New Perspectives of Hydrogels in Chronic Wound Management
Abstract
1. Introduction
2. Chronic Wound Types
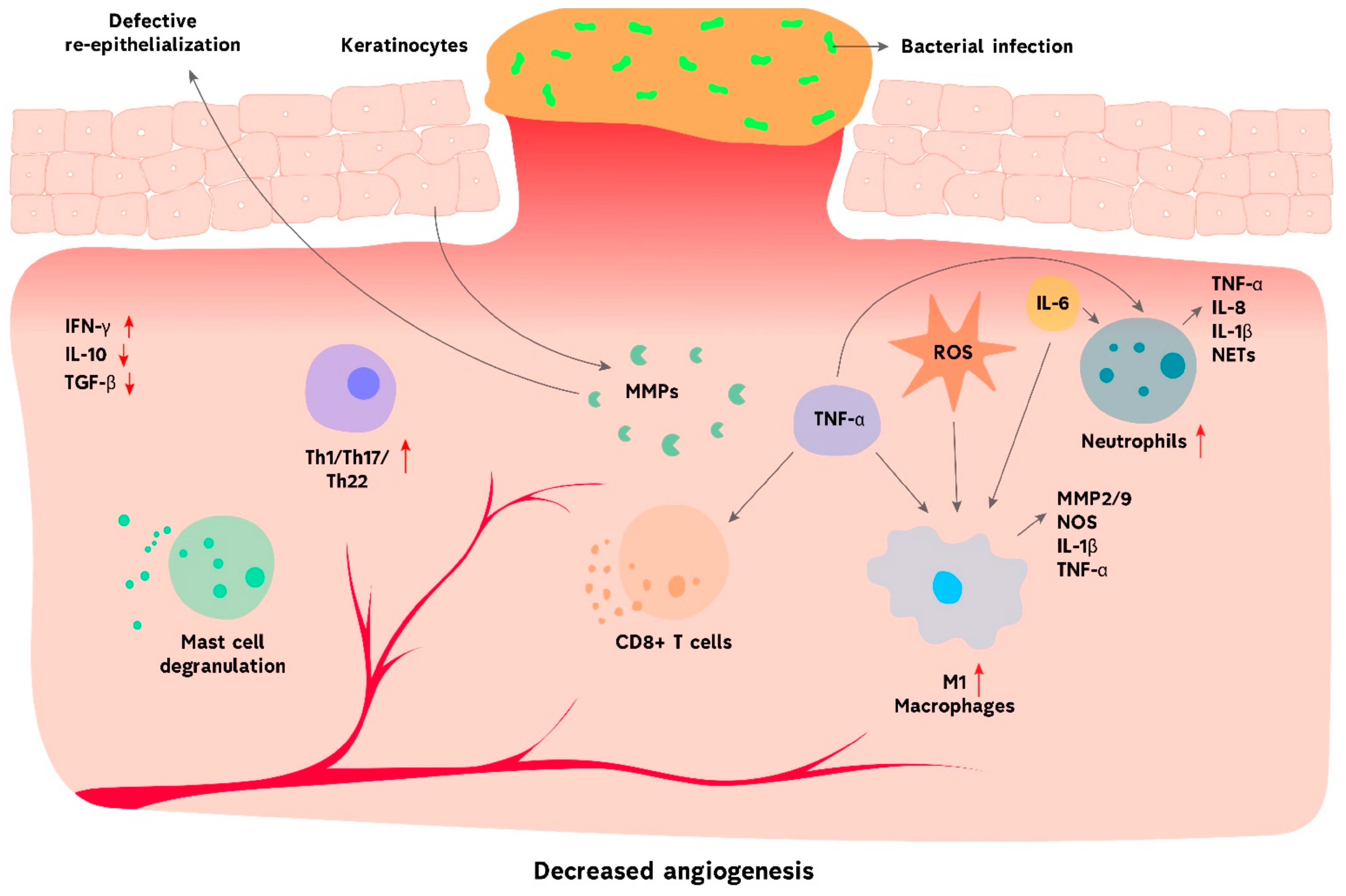
3. Challenges in Chronic Wound Management
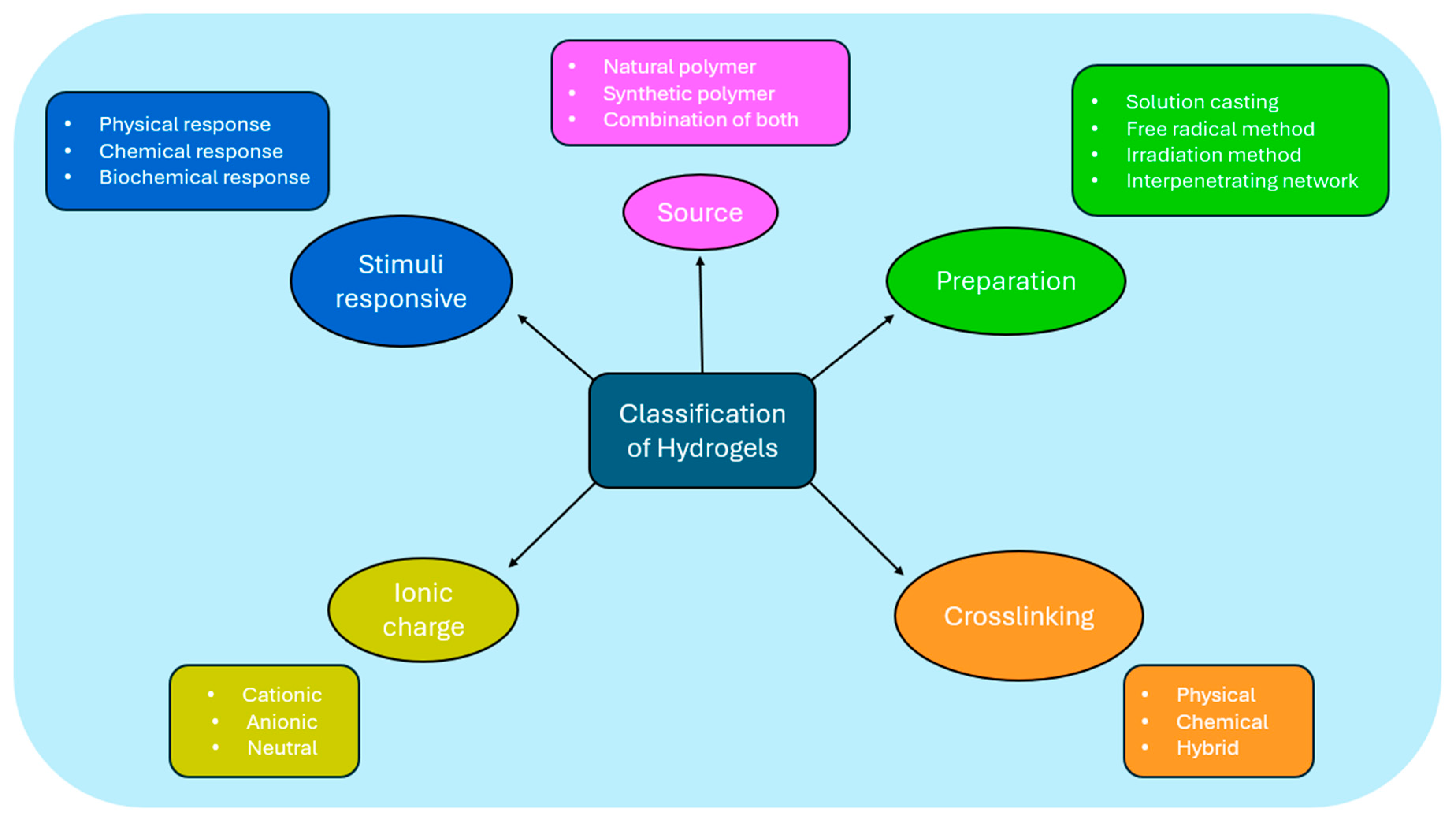
4. Hydrogels: An Overview
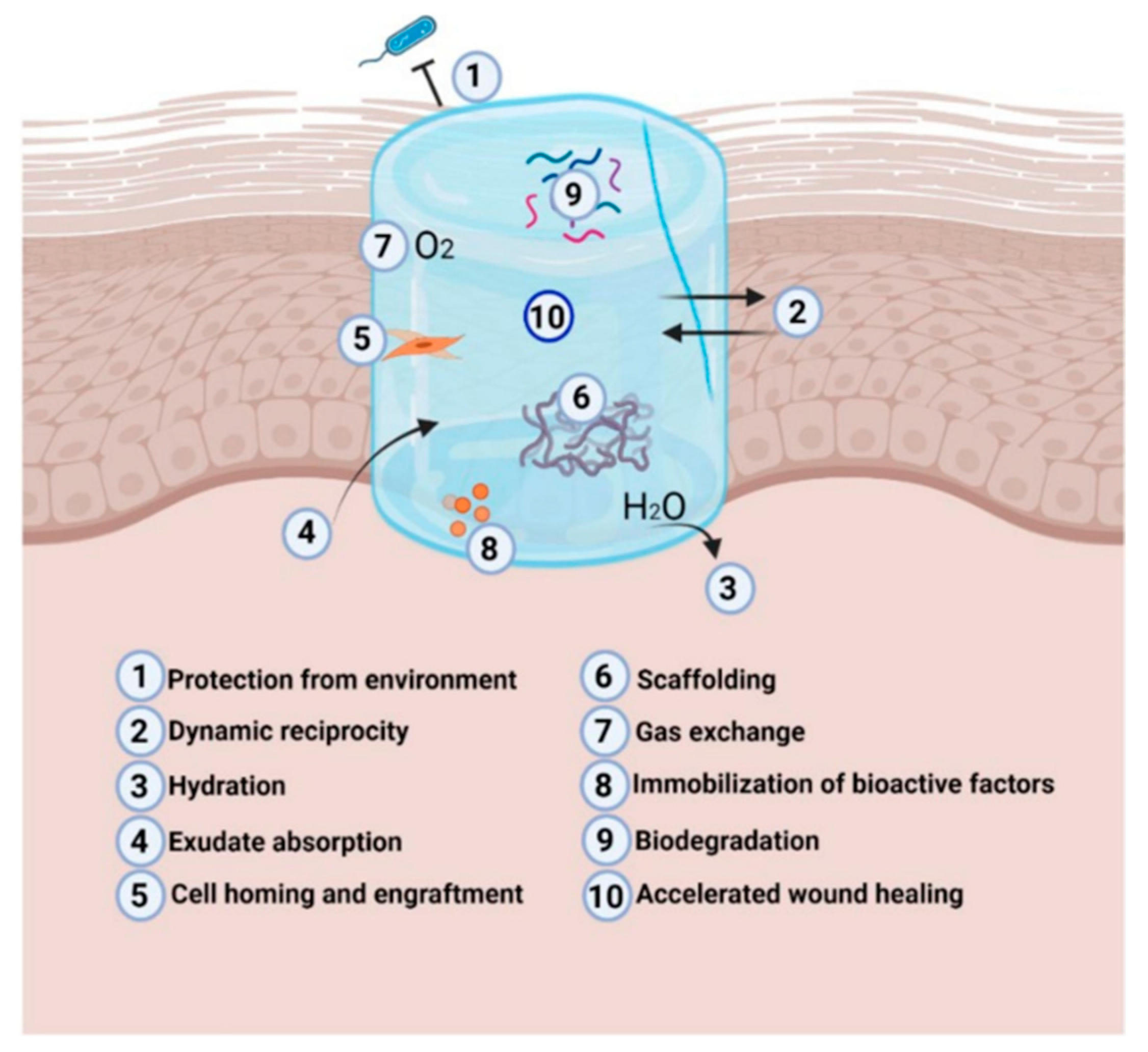
5. Mechanisms of Action in Wound Management
6. Applications of Hydrogels in Chronic Wounds
7. Innovations in Hydrogel Technology
8. Applications of Smart Hydrogels in Real-Time Monitoring
9. Limitations and Challenges of Hydrogels
10. Future Perspectives in Hydrogel Research
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic wounds: Current status, available strategies and emerging therapeutic solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; De Angelis, B.; Pea, F.; Scalise, A.; Stefani, S.; Tasinato, R.; Zanetti, O.; Dalla Paola, L. Challenges in the management of chronic wound infections. J. Glob. Antimicrob. Resist. 2021, 26, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coggeshall, M.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula, W.V.; Bus, S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 2020, 13, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Roy, S. Sociogenomic Approach to Wound Care: A New Patient-Centered Paradigm. Adv. Wound Care 2019, 8, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wound and Its Burden: Updated 2022 Compendium of Estimates. Adv. Wound Care 2023, 12, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.R.; Hwang, C.; Talbot, S.; Hibler, B.; Matoori, S.; Mooney, D.J. Breakthrough treatments for accelerated wound healing. Sci. Adv. 2023, 9, eade7007. [Google Scholar] [CrossRef] [PubMed]
- Kathawala, M.H.; Ng, W.L.; Liu, D.; Naing, M.W.; Yeong, W.Y.; Spiller, K.L.; Van Dyke, M.; Ng, K.W. Healing of Chronic Wounds: An Update of Recent Developments and Future Possibilities. Tissue Eng. Part B Rev. 2019, 25, 429–444. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Rosińczuk, J.; Taradaj, J.; Dymarek, R.; Sopel, M. Mechanoregulation of wound healing and skin homeostasis. Biomed Res. Int. 2016, 2016, 3943481. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.; Ghaffari, A.; Rashidzadeh, H.; Badeleh, S.M.; Maleki, Y. A Systematic Review on Classification, Identification, and Healing Process of Burn Wound Healing. Int. J. Low. Extrem. Wounds 2022, 21, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Negri, S.; Shekha, M.; Faris, P.; Guerra, G. Endothelial Ca2+ signaling, angiogenesis and vasculogenesis: Just what it takes to make a blood vessel. Int. J. Mol. Sci. 2019, 20, 3962. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Gou, M.; Da, L.C.; Zhang, W.Q.; Xie, H.Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Pohlmeier, L.; Ben-Yehuda Greenwald, M.; Haertel, E.; Hiebert, P.; Kopf, M.; Werner, S. Comprehensive characterization of myeloid cells during wound healing in healthy and healing-impaired diabetic mice. Eur. J. Immunol. 2020, 50, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Parikh, U.; Masri, L.; Saravanan, S.; Li, H.; Miao, Q.; Balaji, S. Role of cytokines and chemokines in wound healing; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Li, D.; Peng, H.; Qu, L.; Sommar, P.; Wang, A.; Chu, T.; Li, X.; Bi, X.; Liu, Q.; Gallais Sérézal, I.; et al. miR-19a/b and miR-20a Promote Wound Healing by Regulating the Inflammatory Response of Keratinocytes. J. Investig. Dermatol. 2021, 141, 659–671. [Google Scholar] [CrossRef]
- Nie, X.; Zhao, J.; Ling, H.; Deng, Y.; Li, X.; He, Y. Exploring microRNAs in diabetic chronic cutaneous ulcers: Regulatory mechanisms and therapeutic potential. Br. J. Pharmacol. 2020, 177, 4077–4095. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Wu, D.Q.; Zhu, J.; Han, H.; Zhang, J.Z.; Wu, F.F.; Qin, X.H.; Yu, J.Y. Synthesis and characterization of arginine-NIPAAm hybrid hydrogel as wound dressing: In vitro and in vivo study. Acta Biomater. 2018, 65, 305–316. [Google Scholar] [CrossRef]
- Bus, S.A.; Lavery, L.A.; Monteiro-Soares, M.; Rasmussen, A.; Raspovic, A.; Sacco, I.C.N.; van Netten, J.J. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes. Metab. Res. Rev. 2020, 36, e3269. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, C.-X.; Xu, B.; Yu, Z. Diabetic foot ulcers: Classification, risk factors and management. World J. Diabetes 2022, 13, 1049–1065. [Google Scholar] [CrossRef] [PubMed]
- Subrata, S.A.; Phuphaibul, R. Diabetic foot ulcer care: A concept analysis of the term integrated into nursing practice. Scand. J. Caring Sci. 2019, 33, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L.; Conte, M.S.; Armstrong, D.G.; Pomposelli, F.B.; Schanzer, A.; Sidawy, A.N.; Andros, G. The society for vascular surgery lower extremity threatened limb classification system: Risk stratification based on Wound, Ischemia, and foot Infection (WIfI). J. Vasc. Surg. 2014, 59, 220–234.e2. [Google Scholar] [CrossRef] [PubMed]
- Anthony, D.; Alosoumi, D.; Safari, R. Prevalence Of Pressure Ulcers In Long-Term Care: A Global Review. J. Wound Care 2019, 28, 702–709. [Google Scholar] [CrossRef]
- Boyko, T.V.; Longaker, M.T.; Yang, G.P. Review of the Current Management of Pressure Ulcers. Adv. Wound Care 2018, 7, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Irmak, F. Management and treatment of pressure ulcers: Clinical experience. SiSli Etfal Hastan. Tip Bul./Med. Bull. Sisli Hosp. 2018, 53, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.M.; Lal, B.K.; Durán, W.N.; Pappas, P.J. Pathophysiology of venous ulceration. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Baruah, M.; Bahia, S.S. Diagnosis and management of venous leg ulcers. BMJ 2018, 362, k3115. [Google Scholar] [CrossRef] [PubMed]
- Broderick, C.; Pagnamenta, F.; Forster, R. Dressings and topical agents for arterial leg ulcers. Cochrane Database Syst. Rev. 2020, 2020, 1–42. [Google Scholar] [CrossRef]
