Monitoring Dynamic Changes of Cellular Membrane GSH During Stroke via an ESIPT-Based Near-Infrared Fluorescent Probe
Abstract
1. Introduction
2. Results and Discussion
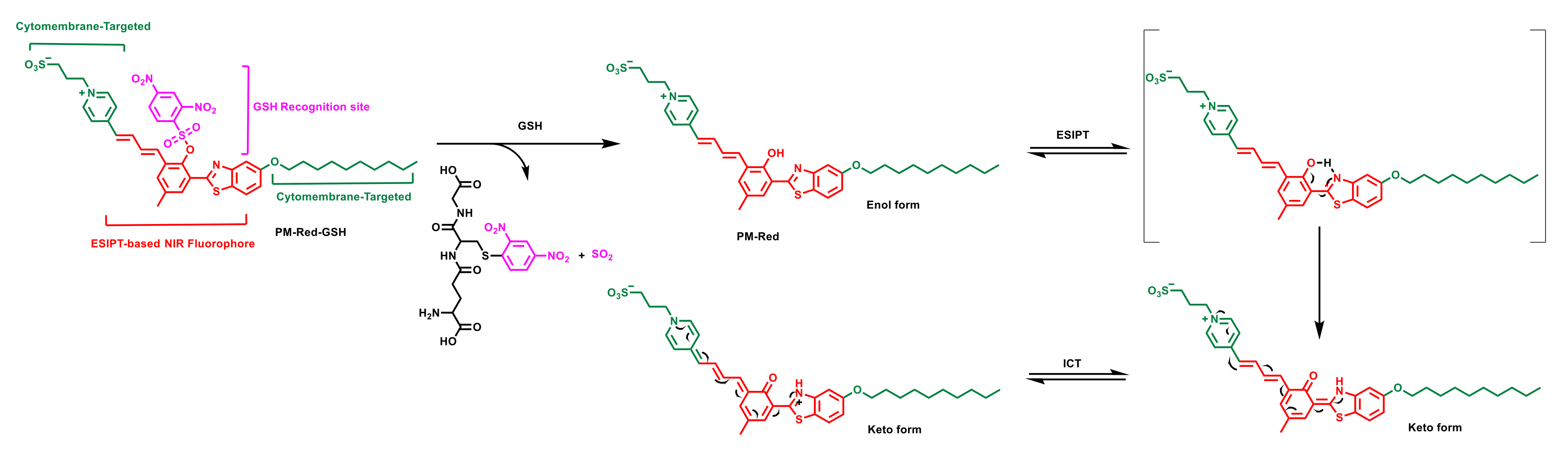
2.1. Design Strategy and Synthesis Process of Fluorescent Probe PM-Red-GSH
2.2. Spectral Response Performances of PM-Red-GSH Toward GSH
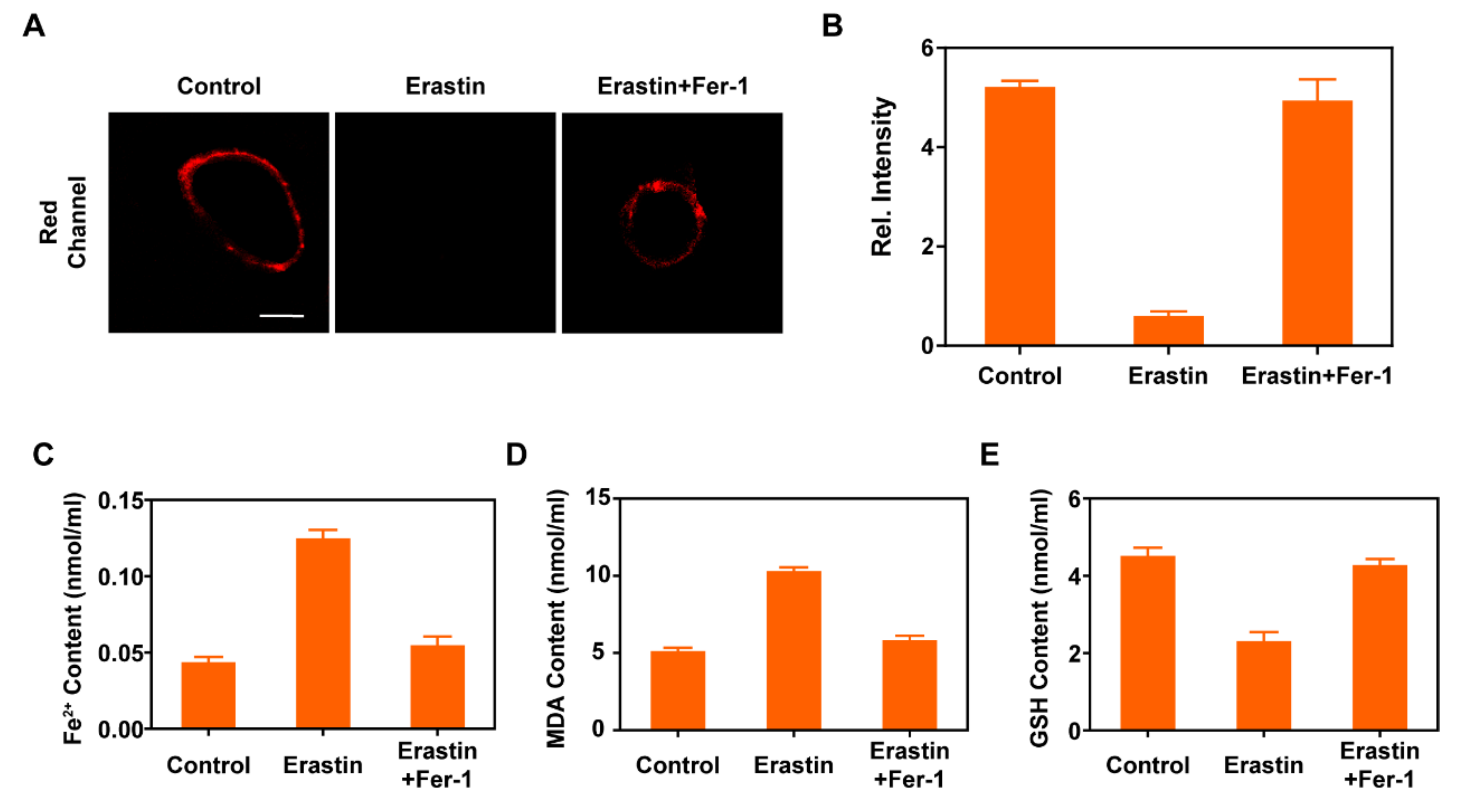
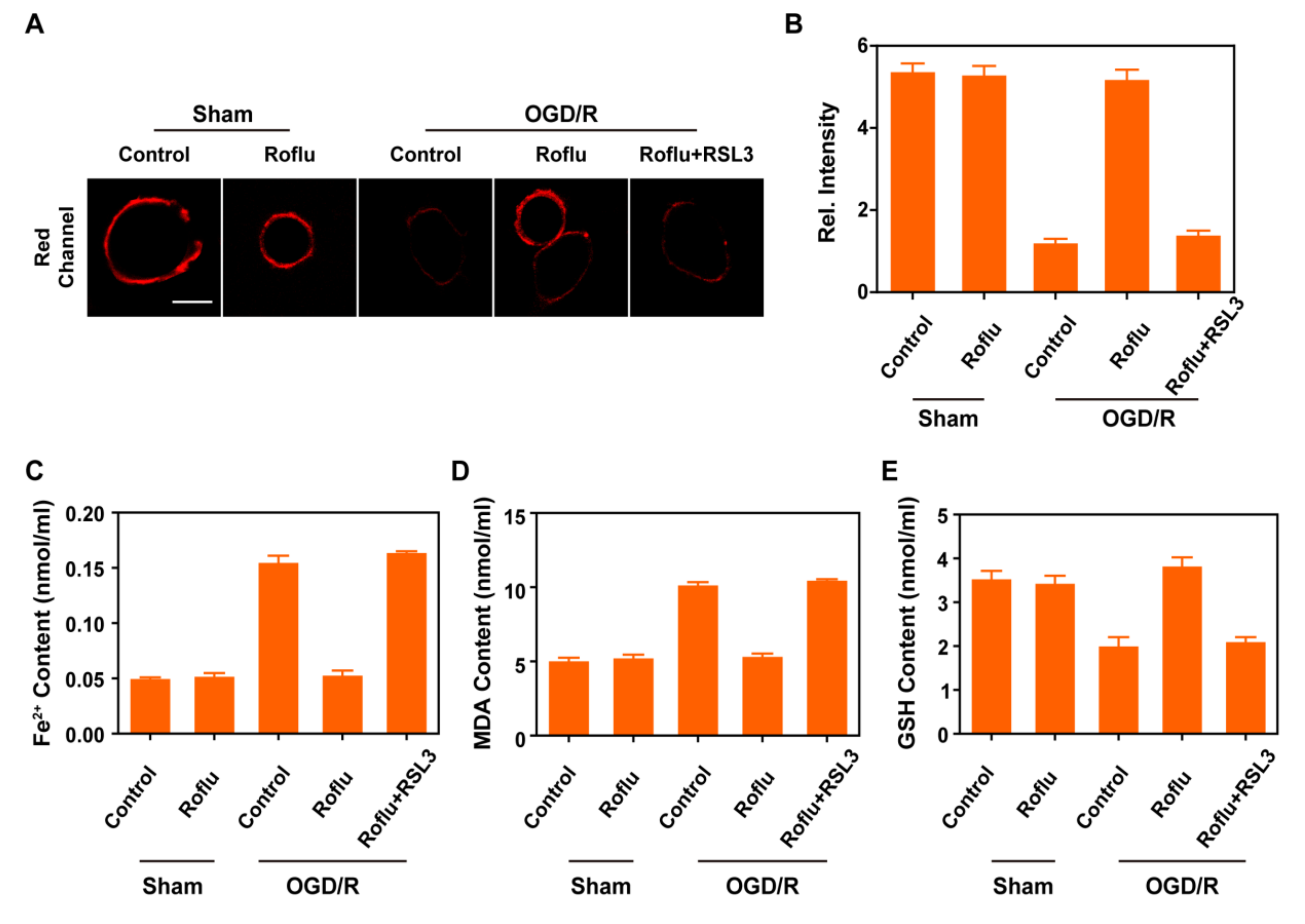
2.3. Bioimaging Application of PM-Red-GSH in Living Cells
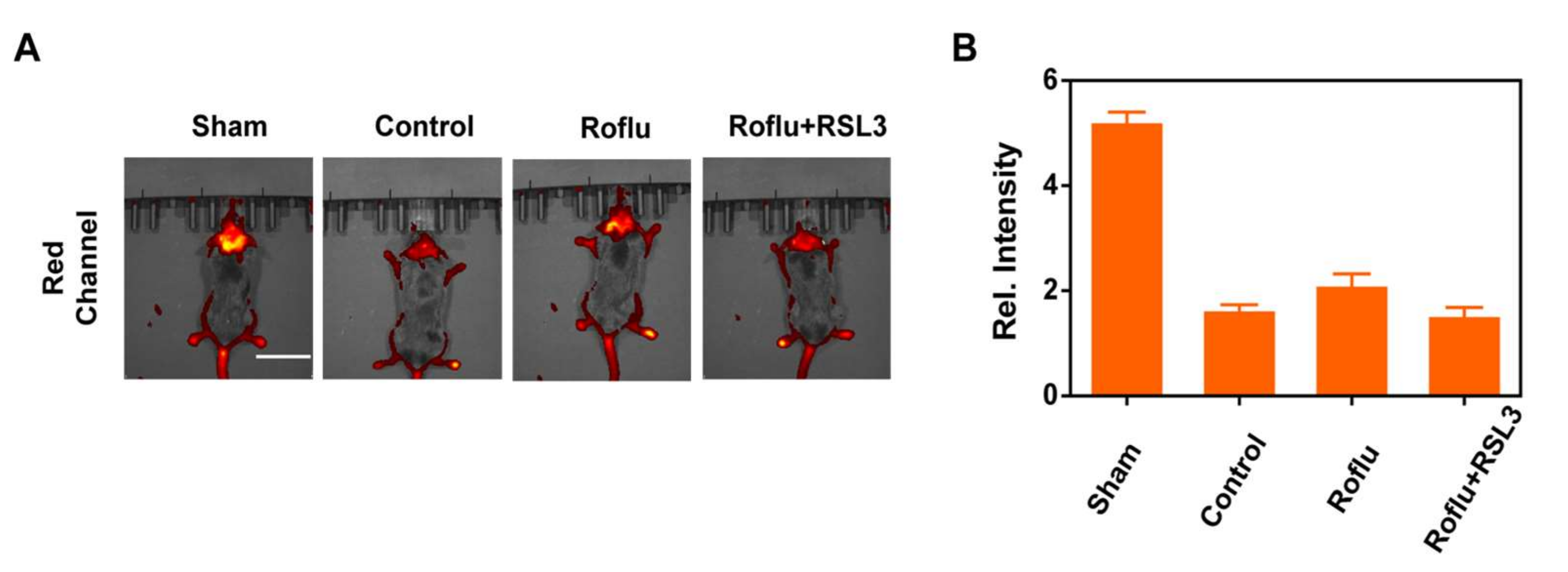
2.4. In Vivo Imaging of Cerebral Apoplexy in Mice
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Joy, M.T.; Carmichael, S.T. Encouraging an excitable brain state: Mechanisms of brain repair in stroke. Nat. Rev. Neurosci. 2021, 22, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, S.; Saha, S. Therapeutic Benefits of Nanoparticles in Stroke. Front. Neurosci. 2015, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprech, M.R. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Guan, Q.; Gao, X. Reaction-based color-convertible fluorescent probe for ferroptosis identification. Anal. Chem. 2018, 90, 9218–9225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Kuang, F.; Liu, J.; Tang, D.; Kang, R. Oxidative damage and antioxidant defense in ferroptosis. Front. Cell Dev. Biol. 2020, 8, 586578. [Google Scholar] [CrossRef]
- Yagoda, N.; von Rechenberg, M.; Zaganjor, E.; Bauer, A.J.; Yang, W.S.; Fridman, D.J.; Wolpaw, A.J.; Smukste, I.J.; Peltier, M.; Boniface, J.J.; et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 2007, 447, 865–869. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Xie, Y.; Chen, B.; Zhu, S. Single fluorescent probe responds to H2O2, NO, and H2O2/NO with three different sets of fluorescence signals. J. Am. Chem. Soc. 2012, 134, 1305–1315. [Google Scholar] [CrossRef]
