Biofunctionalization of Magneto-Plasmonic Fe3O4@SiO2-NH2-Au Heterostructures with the Cellulase from Trichoderma reesei
Abstract
1. Introduction
2. Results and Discussion
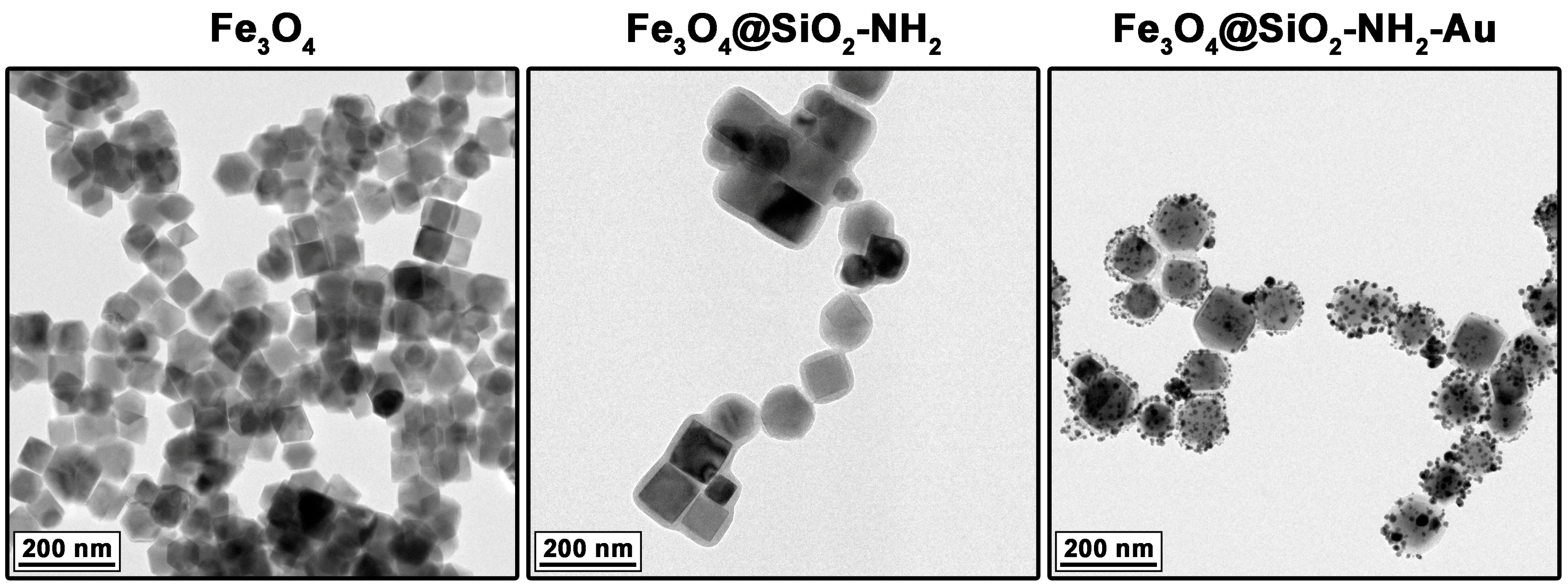
2.1. Characterization of Heterostructures General Physicochemical Properties
2.2. Effects of Cellulase Heterostructure Immobilization
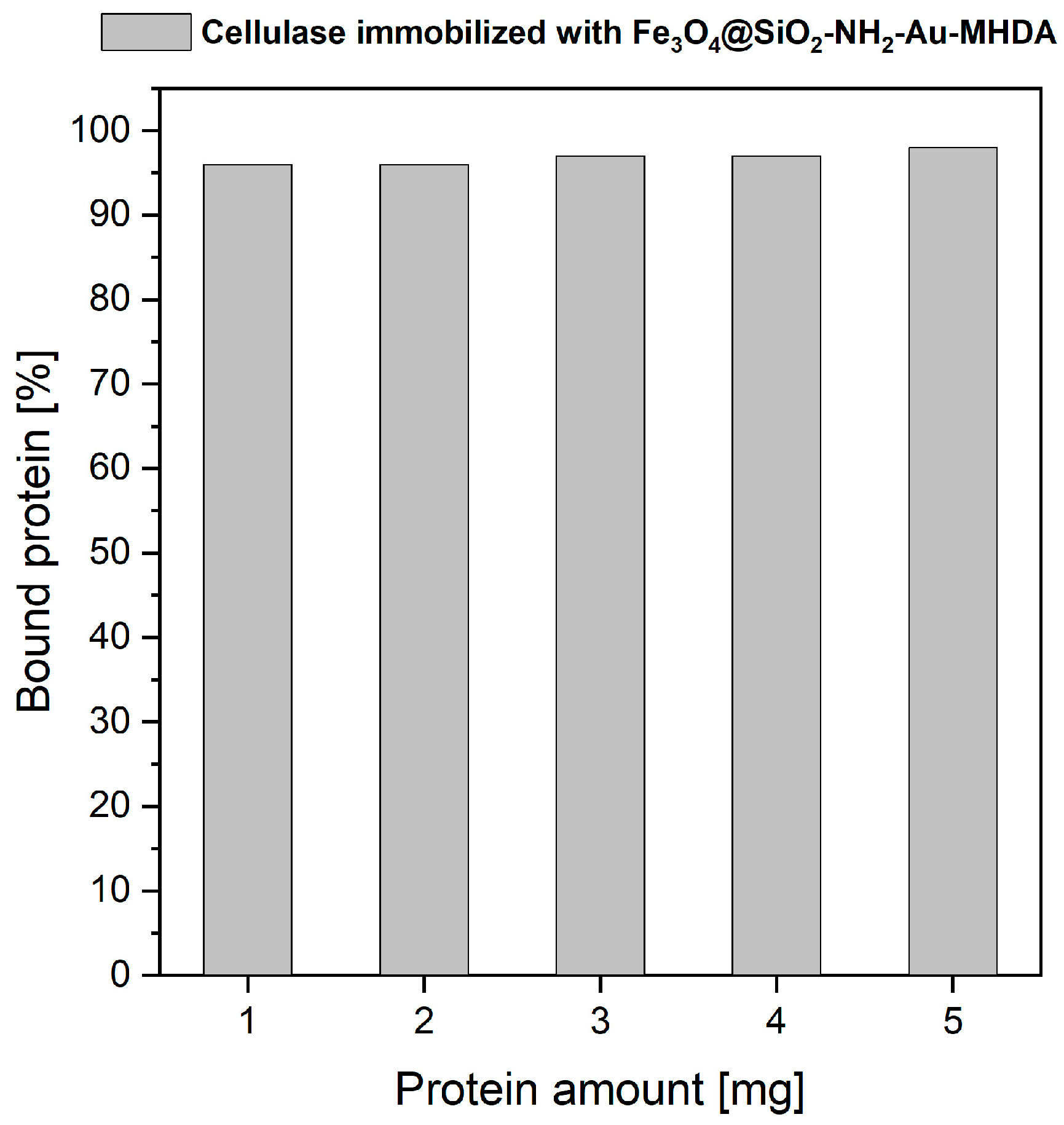
2.2.1. Cellulase Binding Yield
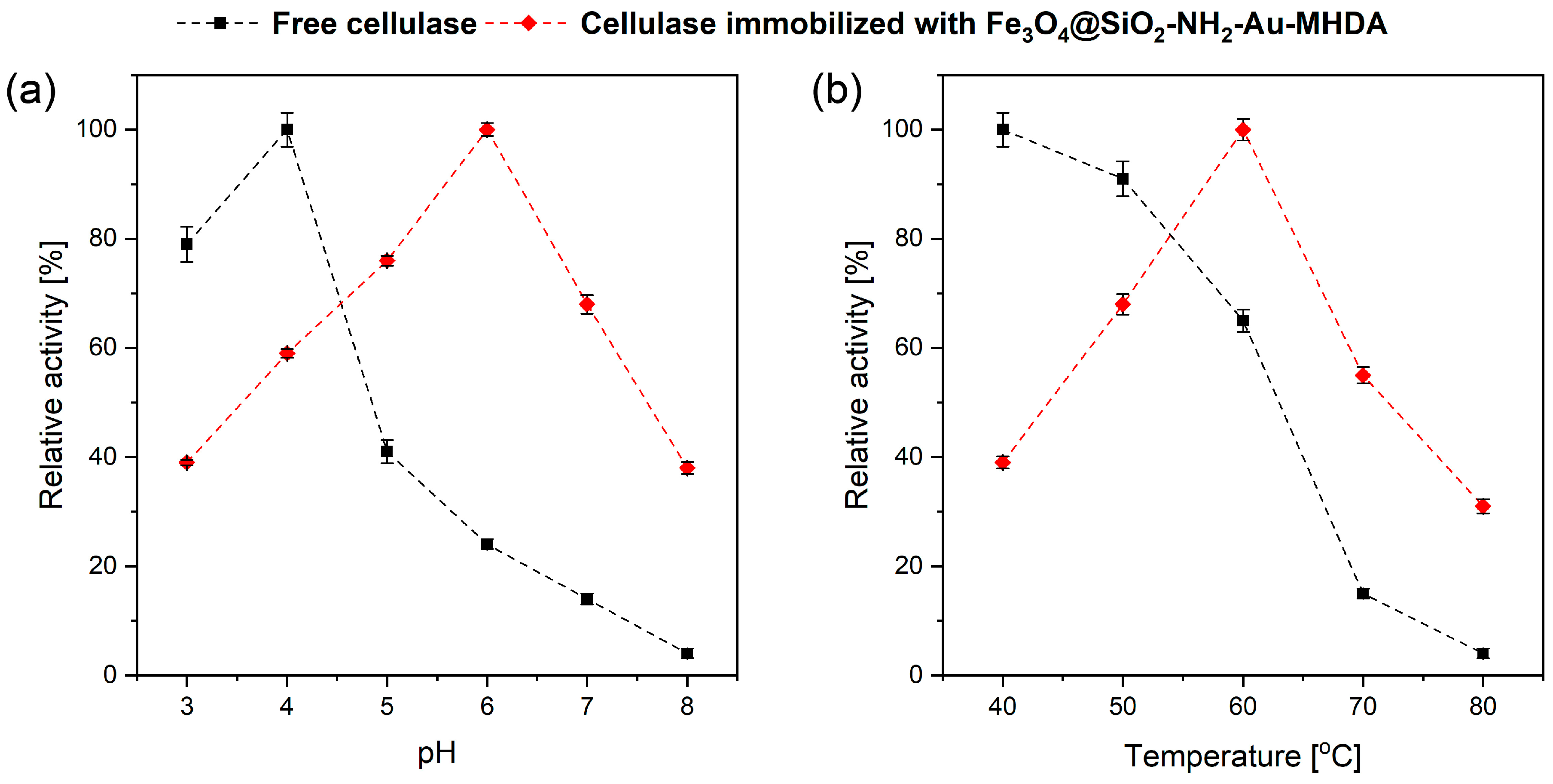
2.2.2. Effect of pH and Temperature on Cellulase Activity
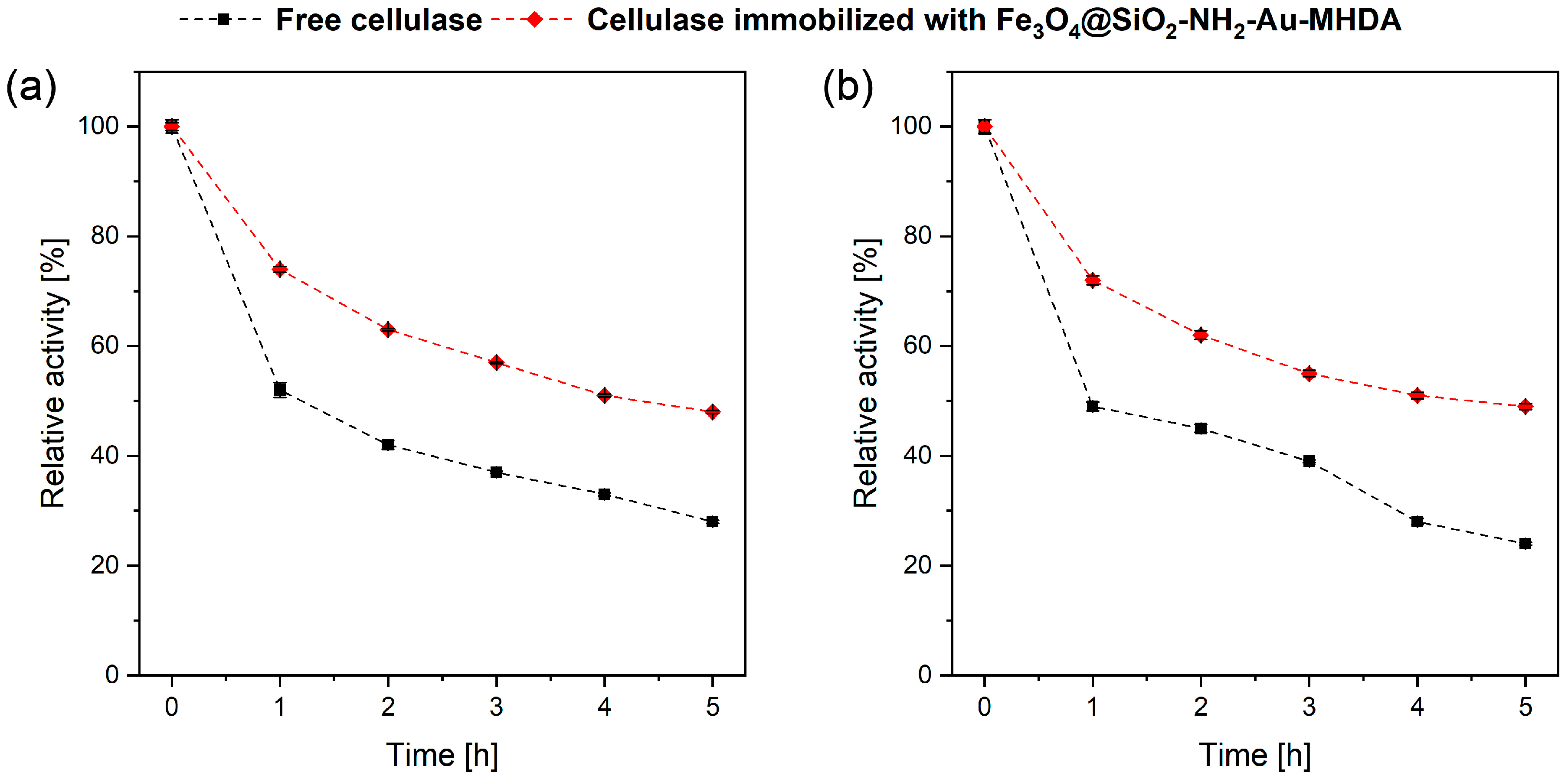
2.2.3. Cellulase Thermal Stability over Time
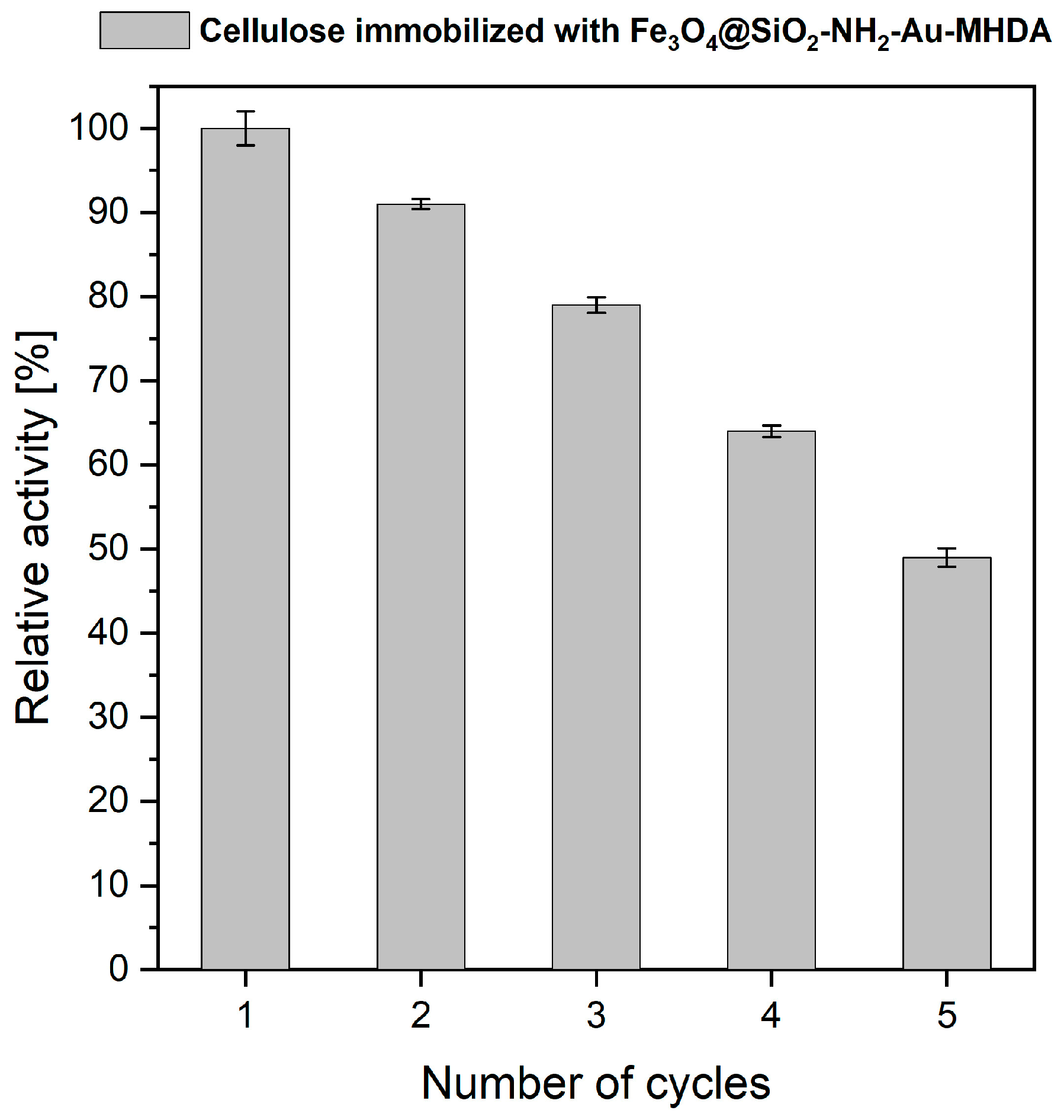
2.3. Reusability of the Cellulase Immobilized Enzyme on Heterostructures
3. Materials and Methods
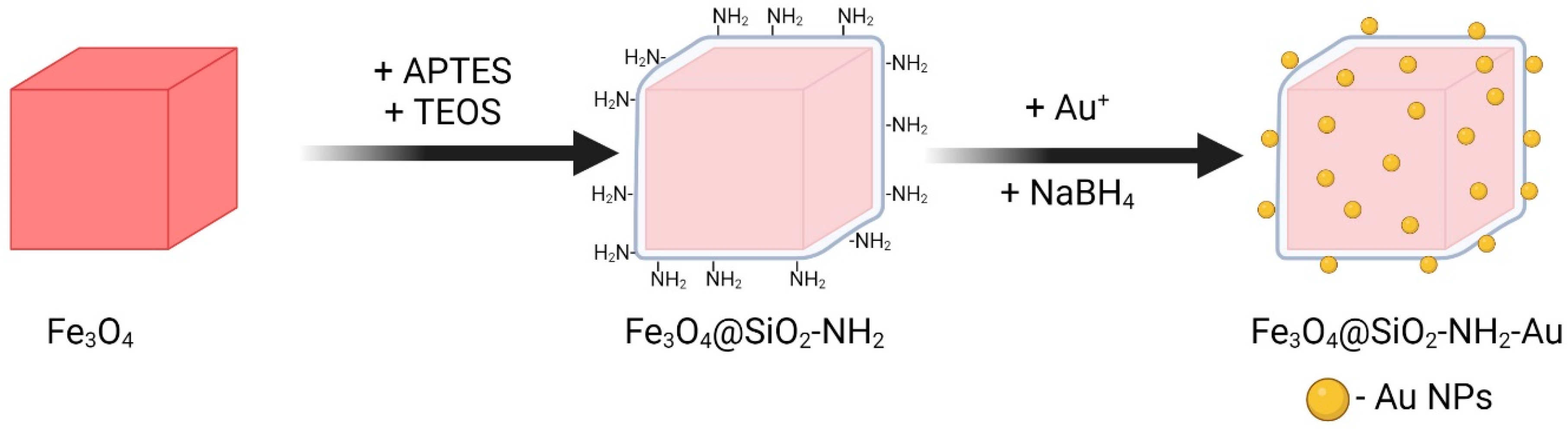
3.1. Synthesis of Magneto-Plasmonic Heterostructures
3.1.1. Synthesis of Cubic Magnetite Particles
3.1.2. Silica Shell Deposition with NH2 Functionalities
3.1.3. Decoration with Gold Nanoparticles
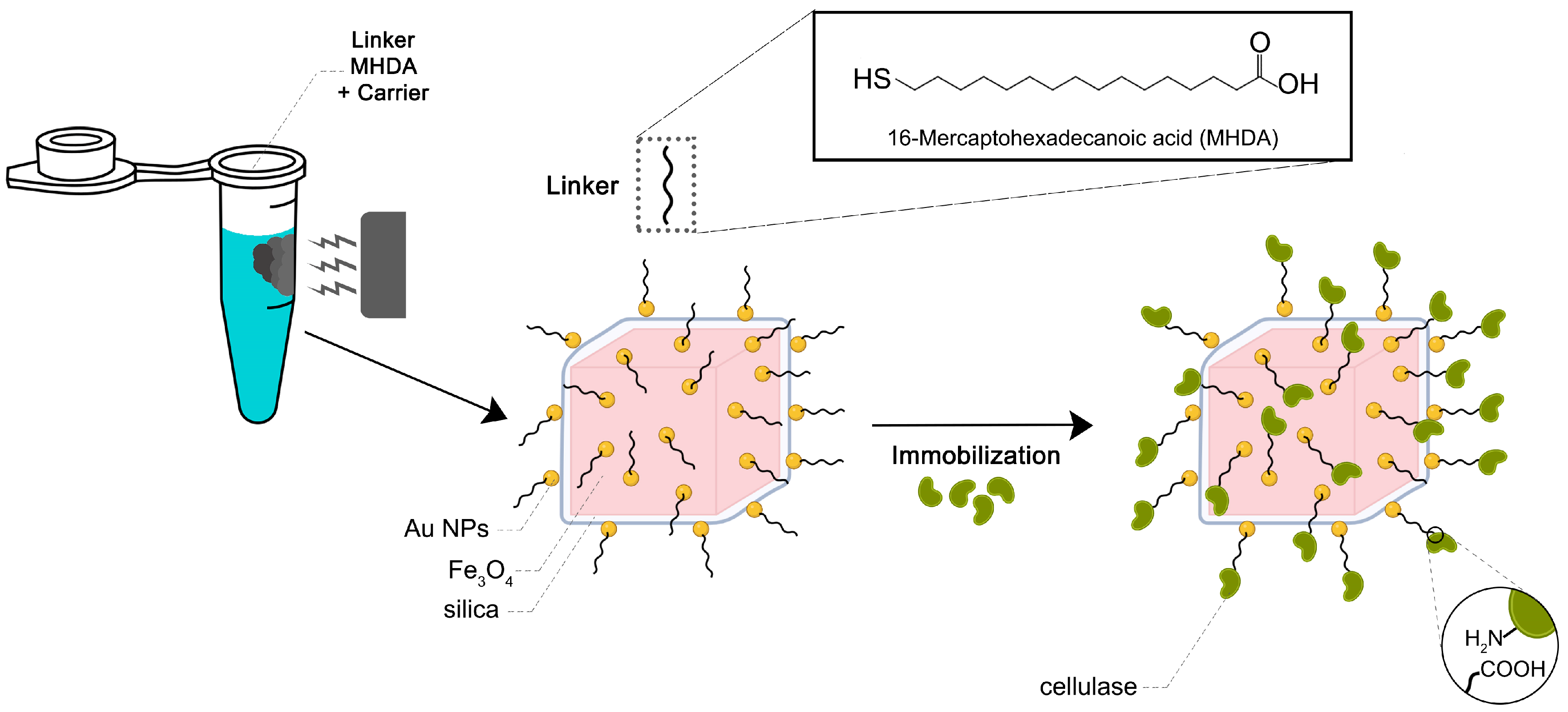
3.1.4. Anchor of the MHDA to Magneto-Plasmonic Heterostructure and Conjugation with the Cellulase Enzyme
3.2. Characterization of Physicochemical Properties of Nanomaterials
3.3. Characterization of the Cellulase Heterostructure Immobilization Conditions
3.3.1. Cellulase Binding Yield, Effect of pH and Temperature
3.3.2. Cellulase Thermal Stability
3.4. Assessment of the Catalytic Activity of Immobilized Cellulase
3.5. Protocol for Enzyme Reusability on Heterostructures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ranjan, R.; Rai, R.; Bhatt, S.B.; Dhar, P. Technological Road Map of Cellulase: A Comprehensive Outlook to Structural, Computational, and Industrial Applications. Biochem. Eng. J. 2023, 198, 109020. [Google Scholar] [CrossRef]
- Korsa, G.; Konwarh, R.; Masi, C.; Ayele, A.; Haile, S. Microbial Cellulase Production and Its Potential Application for Textile Industries. Ann. Microbiol. 2023, 73, 13. [Google Scholar] [CrossRef]
