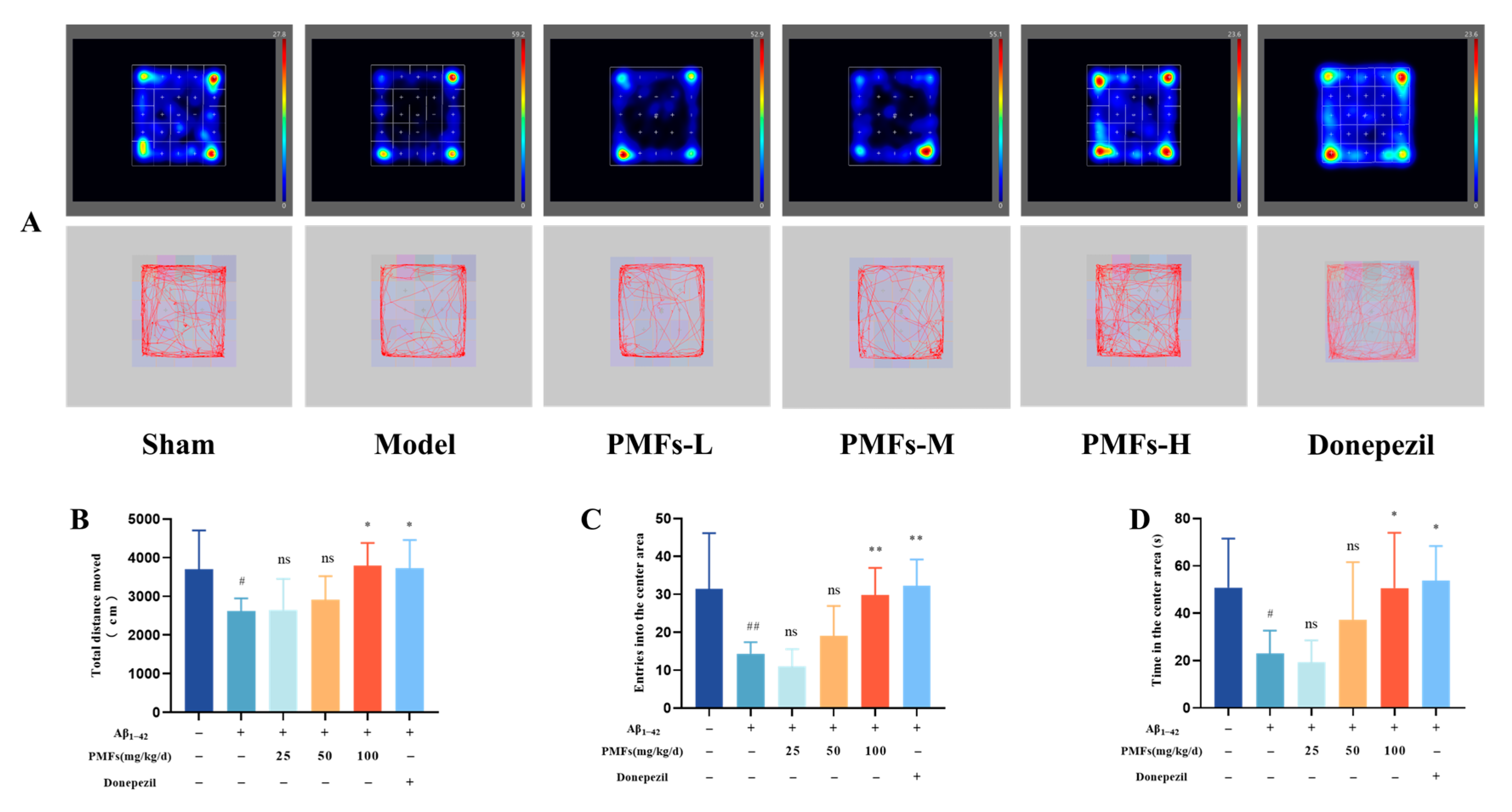
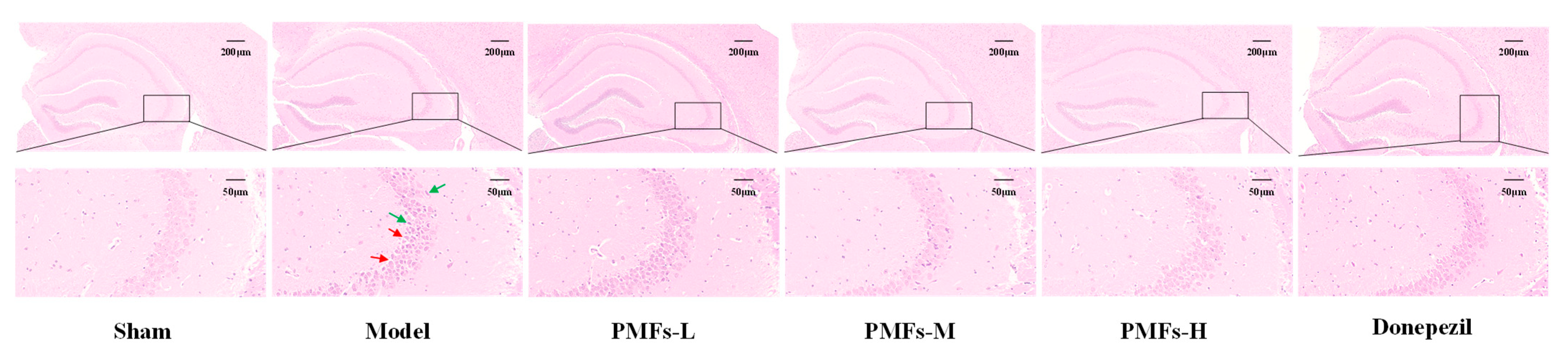
2.3. Effects of PMFs on the AD-like Mice
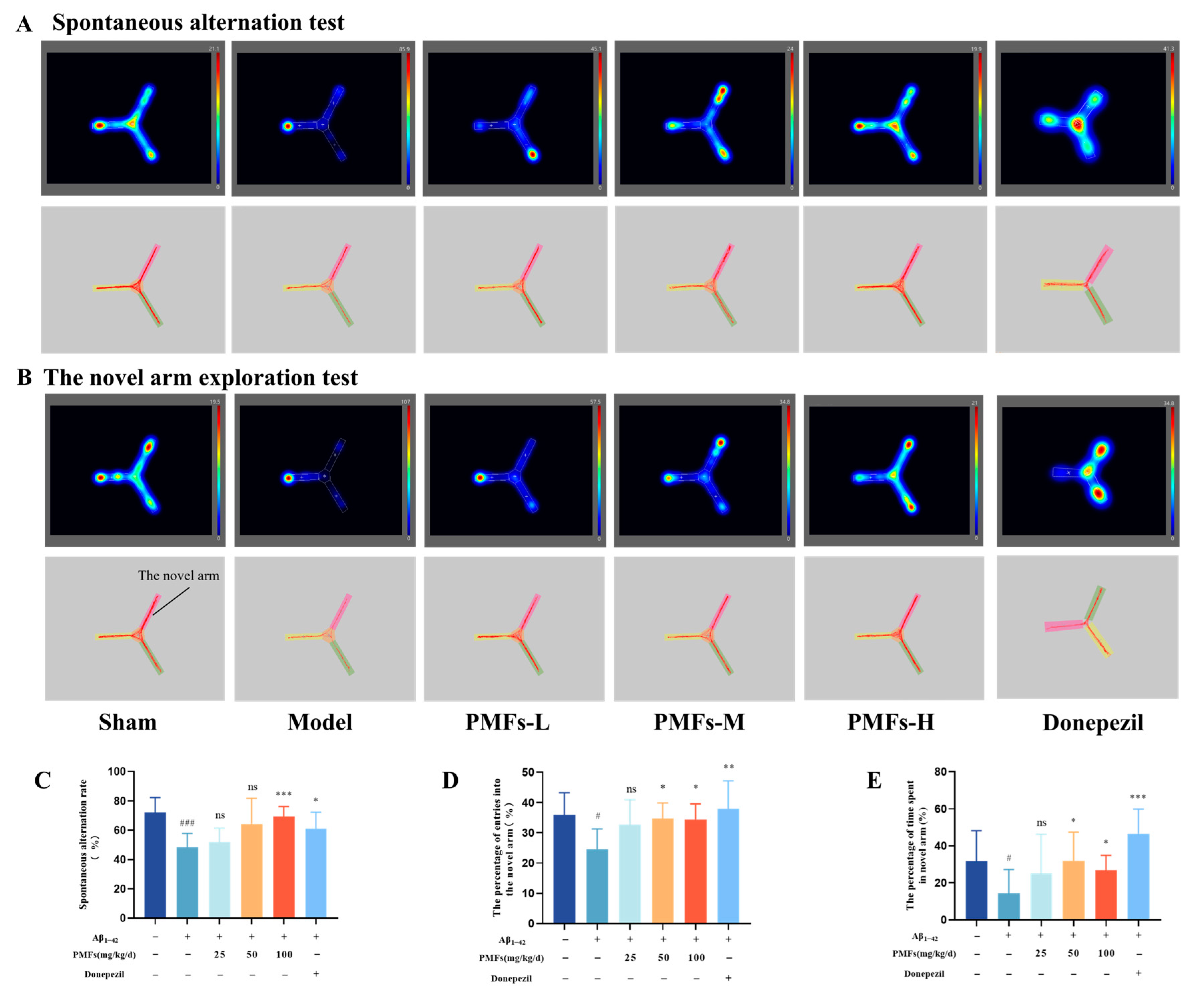
2.3.1. Behavioral Tests
2.3.2. Histological Evaluation
2.3.3. Aβ and P-Tau Measurement
2.3.4. Postsynaptic Density Protein 95 (PSD-95) Measurement
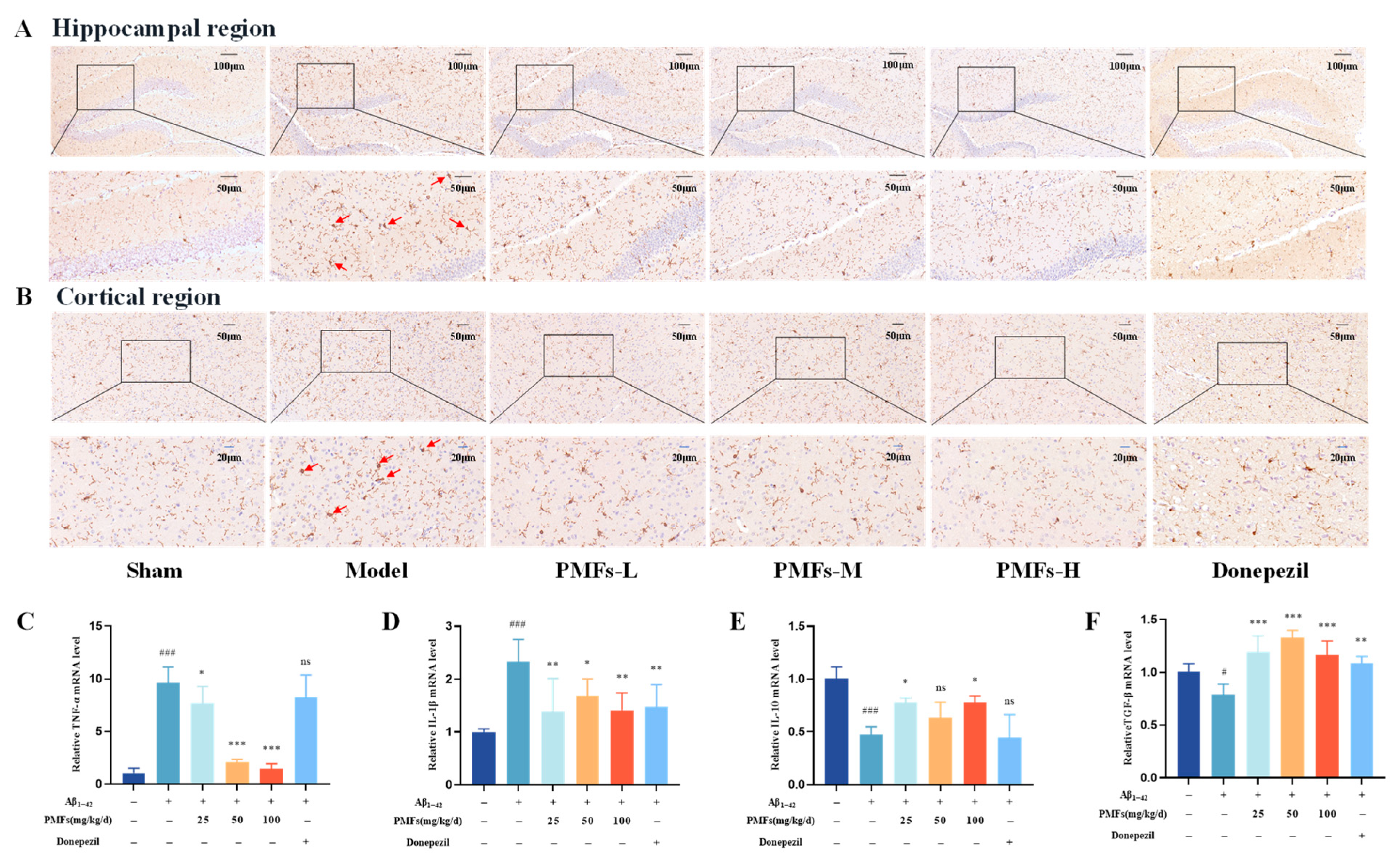
2.3.5. Inflammatory Response in Brain Tissue
2.3.6. BBB Functionality Assessment
2.4. Anti-Inflammatory Effect of PMF-Containing CSF
2.5. Screening for Neuroinflammation Inhibitors from PMFs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of PMFs Extract
4.3. Animals
4.4. Sequential Metabolism of PMFs in CRP
4.4.1. Stability of PMFs Extract in Simulated Gastric Juice
4.4.2. In Vivo Metabolic Experiments
4.4.3. Biological Sample Processing
4.5. UPLC-Q Exactive-Orbitrap HRMS Analysis
4.6. Screening and Identification of Bioactive Compounds in PMFs
4.7. Network Pharmacology Analysis
4.8. Investigation the Effects of PMFs on the AD-like Mice
4.8.1. Groups and Treatment
4.8.2. Y Maze Test
4.8.3. Open Field Test
4.8.4. Tissue Preparation
4.8.5. HE Staining for Histological Evaluation
4.8.6. Activated Microglial Evaluation
4.9. Cell Culture and Treatment
4.10. Cell Viability Assay
4.11. NO and ROS Measurement
4.12. ELISA Analyses
4.13. RT-qPCR Analyses
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Galea, E.; Graeber, M.B. Neuroinflammation: The Abused Concept. ASN Neuro 2023, 15, 17590914231197523. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, F.; Zhang, M. Therapeutic Potential of Plant-Derived Natural Compounds in Alzheimer’s Disease: Targeting Microglia-Mediated Neuroinflammation. Biomed. Pharmacother. 2024, 178, 117235. [Google Scholar] [CrossRef]
- Shen, H.; Pei, H.; Zhai, L.; Guan, Q.; Wang, G. Salvianolic Acid C Improves Cerebral Ischemia Reperfusion Injury through Suppressing Microglial Cell M1 Polarization and Promoting Cerebral Angiogenesis. Int. Immunopharmacol. 2022, 110, 109021. [Google Scholar] [CrossRef]
- Cai, P.; Li, W.; Xu, Y.; Wang, H. Drp1 and Neuroinflammation: Deciphering the Interplay between Mitochondrial Dynamics Imbalance and Inflammation in Neurodegenerative Diseases. Neurobiol. Dis. 2024, 198, 106561. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Lv, J. STAT4 Targets KISS1 to Inhibit the Oxidative Damage, Inflammation and Neuronal Apoptosis in Experimental PD Models by Inactivating the MAPK Pathway. Neurochem. Int. 2024, 175, 105683. [Google Scholar] [CrossRef]
- Bungãu, S.G.; Popa, V.C. Between Religion and Science: Some Aspects: Concerning Illness and Healing in Antiquity. Transylv. Rev. 2024, 24, 3–18. [Google Scholar]
- Yu, X.; Sun, S.; Guo, Y.; Liu, Y.; Yang, D.; Li, G.; Lü, S. Citri Reticulatae Pericarpium (Chenpi): Botany, Ethnopharmacology, Phytochemistry, and Pharmacology of a Frequently Used Traditional Chinese Medicine. J. Ethnopharmacol. 2018, 220, 265–282. [Google Scholar] [CrossRef]
- Yue, L.; Li, N.; Ye, X.; Xiu, Y.; Wang, B. Polymethoxylated Flavones for Modulating Signaling Pathways in Inflammation. Int. Immunopharmacol. 2024, 143, 113522. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z.; Jiang, Z.; Li, Y.; Chen, Q.; Zhou, Z. Polymethoxylated Flavone Variations and in Vitro Biological Activities of Locally Cultivated Citrus Varieties in China. Food Chem. 2025, 463, 141047. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Tait, A.R.; Kitts, D.D. Flavonoid Composition of Orange Peel and Its Association with Antioxidant and Anti-Inflammatory Activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Parkar, N.A.; Bhatt, L.K.; Addepalli, V. Efficacy of Nobiletin, a Citrus Flavonoid, in the Treatment of the Cardiovascular Dysfunction of Diabetes in Rats. Food Funct. 2016, 7, 3121–3129. [Google Scholar] [CrossRef]
