Synthesis and Performance of Epoxy-Terminated Hyperbranched Polymers Based on Epoxidized Soybean Oil
Abstract
1. Introduction
2. Results and Discussion
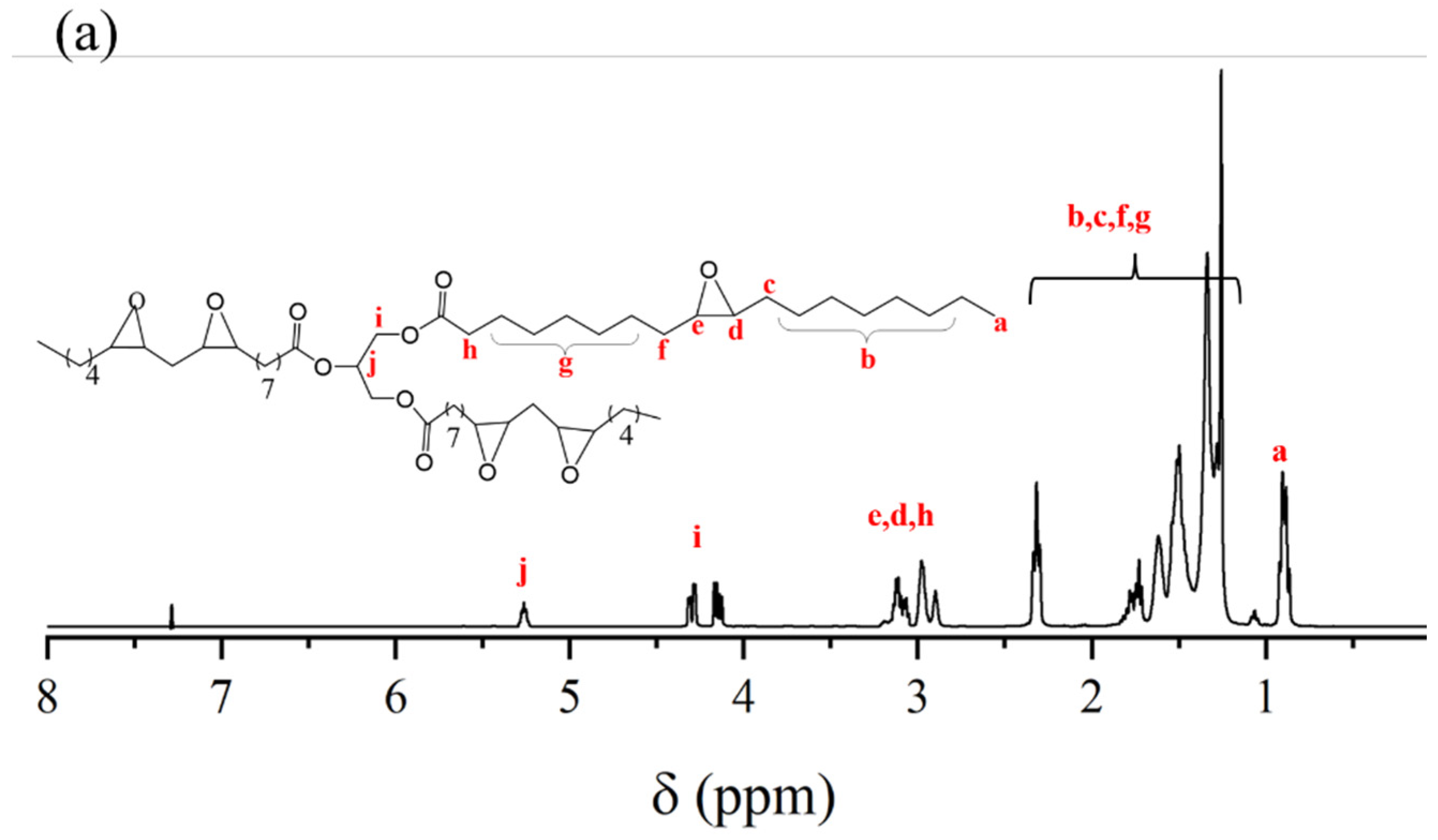
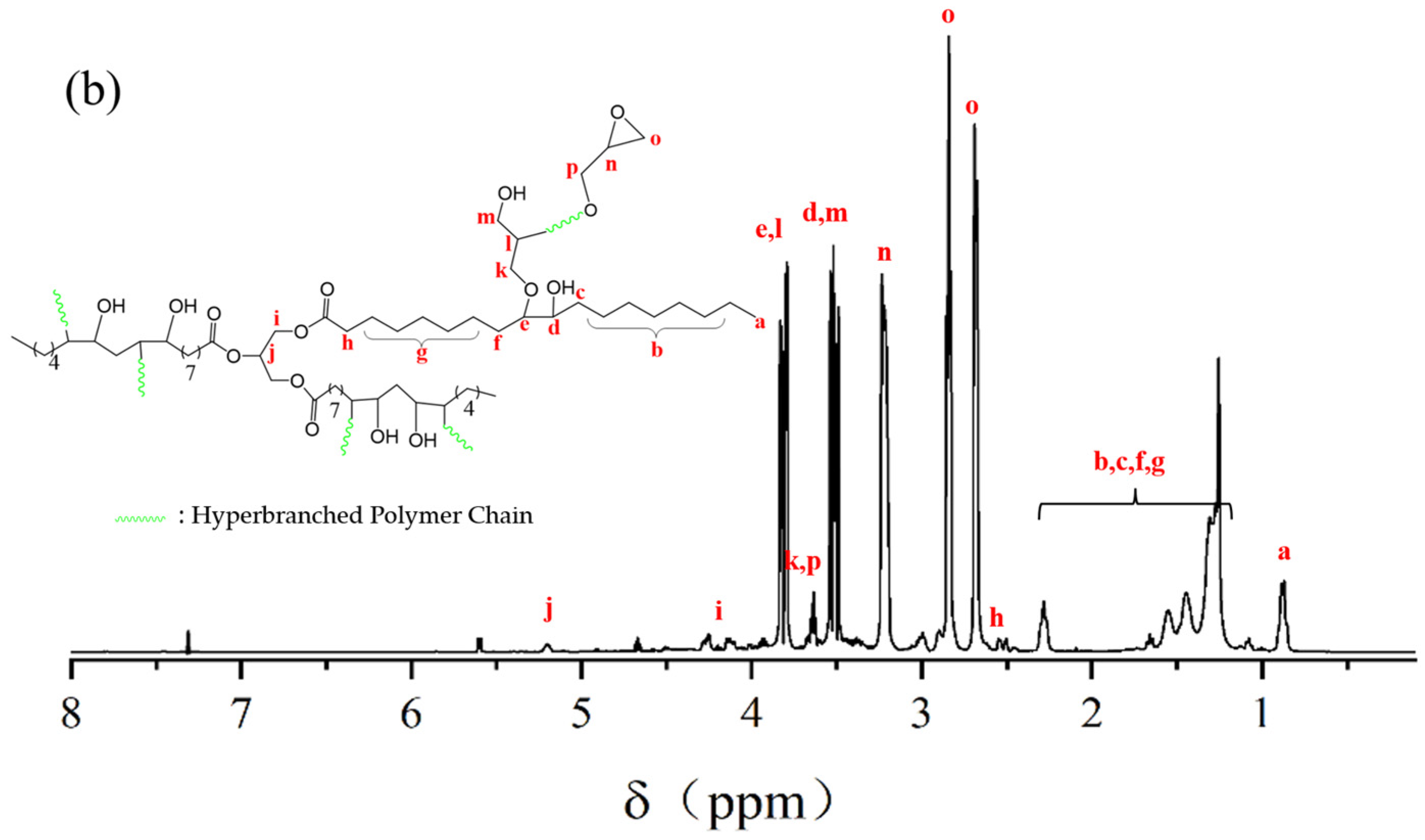
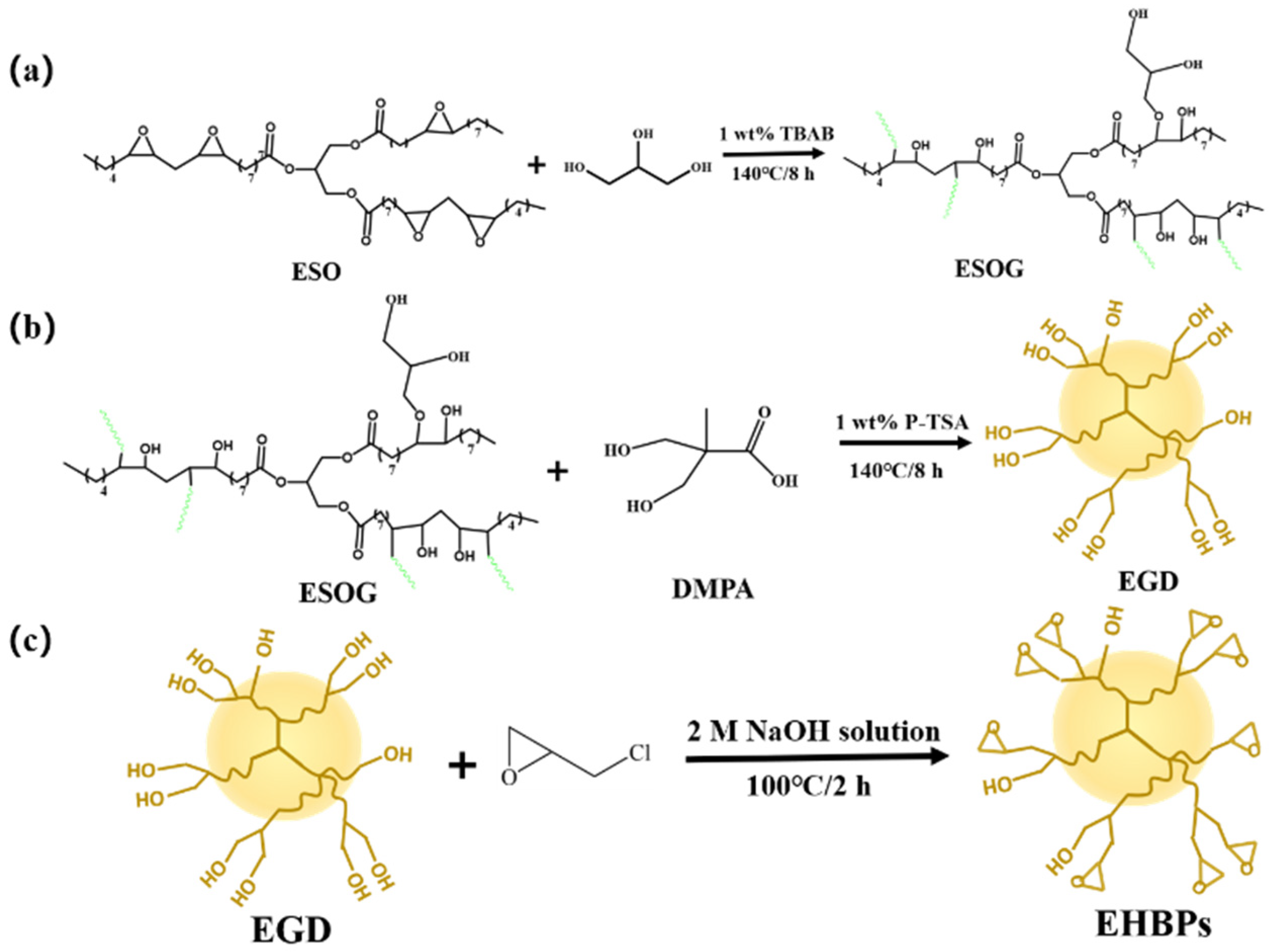
2.1. Composition and Structure of ESO, ESOG, EGD, and EHBPs
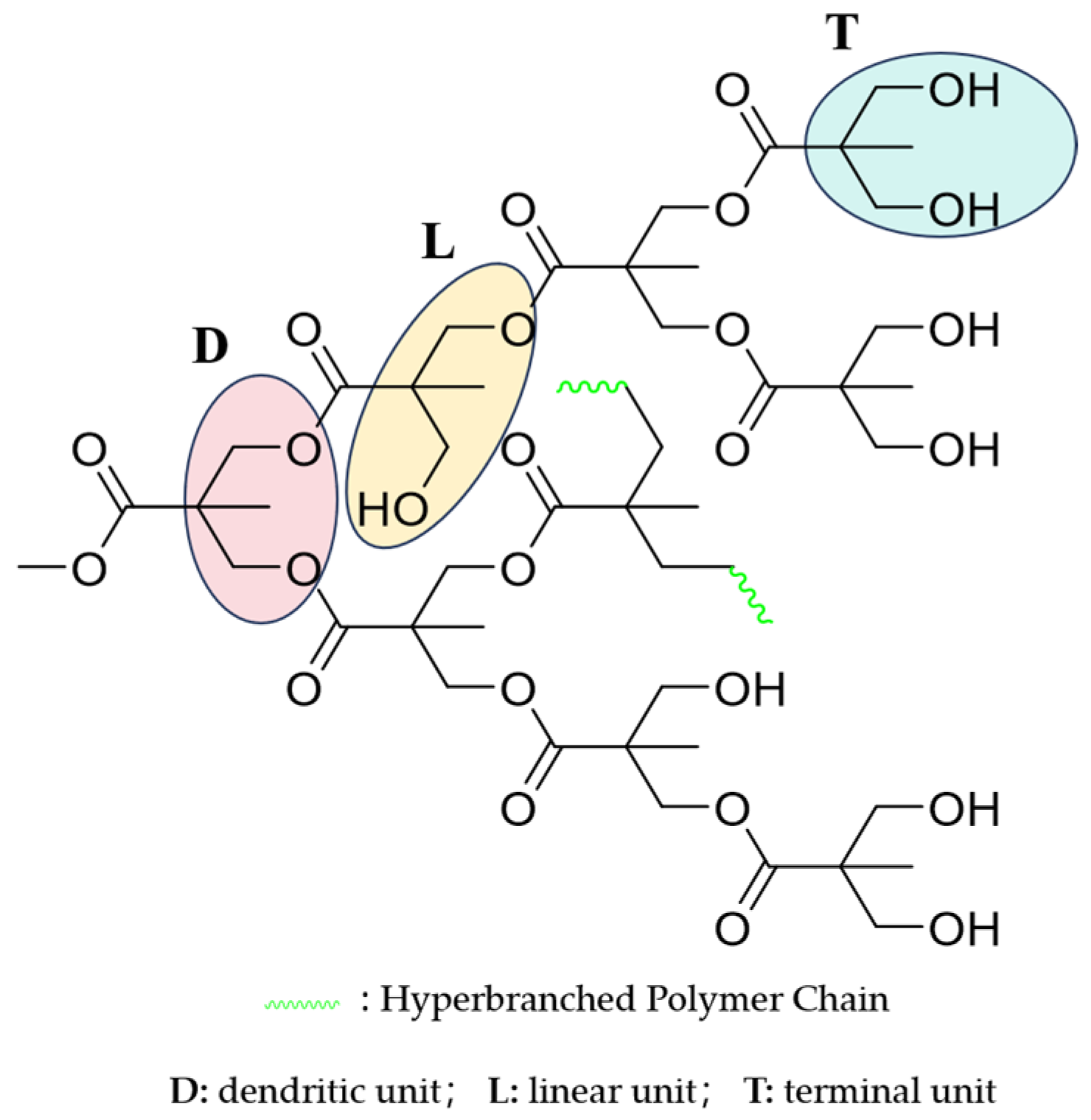
2.2. Degree of Branching of EGD
2.3. Molecular Weight of EHBPs
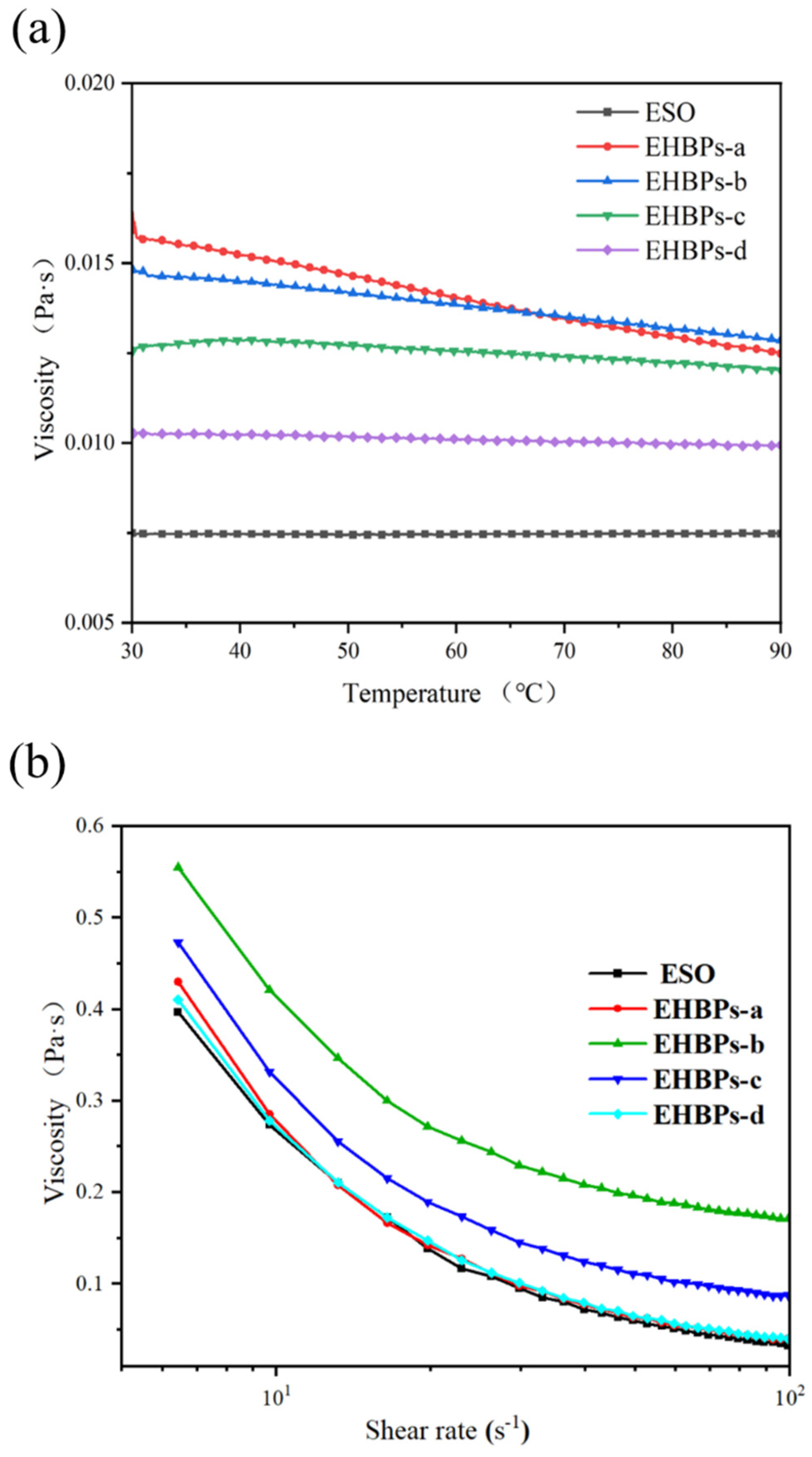
2.4. Rheological Properties of EHBPs
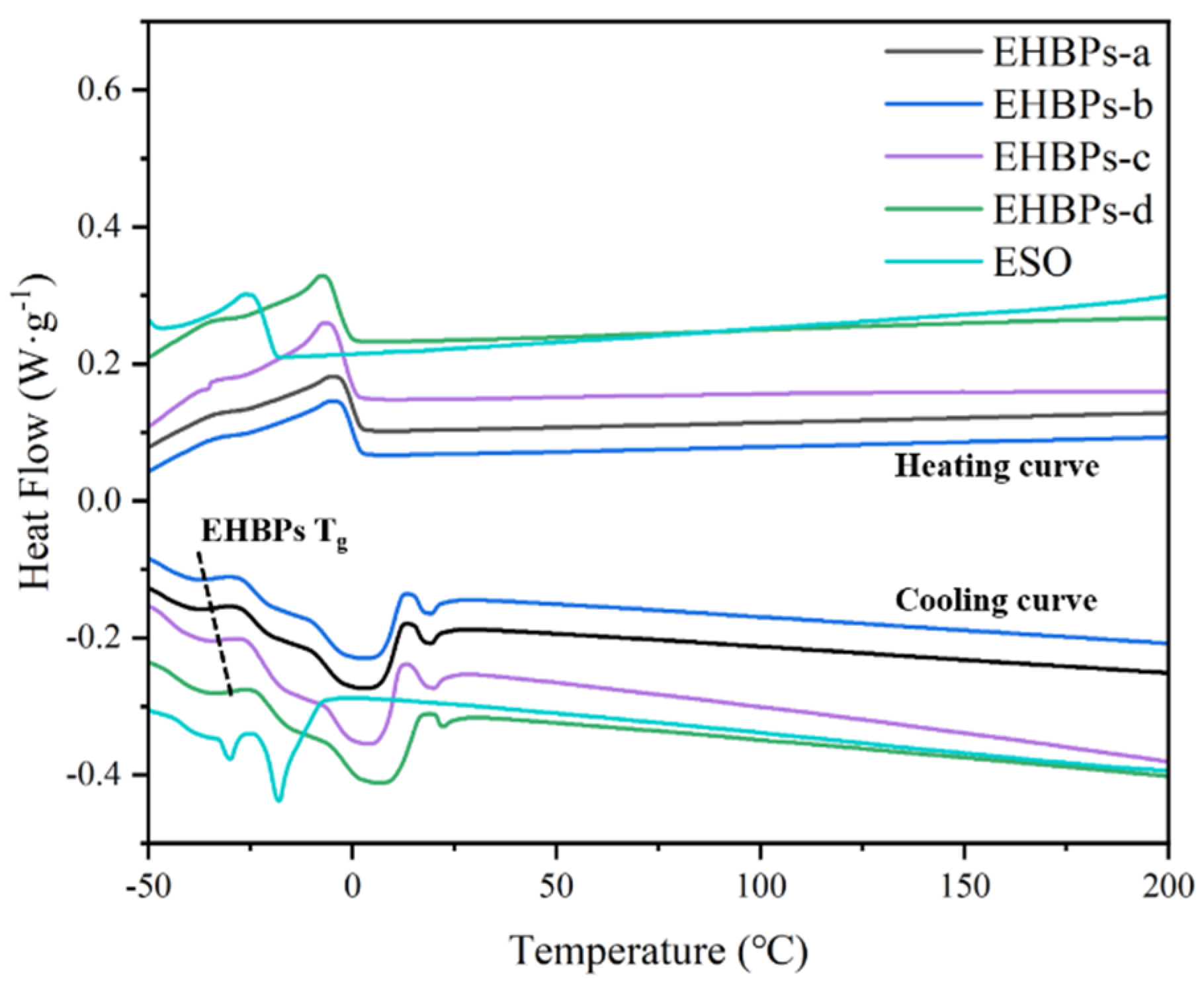
2.5. Thermal Performance of EHBPs
2.6. Water Contact Angle Between EGD and EHBPs
2.7. Adhesion Properties of EGD and EHBPs
3. Materials and Methods
3.1. Materials
3.2. Synthesis of End-Epoxy Hyperbranched Polymers (EHBPs)
3.3. Testing and Characterization
3.3.1. Fourier Transform Infrared Spectroscopy (FT–IR)
3.3.2. 1H NMR Spectra
3.3.3. 13C NMR Spectra
3.3.4. Gel Permeation Chromatography (GPC)
3.3.5. Differential Scanning Calorimeter (DSC)
3.3.6. Rheological Property
3.3.7. Epoxy Value Measurement
3.3.8. Degree of Branching
3.3.9. Water Contact Angle Measurement