- Hedayati, N.; Carson, J.G.; Chi, Y.W.; Link, D. Management of mixed arterial venous lower extremity ulceration: A review. Vasc. Med. 2015, 20, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Diban, F.; Di Lodovico, S.; Di Fermo, P.; D’Ercole, S.; D’Arcangelo, S.; Di Giulio, M.; Cellini, L. Biofilms in Chronic Wound Infections: Innovative Antimicrobial Approaches Using the In Vitro Lubbock Chronic Wound Biofilm Model. Int. J. Mol. Sci. 2023, 24, 1004. [Google Scholar] [CrossRef]
- Thaarup, I.C.; Iversen, A.K.S.; Lichtenberg, M.; Bjarnsholt, T.; Jakobsen, T.H. Biofilm Survival Strategies in Chronic Wounds. Microorganisms 2022, 10, 775. [Google Scholar] [CrossRef]
- Punjataewakupt, A.; Napavichayanun, S.; Aramwit, P. The downside of antimicrobial agents for wound healing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 39–54. [Google Scholar] [CrossRef]
- Han, X.; Ju, L.S.; Irudayaraj, J. Oxygenated Wound Dressings for Hypoxia Mitigation and Enhanced Wound Healing. Mol. Pharm. 2023, 20, 3338–3355. [Google Scholar] [CrossRef] [PubMed]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Thakur, V.K.; Arotiba, O.A. History, Classification, Properties and Application of Hydrogels: An Overview; Springer: Singapore, 2018. [Google Scholar]
- Maleki, B.; Kargar, P.G.; Ashrafi, S.S.; Ghani, M. Perspective Chapter: Introduction to Hydrogels—Definition, Classifications, Applications and Methods of Preparation; IntechOpen: London, UK, 2016; Volume 11, p. 13. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Buckner, C.A.; Lafrenie, R.M.; Dénommée, J.A.; Caswell, J.M.; Want, D.A.; Gan, G.G.; Leong, Y.C.; Bee, P.C.; Chin, E.; Teh, A.K.H.; et al. Polymeric Hydrogels and Nanogels: Classification, Development and Pharmaceutical Applications; IntechOpen: London, UK, 2016; Volume 11, p. 13. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hoffman, A.S. Hydrogels. In Biomaterials Science. An Introduction to Materials in Medicine; Academic Press: Cambridge, MA, USA, 2020; pp. 153–166. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef]
- Tu, H.; Yu, Y.; Chen, J.; Shi, X.; Zhou, J.; Deng, H.; Du, Y. Highly cost-effective and high-strength hydrogels as dye adsorbents from natural polymers chitosan and cellulose. Polym. Chem. 2017, 8, 2913–2921. [Google Scholar] [CrossRef]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Calderon Moreno, J.M.; Musuc, A.M.; Popa, M. Natural Regenerative Hydrogels for Wound Healing. Gels 2024, 10, 547. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Liang, X.; Huang, C.; Liu, H.; Chen, H.; Shou, J.; Cheng, H.; Liu, G. Natural hydrogel dressings in wound care: Design, advances, and perspectives. Chin. Chem. Lett. 2024, 35, 109442. [Google Scholar] [CrossRef]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulou, Z.; Zamboulis, A.; Koumentakou, I.; Michailidou, G.; Noordam, M.J.; Bikiaris, D.N. Biocompatible Synthetic Polymers for Tissue Engineering Purposes. Biomacromolecules 2022, 23, 1841–1863. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Amini, S.; Salehi, H.; Setayeshmehr, M.; Ghorbani, M. Natural and synthetic polymeric scaffolds used in peripheral nerve tissue engineering: Advantages and disadvantages. Polym. Adv. Technol. 2021, 32, 2267–2289. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Ren, J.; Zhao, Y. Bioinspired Multifunctional Hybrid Hydrogel Promotes Wound Healing. Adv. Funct. Mater. 2018, 28, 1801386. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Hybrid-Based Wound Dressings: Combination of Synthetic and Biopolymers. Polymers 2022, 14, 3806. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, Z.; Liu, G.; Huang, J. Novel glucose-responsive antioxidant hybrid hydrogel for enhanced diabetic wound repair. ACS Appl. Mater. Interfaces 2022, 14, 7680–7689. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, T.; Zhou, C.; Jiang, A.; Lu, C.; Yang, G.; Nie, J.; Wang, F.; Yang, X.; Chen, Z. Injectable self-healing chitosan-based POSS-PEG hybrid hydrogel as wound dressing to promote diabetic wound healing. Carbohydr. Polym. 2023, 299, 120198. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Xin, H.; Maruf, D.S.; Akin-Ige, F.; Amin, S. Stimuli-responsive hydrogels for skin wound healing and regeneration. Emergent Mater. 2024. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.Q.; Li, Z.B.; Wu, Y.L.; Li, D.W. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, H.; Wang, C.; Xing, Y.; Liu, S.; Feng, L.; Zhang, X.; Chen, J. Advances in Smart-Response Hydrogels for Skin Wound Repair. Polymers 2024, 16, 2818. [Google Scholar] [CrossRef]
- Li, M.; Xia, W.; Khoong, Y.M.; Huang, L.; Huang, X.; Liang, H.; Zhao, Y.; Mao, J.; Yu, H.; Zan, T. Smart and versatile biomaterials for cutaneous wound healing. Biomater. Res. 2023, 27, 87. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Pei, D.; Wang, Z.; Li, M.; Ma, X.; You, J.; Li, C. Biocompatible and self-healing ionic gel skin as shape-adaptable and skin-adhering sensor of human motions. Chem. Eng. J. 2020, 398, 125540. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Chen, G.; Chen, H.; Zhao, Y. Structural Color Ionic Hydrogel Patches for Wound Management. ACS Nano 2023, 17, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tao, X.; Chen, W.; Mao, J.; Luo, H.; Lin, S.; Zhang, L.; Hao, J. Ionic Hydrogel for Efficient and Scalable Moisture-Electric Generation. Adv. Mater. 2022, 34, 2200693. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, R.; Duan, Z.; Zhu, C.; Deng, J.; Fan, D. Ionic liquid-based non-releasing antibacterial, anti-inflammatory, high-transparency hydrogel coupled with electrical stimulation for infected diabetic wound healing. Compos. Part B Eng. 2022, 236, 109804. [Google Scholar] [CrossRef]
- Liu, P.; Jin, K.; Wong, W.; Wang, Y.; Liang, T.; He, M.; Li, H.; Lu, C.; Tang, X.; Zong, Y.; et al. Ionic liquid functionalized non-releasing antibacterial hydrogel dressing coupled with electrical stimulation for the promotion of diabetic wound healing. Chem. Eng. J. 2021, 415, 129025. [Google Scholar] [CrossRef]