- Ellilä, S.; Fonseca, L.; Uchima, C.; Cota, J.; Goldman, G.H.; Saloheimo, M.; Sacon, V.; Siika-Aho, M. Development of a Low-Cost Cellulase Production Process Using Trichoderma Reesei for Brazilian Biorefineries. Biotechnol. Biofuels 2017, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, V.; Kuila, A. Cellulase Production Using Natural Medium and Its Application on Enzymatic Hydrolysis of Thermo Chemically Pretreated Biomass. 3 Biotech 2016, 6, 139. [Google Scholar] [CrossRef]
- Avcioglu, N.H. Bacterial Cellulose: Recent Progress in Production and Industrial Applications. World J. Microbiol. Biotechnol. 2022, 38, 86. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Shukla, V.K.; Tiwari, A. Algal Cellulases. In New and Future Developments in Microbial Biotechnology and Bioengineering; Srivastava, N., Srivastava, M., Mishra, P.K., Ramteke, P.W., Singh, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–295. ISBN 9780444642233. [Google Scholar]
- Liu, L.; Huang, W.C.; Liu, Y.; Li, M. Diversity of Cellulolytic Microorganisms and Microbial Cellulases. Int. Biodeterior. Biodegrad. 2021, 163, 105277. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Agrawal, K.; Verma, P. Current Perspective on Production and Applications of Microbial Cellulases: A Review. Bioresour. Bioprocess. 2021, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Ilić, N.; Milić, M.; Beluhan, S.; Dimitrijević-Branković, S. Cellulases: From Lignocellulosic Biomass to Improved Production. Energies 2023, 16, 3598. [Google Scholar] [CrossRef]
- Cavaco-Paulo, A. Mechanism of Cellulase Action in Textile Processes. Carbohydr. Polym. 1998, 37, 273–277. [Google Scholar] [CrossRef]