- Keith, C.T.; Borisy, A.A.; Stockwell, B.R. Multicomponent Therapeutics for Networked Systems. Nat. Rev. Drug Discov. 2005, 4, 71–78. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Banks, W.A. Drug Delivery to the Brain in Alzheimer’s Disease: Consideration of the Blood-Brain Barrier. Adv. Drug Delivery Rev. 2012, 64, 629–639. [Google Scholar] [CrossRef]
- Lei, J.; Xue, Y.; Liu, Y.-M.; Liao, X. Characterization of Major Metabolites of Polymethoxylated Flavonoids in Pericarpium Citri Reticulatae Using Liver Microsomes Immobilized on Magnetic Nanoparticles Coupled with UPLC/MS-MS. Chem. Cent. J. 2017, 11, 13. [Google Scholar] [CrossRef]
- Zeng, S.-L.; Duan, L.; Chen, B.-Z.; Li, P.; Liu, E.-H. Chemicalome and Metabolome Profiling of Polymethoxylated Flavonoids in Citri Reticulatae Pericarpium Based on an Integrated Strategy Combining Background Subtraction and Modified Mass Defect Filter in a Microsoft Excel Platform. J. Chromatogr. A 2017, 1508, 106–120. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Han, X.; Yang, W.; Wang, G.; Wang, J.; Jiang, X.; Sen, M.; Li, X.; Yu, G.; et al. Mechanism of Paeoniae Radix Alba in the Treatment of Non-Alcoholic Fatty Liver Disease Based on Sequential Metabolites Identification Approach, Network Pharmacology, and Binding Affinity Measurement. Front. Nutr. 2021, 8, 677659. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Liu, Y.; Dong, H.; Lv, B.; Fang, M.; Zhao, H. Metabolic Routes along Digestive System of Licorice: Multicomponent Sequential Metabolism Method in Rat. Biomed. Chromatogr. BMC 2016, 30, 902–912. [Google Scholar] [CrossRef]
- Niu, B.; Xie, X.; Xiong, X.; Jiang, J. Network Pharmacology-Based Analysis of the Anti-Hyperglycemic Active Ingredients of Roselle and Experimental Validation. Comput. Biol. Med. 2022, 141, 104636. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, S.; Tan, H.Y.; Cheung, F.; Wang, N.; Huang, J.; Feng, Y. A Network-Based Pharmacology Study of the Herb-Induced Liver Injury Potential of Traditional Hepatoprotective Chinese Herbal Medicines. Molecules 2017, 22, 632. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.-D.; Zhou, P.; Yang, H.; Li, Y.-S.; Li, P.; Liu, E.-H. Rapid Resolution Liquid Chromatography-Electrospray Ionisation Tandem Mass Spectrometry Method for Identification of Chemical Constituents in Citri Reticulatae Pericarpium. Food Chem. 2013, 136, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.T.; Zhao, X.J.; Zhang, Y.D.; Li, Y.F. Fast Separation and Sensitive Quantitation of Polymethoxylated Flavonoids in the Peels of Citrus Using UPLC-Q-TOF-MS. J. Agric. Food Chem. 2017, 65, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Y.; Zeng, X.; Peng, W.; Wu, Z.; Su, W.-W. Characterisation and Classification of Citri Reticulatae Pericarpium Varieties Based on UHPLC-Q-TOF-MS/MS Combined with Multivariate Statistical Analyses. Phytochem. Anal. PCA 2019, 30, 278–291. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, X.; Li, T.; Song, Y.-L.; Li, J.; Zhao, Y.-F.; Song, Q.-Q.; Tu, P.-F. Chemome Profiling of Citri Reticulatae Pericarpium Using UHPLC-IT-TOF-MS. China J. Chin. Mater. Med. 2020, 45, 899–909. [Google Scholar] [CrossRef]
- Xu, L.-L.; He, Y.-Q.; Guo, X.; Lu, Y.-H.; Wang, C.-H.; Wang, Z.-T. Identification of Metabolites of Nobiletin in Rats Using Ultra-Performance Liquid Chromatography Coupled with Triple-Quadrupole Mass Spectrometry. Acta Pharm. Sin. 2011, 46, 1483–1487. [Google Scholar]
- Li, Z.; Wang, Y.-Q.; Wu, D.-X.; Li, S.-F.; Li, Y.-N.; Wang, S.-P.; Zhang, J.-Y.; Dai, L. Analysis of Metabolites of Chuan Pi Chen Pin in Rats Based on Characteristic Ion Analysis. Chin. Tradit. Pat. Med. 2024, 46, 1800–1809. [Google Scholar]
- Du, L.; Chen, J.; Yan, J.; Xie, H.; Wang, L.; Wang, R.; Han, X.; Wang, Y. Lingguizhugan Decoction Ameliorates Cognitive Impairment in AD-like Mice by Influencing the Microbiome-Gut-Brain Axis Mediated by SCFAs. Phytomed. Int. J. Phytother. Phytopharm. 2024, 133, 155942. [Google Scholar] [CrossRef]