- Drakulich, A. Pharma innovation. Pharm. Technol. 2012, 36, 32–37. [Google Scholar]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, F.; Chen, G.; Liu, J.; Li, X.L.; Cheng, B.; Mo, X.M.; Chen, C.; Pan, J.F. Moist-Retaining, Self-Recoverable, Bioadhesive, and Transparent in Situ Forming Hydrogels to Accelerate Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.H.; Chen, X.Y.; Fu, L.Q.; Du, W.L.; Yang, X.; Mou, X.Z.; Hu, P.Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef] [PubMed]
- Kruczkowska, W.; Gałęziewska, J.; Grabowska, K.; Liese, G.; Buczek, P.; Kłosiński, K.K.; Kciuk, M.; Pasieka, Z.; Kałuzińska-Kołat, Ż.; Kołat, D. Biomedical Trends in Stimuli-Responsive Hydrogels with Emphasis on Chitosan-Based Formulations. Gels 2024, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing. Adv. Mater. 2021, 33, 2100176. [Google Scholar] [CrossRef]
- Güiza-Argüello, V.R.; Solarte-David, V.A.; Pinzón-Mora, A.V.; Ávila-Quiroga, J.E.; Becerra-Bayona, S.M. Current Advances in the Development of Hydrogel-Based Wound Dressings for Diabetic Foot Ulcer Treatment. Polymers 2022, 14, 2764. [Google Scholar] [CrossRef]
- Lei, J.; Chen, P.; Li, Y.; Wang, X.; Tang, S. Collagen hydrogel dressing for wound healing and angiogenesis in diabetic rat models. Int. J. Clin. Exp. Med. 2017, 10, 16319–16327. [Google Scholar]
- Tellechea, A.; Silva, E.A.; Min, J.; Leal, E.C.; Auster, M.E.; Pradhan-Nabzdyk, L.; Shih, W.; Mooney, D.J.; Veves, A. Alginate and DNA gels are suitable delivery systems for diabetic wound healing. Int. J. Low. Extrem. Wounds 2015, 14, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Liu, C.; Zheng, Q.; Chu, G.; Wang, H.; Jin, J.; Wu, H.; Chen, J.; Huang, Q.; Deng, Z.; et al. Exosome-laden injectable self-healing hydrogel based on quaternized chitosan and oxidized starch attenuates disc degeneration by suppressing nucleus pulposus senescence. Int. J. Biol. Macromol. 2023, 232, 123479. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- Xu, Q.; Sigen, A.; Gao, Y.; Guo, L.; Creagh-Flynn, J.; Zhou, D.; Greiser, U.; Dong, Y.; Wang, F.; Tai, H.; et al. A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomater. 2018, 75, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater. Sci. Eng. C 2020, 110, 110686. [Google Scholar] [CrossRef] [PubMed]
- Khampieng, T.; Wongkittithavorn, S.; Chaiarwut, S.; Ekabutr, P.; Pavasant, P.; Supaphol, P. Silver nanoparticles-based hydrogel: Characterization of material parameters for pressure ulcer dressing applications. J. Drug Deliv. Sci. Technol. 2018, 44, 91–100. [Google Scholar] [CrossRef]
- Vijayakanth, T.; Shankar, S.; Finkelstein-Zuta, G.; Rencus-Lazar, S.; Gilead, S.; Gazit, E. Perspectives on recent advancements in energy harvesting, sensing and bio-medical applications of piezoelectric gels. Chem. Soc. Rev. 2023, 52, 6191–6220. [Google Scholar] [CrossRef]
- Zhu, Y.; Hoshi, R.; Chen, S.; Yi, J.; Duan, C.; Galiano, R.D.; Zhang, H.F.; Ameer, G.A. Sustained release of stromal cell derived factor-1 from an antioxidant thermoresponsive hydrogel enhances dermal wound healing in diabetes. J. Control. Release 2016, 238, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shi, J.; Zhang, M.; Chen, Y.; Wang, X.; Zhang, L.; Tian, Z.; Yan, Y.; Li, Q.; Zhong, W.; et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci. Rep. 2015, 5, 18104. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of Apigenin loaded gellan gum–chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. View 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Neumann, M.; di Marco, G.; Iudin, D.; Viola, M.; van Nostrum, C.F.; van Ravensteijn, B.G.P.; Vermonden, T. Stimuli-Responsive Hydrogels: The Dynamic Smart Biomaterials of Tomorrow. Macromolecules 2023, 56, 8377–8392. [Google Scholar] [CrossRef]
- Michalicha, A.; Belcarz, A.; Giannakoudakis, D.A.; Staniszewska, M.; Barczak, M. Designing Composite Stimuli-Responsive Hydrogels for Wound Healing Applications: The State-of-the-Art and Recent Discoveries. Materials 2024, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, Y.; Zhu, Y.; Zhao, J.; Ren, K.; Lu, Z.; Li, J.; Hao, Z. Stimuli-Responsive Protein Hydrogels: Their Design, Properties, and Biomedical Applications. Polymers 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nakahata, M.; Linke, P.; Kaufmann, S. Stimuli-responsive hydrogels as a model of the dynamic cellular microenvironment. Polym. J. 2020, 52, 861–870. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, Z.; Li, Z.; Luan, H.; Yu, Y.; Zhu, A.T.; Ding, S.; Gao, W.; Fang, R.H.; Zhang, L.; et al. Biohybrid microrobots locally and actively deliver drug-loaded nanoparticles to inhibit the progression of lung metastasis. Sci. Adv. 2024, 10, eadn6157. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Li, L.; Liu, X.; Zhu, Y.; Yu, S.; Wang, H.; Wang, L. Hydrogel microrobots for biomedical applications. Front. Chem. 2024, 12, 1416314. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Heydarpour, R.; Tehrani, Z.M. Multi-stimuli-responsive hydrogels and their medical applications. New J. Chem. 2021, 45, 15705–15717. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, D.; Hui, Q.; Bi, J.; Yu, B.; Huang, Z.; Hu, S.; Wang, Z.; Caranasos, T.; Rossi, J.; et al. Injection of ROS-Responsive Hydrogel Loaded with Basic Fibroblast Growth Factor into the Pericardial Cavity for Heart Repair. Adv. Funct. Mater. 2021, 31, 2004377. [Google Scholar] [CrossRef]
- Xu, C.; Guan, S.; Wang, S.; Gong, W.; Liu, T.; Ma, X.; Sun, C. Biodegradable and electroconductive poly(3,4-ethylenedioxythiophene)/carboxymethyl chitosan hydrogels for neural tissue engineering. Mater. Sci. Eng. C 2018, 84, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef]
- Levingstone, T.; Ali, B.; Kearney, C.; Dunne, N. Hydroxyapatite sonosensitization of ultrasound-triggered, thermally responsive hydrogels: An on-demand delivery system for bone repair applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1622–1633. [Google Scholar] [CrossRef]
- Qu, M.; Jiang, X.; Zhou, X.; Wang, C.; Wu, Q.; Ren, L.; Zhu, J.; Zhu, S.; Tebon, P.; Sun, W.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Healthc. Mater. 2020, 9, 1901714. [Google Scholar] [CrossRef]
