1. Introduction
Metal–organic frameworks (MOFs) are inherently porous materials with large specific surface areas [
1,
2,
3]. This feature promotes enhanced interaction with electrolytes and shortens ion transport pathways, significantly benefiting applications like lithium-ion batteries (LIBs) and electrocatalysis [
4,
5,
6,
7,
8,
9]. NiCo-based MOFs are particularly intriguing due to their unique combination of chemical, structural, and electrochemical properties that make them valuable in various applications, including energy storage, electrocatalysis, and solid-state batteries [
10,
11,
12,
13]. NiCo MOFs leverage the synergistic properties of Ni and Co, enhancing electronic conductivity and catalytic activity. The combination of these metals often provides improved performance compared to single-metal systems. The structure of NiCo MOFs allows for unsaturated metal sites and tunable pore sizes, providing abundant active sites for redox reactions and catalytic processes. Further, MOF-derived materials, such as NiCo₂O₄ from NiCo MOFs, exhibit high specific capacities and cycling stability in batteries [
14,
15,
16]. These characteristics are attributed to their spinel structures and large surface areas, which enhance lithium-ion intercalation and diffusion [
16].
Gunasekaran et al. reported that NiCo MOF-derived porous NiCo₂O₄ nanofile arrays used as an anode exhibited exceptional electrochemical performance, delivering a discharge-specific capacity of 1120 mAh/g at 0.1 C and 210 mAh/g at 2 C after 100 cycles [
17]. This impressive performance was attributed to its pseudocapacitive behavior and efficient Li⁺ transport, underscoring its potential as a high-capacity and cycling-stable anode material for rechargeable LIBs. Zhang et al. introduced a synthesis strategy for four distinct NiCo-MOFs with unique structures, achieving high initial discharge capacities ranging from 1770 to 1877.5 mAh/g at 100 mA/g, along with excellent cycling stability [
10]. Cheng et al. developed a 3D hierarchical porous Ti₃C₂/NiCo-MOF nanoarchitecture by integrating Ti₃C₂ nanosheets with NiCo-MOF nanoflakes using a vacuum-assisted filtration method [
18]. The optimized Ti₃C₂/NiCo-MOF electrode demonstrated outstanding electrochemical properties, including a high reversible capacity of 402 mAh/g at 0.1 A/g after 300 cycles, superior rate capability, and 85.7% capacity retention after 400 cycles. These results highlight the material’s potential for advanced energy storage applications, offering superior performance compared to pristine Ti₃C₂ MXene electrodes.
NiCo MOFs and their derivatives are highly versatile materials for advanced applications, combining the structural advantages of MOF architectures with the unique properties of Ni and Co. While numerous studies have highlighted their potential in energy conversion, storage, and catalysis, the intrinsic electrochemical properties of NiCo-MOF remain relatively underexplored [
19,
20,
21,
22].
In this study, additive-free NiCo-MOF films were employed as LIB anodes, fabricated using the alternating current electrophoretic deposition (AC-EPD) method, to explore the intrinsic electrochemical properties of MOF-derived NiCo₂O₄ electrodes without the influence of conductive agents or binders. The use of NiCo-based MOFs as anodes through AC-EPD offers several advantages, combining the unique properties of ultrathin MOFs with the precision and versatility of the EPD technique. The two-dimensional nanosheet structure of NiCo MOFs provides a high surface-to-volume ratio and abundant exposed active sites, facilitating efficient lithium-ion storage and improved reaction kinetics.
The EPD process enables the fabrication of highly uniform and thin films, ensuring greater accessibility of active sites to lithium ions during charge/discharge cycles. NiCo MOFs inherently exhibit enhanced electrical conductivity due to the synergistic interaction between Ni and Co, which is further optimized by EPD, enabling close contact between the material and current collectors.
NiCo-MOF has an intrinsic microporous and mesoporous framework, characteristic of MOF materials, which provides a high surface-to-volume ratio. This structure allows for more exposed active sites, enhancing interaction with ions and reactants. NiCo-MOF is often synthesized in nanosheet or nanoflake forms, further increasing the available surface area for electrochemical reactions. For example, the metal nodes (Ni and Co) combined with organic linkers create a periodic, well-defined porous structure, optimizing surface accessibility.