- Xiong, K.; Huo, F.; Chao, J.; Zhang, Y.; Yin, C. Colorimetric and NIR fluorescence probe with multiple binding sites for distinguishing detection of Cys/Hcy and GSH in vivo. Anal. Chem. 2018, 91, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Huo, F.; Ning, P.; Zhang, Y.; Chao, J.; Meng, X.; Yin, C. Dual-site fluorescent probe for visualizing the metabolism of cys in living cells. J. Am. Chem. Soc. 2017, 139, 3181–3185. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.Y.; Chen, Y.Z.; Zheng, H.R.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. Design strategies of fluorescent probes for selective detection among biothiols. Chem. Soc. Rev. 2015, 44, 6143–6160. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ma, Y.; Yin, J.; Lin, W. Strategies for designing organic fluorescent probes for biological imaging of reactive carbonyl species. Chem. Soc. Rev. 2019, 48, 4036–4048. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, J.; Bajic, A.; Zhang, C.; Wang, J. Quantitative real-time imaging of glutathione. Nat. Commun. 2017, 8, 16087. [Google Scholar] [CrossRef]
- Jian, Z.; Ye, Y.; Yu, K.; Zhu, W.H.; Wang, J.; Xiong, X.; Li, C.; Gu, L. Ratiometric two-photon fluorescent probe for imaging hydrogen peroxide during stroke-induced ferroptosis. Sens. Actuators B Chem. 2024, 417, 136064. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.Y.; Zhao, S.; Gao, W.S.; Chen, B.; He, L.W.; Zhu, S.S. A unique approach to development of near-infrared flu-orescent sensors for in vivo imaging. J. Am. Chem. Soc. 2012, 134, 13510–13523. [Google Scholar] [CrossRef]
- Wang, J.P.; Zhang, F.; Yang, L.; Wang, B.H.; Song, X.Z. A red-emitting fluorescent probe for sensing and imaging biothiols in living cells. J. Lumin. 2021, 234, 117994. [Google Scholar] [CrossRef]