- Chang, S.; Wang, S.; Liu, Z.; Wang, X. Advances of Stimulus-Responsive Hydrogels for Bone Defects Repair in Tissue Engineering. Gels 2022, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, C.; Ma, Z. Stimuli-responsive biomaterials for cardiac tissue engineering and dynamic mechanobiology. APL Bioeng. 2021, 5, 011506. [Google Scholar] [CrossRef]
- Fan, C.; Shi, J.; Zhuang, Y.; Zhang, L.; Huang, L.; Yang, W.; Chen, B.; Chen, Y.; Xiao, Z.; Shen, H.; et al. Myocardial-Infarction-Responsive Smart Hydrogels Targeting Matrix Metalloproteinase for On-Demand Growth Factor Delivery. Adv. Mater. 2019, 31, 1902900. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Duan, L.; Kong, M.; Wen, X.; Guan, F.; Ma, S. Applications and Mechanisms of Stimuli-Responsive Hydrogels in Traumatic Brain Injury. Gels 2022, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.R.; Irani, A.S.; Ander, E.W.; Ludolph, C.M.; Venkataraman, A.K.; Zhong, J.X.; Peppas, N.A. Synthetic networks with tunable responsiveness, biodegradation, and molecular recognition for precision medicine applications. Sci. Adv. 2019, 5, eaax7946. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Sadeghian, R.B.; Salehi, S.; Ostrovidov, S.; Bae, H.; Ramalingam, M.; Khademhosseini, A. Bioconjugated Hydrogels for Tissue Engineering and Regenerative Medicine. Bioconjug. Chem. 2015, 26, 1984–2001. [Google Scholar] [CrossRef]
- Wang, C.; Shirzaei Sani, E.; Shih, C.D.; Lim, C.T.; Wang, J.; Armstrong, D.G.; Gao, W. Wound management materials and technologies from bench to bedside and beyond. Nat. Rev. Mater. 2024, 9, 550–566. [Google Scholar] [CrossRef]
- Feng, C.; Ouyang, J.; Tang, Z.; Kong, N.; Liu, Y.; Fu, L.Y.; Ji, X.; Xie, T.; Farokhzad, O.C.; Tao, W. Germanene-Based Theranostic Materials for Surgical Adjuvant Treatment: Inhibiting Tumor Recurrence and Wound Infection. Matter 2020, 3, 127–144. [Google Scholar] [CrossRef]
- Bercea, M.; Lupu, A. Recent Insights into Glucose-Responsive Concanavalin A-Based Smart Hydrogels for Controlled Insulin Delivery. Gels 2024, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Said, S.S.; Campbell, S.; Hoare, T. Externally Addressable Smart Drug Delivery Vehicles: Current Technologies and Future Directions. Chem. Mater. 2019, 31, 4971–4989. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.L. Review of applications and future prospects of stimuli-responsive hydrogel based on thermo-responsive biopolymers in drug delivery systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef] [PubMed]
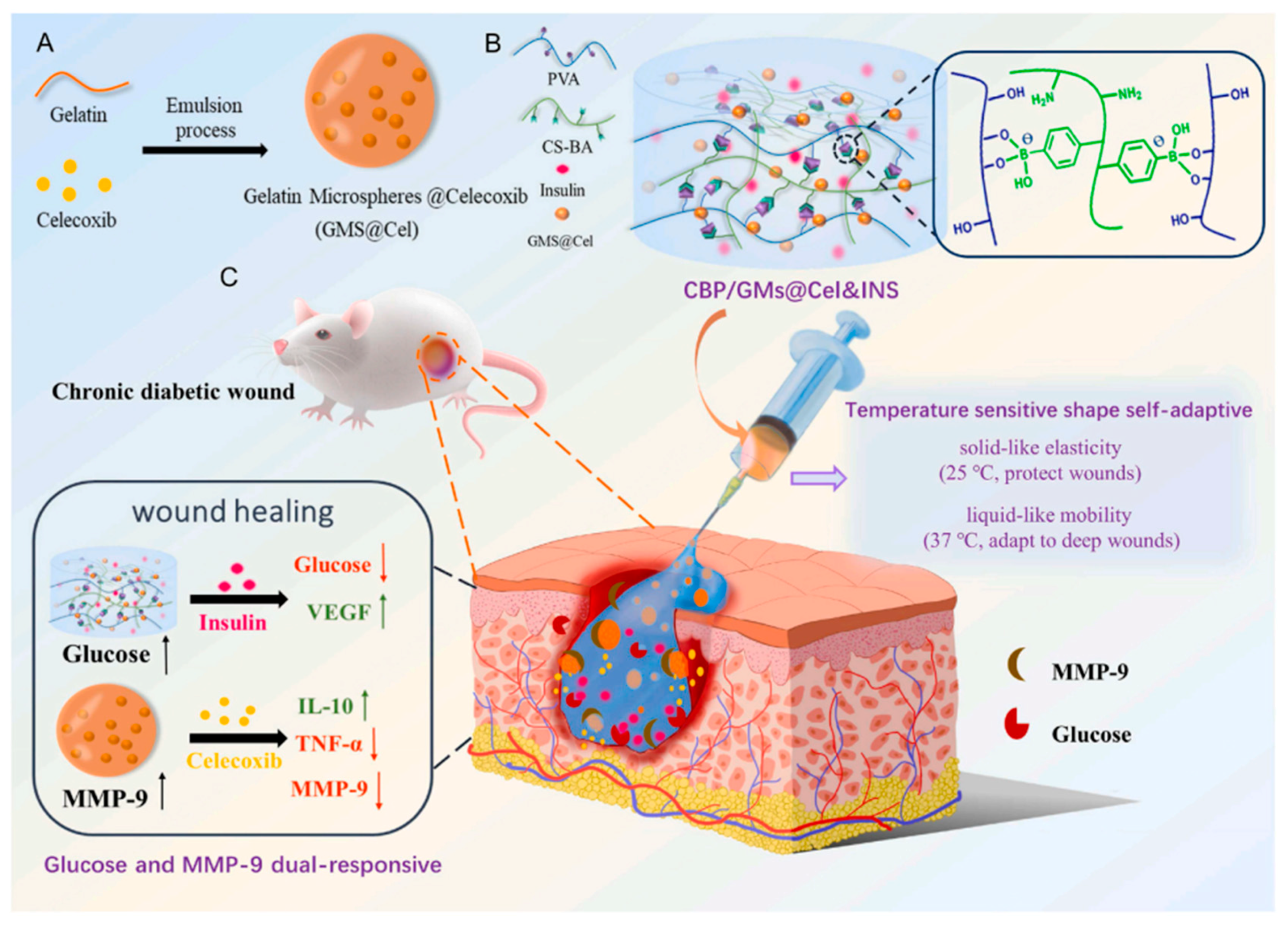
- Zhou, W.; Duan, Z.; Zhao, J.; Fu, R.; Zhu, C.; Fan, D. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact. Mater. 2022, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhou, Y.; Liu, X.; Chen, Y.; Duan, S.; Zhu, R.; Liu, Y.; Yin, L. Recent Advances on Reactive Oxygen Species-Responsive Delivery and Diagnosis System. Biomacromolecules 2019, 20, 2441–2463. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, K.; Hosseinzadeh, R.; Esfahani, H.S.; Zandsalimi, K.; Shahidi, F.K.; Abrahamse, H. Accelerating skin regeneration and wound healing by controlled ROS from photodynamic treatment. Inflamm. Regen. 2022, 42, 40. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Yu, H.; Wang, L.; Huang, Y.; Lu, H.; Zhou, H.; Liu, Q. Multistage ROS-Responsive and Natural Polyphenol-Driven Prodrug Hydrogels for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 52643–52658. [Google Scholar] [CrossRef]
- Cui, T.; Yu, J.; Wang, C.F.; Chen, S.; Li, Q.; Guo, K.; Qing, R.; Wang, G.; Ren, J. Micro-Gel Ensembles for Accelerated Healing of Chronic Wound via pH Regulation. Adv. Sci. 2022, 9, 2201254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ochoa, M.; Waimin, J.F.; Rahimi, R.; Ziaie, B. A pH-regulated drug delivery dermal patch for targeting infected regions in chronic wounds. Lab Chip 2019, 19, 2265–2274. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Campo Dall′ Orto, V.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef]
- Shen, K.H.; Lu, C.H.; Kuo, C.Y.; Li, B.Y.; Yeh, Y.C. Smart near infrared-responsive nanocomposite hydrogels for therapeutics and diagnostics. J. Mater. Chem. B 2021, 9, 7100–7116. [Google Scholar] [CrossRef]
- Karmakar, A.; Silswal, A.; Koner, A.L. Review of NIR-responsive ‘“Smart”’ carriers for photothermal chemotherapy. J. Mater. Chem. B 2024, 12, 4785–4808. [Google Scholar] [CrossRef] [PubMed]
- Azeera, M.; Vaidevi, S.; Ruckmani, K. Characterization Techniques of Hydrogel and Its Applications. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2019; pp. 737–761. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Z.; Liu, K.; Ji, X.; Fatehi, P.; Chen, J. A cellulose nanofibril-reinforced hydrogel with robust mechanical, self-healing, pH-responsive and antibacterial characteristics for wound dressing applications. J. Nanobiotechnol. 2022, 20, 312. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; She, W.; Luo, Y.; He, D.; Chen, J.; Ning, N.; Yu, Y.; De Beer, S.; Zhang, S. One-pot, self-catalyzed synthesis of self-adherent hydrogels for photo-thermal, antimicrobial wound treatment. J. Mater. Chem. B 2021, 9, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Laurano, R.; Torchio, A.; Ciardelli, G.; Boffito, M. In Situ Forming Bioartificial Hydrogels with ROS Scavenging Capability Induced by Gallic Acid Release with Potential in Chronic Skin Wound Treatment. Gels 2023, 9, 731. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C 2019, 104, 109930. [Google Scholar] [CrossRef]
- Cao, J.; Wu, P.; Cheng, Q.; He, C.; Chen, Y.; Zhou, J. Ultrafast Fabrication of Self-Healing and Injectable Carboxymethyl Chitosan Hydrogel Dressing for Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 24095–24105. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Liu, H.; Ren, M.; Wang, Z.; Wang, X.; Liu, H.; Feng, Y.; Lin, Q.; Wang, C.; et al. pH-responsive hydrogel loaded with insulin as a bioactive dressing for enhancing diabetic wound healing. Mater. Des. 2021, 210, 110104. [Google Scholar] [CrossRef]
