The Preparation of Cyclic Binary Block Polymer Using Bimolecular Homodifunctional Coupling Reaction and Characterization of Its Performance as a Drug Carrier
Abstract
1. Introduction
2. Results
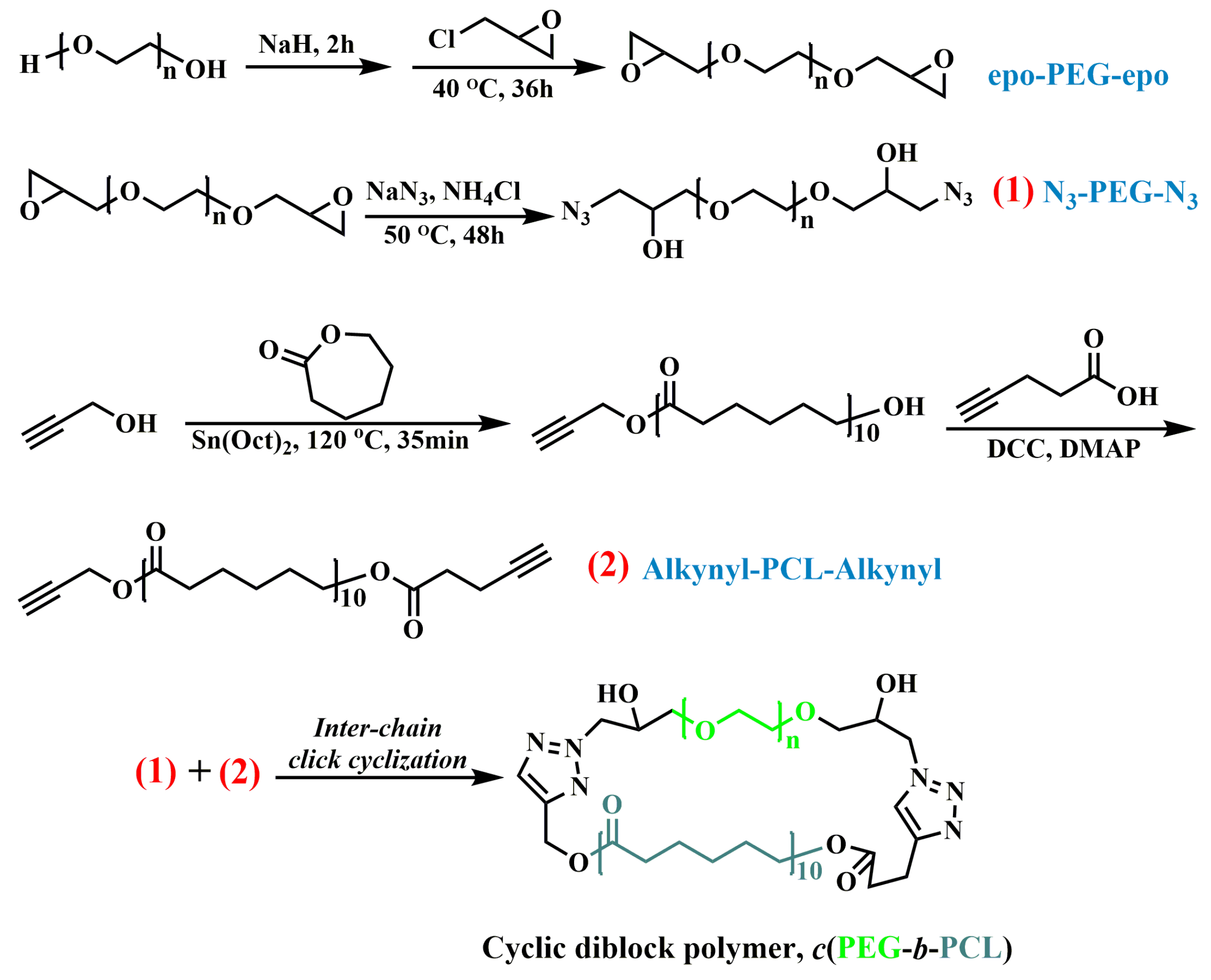
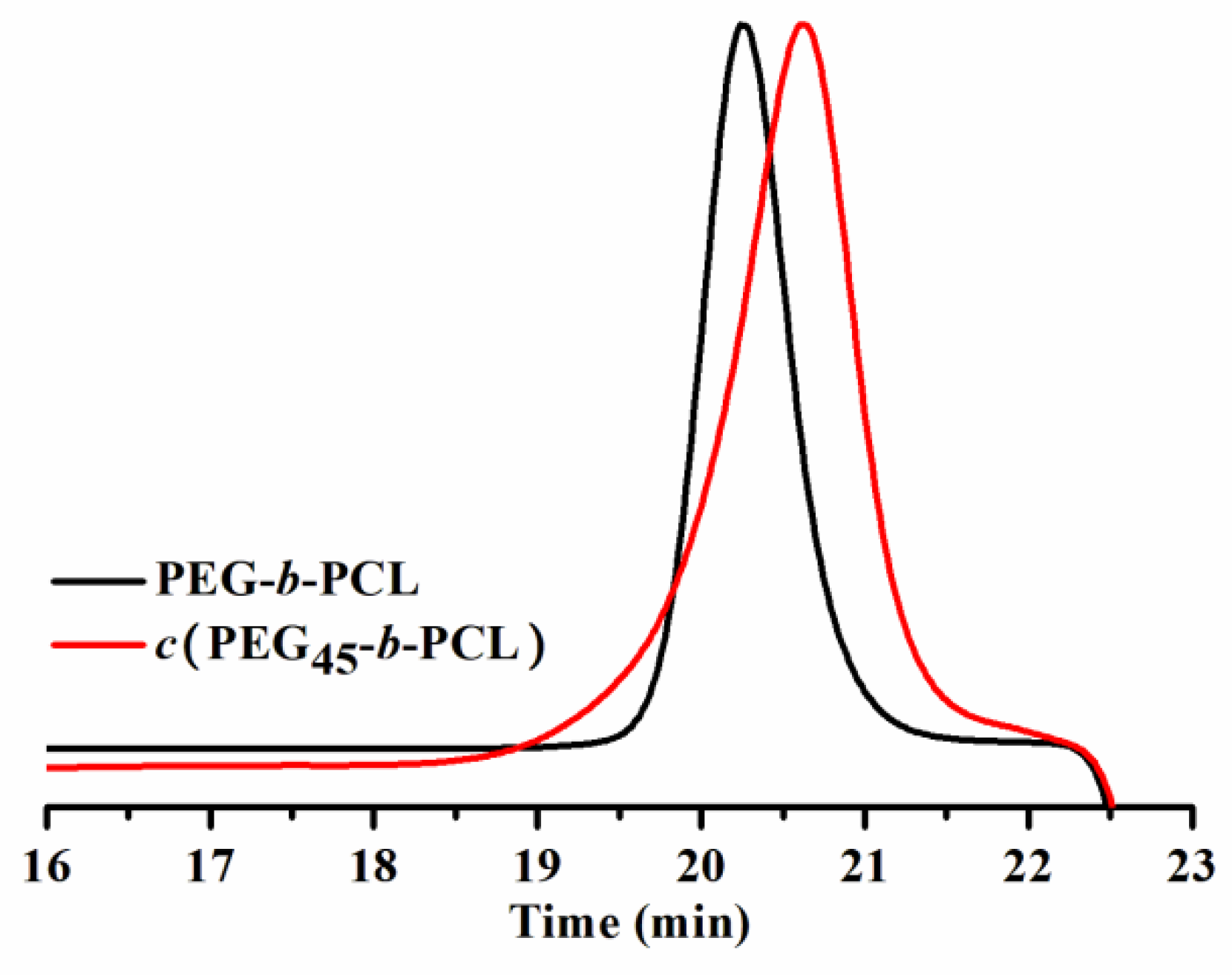
2.1. Synthesis and Characterization of Cyclic Binary Block Polymer c(PEG-b-PCL) and Linear Analog PEG-b-PCL
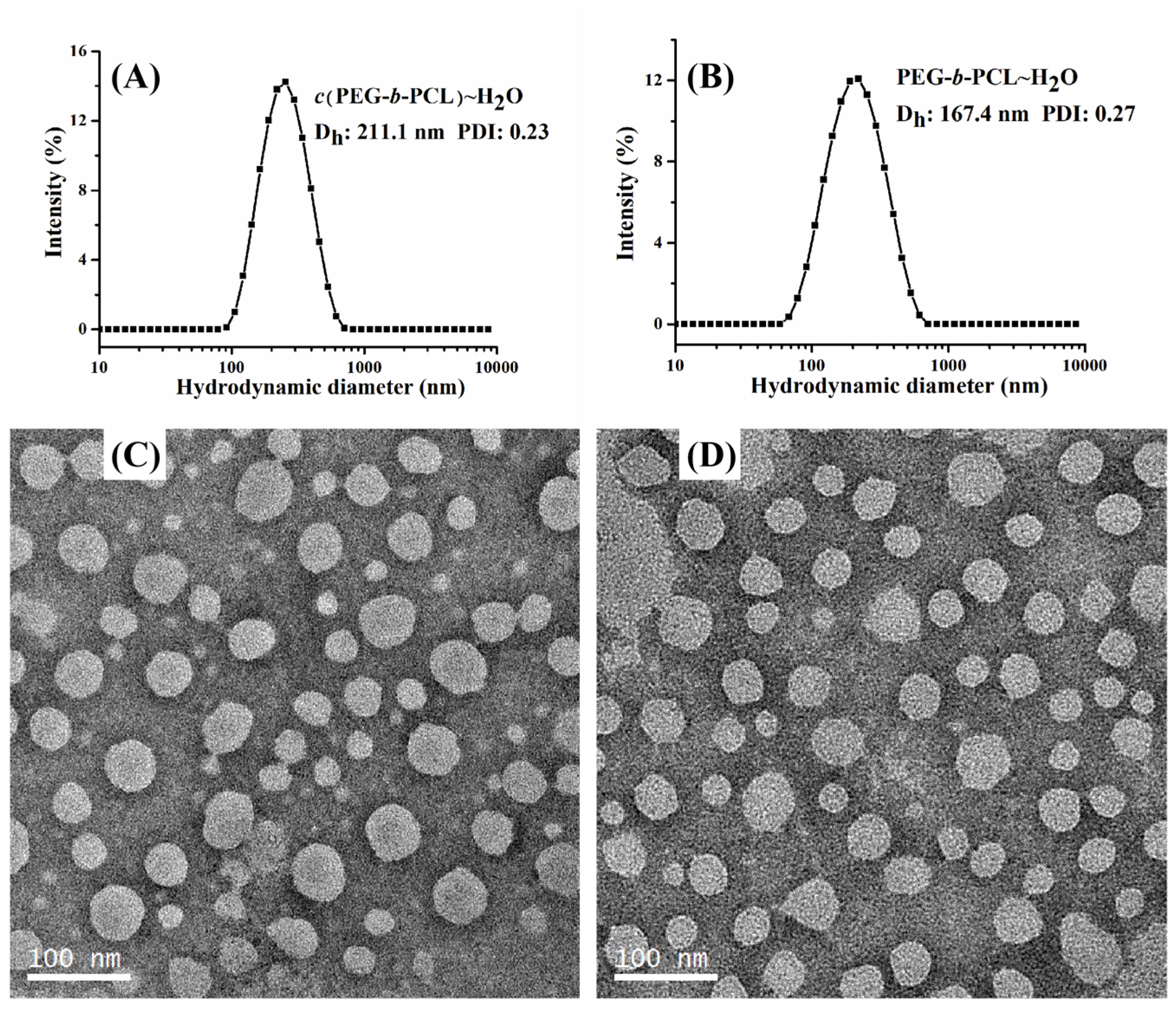
2.2. Characterization of Self-Assembled Micelles
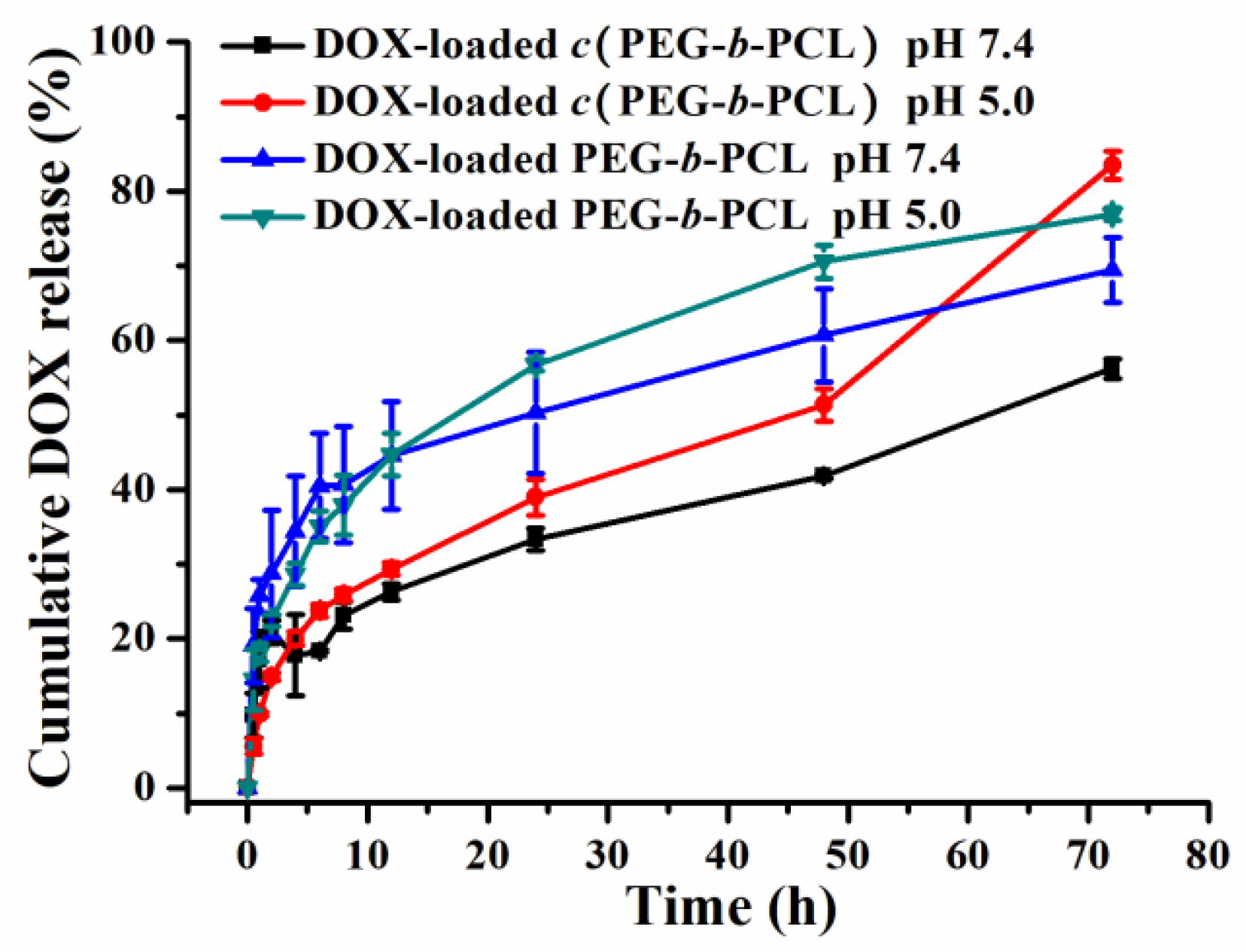
2.3. In Vitro Drug Loading and Drug Release Study
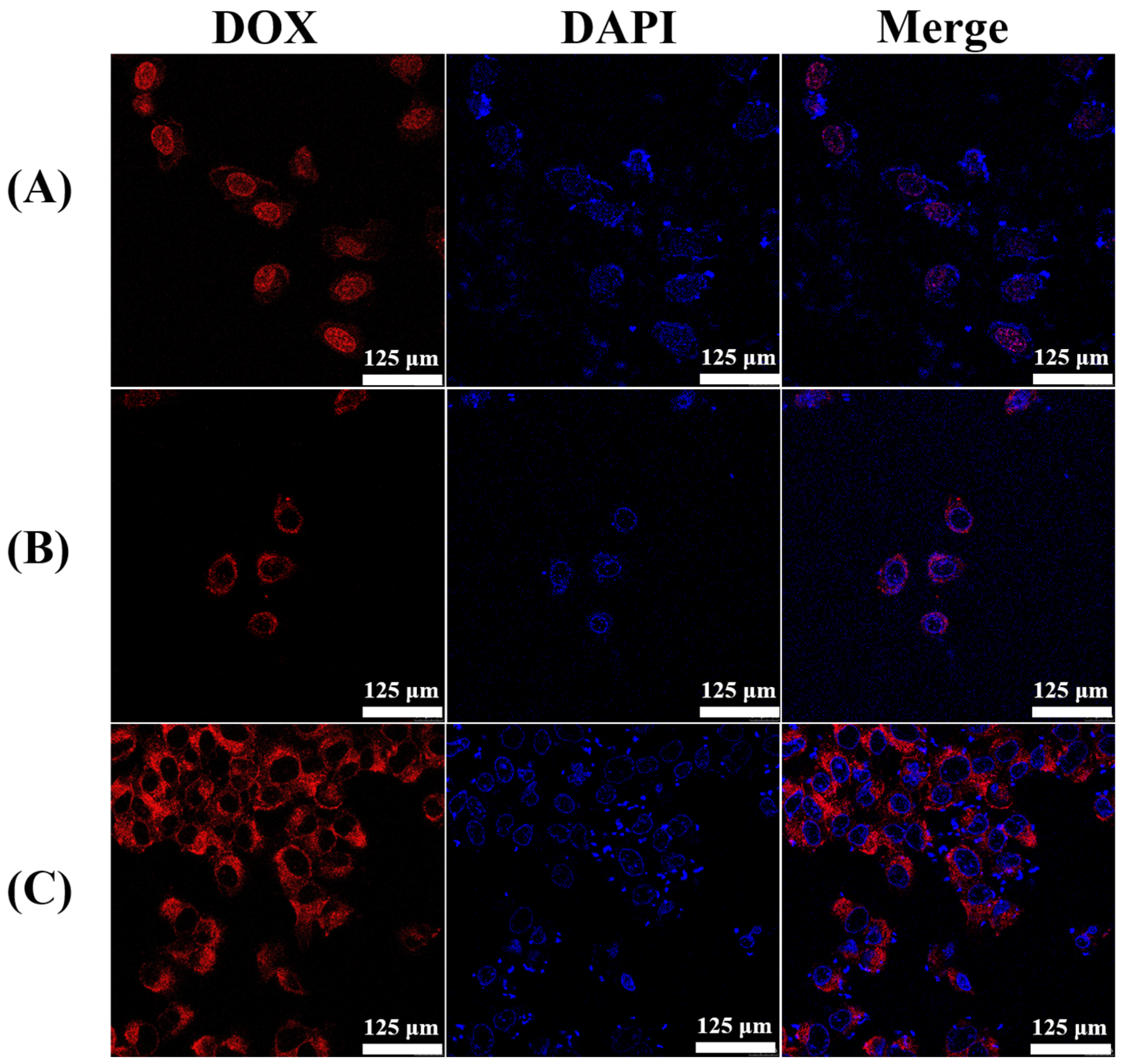
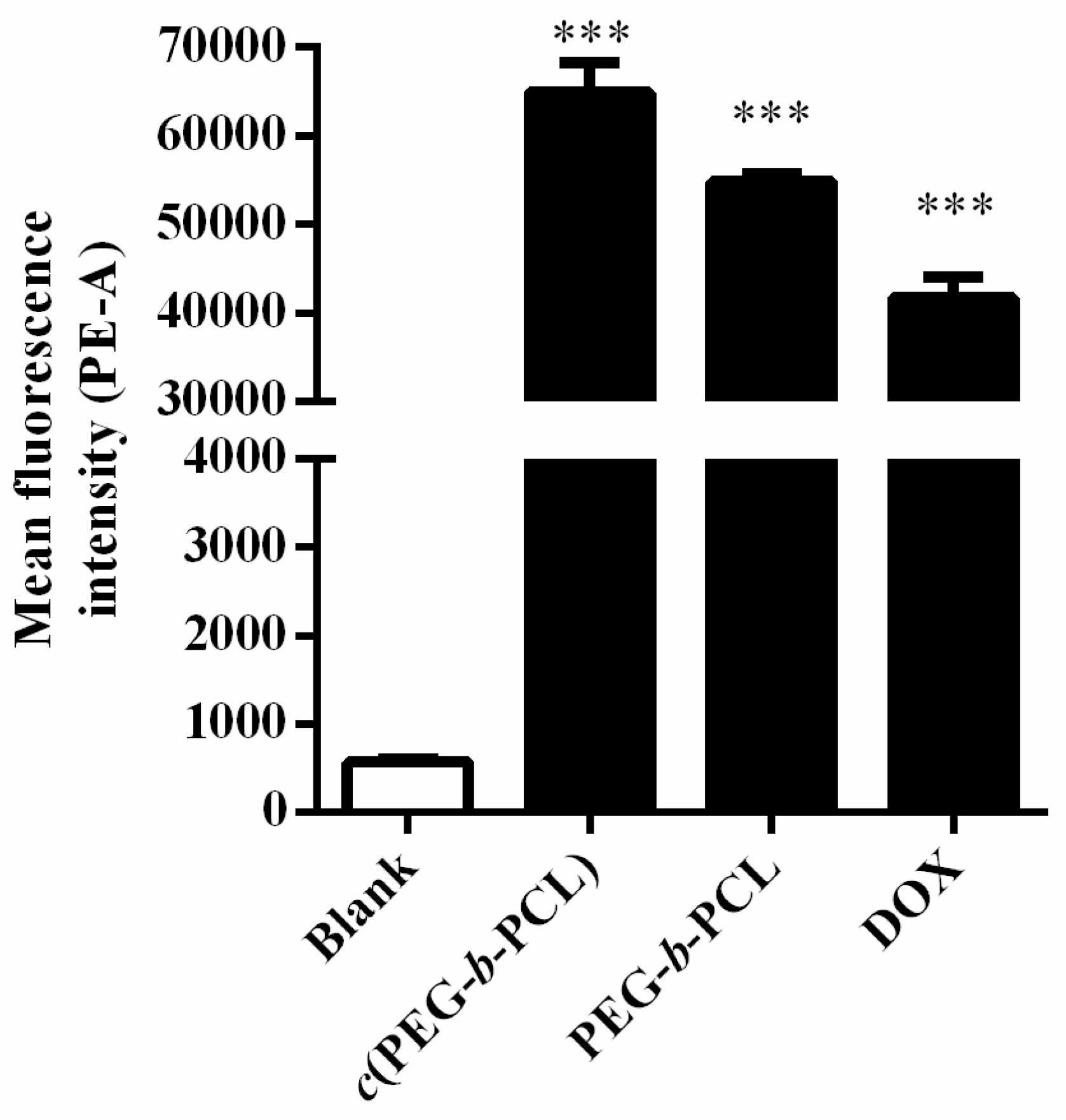
2.4. In Vitro Cellular Uptake and Cytotoxicity Studies
2.5. In Vitro Degradation Study
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Yang, J.; Ishaq, M.W.; Li, L. Study of Linear and Cyclic Graft Polystyrenes with Identical Backbone Contour in Dilute Solutions: Preparation, Characterization, and Conformational Properties. Macromolecules 2022, 55, 1398–1411. [Google Scholar] [CrossRef]
- Hadziioannou, G.; Cotts, P.M.; Ten Brinke, G.; Han, C.C.; Lutz, P.; Strazielle, C.; Rempp, P.; Kovacs, A.J. Thermodynamic and Hydrodynamic Properties of Dilute Solutions of Cyclic and Linear Polystyrenes. Macromolecules 1987, 20, 493–497. [Google Scholar] [CrossRef]
- Alberty, K.A.; Hogen-Esch, T.E.; Carlotti, S. Synthesis and Characterization of Macrocyclic Vinyl-Aromatic Polymers. Macromol. Chem. Phys. 2005, 206, 1035–1042. [Google Scholar] [CrossRef]