3.3.10. Adhesion Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carlmark, A.; Hawker, C.; Hult, A.; Malkoch, M. New methodologies in the construction of dendritic materials. Chem. Soc. Rev. 2009, 38, 352–362. [Google Scholar] [CrossRef]
- Voit, B.I.; Lederer, A. ChemInform Abstract: Hyperbranched and Highly Branched Polymer Architectures—Synthetic Strategies and Major Characterization Aspects. ChemInform 2010, 41, chin.201013273. [Google Scholar] [CrossRef]
- Inoue, K. Functional dendrimers, hyperbranched and star polymers. Prog. Polym. Sci. 2000, 25, 453–571. [Google Scholar] [CrossRef]
- Yan, D.; Gao, C.; Frey, H. Hyperbranched Polymers: Synthesis, Properties, and Applications. John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; ISBN 978-0-471-78014-4. [Google Scholar]
- Fan, J.; Ren, N.; Zhang, C.; Li, M.; Zhu, X.; Tong, G. Synthesis of hyperbranched polyolefin with well-defined terminal functional group. Polymer 2022, 242, 124571. [Google Scholar] [CrossRef]
- Alfurhood, J.A.; Sun, H.; Bachler, P.R.; Sumerlin, B.S. Hyperbranched poly(N-(2-hydroxypropyl)methacrylamide) via RAFT self-condensing vinyl polymerization. Polym. Chem. 2016, 7, 2099–2104. [Google Scholar] [CrossRef]