- Kraehenbuehl, T.P.; Ferreira, L.S.; Zammaretti, P.; Hubbell, J.A.; Langer, R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials 2009, 30, 4318–4324. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, J.; Cao, L.; Jiao, Q.; Zhou, J.; Yang, L.; Zhang, H.; Wei, Y. Antifouling Antioxidant Zwitterionic Dextran Hydrogels as Wound Dressing Materials with Excellent Healing Activities. ACS Appl. Mater. Interfaces 2021, 13, 7060–7069. [Google Scholar] [CrossRef]
- Oh, G.W.; Kim, S.C.; Kim, T.H.; Jung, W.K. Characterization of an oxidized alginate-gelatin hydrogel incorporating a COS-salicylic acid conjugate for wound healing. Carbohydr. Polym. 2021, 252, 117145. [Google Scholar] [CrossRef]
- Ahmed, H.E.; Iqbal, Y.; Aziz, M.H.; Atif, M.; Batool, Z.; Hanif, A.; Yaqub, N.; Farooq, W.A.; Ahmad, S.; Fatehmulla, A.; et al. Green synthesis of CeO2 nanoparticles from the abelmoschus esculentus extract: Evaluation of antioxidant, anticancer, antibacterial, and wound-healing activities. Molecules 2021, 26, 4659. [Google Scholar] [CrossRef]
- Peng, Y.; He, D.; Ge, X.; Lu, Y.; Chai, Y.; Zhang, Y.; Mao, Z.; Luo, G.; Deng, J.; Zhang, Y. Construction of heparin-based hydrogel incorporated with Cu5.4O ultrasmall nanozymes for wound healing and inflammation inhibition. Bioact. Mater. 2021, 6, 3109–3124. [Google Scholar] [CrossRef]
- Bonetti, L.; Fiorati, A.; D’agostino, A.; Pelacani, C.M.; Chiesa, R.; Farè, S.; De Nardo, L. Smart Methylcellulose Hydrogels for pH-Triggered Delivery of Silver Nanoparticles. Gels 2022, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Suo, H.; Xie, Y.; Wang, K.; Wang, H.; Hou, Z.; Gao, Y.; Zhang, L.; Tao, J.; Jiang, H.; et al. Dopamine-Substituted Multidomain Peptide Hydrogel with Inherent Antimicrobial Activity and Antioxidant Capability for Infected Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 29380–29391. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, W.; Liu, J.; Han, Y.; Yan, Q.; Dong, Y.; Liu, X.; Yang, D.; Ma, G.; Cao, H. A novel sprayable thermosensitive hydrogel coupled with zinc modified metformin promotes the healing of skin wound. Bioact. Mater. 2023, 20, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Bean, J.E.; Alves, D.R.; Laabei, M.; Esteban, P.P.; Thet, N.T.; Enright, M.C.; Jenkins, A.T.A. Triggered release of Bacteriophage K from agarose/hyaluronan hydrogel matrixes by Staphylococcus aureus virulence factors. Chem. Mater. 2014, 26, 7201–7208. [Google Scholar] [CrossRef]
- Hathaway, H.; Alves, D.R.; Bean, J.; Esteban, P.P.; Ouadi, K.; Mark Sutton, J.; Jenkins, A.T.A. Poly(N-isopropylacrylamide-co-allylamine) (PNIPAM-co-ALA) nanospheres for the thermally triggered release of Bacteriophage K. Eur. J. Pharm. Biopharm. 2015, 96, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Gondil, V.S.; Chhibber, S. A novel wound dressing consisting of PVA-SA hybrid hydrogel membrane for topical delivery of bacteriophages and antibiotics. Int. J. Pharm. 2019, 572, 118779. [Google Scholar] [CrossRef]
- Kumari, S.; Harjai, K.; Chhibber, S. Bacteriophage versus antimicrobial agents for the treatment of murine burn wound infection caused by Klebsiella pneumoniae B5055. J. Med. Microbiol. 2011, 60, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Dharmaraj, T.; Chen, Q.; Echterhof, A.; Manasherob, R.; Zhang, L.; Leeuw, C.; Peterson, N.; Stannard, W.; Li, Z.; et al. Optimized Dosing and Delivery of Bacteriophage Therapy for Wound Infections. bioRxiv 2024. [Google Scholar] [CrossRef]
- Meireles Gouvêa Boggione, D.; Boggione Santos, I.J.; Menezes de Souza, S.; Santos Mendonça, R.C. Preparation of polyvinyl alcohol hydrogel containing bacteriophage and its evaluation for potential use in the healing of skin wounds. J. Drug Deliv. Sci. Technol. 2021, 63, 102484. [Google Scholar] [CrossRef]
- Khazani Asforooshani, M.; Elikaei, A.; Abed, S.; Shafiei, M.; Barzi, S.M.; Solgi, H.; Badmasti, F.; Sohrabi, A. A novel Enterococcus faecium phage EF-M80: Unveiling the effects of hydrogel-encapsulated phage on wound infection healing. Front. Microbiol. 2024, 15, 1416971. [Google Scholar] [CrossRef] [PubMed]
- Cobb, L.H.; Park, J.Y.; Swanson, E.A.; Beard, M.C.; McCabe, E.M.; Rourke, A.S.; Seo, K.S.; Olivier, A.K.; Priddy, L.B. CRISPR-Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLoS ONE 2019, 14, e0220421. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Bukhari, S.N.A.; Hussain, M.A.; Ejaz, H.; Munir, M.U.; Amjad, M.W. Nanoparticles incorporated hydrogels for delivery of antimicrobial agents: Developments and trends. RSC Adv. 2024, 14, 13535–13564. [Google Scholar] [CrossRef]
- Carnero Canales, C.S.; Marquez Cazorla, J.I.; Marquez Cazorla, R.M.; Roque-Borda, C.A.; Polinário, G.; Figueroa Banda, R.A.; Sábio, R.M.; Chorilli, M.; Santos, H.A.; Pavan, F.R. Breaking barriers: The potential of nanosystems in antituberculosis therapy. Bioact. Mater. 2024, 39, 106–134. [Google Scholar] [CrossRef]
- Li, X.; Fan, R.; Tong, A.; Yang, M.; Deng, J.; Zhou, L.; Zhang, X.; Guo, G. In situ gel-forming AP-57 peptide delivery system for cutaneous wound healing. Int. J. Pharm. 2015, 495, 560–571. [Google Scholar] [CrossRef]
