Towards Improved Bioavailability of Cereal Inositol Phosphates, Myo-Inositol and Phenolic Acids
Abstract
1. Introduction
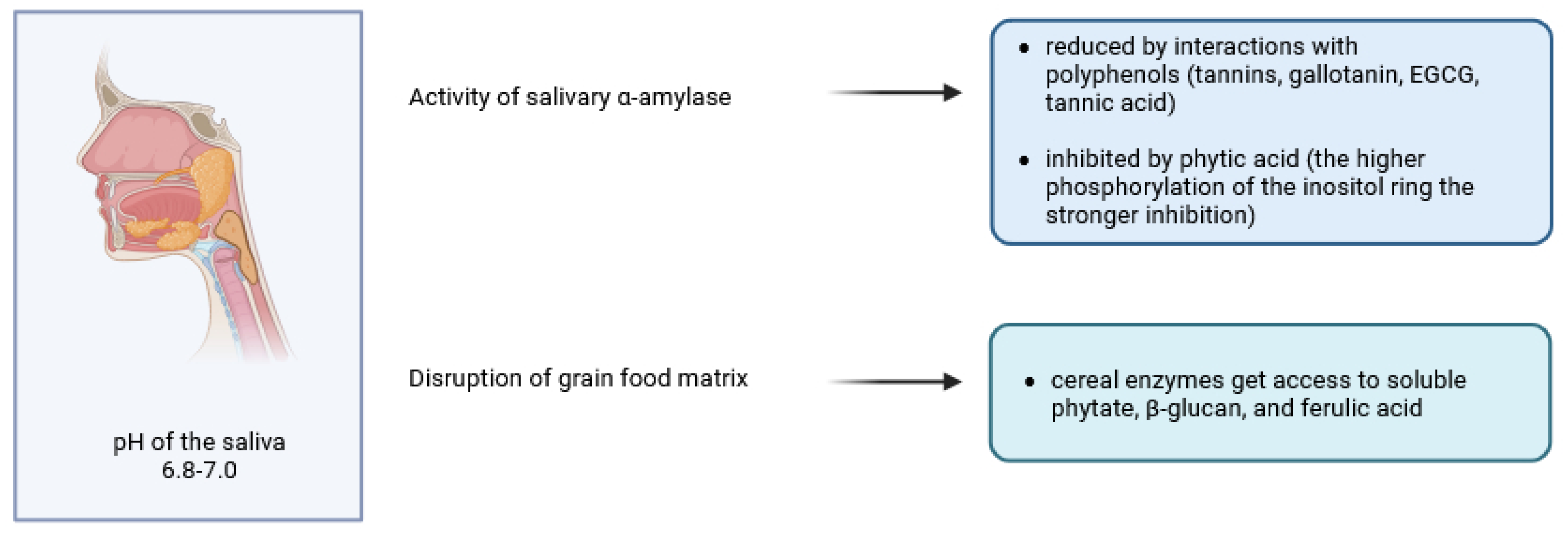
2. The Breakdown of Cereal Phytates and Polyphenols in Oral Cavity
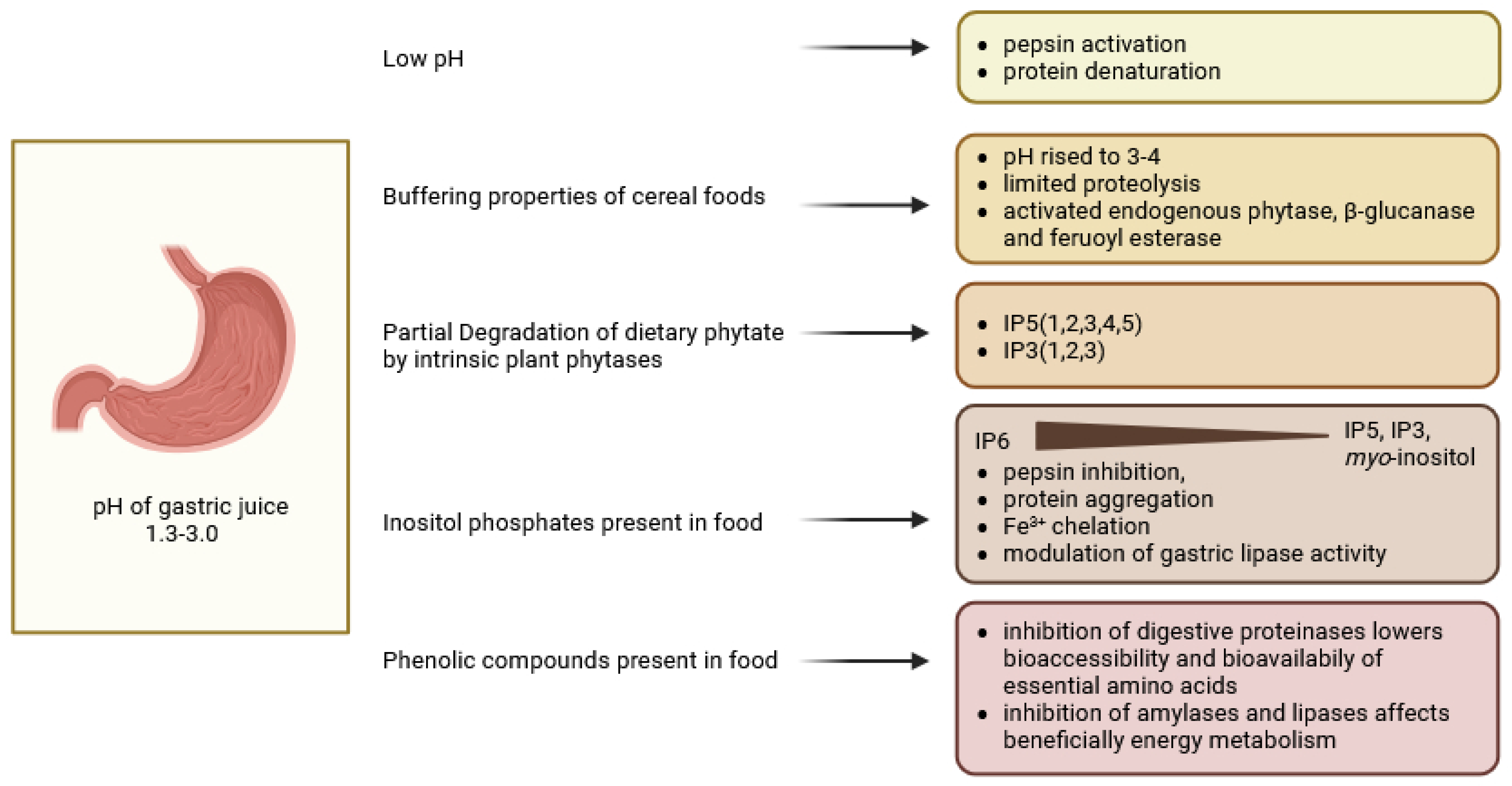
3. Digestion in the Stomach
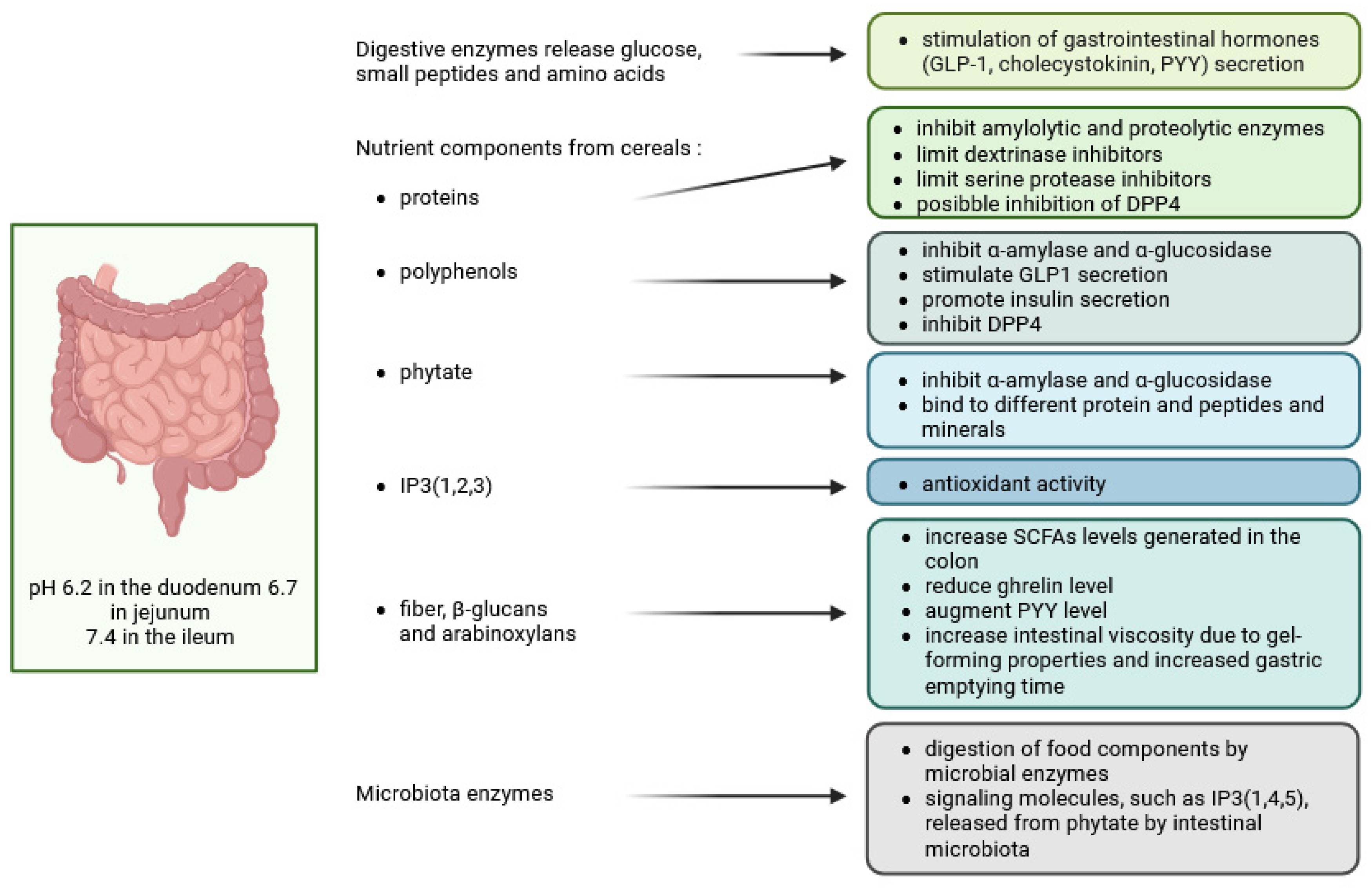
4. Digestion in the Small Intestine
4.1. Regulation of Gut Hormones—Incretins (GIP, GLP-1) by Phenols and Inositol Phosphates and Inhibitory Effect of These Compounds on α-Amylase and α-Glucosidase Activities
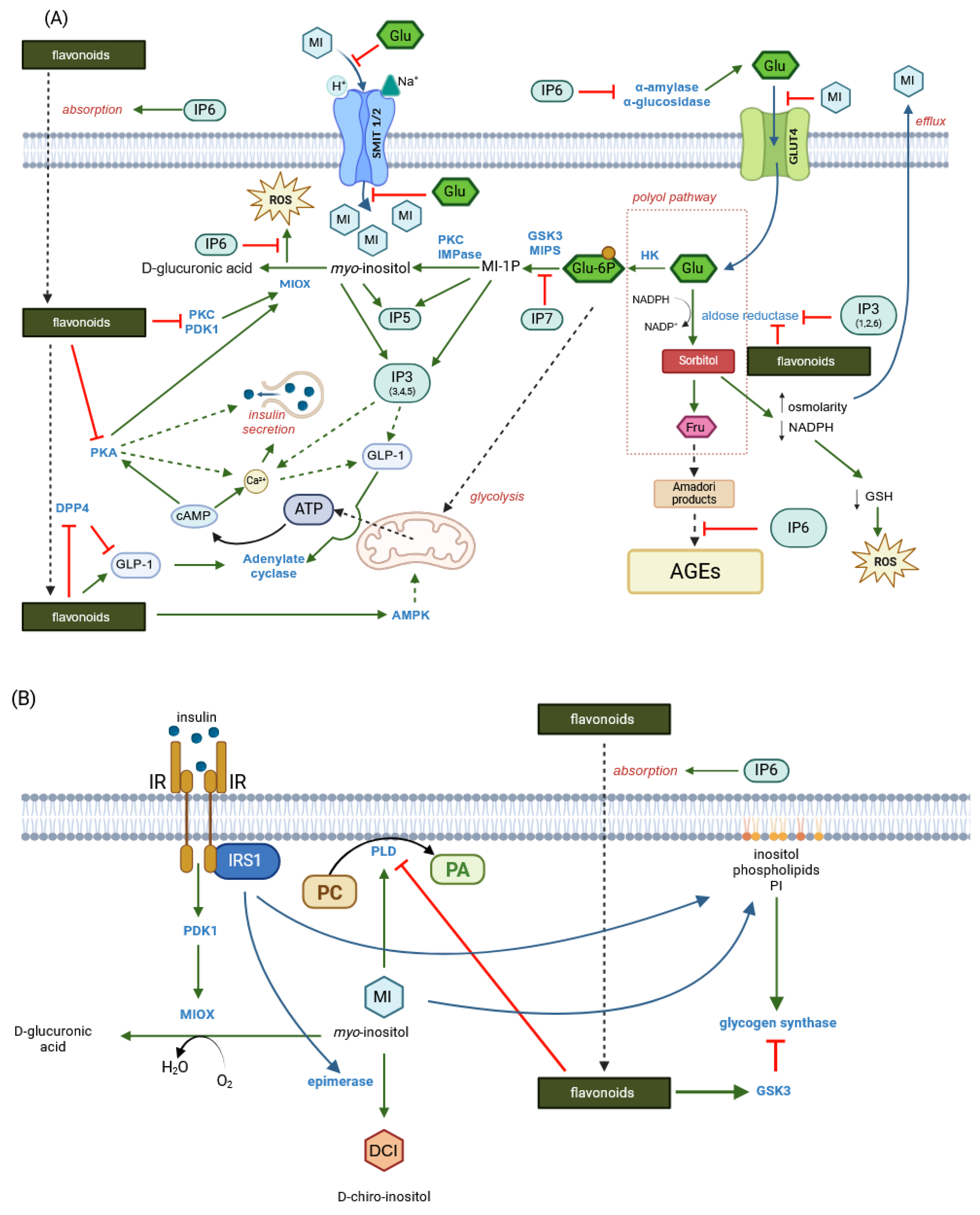
4.2. Intestinal Absorption Phenomena and Regulation of Tight Junction Transport—Two Mechanisms: Tight Junction Modulation and P-Glycoprotein Inhibition
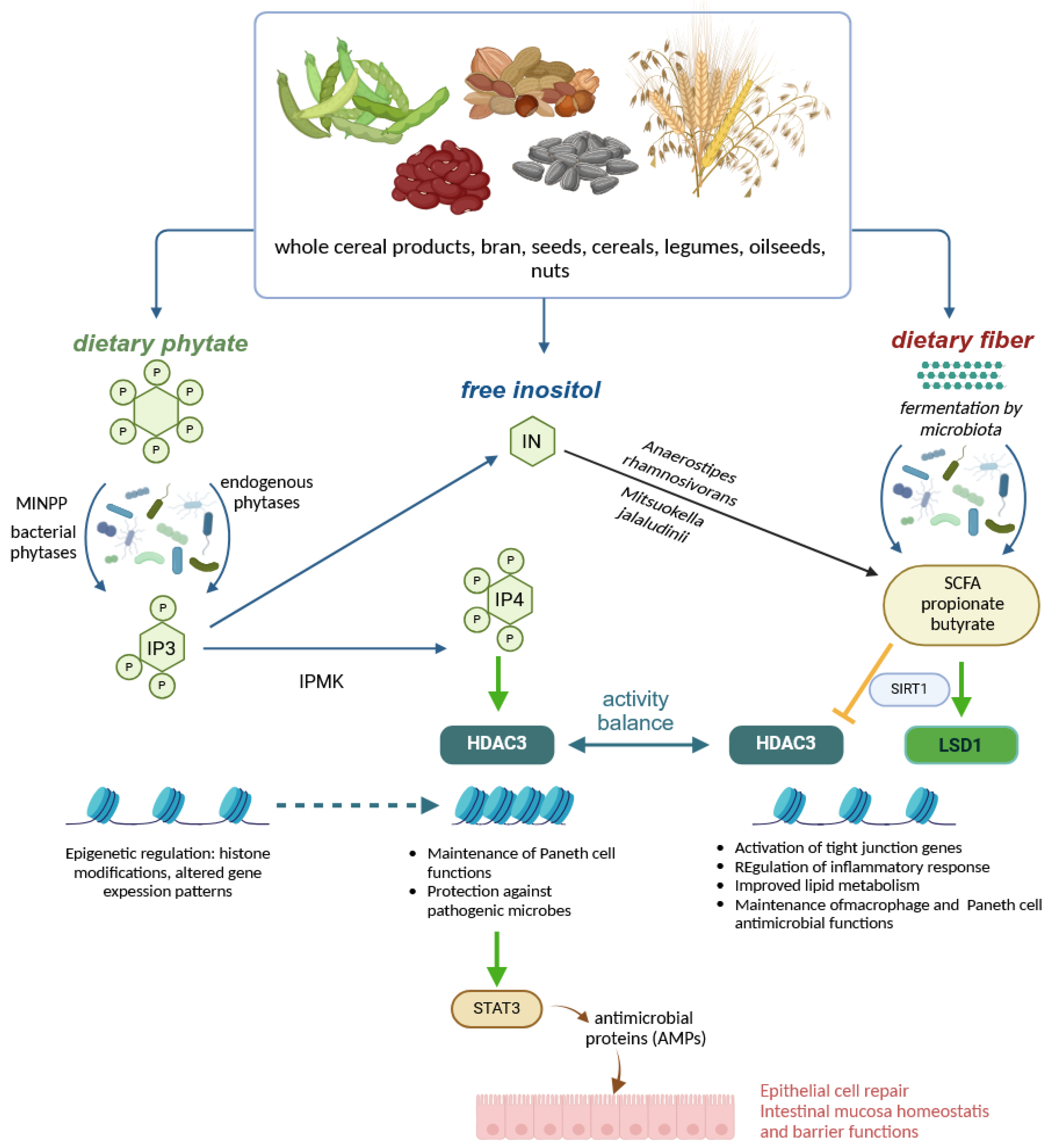
5. Colonic Fermentations, Regulation, Breakdown of Polyphenols and Inositol Phosphates
6. Myo-Inositol
7. Conclusions
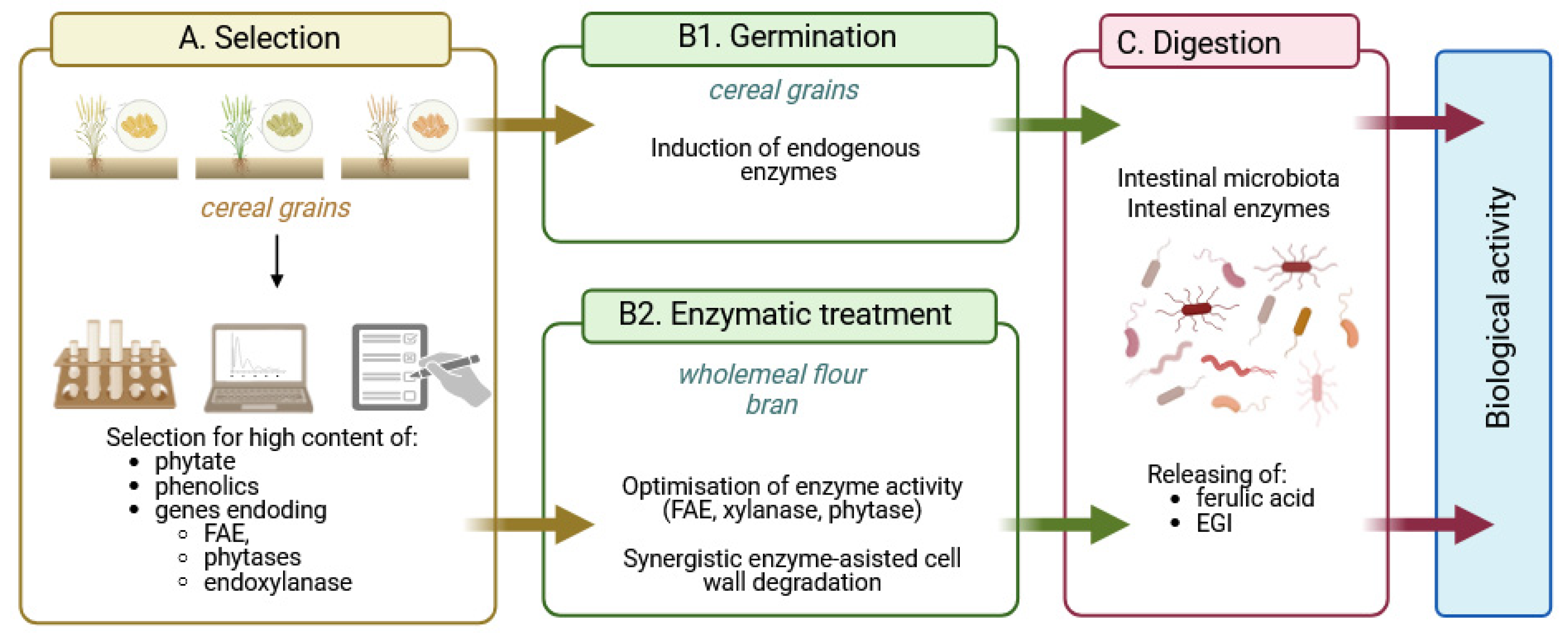
- Current knowledge provides strong evidence for many possible synergistic effects between phytic acid, myo-inositol phosphates, free myo-inositol and ferulic acid. For example, both IP6 and FA exert potent and complementary antioxidant effects in the intestinal tract, maintaining cell barrier integrity and epithelial antibacterial immunity. IP6 enhances the absorption of nutrients, including phenolics, by regulating tight junctions and may act as a non-competitive P-glycoprotein inhibitor, while ferulic acid has been shown to reverse P-glycoprotein-mediated resistance and increase nutrient bioavailability. Ferulic acid, together with various myo-inositol phosphates resulting from increased hydrolysis of IP6 by endogenous and microbial phytases, would affect pepsin and gastric lipase activity and then incretin secretion, affecting insulin and glucagon release. Ferulic acid was found to bind to the GLP-1 molecule in a 1:1 ratio, effectively preventing hydrolysis of the hormone by DPP4, while IP3, a product of IP6 hydrolysis, may promote GLP-1 synthesis. Inositol phosphates, especially IP3(1,2,6), together with free myo-inositol generated by prolonged hydrolysis of phytate, would inhibit the aldose reductase enzyme and thus prevent various diabetic complications.
- The bran fraction of wheat, maize, brown rice and other cereals contains not only high levels of phytate, free and total phenolics, but also endogenous enzymes such as amylases, phytase, xylanase, β-glucanase and feruloyl esterase, whose activities can be increased by germination. In addition, cereals in the form of wholemeal flour or bran can be subjected to enzymolysis by exogenous enzyme preparations to achieve advanced hydrolysis of cereal phytate and phenolic compounds, further enhanced by biotransformations from gut microbiota.
- Here, we have proposed a novel strategy of selecting cereals with high phytate, phenolic and endogenous phytase, ferulic esterase and endoxylanase activities as a starting point for strategies to produce value-added health-promoting foods. The key assumption of the strategy is the advanced hydrolysis of phytate and phenolic compounds by cereal and/or microbial enzymes, which would generate substantial amounts of “enzymatically generated inositol” (EGI), which includes phytic acid, myo-inositol phosphates and myo-inositol, the compounds that, together with free ferulic acid, provide enhanced bioavailability of cereal nutrients through multiple synergistic effects.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Skeie, G.; Fadnes, L.T. Cereals and cereal products—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2024, 68, 10457. [Google Scholar] [CrossRef]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, W.; Xu, O.; Reddy, M.B. Neuroprotection of phytic acid in Parkinson’s and Alzheimer’s disease. J. Funct. Food. 2023, 110, 105856. [Google Scholar] [CrossRef]
- Bloot, A.P.M.; Kalschne, D.L.; Amaral, J.A.S.; Baraldi, I.J.; Canan, C. A review of phytic acid sources, obtention, and applications. Food Rev. Int. 2021, 39, 73–92. [Google Scholar] [CrossRef]
- Chen, W.; Xu, D. Phytic acid and its interactions in food components, health benefits, and applications: A comprehensive review. Trends Food Sci. Technol. 2023, 141, 104201. [Google Scholar] [CrossRef]
- Tian, S.; Sun, Y.; Chen, Z.; Yang, Y.; Wang, Y. Functional properties of polyphenols in grains and effects of physicochemical processing on polyphenols. J. Food Qual. 2019, 2019, 2793973. [Google Scholar] [CrossRef]
- Ed Nignpense, B.; Latif, S.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and Antioxidant Activity of Polyphenols from Pigmented Barley and Wheat. Foods 2022, 11, 3697. [Google Scholar] [CrossRef] [PubMed]
- López-Perea, P.; Gunzmán-Ortiz, F.A.; Román-Gutiérrez, A.D.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Rodríguez-Marín, M.L.; Falfán-Cortés, R.N.; Gonzáles-Olivares, L.G.; Torruco-Uco, J.G. Bioactive compounds and antioxidative activity of wheat bran and barley husk in the extracts with different polarity. Int. J. Food Prop. 2019, 22, 646–658. [Google Scholar] [CrossRef]
- Tiozon, R.J.N.; Sartagoda, K.J.D.; Serrano, L.M.N.; Fernie, A.R.; Sreenivasulu, N. Metabolomics based inferences to unravel phenolic compound diversity in cereals and its implications for human gut health. Trends Food Sci. Technol. 2022, 127, 14–25. [Google Scholar] [CrossRef]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiasvaroli, L.; Bighenti, F.; Del Rio, D.; Martini, D. Bioaccessibility and bioavailability of phenolic compounds in bread: A review. Food Funct. 2017, 8, 2368–2393. [Google Scholar] [CrossRef]
