Studies of the Synthesis of Fused Isoxazoline/Isoquinolinones and Evaluation of the Antifungal Activity of Isoxazole-like Benzamide and Isoquinolinone Hybrids
Abstract
1. Introduction
2. Results and Discussion
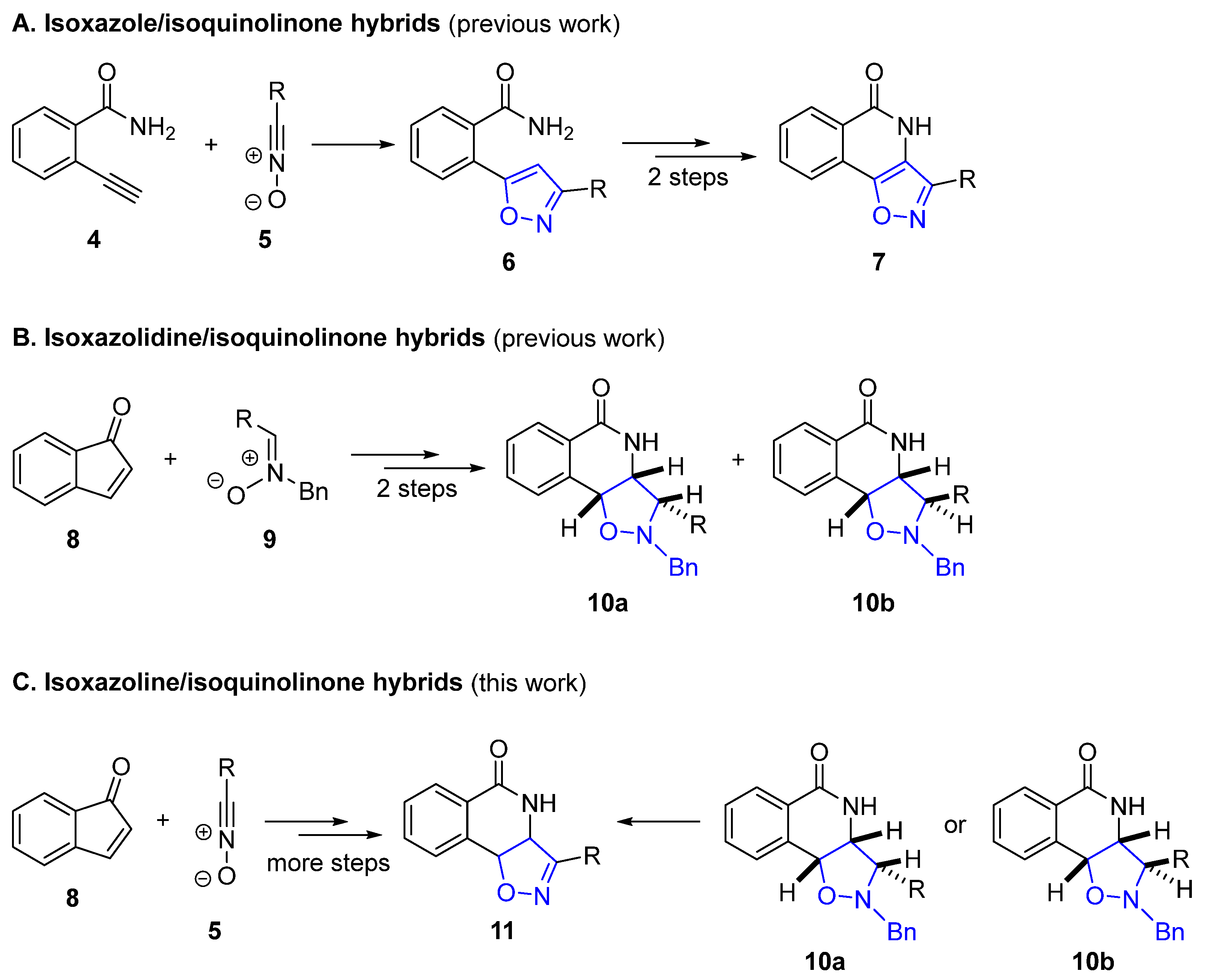
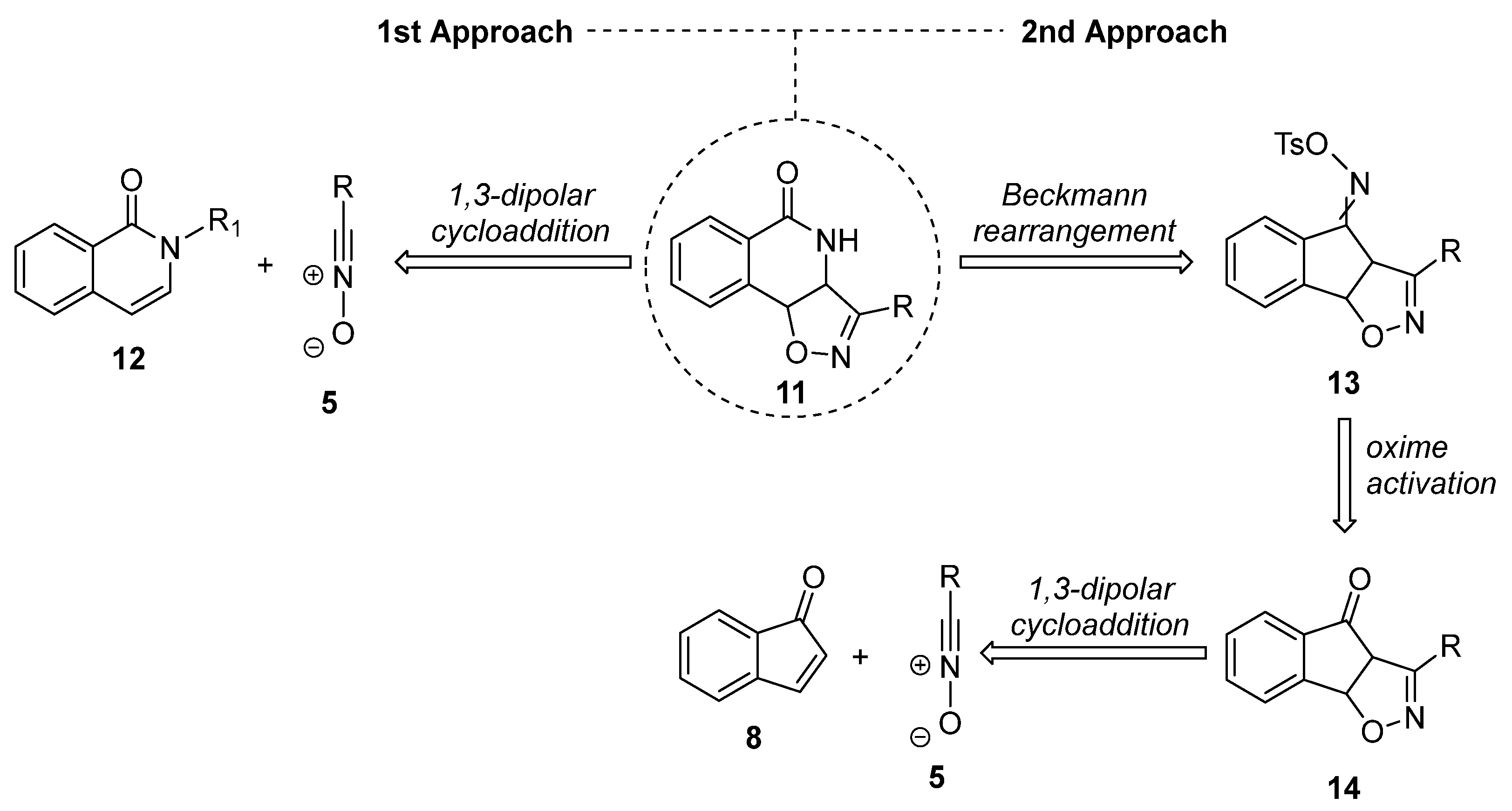
2.1. Retrosynthetic Plans for the Targeted Hybrids
2.2. Attempted Synthesis Directly from Isoquinolinone
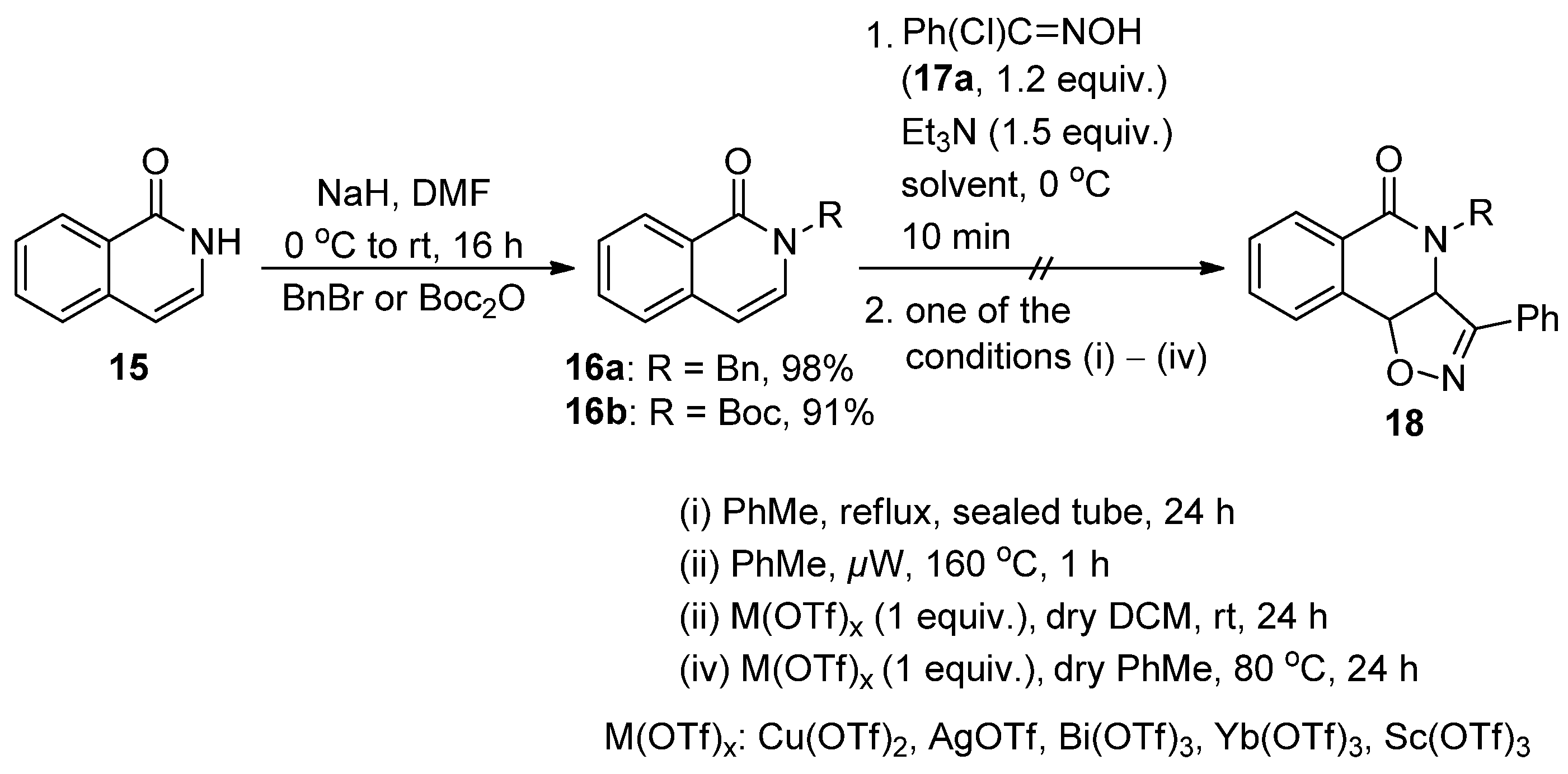
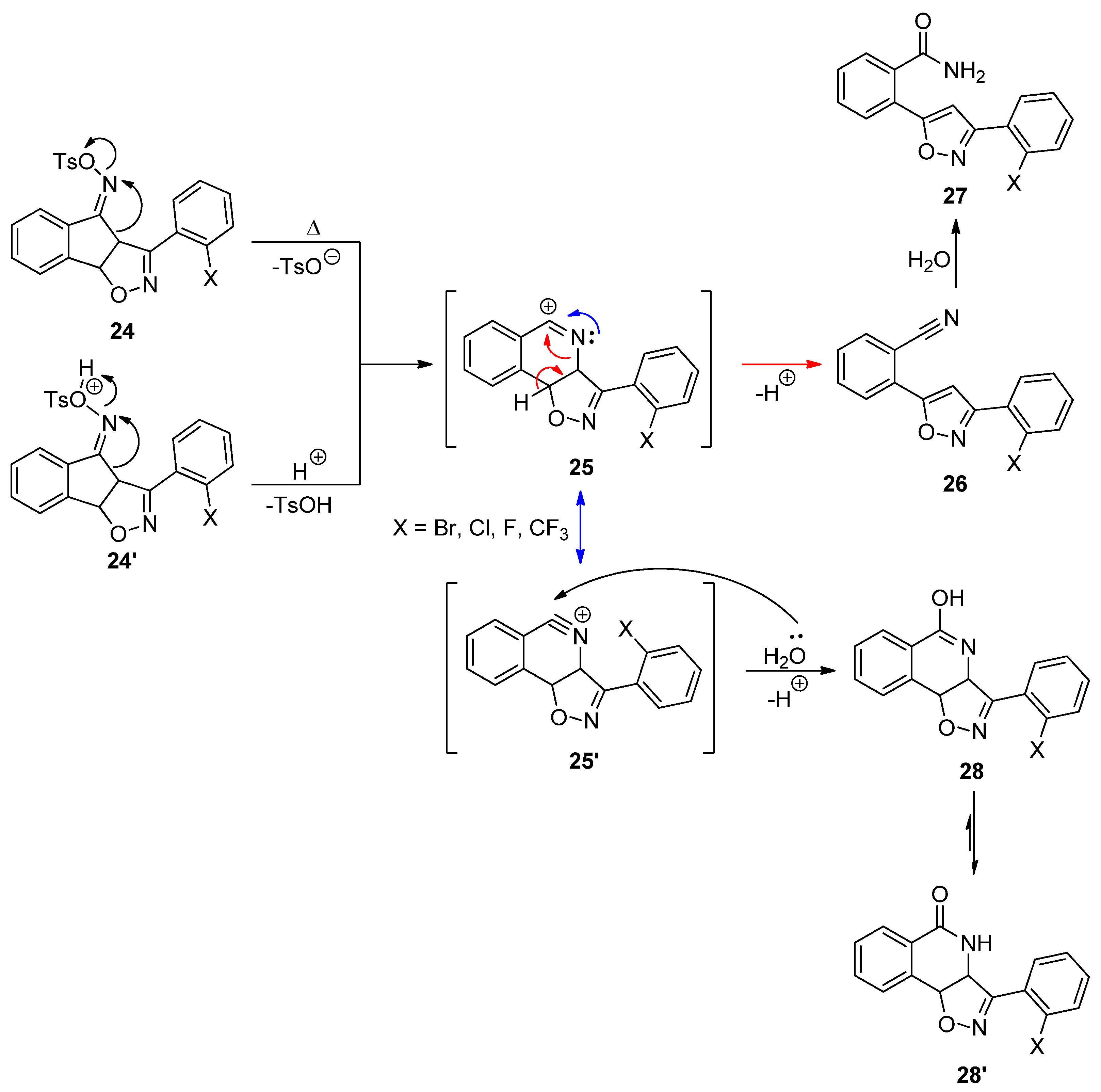
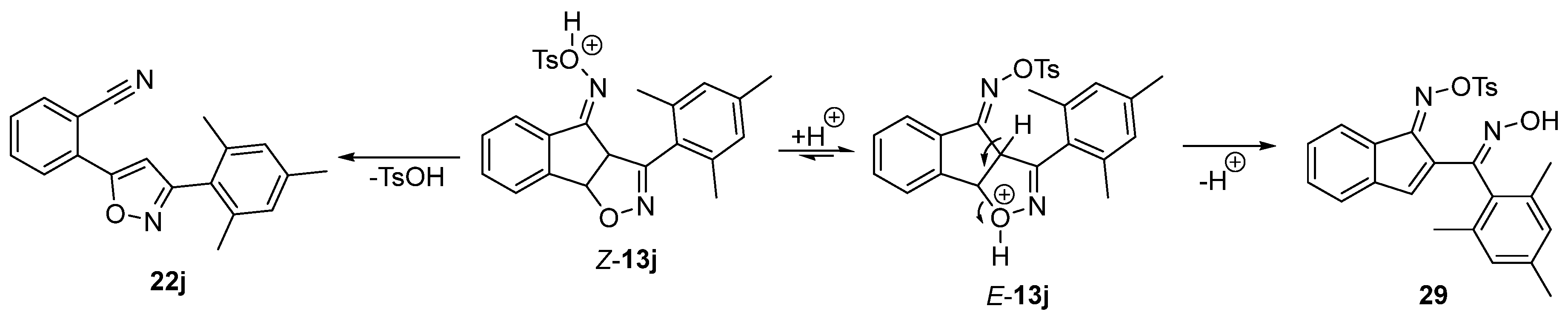
2.3. Exploring the Synthesis of Isoxazoline/Isoquinolinone Hybrids via Beckmann Rearrangement
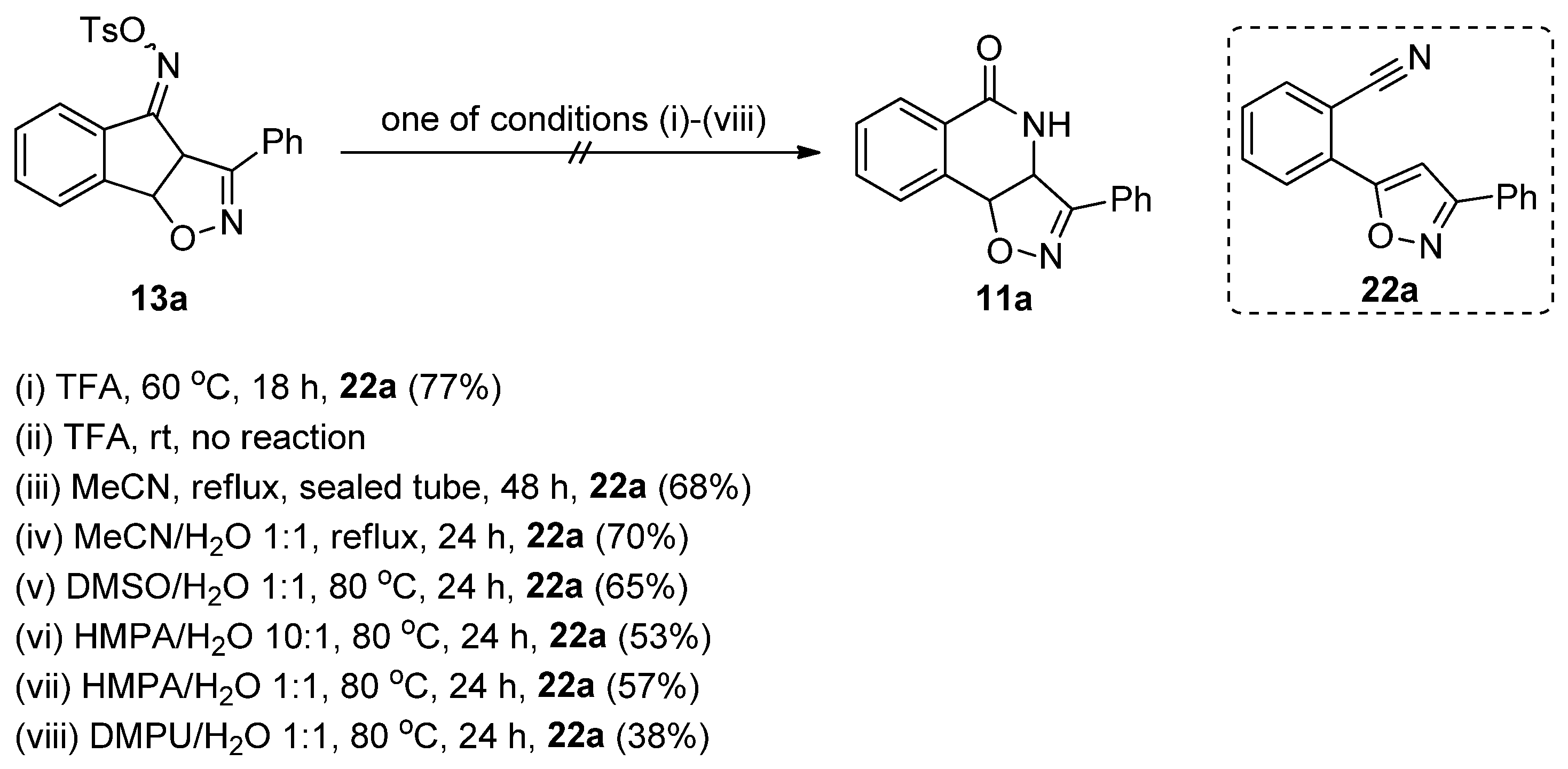
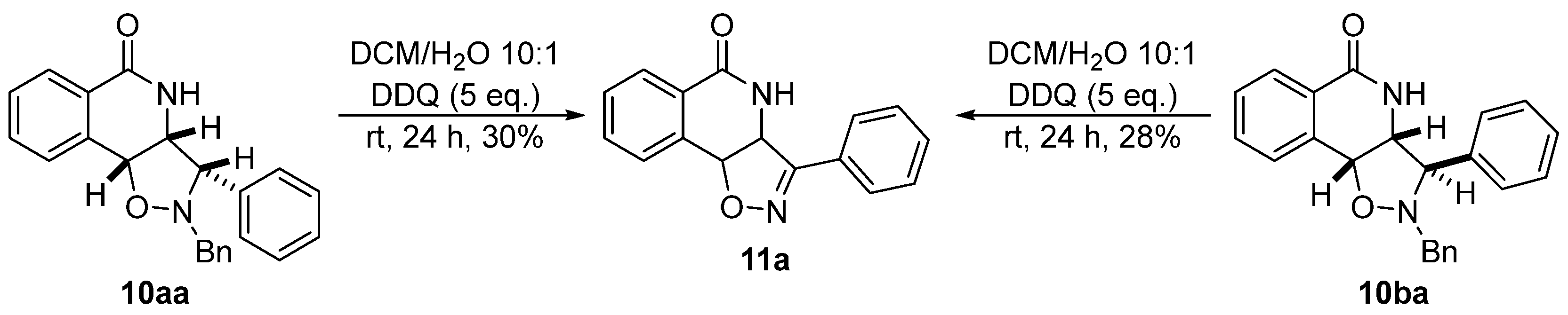
2.4. Isoxazoline/Isoquinolinone Hybrids via Oxidation of the Corresponding Isoxazolidines
2.5. Evaluation of Antifungal Activity
| Compound | Strain | MIC1° (Compound) | MIC2° (K 1) | FIC1°/FIC2° (Compound/K) | FICI |
|---|---|---|---|---|---|
| 23j | C. albicans | 0.08 | 0.0125 | 0.005/0.0125 | 1.06 |
| C. krusei | 0.16 | 0.0004 | 0.08/0.0008 | 2.50 | |
| A. fumigatus | 0.08 | 0.025 | 0.04/0.00625 | 0.75 | |
| A.niger | 0.04 | 0.10 | 0.04/0.10 | 2.00 | |
| P. funiculosum | 0.04 | 0.0125 | 0.04/0.00315 | 1.25 | |
| P. chrysogenum | 0.08 | 0.05 | 0.02/0.025 | 0.75 | |
| 11l | C. albicans | 0.16 | 0.0125 | 0.16/0.0125 | 2 |
