TQFL19, a Novel Derivative of Thymoquinone (TQ), Plays an Essential Role by Inhibiting Cell Growth, Metastasis, and Invasion in Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Results
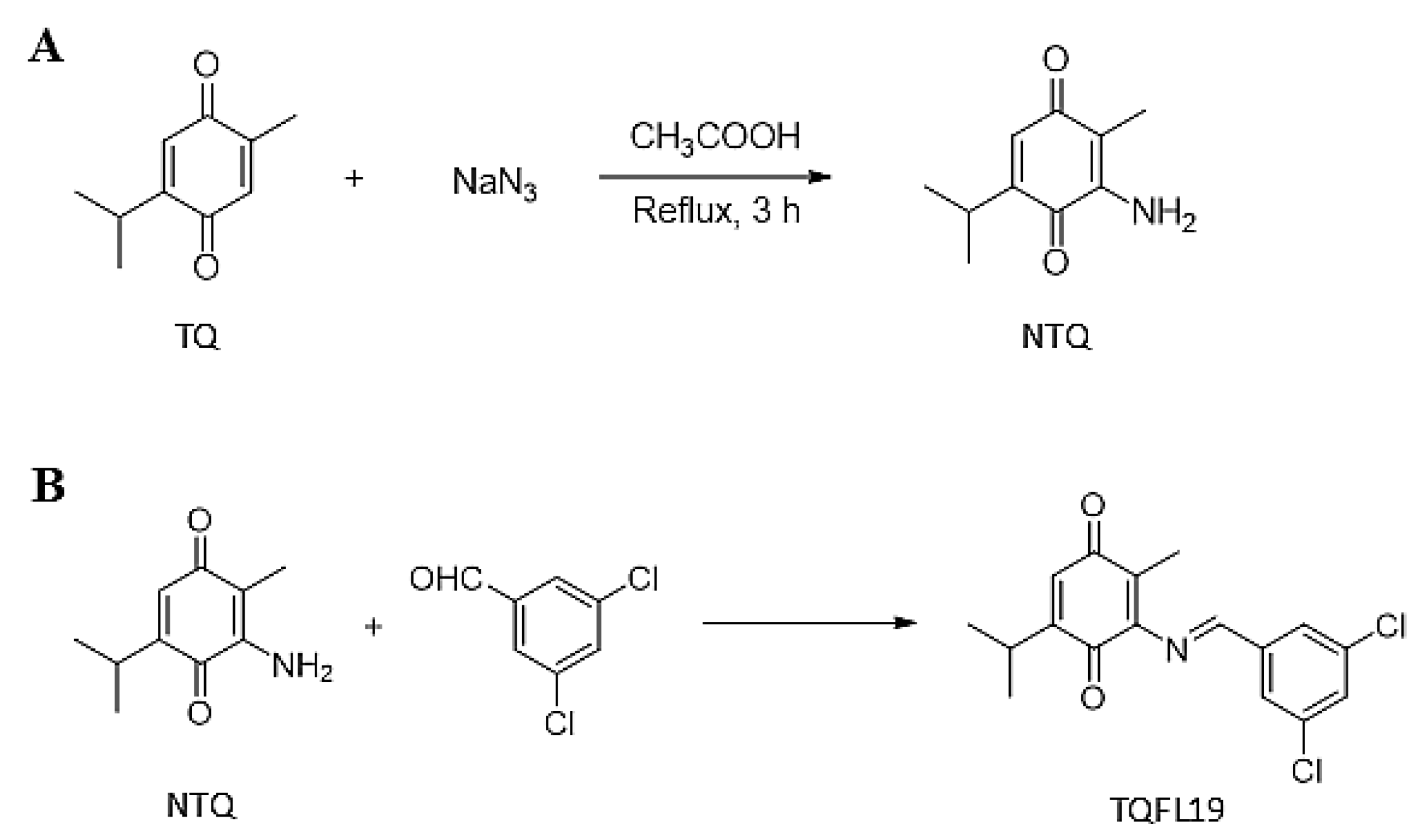
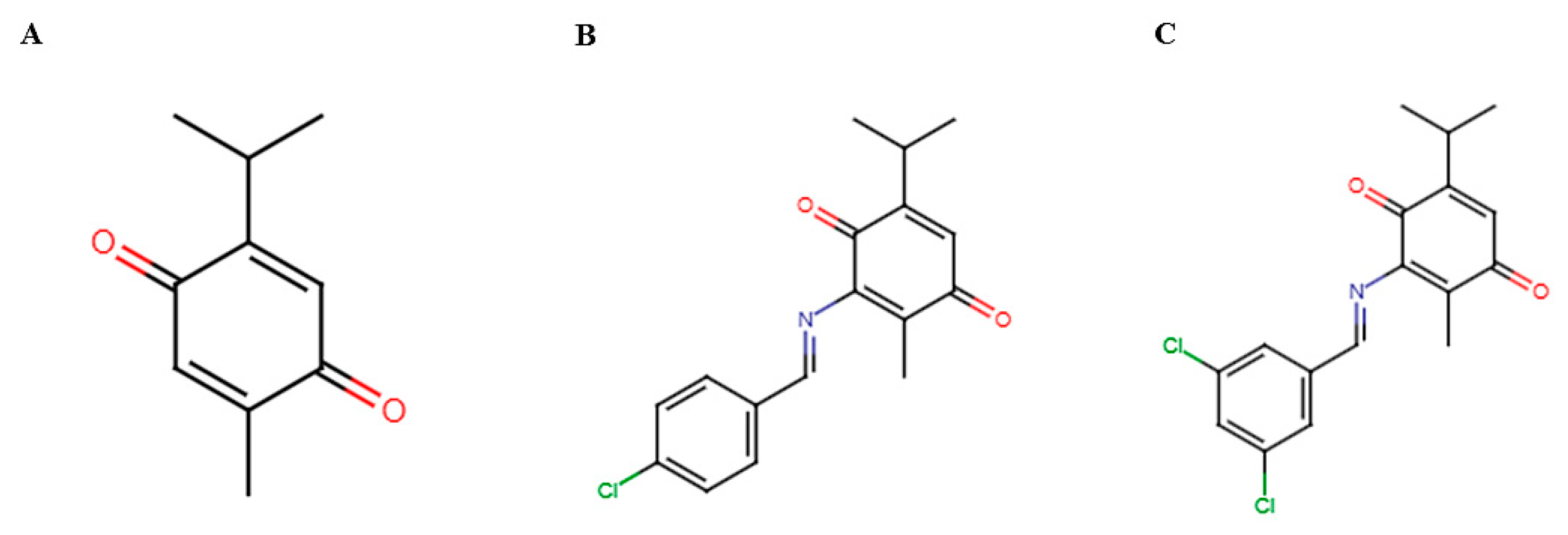
2.1. Synthesis of TQFL19
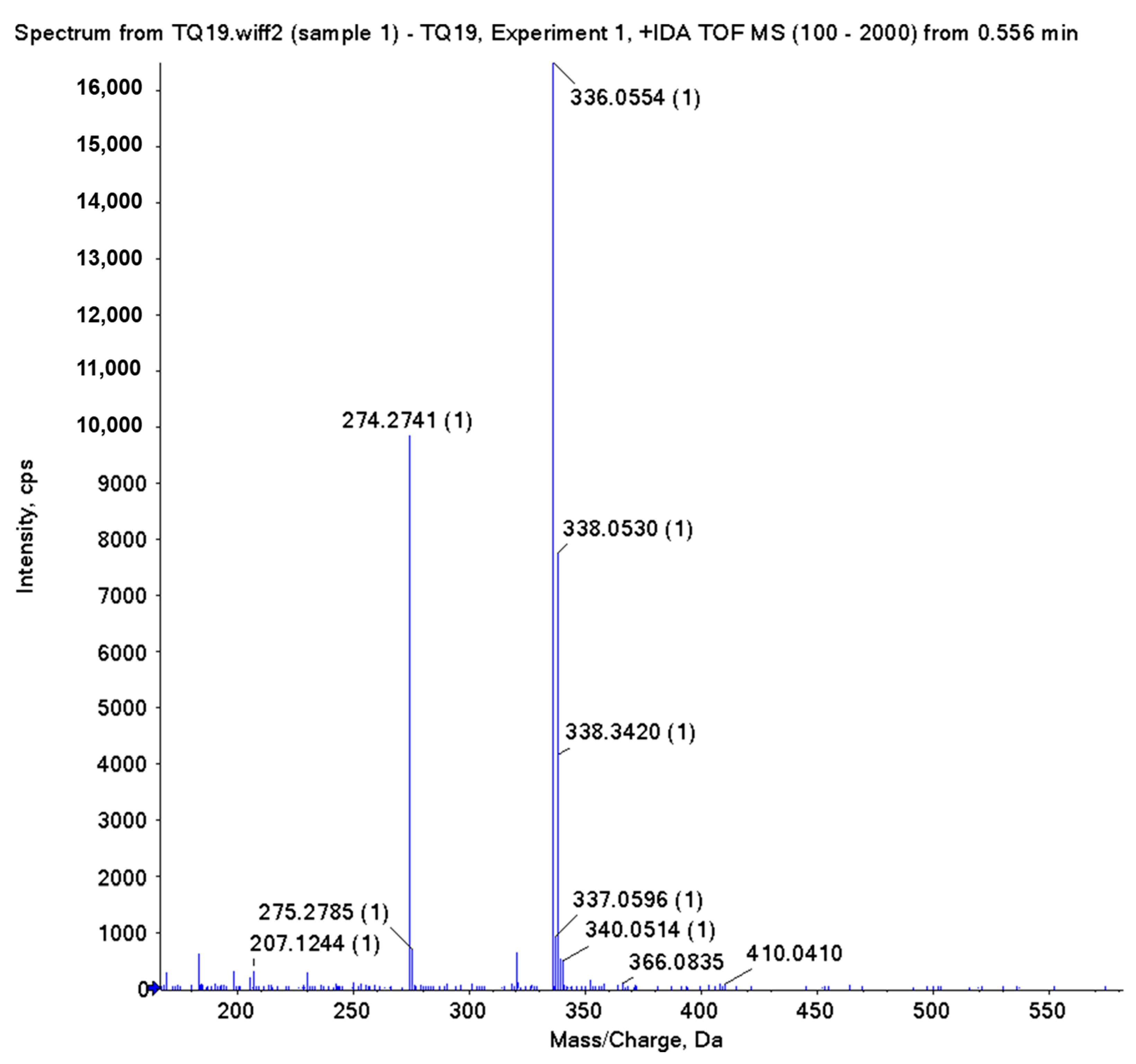
2.2. Characterization and Purity Analyses
2.3. Prediction Results of ADMET Properties
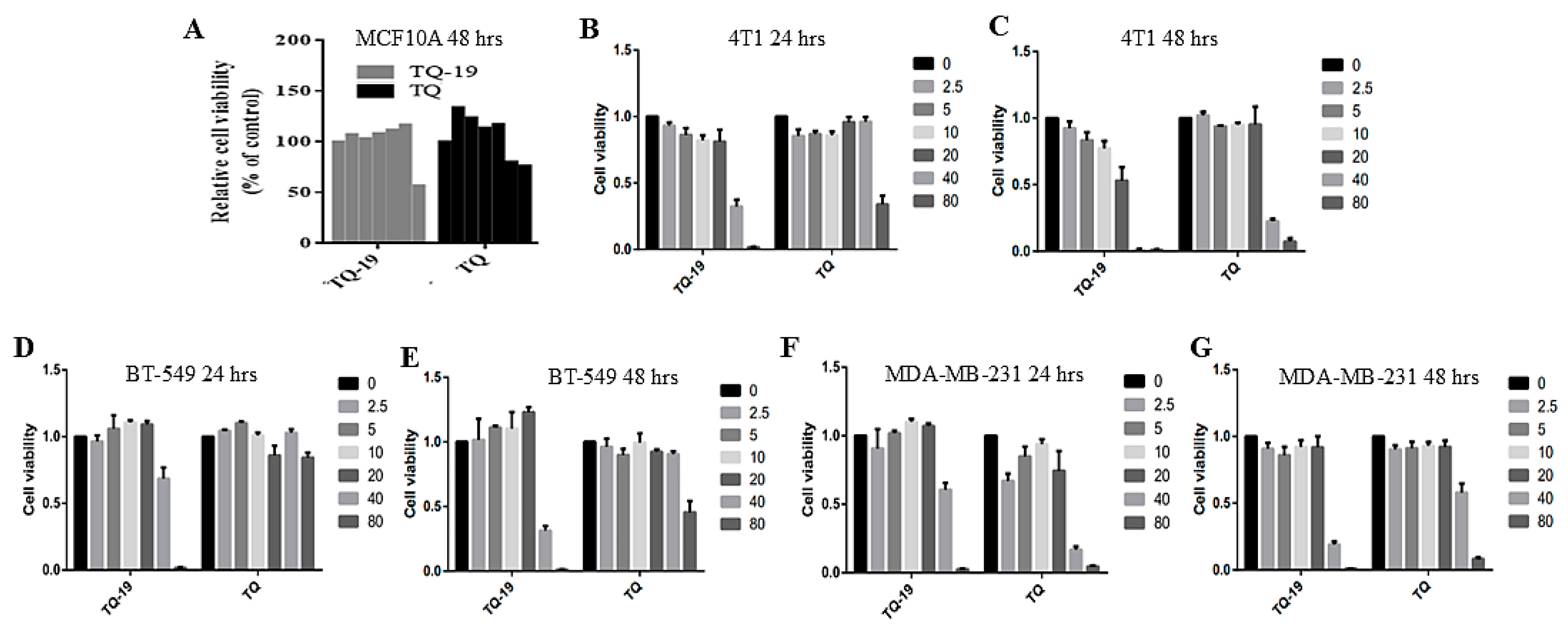
2.4. Cytotoxicity and IC50s of TQ and TQFL19
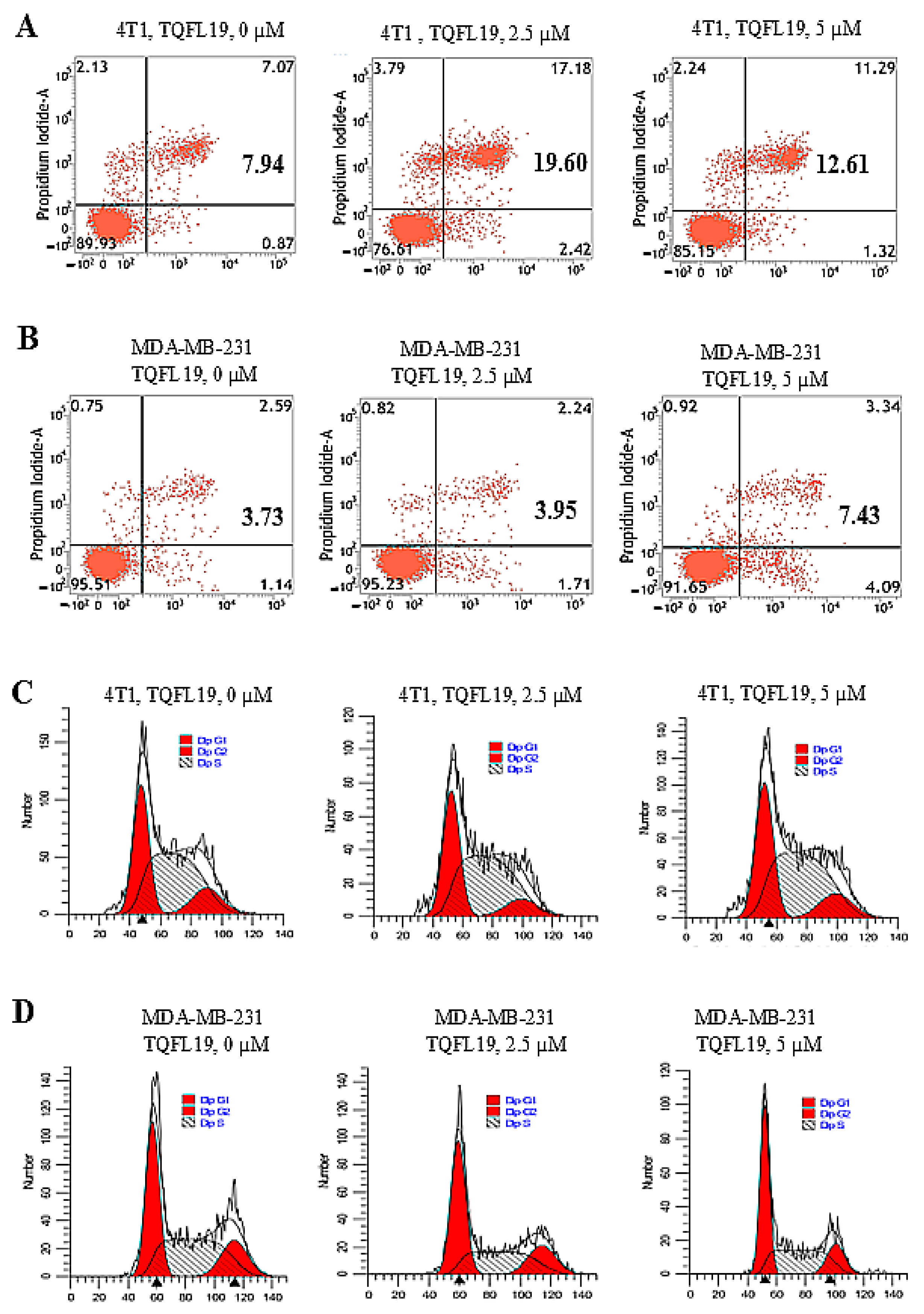
2.5. Apoptosis and Cell Cycle Effects of TQFL19
2.6. TQFL19 Inhibits Cell Growth, Migration, and Invasion In Vitro
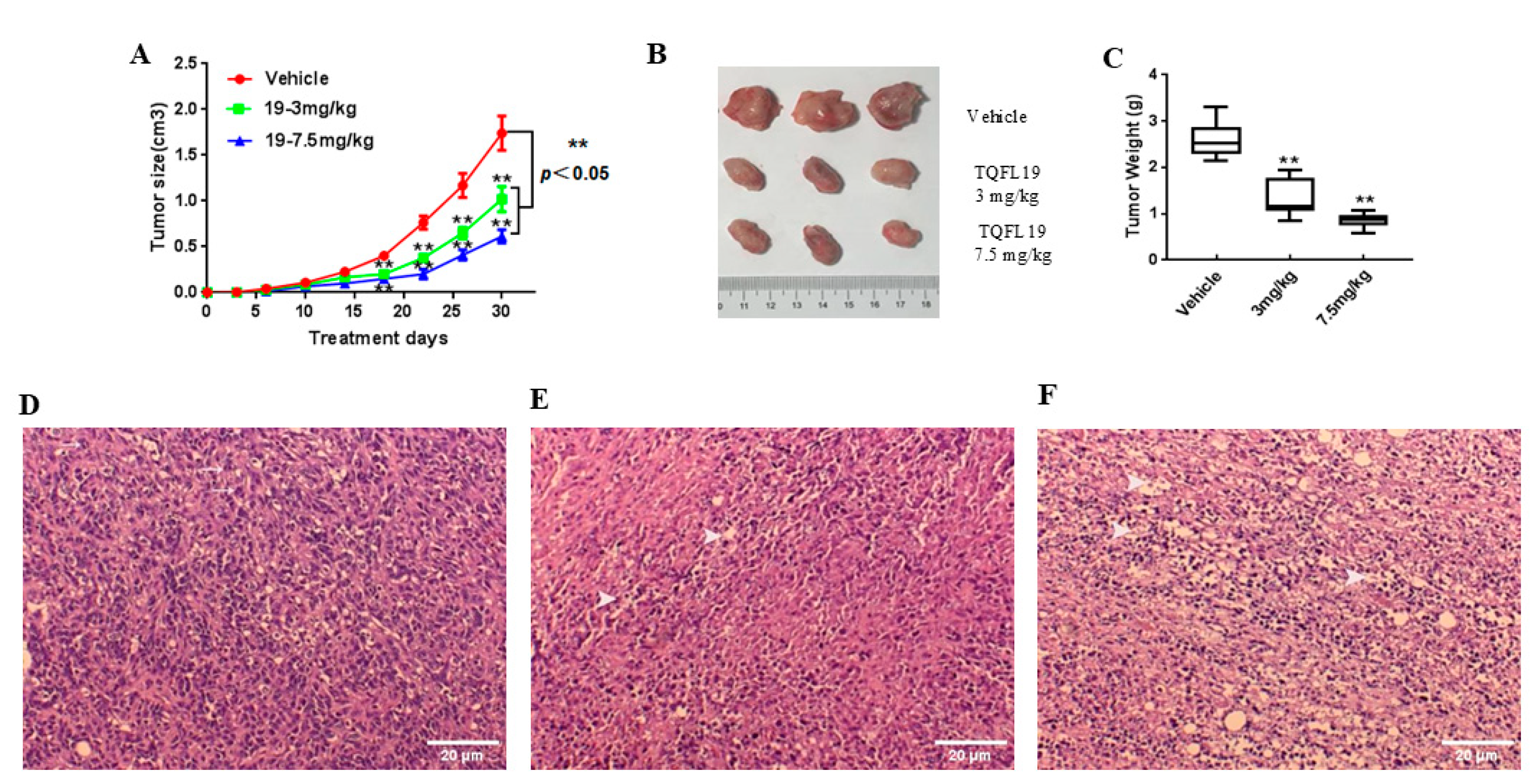
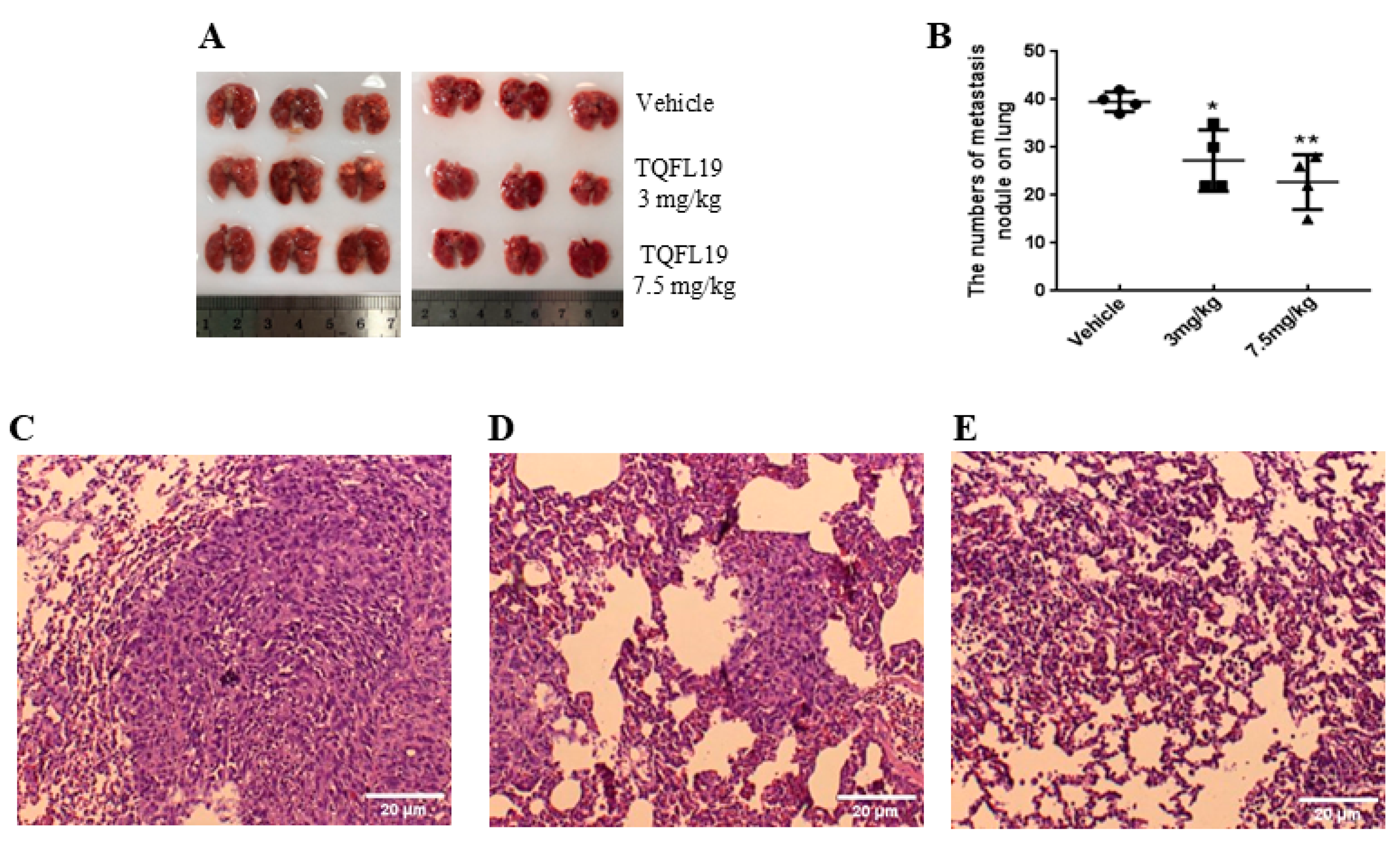
2.7. Mouse Allograft Assessment
3. Materials and Methods
3.1. Ethical Statement
3.2. Reagents and Cell Lines
3.3. Chemical Synthesis and Characterization and Purity Analyses
3.4. Prediction of ADMET Properties
3.5. Cell Counting Kit-8 (CCK8) Assays
3.6. Apoptosis and Cell Cycle Assays
3.7. Cell Growth, Migration, and Invasion Assays
3.8. Mouse Allograft Model
3.9. Histopathology Assessment
3.10. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDKs | Cyclin-dependent kinases. |
| CKIs | cyclin-dependent kinase inhibitors. |
| DMSO | dimethyl sulfoxide. |
| ESI | Electrospray ionization. |
| NMR | Nuclear magnetic resonance. |
| NTQ | 3-amino-thymoquinone. |