- Longin, C.F.H.; Afzal, M.; Pfannstiel, J.; Bertsche, U.; Melzer, T.; Ruf, A.; Heger, C.; Pfaff, T.; Schollenberger, M.; Rodehutscord, M. Mineral and Phytic Acid Content as Well as Phytase Activity in Flours and Breads Made from Different Wheat Species. Int. J. Mol. Sci. 2023, 24, 2770. [Google Scholar] [CrossRef]
- Brouns, F. Phytic Acid and Whole Grains for Health Controversy. Nutrients 2022, 14, 25. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, V.K.; Sultan, Z.; Singh, R.; Dar, A.H. Classification, benefits, and application of various anti-nutritional factors present in edible crops. J. Agric. Food Res. 2023, 14, 100902. [Google Scholar] [CrossRef]
- Abdulwaliyu, I.; Arekemase, S.O.; Adudu, J.A.; Batari, M.L.; Egbule, M.N.; Okoduwa, S.I.R. Investigations of the medical significance of phytic acid as an indispensable antinutrient in diseases. Clin. Nutr. Exp. 2019, 28, 42–61. [Google Scholar] [CrossRef]
- Vucenik, I. Anticancer properties of inositol hexaphosphate and inositol: An overview. J. Nutr. Sci. Vitaminol. 2019, 65, S18–S22. [Google Scholar] [CrossRef]
- Brehm, M.A.; Windhorst, S. New options of cancer treatment employing InsP6. Biochem. Pharmacol. 2019, 163, 206–214. [Google Scholar] [CrossRef]
- Khurana, S.; Balseo, C.; Joseph, R.W. Inositol hexaphosphate plus inositol induced complete remission in stage IV melanoma: A case report. Melanoma Res. 2019, 29, 322–324. [Google Scholar] [CrossRef]
- Vucenik, I.; Druzijanic, A.; Druzijanic, N. Inositol Hexaphosphate (IP6) and Colon Cancer: From Concepts and First Experiments to Clinical Application. Molecules 2020, 25, 5931. [Google Scholar] [CrossRef] [PubMed]
- Matsuoto, H.; Ito, K.; Yonekura, K.; Tsuda, T.; Ichiyanagi, T.; Hirayama, M.; Konishi, T. Enhanced absorption of anthocyanins after oral administration of phytic acid in rats and humans. J. Agric. Food Chem. 2007, 55, 2489–2496. [Google Scholar] [CrossRef]
- Xie, Y.; Luo, H.; Duan, J.; Hong, C.; Ma, P.; Li, G.; Zhang, T.; Wu, T.; Ji, G. Phytic acid enhances the oral absorption of isorhamnetin, quercetin, and kaempferol in total flavones of Hippophae rhamnoides L. Fitoterapia 2014, 93, 216–225. [Google Scholar] [CrossRef]
- Pei, Y.; Ai, T.; Deng, Z.; Wu, D.; Liang, H.; McClements, D.; Li, B. Impact of plant extract on the gastrointestinal fate of nutraceutical-loaded nanoemulsions: Phytic acid inhibits lipid digestion but enhances curcumin bioaccessibility. Food Funct. 2019, 10, 3344–3355. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, H.; Xia, M.; Deng, B.; Shen, H.; Ji, G.; Li, G.; Xie, Y. The effect of phytic acid on tight junctions in the human intestinal Caco-2 cell line and its mechanism. Eur. J. Pharm. Sci. 2015, 80, 1–8. [Google Scholar] [CrossRef]
- Yang, G.; Bibi, S.; Du, M.; Suzuli, T.; Zhu, M.-J. Regulation of the intestinal tight junctions by natural polyphenols: A mechanistic perspective. Crit. Rev. Food Sci. Nutr. 2016, 57, 3830–3839. [Google Scholar] [CrossRef]
- Panwar, S.; Sharma, S.; Tripathi, P. Role of barrier integrity and dysfunctions in maintaining the healthy gut and their health outcomes. Front. Physiol. 2021, 12, 715611. [Google Scholar] [CrossRef]
- Hashimoto-Hill, S.; Calapietro, L.; Woo, V.; Antonacci, S.; Whitt, J.; Engleman, L.; Alenghat, T. Dietary phytate primes epithelial antibacterial immunity in the intestine. Front. Immunol. 2022, 13, 952994. [Google Scholar] [CrossRef]
- Drucker, D.J.; Holst, J.J. The expanding incretin universe; from basic biology to clinical translation. Diabetologia 2023, 66, 1765–1779. [Google Scholar] [CrossRef]
- Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; Feo, V.D.; Zia-Ul-Haq, M. Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition. Molecules 2017, 22, 871. [Google Scholar] [CrossRef]
- Li, L.; Fu, Q.; Xia, M.; Xin, L.; Shen, H.; Li, G.; Ji, G.; Meng, Q.; Xie, Y. Inhibition of P-glycoprotein mediated efflux in Caco-2 by phytic acid. J. Agric. Food Chem. 2018, 66, 988–998. [Google Scholar] [CrossRef]
- Mosca, A.C.; Chen, J. Food-saliva interactions: Mechanisms and implications. Trends Food Sci. Technol. 2017, 66, 125–134. [Google Scholar] [CrossRef]
- Çelebioğlu, H.Y.; Lee, S.; Chronakis, J.S. Interactions of salivary mucins with food proteins: A review. Crit. Rev. Food Sci. Technol. 2020, 6091, 64–83. [Google Scholar] [CrossRef]
- Peyrot des Ganchos, C.; Breslin, P.A.S. Salivary amylase: Digestion and metabolic syndrome. Curr. Diab. Rep. 2016, 16, 102. [Google Scholar] [CrossRef]
- Morzel, M.; Canon, F.; Guyot, S. Interactions between salivary proteins and dietary polyphenols: Potential consequences on gastrointestinal digestive events. J. Agric. Food Chem. 2022, 70, 6317–6327. [Google Scholar] [CrossRef]
- Nyambe-Silavwe, H.; Williamson, G. Chlorogenic and phenolic acids are only very week inhibitors of human salivary α-amylase and rat intestinal maltase activities. Food Res. Int. 2018, 113, 452–455. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Lucini, G. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated review. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, S.; Huang, D.; Tao, X.; Li, Y. Inhibition of starch digestion by phenolic acid with a cinnamic acid backbone: Structural requirements for the inhibition of α-amylase and α-glucosidase. Food Chem. 2004, 435, 137499. [Google Scholar] [CrossRef]