- Abdulbasit, A.; Stephen Michael, F.; Shukurat Onaopemipo, A.; Abdulmusawwir, A.-O.; Aminu, I.; Nnaemeka Tobechukwu, A.; Wahab Imam, A.; Oluwaseun Aremu, A.; Folajimi, O.; Bilikis Aderonke, A.; et al. Glucocorticoid Receptor Activation Selectively Influence Performance of Wistar Rats in Y-Maze. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2018, 25, 41–50. [Google Scholar] [CrossRef]
- Wan, Y.-C.; Yang, Y.; Pang, S.; Kong, Z.-L. A Novel Derivative of Evodiamine Improves Cognitive Impairment and Synaptic Integrity in AD Mice. Biomed. Pharmacother. 2024, 177, 117103. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Yang, Y.; Cheng, Y.; Sun, D.; Wang, X.; Khanna, R.; Ju, W. Nasal Delivery of a CRMP2-Derived CBD3 Adenovirus Improves Cognitive Function and Pathology in APP/PS1 Transgenic Mice. Mol. Brain. 2020, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Purushothuman, S.; Johnstone, D.M.; Nandasena, C.; Mitrofanis, J.; Stone, J. Photobiomodulation with near Infrared Light Mitigates Alzheimer’s Disease-Related Pathology in Cerebral Cortex—Evidence from Two Transgenic Mouse Models. Alzheimer’s Res. Ther. 2014, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Dore, K.; Carrico, Z.; Alfonso, S.; Marino, M.; Koymans, K.; Kessels, H.W.; Malinow, R. PSD-95 Protects Synapses from β-Amyloid. Cell Rep. 2021, 35, 109194. [Google Scholar] [CrossRef]
- Wu, A.-G.; Zhou, X.-G.; Qiao, G.; Yu, L.; Tang, Y.; Yan, L.; Qiu, W.-Q.; Pan, R.; Yu, C.-L.; Law, B.Y.-K.; et al. Targeting Microglial Autophagic Degradation in NLRP3 Inflammasome-Mediated Neurodegenerative Diseases. Ageing Res. Rev. 2021, 65, 101202. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Su, Y.; Yin, G.; Wang, S.; Yu, B.; Li, H.; Qi, J.; Chen, H.; Zeng, W.; et al. Activation of Wnt/β-Catenin Pathway Mitigates Blood-Brain Barrier Dysfunction in Alzheimer’s Disease. Brain J. Neurol. 2022, 145, 4474–4488. [Google Scholar] [CrossRef]
- Iram, T.; Kern, F.; Kaur, A.; Myneni, S.; Morningstar, A.R.; Shin, H.; Garcia, M.A.; Yerra, L.; Palovics, R.; Yang, A.C.; et al. Young CSF Restores Oligodendrogenesis and Memory in Aged Mice via Fgf17. Nature 2022, 605, 509–515. [Google Scholar] [CrossRef]
- Razani, E.; Pourbagheri-Sigaroodi, A.; Safaroghli-Azar, A.; Zoghi, A.; Shanaki-Bavarsad, M.; Bashash, D. The PI3K/Akt Signaling Axis in Alzheimer’s Disease: A Valuable Target to Stimulate or Suppress? Cell Stress Chaperones 2021, 26, 871–887. [Google Scholar] [CrossRef]
- Nakajima, K.; Kohsaka, S. Microglia: Activation and Their Significance in the Central Nervous System. J. Biochem. 2001, 130, 169–175. [Google Scholar] [CrossRef]
- Wright, B.; King, S.; Suphioglu, C. The Importance of Phosphoinositide 3-Kinase in Neuroinflammation. Int. J. Mol. Sci. 2024, 25, 11638. [Google Scholar] [CrossRef]
- Lu, T.; Ding, L.; Zheng, X.; Li, Y.; Wei, W.; Liu, W.; Tao, J.; Xue, X. Alisol a Exerts Neuroprotective Effects against HFD-Induced Pathological Brain Aging via the SIRT3-NF-κB/MAPK Pathway. Mol. Neurobiol. 2024, 61, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.M.; Emerson, C.P.; Owens, J.; Christoforou, N. P38 MAPKs—Roles in Skeletal Muscle Physiology, Disease Mechanisms, and as Potential Therapeutic Targets. JCI Insight 2021, 6, e149915. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yao, X.; Jiang, W.; Li, W.; Zhu, S.; Liao, C.; Zou, L.; Ding, R.; Chen, J. Advanced Oxidation Protein Products Induce Microglia-Mediated Neuroinflammation via MAPKs-NF-κB Signaling Pathway and Pyroptosis after Secondary Spinal Cord Injury. J. Neuroinflamm. 2020, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Kim, D.H.; Lee, J.S. FoxO Transcription Factors: Applicability as a Novel Immune Cell Regulators and Therapeutic Targets in Oxidative Stress-Related Diseases. Int. J. Mol. Sci. 2022, 23, 11877. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric Oxide in Immunity and Inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Ozben, T.; Ozben, S. Neuro-Inflammation and Anti-Inflammatory Treatment Options for Alzheimer’s Disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