| QTOF | quadrupole tandem time-of-flight. |
| TNBC | Triple-negative breast cancer. |
| TQ | Thymoquinone. |
References
- Sedeta, E.T.; Jobre, B.; Avezbakiyev, B. Breast Cancer: Global Patterns of Incidence, Mortality, and Trends. J. Clin. Oncol. 2023, 41, 10528. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Wang, X. The Metabolic Mechanisms of Breast Cancer Metastasis. Front. Oncol. 2021, 10, 602416. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Jung, W.H.; Koo, J.S. Differential Expression of Enzymes Associated with Serine/Glycine Metabolism in Different Breast Cancer Subtypes. PLoS ONE 2014, 9, e101004. [Google Scholar] [CrossRef] [PubMed]
- Zagami, P.; Carey, L.A. Triple Negative Breast Cancer: Pitfalls and Progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Trapani, D.; Viale, G.; Criscitiello, C.; Curigliano, G. Practical Classification of Triple-Negative Breast Cancer: Intratumoral Heterogeneity, Mechanisms of Drug Resistance, and Novel Therapies. NPJ Breast Cancer 2020, 6, 54. [Google Scholar] [CrossRef]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Henry, N.L. Optimising Therapy and Avoiding Overtreatment in Breast Cancer. Lancet Oncol. 2024, 26, 2–3. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.-G. Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives. Breast Cancer: Targets Ther. 2023, 15, 721–730. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Khaiwa, N.; Maarouf, N.R.; Darwish, M.H.; Alhamad, D.W.M.; Sebastian, A.; Hamad, M.; Omar, H.A.; Orive, G.; Al-Tel, T.H. Camptothecin’s Journey from Discovery to WHO Essential Medicine: Fifty Years of Promise. Eur. J. Med. Chem. 2021, 223, 113639. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E. Camptothecin and Taxol: Discovery to Clinic. Med. Res. Rev. 1998, 18, 299–314. [Google Scholar] [CrossRef]
- Abukhader, M.M. Thymoquinone in the Clinical Treatment of Cancer: Fact or Fiction? Pharmacogn. Rev. 2013, 7, 117–120. [Google Scholar] [CrossRef]
- Woo, C.C.; Hsu, A.; Kumar, A.P.; Sethi, G.; Tan, K.H.B. Thymoquinone Inhibits Tumor Growth and Induces Apoptosis in a Breast Cancer Xenograft Mouse Model: The Role of P38 MAPK and ROS. PLoS ONE 2013, 8, e75356. [Google Scholar] [CrossRef]
- Effenberger-Neidnicht, K.; Schobert, R. Combinatorial Effects of Thymoquinone on the Anti-Cancer Activity of Doxorubicin. Cancer Chemother. Pharmacol. 2011, 67, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Zheng, M.; Fu, J.J.; Tania, M.; Li, J.; Fu, J.J. Thymoquinone Upregulates IL17RD in Controlling the Growth and Metastasis of Triple Negative Breast Cancer Cells in Vitro. BMC Cancer 2022, 22, 707. [Google Scholar] [CrossRef]
- Mohammadabadi, M.R.; Mozafari, M.R. Enhanced Efficacy and Bioavailability of Thymoquinone Using Nanoliposomal Dosage Form. J. Drug Deliv. Sci. Technol. 2018, 47, 445–453. [Google Scholar] [CrossRef]