- Thompson, L.U.; Yoon, J.H. Starch digestibility as affected by polyphenols and phytic acid. J. Food Sci. 1984, 49, 1228–1229. [Google Scholar] [CrossRef]
- Knuckles, B.E.; Betschart, A.A. Effect of phytate and other myo-inositol phosphate esters on α-amylase digestion of starch. J. Food Sci. 1987, 52, 719–721. [Google Scholar] [CrossRef]
- Kumar, A.; Sahu, C.; Panda, P.A.; Biswal, M.; Sah, R.P.; Lal, M.K.; Baig, M.J.; Swain, P.; Behera, L.; Chattopadhyay, K.; et al. Phytic acid content may affect starch digestibility and glycemic index value of rice (Oryza sativa L.). J. Sci. Food Agric. 2019, 100, 1598–1607. [Google Scholar] [CrossRef]
- Kumar Lal, M.; Singh, B.; Sharma, S.; Singh, M.P.; Kumar, A. Glycemic index of starchy crops and factors affecting its digestibility: A review. Trends Food Sci. Technol. 2021, 111, 741–745. [Google Scholar] [CrossRef]
- Rashid, U.; Rehman, H.U.; Baloch, A.H. Effect of phytic acid on the enzymatic activities of starch degrading human salivary α-amylase. J. Org. Chem. Chem. Sci. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Rogozinska, M.; Biesaga, M. Decomposition of flavonols in the presence of saliva. Appl. Sci. 2020, 10, 7511. [Google Scholar] [CrossRef]
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Rabail, R.; Munir, S.; Jan, H.; Fernández-Lázaro, D.; Aadil, R.M. Impact of oats on appetite hormones and body weight management. A review. Curr. Nutr. Rep. 2023, 12, 66–82. [Google Scholar] [CrossRef]
- Mandel, A.L.; Breslin, P.A.S. High endogenous salivary amylase activity is associated with improved glycemic homeostasis following starch ingestion in adults. J. Nutr. 2012, 142, 853–858. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Nadia, J.; Olenskyj, A.G.; Subramanian, P.; Hodgkinson, S.; Stroebinger, N.; Estevez, T.G.; Singh, R.P.; Singh, H.; Bornhorst, G.M. Influence of food macrostructure on the kinetics of acidification of pig stomach after the consumption of rice- and wheat-based foods: Implications for starch hydrolysis and starch emptying rate. Food Chem. 2022, 394, 133410. [Google Scholar] [CrossRef]
- Selle, P.H.; Cowieson, A.J.; Cowieson, N.P.; Ravindran, V. Protein-phytate interactions in pig and poultry nutrition: A reappraisal. Nutr. Res. Rev. 2012, 25, 1–17. [Google Scholar] [CrossRef]
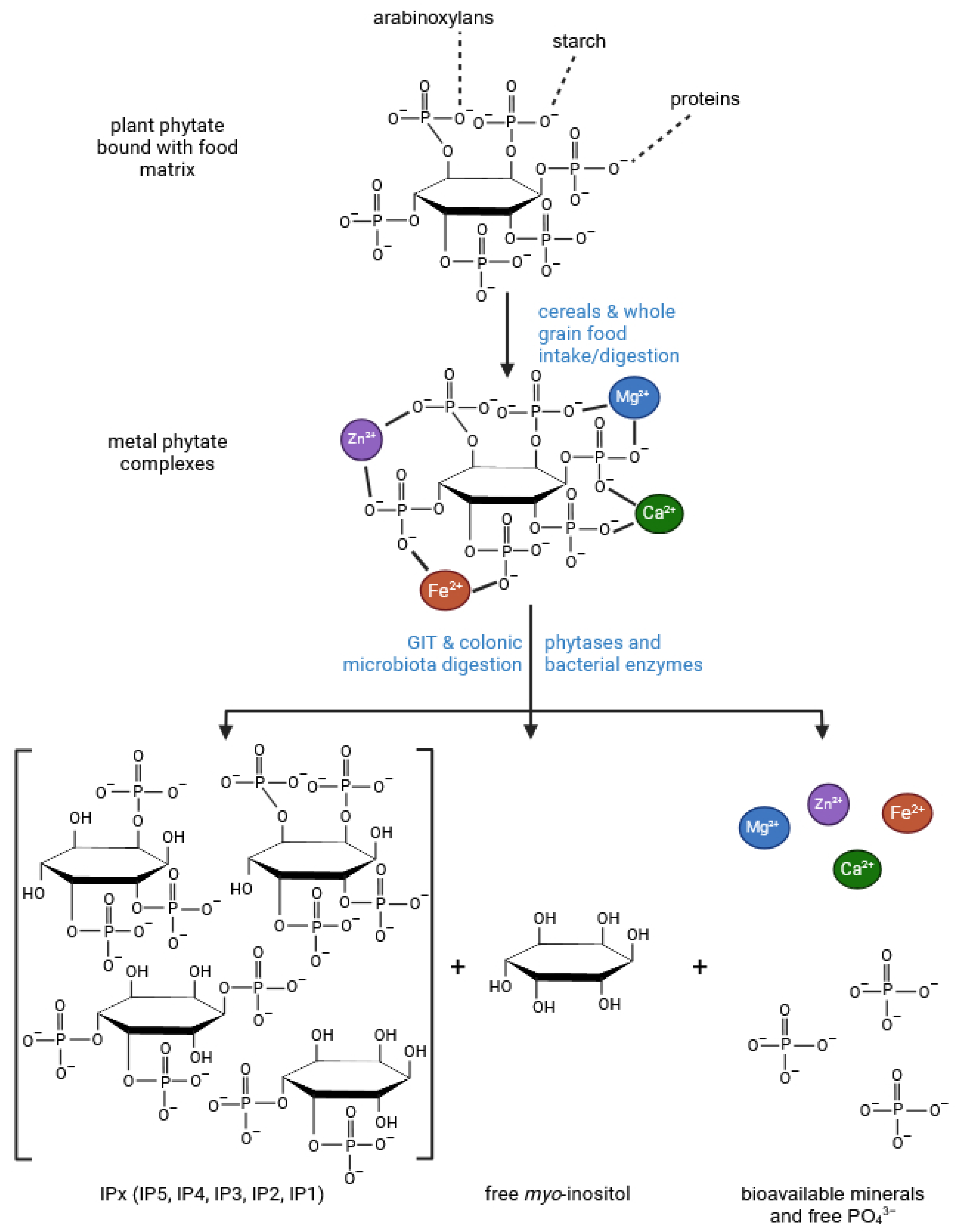
- Schlemmer, U.; Frølich, W.; Prieto, R.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef]
- Yu, S.; Cowieson, A.; Gilbert, C.; Plumstead, P.; Dalsgaard, S. Interaction of phytate and myo-inositol phosphate esters (IP1-5) including IP5 isomers with dietary protein and iron and inhibition of pepsin. J. Anim. Sci. 2012, 90, 1824–1832. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. Effect of some phenolics compounds and beverages on pepsin activity during simulated gastric digestion. J. Agric. Food Chem. 2005, 53, 8706–8713. [Google Scholar] [CrossRef]
- Raeessi-Babaheydari, E.; Farhadian, S.; Shareghi, B. Evaluation of interaction between citrus flavonoid, naringenin, and pepsin using spectroscopic analysis and docking simulation. J. Mol. Liq. 2021, 339, 116763. [Google Scholar] [CrossRef]
- Dragičević, V.; Simić, M.; Kandić Raftery, V.; Vukadinović, J.; Dodevska, M.; Durović, S.; Brankov, M. Screening of nutritionally important components in standard and ancient cereals. Foods 2024, 13, 4116. [Google Scholar] [CrossRef]
- Cirkovic Velikovic, T.D.; Stanic-Vucinic, D.J. The role of dietary phenolic compounds in protein digestion and processing technologies to improve their antinutritive properties. Compr. Rev. Food Sci. Food Saf. 2017, 17, 82–103. [Google Scholar] [CrossRef]
- Shahidi, F.; Pan, Y. Influence of food matrix and food processing on the chemical interaction and bioaccessibility of dietary phytochemicals. A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 6421–6445. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible side effects of polyphenols and their interactions with medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Li, R.; Song, T.; Kang, R.; Ma, W.; Zhang, M.; Ren, F. Investigating the impact of ultrasound assisted cellulase pretreatment on the nutrients, phytic acid, and phenolics bioaccessibility in sprouted brown rice. Ultrason. Sonochem. 2024, 106, 106878. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.; Kanojiya, N.; Bhingardeve, N.; Ahire, J.J.; Saroj, D. In vitro gastrointestinal tract simulation systems: A panoramic view. Probiotics Antimicrob. Proteins 2024, 16, 501–518. [Google Scholar] [CrossRef]
- Deng, L.; Golding, M.; Lentle, R.; MacGibbon, A.; Matia-Merino, L. The role of gastric lipase and pepsin in lipid digestion of a powder infant formula using a simulated neonatal gastric system. Food Biophys. 2024, 19, 369–385. [Google Scholar] [CrossRef]
- Okuro, P.K.; Viau, M.; Marze, S.; Laurent, S.; Cunha, R.L.; Berton-Carabin, C.; Meynier, A. In vitro digestion of high-lipid emulsions; towards a critical interpretation of lipolysis. Food Funct. 2023, 14, 10868. [Google Scholar] [CrossRef]
- Vaquero, M.R.; Yáñez-Gascón, M.-J.; Villalba, R.G.; Larrosa, M.; Fromentin, E.; Ibarra, A.; Roller, M.; Tomás-Barberán, F.; de Gea, J.C.E.; García-Conesa, M.-T. Inhibition of gastric lipase as a mechanism for body weight and plasma lipids reduction in Zucker rats fed a rosemary extract rich in carnosic acid. PLoS ONE 2012, 7, e39773. [Google Scholar] [CrossRef] [PubMed]