- Gao, L.; Oh, J.; Tu, Y.; Chang, T.; Li, C.Y. Glass Transition Temperature of Cyclic Polystyrene and the Linear Counterpart Contamination Effect. Polymers 2019, 170, 198–203. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, T.; Zhang, S.; Zhou, K.; Zhang, W.; Hu, Z. Effects of Chain Ends and Densities on the Glass Transition of Polymer Thin Films Probed by Linear and Cyclic Polystyrene. Polymer 2022, 253, 124986. [Google Scholar] [CrossRef]
- Kammiyada, H.; Ouchi, M.; Sawamoto, M. A Study on Physical Properties of Cyclic Poly(vinyl ether)s Synthesized via Ring-Expansion Cationic Polymerization. Macromolecules 2017, 50, 841–848. [Google Scholar] [CrossRef]
- Liu, R.-J.; Zhou, Z.-P.; Liu, Y.; Liang, Z.-P.; Ming, Y.-Q.; Hao, T.-F.; Nie, Y.-J. Differences in Crystallization Behaviors between Cyclic and Linear Polymer Nanocomposites. Chin. J. Polym. Sci. 2020, 38, 1034–1044. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Li, H.; Liu, B.; Chen, J.; Jiang, S. The Role of Entanglement in Crystallization and Melting of Cyclic Poly(ε-caprolactone)s. CrystEngComm. 2023, 25, 1383–1392. [Google Scholar] [CrossRef]
- Zaldua, N.; Liénard, R.; Josse, T.; Zubitur, M.; Mugica, A.; Iturrospe, A.; Arbe, A.; De Winter, J.; Coulembier, O.; Müller, A.J. Influence of Chain Topology (Cyclic versus Linear) on the Nucleation and Isothermal Crystallization of Poly(l-lactide) and Poly(d-lactide). Macromolecules 2018, 51, 1718–1732. [Google Scholar] [CrossRef]