| C. krusei | 0.16 | 0.0004 | 0.32/0.0008 | 4 | |
| A. fumigatus | 0.16 | 0.025 | 0.01/0.025 | 1.06 | |
| A.niger | 0.01 | 0.10 | 0.005/0.05 | 1 | |
| P. funiculosum | 0.04 | 0.0125 | 0.08/0.00315 | 2.25 | |
| P. chrysogenum | 0.16 | 0.05 | 0.01/0.0125 | 0.375 | |
| 10bm | C. albicans | 0.16 | 0.0125 | 0.16/0.0125 | 2 |
| C. krusei | 0.16 | 0.0004 | 0.01/0.0002 | 0.28 | |
| A. fumigatus | 0.16 | 0.025 | 0.08/0.00625 | 0.75 | |
| A.niger | 0.005 | 0.10 | 0.0025/0.025 | 0.75 | |
| P. funiculosum | 0.04 | 0.0125 | 0.04/0.00625 | 1.50 | |
| P. chrysogenum | 0.16 | 0.05 | 0.02/0.025 | 0.625 |

3. Experimental Section
3.1. General Information
3.2. General Procedure for the Synthesis of N-Substituted Isoquinolinones 16
3.3. General Procedure for the Preparation of Hydroxamoyl Chlorides 17
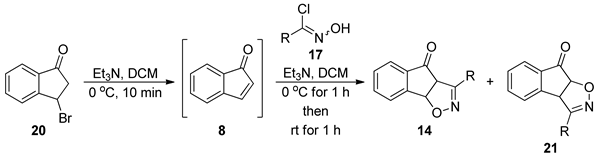
3.4. Synthesis of 3-Bromo-2,3-dihydro-1H-inden-1-one 20
3.5. General Procedure for the Preparation of Isoxazoline Adducts 14 and 21
3.6. General Procedure for the One-Pot Synthesis of Tosyl Oxime 13
3.7. General Procedure for the Beckmann Rearrangement/Fragmentation
3.8. Synthesis of Compounds 23, 30, and 10
3.9. General Procedure for the Hydrolysis of Nitrile 22a
3.10. General Procedure for the DDQ-Mediated Synthesis of Isoxazoline Isoquinolinone Hybrid 11
3.11. In Vitro Antifungal Assay