- Dalbaere, K.; Roegiers, I.; Bron, A.; Durif, C.; Van de Wiele, T.; Blanquet-Diot, S.; Marinelli, L. The small intestine: Dining table of host-microbiota meetings. FEMS Microbiol. Rev. 2023, 47, fuad022. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y.; Li, S.; Yi, X.; Shao, S.; Yu, W.; Li, E. Biological factors controlling starch digestibility in human digestive system. Food Sci. Hum. Well. 2023, 12, 351–358. [Google Scholar] [CrossRef]
- Picariello, G.; Siano, F.; Di Stasio, L.; Mamone, G.; Addeo, F.; Ferranti, P. Structural properties of food proteins underlying stability or susceptibility to human gastrointestinal digestion. Curr. Opin. Food Sci. 2023, 50, 100992. [Google Scholar] [CrossRef]
- Loveday, S.M. Protein digestion and absorption: The influence of food processing. Nutr. Res. Rev. 2023, 36, 544–559. [Google Scholar] [CrossRef]
- Kårlund, A.; Paukkonen, I.; Gómez-Gallego, C.; Kolehmainen, M. Intestinal exposure to food-derived protease inhibitors: Digestion physiology- and gut health-related effects. Healthcare 2021, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.; Schmid, B.; Bosa, L.; Morlock, G.E. Screening of α-amylase/trypsin inhibitor activity in wheat, spelt and einkorn by high-performance thin-layer chromatography. Anal. Methods 2024, 16, 2997. [Google Scholar] [CrossRef] [PubMed]
- Mat, D.J.L.; Souchon, I.; Michon, C.; Le Feunteun, S. Gastro-intestinal in vitro digestions of protein emulsions monitored by pH-stat: Influence of structural properties and interplay between proteolysis and lipolysis. Food Chem. 2020, 311, 125946. [Google Scholar] [CrossRef]
- Kłosowska, K.; de Castillo-Santaella, T.; Moldonado-Valderrama, J.; Macierzanka, A. The bile salt/phospholipid ratio determines the extent of in vitro intestinal lipolysis of triglycerides: Interfacial and emulsion studies. Food Res. Int. 2024, 187, 114421. [Google Scholar] [CrossRef]
- Sirvi, A.; Debaje, S.; Guleria, K.; Sangamwar, A.T. Critical aspects involved in lipid dispersion and digestion: Emphasis on in vitro models and factors influencing lipolysis of oral lipid based formulations. Adv. Colloid Interface Sci. 2023, 321, 103028. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Andújar, I.; Schinella, G.R.; Francini, F. Modulation of diabetes by natural products and medicinal plants via incretins. Planta Med. 2019, 85, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Bloom, S.R.; Buenaventura, T.; Tomas, A.; Rutter, G.A. Control of insulin secretion by GLP-1. Peptides 2018, 100, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B. Clinical use of DPP-4 inhibitors. Frontiers Endocrinol. 2019, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Rostami, Z.; Ghobadi, S.; Faghih, S. Effects of whole grain intake on glucagon-like peptide 1 and glucose-dependent insulinotropic peptide: A systematic review and meta-analysis. Nutr. Rev. 2023, 81, 384–396. [Google Scholar] [CrossRef]
- Kabisch, S.; Weickert, M.O.; Pfeiffer, A.F.H. The role of cereal soluble fiber in the beneficial modulation of glycometabolic gastrointestinal hormones. Crit. Rev. Food Sci. Nutr. 2024, 64, 4331–4347. [Google Scholar] [CrossRef]
- Gotteland, M.; Zazueta, A.; Pino, J.L.; Fresard, A.; Sambra, V.; Codoceo, J.; Cires, M.J.; López, X.; Vivanco, J.P.; Magne, F. Modulation of postprandial plasma concentrations of digestive hormones and gut microbiota by foods containing oat β-glucans in healthy volunteers. Foods 2023, 12, 700. [Google Scholar] [CrossRef]
- Yilmaz, B.; Sirbu, A.; Başar, H.B.A.; Goksen, G.; Chabi, I.B.; Kumaga, H.; Ozogul, F. Potential role of cereal bioactive compounds in the prevention and treatment of type 2 diabetes: A review of the current knowledge. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Møller, M.S.; Svensson, B. Structure, function and protein engineering of cereal-type inhibitors acting on amylolytic enzymes. Front. Mol. Biosci. 2022, 9, 868568. [Google Scholar] [CrossRef] [PubMed]
- Gebre, B.A.; Liu, X.; Zhang, C.; Ma, M.; Mekonnen, S.A.; Yao, T.; Sui, Z. Exploring the therapeutic potential of barley grain in type 2 diabetes management: A review. Int. J. Food Sci. Technol. 2024, 59, 4393–4402. [Google Scholar] [CrossRef]
- Domínguez Avila, J.A.; Rodrigo García, J.; González Aguilar, G.A.; De la Rosa, L.A. The Antidiabetic Mechanisms of Polyphenols Related to Increased Glucagon-Like Peptide-1 (GLP1) and Insulin Signaling. Molecules 2017, 22, 903. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Xiaoqin, L.; Li, S.; Tian, J.; Fan, X.; Cui, K.; Zhang, L.; Li, Z. Polyphenols from whole millet grain (Setaria italica) alleviate glucose and lipid homeostasis in diet-induced obese mice by increasing endogenous GLP-1. J. Sci. Food Agric. 2023, 103, 7785–7797. [Google Scholar] [CrossRef]
- An, Y.; Zhang, L.; Fan, X.; Cui, K.; He, S.; Ji, P.; Tian, J.; Li, Z. Ferulic acid reduces GLP-1 degradation to ameliorate diet-induced obesity-associated hepatic steatosis. Food Front. 2024, 5, 691–707. [Google Scholar] [CrossRef]
- Fang, W.; Peng, W.; Oi, W.; Zhang, J.; Song, G.; Pang, S. Ferulic acid combined with different dietary fibers improve glucose metabolism and intestinal barrier function by regulating gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2024, 112, 105919. [Google Scholar] [CrossRef]
- Paventi, G.; Di Martino, C.; Crawford, T.W., Jr.; Iorizzo, M. Enzymatic activities of Lactiplantibacillus plantarum: Technological and functional role in food processing and human nutrition. Food Biosci. 2024, 61, 104944. [Google Scholar] [CrossRef]