- Pasquino, R.; Vasilakopoulos, T.C.; Jeong, Y.C.; Lee, H.; Rogers, S.; Sakellariou, G.; Allgaier, J.; Takano, A.; Brás, A.R.; Chang, T.; et al. Viscosity of Ring Polymer Melts. ACS Macro Lett. 2013, 2, 874–878. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, J. Nucleation and Crystallization of Polymer Melts under Cyclic Stretching: Entanglement Effect. Macromolecules 2023, 56, 5490–5501. [Google Scholar] [CrossRef]
- Roovers, J. Viscoelastic Properties of Polybutadiene Rings. Macromolecules 1988, 21, 1517–1521. [Google Scholar] [CrossRef]
- Chen, D.; Molnar, K.; Kim, H.; Helfer, C.A.; Kaszas, G.; Puskas, J.E.; Kornfield, J.A.; McKenna, G.B. Linear Viscoelastic Properties of Putative Cyclic Polymers Synthesized by Reversible Radical Recombination Polymerization (R3P). Macromolecules 2023, 56, 1013–1032. [Google Scholar] [CrossRef]
- Honda, S.; Yamamoto, T.; Tezuka, Y. Topology-Directed Control on Thermal Stability: Micelles Formed from Linear and Cyclized Amphiphilic Block Copolymers. J. Am. Chem. Soc. 2010, 132, 10251–10253. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Yamamoto, T.; Tezuka, Y. Tuneable Enhancement of the Salt and Thermal Stability of Polymeric Micelles by Cyclized Amphiphiles. Nat. Commun. 2013, 4, 1574. [Google Scholar] [CrossRef]
- Yamamoto, T.; Masuda, Y.; Tezuka, Y.; Korchagina, E.; Winnik, F.M. Comparative Thermodynamic Studies of the Micellization of Amphiphilic Block Copolymers before and after Cyclization. Langmuir 2022, 38, 5033–5039. [Google Scholar] [CrossRef]