- Ai, X.; Pan, J.; Xie, Q.; Ma, C.; Zhang, G. UV-curable hyperbranched poly(ester-co-vinyl) by radical ring-opening copo ymerization for antifouling coatings. Polym. Chem. 2021, 12, 4524–4531. [Google Scholar] [CrossRef]
- Indumathy, B.; Sathiyanathan, P.; Prasad, G.; Reza, M.S.; Prabu, A.A.; Kim, H. A Comprehensive Review on Processing, Development and Applications of Organofunctional Silanes and Silane-Based Hyperbranched Polymers. Polymers 2023, 15, 2517. [Google Scholar] [CrossRef]
- Patil, A.M.; Shirale, D.J.; Jirimali, H.D.; Jagtap, R.N. An A2 + CB2 approach to the synthesis of hyperbranched polyester polyol and application in PU coatings. J. Coat. Technol. Res. 2024, 21, 1085–1095. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Synthesis of Renewable Thermoset Polymers through Successive Lignin Modification Using Lignin-Derived Phenols. ACS Sustain. Chem. Eng. 2017, 5, 5059–5066. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ibrahim, A.A.; Ali, G.A.M. Cutting-edge development in dendritic polymeric materials for biomedical and energy applications. Eur. Polym. J. 2021, 160, 110770. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, S.; Liu, X.; Jiang, Y.; Zhang, D. Preparation of high-performance polyurethane-polyacrylate coatings based on hyperbranched structure and application in anti-ultraviolet film. Prog. Org. Coat. 2023, 182, 107614. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Xu, Z.; Cheng, J.; Chen, S.; Zhang, J.; Miao, M. Preparation of epoxy-ended hyperbranched polymers with precisely controllable degree of branching by thiol-ene Michael addition. J. Appl. Polym. Sci. 2016, 133, 44277. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Zhang, W.; Liang, L.; Yong, Q. Development of biobased polyol from epoxidized soybean oil for polyurethane anti-smudge coatings. J. Appl. Polym. Sci. 2022, 139, e53101. [Google Scholar] [CrossRef]
- Alregeb, F.; Khalili, F.; Sweileh, B.; Ali, D.K. Synthesis and Characterization of Chelating Hyperbranched Polyester Nanoparticles for Cd(II) Ion Removal from Water. Molecules 2022, 27, 3656. [Google Scholar] [CrossRef]
- Fei, J.; Tang, T.; Zhou, L.; He, H.; Ma, M.; Shi, Y.; Chen, S.; Wang, X. High-Toughness and Biodegradable Superabsorbent Hydrogels Based on Dual Functional Crosslinkers. ACS Appl Polym Mater. ACS Appl. Polym. 2023, 5, 3686–3697. [Google Scholar] [CrossRef]
- Yu, L.; Hu, Q.; Li, T.; Zhang, J.; Chen, S.; Xu, Z.; Chen, S.; Zhang, D. Ultrahigh Flowability and Excellent Mechanical Performance of Glass Fiber/PA6 Composites Prepared by Hyperbranched Polymers. Macromol. Mater. Eng. 2023, 308, 2300012. [Google Scholar] [CrossRef]
- Lu, S.; Wu, G.; Niu, F.; Liu, Y.; Liu, X.; Xu, W.; Chen, Z. Toughening modification of epoxy resins by epoxy-terminated hyperbranched polyethers fabricated by doping sorbitol with inositol. J. Appl. Polym. Sci. 2023, 140, e54551. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y. Synthesis and Characterization of Hyperbranched Polyester-Type Epoxy Resins. Thermosetting Resin. 2003, 2, 12–14. (In Chinese) [Google Scholar]
- Gong, C.; Fréchet, J.M.J. End functionalization of hyperbranched poly(siloxysilane): Novel crosslinking agents and hyperbranched–linear star block copolymers. J. Polym. Sci. Polym. Chem. 2000, 38, 2970–2978. [Google Scholar] [CrossRef]
- Xia, M.; Luo, Y. Synthesis and Application of Hyperbranched Epoxy Compounds. Eng. Plast. Appl. 2006, 5, 69–71. (In Chinese) [Google Scholar]
- Emrick, T.; Chang, H.T.; Fréchet, J.M.J.; Woods, J.; Baccei, L. Hyperbranched aromatic epoxies in the design of adhesive materials. Polym. Bull. 2000, 45, 1–7. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Zhang, D. Synthesis and application of epoxy-ended hyperbranched polymers. Chem. Eng. J. 2018, 343, 283–302. [Google Scholar] [CrossRef]