- Hoffman, S.; Adeli, K. Glucagon-like peptide (GLP)-1 regulation of lipid and lipoprotein metabolism. Med. Rev. 2024, 4, 301–311. [Google Scholar] [CrossRef]
- Reimann, F.; Ward, P.S.; Gribble, F.M. Signaling mechanisms underlying the release of glucagon-like peptide 1. Diabetes 2006, 55 (Suppl. S2), S78–S85. [Google Scholar] [CrossRef]
- Yamashita, Y. Physiological functions of poorly absorbed polyphenols via glucagon-like peptide 1. Biosci. Biotechnol. Biochem. 2024, 88, 493–498. [Google Scholar] [CrossRef]
- Barker, C.J.; Wright, J.; Hughes, P.J.; Kirk, C.J.; Michell, R.H. Complex changes in cellular inositol phosphate complement accompany transit through the cell cycle. Biochem. J. 2004, 380 Pt 2, 465–473. [Google Scholar] [CrossRef]
- Manning, B.D. Insulin signaling: Inositol phosphates get into the Akt. Cell 2010, 143, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-H.; Chao, H.-N.; Walker, C.S.; Choong, S.-Y.; Phillips, A.; Loomes, K.M. Renal depletion of myo-inositol is associated with its increased degradation in animal models of metabolic disease. Am. J. Physiol. Renal Physiol. 2015, 309, F755–F763. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Bizzarri, M. Inositols in insulin signaling and glucose metabolism. Int. J. Endocrinol. 2018, 2018, 1968450. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Monti, N.; Piombarolo, A.; Angeloni, A.; Verna, R. Myo-Inositol and D-Chiro-Inositol as Modulators of Ovary Steroidogenesis: A Narrative Review. Nutrients 2023, 15, 1875. [Google Scholar] [CrossRef] [PubMed]
- Chakkour, M.; Greenberg, M.L. Insights into the roles of inositol hexakisphosphate kinase 1 (IP6K1) in mammalian cellular processes. J. Biol. Chem. 2024, 300, 107116. [Google Scholar] [CrossRef]
- Formoso, G.; Baldassarre, M.P.A.; Ginestra, F.; Carlucci, M.A.; Bucci, I.; Consoli, A. Inositol and antioxidant supplementation: Safety and efficacy in pregnancy. Diabetes/Metab. Res. Rev. 2019, 35, e3154. [Google Scholar] [CrossRef]
- Dinicola, S.; Minini, M.; Unfer, V.; Verna, R.; Cucina, A.; Bizzarri, M. Nutritional and Acquired Deficiencies in Inositol Bioavailability. Correlations with Metabolic Disorders. Int. J. Mol. Sci. 2017, 18, 2187. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Shimayoshi, T.; Holtz, G.G.; Noma, A. Modeling analysis of inositol 1,4,5-trisphosphatereceptor-mediated Ca2+ mobilization under the control of glucagon-like peptide-1 in mouse pancreatic beta-cells. Am. J. Physiol. Cell Physiol. 2016, 310, C337–C345. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hashimoto-Hill, S.; Woo, V.; Eshleman, E.M.; Whitt, J.; Engleman, L.; Karns, R.; Denson, L.A.; Haslam, D.B.; Alenghat, T. Microbiota-derived metabolite promotes HDAC3 activity in the gut. Nature 2020, 586, 108–112. [Google Scholar] [CrossRef]
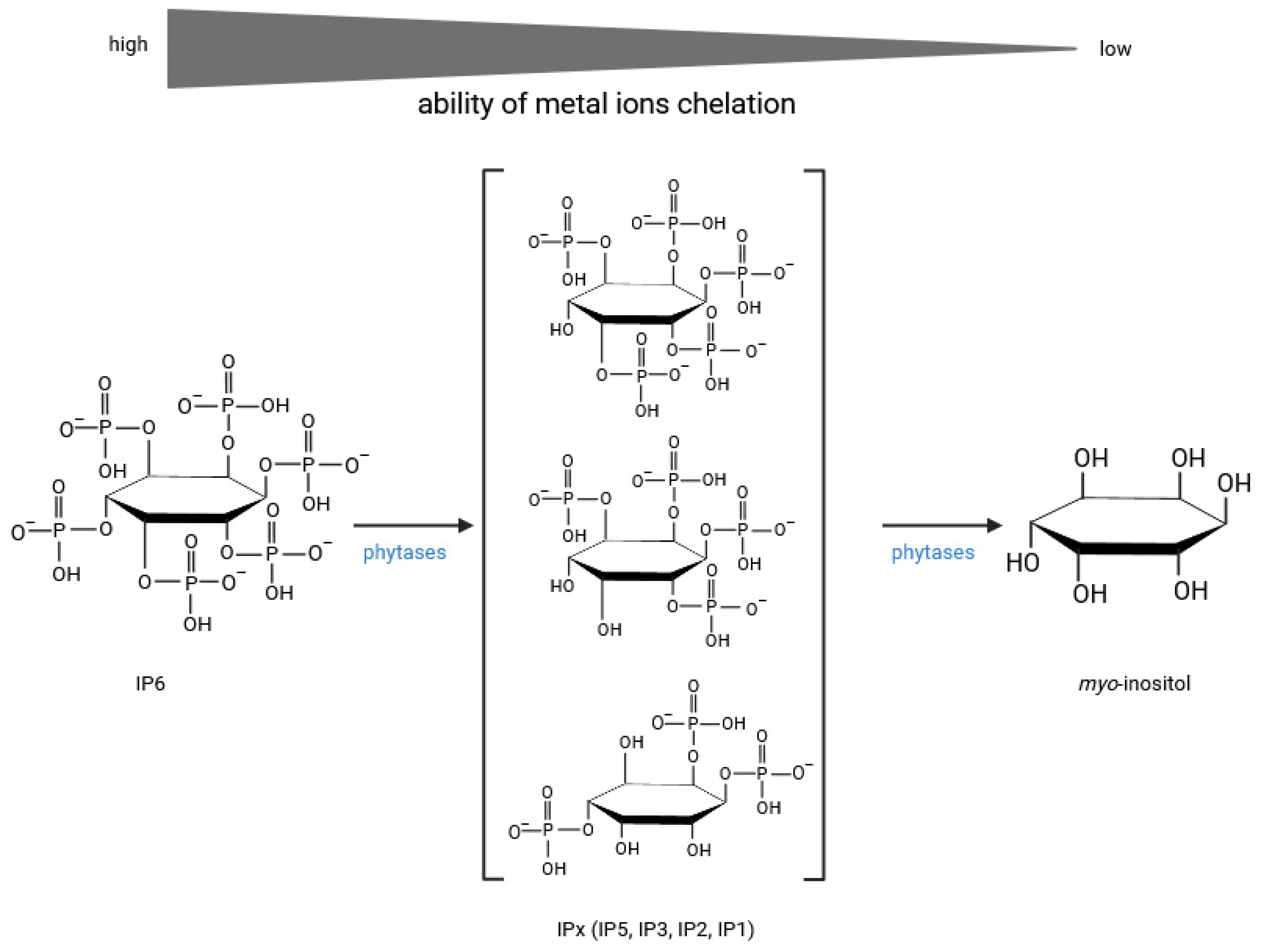
- Phillippy, B.Q.; Graf, E. Antioxidant functions of inositol 1,2,3-trisphosphate and inositol 1,2,3,6-tetrakisphosphate. Free Rad. Biol. Med. 1997, 22, 939–946. [Google Scholar] [CrossRef]
- Phillippy, B.Q. Inositol phosphates in foods. Adv Food Nutr Res. 2003, 45, 1–60. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Lu, Y.; Tian, X.; Chen, S.; Wu, B.; Du, J.; Xiao, Y.; Cai, W. Inositol hexaphosphate promotes intestinal adaptation in short bowel syndrome via an HDAC3-mediated epigenetic pathway. Food Nutr. Res. 2023, 67, 8694. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Abdel-Halim, N.H.M.; El-Shenbaby, I.; Helmy, J.M.A.; Hammad, M.O.; Habotta, O.A.; El Nashar, E.M.; Alghamdi, M.A.; Aldahham, R.A.; Al-Khater, K.M.; et al. Phytic acid attenuates acetaminophen-induced hepatoxicity via modulating iron-mediated oxidative stress and SIRT-1 expression in mice. Front. Pharmacol. 2024, 15, 1384834. [Google Scholar] [CrossRef]
- Watson, P.J.; Millard, C.J.; Riley, A.M.; Robertson, N.S.; Wright, L.C.; Godage, H.Y.; Cowley, S.M.; Jamieson, A.G.; Potter, B.V.L.; Schwabe, J.W.L. Insights into the activation mechanism of class I HDAC complexes by inositol phosphates. Nat. Commun. 2016, 7, 11262. [Google Scholar] [CrossRef]
- Sowd, G.A.; Stivison, E.A.; Chapagain, P.; Hale, A.T.; Poland, J.C.; Rameh, L.E.; Blind, R.D. IMPK regulates HDAC3 activity and histone acetylation in human cells. bioRxiv 2024. [Google Scholar] [CrossRef]
- Bui, T.P.N.; Mannerås-Holm, L.; Puschmann, R.; Wu, H.; Troise, A.D.; Nijsse, B.; Boeren, S.; Bäckhed, F.; Fiedler, D.; DeVos, W.M. Conversion of dietary inositol into propionate and acetate by commensal Anaerostipes associates with host health. Nat. Commun. 2021, 12, 4798. [Google Scholar] [CrossRef]