3.12. In Vitro 2D Checkerboard Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, S.; He, J.; Sun, Y.; He, L.; She, X. Efficient and Regioselective Synthesis of 5-Hydroxy-2-isoxazolines: Versatile Synthons for Isoxazoles, β-Lactams, and γ-Amino Alcohols. J. Org. Chem. 2010, 75, 1961–1966. [Google Scholar] [CrossRef]
- Berthet, M.; Cheviet, T.; Dujarin, G.; Parrot, I.; Marinez, J. Isoxazolidine: A Privileged Scaffold for Organic and Medicinal Chemistry. Chem. Rev. 2016, 116, 15235–15283. [Google Scholar] [CrossRef] [PubMed]
- Duc, D.X.; Dung, V.C. Recent Progress in the Synthesis of Isoxazoles. Curr. Org. Chem. 2021, 25, 2938–2989. [Google Scholar] [CrossRef]
- Kleinbeck, F.; Carreira, E.M. Total Synthesis of Bafilomycin A1. Angew. Chem. Int. Ed. 2009, 48, 578–581. [Google Scholar] [CrossRef]
- Kudryavtseva, T.N.; Lamanov, A.Y.; Sysoev, P.I.; Klimova, L.G. Synthesis and Antibacterial Activity of New Acridone Derivatives Containing an Isoxazoline Fragment. Russ. J. Gen. Chem. 2020, 90, 45–49. [Google Scholar] [CrossRef]
- Pandhurnekar, C.P.; Pandhurnekar, H.C.; Mungole, A.J.; Butoliya, S.S.; Yadao, B.G. A Review of Recent Synthetic Strategies and Biological Activities of Isoxazole. J. Heterocycl. Chem. 2023, 60, 537–565. [Google Scholar] [CrossRef]