- Liu, K.Y.; Shang, H.M.; Kong, X.Q.; Lin, W.Y. A novel near-infrared fluorescent probe with large Stokes shift for biothiols and the application for in vitro and in vivo fluorescence imaging. J. Mater. Chem. B 2017, 5, 3836–3841. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, L.W.; Wang, J.Y. A NIR fluorescent sensor for biothiols based on a dicyanoisophorone derivative with a large Stokes shift and high quantum yield. New J. Chem. 2019, 43, 9614–9622. [Google Scholar] [CrossRef]
- Wang, K.; Leng, T.H.; Liu, Y.J.; Wang, C.Y.; Shi, P.; Shen, Y.J.; Zhu, W.H. A novel near-infrared fluorescent probe with a large stokes shift forthe detection and imaging of biothiols. Sens. Actuators B Chem. 2017, 248, 338–345. [Google Scholar] [CrossRef]
- Li, M.; Wu, X.M.; Wang, Y.; Li, Y.S.; Zhu, W.H.; James, T.D. A near-infrared colorimetric fluorescent chemodosimeter for the detection of glutathione in living cells. Chem. Commun. 2014, 50, 1751–1753. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.H.; Yan, D.L.; Hou, P.; Liu, X.B.; Wang, H.; Xia, C.H.; Li, G.; Chen, S. Bioimaging and sensing thiols in vivo and in tumor tissues based on a near-infrared fluorescent probe with large Stokes shift. Molecules 2023, 28, 5702. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.P.; Liu, X.J.; Yang, L.; Yang, L.; Chen, W.Q.; Song, X.Z. A red-emitting fluorescent probe for biothiols detection with a large Stokes shift. Tetrahedron 2016, 72, 6909–6913. [Google Scholar] [CrossRef]
- Chen, D.G.; Long, Z.; Sun, Y.M.; Luo, Z.J.; Lou, X.D. A red-emission probe for intracellular biothiols imaging with a large Stokes shift. J. Photochem. Photobiol. A 2019, 368, 90–96. [Google Scholar] [CrossRef]