- Hoskins, J.N.; Grayson, S.M. Synthesis and Degradation Behavior of Cyclic Poly(ε-caprolactone). Macromolecules 2009, 42, 6406–6413. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Li, Y.; Hoskins, J.N.; Grayson, S.M. Exploring the Effect of Amphiphilic Polymer Architecture: Synthesis, Characterization, and Self-Assembly of Both Cyclic and Linear Poly(ethylene gylcol)-b-polycaprolactone. ACS Macro Lett. 2013, 2, 845–848. [Google Scholar] [CrossRef]
- Gillies, E.R.; Dy, E.; Fréchet, J.M.J.; Szoka, F.C. Biological Evaluation of Polyester Dendrimer: Poly(ethylene oxide) “Bow-Tie” Hybrids with Tunable Molecular Weight and Architecture. Mol. Pharm. 2005, 2, 129–138. [Google Scholar] [CrossRef]
- Chen, B.; Jerger, K.; Fréchet, J.M.J.; Szoka, F.C. The Influence of Polymer Topology on Pharmacokinetics: Differences between Cyclic and Linear PEGylated Poly(acrylic acid) Comb Polymers. J. Control. Release 2009, 140, 203–209. [Google Scholar] [CrossRef]
- Wang, Y.; Quinsaat, J.E.Q.; Ono, T.; Maeki, M.; Tokeshi, M.; Isono, T.; Tajima, K.; Satoh, T.; Sato, S.-i.; Miura, Y.; et al. Enhanced Dispersion Stability of Gold Nanoparticles by the Physisorption of Cyclic Poly(ethylene glycol). Nat. Commun. 2020, 11, 6089. [Google Scholar] [CrossRef] [PubMed]
- Nasongkla, N.; Chen, B.; Macaraeg, N.; Fox, M.E.; Fréchet, J.M.J.; Szoka, F.C. Dependence of Pharmacokinetics and Biodistribution on Polymer Architecture: Effect of Cyclic versus Linear Polymers. J. Am. Chem. Soc. 2009, 131, 3842–3843. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, S.; Ma, L.-W.; Yu, C.-Y.; Wei, H. Multicyclic Topology-Enhanced Micelle Stability and pH-Sensitivity. Eur. Polym. J. 2022, 177, 111446. [Google Scholar] [CrossRef]