- Wei, C.; Zou, H.; Xiao, T.; Liu, X.; Wang, Q.; Cheng, J.; Fu, S.; Peng, J.; Xie, X.; Fu, J. TQFL12, a Novel Synthetic Derivative of TQ, Inhibits Triple-Negative Breast Cancer Metastasis and Invasion through Activating AMPK/ACC Pathway. J. Cell Mol. Med. 2021, 25, 10101–10110. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Shen, S.; Wei, C.; Fu, J. RNA-Sequencing Reveals Heat Shock 70-KDa Protein 6 (HSPA6) as a Novel Thymoquinone-Upregulated Gene That Inhibits Growth, Migration, and Invasion of Triple-Negative Breast Cancer Cells. Front. Oncol. 2021, 11, 667995. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; La Porta, R.; Napolitano, M.; Galletti, P.; Quagliuolo, L.; Boccellino, M. Effect of Annurca Apple Polyphenols on Human HaCaT Keratinocytes Proliferation. J. Med. Food 2012, 15, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Almajali, B.; Al-Jamal, H.A.N.; Taib, W.R.W.; Ismail, I.; Johan, M.F.; Doolaanea, A.A.; Ibrahim, W.N. Thymoquinone, as a Novel Therapeutic Candidate of Cancers. Pharmaceuticals 2021, 14, 369. [Google Scholar] [CrossRef] [PubMed]
- Almajali, B.; Al-Jamal, H.A.N.; Wan Taib, W.R.; Ismail, I.; Johan, M.F.; Doolaanea, A.A.; Ibrahim, W.N.; Tajudin, S.A. Thymoquinone Suppresses Cell Proliferation and Enhances Apoptosis of HL60 Leukemia Cells through Re-Expression of JAK/STAT Negative Regulators. Asian Pac. J. Cancer Prev. 2021, 22, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Tania, M.; Wei, C.; Mei, Z.; Fu, S.; Cheng, J.; Xu, J.; Fu, J. Thymoquinone Inhibits Cancer Metastasis by Downregulating TWIST1 Expression to Reduce Epithelial to Mesenchymal Transition. Oncotarget 2015, 6, 19580–19591. [Google Scholar] [CrossRef] [PubMed]
- Ünal, T.D.; Hamurcu, Z.; Delibaşı, N.; Çınar, V.; Güler, A.; Gökçe, S.; Nurdinov, N.; Ozpolat, B. Thymoquinone Inhibits Proliferation and Migration of MDA-MB-231 Triple Negative Breast Cancer Cells by Suppressing Autophagy, Beclin-1 and LC3. Anticancer Agents Med. Chem. 2021, 21, 355–364. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell Cycle Control in Cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Ziegler, D.V.; Huber, K.; Fajas, L. The Intricate Interplay between Cell Cycle Regulators and Autophagy in Cancer. Cancers 2021, 14, 153. [Google Scholar] [CrossRef]
- Adinew, G.M.; Messeha, S.S.; Taka, E.; Badisa, R.B.; Antonie, L.M.; Soliman, K.F.A. Thymoquinone Alterations of the Apoptotic Gene Expressions and Cell Cycle Arrest in Genetically Distinct Triple-Negative Breast Cancer Cells. Nutrients 2022, 14, 2120. [Google Scholar] [CrossRef]
- El-Shehawy, A.A.; Elmetwalli, A.; El-Far, A.H.; Mosallam, S.A.E.