- Wu, Z.J.; Zhao, T.T.; Jiang, X.Y.; Xia, X.F.; Zhang, D.; Wang, F.Y.; Ren, X.M.; Wang, Z.; Ren, J.; Wang, E.F. A near-infrared fluores-cent probe with a large Stokes shift for the detection and imaging of biothiols in vitro and in vivo. Anal. Bioanal. Chem. 2024, 416, 6485–6495. [Google Scholar] [CrossRef]
- Li, H.; Deng, C.; Zhu, N.; Zhang, C.J.; Zeng, Q.; Qin, L. An ultrasensitive GSH-specific fluorescent probe unveils celastrol-induced ccRCC ferroptosis. Bioorg. Chem. 2023, 134, 106454. [Google Scholar] [CrossRef]
- He, R.; Tang, D.D.; Xu, N.G.; Liu, H.; Dou, K.; Zhou, X.J.; Yu, F.B. Evaluation of erastin synergized cisplatin anti-nasopharyngeal carcinoma effect with a glutathione-activated near-infrared fluorescent probe. Chin. Chem. Lett. 2024, 35, 108658. [Google Scholar] [CrossRef]
- Wang, Y.T.; Yan, H.M.; Yue, Y.K.; Zhang, Y.B.; Huo, F.J.; Cheng, F.Q.; Yin, C.X. GSH and H2O2 dynamic correlation in the ferroptosis pathways revealed by engineered probe in tumor and kidney injury. Chem. Eng. J. 2023, 464, 142496. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhang, Y.R.; Zheng, M.H.; Wang, X.; Wu, X.; Jin, J.Y. A single fluorescent probe reveals changes in endoplasmic reticulum–mitochondria contact in hepatocytes during ferroptosis. Chem. Eng. J. 2023, 466, 143104. [Google Scholar] [CrossRef]
- Jia, T.T.; Guo, D.D.; Meng, X.; Du, H.T.; Qin, F.Y.; Chen, J.L.; Niu, H.W. Development of a fast fluorescent probe for sensitive detection of glutathione in 100% aqueous solution and its applications in real samples, oxidative stress model and ferroptosis model. Food Chem. 2025, 463, 141073. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.X.; Wang, Y.; Yue, T.; Wei, H.; Li, S.J.; Dong, B.L. Fluorescence imaging of intracellular Glutathione levels in the endoplasmic reticulum to reveal the inhibition effect of rutin on ferroptosis. Anal. Chem. 2023, 95, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cheung, S.; Sampedro, G.; Chen, Z.L.; Cahill, R.A.; O’Shea, D.F. A DIE responsive NIR-fluorescent cell membrane probe. BBA Biomembr. 2018, 11, 1860. [Google Scholar] [CrossRef]
- Wang, K.N.; Qi, G.; Chu, H.; Chao, X.J.; Liu, L.Y.; Li, G.; Cao, Q.; Mao, Z.; Liu, B. Probing Cell Membrane Damage using Molecular Rotor Probe with Membrane-to-Nucleus Translocation. Mater. Horiz. 2020, 7, 3226–3233. [Google Scholar] [CrossRef]
- Li, Q.H.; Zhu, W.L.; Gong, S.Y.; Jiang, S.Y.; Feng, G.Q. Selective Visualization of Tumor Cell Membranes and Tumors with a Viscosity-Sensitive Plasma Membrane Probe. Anal. Chem. 2023, 95, 7254–7261. [Google Scholar] [CrossRef]
- Yu, L.; Xie, M.L.; Chen, M.; Yang, H.R.; Chen, L.; Xing, P.F.; Tian, Z.Y.; Wang, C.J. An ortho-activation strategy to develop NIR fluorescent probe for rapid imaging of biothiols in vivo. Talanta 2024, 266, 125110. [Google Scholar] [CrossRef]
- Yuan, H.; Han, Z.; Chen, Y.; Qi, F.; Fang, H.; Guo, Z.; Zhang, S.; He, W. Ferroptosis photoinduced by new cyclometalated iri-ium(III) complexes and its synergism with apoptosis in tumor cell inhibition. Angew. Chem. 2021, 60, 8174–8181. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Ru, F.; Gan, Y.; Li, B.; Xia, W.; Dai, G.; He, Y.; Chen, Z. Liproxstatin-1 attenuates unilateral ureteral obstruction-induced renal fibrosis by inhibiting renal tubular epithelial cells ferroptosis. Cell Death Dis. 2021, 12, 843. [Google Scholar] [CrossRef]