Further, Ni and Co are transition metals with partially filled d-orbitals, which facilitate electron transfer and enhance the electrical conductivity of the framework. The combination of Ni and Co within the MOF structure provides a synergistic effect, improving electron mobility through the framework. When annealed, NiCo-MOF converts into conductive metal oxides like NiCo₂O₄, further enhancing electrical conductivity while retaining its high surface area. These features make NiCo-MOF an excellent material for energy storage and conversion applications, as it combines efficient ion accessibility with rapid electron transport.
In contrast to conventional DC-EPD, which often results in uneven particle distribution and higher porosity, AC-EPD significantly reduces particle agglomeration by employing a bidirectional electric field, resulting in a smooth, defect-free, and densely packed film [
23,
24,
25].
By leveraging AC-EPD, the uniformity, density, and adhesion of NiCo-MOF coatings on the substrate were significantly improved. The resulting NiCo-MOF films, particularly those derived into NiCo₂O₄, demonstrated excellent specific capacities attributable to their spinel structures and the mixed-valence states of Ni and Co. Furthermore, the intimate contact between the MOF layer and the current collector achieved through EPD minimized interfacial resistance, enabling superior rate capabilities and high-performance cycling stability.
2. Results and Discussion
Figure 1 depicts the AC-EPD apparatus, specifically designed for the deposition of battery electrodes, where the setup utilizes an AC voltage operating at a frequency of 4 Hz, ensuring the NiCo MOF powders are evenly distributed onto the SS foil substrate. This configuration is engineered to produce a highly uniform and well-adhered layer, a critical factor for maximizing battery performance.
The alternating nature of the electric field in AC-EPD is instrumental in achieving a homogeneous particle distribution across the substrate. This dynamic electric field prevents the formation of clusters and promotes a smoother coating, as highlighted by the SEM micrograph of the deposited layer in
Figure 1. The SEM image depicts the surface morphology of the NiCo₂O₄ film derived from NiCo-MOF, deposited on SS foil via AC-EPD. The individual particles are sub-micrometers in size, consistent with the nanoscale features expected from MOF-derived materials. The visible voids and gaps between the particles suggest a high degree of porosity in the film with a Brunauer–Emmett–Teller surface area of 31 m
2/g. Smaller particle sizes enhance the electrochemical performance by reducing the ion diffusion path lengths and increasing the number of active sites for lithium-ion storage.
In AC-EPD, the oscillating electric field reverses direction at 4 Hz, influencing the movement of particles towards the substrate in a bidirectional manner. Unlike the unidirectional push in DC-EPD, this alternating motion provides particles multiple opportunities to settle optimally on the surface. This mechanism reduces the likelihood of particles adhering prematurely in suboptimal positions, a common challenge with DC-EPD. Moreover, the bidirectional field prevents the particles from clustering around specific regions of the electrode, leading to a more even and defect-free layer. This improved distribution enhances the structural integrity and bonding strength of the electrode layer, contributing to better electrochemical performance.
The XRD pattern shown in
Figure 2 illustrates the structural characteristics of the NiCo-MOF-derived material deposited on an SS foil via AC-EPD and annealed in a two-step process. Peaks corresponding to NiCo₂O₄ and Co₃O₄ are observed, with their characteristic reflections indexed as (311) and (400) [
26]. These peaks indicate the successful formation of spinel-phase materials after the annealing process.
The formation of the Co₃O₄ phase after annealing Ni-Co MOF can occur due to the thermal decomposition and oxidation of cobalt species in the MOF structure during the annealing process. During annealing, the organic ligands in the Ni-Co MOF are thermally decomposed, releasing gases such as CO₂ and H₂O. This exposes the metal centers (Co and Ni) to the environment, enabling phase transformations. At elevated temperatures, cobalt in the MOF can undergo oxidation, forming Co3⁺ from Co2⁺ through reactions with oxygen from the air during annealing (2Co2+ + O2 → 2Co3+). Co3⁺ and Co2⁺ coexist in a stable spinel Co₃O₄ structure (containing both Co2⁺ and Co3⁺), leading to the formation of this phase.