- Jin, Y.; Sima, Y.; Weng, Y.; Men, S.; Huang, Z. Simultaneously reinforcing and toughening of poly(propylene carbonate) by epoxy-terminated hyperbranched polymer(EHBP) through micro-crosslinking. Polym. Bull. 2019, 76, 5733–5749. [Google Scholar] [CrossRef]
- Roy, B.; Karak, N. Synthesis and characterization of thermostable hyperbranched epoxy resin for surface coating applications. J. Mater. Res. 2012, 27, 1806–1814. [Google Scholar] [CrossRef]
- Sun, Y. Research progress on synthesis and applications of hyperbranched polymers. Appl. Chem. Eng. 2023, 7, 234–239. [Google Scholar] [CrossRef]
- Tabatabaei, S.H.; Carreau, P.J.; Ajji, A. Rheological properties of blends of linear and long-chain branched polypropylenes. Polym. Eng. Sci. 2010, 50, 191–199. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, K.; Su, Z.; Qiu, Z. Thermal behavior, mechanical and rheological properties, and hydrolytic degradation of novel branched biodegradable poly(ethylene succinate) copolymers. Polym. Test. 2018, 66, 64–69. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Guo, W.; Miao, M.; Zhang, D. The precise effect of degree of branching of epoxy-ended hyperbranched polymers on intrinsic property and performance. Prog. Org. Coat. 2019, 127, 157–167. [Google Scholar] [CrossRef]
- Siau, S.; Vervaet, A.; Van Vaeck, L.; Schacht, E.; Demeter, U.; Van Calster, A. Adhesion Strength of the Epoxy Polymer/Copper Interface for Use in Microelectronics. J. Electrochem. 2005, 152, C442. [Google Scholar] [CrossRef]
- GB/T 1677−2023; Determinating the Epoxy Value of Plasticizers. SAC/TC 35: Beijing, China, 2023.


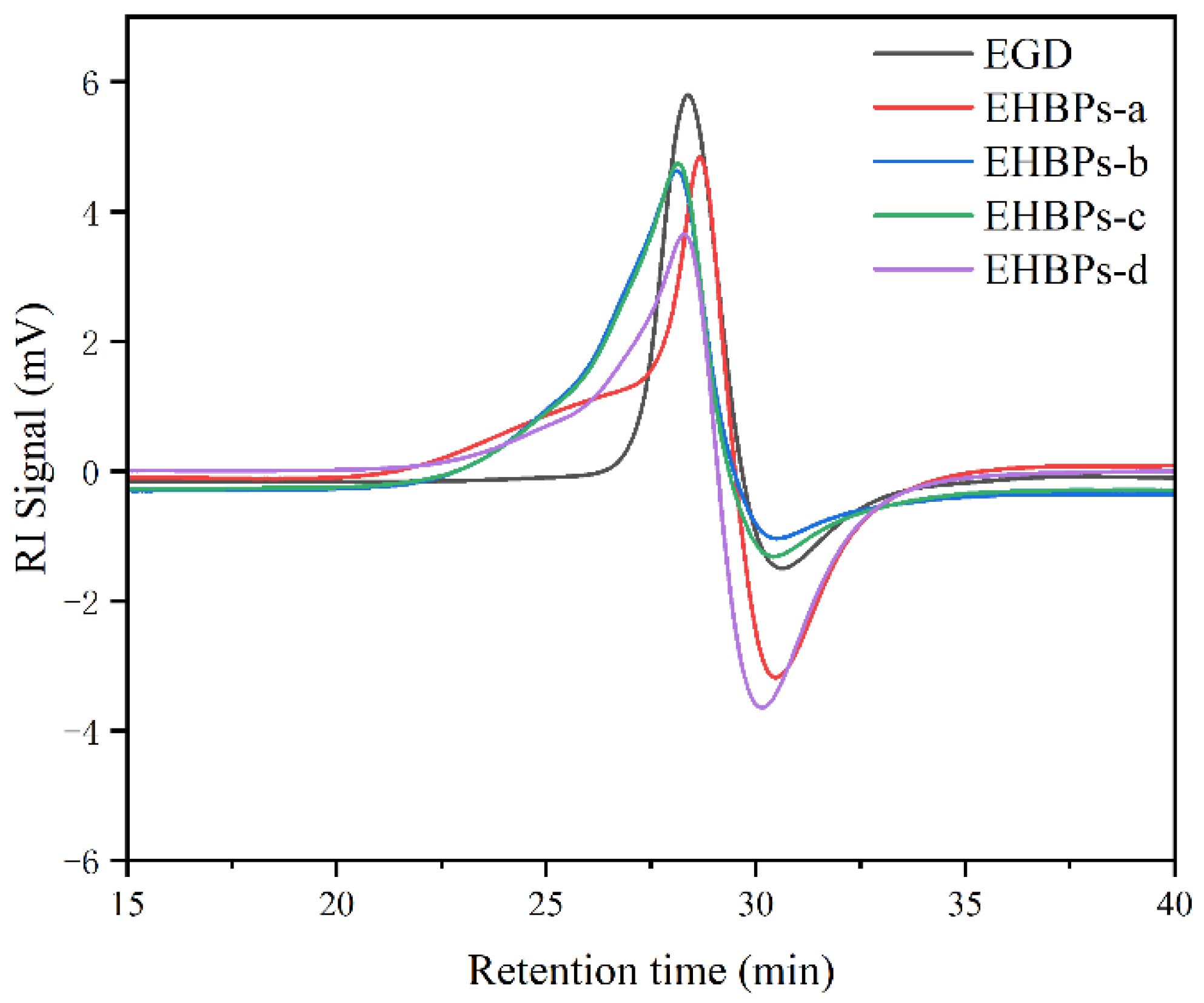


| Samples | n (EGD): n (ECH) | Epoxy Value (mol/100g) | Mn (Da) | PDI |
|---|---|---|---|---|
| ESO | - | 0.375 | 1050 | 1.3 |
| EGD | - | - | 2100 | 1.04 |
| EHBPs-a | 1:3 | 0.73 | 2650 | 1.37 |
| EHBPs-b | 1:4 | 0.79 | 2900 | 1.22 |
| EHBPs-c | 1:5 | 0.82 | 2950 | 1.25 |
| EHBPs-d | 1:6 | 0.89 | 3300 | 1.20 |
| Samples | Tm/°C | ΔHm/(J·g−1) | Tc/°C | Tg/°C | |
|---|---|---|---|---|---|
| 1 | 2 | ||||
| EHBPs-a | 3.28 | 18.65 | 6.68 | −4.29 | −45.70 |
| EHBPs-b | 3.46 | 19.19 | 6.96 | −4.21 | −44.97 |
| EHBPs-c | 3.54 | 19.48 | 7.82 | −5.97 | −43.56 |