- Singh, S.; Singh, V.K.; Aamir, M.; Dubey, M.K.; Patel, J.S.; Upadhyay, R.S.; Gupta, V.K. Cellulase in Pulp and Paper Industry. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Cellulase System Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 152–162. [Google Scholar] [CrossRef]
- Oliveira, T.J.; Segato, T.C.M.; Machado, G.P.; Grotto, D.; Jozala, A.F. Evolution of Bacterial Cellulose in Cosmetic Applications: An Updated Systematic Review. Molecules 2022, 27, 8341. [Google Scholar] [CrossRef]
- Mbituyimana, B.; Liu, L.; Ye, W.; Ode Boni, B.O.; Zhang, K.; Chen, J.; Thomas, S.; Vasilievich, R.V.; Shi, Z.; Yang, G. Bacterial Cellulose-Based Composites for Biomedical and Cosmetic Applications: Research Progress and Existing Products. Carbohydr. Polym. 2021, 273, 118565. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.V.; Ananthanarayan, L. Enzyme Stability and Stabilization-Aqueous and Non-Aqueous Environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Lee, C.-K.; Au-Duong, A.-N. Enzyme Immobilization on Nanoparticles: Recent Applications. In Emerging Areas in Bioengineering; Chang, H.M., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 67–80. [Google Scholar]
- Khoshnevisan, K.; Poorakbar, E.; Baharifar, H.; Barkhi, M. Recent Advances of Cellulase Immobilization onto Magnetic Nanoparticles: An Update Review. Magnetochemistry 2019, 5, 36. [Google Scholar] [CrossRef]
- Zanuso, E.; Ruiz, H.A.; Domingues, L.; Teixeira, J.A. Magnetic Nanoparticles as Support for Cellulase Immobilization Strategy for Enzymatic Hydrolysis Using Hydrothermally Pretreated Corn Cob Biomass. Bioenergy Res. 2022, 15, 1946–1957. [Google Scholar] [CrossRef]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of Magnetic Nanoparticles in Smart Enzyme Immobilization. Biotechnol. Lett. 2016, 38, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Ganapathe, L.S.; Mohamed, M.A.; Yunus, R.M.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Marín, T.; Montoya, P.; Arnache, O.; Calderón, J. Influence of Surface Treatment on Magnetic Properties of Fe3O4 Nanoparticles Synthesized by Electrochemical Method. J. Phys. Chem. B 2016, 120, 6634–6645. [Google Scholar] [CrossRef]