- Kumar, G.; Shankar, R. 2-Isoxazolines: A Synthetic and Medicinal Overview. ChemMedChem 2021, 16, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Kitao, S.; Okita, T.; Shoji, T. Total Synthesis and Assignment of the Double-Bond Position and Absolute Configuration of (−)-Pyrinodemin A. Org. Lett. 2003, 5, 2611–2614. [Google Scholar] [CrossRef]
- Wei, H.; Qiao, C.; Liu, G.; Yang, Z.; Li, C.-C. Stereoselective Total Syntheses of (−)-Flueggine A and (+)-Virosaine B. Angew. Chem. 2013, 125, 648–652. [Google Scholar] [CrossRef]
- Serna, A.V.; Kürti, L.; Siitonen, J.H. Synthesis of (−)-Setigerumine I: Biosynthetic Origins of the Elusive Racemic Papaveraceae Isoxazolidine Alkaloids. Angew. Chem. Int. Ed. 2021, 60, 27236–27240. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Q.; Tang, H.; Pan, X. Isoxazole/Isoxazoline Skeleton in the Structural Modification of Natural Products: A Review. Pharmaceuticals 2023, 16, 228. [Google Scholar] [CrossRef] [PubMed]
- Algethami, F.K.; Saidi, I.; Abdelhamid, H.N.; Elamin, M.R.; Abdulkhair, B.Y.; Chrouda, A.; Jannet, H.B. Trifluoromethylated Flavonoid-Based Isoxazoles as Antidiabetic and Anti-Obesity Agents: Synthesis, In Vitro a-Amylase Inhibitory Activity, Molecular Docking and Structure–Activity Relationship Analysis. Molecules 2021, 26, 5214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mo, J.; Lin, H.; Sun, H. The recent progress of isoxazole in medicinal chemistry. Bioorg. Med. Chem. 2018, 26, 3065–3075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Z.; Zhao, Z.-L.; Zhou, C.-H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef]