- Wenzel, T.J.; Klegeris, A. Novel Multi-Target Directed Ligand-Based Strategies for Reducing Neuroinflammation in Alzheimer’s Disease. Life Sci. 2018, 207, 314–322. [Google Scholar] [CrossRef]
- Kasza, Á.; Penke, B.; Frank, Z.; Bozsó, Z.; Szegedi, V.; Hunya, Á.; Németh, K.; Kozma, G.; Fülöp, L. Studies for Improving a Rat Model of Alzheimer’s Disease: Icv Administration of Well-Characterized β-Amyloid 1-42 Oligomers Induce Dysfunction in Spatial Memory. Molecules 2017, 22, 2007. [Google Scholar] [CrossRef]
- Gou, X.; Chen, J.; Ran, X.; Deng, L.; Deng, Y.; Liu, C.; Long, S.; Xie, J.; Peng, T.; Zhang, X. Konjac Oligo-Glucomannan Ameliorate Cognition Impairments of Aβ1-42 Induced Alzheimer’s Disease in Mice by Targeting Microbiota-SCFAs-Brain Axis. J. Funct. Foods. 2024, 122, 106469. [Google Scholar] [CrossRef]
- Egger, F.; Jakab, M.; Fuchs, J.; Oberascher, K.; Brachtl, G.; Ritter, M.; Kerschbaum, H.H.; Gaisberger, M. Effect of Glycine on BV-2 Microglial Cells Treated with Interferon-γ and Lipopolysaccharide. Int. J. Mol. Sci. 2020, 21, 804. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Ishiwa, S.; Lin, X.; Nakazawa, Y.; Tago, K.; Funakoshi-Tago, M. The Citrus Flavonoid, Nobiletin Inhibits Neuronal Inflammation by Preventing the Activation of NF-κB. Neurochem. Int. 2023, 171, 105613. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Lee, E.-J.; Park, J.-S.; Jang, S.-E.; Kim, D.-H.; Kim, H.-S. Anti-Inflammatory and Antioxidant Mechanism of Tangeretin in Activated Microglia. J. Neuroimmune Pharmacol. 2016, 11, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Wang, M.; Peng, Y.; Chen, D.; Li, X. Simulated Gastrointestinal Tract Metabolism and Pharmacological Activities of Water Extract of Scutellaria baicalensis Roots. J. Ethnopharmacol. 2014, 152, 183–189. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, X.; Liu, Y.; Lu, L.; Yang, R.; Yu, G.; Sun, M.; Xin, S.; Tian, S.; Chen, X.; et al. An Approach to Characterizing the Complicated Sequential Metabolism of Salidroside in Rats. Molecules 2016, 21, 706. [Google Scholar] [CrossRef]
- Liu, H.; Yu, S.; Li, X.; Wang, X.; Qi, D.; Pan, F.; Chai, X.; Wang, Q.; Pan, Y.; Zhang, L.; et al. Integration of Deep Learning and Sequential Metabolism to Rapidly Screen Dipeptidyl Peptidase (DPP)-IV Inhibitors from Gardenia jasminoides Ellis. Molecules 2023, 28, 7381. [Google Scholar] [CrossRef]
- Wu, J.; Cai, Y.; Wu, X.; Ying, Y.; Tai, Y.; He, M. Transcardiac Perfusion of the Mouse for Brain Tissue Dissection and Fixation. Bio-Protoc. 2021, 11, e3988. [Google Scholar] [CrossRef]
- Zborowski, V.A.; Martins, C.C.; Marques, L.S.; Heck, S.O.; Nogueira, C.W. A Chloro Substituted Organoselenium Mitigates Stress-Associated Memory Impairment and Hippocampal Glutamatergic Function in a Repeated Forced Swim Stress Model. Neuroscience 2024, 563, 110–116. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Ma, C.; Ma, X.; Meng, W.; Yin, F.; Yang, Y. Tactile Cues Are Important to Environmental Novelty during Repeated Open Field Tests. Behav. Process. 2023, 204, 104796. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Wu, Q.; Wang, S.; Yang, S.; Li, X.; Chen, N.; Li, L.; Zhang, L. Tetrahydroxy Stilbene Glycoside Ameliorates Alzheimer’s Disease in APP/PS1 Mice via Glutathione Peroxidase Related Ferroptosis. Int. Immunopharmacol. 2021, 99, 108002. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, Z.; Ding, W.; Chen, D.; Ma, S.; Wen, C.; Wang, X.; Jin, Y.; Zhi, Y. Determination of Nobiletin and Tangeretin in Rat Plasma by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry and Study of Their Pharmacokinetics. Acta Chromatogr. 2024, 36, 413–419. [Google Scholar] [CrossRef]