- Pazik, R.; Lewinska, A.; Adamczyk-Grochala, J.; Kulpa-Greszta, M.; Kłoda, P.; Tomaszewska, A.; Dziedzic, A.; Litwinienko, G.; Noga, M.; Sikora, D.; et al. Energy Conversion and Biocompatibility of Surface Functionalized Magnetite Nanoparticles With Phosphonic Moieties. J. Phys. Chem. B 2020, 124, 4931–4948. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of Functionalization: Strategies to Explore Potential Nano-Bio Applications of Magnetic Nanoparticles. RSC Adv. 2016, 6, 43989–44012. [Google Scholar] [CrossRef]
- Mukha, I.; Chepurna, O.; Vityuk, N.; Khodko, A.; Storozhuk, L.; Dzhagan, V.; Zahn, D.R.T.; Ntziachristos, V.; Chmyrov, A.; Ohulchanskyy, T.Y. Multifunctional Magneto-Plasmonic Fe3O4/Au Nanocomposites: Approaching Magnetophoretically-Enhanced Photothermal Therapy. Nanomaterials 2021, 11, 1113. [Google Scholar] [CrossRef]
- Kulpa-Greszta, M.; Tomaszewska, A.; Dziedzic, A.; Rzeszutek, I.; Pązik, R. Heat Generation on Fe3O4@SiO2@Au Core-Shell Structures Using the Synergy of an Alternating Magnetic Field and NIR Laser Light within Ist Biological Optical Window. Mater. Today Commun. 2023, 35, 105513. [Google Scholar] [CrossRef]
- Kulpa-Greszta, M.; Tomaszewska, A.; Michalicha, A.; Sikora, D.; Dziedzic, A.; Wojnarowska-Nowak, R.; Belcarz, A.; Pązik, R. Alternating Magnetic Field and NIR Energy Conversion on Magneto-Plasmonic Fe3O4@APTES-Ag Heterostructures with SERS Detection Capability and Antimicrobial Activity. RSC Adv. 2022, 12, 27396–27410. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying Thiol-Gold Interactions towards the Efficient Strength Control. Nat. Commun. 2014, 5, 4348. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Lai, F.J.; Chou, H.L.; Lu, W.T.; Hwang, B.J.; Brolo, A.G. Surface-Enhanced Raman Scattering (SERS) from Au:Ag Bimetallic Nanoparticles: The Effect of the Molecular Probe. Chem. Sci. 2013, 4, 509–515. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman Spectroscopy to Unravel the Magnetic Properties of Iron Oxide Nanocrystals for Bio-Related Applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef]
- Nguyen, X.S.; Zhang, G.; Yang, X. Mesocrystalline Zn-Doped Fe3O4 Hollow Submicrospheres: Formation Mechanism and Enhanced Photo-Fenton Catalytic Performance. ACS Appl. Mater. Interfaces 2017, 9, 8900–8909. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Silva, T.; Keijok, W.J.; Guimarães, M.C.C.; Cassini, S.T.A.; de Oliveira, J.P. Impact of Immobilization Strategies on the Activity and Recyclability of Lipases in Nanomagnetic Supports. Sci. Rep. 2022, 12, 6815. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Matamoros, D.; Castro-García, S.; Balado, M.; Matamoros-Veloza, A.; Camargo-Valero, M.A.; Cespedes, O.; Rodríguez, J.; Lemos, M.L.; Jiménez, C. Preparation of Functionalized Magnetic Nanoparticles Conjugated with Feroxamine and Their Evaluation for Pathogen Detection. RSC Adv. 2019, 9, 13533–13542. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowska, R.; Polit, J.; Broda, D.; Gonchar, M.; Sheregii, E. Gold Nanoparticles like a Matrix for Covalent Immobilization of Cholesterol Oxidase—Application for Biosensing. Arch. Metall. Mater. 2015, 60, 2289–2296. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, Y.; Xu, C.; Lin, K.; Ye, Q.; Wang, X.; Xie, S.; Chen, R.; Lin, J. Label-Free Detection of Nasopharyngeal and Liver Cancer Using Surface-Enhanced Raman Spectroscopy and Partial Lease Squares Combined with Support Vector Machine. Biomed. Opt. Express 2018, 9, 6053. [Google Scholar] [CrossRef] [PubMed]