These phases are known for their excellent electrochemical activity due to mixed-valence states of Ni and Co, which contribute to the redox reactions during lithium-ion storage, which will be discussed in
Figure 3 and
Figure 4. Peaks labeled γ (111) and α (110) correspond to the austenitic and ferritic SS foil substrate, which may arise from the substrate material or interaction during annealing. The sharpness and intensity of the spinel peaks (e.g., (311) and (400)) suggest good crystallinity of the NiCo₂O₄/Co₃O₄ phases, which is likely due to the high-temperature annealing process. Annealing at 400 °C in air and 600 °C in argon promotes phase formation and removes organic residues from the NiCo-MOF precursor, resulting in crystalline oxide phases. The NiCo₂O₄ spinel structure contributes to high redox activity due to the presence of Ni
2⁺/Ni
3⁺ and Co
2⁺/Co
3⁺ redox couples. The Co₃O₄ phase enhances the overall electrochemical performance by providing additional active sites for lithium-ion storage. The crystallite size of the NiCo₂O₄ spinel structure was calculated to be 244 nm from the Debye–Scherrer method.
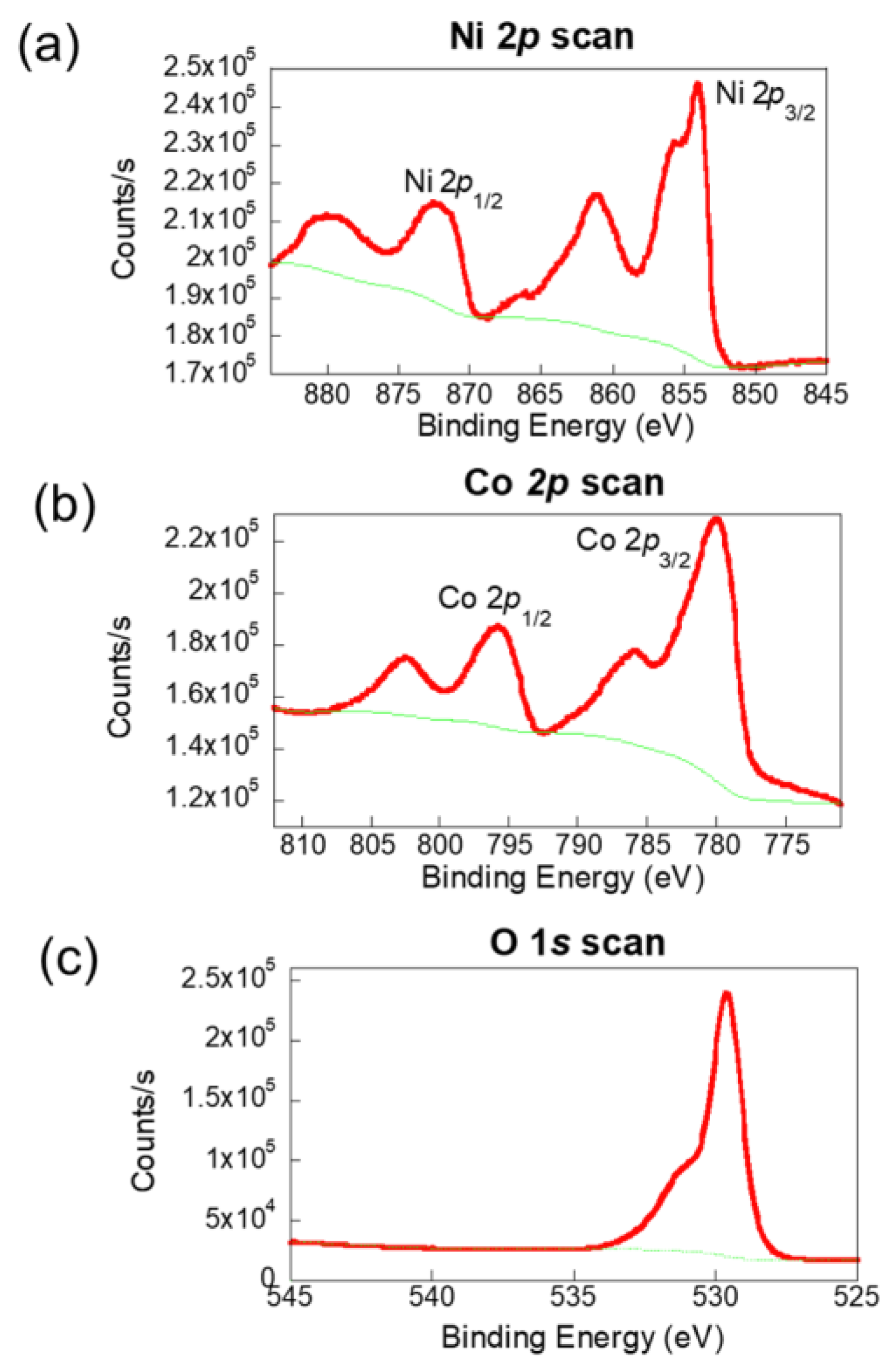
The mixed valence states of Ni and Co in NiCo₂O₄ were investigated by using XPS to analyze the oxidation states of the MOF-derived NiCo₂O₄ film, with the results presented in
Figure 3. As shown in
Figure 3a, the high-resolution Ni 2p spectrum reveals distinct peaks at 854 and 872 eV, corresponding to the spin-orbit splitting of Ni
3⁺ 2p₃/₂ and Ni
3⁺ 2p₁/₂, respectively. Additionally, peaks at 861 and 879 eV are attributed to Ni
2⁺ 2p₃/₂ and Ni
2⁺ 2p₁/₂. The Co 2p spectrum, illustrated in
Figure 3b, shows two main peaks at 780 and 796 eV, which are indicative of the spin-orbit components Co
3⁺ 2p₃/₂ and Co
2⁺ 2p₁/₂. These are accompanied by two satellite peaks, confirming the presence of both Co
3⁺ and Co
2⁺ oxidation states. In
Figure 3c, the O 1s spectrum highlights a peak at 529 eV, corresponding to the metal–oxygen bonds in the lattice structure. Another peak at 531 eV is associated with surface-adsorbed water molecules. These results collectively validate the mixed-valence states of Ni and Co in NiCo₂O₄ [