- Agrawal, N.; Mishra, P. The synthetic and therapeutic expedition of isoxazole and its analogs. Med. Chem. Res. 2018, 27, 1309–1344. [Google Scholar] [CrossRef]
- Shinde, Y.; Khairnar, B.; Bangale, S. Exploring the Diverse Biological Frontiers of Isoxazole: A Comprehensive Review of its Pharmacological Significance. ChemistrySelect 2024, 9, e202401423. [Google Scholar] [CrossRef]
- Ouzounthanasis, K.A.; Rizos, S.R.; Koumbis, A.E. A Convenient Synthesis of Novel Isoxazolidine and Isoxazole Isoquinolinones Fused Hybrids. Molecules 2024, 29, 91. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.B.; Sreenivasulu, C.; Kishore, D.R.; Satyanarayana, G. Trending Strategies for the Synthesis of Quinolinones and Isoquinolinones. Tetrahedron 2022, 127, 133093. [Google Scholar] [CrossRef]
- Mesaros, E.F.; Cole, A.G.; Kultgen, S.G.; Mani, N.; Fan, K.Y.; Dugan, B.J.; Ardzinski, A.; Stever, K.; Micolochick Steuer, H.M.; Graves, I.; et al. Conformationally Constrained Isoquinolinones as Orally Efficacious Hepatitis B Capsid Assembly Modulators. ACS Med. Chem. Lett. 2024, 15, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://iris.who.int/handle/10665/332081 (accessed on 28 December 2024).