- Tuma, R. Raman Spectroscopy of Proteins: From Peptides to Large Assemblies. J. Raman Spectrosc. 2005, 36, 307–319. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman Spectroscopy of Proteins: A Review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Abraham, R.E.; Verma, M.L.; Barrow, C.J.; Puri, M. Suitability of Magnetic Nanoparticle Immobilised Cellulases in Enhancing Enzymatic Saccharification of Pretreated Hemp Biomass. Biotechnol. Biofuels 2014, 7, 90. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of Enzymes via Immobilization: Multipoint Covalent Attachment and Other Stabilization Strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Lindberg, D.; De La Revenga, M.F.; Widersten, M. Temperature and PH Dependence of Enzyme-Catalyzed Hydrolysis of Trans-Methylstyrene Oxide. A Unifying Kinetic Model for Observed Hysteresis, Cooperativity, and Regioselectivity. Biochemistry 2010, 49, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Hegedús, I.; Hancsók, J.; Nagy, E. Stabilization of the Cellulase Enzyme Complex as Enzyme Nanoparticle. Appl. Biochem. Biotechnol. 2012, 168, 1372–1383. [Google Scholar] [CrossRef]
- Abbaszadeh, M.; Hejazi, P. Metal Affinity Immobilization of Cellulase on Fe3O4 Nanoparticles with Copper as Ligand for Biocatalytic Applications. Food Chem. 2019, 290, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, N.M.; Wong, J.R.; Tan, K.W.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Immobilization of Cellulase Enzyme on Functionalized Multiwall Carbon Nanotubes. J. Mol. Catal. B Enzym. 2014, 107, 124–131. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; Guo, R.; Ma, Y.; Yuan, Y.; Liu, Y. Cellulase Immobilized by Sodium Alginate-Polyethylene Glycol-Chitosan for Hydrolysis Enhancement of Microcrystalline Cellulose. Process Biochem. 2021, 107, 38–47. [Google Scholar] [CrossRef]