- Moorcroft, S.C.T.; Roach, L.; Jayne, D.G.; Ong, Z.Y.; Ong, Z.Y.; Evans, S.D. Nanoparticle-Loaded Hydrogel for the Light-Activated Release and Photothermal Enhancement of Antimicrobial Peptides. ACS Appl. Mater. Interfaces 2020, 12, 24544–24554. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Del Giudice, A.; Mignardi, S.; Amato, F.; Marrani, A.G.; Sivori, F.; Cavallo, I.; Di Domenico, E.G.; Palocci, C.; Chronopoulou, L. Green In Situ Synthesis of Silver Nanoparticles-Peptide Hydrogel Composites: Investigation of Their Antibacterial Activities. Gels 2022, 8, 700. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Guo, B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering; Springer: Singapore, 2022; Volume 14. [Google Scholar]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.; Sahoo, P.K.; Li, K.; Johnson, S.; Raxworthy, M.J.; Krauss, T.F. Nanophotonic and hydrogel-based diagnostic system for the monitoring of chronic wounds. Biosens. Bioelectron. 2023, 242, 115743. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, J.; Li, H.; Wu, J.; Wan, Q.; Chen, T.; Liu, W.; Peng, H.; Zhang, H.; Luo, Y. Smart Hydrogel Sensors for Health Monitoring and Early Warning. Adv. Sens. Res. 2024, 3, 2400003. [Google Scholar] [CrossRef]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1700718. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-Based Sensor Networks: Compositions, Properties, and Applications—A Review. ACS Appl. Bio Mater. 2021, 4, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Achavananthadith, S.; Lian, S.; Edward Madden, L.; Ong, Z.X.; Chua, W.; Kalidasan, V.; Li, Z.; Liu, Z.; Singh, P.; et al. A wireless and battery-free wound infection sensor based on DNA hydrogel. Sci. Adv. 2021, 7, eabj1617. [Google Scholar] [CrossRef]
- Pang, Q.; Lou, D.; Li, S.; Wang, G.; Qiao, B.; Dong, S.; Ma, L.; Gao, C.; Wu, Z. Smart Flexible Electronics-Integrated Wound Dressing for Real-Time Monitoring and On-Demand Treatment of Infected Wounds. Adv. Sci. 2020, 7, 1902673. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Tong, Y.; Zhang, S.; He, R.; Xiao, L.; Iqbal, Z.; Zhang, Y.; Gao, J.; Zhang, L.; Jiang, L.; et al. Flexible Bicolorimetric Polyacrylamide/Chitosan Hydrogels for Smart Real-Time Monitoring and Promotion of Wound Healing. Adv. Funct. Mater. 2021, 31, 2102599. [Google Scholar] [CrossRef]
- Chen, F.; Wu, M.; Dong, Q.; Ke, M.; Liang, X.; Ai, J.; Cheng, Q.; Cai, L.; Tong, Z.; Chen, Y. Arbitrarily shapeable and conductive hydrogel with “Magic Cube” like structure for real-time monitoring and promoting wound healing. Compos. Part B Eng. 2022, 238, 109903. [Google Scholar] [CrossRef]
- Swisher, S.L.; Lin, M.C.; Liao, A.; Leeflang, E.J.; Khan, Y.; Pavinatto, F.J.; Mann, K.; Naujokas, A.; Young, D.; Roy, S.; et al. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat. Commun. 2015, 6, 6575. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shirzaei Sani, E.; Gao, W. Wearable Bioelectronics for Chronic Wound Management. Adv. Funct. Mater. 2022, 32, 2111022. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.; Rahimi, R.; Zhou, J.; Jiang, H.; Yoon, C.K.; Maddipatla, D.; Narakathu, B.B.; Jain, V.; Oscai, M.M.; Morken, T.J.; et al. Integrated sensing and delivery of oxygen for next-generation smart wound dressings. Microsyst. Nanoeng. 2020, 6, 46. [Google Scholar] [CrossRef]
- Wu, Q.; Qiao, Y.; Guo, R.; Naveed, S.; Hirtz, T.; Li, X.; Fu, Y.; Wei, Y.; Deng, G.; Yang, Y.; et al. Triode-Mimicking Graphene Pressure Sensor with Positive Resistance Variation for Physiology and Motion Monitoring. ACS Nano 2020, 14, 10104–10114. [Google Scholar] [CrossRef]
- Abbott, C.A.; Chatwin, K.E.; Foden, P.; Hasan, A.N.; Sange, C.; Rajbhandari, S.M.; Reddy, P.N.; Vileikyte, L.; Bowling, F.L.; Boulton, A.J.M.; et al. Innovative intelligent insole system reduces diabetic foot ulcer recurrence at plantar sites: A prospective, randomised, proof-of-concept study. Lancet Digit. Health 2019, 1, e308–e318. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Healthc. Mater. 2020, 9, 1901648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Niu, C.; Zhang, L.; Li, G.; Yang, Y. Construction of polyacrylamide/graphene oxide/gelatin/sodium alginate composite hydrogel with bioactivity for promoting Schwann cells growth. J. Biomed. Mater. Res. Part A 2018, 106, 1951–1964. [Google Scholar] [CrossRef]
- Vieira de Souza, T.; Malmonge, S.M.; Santos, A.R. Development of a chitosan and hyaluronic acid hydrogel with potential for bioprinting utilization: A preliminary study. J. Biomater. Appl. 2021, 36, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Gensini, L.; Serna, J.A.; Cifuentes, J.; Cruz, J.C.; Muñoz-Camargo, C. Graphene Oxide-Embedded Extracellular Matrix-Derived Hydrogel as a Multiresponsive Platform for 3D Bioprinting Applications. Int. J. Bioprinting 2021, 7, 353. [Google Scholar] [CrossRef]
- Placone, J.K.; Navarro, J.; Laslo, G.W.; Lerman, M.J.; Gabard, A.R.; Herendeen, G.J.; Falco, E.E.; Tomblyn, S.; Burnett, L.; Fisher, J.P. Development and Characterization of a 3D Printed, Keratin-Based Hydrogel. Ann. Biomed. Eng. 2017, 45, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Pasini, C.; Pandini, S.; Ramorino, G.; Sartore, L. Tailoring the properties of composite scaffolds with a 3D-Printed lattice core and a bioactive hydrogel shell for tissue engineering. J. Mech. Behav. Biomed. Mater. 2024, 150, 106305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ciftci, S.; Barthes, J.; Knopf-Marques, H.; Muller, C.B.; Debry, C.; Vrana, N.E.; Ghaemmaghami, A.M. A composite Gelatin/hyaluronic acid hydrogel as an ECM mimic for developing mesenchymal stem cell-derived epithelial tissue patches. J. Tissue Eng. Regen. Med. 2020, 14, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Diba, M.; Yang, S.; Freedman, B.R.; Elosegui-Artola, A.; Mooney, D.J. Hydrogel viscoelasticity modulates migration and fusion of mesenchymal stem cell spheroids. Bioeng. Transl. Med. 2023, 8, e10464. [Google Scholar] [CrossRef] [PubMed]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Chong, C.; Yap, R.; Tay, K.; Hang, T.; Edwin, T.H.T.; Olivier, A.A.; Yap, C.C.R.; Tay, B.K.; et al. Mechanically strengthened hybrid peptide-polyester hydrogel and potential application in spinal cord injury repair. Mater. Today Proc. 2019, 22, 16–20. [Google Scholar]
- Ding, L.; He, L.; Wang, Y.; Zhao, X.; Ma, H.; Luo, Y.; Guan, F.; Xiong, Y. Research progress and challenges of composite wound dressings containing plant extracts. Cellulose 2023, 30, 11297–11322. [Google Scholar] [CrossRef]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings. Mini-Reviews Med. Chem. 2017, 18, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomed. 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Sachdeo, R.A.; Khanwelkar, C.; Shete, A. 3D Printing in Wound Healing: Innovations, Applications, and Future Directions. Cureus 2024, 16, e75331. [Google Scholar] [CrossRef]