- Janbon, G.; Quintin, J.; Lanternier, F.; d’Enfert, C. Studying fungal pathogens of humans and fungal infections: Fungal diversity and diversity of approaches. Microbes Infect. 2019, 21, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R.; Cox, G.M.; Lee, J.Y.; Kauffman, C.A.; de Repentigny, L.; Chapman, S.W.; Morrison, V.A.; Pappas, P.; Hiemenz, J.W.; Stevens, D.A. The impact of culture isolation of Aspergillus species: A hospital-based survey of aspergillosis. Clin. Infect. Dis. 2001, 33, 1824–1833. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The virulence factors and clinical manifestations of infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, A.P.; Bush, R.K.; Demain, J.G.; Denning, D.W.; Dixit, A.; Fairs, A.; Greenberger, P.A.; Kariuki, B.; Kita, H.; Kurup, V.P.; et al. Fungi and allergic lower respiratory tract diseases. J. Allergy Clin. Immunol. 2012, 129, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, G.; Tang, S.; Pan, Y.; Shao, H.; Jiao, W. Dess–Martin Periodinane-Mediated Oxidative Coupling Reaction of Isoquinoline with Benzyl Bromide. Molecules 2023, 28, 923. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Martel, A.; Dhal, R.; Dujardin, G. 1,3-Dipolar Cycloaddition of N-Substituted Dipolarophiles and Nitrones: Highly Efficient Solvent-Free Reaction. J. Org. Chem. 2008, 73, 2621–2632. [Google Scholar] [CrossRef]
- Maiuolo, L.; Bortolini, O.; De Nino, A.; Russo, B.; Gavioli, R.; Sforza, F. Modified N,O-Nucleosides: Design, Synthesis, and Anti-tumour Activity. Aust. J. Chem. 2014, 67, 670–674. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Li, R.; Lu, Z.; Pitsinos, E.N.; Alemany, L.B.; Aujay, M.; Lee, C.; Sandoval, J.; Gavrilyuk, J. Streamlined Total Synthesis of Shishijimicin A and Its Application to the Design, Synthesis, and Biological Evaluation of Analogues thereof and Practical Syntheses of PhthNSSMe and Related Sulfenylating Reagents. J. Am. Chem. Soc. 2018, 140, 12120–12136. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Srivastava, S. Beckmann Rearrangement Catalysis: A Review of Recent Advances. New J. Chem. 2020, 44, 18530–18572. [Google Scholar] [CrossRef]
- Ning, Y.; Fukuda, T.; Ikeda, H.; Otani, Y.; Kawahata, M.; Yamaguchi, K.; Ohwada, T. Revisiting Secondary Interactions in Neighboring Group Participation, Exemplified by Reactivity Changes of Iminylium Intermediates. Org. Biomol. Chem. 2017, 15, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Touchette, S.J.; Dunkley, E.M.; Lowder, L.L.; Wu, J. Nucleophile-Intercepted Beckmann Fragmentation Reactions. Chem. Sci. 2019, 10, 7812–7815. [Google Scholar] [CrossRef]
- Arnett, E.M.; Bushick, R.D. Solvent Effects in Organic Chemistry. III. Solvation of Stable Carbonium and Ammonium Ions in Water. The Temperature Coefficient of the HR Acidity Scale. J. Am. Chem. Soc. 1964, 86, 1564–1571. [Google Scholar] [CrossRef]
- Hansen, T.; Vermeeren, P.; Bickelhaupt, F.M.; Hamlin, T.A. Stability of Alkyl Carbocations. Chem. Commun. 2022, 58, 12050–12053. [Google Scholar] [CrossRef]
- Stradling, S.S.; Hornick, D.; Lee, J.; Riley, J. A Study of Stereospecificity: The Beckmann Rearrangement. J. Chem. Educ. 1983, 60, 502–503. [Google Scholar] [CrossRef]
- Crosby, I.T.; Shin, J.K.; Capuano, B. The Application of the Schmidt Reaction and Beckmann Rearrangement to the Synthesis of Bicyclic Lactams: Some Mechanistic Considerations. Aust. J. Chem. 2010, 63, 211–226. [Google Scholar] [CrossRef]
- Zhao, Z.-N.; He, F.-K.; Wang, Y.-H.; Li, Y.-C.; Li, Z.-H.; Yang, X.-Y.; Schneider, U.; Huang, Y.-Y. Asymmetric Clicking of Alkynyl Dipolarophiles and Nitrones Catalyzed by a Well-Defined Chiral Iron Complex. ACS Catal. 2024, 14, 13291–13302. [Google Scholar] [CrossRef]
- Stotani, S.; Gatta, V.; Medda, F.; Padmanaban, M.; Karawajczyk, A.; Tammela, P.; Giordanetto, F.; Tzalis, D.; Collina, S. A Versatile Strategy for the Synthesis of 4,5-Dihydroxy-2,3-Pentanedione (DPD) and Related Compounds as Potential Modulators of Bacterial Quorum Sensing. Molecules 2018, 23, 2545. [Google Scholar] [CrossRef]
- Lu, X.; Schneider, U. Aza-Morita-Baylis-Hillman Reactions Catalyzed by a Cyclopropenylidene. Chem. Commun. 2016, 52, 12980–12983. [Google Scholar] [CrossRef] [PubMed]
- M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed. Clinical and Laboratory Standards Insitute—CLSI: Wayne, PA, USA, 2018.