- Yasmin Hasnol Azahari, N.; Sandrakesaran, N.; Chin Gaik Connie, C.; Balakrishnan, K. Immobilization of Cellulase from Trichoderma Reesei on Multiwall Carbon Nanotubes (MWCNTs). IOP Conf. Ser. Mater. Sci. Eng. 2020, 864, 012171. [Google Scholar] [CrossRef]
- Lima, J.S.; Araújo, P.H.H.; Sayer, C.; Souza, A.A.U.; Viegas, A.C.; de Oliveira, D. Cellulase Immobilization on Magnetic Nanoparticles Encapsulated in Polymer Nanospheres. Bioprocess Biosyst. Eng. 2017, 40, 511–518. [Google Scholar] [CrossRef]
- Van Der Kamp, M.W.; Prentice, E.J.; Kraakman, K.L.; Connolly, M.; Mulholland, A.J.; Arcus, V.L. Dynamical Origins of Heat Capacity Changes in Enzyme-Catalysed Reactions. Nat. Commun. 2018, 9, 1177. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Kumar, C.S.S.R.; Theegala, C. Preparation and Characterization of Cellulase-Bound Magnetite Nanoparticles. J. Mol. Catal. B Enzym. 2011, 68, 139–146. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, J.; Martínez-Hernández, J.L.; Segura-Ceniceros, P.; López, G.; Saade, H.; Medina-Morales, M.A.; Ramos-González, R.; Aguilar, C.N.; Ilyina, A. Cellulases Immobilization on Chitosan-Coated Magnetic Nanoparticles: Application for Agave Atrovirens Lignocellulosic Biomass Hydrolysis. Bioprocess Biosyst. Eng. 2017, 40, 9–22. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Wang, G.; Zhao, C.; Ma, Y.; Yang, W. Immobilization of Cellulase on Styrene/Maleic Anhydride Copolymer Nanoparticles with Improved Stability against pH Changes. Chem. Eng. J. 2018, 336, 152–159. [Google Scholar] [CrossRef]
- Song, Q.; Mao, Y.; Wilkins, M.; Segato, F.; Prade, R. Cellulase Immobilization on Superparamagnetic Nanoparticles for Reuse in Cellulosic Biomass Conversion. AIMS Bioeng. 2016, 3, 264–276. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]

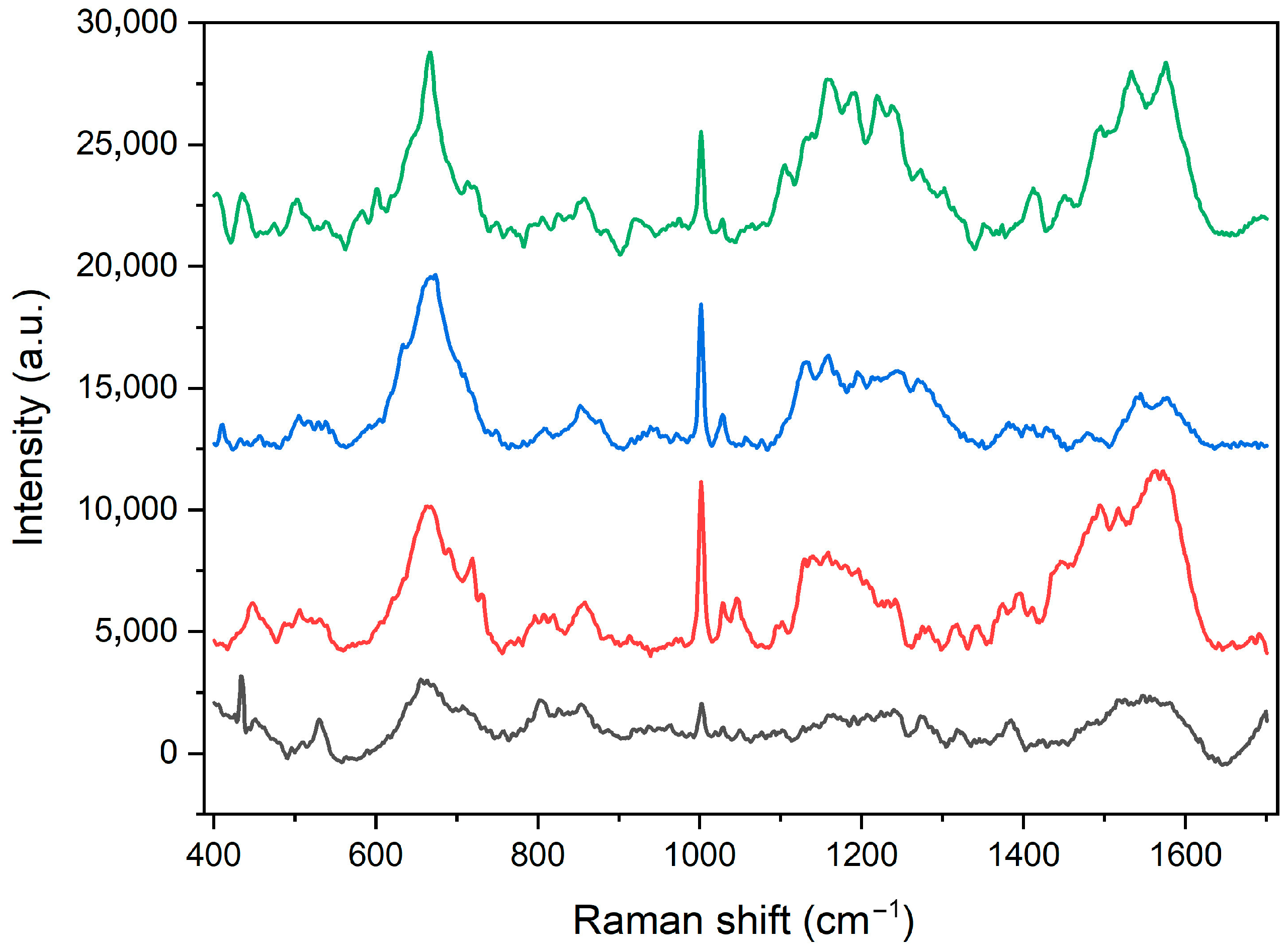


| Peak Positions (cm−1) | Band Identification |
|---|---|
| 1560 | tryptophan: ν(C=C) |
| 1530 | amide II: ν(C-N), δ(N-H) |