17].
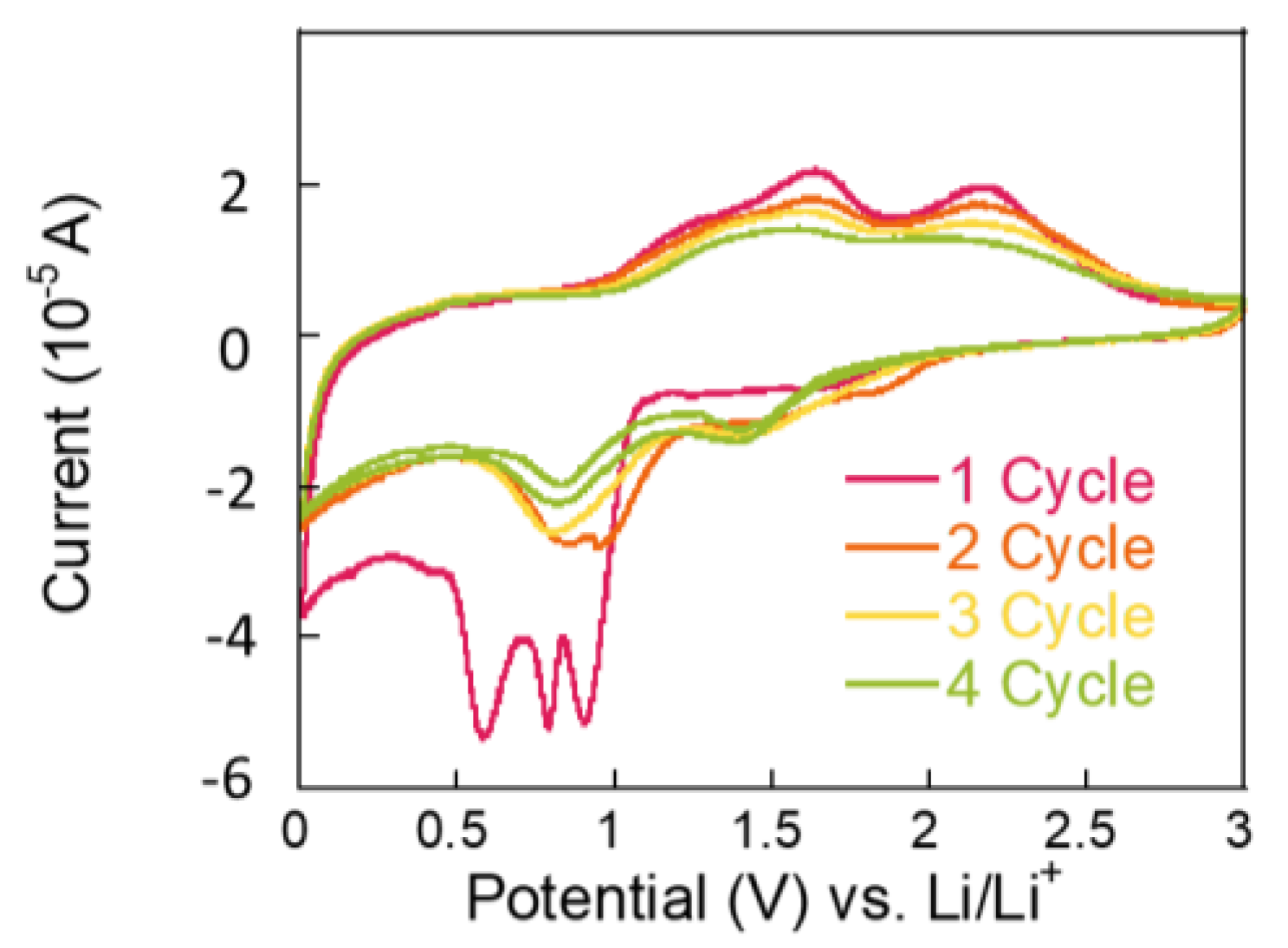
To investigate the origin of charge storage, redox peaks were analyzed using CV at a scan rate of 0.1 mV s⁻
1 within a voltage range of 0.01~3 V vs. Li/Li⁺ during the battery charging/discharging process. As shown in
Figure 4, the initial scan reveals a reduction peak at approximately 0.6 V, attributed to the reduction in Co₃O₄ to Co during the discharge process. Additionally, sharp reduction (cathodic) peaks between 0.8 and 0.9 V are associated with the decomposition of NiCo₂O₄ into Ni and Co ions, as well as the formation of a solid electrolyte interphase (SEI) layer. In subsequent scans, oxidation of metallic Ni to Ni
2⁺ and Co to Co
3⁺ is observed at 1.7 V and 2.2 V, respectively [
17,
19]. Notably, the charge storage capacity is primarily derived from the conversion reactions, as confirmed by the XRD and CV data. The redox reactions involving NiCo₂O₄ and Co₃O₄ significantly contribute to the overall charge storage capacity, consistent with the proposed Equations (1)–(4).