- De Vos, W.M.; Trung, M.N.; Davids, M.; Liu, G.; Rios-Morales, M.; Jessen, H.; Fiedler, D.; Nieuwdorp, M.; Bui, T.P.N. Phytate metabolism is mediated by microbial cross-feeding in the gut. Nat. Microbiol. 2024, 9, 1812–1827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Wang, X.; Zhang, M.; Du, M.; Jiang, W.; Li, C. Butyrate and propionate are negatively correlated with obesity and glucose levels in patients with type 2 diabetes and obesity. Diabetes Metab. Syndr. Obes. 2024, 17, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dernst, A.; Martin, B.; Lorenzi, L.; Cadefau, M.; Phulphager, K.; Wagener, A.; Budden, C.; Stair, N.; Wagner, T.; et al. Butyrate and propionate are microbial danger signals that activate the NLRP3-inflammasome in human macrophages in the presence of TLR stimulation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Stentz, R.; Osborne, S.; Horn, M.; Li, A.W.H.; Hautefort, I.; Bongaerts, R.; Rouyer, M.; Bailey, P.; Shears, S.B.; Hemmings, A.M.; et al. A bacterial homolog of a eukaryotic inositol phosphate signaling enzyme mediates cross-kingdom dialog in the mammalian gut. Cell Rep. 2014, 6, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Alenghat, T.; Osborne, L.C.; Saenz, S.A.; Kobuley, D.; Ziegler, C.G.K.; Mullican, S.E.; Choi, I.; Grunberg, S.; Sinha, R.; Wynosky-Dolfi, M.; et al. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature 2013, 504, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.F.; Vinolo, M.A.R. Histone acylations as a mechanism for regulation of intestinal epithelial cells. Dig. Med. Res. 2024, 7, 4. [Google Scholar] [CrossRef]
- Li, X.; Lau, H.C.H.; Yu, J. Microbiota-mediated phytate metabolism activates HDAC3 to contribute intestinal homeostasis. Signal Transduct. Target. Ther. 2020, 5, 211. [Google Scholar] [CrossRef]
- Di Vincezo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Simons, K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986, 5, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Furuse, M. The tight junction structure and function revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef]
- Pat, Y.; Yazici, D.; D’Avino, P.; Li, M.; Ardicli, S.; Ardicli, O.; Mitamura, Y.; Akdis, M.; Dhir, R.; Nadeau, K.; et al. Recent advances in the epithelial barrier theory. Int. Immunol. 2024, 36, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A. α-Lactalbumin, amazing calcium-binding protein. Biomolecules 2020, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Monastra, G.; Sambuy, Y.; Ferruzza, S.; Ferrari, D.; Rinaldi, G. Alpha lactalbumin effect on myo-inositol intestinal absorption: In vivo and in vitro. Curr. Drug Deliv. 2018, 15, 1305–1311. [Google Scholar] [CrossRef]
- Żyła, K.; Mika, M.; Duliński, R.; Świątkiewicz, W.S.; Koreleski, J.; Pustkowiak, H.; Piironen, J. Effects of inositol, inositol-generating phytase B alone, and in combination with 6-phytase A to phosphorus-deficient diets on laying performance, eggshell quality, yolk cholesterol, and fatty acid deposition in laying hens. Poult. Sci. 2012, 91, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.; Ragupathy, S.; Borchard, G. Target specific tight junction modulators. Adv. Drug Deliv. Rev. 2021, 171, 266–288. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Yang, F.; Li, X.; Cheng, L.; Song, Y. Phytic acid improves intestinal mucosal barrier damage and reduces serum levels of proinflammatory cytokines in a 1,2-dimethylhydrazine-induced rat colorectal cancer model. Br. J. Nutr. 2018, 120, 121–130. [Google Scholar] [CrossRef]
- Reddy, C.S.; Vani, K.; Pandey, S.; Gupta, G.; Vijaylakshmi, M.; Obul Reddy, P.C.; Kaul, T. Manipulating microbial phytases for heterologous expression in crops for sustainable nutrition. Ann. Plant Sci. 2013, 02, 436–454. [Google Scholar]
- Vashishth, A.; Ram, S.; Beniwal, V. Cereal phytases and their importance in improvement of micronutrients bioavailability. 3 Biotech 2017, 7, 42. [Google Scholar] [CrossRef]
- Majumder, S.; Datta, K.; Datta, S.K. Rice Biofortification: High Iron, Zinc, and Vitamin-A to Fight against “Hidden Hunger”. Agronomy 2019, 9, 803. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Biofortification and bioavailability of Zn, Fe and Se in wheat: Present status and future prospects. Theor. Appl. Genet. 2021, 134, 1–35. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Role of flavonoids in intestinal tight junction regulation. J. Nutr. Biochem. 2011, 22, 401–408. [Google Scholar] [CrossRef]
- Vaghari-Tabari, M.; Alemi, F.; Zokaei, M.; Moein, S.; Qujeq, D.; Yuosefi, B.; Farzami, P.; Hosseininasab, S. Polyphenols and inflammatory bowel disease: Natural products with therapeutic effects? Crit. Rev. Food Sci. Nutr. 2022, 64, 4155–4178. [Google Scholar] [CrossRef]
- Li, L.; Peng, P.; Ding, N.; Jia, W.; Huang, C.; Tang, Y. Oxidative stress, inflammation, gut dysbiosis: What can polyphenols do in inflammatory bowel disease? Antioxidants 2023, 17, 967. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Hur, G.; Jang, H.-j.; Jeong, S.; Lee, S.W.; Lee, S.-J.; Rho, M.-C.; Kim, S.-H.; Lee, S. Ferulic acid derivatives ameliorate intestine barrier destruction by alleviating inflammatory response in dextran sulfate sodium-induced inflammatory bowel disease. Toxics 2024, 12, 268. [Google Scholar] [CrossRef]
- Wen, X.; Peng, H.; Zhang, H.; He, Y.; Guo, F.; Bi, X.; Liu, J.; Sun, Y. Wheat bran polyphenols ameliorate DSS-induced ulcerative colitis in mice by suppressing MAPK/NF-κB inflammasome pathways and regulating intestinal microbiota. Foods 2024, 13, 225. [Google Scholar] [CrossRef]
- Fan, L.; Ma, S.; Li, L.; Huang, J. Fermentation biotechnology applied to wheat bran for the degradation of cell wall fiber and its potential health benefits. A review. Int. J. Biol. Macromol. 2024, 275, 133529. [Google Scholar] [CrossRef]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, B.; Huang, K.; Li, S.; Cao, H.; Guan, X. Bound polyphenols of oat bran released by gut microbiota mitigate high fat diet-induced oxidative stress and strengthen the gut barrier via the colonic ROS/Akt/Nrf2 pathway. J. Agric. Food Chem. 2024, 72, 13099–13110. [Google Scholar] [CrossRef]