-R.; Salama, A.F.; Babalghith, A.O.; Mahmoud, M.A.; Mohany, H.; Gaber, M.; El-Sewedy, T. Thymoquinone, Piperine, and Sorafenib Combinations Attenuate Liver and Breast Cancers Progression: Epigenetic and Molecular Docking Approaches. BMC Complement. Med. Ther. 2023, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Dang, S. Evaluation of Enhanced Cytotoxicity Effect of Repurposed Drug Simvastatin/Thymoquinone Combination against Breast Cancer Cell Line. Cardiovasc. Hematol. Agents Med. Chem. 2024, 22, 348–366. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, B.; Elderdery, A.Y.; Alsrhani, A.; Alzerwi, N.A.N.; Althobiti, M.M.; Elkhalifa, A.M.E.; Rayzah, M.; Idrees, B.; Kumar, S.S.; Mok, P.L. Sodium Alginate Encapsulated Iron Oxide Decorated with Thymoquinone Nanocomposite Induces Apoptosis in Human Breast Cancer Cells via PI3K-Akt-MTOR Pathway. Int. J. Biol. Macromol. 2023, 244, 125054. [Google Scholar] [CrossRef]
- Ireson, C.R.; Alavijeh, M.S.; Palmer, A.M.; Fowler, E.R.; Jones, H.J. The Role of Mouse Tumour Models in the Discovery and Development of Anticancer Drugs. Br. J. Cancer 2019, 121, 101–108. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ghosh, A.; Maiti, S.; Ahir, M.; Debnath, G.H.; Gupta, P.; Bhattacharjee, M.; Ghosh, S.; Chattopadhyay, S.; Mukherjee, P.; et al. Delivery of Thymoquinone through Hyaluronic Acid-Decorated Mixed Pluronic® Nanoparticles to Attenuate Angiogenesis and Metastasis of Triple-Negative Breast Cancer. J. Control. Release 2020, 322, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Kabil, N.; Bayraktar, R.; Kahraman, N.; Mokhlis, H.A.; Calin, G.A.; Lopez-Berestein, G.; Ozpolat, B. Thymoquinone