- Tsukatani, T.; Suenaga, H.; Shiga, M.; Noguchi, K.; Ishiyama, M.; Ezoe, T.; Matsumoto, K. Comparison of the WST-8 colorimetric method and the CLSI broth microdilution method for susceptibility testing against drug-resistant bacteria. J. Microbiol. Methods 2012, 90, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, L.; He, C.; Wu, Q.; Li, M.; Zeng, T. Givinostat exhibits in vitro synergy with posaconazole against Aspergillus spp. Med. Mycol. 2017, 55, 798–802. [Google Scholar] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]





 | ||||
| Entry | R = | 14 (Major Cycloadduct) | 21 (Minor Cycloadduct) | Yield 1 (Comb.) |
| 1 |  | 88% (14a) | 6% (21a) | 94% |
| 2 |  | 82% (14b) | 7% (21b) | 89% |
| 3 |  | 86% (14c) | 5% (21c) | 91% |
| 4 | 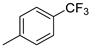 | 85% (14d) | 4% (21d) | 89% |
| 5 |  | 88% (14e) | 5% (21e) | 93% |
| 6 | 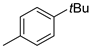 | 83% (14f) | 6% (21f) | 89% |
| 7 | 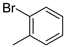 | 84% (14g) | 6% (21g) | 90% |
| 8 | 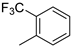 | 87% (14h) | 3% (21h) | 90% |
| 9 | 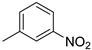 | 89% (14i) | 3% (21i) | 92% |
| 10 | 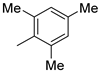 | 87% (14j) | 3% (21j) | 90% |
| 11 | 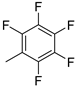 | 80% (14k) | - | 80% |
| 12 |  | 89% (14l) | 4% (21l) | 93% |
| 13 | 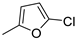 | 70% (14m) | - | 70% |
| 14 | 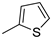 | 64% (14n) | - | 64% |
| 15 |  | 92% (14o) | - | 92% |
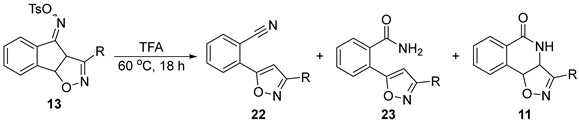 | |||||
| Entry | R = | 22 | 23 | 11 | Yield 1 (Comb.) |
| 1 |  | 80% (22a) | 10% (23a) | - | 80% |
| 2 |  | 76% (22b) | 9% (23b) | - | 85% |
| 3 | 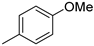 | 75% (22c) | 13% (23c) | - | 88% |
| 4 |  | 72% (22d) | 15% (23d) | - | 87% |
| 5 |  | 81% (22e) | 8% (23e) | - | 89% |
| 6 |  | 85% (22f) | 6% (23f) | - | 91% |
| 7 | 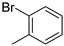 | 57% (22g) | 12% (23g) | 10% (11g) | 79% |
| 8 |  | 59% (22h) | 22% (23h) | 11% (11h) | 92% |
| 9 |  | 86% (22i) | - | - | 86% |
| 10 |  | 12% (22j) | - | - | 77% 2 |
| 11 |  | 54% (22k) | 29% (23k) | 13% (11k) | 96% |
| 12 | 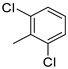 | 53% (22l) | 32% (23l) | 6% (11l) | 91% |
| 13 | 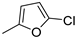 | 71% (22m) | 12% (23m) | - | 83% |
| 14 | 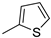 | 70% (22n) | 9% (23n) | - | 79% |
| 15 |  | 82% (22o) | 9% (23o) | - | 91% |
 | ||
| Entry | Reagents | Yield 1 |
| 1 | 96% H2SO4 | 97% |
| 2 | TFA, H2O (1 equiv.) | 0% |
| 3 | TFA, p-TsOH (1 equiv.), H2O (1 equiv.) | 9% |
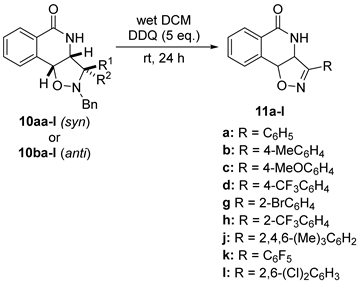 | |||||
| Entry | SM 1 | R1 = | R2 = | Product 2 | BRSM Yield 3 |
| 1 | 10aa | H | 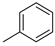 | 45% (11a) | 97% |
| 2 | 10ba |  | H | 48% (11a) | 96% |
| 3 | 10ab | H | 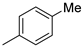 | 44% (11b) | 94% |
| 4 | 10bb |  | H | 47% (11b) | 92% |
| 5 | 10ac | H |  | 53% (11c) | 91% |
| 6 | 10bc |  | H | 50% (11c) | 95% |
| 7 | 10ad | H |  | 42% (11d) | 90% |
| 8 | 10bd | 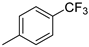 | H | 41% (11d) | 89% |
| 9 | 10ag | H | 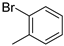 | 48% (11g) | 93% |
| 10 | 10bg | 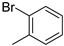 | H | 51% (11g) | 97% |
| 11 | 10ah | H |  | 48% (11h) | 93% |
| 12 | 10bh | 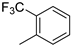 | H | 45% (11h) | 95% |
| 13 | 10aj | H |  | 75% (11j) | 93% |
| 14 | 10ak | H | 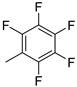 | 46% (11k) | 96% |
| 15 | 10bk |  | H | 48% (11k) | 93% |
| 16 | 10al | H | 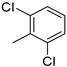 | 49% (11l) | 95% |
| 17 | 10bl | 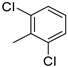 | H | 49% (11l) | 92% |
| Compound 1 | C. albicans | C. krusei | A. fumigatus | A. niger | P. funiculosum | P. chrysogenum | |
|---|---|---|---|---|---|---|---|
| 23a | MIC | 0.08 | 0.16 | 0.24 | 0.16 | 0.08 | 0.16 |
| MFC | 0.16 | 0.24 | 0.32 | 0.24 | 0.16 | 0.24 | |
| 23b | MIC | - | - | 0.16 | 0.08 | 0.04 | 0.08 |
| MFC | - | - | 0.24 | 0.16 | 0.08 | 0.16 | |
| 23c | MIC | - | 0.16 | 0.16 | 0.08 | 0.04 | 0.16 |
| MFC | - | 0.24 | 0.24 | 0.16 | 0.08 | 0.24 | |
| 23d | MIC | - | 0.08 | 0.24 | 0.04 | 0.04 | 0.08 |
| MFC | - | 0.16 | 0.32 | 0.08 | 0.08 | 0.16 | |