- Singh, S.P.; Wahajuddin, N.; Tewari, D.; Patel, K.; Jain, G.K. Permeability Determination and Pharmacokinetic Study of Nobiletin in Rat Plasma and Brain by Validated High-Performance Liquid Chromatography Method. Fitoterapia 2011, 82, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, D.; Yu, C.; Lv, B.; Peng, J.; Wang, J.; Lin, Y. Citrus Nobiletin Ameliorates Experimental Colitis by Reducing Inflammation and Restoring Impaired Intestinal Barrier Function. Mol. Nutr. Food Res. 2015, 59, 829–842. [Google Scholar] [CrossRef]
- Yang, G.; Li, S.; Yuan, L.; Yang, Y.; Pan, M.-H. Effect of Nobiletin on the MAPK/NF-κB Signaling Pathway in the Synovial Membrane of Rats with Arthritis Induced by Collagen. Food Funct. 2017, 8, 4668–4674. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Assini, J.M.; Lee, J.K.; Allister, E.M.; Sutherland, B.G.; Koppes, J.B.; Sawyez, C.G.; Edwards, J.Y.; Telford, D.E.; Charbonneau, A.; et al. Nobiletin Attenuates VLDL Overproduction, Dyslipidemia, and Atherosclerosis in Mice with Diet-Induced Insulin Resistance. Diabetes 2011, 60, 1446–1457. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Gao, Z.; Sun, Y.; Wang, M.; Li, F.; Zheng, J.; Xiao, H. Nobiletin and Its Colonic Metabolites Suppress Colitis-Associated Colon Carcinogenesis by down-Regulating iNOS, Inducing Antioxidative Enzymes and Arresting Cell Cycle Progression. J. Nutr. Biochem. 2017, 42, 17–25. [Google Scholar] [CrossRef]
- Kim, E.; Nohara, K.; Wirianto, M.; Escobedo, G.; Lim, J.Y.; Morales, R.; Yoo, S.-H.; Chen, Z. Effects of the Clock Modulator Nobiletin on Circadian Rhythms and Pathophysiology in Female Mice of an Alzheimer’s Disease Model. Biomolecules 2021, 11, 1004. [Google Scholar] [CrossRef]
- Chai, W.; Zhang, J.; Xiang, Z.; Zhang, H.; Mei, Z.; Nie, H.; Xu, R.; Zhang, P. Potential of Nobiletin against Alzheimer’s Disease through Inhibiting Neuroinflammation. Metab. Brain Dis. 2022, 37, 1145–1154. [Google Scholar] [CrossRef]
- Hung, W.-L.; Chang, W.-S.; Lu, W.-C.; Wei, G.-J.; Wang, Y.; Ho, C.-T.; Hwang, L.S. Pharmacokinetics, Bioavailability, Tissue Distribution and Excretion of Tangeretin in Rat. J. Food Drug Anal. 2018, 26, 849–857. [Google Scholar] [CrossRef]
- Elhennawy, M.G.; Lin, H.-S. Determination of Tangeretin in Rat Plasma: Assessment of Its Clearance and Absolute Oral Bioavailability. Pharmaceutics 2017, 10, 3. [Google Scholar] [CrossRef]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Tangeretin, a Polymethoxylated Flavone, Modulates Lipid Homeostasis and Decreases Oxidative Stress by Inhibiting NF-κB Activation and Proinflammatory Cytokines in Cardiac Tissue of Streptozotocin-Induced Diabetic Rats. J. Funct. Foods 2015, 16, 315–333. [Google Scholar] [CrossRef]
- Arab, H.H.; Mohamed, W.R.; Barakat, B.M.; Arafa, E.-S.A. Tangeretin Attenuates Cisplatin-Induced Renal Injury in Rats: Impact on the Inflammatory Cascade and Oxidative Perturbations. Chem. Biol. Interact. 2016, 258, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Yu, Y.; Lee, J.; Jeong, W.-S.; Ho, C.-T.; Jun, M. Polymethoxyflavones: Novel β-Secretase (BACE1) Inhibitors from Citrus Peels. Nutrients 2017, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cao, A.; Shi, J.; Yin, P.; Wang, L.; Ji, G.; Xie, J.; Wu, D. Tangeretin, a Citrus Polymethoxyflavonoid, Induces Apoptosis of Human Gastric Cancer AGS Cells through Extrinsic and Intrinsic Signaling Pathways. Oncol. Rep. 2014, 31, 1788–1794. [Google Scholar] [CrossRef]
- Guo, Z.; Li, B.; Gu, J.; Zhu, P.; Su, F.; Bai, R.; Liang, X.; Xie, Y. Simultaneous Quantification and Pharmacokinetic Study of Nine Bioactive Components of Orthosiphon stamineus Benth. Extract in Rat Plasma by UHPLC-MS/MS. Molecules 2019, 24, 3057. [Google Scholar] [CrossRef]