Inhibits Cell Proliferation, Migration, and Invasion by Regulating the Elongation Factor 2 Kinase (EEF-2K) Signaling Axis in Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2018, 171, 593–605. [Google Scholar] [CrossRef] [PubMed]

| Thymoquinone (TQ) | TQFL19 | ||
|---|---|---|---|
| ADEMT | Solubility (log mol/L) | −3.021 | −5.902 |
| Solubility level | 3 | 2 | |
| AD-MET-BBB (log BB) | 0.006 | 0.639 | |
| PPB prediction | True | True | |
| Hepatotoxicity prediction | True | True | |
| Ames’s toxicity | Ames’s prediction | Non-Mutagen | Non-Mutagen |
| Ames’s probability | 0.329 | 0.330 | |
| Ames’s score | −11.192 | −11.160 | |
| Molecular properties | H-donors | 0 | 0 |
| H-acceptors | 2 | 3 | |
| Rotatable bonds | 1 | 3 | |
| Rings | 1 | 2 | |
| Aromatic rings | 0 | 1 | |
| Polar surface area | 0.179 | 0.179 | |
| Pharmacokinetics | GI absorption | High | High |
| P-gp substrate | No | No | |
| CYP1A2 inhibitor | No | Yes | |
| CYP2C19 inhibitor | No | Yes | |
| CYP2C9 inhibitor | No | Yes | |
| CYP2D6 inhibitor | No | No | |
| CYP3A4 inhibitor | No | Yes | |
| Log Kp (skin permeation) | −5.74 cm/s | −5.29 cm/s |
| IC50 Values at 24 h (μM) | IC50 Values at 48 h (μM) | |||
|---|---|---|---|---|
| Cell lines | TQFL19 | TQ | TQFL19 | TQ |
| 4T1 | 26.169 | 72.05 | 15.478 | 32.916 |
| BT549 | 44.271 | >80 | 37.222 | >80 |