| 23e | MIC | - | 0.16 | 0.16 | 0.16 | 0.16 | - |
| MFC | - | 0.24 | 0.24 | 0.24 | 0.32 | - | |
| 23f | MIC | - | 0.16 | 0.16 | 0.04 | 0.04 | 0.16 |
| MFC | - | 0.24 | 0.24 | 0.16 | 0.08 | 0.24 | |
| 23g | MIC | - | 0.16 | 0.16 | 0.08 | 0.16 | 0.16 |
| MFC | 0.24 | 0.24 | 0.16 | 0.32 | 0.24 | ||
| 23h | MIC | 0.16 | 0.16 | 0.16 | 0.16 | 0.08 | 0.16 |
| MFC | 0.24 | 0.24 | - | 0.32 | 0.16 | 0.24 | |
| 23i | MIC | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | - |
| MFC | 0.24 | 0.24 | 0.24 | 0.32 | 0.24 | - | |
| 23j | MIC | 0.08 | 0.16 | 0.08 | 0.04 | 0.04 | 0.08 |
| MFC | 0.16 | 0.24 | 0.24 | 0.16 | 0.08 | 0.16 | |
| 23k | MIC | 0.24 | - | 0.16 | 0.16 | 0.04 | 0.08 |
| MFC | 0.32 | - | 0.24 | 0.24 | 0.08 | 0.16 | |
| 23l | MIC | 0.08 | 0.08 | 0.24 | 0.04 | 0.16 | 0.16 |
| MFC | - | 0.16 | 0.32 | 0.08 | 0.24 | 0.24 | |
| 23m | MIC | 0.08 | 0.16 | 0.08 | 0.08 | 0.04 | 0.04 |
| MFC | 0.16 | 0.24 | 0.16 | 0.16 | 0.08 | 0.08 | |
| 23n | MIC | 0.16 | - | 0.16 | 0.16 | 0.04 | 0.16 |
| MFC | 0.24 | - | 0.24 | 0.24 | 0.16 | 0.24 | |
| 23o | MIC | - | 0.24 | 0.24 | 0.08 | 0.08 | 0.16 |
| MFC | - | 0.32 | 0.32 | 0.16 | 0.16 | 0.24 | |
| 30a | MIC | 0.16 | 0.08 | 0.12 | 0.04 | 0.16 | 0.08 |
| MFC | 0.32 | 0.16 | 0.16 | 0.08 | 0.32 | 0.16 | |
| 30b | MIC | 0.16 | 0.16 | 0.16 | 0.02 | 0.08 | 0.16 |
| MFC | 0.32 | 0.32 | 0.24 | 0.04 | 0.16 | 0.32 | |
| 30c | MIC | 0.16 | 0.16 | 0.16 | 0.04 | 0.08 | 0.08 |
| MFC | 0.32 | 0.32 | 0.32 | 0.08 | 0.16 | 0.16 | |
| 30d | MIC | 0.16 | 0.08 | 0.16 | 0.04 | 0.16 | 0.16 |
| MFC | 0.32 | 0.24 | 0.24 | 0.08 | 0.24 | 0.32 | |
| K 2 | MIC | 0.0125 | 0.0004 | 0.025 | 0.10 | 0.0125 | 0.05 |
| MFC | 0.025 | 0.0008 | 0.05 | 0.20 | 0.025 | 0.10 | |
| Compound 1 | C. albicans | C. krusei | A. fumigatus | A. niger | P. funiculosum | P. chrysogenum | |
|---|---|---|---|---|---|---|---|
| 11g | MIC | 0.08 | 0.16 | 0.16 | 0.08 | 0.16 | 0.16 |
| MFC | 0.16 | 0.32 | 0.24 | 0.16 | 0.32 | 0.24 | |
| 11h | MIC | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| MFC | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | |
| 11k | MIC | 0.16 | 0.08 | 0.08 | 0.04 | 0.04 | 0.08 |
| MFC | 0.32 | 0.16 | 0.16 | 0.08 | 0.08 | 0.16 | |
| 11l | MIC | 0.16 | 0.16 | 0.16 | 0.01 | 0.04 | 0.16 |
| MFC | 0.32 | 0.32 | 0.32 | 0.02 | 0.08 | 0.32 | |
| 10ad | MIC | 0.08 | 0.16 | 0.16 | 0.08 | 0.16 | 0.16 |
| MFC | 0.16 | 0.32 | 0.32 | 0.16 | 0.32 | 0.24 | |
| 10bd | MIC | 0.16 | 0.16 | 0.16 | 0.04 | 0.16 | 0.08 |
| MFC | 0.32 | 0.32 | 0.32 | 0.08 | 0.24 | 0.16 | |
| 10aj | MIC | 0.16 | 0.16 | 0.16 | 0.02 | 0.16 | 0.04 |
| MFC | 0.32 | 0.32 | 0.32 | 0.04 | 0.24 | 0.08 | |
| 10al | MIC | 0.16 | 0.08 | 0.16 | 0.01 | 0.16 | 0.16 |
| MFC | 0.32 | 0.24 | 0.32 | 0.02 | 0.32 | 0.32 | |
| 10am | MIC | 0.16 | 0.16 | 0.12 | 0.02 | 0.08 | 0.16 |
| MFC | 0.32 | 0.32 | 0.24 | 0.04 | 0.16 | 0.32 | |
| 10bm | MIC | 0.16 | 0.16 | 0.16 | 0.005 | 0.04 | 0.16 |
| MFC | 0.32 | 0.32 | 0.32 | 0.01 | 0.08 | 0.32 | |
| K 2 | MIC | 0.0125 | 0.0004 | 0.025 | 0.10 | 0.0125 | 0.05 |
| MFC | 0.025 | 0.0008 | 0.05 | 0.20 | 0.025 | 0.10 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouzounthanasis, K.A.; Glamočlija, J.; Ćirić, A.; Koumbis, A.E. Studies of the Synthesis of Fused Isoxazoline/Isoquinolinones and Evaluation of the Antifungal Activity of Isoxazole-like Benzamide and Isoquinolinone Hybrids. Molecules 2025, 30, 589. https://doi.org/10.3390/molecules30030589
Ouzounthanasis KA, Glamočlija J, Ćirić A, Koumbis AE. Studies of the Synthesis of Fused Isoxazoline/Isoquinolinones and Evaluation of the Antifungal Activity of Isoxazole-like Benzamide and Isoquinolinone Hybrids. Molecules. 2025; 30(3):589. https://doi.org/10.3390/molecules30030589
Chicago/Turabian StyleOuzounthanasis, Konstantinos A., Jasmina Glamočlija, Ana Ćirić, and Alexandros E. Koumbis. 2025. "Studies of the Synthesis of Fused Isoxazoline/Isoquinolinones and Evaluation of the Antifungal Activity of Isoxazole-like Benzamide and Isoquinolinone Hybrids" Molecules 30, no. 3: 589. https://doi.org/10.3390/molecules30030589
APA StyleOuzounthanasis, K. A., Glamočlija, J., Ćirić, A., & Koumbis, A. E. (2025). Studies of the Synthesis of Fused Isoxazoline/Isoquinolinones and Evaluation of the Antifungal Activity of Isoxazole-like Benzamide and Isoquinolinone Hybrids. Molecules, 30(3), 589. https://doi.org/10.3390/molecules30030589