- Wang, S.; Yang, X.X.; Li, T.J.; Tian, X.M.; Wang, Y.L.; Bai, G.; Bao, Y.R.; Meng, X.S. Metabolic Regularity of Bioactive Compounds in Bufei Jianpi Granule in Rats Using Ultra-High-Performance Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry Analysis Technology. Biomed. Chromatogr. BMC 2023, 37, e5740. [Google Scholar] [CrossRef]
- Zhu, Y.; Zuo, F.; Ouyang, H.; Chen, L.; Zhang, M.; Shang, Y.; Lv, Z.; Chang, Y.; He, J. Determination of Eleven Components in Rat Plasma by UPLC-MS/MS and GC-MS for Pharmacokinetic Studies after Oral Administration of Citri Reticulatae Pericarpium Extract. J. Pharm. Biomed. Anal. 2024, 248, 116315. [Google Scholar] [CrossRef]
- Li, J.; Jie, X.; Liang, X.; Chen, Z.; Xie, P.; Pan, X.; Zhou, B.; Li, J. Sinensetin Suppresses Influenza a Virus-Triggered Inflammation through Inhibition of NF-κB and MAPKs Signalings. BMC Complement. Med. Ther. 2020, 20, 135. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Li, S.; Wu, Y.; Yu, C.; Ni, L.; Xiao, J.; Shao, Z.; Zhu, H.; Wang, J.; et al. Tangeretin Suppresses Osteoarthritis Progression via the Nrf2/NF-κB and MAPK/NF-κB Signaling Pathways. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 98, 153928. [Google Scholar] [CrossRef]
- You, Q.; Li, D.; Ding, H.; Chen, H.; Hu, Y.; Liu, Y. Pharmacokinetics and Metabolites of 12 Bioactive Polymethoxyflavones in Rat Plasma. J. Agric. Food Chem. 2021, 69, 12705–12716. [Google Scholar] [CrossRef]
- Wu, L.; Liu, H.; Li, L.; Liu, H.; Yang, K.; Liu, Z.; Huang, H. 5,7,3’,4’-Tetramethoxyflavone Exhibits Chondroprotective Activity by Targeting β-Catenin Signaling in Vivo and in Vitro. Biochem. Biophys. Res. Commun. 2014, 452, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Sun, J.; Zheng, Y.; Wang, L.; Zhang, J.; Chen, R.; Ye, W. Determination of Isosinensetin in Rat Plasma by UHPLC-MS/MS: Application to Oral and Intravenous Pharmacokinetic Study in Healthy Rats. J. Pharm. Biomed. Anal. 2020, 184, 113210. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Sale, S.; Britton, R.G.; Brown, K.; Steward, W.P.; Gescher, A.J. Pharmacokinetics in Mice and Metabolism in Murine and Human Liver Fractions of the Putative Cancer Chemopreventive Agents 3′,4′,5′,5,7-Pentamethoxyflavone and Tricin (4′,5,7-Trihydroxy-3′,5′-Dimethoxyflavone). Cancer Chemother. Pharmacol. 2011, 67, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Mekjaruskul, C.; Jay, M.; Sripanidkulchai, B. Pharmacokinetics, Bioavailability, Tissue Distribution, Excretion, and Metabolite Identification of Methoxyflavones in Kaempferia parviflora Extract in Rats. Drug Metab. Dispos. Biol. Fate Chem. 2012, 40, 2342–2353. [Google Scholar] [CrossRef]
- Okuyama, S.; Morita, M.; Miyoshi, K.; Nishigawa, Y.; Kaji, M.; Sawamoto, A.; Terugo, T.; Toyoda, N.; Makihata, N.; Amakura, Y.; et al. 3,5,6,7,8,3’,4’-Heptamethoxyflavone, a Citrus Flavonoid, on Protection against Memory Impairment and Neuronal Cell Death in a Global Cerebral Ischemia Mouse Model. Neurochem. Int. 2014, 70, 30–38. [Google Scholar] [CrossRef]
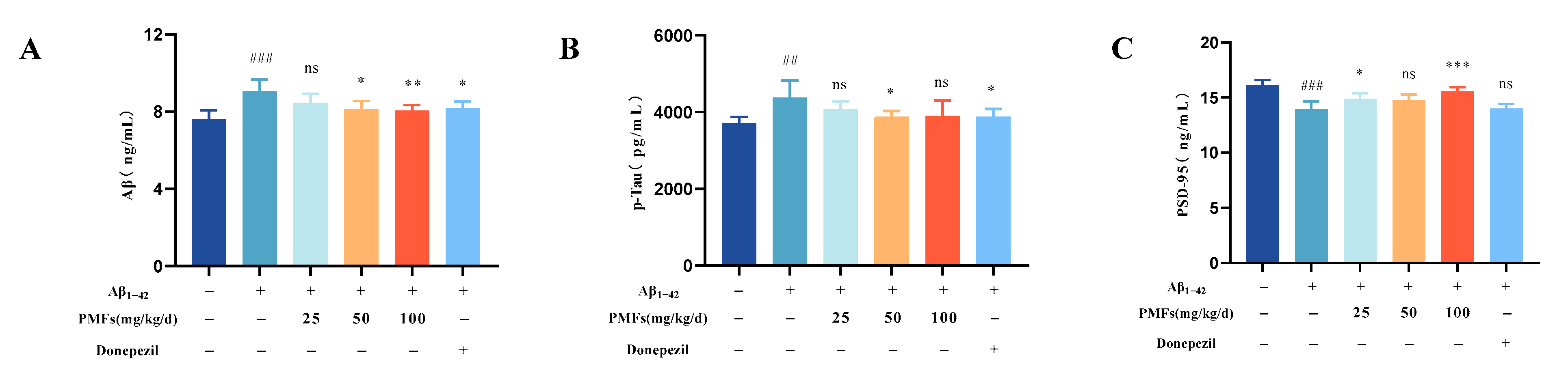



| No. | tR /min | Observed Mass | Error /ppm | MS/MS Fragments | Molecular Formula | Proposed Compounds | PMFs | SGJ | MB-W | MB-F | FVB | AA | CSF | BT | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12.20 | [M + H]+ 375.1434 | −1.065 | 211.0604, 196.0369, 168.0415, 150.0314 | C20H22O7 | 5,6,7,3′,4′-pentamethoxyflavanone | Y | Y | Y | Y | Y | N | N | N | [23] |
| 2 | 13.07 | [M + H]+ 373.1282 | 0.136 | 358.1048, 343.0814, 315.0865, 181.0132, 153.0183 | C20H20O7 | Isosinensetin | Y | Y | Y | Y | Y | Y | Y | Y | [24] |
| 3 | 13.72 | [M + H]+ 403.1389 | 0.106 | 388.1147, 373.0921, 327.0861 | C21H22O8 | 5,7,8,3′,4′,5′-hexamethoxyflavone | Y | Y | Y | Y | Y | Y | N | Y | [23] |