| MDA-MB-231 | 28.15 | 48.76 | 27.30 | 73.21 |
| MDA-MB-468 | 57.23 | >100 | 40.00 | 83.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Far, A.; Liu, X.; Xiao, T.; Du, J.; Du, X.; Wei, C.; Cheng, J.; Zou, H.; Fu, J. TQFL19, a Novel Derivative of Thymoquinone (TQ), Plays an Essential Role by Inhibiting Cell Growth, Metastasis, and Invasion in Triple-Negative Breast Cancer. Molecules 2025, 30, 773. https://doi.org/10.3390/molecules30040773
El-Far A, Liu X, Xiao T, Du J, Du X, Wei C, Cheng J, Zou H, Fu J. TQFL19, a Novel Derivative of Thymoquinone (TQ), Plays an Essential Role by Inhibiting Cell Growth, Metastasis, and Invasion in Triple-Negative Breast Cancer. Molecules. 2025; 30(4):773. https://doi.org/10.3390/molecules30040773
Chicago/Turabian StyleEl-Far, Ali, Xiaoyan Liu, Ting Xiao, Jun Du, Xinwei Du, Chunli Wei, Jingliang Cheng, Hui Zou, and Junjiang Fu. 2025. "TQFL19, a Novel Derivative of Thymoquinone (TQ), Plays an Essential Role by Inhibiting Cell Growth, Metastasis, and Invasion in Triple-Negative Breast Cancer" Molecules 30, no. 4: 773. https://doi.org/10.3390/molecules30040773
APA StyleEl-Far, A., Liu, X., Xiao, T., Du, J., Du, X., Wei, C., Cheng, J., Zou, H., & Fu, J. (2025). TQFL19, a Novel Derivative of Thymoquinone (TQ), Plays an Essential Role by Inhibiting Cell Growth, Metastasis, and Invasion in Triple-Negative Breast Cancer. Molecules, 30(4), 773. https://doi.org/10.3390/molecules30040773