- Haque, F.M.; Grayson, S.M. The Synthesis, Properties and Potential Applications of Cyclic Polymers. Nat. Chem. 2020, 12, 433–444. [Google Scholar] [CrossRef]
- Chen, C.; Weil, T. Cyclic Polymers: Synthesis, Characteristics, and Emerging Applications. Nanoscale Horiz. 2022, 7, 1121–1135. [Google Scholar] [CrossRef]
- Gao, R.-T.; Xu, L.; Li, S.-Y.; Liu, N.; Chen, Z.; Wu, Z.-Q. Cyclic Polymers: Controlled Synthesis, Properties and Perspectives. Chem.–A Eur. J. 2023, 29, e202300916. [Google Scholar] [CrossRef]
- Geiser, D.; Höcker, H. Synthesis and Investigation of Macrocyclic Polystyrene. Macromolecules 1980, 13, 653–656. [Google Scholar] [CrossRef]
- Hild, G.; Kohler, A.; Rempp, P. Synthesis of Ring-Shaped Macromolecules. Eur. Polym. J. 1980, 16, 525–527. [Google Scholar] [CrossRef]
- Laurent, B.A.; Grayson, S.M. An Efficient Route to Well-Defined Macrocyclic Polymers via “Click” Cyclization. J. Am. Chem. Soc. 2006, 128, 4238–4239. [Google Scholar] [CrossRef]
- Kang, G.; Sun, L.; Liu, Y.; Meng, C.; Ma, W.; Wang, B.; Ma, L.; Yu, C.; Wei, H. Micelles with Cyclic Poly(ε-caprolactone) Moieties: Greater Stability, Larger Drug Loading Capacity, and Slower Degradation Property for Controlled Drug Release. Langmuir 2019, 35, 12509–12517. [Google Scholar] [CrossRef]
- Laurent, B.A.; Grayson, S.M. Synthetic Approaches for the Preparation of Cyclic Polymers. Chem. Soc. Rev. 2009, 38, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Liénard, R.; De Winter, J.; Coulembier, O. Cyclic Polymers: Advances in their Synthesis, Properties, and Biomedical Applications. J. Polym. Sci. 2020, 58, 1481–1502. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Z.; Xie, J.; Wang, W.; Zhu, H.; Zhang, E.; Cao, Z. Micelles with Ultralow Critical Micelle Concentration as Carriers for Drug Delivery. Nat. Biomed. Eng. 2018, 2, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-Y.; Ma, W.; Liu, M.-Z.; Luo, H.-X.; Yu, C.-Y.; Wei, H. Expanding Cyclic Topology-Based Biomedical Polymer Panel: Universal Synthesis of Hetero-”8”-Shaped Copolymers and Topological Modulation of Polymer Degradation. Macromol. Rapid Commun. 2021, 42, 2100298. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Liu, Y.; Li, L.; Sun, L.; Ma, W.; Meng, C.; Ma, L.; Zheng, G.; Chang, C.; Wei, H. Modulation of Cyclic Topology toward Enhanced Drug Delivery, from Linear and Tadpole-Like to Dumbbell-Shaped Copolymers. Chem. Commun. 2020, 56, 3003–3006. [Google Scholar] [CrossRef]
- Dong, Y.-Q.; Tong, Y.-Y.; Dong, B.-T.; Du, F.-S.; Li, Z.-C. Preparation of Tadpole-Shaped Amphiphilic Cyclic PS-b-linear PEO via ATRP and Click Chemistry. Macromolecules 2009, 42, 2940–2948. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Y.; Song, T.; Cui, J.; Fan, J. Topology-Directed Coassembly of Linear and Cyclic Amphiphilic Diblock Copolymers: A Monte Carlo Study. Langmuir 2024, 40, 16103–16112. [Google Scholar] [CrossRef]
- Chang, C.; Wei, H.; Feng, J.; Wang, Z.-C.; Wu, X.-J.; Wu, D.-Q.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Temperature and pH Double Responsive Hybrid Cross-Linked Micelles Based on P(NIPAAm-co-MPMA)-b-P(DEA): RAFT Synthesis and “Schizophrenic” Micellization. Macromolecules 2009, 42, 4838–4844. [Google Scholar] [CrossRef]