- Veiga-Matos, J.; Morales, A.I.; Prieto, M.; Remião, F.; Silva, R. Study models of drug-drug interactions involving P-glycoprotein: The potential benefit of P-glycoprotein modulations at the kidney and intestinal levels. Molecules 2023, 28, 7532. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, J.; Zhao, S.; Li, H.; Guo, Z.; Liang, Z.; Li, J.; Qu, Y.; Chen, D.; Liu, L. Phytic acid-modified CeO2 as Ca2+ inhibitor for a security reversal of tumor drug resistance. Nano Res. 2022, 15, 4334–4343. [Google Scholar] [CrossRef]
- Fang, Y.; Cao, W.; Xia, M.; Pan, S.; Xu, X. Transport and interactions of co-incubated bi-functional flavonoids through inhibiting the function of P-glycoprotein (Pgp) using KB/multidrug –resistant (MDR) cells and rat everted gut sacs. J. Agric. Food Chem. 2022, 70, 1923–1933. [Google Scholar] [CrossRef]
- Muthusamy, G.; Gunaseelan, S.; Prasad, N.R. Ferulic acid reverses P-glycoprotein-mediated multidrug resistance via inhibition of PI3K/Akt/NFκB signaling pathway. J. Nutr. Biochem. 2019, 63, 62–71. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Wang, P.; Cai, M.; Neng, J.; Hu, J.; Xia, D.; Cao, W.; Yang, K.; Sun, P. Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet-induced mice. Eur. J. Nutr. 2022, 61, 3767–3783. [Google Scholar] [CrossRef]
- Grant, E.T.; De Franco, H.; Desai, M.S. Non-SCFA microbial metabolites associated with fiber fermentation and human host. Trends Endocrinol. Metab. 2025, 36, 70–82. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Cante, R.C.; Nigro, F.; Passannanti, F.; Lentini, G.; Gallo, M.; Nigro, R.; Budelli, A.L. Gut health benefits and associated systemic effects provided by functional components from the fermentation of natural matrices. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13356. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Pires, S.M.G.; Reis, R.S.; Cardoso, S.M.; Pezzani, R.; Paredes-Osses, E.; Seilkhan, A.; Ydyrys, A.; Mortorell, M.; Gürer, E.S.; Setzer, W.N.; et al. Phytates as a natural source for health promotion: A critical evaluation of clinical trials. Front. Chem. 2023, 11, 1174109. [Google Scholar] [CrossRef]
- Pyrzyńska, K. Ferulic acid—A brief review of its extraction, bioavailability and biological activity. Separations 2024, 11, 204. [Google Scholar] [CrossRef]
- Alazouni, A.S.; Dkhil, M.A.; Gadelmawla, M.H.A.; Gabri, M.S.; Farag, A.H.; Hassan, B.N. Ferulic acid as anticarcinogenic agent against 1,2-dimethylhydrazine induced colon cancer in rats. J. King Saud Univ. Sci. 2021, 33, 101354. [Google Scholar] [CrossRef]
- Feng, J.; Liu, Y.; Zhang, C.; Ji, M.; Li, C. Phytic acid regulates proliferation of colorectal cancer cells by downregulating NF-κB and β-catenin signalling. Eur. J. Inflamm. 2023, 21, 1721727x231182622. [Google Scholar] [CrossRef]
- Graf, E.; Eaton, J.W. Antioxidant function of phytic acid. Free Rad. Biol. Med. 1990, 8, 61–69. [Google Scholar] [CrossRef]
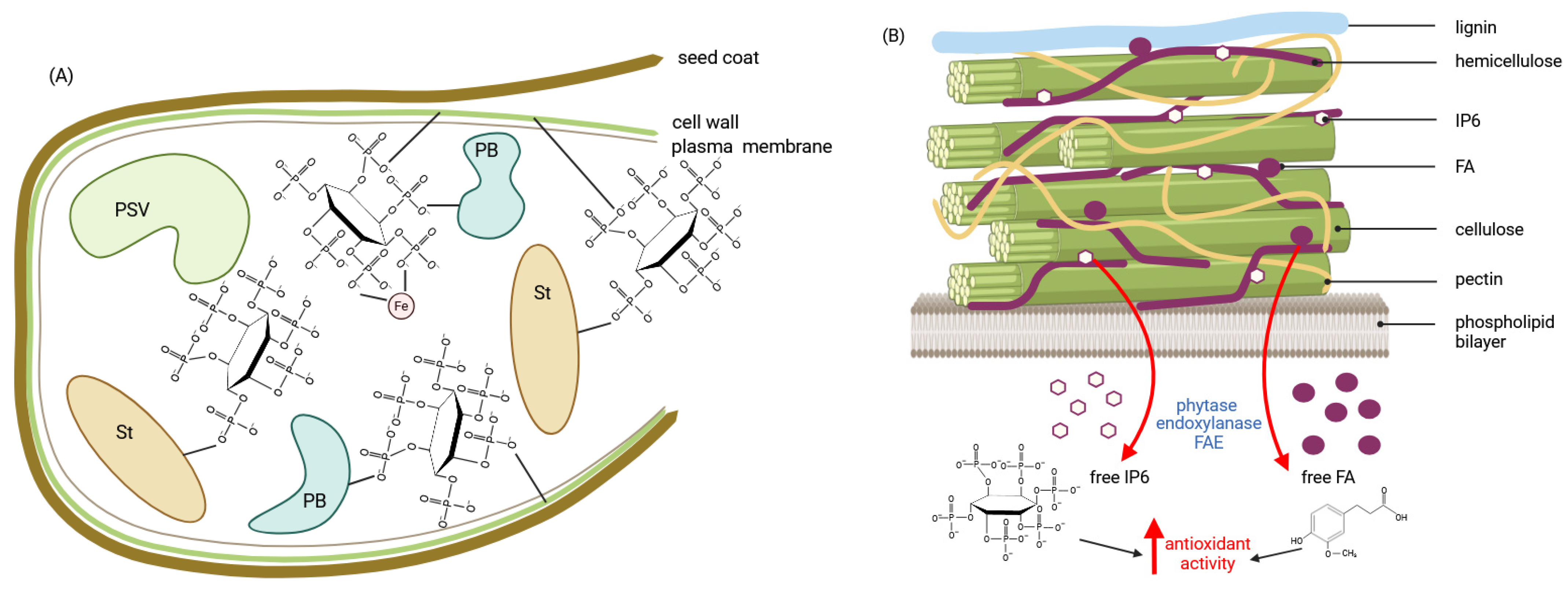
- Roustan, V.; Hilscher, J.; Weidinger, M.; Siegfried Reipert, S.; Shabrangy, A.; Gebert, C.; Dietrich, B.; Dermendjiev, G.; Schnurer, M.; Roustan, P.-J.; et al. Protein sorting into protein bodies during barley endosperm development is putatively regulated by cytoskeleton members, MVBs and the HvSNF7s. Sci. Rep. 2020, 10, 1864. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Fan, M.; Li, T.; Li, Y.; Qian, H.; Wang, L.; Zhang, H.; Qi, X.; Rao, Z. Cereal-derived arabinoxylans: Structural features and structure–activity correlations. Trends Food Sci. Technol. 2020, 96, 157–165. [Google Scholar] [CrossRef]
- Sanz-Penella, J.M.; Haros, M. Whole grain and phytate-degrading human Bifidobacteria. In Wheat and Rice in Disease Prevention and Health Benefits; Watson, R.S., Preedy, V.R., Zibadi, S., Eds.; Academic Press: London, UK; Waltham, MA, USA; San Diego, CA, USA, 2014; pp. 17–31. [Google Scholar]
- Wei, L.; Wang, B.; Bai, J.; Zhang, Y.; Liu, C.; Suo, H.; Wang, C. Postbiotics are a candidate for new functional foods. Food Chem. X 2024, 23, 101650. [Google Scholar] [CrossRef]
- Tomasik, P.; Tomasik, P. Probiotics, Non-Dairy Prebiotics and Postbiotics in Nutrition. Appl. Sci. 2020, 10, 1470. [Google Scholar] [CrossRef]
- Sanz-Penell, J.M.; Frontela, C.; Ros, G.; Martinez, C.; Monedero, V.; Haros, M. Application of bifidobacterial phytases in infant cereals: Effect on phytate contents and mineral dialyzability. J. Agric. Food Chem. 2012, 60, 11787–11792. [Google Scholar] [CrossRef] [PubMed]
- Kunnummal, S.P.; Sori, N.; Khan, M.A.; Khan, M. Plant-based nutraceutical formulation modulates the human gut microbiota and ferulic acid esterase activity during in vitro fermentation. Curr. Microbiol. 2024, 81, 3. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Esa, N.M. Phytic acid (myo-inositol hexaphosphate)—A promising pharmaceutical agent: A review. Asian J. Pharm. Clin. Res. 2018, 11, 42–46. [Google Scholar] [CrossRef]
- Alim, Z.; Kilinç, N.; Şengül, B.; Beydemir, Ş. Inhibition behaviours of some phenolic acids on rat kidney aldolase reductase enzyme: An in vitro study. J. Enzyme Inhib. Med. Chem. 2017, 32, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.M.; Bhuia, M.S.; Chowdhury, R.; Sheikh, S.; Ajmee, A.; Mollah, F.; Al Hasan, M.S.; Coutinho, H.D.M.; Islam, M.T. Potential utilization of ferulic acid and its derivatives in the management of metabolic diseases and disorders: An insight into mechanisms. Cell. Signal. 2024, 121, 111291. [Google Scholar] [CrossRef]