| EHBPs-d | 3.76 | 20.33 | 8.07 | −6.31 | −42.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.-Z.; Wang, Q.; Zhu, C.; Zhang, S.; Wang, F.; Tao, L.; Jiang, Y.; Zhang, Q.; Wang, W.; Han, R. Synthesis and Performance of Epoxy-Terminated Hyperbranched Polymers Based on Epoxidized Soybean Oil. Molecules 2025, 30, 583. https://doi.org/10.3390/molecules30030583
Li G-Z, Wang Q, Zhu C, Zhang S, Wang F, Tao L, Jiang Y, Zhang Q, Wang W, Han R. Synthesis and Performance of Epoxy-Terminated Hyperbranched Polymers Based on Epoxidized Soybean Oil. Molecules. 2025; 30(3):583. https://doi.org/10.3390/molecules30030583
Chicago/Turabian StyleLi, Guang-Zhao, Qiuhong Wang, Chongyu Zhu, Shuai Zhang, Fumei Wang, Lei Tao, Youqi Jiang, Qiang Zhang, Wenyan Wang, and Rui Han. 2025. "Synthesis and Performance of Epoxy-Terminated Hyperbranched Polymers Based on Epoxidized Soybean Oil" Molecules 30, no. 3: 583. https://doi.org/10.3390/molecules30030583
APA StyleLi, G.-Z., Wang, Q., Zhu, C., Zhang, S., Wang, F., Tao, L., Jiang, Y., Zhang, Q., Wang, W., & Han, R. (2025). Synthesis and Performance of Epoxy-Terminated Hyperbranched Polymers Based on Epoxidized Soybean Oil. Molecules, 30(3), 583. https://doi.org/10.3390/molecules30030583