- Ma, W.; Kang, G.-Y.; Sun, L.; Meng, C.; Liu, Y.; Zheng, Z.; Jiang, M.-C.; Wang, D.; Pun, S.H.; Yu, C.-Y.; et al. Multicyclic Topology-Enhanced Anticancer Drug Delivery. J. Control. Release 2022, 345, 278–291. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Ma, Z.; Tu, X.; Zhao, S.; Wasng, B.; Ma, L.; Wei, H. Promotion of Micelle Stability via A Cyclic Hydrophilic Moiety. Polym. Chem. 2018, 9, 2569–2573. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Zhang, X.; Tu, X.; Wang, M.; Ma, L.; Wang, B.; He, J.; Ni, P.; Wei, H. Fabrication of Cyclic Brush Copolymers with Heterogeneous Amphiphilic Polymer Brushes for Controlled Drug Release. Macromolecules 2018, 51, 7672–7679. [Google Scholar] [CrossRef]
- Golba, B.; Benetti, E.M.; De Geest, B.G. Biomaterials Applications of Cyclic Polymers. Biomaterials 2021, 267, 120468. [Google Scholar] [CrossRef] [PubMed]
- Minatti, E.; Viville, P.; Borsali, R.; Schappacher, M.; Deffieux, A.; Lazzaroni, R. Micellar Morphological Changes Promoted by Cyclization of PS-b-PI Copolymer: DLS and AFM Experiments. Macromolecules 2003, 36, 4125–4133. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Li, N.; Guo, C.; Li, S. The Effect of Topology on Block Copolymer Nanoparticles: Linear versus Star Block Copolymers in Toluene. Polymers 2022, 14, 3691. [Google Scholar] [CrossRef]