- Gupta, J.K. The role of aldose reductase in polyol pathway: An emerging pharmacological target in diabetic complications and associated morbidities. Curr. Pharm. Biotechnol. 2024, 25, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ko, A.-L.A.; Saiardi, A. Regulations of myo-inositol homeostasis: Mechanisms, implications, and perspectives. Adv. Biol. Regul. 2023, 87, 100921. [Google Scholar] [CrossRef]
- Imelda, E.; Idroes, R.; Khairan, K.; Lubis, R.R.; Abas, A.H.; Nursalim, A.J.; Rafi, M.; Tallei, T.E. Natural antioxidant activities of plants in preventing cataractogenesis. Antioxidants 2022, 11, 1285. [Google Scholar] [CrossRef]
- Concerto, C.; Chiarenza, C.; Di Francesco, A.; Natale, A.; Privitera, I.; Rodolico, A.; Trovato, A.; Aguglia, A.; Fisicaro, F.; Pennisi, M.; et al. Neurobiology and implications of inositol in psychiatry: A narrative review. Curr. Issues Mol. Biol. 2023, 45, 1762–1778. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. Myo-inositol for insulin resistance, metabolic syndrome, polycystic ovary syndrome and gestational diabetes. Open Heart 2022, 9, e0011989. [Google Scholar] [CrossRef] [PubMed]
- Pujol, A.; Sanchis, P.; Grases, F.; Masmiquel, L. Phytate intake, health and disease: “Let thy food be thy medicine and medicine be thy food”. Antioxidants 2023, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Żyła, K.; Grabacka, M.; Pierzchalska, M.; Duliński, R.; Starzyńska-Janiszewska, A. Effect of inositol and phytases on hematological indices and α-1 acid glycoprotein levels in lying hens fed phosphorus-deficient corn-soybean meal-based diets. Poult. Sci. 2013, 92, 199–204. [Google Scholar] [CrossRef]
- Kim, S.; Bhandari, R.; Brearley, C.A.; Saiardi, A. The inositol phosphate signaling network in physiology and disease. Trends Biochem. Sci. 2024, 49, 969–985. [Google Scholar] [CrossRef]
- Sprigg, C.; Whitfield, H.; Burton, E.; Scholey, D.; Bedford, M.R.; Brearley, C.A. Phytase dose-dependent response of kidney inositol phosphate levels in poultry. PLoS ONE 2022, 17, e0275742. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Huang, Q.; Song, T.; Dai, Z. Myo-inositol rescued insulin resistance and dyslipidemia in db/db mice. J. Appl. Biomed. 2024, 22, 74–80. [Google Scholar] [CrossRef]
- Aghajani, T.; Arefhosseini, S.; Ebrahimi-Mameghani, M.; Safaralizadeh, R. The effect of myo-inositol supplementation on AMPK/PI3K/AKT pathway and insulin resistance in patients with NAFLD. Food Sci. Nutr. 2024, 12, 7177–7185. [Google Scholar] [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid exerts its diabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Olofinsan, K.O.; Bharuth, V.; Ijomone, O.M.; Islam, S. Ferulic acid improves glucose homeostasis by modulation of key diabetogenic activities and restoration of pancreatic architecture in diabetic rats. Fundam. Clin. Pharmacol. 2023, 37, 324–339. [Google Scholar] [CrossRef] [PubMed]
- Kulathunga, J.; Simsek, S. Stone milling conditions and starter culture source influence phytic acid content and antioxidant activity in whole-grain sourdough bread. Cereal Chem. 2024, 101, 313–322. [Google Scholar] [CrossRef]
- Clements, R.S.; Darnell, B. Myo-inositol content of common foods: Development of a high myo-inositol diet. Am. J. Clin. Nutr. 1980, 33, 1954–1967. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, I.; Gao, R.; Ding, L.; Yang, Y.; Zhao, W.; Cui, X.; Lu, W.; Wang, J.; Li, Y. Estimating effects of whole grain consumption on type 2 diabetes, colorectal cancer and cardiovascular disease: A burden of proof study. Nutr. J. 2024, 23, 49. [Google Scholar] [CrossRef]
- Ying, T.; Zheng, J.; Kan, J.; Li, W.; Xue, K.; Du, J.; Liu, Y.; He, G. Effects of whole grains on glycemic control: A systematic review and dose-response meta-analysis of prospective cohort studies and randomized controlled trials. Nutr. J. 2024, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, F.; Zhong, H.; Hong, J.; Lin, H.; Zong, M.; Ren, H.; Zhao, S.; Chen, Y.; Shi, Z.; et al. Obesity-enriched gut microbe degrades myo-inositol and promotes lipid absorption. Cell Host Microbe 2024, 32, 1301–1314.e9. [Google Scholar] [CrossRef]
- EL Houssni, I.; Zahidi, A.; Khedid, K.; Hassikou, R. Review of processes for improving bioaccessibility of minerals by reducing the harmful effect of phytic acid in wheat. Food Chem. Adv. 2024, 4, 100568. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Y.; Wen, L.; Yang, B.; Farag, M.A.; Jiang, Y. The occurrence, role, and management strategies for phytic acid in foods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żyła, K.; Duda, A. Towards Improved Bioavailability of Cereal Inositol Phosphates, Myo-Inositol and Phenolic Acids. Molecules 2025, 30, 652. https://doi.org/10.3390/molecules30030652
Żyła K, Duda A. Towards Improved Bioavailability of Cereal Inositol Phosphates, Myo-Inositol and Phenolic Acids. Molecules. 2025; 30(3):652. https://doi.org/10.3390/molecules30030652
Chicago/Turabian StyleŻyła, Krzysztof, and Aleksandra Duda. 2025. "Towards Improved Bioavailability of Cereal Inositol Phosphates, Myo-Inositol and Phenolic Acids" Molecules 30, no. 3: 652. https://doi.org/10.3390/molecules30030652
APA StyleŻyła, K., & Duda, A. (2025). Towards Improved Bioavailability of Cereal Inositol Phosphates, Myo-Inositol and Phenolic Acids. Molecules, 30(3), 652. https://doi.org/10.3390/molecules30030652