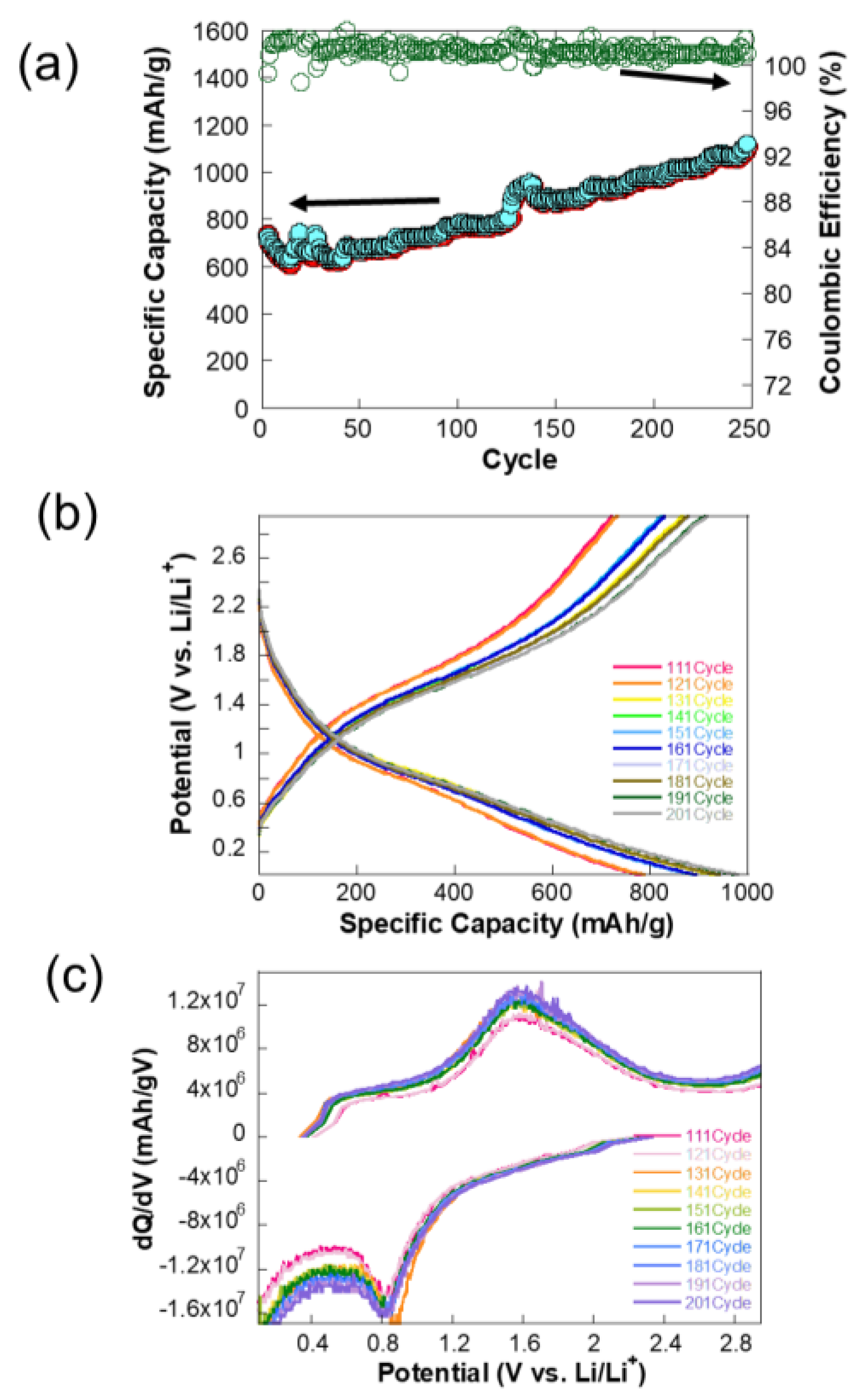
The plot in
Figure 5a illustrates the charge–discharge cycling performance of a NiCo-MOF electrode, tested at a constant specific current of 2.4 A/g. Interestingly, the discharge capacity increases slightly over cycles, which might be due to the activation of additional electroactive sites or gradual improvement in electrode kinetics. After the initial fluctuations, the specific capacity stabilizes around 1100–1200 mAh/g. The Coulombic efficiency is close to 100% throughout the cycling process, indicating minimal irreversible capacity loss and excellent cycling stability. This high efficiency suggests that the charge–discharge process is highly reversible, with minimal degradation of the electrode material.
The initial increase in capacity could be attributed to the formation of a stable SEI or enhanced accessibility of electroactive sites within the NiCo-MOF structure. Over 250 cycles, the capacity remains consistent, indicating the robustness of the NiCo-MOF electrode and its ability to withstand high current densities without significant degradation. The testing current density of 2.4 A/g is relatively high, yet the electrode maintains excellent capacity and stability. This demonstrates the NiCo-MOF electrode’s suitability for high-rate applications, likely due to its high surface area, good conductivity, and structural integrity. The plot highlights the excellent cycling stability, high specific capacity, and outstanding Coulombic efficiency of the NiCo-MOF electrode, even under the demanding conditions of high current density.