- Liu, L.; Yang, C.; Zhu, L.; Wang, Y.; Zheng, F.; Liang, L.; Cao, P.F.; Liu, J.; Han, X.; Zhang, J. RSL3 enhances ROS-mediated cell apoptosis of myelodysplastic syndrome cells through MYB/Bcl-2 signaling pathway. Cell Death Dis. 2024, 15, 465. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, J.; Xie, X.L.; Li, M.M.; Niu, J.Y.; Tong, L.L.; Tang, B. Fluorescent Probe Based on Azobenzene-Cyclopalladium for the Selective Imaging of Endogenous Carbon Monoxide under Hypoxia Conditions. Anal. Chem. 2016, 88, 11154–11159. [Google Scholar] [CrossRef]
- Liang, T.; Qiang, T.; Ren, L.; Cheng, F.; Wang, B.; Li, M.; Hu, W.; James, T.D. Near-infrared fluorescent probe for hydrogen sulfide: High-fidelity ferroptosis evaluation in vivo during stroke. Chem. Sci. 2022, 13, 2992–3001. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Han, X.Y.; Zhang, L.W.; Chen, L.X. Evaluating the Protective Effects of Mitochondrial Glutathione on Cerebral Ischemia/Reperfusion Injury via Near-Infrared Fluorescence Imaging. Anal. Chem. 2019, 91, 14728–14736. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, Z. Monitoring Dynamic Changes of Cellular Membrane GSH During Stroke via an ESIPT-Based Near-Infrared Fluorescent Probe. Molecules 2025, 30, 592. https://doi.org/10.3390/molecules30030592
Gao Y, Wang Z. Monitoring Dynamic Changes of Cellular Membrane GSH During Stroke via an ESIPT-Based Near-Infrared Fluorescent Probe. Molecules. 2025; 30(3):592. https://doi.org/10.3390/molecules30030592
Chicago/Turabian StyleGao, Yue, and Zhao Wang. 2025. "Monitoring Dynamic Changes of Cellular Membrane GSH During Stroke via an ESIPT-Based Near-Infrared Fluorescent Probe" Molecules 30, no. 3: 592. https://doi.org/10.3390/molecules30030592
APA StyleGao, Y., & Wang, Z. (2025). Monitoring Dynamic Changes of Cellular Membrane GSH During Stroke via an ESIPT-Based Near-Infrared Fluorescent Probe. Molecules, 30(3), 592. https://doi.org/10.3390/molecules30030592