- Guptha, P.M.; Kanoujia, J.; Kishore, A.; Raina, N.; Wahi, A.; Gupta, P.K.; Gupta, M. A comprehensive review of the application of 3D-bioprinting in chronic wound management. Expert Opin. Drug Deliv. 2024, 21, 1573–1594. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.T.; Liang, K.; Ngo, Z.H.; Dube, C.T.; Lim, C.Y. Application of 3d bioprinting technologies to the management and treatment of diabetic foot ulcers. Biomedicines 2020, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Bajuri, M.Y.; Kim, J.; Yu, Y.; Shahul Hameed, M.S. New Paradigm in Diabetic Foot Ulcer Grafting Techniques Using 3D-Bioprinted Autologous Minimally Manipulated Homologous Adipose Tissue (3D-AMHAT) with Fibrin Gel Acting as a Biodegradable Scaffold. Gels 2023, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Bita, B. Exploring the Potential of Artificial Intelligence for Hydrogel Development—A Short Review. Gels 2023, 9, 845. [Google Scholar] [CrossRef]
- Wang, Y.; Wallmersperger, T.; Ehrenhofer, A. Application of back propagation neural networks and random forest algorithms in material research of hydrogels. Pamm 2023, 23, e202200278. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Boztepe, C.; Künkül, A.; Yüceer, M. Application of artificial intelligence in modeling of the doxorubicin release behavior of pH and temperature responsive poly(NIPAAm-co-AAc)-PEG IPN hydrogel. J. Drug Deliv. Sci. Technol. 2020, 57, 101603. [Google Scholar] [CrossRef]


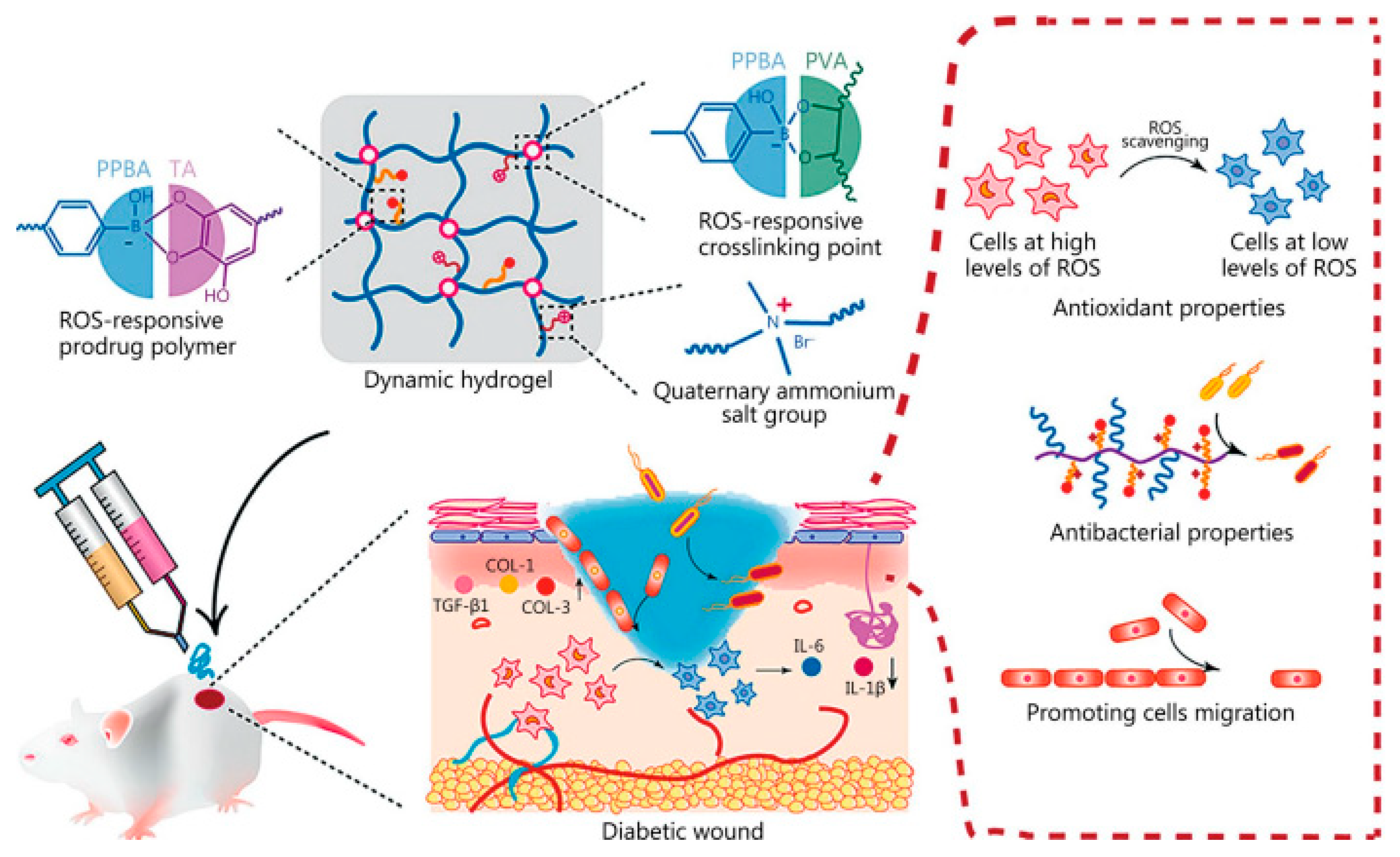



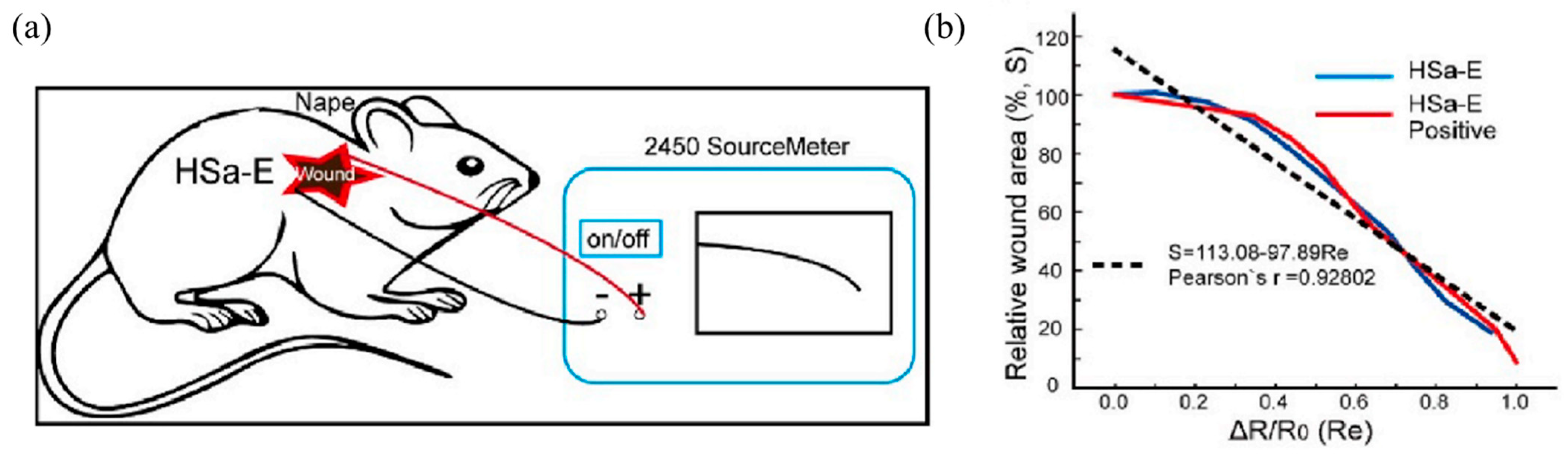
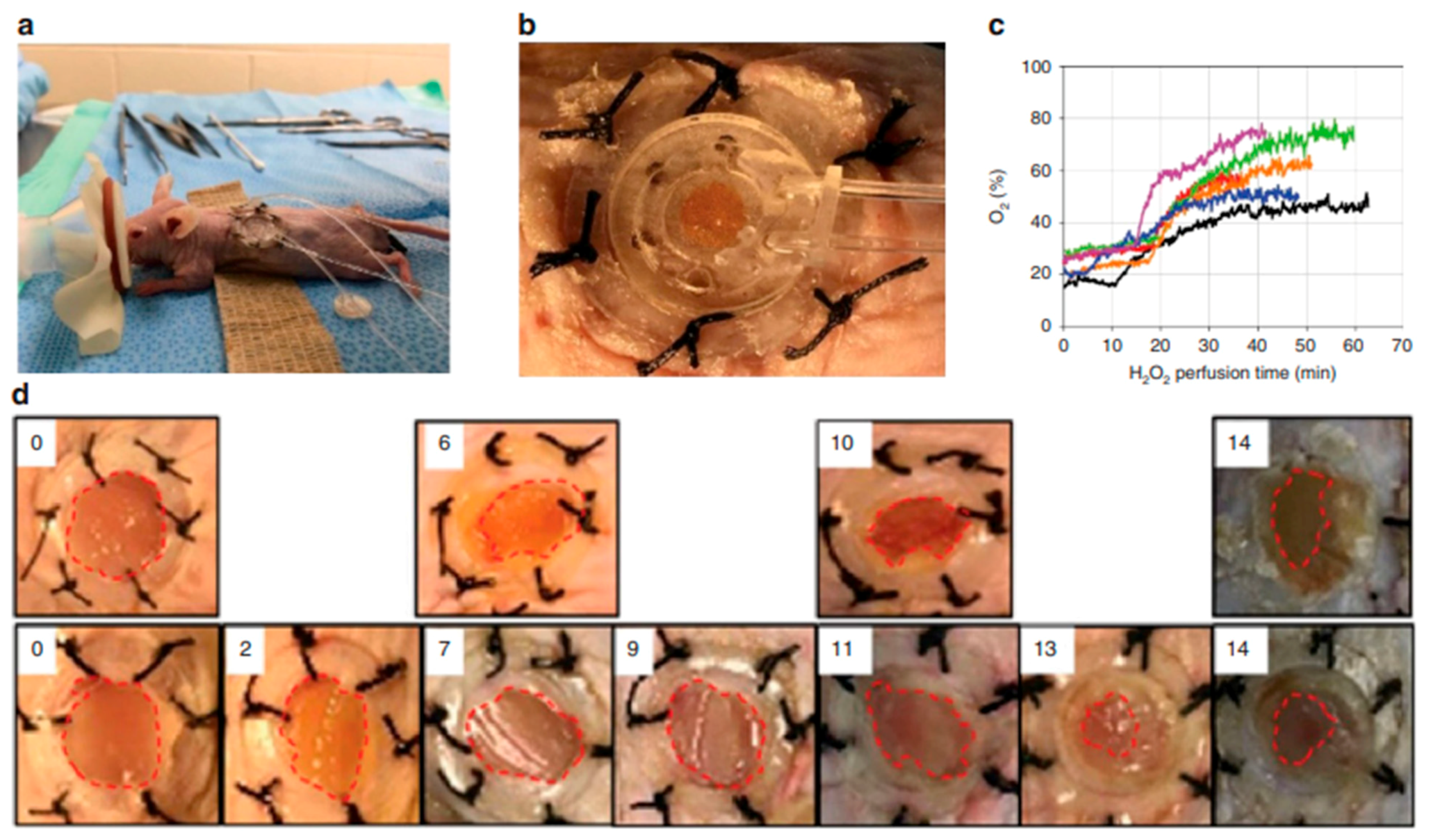
| Type of Hydrogel | Biocompatibility | Cost | Effectiveness | Advantages | Disadvantages/Limitations | Refs. |
|---|---|---|---|---|---|---|
| Natural hydrogels | High | Low | Excellent for wound healing and tissue engineering. | High biocompatibility and biodegradability. Mimics natural extracellular matrix. Support cell adhesion and growth. Renewable and environmentally friendly. Intrinsic bioactivity (cell signaling, antimicrobial properties in some cases). Easy to crosslink with mild conditions (ionic or thermal processes) | Low mechanical strength and elasticity. Limited control over degradation rate. Batch-to-batch fluctuations brought on by natural sources. Susceptible to microbial contamination or degradation without proper treatment. Short shelf-life. Could require chemical modifications to enhance stability and performance. | [58,59,60,61,62,63,64] |
| Synthetic hydrogels | Moderate | Moderate | High mechanical stability and tunable properties. Less bioactive without modification. | Stable and reproducible. Versatile in drug delivery and tissue regeneration. Easy to tailor physical and chemical properties. Minimal degradation. Can be modified for controlled release or specific bioactivity. | Limited bioactivity without modification. Potential cytotoxicity or inflammatory response depending on the polymer type. Some polymers are non-biodegradable (PHEMA). Can require chemical crosslinking, which can be toxic. Hydrophobicity involves surface modification to improve interactions with biological systems. | [56,65,66,67,68,69] |
| Hybrid hydrogels | High | High | Combines biocompatibility, bioactivity, and mechanical strength from natural and synthetic hydrogels. Suitable for advanced biomedical applications (drug delivery, regenerative medicine, and tissue scaffolds). | Customizable properties to suit specific applications. Can achieve controlled degradation and release rates. Suitable for multifunctional uses, including 3D bioprinting and personalized medicine. | High manufacturing complexity and cost. Requires advanced synthesis technologies. Potential compatibility issues between natural and synthetic components. Limited scalability due to sophisticated production methods. Can require modification strategies in order to enhance biocompatibility. | [70,71,72,73,74,75] |
| Smart hydrogels | High | High | Highly effective in controlled drug delivery and tissue engineering due to their responsiveness to stimuli. Effectiveness varies based on the type of stimuli (pH, temperature, light, ROS, etc.) | Responds to specific stimuli, enabling precise control over drug release, swelling, or structural changes. Can adapt dynamically to changing environment (i.e., temperature-sensitive for wound healing). Enable innovation in targeted therapies and personalized medicine. | Limited scalability for industrial or large-scale production. Requires specialized materials and techniques. Stability and performance can degrade over repeated stimuli cycles. Modeling in vivo release profiles is necessary before commercialization. | [76,77,78,79,80,81] |
| Ionic hydrogels | Moderate | Low | Efficient for tissue repair, drug delivery, and wound healing due to ionic interactions that enhance biocompatibility and adhesion. | Excellent biocompatibility with biological systems due to ionic interactions. Suitable for wound healing and tissue repair applications, providing good adhesion. They can self-heal and reassemble under certain conditions, making them reusable in some cases. | Sensitive to ionic strength and pH changes in the environment, which can destabilize their performance. Limited long-term stability, especially in dynamic biological environments. May request reinforcement to improve mechanical strength. | [82,83,84,85,86] |
| Hydrogel Material | Additional Bioactive Components | Testing Stage | Experimental Results | Ref. |
|---|---|---|---|---|
| Collagen hydrogel | Hydroxypropyl methylcellulose (HPMC) and Polyvinyl alcohol (PVA) | In vivo (rats) | The experimental group had a larger healing area than the positive control group on days 14 and 21. In diabetic rats, collagen gel dressing can accelerate and improve the quality of full-thickness wound healing. | [100] |