| 4 | 13.79 | [M + H]+ 389.1234 | 0.658 | 374.1000, 359.0761, 328.0958, 313.0702 | C20H20O8 | 3′(4′)-monohydroxy-5,6,7,8,4′(5,6,7,8,3′)-pentamethoxyflavone | Y | Y | Y | Y | Y | Y | N | Y | [23] |
| 5 | 14.17 | [M + H]+ 373.1281 | −0.266 | 358.1042, 343.0814, 312.0993, 329.1021, 357.0967 | C20H20O7 | Sinensetin * | Y | Y | Y | Y | Y | Y | Y | Y | * |
| 6 | 14.30 | [M + H]+ 375.1441 | 0.641 | 211.0604, 196.0369 | C20H22O7 | 5,7,8,3′,4′-Pentametho-xyflavanone | Y | Y | Y | Y | Y | Y | N | Y | [23] |
| 7 | 14.51 | [M + H]+ 389.1233 | 0.427 | 374.1002, 359.0761, 341.0656, 316.0576 | C20H20O8 | Monohydroxy-pentamethoxyflavone | Y | Y | Y | Y | Y | Y | N | N | [25] |
| 8 | 14.78 | [M + H]+ 403.1387 | 0.016 | 388.1135, 373.0919, 327.0866 | C21H22O8 | 5,6,7,3′,4′,5′-hexamethoxyflavone | Y | Y | Y | Y | Y | Y | Y | Y | [23] |
| 9 | 15.09 | [M + H]+ 403.1387 | −0.074 | 388.1151, 373.0919, 355.0811, 327.0863 | C21H22O8 | Nobiletin * | Y | Y | Y | Y | Y | Y | Y | Y | * |
| 10 | 15.43 | [M + H]+ 343.1176 | −0.014 | 328.0937, 313.0706, 285.0758, 181.0133, 153.0184, 133.0649 | C19H18O6 | 5,6,7,4′-tetramethoxyflavone | Y | Y | Y | Y | Y | Y | Y | Y | [23] |
| 11 | 15.67 | [M + H]+ 433.1494 | 0.234 | 418.1245, 403.1024, 388.0774, 360.0845, 385.0923, 345.0604 | C22H24O9 | 3,5,6,7,8,3′,4′-heptemethoxyflavone * | Y | Y | Y | Y | Y | Y | Y | Y | * |
| 12 | 15.70 | [M + H]+ 389.1231 | −0.036 | 374.1004, 359.0761, 356.0887, 341.0656, 197.0883, 169.0132 | C20H20O8 | Monohydroxy-pentamethoxyflavone | Y | Y | Y | Y | Y | Y | N | Y | [25] |
| 13 | 16.28 | [M + H]+ 373.1282 | 0.136 | 358.1043, 343.0813, 325.0714, 300.0622, 297.0757 | C20H20O7 | Tangeretin * | Y | Y | Y | Y | Y | Y | Y | Y | * |
| 14 | 16.38 | [M + H]+ 419.1330 | −1.572 | 404.1095, 389.0865, 371.0760, 361.0908 | C21H22O9 | 5-monohydroxy-6,7,8,3′,4′,5′-hexamethoxyflavone | Y | Y | Y | Y | N | N | N | N | [23] |
| 15 | 16.55 | [M + H]+ 359.1124 | −0.277 | 344.0887, 329.0660, 311.0545, 301.0695, 163.0755, 138.9975 | C19H18O7 | Monohydroxy-tetramethoxyflavone | Y | Y | Y | Y | Y | Y | N | Y | [25] |
| 16 | 17.03 | [M + H]+ 403.1388 | 0.076 | 388.1156, 373.0919, 327.0872 | C21H22O8 | Hexamethoxyflavone | Y | Y | Y | Y | Y | Y | N | Y | [23] |
| 17 | 17.41 | [M + H]+ 389.1231 | −0.036 | 374. 1000, 359. 0761, 341. 0655, 331. 0811, 197. 0082 | C20H20O8 | 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone | Y | Y | Y | Y | Y | Y | N | Y | [26] |
| 18 | 17.92 | [M + H]+ 419.1338 | 0.313 | 404.1100, 389.0868, 371.0758 | C21H22O9 | Monohydroxy-hexamethoxyflavone | Y | Y | Y | Y | Y | N | N | N | [25] |
| No. | tR /min | Observed Mass | Error /ppm | Molecular Formula | MS/MS Fragments | SGJ | MB- W | MB- F | FVB | AA | CSF | BT | Metabolites | Structure of Prediction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | 8.78 | [M + H]+ 595.1661 | 0.661 | C27H30O15 | 419.1342, 389.0872, 404.1107, 371.0768, 595.1672, 403.1023, 346.0687, 165.0548 | N | Y | N | Y | N | N | N | Hydroxylation + Glucuronidation | C15H12O2+6OCH3+OH+GluA-12H |
| M2 | 9.44 | [M + H]+ 595.1663 | 0.964 | C27H30O15 | 419.1342, 389.0870, 404.1104, 371.0764, 346.0688, 343.0444, 374.0628, 403.1026 | N | Y | N | N | N | N | N | Hydroxylation + Glucuronidation | C15H12O2+6OCH3+OH+GluA-12H |
| M3 | 9.54 | [M + H]+ 537.1243 | 0.835 | C24H24O14 | 211.0604, 361.1285, 196.0369, 183.0291, 360.1165, 167.0339, 330.0693, 177.0547 | N | N | Y | N | N | N | N | Demethylation + Glucuronidation | C15H10O2+3OCH3+3OH+GluA-8H |
| M4 | 9.68 | [M + H]+ 565.1556 | 0.704 | C26H28O14 | 389.1234, 359.0763, 257.081, 565.1561, 85.0291, 565.372, 390.127 | N | N | Y | Y | N | N | N | Demethylation + Glucuronidation | C15H10O2+5OCH3+OH+GluA-8H |
| M5 | 10.92 | [M + H]+ 551.1401 | 1.104 | C25H26O14 | 375.108, 345.0609, 342.0739, 360.0839, 376.1107, 359.0764, 85.0291, 270.0887 | N | Y | N | N | N | N | N | Demethylation + Glucuronidation | C15H10O2+4OCH3+2OH+GluA-8H |