- Rodichkin, I.D.; Gumerov, R.A.; Potemkin, I.I. Self-Assembly of Miktoarm Palm Tree-Like Star Copolymers in A Selective Solvent. J. Colloid Interface Sci. 2022, 606, 1966–1973. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, Y.; Huang, Y.; Xu, Y.; You, D.; Lu, W.; Yu, J. Rod-Shaped Micelles Based on PHF-g-(PCL-PEG) with pH-Triggered Doxorubicin Release and Enhanced Cellular Uptake. Biomacromolecules 2019, 20, 1167–1177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, G.; Lu, M.; Zhou, K.; Yu, C.; Wei, H. The Preparation of Cyclic Binary Block Polymer Using Bimolecular Homodifunctional Coupling Reaction and Characterization of Its Performance as a Drug Carrier. Molecules 2025, 30, 599. https://doi.org/10.3390/molecules30030599
Kang G, Lu M, Zhou K, Yu C, Wei H. The Preparation of Cyclic Binary Block Polymer Using Bimolecular Homodifunctional Coupling Reaction and Characterization of Its Performance as a Drug Carrier. Molecules. 2025; 30(3):599. https://doi.org/10.3390/molecules30030599
Chicago/Turabian StyleKang, Guiying, Muxin Lu, Kang Zhou, Cuiyun Yu, and Hua Wei. 2025. "The Preparation of Cyclic Binary Block Polymer Using Bimolecular Homodifunctional Coupling Reaction and Characterization of Its Performance as a Drug Carrier" Molecules 30, no. 3: 599. https://doi.org/10.3390/molecules30030599
APA StyleKang, G., Lu, M., Zhou, K., Yu, C., & Wei, H. (2025). The Preparation of Cyclic Binary Block Polymer Using Bimolecular Homodifunctional Coupling Reaction and Characterization of Its Performance as a Drug Carrier. Molecules, 30(3), 599. https://doi.org/10.3390/molecules30030599