| 1446 | σ(CH2), σ(CH3) |
| 1342 | tryptophan: CH2/CH3 wagging, twisting and/or bending mode |
| 1270 | amide III (ν(C-N), δ(N-H), ν(CH3-C)), ν(P=O)(FAD) |
| 1130 | δ(CCH), ν(CC), ν(C-N) |
| 1057 | ν(C-N), ν(C-O) |
| 1029 | phenylalanine: δ(C-H) |
| 1002 | phenylalanine: ring breathing |
| 914 | proline/valine/protein backbone (α-helix conformation): ν(C-C) |
| 871 | hydroxyproline, tryptophan: β(CH) ring |
| 857 | tyrosine: ν(CC) ring |
| 820 | tyrosine: ν(CC) ring, β(CH) ring, γr(CH2) |
| 765 | tyrosine: ring breathing, γ(N-H), |
| 644 | amid IV (δ(O=C-N)), δ(CCN) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewska, A.; Kulpa-Greszta, M.; Hryców, O.; Niemczyk, K.; Wojnarowska-Nowak, R.; Broda, D.; Pazik, R. Biofunctionalization of Magneto-Plasmonic Fe3O4@SiO2-NH2-Au Heterostructures with the Cellulase from Trichoderma reesei. Molecules 2025, 30, 756. https://doi.org/10.3390/molecules30030756
Tomaszewska A, Kulpa-Greszta M, Hryców O, Niemczyk K, Wojnarowska-Nowak R, Broda D, Pazik R. Biofunctionalization of Magneto-Plasmonic Fe3O4@SiO2-NH2-Au Heterostructures with the Cellulase from Trichoderma reesei. Molecules. 2025; 30(3):756. https://doi.org/10.3390/molecules30030756
Chicago/Turabian StyleTomaszewska, Anna, Magdalena Kulpa-Greszta, Oliwia Hryców, Klaudia Niemczyk, Renata Wojnarowska-Nowak, Daniel Broda, and Robert Pazik. 2025. "Biofunctionalization of Magneto-Plasmonic Fe3O4@SiO2-NH2-Au Heterostructures with the Cellulase from Trichoderma reesei" Molecules 30, no. 3: 756. https://doi.org/10.3390/molecules30030756
APA StyleTomaszewska, A., Kulpa-Greszta, M., Hryców, O., Niemczyk, K., Wojnarowska-Nowak, R., Broda, D., & Pazik, R. (2025). Biofunctionalization of Magneto-Plasmonic Fe3O4@SiO2-NH2-Au Heterostructures with the Cellulase from Trichoderma reesei. Molecules, 30(3), 756. https://doi.org/10.3390/molecules30030756