| M6 | 10.98 | [M + H]+ 565.1559 | 1.341 | C26H28O14 | 389.1236, 331.0816, 356.0894, 313.0708, 374.0999, 390.12732, 359.0766, 328.0945 | N | Y | N | N | N | N | N | Demethylation + Glucuronidation | C15H10O2+5OCH3+OH+GluA-8H |
| M7 | 12.30 | [M + H]+ 389.1234 | 0.658 | C20H20O8 | 389.1237, 359.0766, 169.0134, 331.0817, 341.066, 390.1272, 316.0580, 374.1003 | N | Y | Y | Y | Y | N | N | Demethylation + Hydroxylation | C15H10O2+5OCH3+OH-6H |
| M8 | 15.88 | [M + H]+ 389.1231 | −0.113 | C20H20O8 | 389.1234, 331.0814, 356.0893, 313.0709, 359.0765, 341.0660, 328.0945, 390.1264 | N | Y | Y | N | Y | N | Y | Demethylation + Hydroxylation | C15H10O2+5OCH3+OH-6H |
| M9 | 15.91 | [M + H]+ 375.1074 | 0.336 | C19H18O8 | 375.1077, 345.0607, 356.0892, 317.0657, 313.0707, 331.0813, 360.0830, 374.0966 | N | N | Y | N | N | N | N | Demethylation | C15H10O2+4OCH3+2OH-6H |
| M10 | 17.38 | [M + H]+ 389.1231 | −0.113 | C20H20O8 | 389.1235, 359.0764, 341.0661, 197.0083, 169.0134, 163.0756, 390.1267, 316.0581 | N | N | N | Y | Y | N | Y | Demethylation + Hydroxylation | C15H10O2+5OCH3+OH-6H |
| M11 | 17.50 | [M − H]− 467.0661 | 3.943 | C20H20O11S | 387.1093, 372.0857, 357.0623, 342.0385, 467.0659, 327.0153, 299.0203, 388.1125 | N | N | N | Y | N | N | N | Demethylation + Sulfation | C15H10O2+5OCH3+OH+SO3-6H |
| M12 | 20.78 | [M − H]− 453.0505 | 4.065 | C19H18O11S | 373.0933, 343.0463, 358.0699, 328.023, 374.0968, 453.0513, 300.0279, 359.0729 | N | Y | N | Y | N | N | N | Demethylation + Sulfation | C15H10O2+4OCH3+2OH+SO3-6H |
| Molecule Name | Structure |
|---|---|
| Isosinensetin |  |
| Sinensetin | 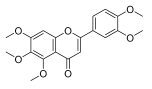 |
| 5,6,7,3′,4′,5′-hexamethoxyflavone | 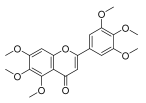 |
| Nobiletin | 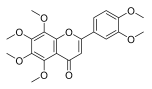 |
| 5,6,7,4′-tetramethoxyflavone | 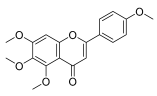 |
| 3,5,6,7,8,3′,4′-heptemethoxyflavone | 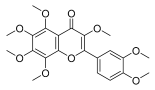 |
| Tangeretin | 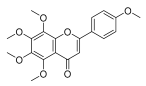 |
| Gene Symbol | Forward Primer | Reverse Primer |
|---|---|---|
| TNF-α | CCCTCACACTCACAAACCAC | ACAAGGTACAACCCATCGGC |
| IL-1β | CACAGCTCTGGAGATGGTGA | CTTTCAAGCTTGGGCACTTC |
| IL-10 | GGTTGCCAAGCCTTATCGGA | GACACCTTGGTCTTGGAGCTTA |
| TGF-β | CCTCGAGACAGGCCATTTGT | GCCAGCTGACTGCTTTTCTG |
| MMP-9 | TCTAGGCCCAGAGGTAACCC | AGTCGAATCTCCAGACACGC |
| Claudin-5 | GTTAAGGCACGGGTAGCACT | TACTTCTGTGACACCGGCAC |
| ZO-1 | CTCAAGTTCCTGAAGCCCGT | GCAAAAGACCAACCGTCAGG |
| GAPDH | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yi, Z.; Zhang, Y.; Zhang, J.; Li, X.; Qi, D.; Wang, Q.; Chai, X.; Liu, H.; Wang, G.; et al. Identification and Therapeutic Potential of Polymethoxylated Flavones in Citri Reticulatae Pericarpium for Alzheimer’s Disease: Targeting Neuroinflammation. Molecules 2025, 30, 771. https://doi.org/10.3390/molecules30040771
Wang X, Yi Z, Zhang Y, Zhang J, Li X, Qi D, Wang Q, Chai X, Liu H, Wang G, et al. Identification and Therapeutic Potential of Polymethoxylated Flavones in Citri Reticulatae Pericarpium for Alzheimer’s Disease: Targeting Neuroinflammation. Molecules. 2025; 30(4):771. https://doi.org/10.3390/molecules30040771
Chicago/Turabian StyleWang, Xinyu, Zirong Yi, Yiming Zhang, Jing Zhang, Xueyan Li, Dongying Qi, Qianqian Wang, Xiaoyu Chai, Huan Liu, Guopeng Wang, and et al. 2025. "Identification and Therapeutic Potential of Polymethoxylated Flavones in Citri Reticulatae Pericarpium for Alzheimer’s Disease: Targeting Neuroinflammation" Molecules 30, no. 4: 771. https://doi.org/10.3390/molecules30040771
APA StyleWang, X., Yi, Z., Zhang, Y., Zhang, J., Li, X., Qi, D., Wang, Q., Chai, X., Liu, H., Wang, G., Pan, Y., Liu, Y., & Yu, G. (2025). Identification and Therapeutic Potential of Polymethoxylated Flavones in Citri Reticulatae Pericarpium for Alzheimer’s Disease: Targeting Neuroinflammation. Molecules, 30(4), 771. https://doi.org/10.3390/molecules30040771