| Poly(polyethylene glycol citrate co-N-isopropylacrylamide) (PPCN) hydrogel | Stromal cell-derived factor-1 (SDF-1) | In vitro and in vivo (mice) | DFU wounds treated with PPCN + SDF-1 showed faster healing (24 days), increased granulation tissue development, epithelial maturation, and the most perfused blood vessels. | [108] |
| Multi-arm thiolated polyethylene glycol (SH-PEG) with silver nitrate (AgNO3) hydrogel (Ag-SH-PEG hydrogel) | Desferrioxamine (DFO) | In vitro and in vivo (mice) | The hydrogel effectively treated diabetic skin lesions with minimal bacterial infection and increased angiogenic activity. | [103] |
| N-isopropylacrylamide (NIPAM)-based, thermosensitive hydrogel | Bone marrow mesenchymal stem cells (BMSCs) | In vitro and in vivo (mice) | On day 7, the hydrogel-loaded MSC group’s average unhealed area in type II diabetic mice was 24.6 ± 4.21%, much less than the mean of the untreated control group (79.54 ± 5.92%), suggesting that hydrogel stimulated angiogenesis, granulation tissue creation, re-epithelialization, and even hair follicle and sebaceous gland regeneration. | [109] |
| Gellan gum-PEG-chitosan hydrogel (GGCH-HGs) | Apigenin (APN) | In vivo (mice) | APN-loaded GGCH-HGs had a strong antioxidant impact and a greater wound-healing effect in both diabetic and healthy wound tissues. | [110] |
| Alginate (Alg) hydrogel | human umbilical cord-derived outgrowth endothelial cells (OECs), substance P and neurotensin | In vitro and in vivo (mice) | The hydrogel accelerated wound closure when compared to alginate gel alone, and the combination of OEC and SP proved to be the most effective. | [101] |
| Quaternized chitosan (QCS)-oxidized starch (OST) hydrogel | Exosomes | In vivo (mice) | Nucleus pulposus (NP) cell senescence was rejuvenated by QCS-OST/Exos hydrogel, encouraging extracellular matrix (ECM) remodeling and partially restoring the NP and annulus fibrosis structures. | [102] |
| Hydrogel made of hyperbranched multi-acrylated poly(ethylene glycol) macromers (HP-PEGs) and thiolated hyaluronic acid (HA-SH) | Adipose-derived stem cells (ADSCs) | In vitro and in vivo (subcutaneous implantation and mice) | This adaptable hydrogel system with ADSCs demonstrated an accelerated diabetic wound healing process by reducing inflammation and encouraging angiogenesis and re-epithelialization. | [104] |
| PVA-Alg hydrogel (H) | Green tea polyphenol nanospheres (TPN) | In vitro and in vivo (mice) | TPN@H promotes wound healing and regulates immune response by controlling the PI3K/AKT signaling pathway. | [105] |
| Poly(vinyl pyrrolidone) (PVP)/Alginate/Chitosan hydrogel | Silver nanoparticles | In vitro | The results demonstrate that the 10 mM AgNP-based hydrogel has the best antibacterial properties while maintaining non-cytotoxicity, confirming its suitability for use in pressure ulcer therapy cases. | [106] |
| Hydrogel-Modifying Substance | Main Characteristics of Modified Matrices | Refs. |
|---|---|---|
| Polyphenols |
| [148,149,150] |
| Chitosan |
| [151,152] |
| Peptides, polypeptides, proteins, and amino acids |
| [153,154] |
| Polysaccharides |
| [155,156] |
| Metal oxides |
| [157,158] |
| Silver nanoparticles |
| [159,160] |
| Synthetic polymer materials |
| [161,162] |
| Antibiotics |
| [163,164] |
| Therapeutic Use | Hydrogel | Target Bacteria | Phages | Testing Level | Findings | Refs. |
|---|---|---|---|---|---|---|
| Treating wounds related to burn injuries | HPMC hydrogel | K. pneumoniae | Kpn5 | In vivo (mice) | The greatest survival rate in contrast to gentamicin and silver nitrate after 7 days. | [168] |
| PVA-SA hydrogel | S. aureus P. aeruginosa K. pneumoniae | MR10 PA5 Kpn5 | In vitro and in vivo (mice) | Demonstrated a decrease in inflammation with wound contraction and a significant reduction (>1 log reduction) in resistant burn wound infection. | [167] | |
| Treatment for skin infections | Agarose-HAMA hydrogel | S. aureus | Phage K | In vitro | Hyaluronidase release of phage K damages the HAMA layer and inhibits bacterium development. | [165] |
| HA-PEG hydrogel | P. aeruginosa | PAML-31-1 LPS-5 Luz24 | In vitro and in vivo (mice) | Hydrogel-based sustained phage administration provides a useful, well-tolerated alternative for topical treatment while improving phage therapy’s effectiveness. | [169] | |
| PVA hydrogel | E. coli | UFV-AREG1 | In vitro | Compared to the PVA control, the PVA-phage inhibition zone was more significant (p < 0.05), suggesting that it can be used for the treatment of skin infections. | [170] | |
| Treatment for skin and/or soft tissue infection | PNIPAM-co-ALA hydrogel | S. aureus | Phage K | In vitro | At 37 °C, PNIPAM-co-ALA nanogels coupled to phage K demonstrated thermally induced bacterial lysis of S. aureus. | [166] |
| Sodium alginate (SA)-Carboxymethyl cellulose (CMC) -Hyaluronic acid (HA) hydrogel | E. faecium | EF-M80 | In vivo (mice) | It has been shown that the EF-M80 phage maintained its antibacterial qualities, lysing E. faecium efficiently in the host environment. Its ability to promote wound healing was further increased by encapsulating it in a hydrogel delivery method. | [171] | |
| Alginate hydrogel | S. aureus | Genetically modified phage | In vitro and in vivo (rat) | Significantly reduced soft tissue infection (>0.5 log reduction). | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. New Perspectives of Hydrogels in Chronic Wound Management. Molecules 2025, 30, 686. https://doi.org/10.3390/molecules30030686
Alberts A, Bratu AG, Niculescu A-G, Grumezescu AM. New Perspectives of Hydrogels in Chronic Wound Management. Molecules. 2025; 30(3):686. https://doi.org/10.3390/molecules30030686
Chicago/Turabian StyleAlberts, Adina, Andreea Gabriela Bratu, Adelina-Gabriela Niculescu, and Alexandru Mihai Grumezescu. 2025. "New Perspectives of Hydrogels in Chronic Wound Management" Molecules 30, no. 3: 686. https://doi.org/10.3390/molecules30030686
APA StyleAlberts, A., Bratu, A. G., Niculescu, A.-G., & Grumezescu, A. M. (2025). New Perspectives of Hydrogels in Chronic Wound Management. Molecules, 30(3), 686. https://doi.org/10.3390/molecules30030686