The potential profiles in
Figure 5b reveal a gradual increase in capacity over cycling, particularly within the voltage range of 0.8 V to 1.7 V. During the discharge process at approximately 0.8 V, this increase is likely attributed to the decomposition of NiCo₂O₄ into metallic Ni and Co ions, where Li⁺ ions react with the metal oxide, leading to its breakdown into metallic components and lithium oxide (Li₂O). Conversely, during the charge process at around 1.7 V, the oxidation of metallic Ni and Co back into Ni
2⁺ and Co
3⁺ is observed, corresponding to the delithiation process. This redox activity is characteristic of the spinel NiCo₂O₄ structure. Over successive cycles, the redox reactions in these regions become more pronounced, as evidenced by the increasing capacity. This suggests that repeated cycling activates additional electroactive sites or improves the kinetics of these redox reactions, a phenomenon that will be explored in detail later.
The dQ/dV plot in
Figure 5c offers valuable insights into the underlying electrochemical processes. The peak observed around 0.8 V is associated with the lithiation reaction, during which NiCo₂O₄ decomposes into Ni and Co ions. Over successive cycles, the increasing intensity of this peak suggests enhanced lithiation activity, likely due to improved accessibility of electroactive sites or favorable structural adjustments. Similarly, a peak at approximately 1.7 V corresponds to the oxidation of metallic Ni and Co into their higher oxidation states (Ni
2⁺ and Co
3⁺) during delithiation. The growing intensity of this peak with continued cycling indicates enhanced reversibility and improved kinetics of the delithiation process.
During early cycles, some active sites within the NiCo₂O₄ structure may not be fully utilized. As cycling progresses, these sites become accessible, contributing to the increased redox activity and overall capacity. The formation and stabilization of an SEI layer may also improve the electrode’s performance by reducing side reactions. Repeated cycling may induce beneficial structural changes in the NiCo₂O₄ material, such as enhanced porosity or breakdown of larger particles, increasing the surface area and enabling more efficient Li-ion transport, which has not been observed in thick electrodes with additives. The enhanced redox peaks in the dQ/dV plot suggest that the lithiation/delithiation kinetics improve over cycles, possibly due to better ionic conductivity or reduced charge-transfer resistance at the electrode–electrolyte interface.
The increase in capacity with cycling is primarily attributed to the improved electrochemical activity at ~0.8 V and ~1.7 V, as evidenced by the potential profile and dQ/dV plot. These regions correspond to the decomposition of NiCo₂O₄ into metallic Ni and Co during lithiation and their re-oxidation during delithiation, as mentioned earlier. Based on the correlation, it is believed that the activation of additional electroactive sites, structural adjustments, and enhanced kinetics over time collectively contribute to this behavior, highlighting the dynamic nature of NiCo₂O₄ as a high-performance electrode material.
The analysis of
b-values derived from scan rate-dependent CV measurements revealed that surface capacitive contributions dominate the charge storage mechanism at both oxidation (
b = 0.89) and reduction (
b = 0.98) potentials [
27,
28,
29]. A
b-value close to 1.0 indicates that the current is primarily determined by surface-controlled processes, such as pseudocapacitive behavior, rather than diffusion-limited ion intercalation. Indeed, a ratio of 0.37 between diffusive and capacitive charge storage was determined from CV measurements performed at a scan rate of 0.5 mV/s. This highlights the significant role of surface reactions in facilitating rapid charge storage and discharge kinetics in the electrode.
This study presents significant advancements over previous research, underscoring the novelty of our approach [
17,
30,
31]. First, we employed ultrathin NiCo-MOF films, which exhibit exceptional sensitivity to surface electrochemical reactions during charge/discharge processes. This facilitated the development of an advanced platform to probe the electrode/electrolyte interface—an accomplishment unattainable with conventional slurry-cast electrodes due to their bulk nature. Second, we implemented the AC-EPD technique, which has not been previously utilized for NiCo-MOF battery electrodes. This method enabled the fabrication of high-performance electrodes by capitalizing on the pseudocapacitive charge storage mechanism, thereby enhancing energy storage efficiency and rate capability. These innovations highlight the potential of ultrathin NiCo-MOF films and AC-EPD to revolutionize battery electrode design, providing a novel approach to interface analysis and superior electrochemical performance.