Ultrasonic Activation of Au Nanoclusters/TiO2: Tuning Hydroxyl Radical Production Through Frequency and Nanocluster Size
Abstract
1. Introduction
2. Results and Discussion
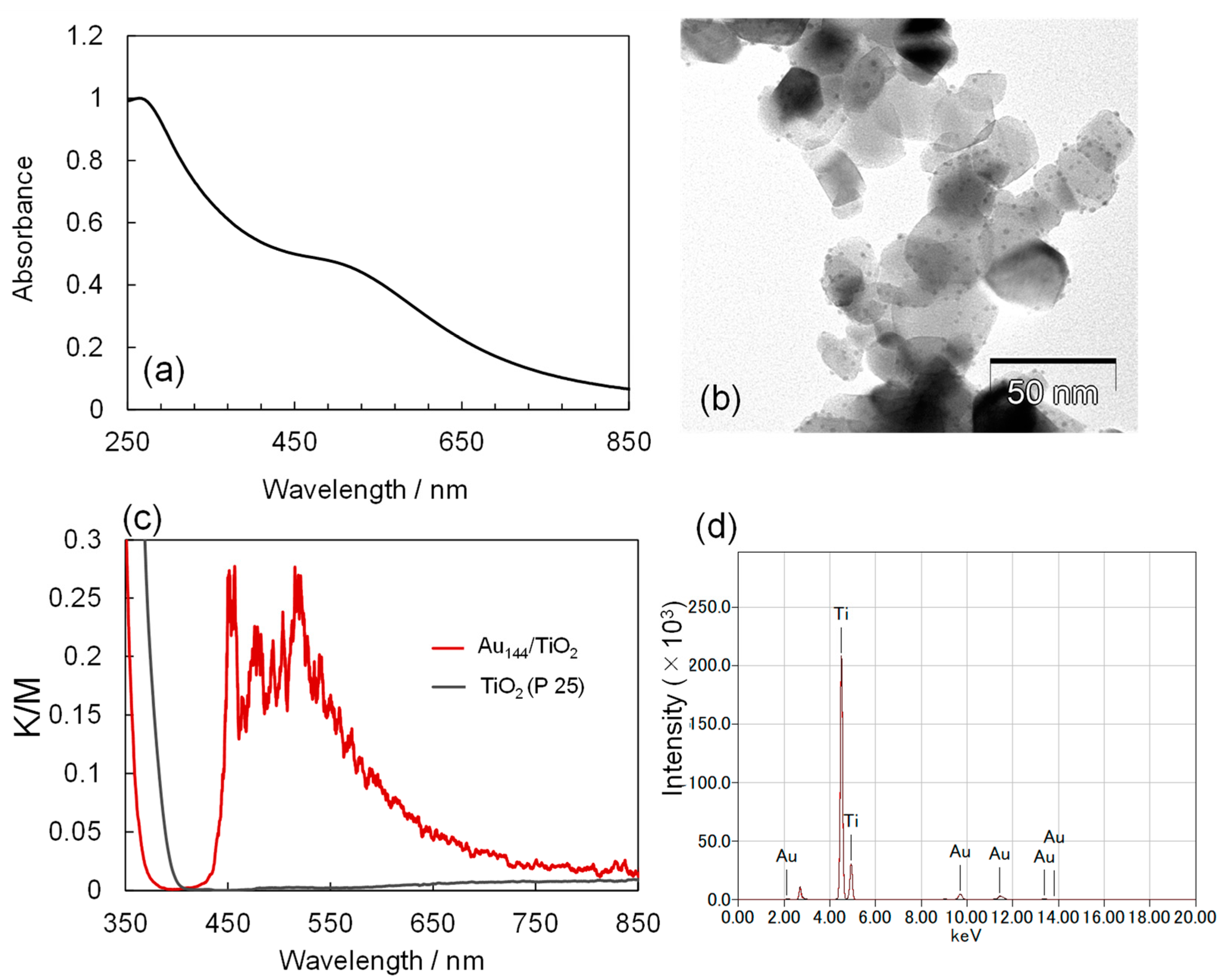
2.1. Preparation of Au144/TiO2 Nanocomposite
2.2. Sonocatalysis of TiO2 vs. Au144/TiO2
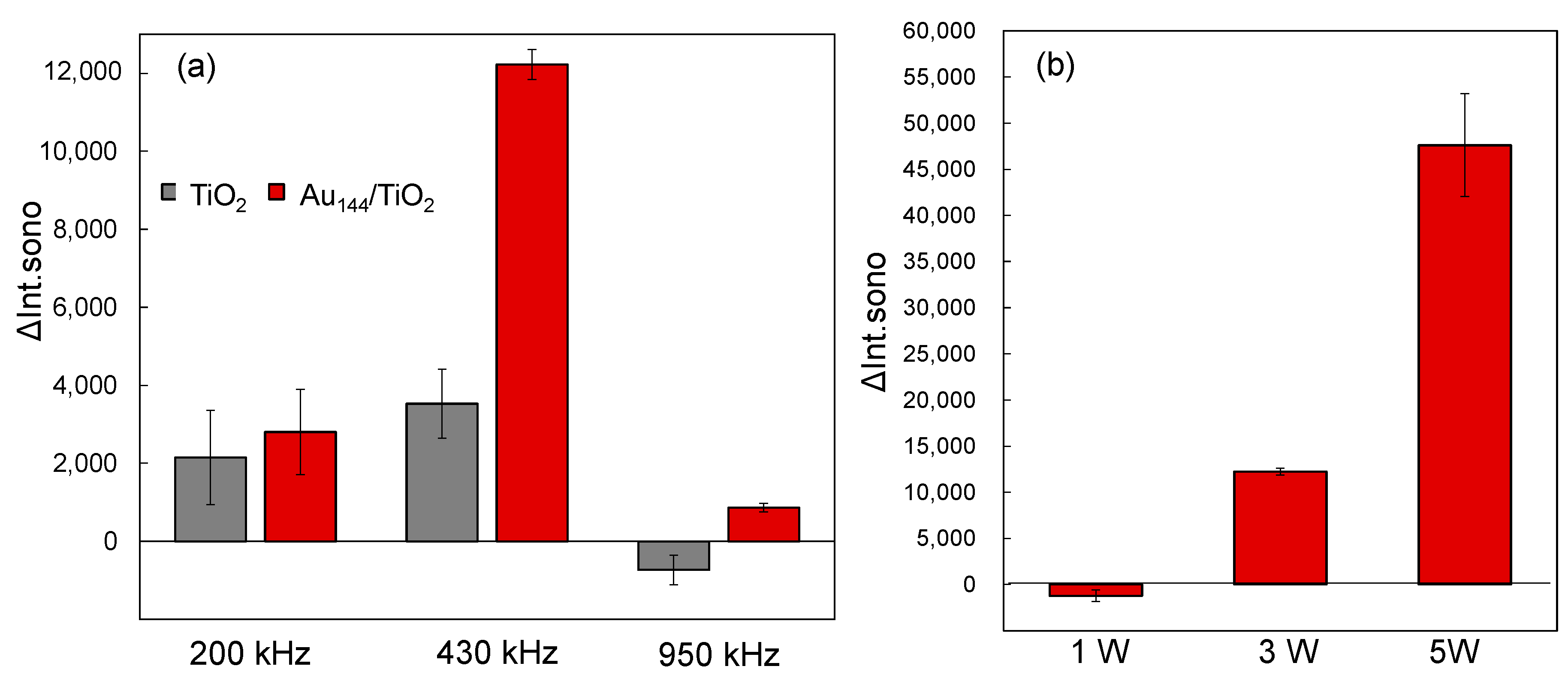
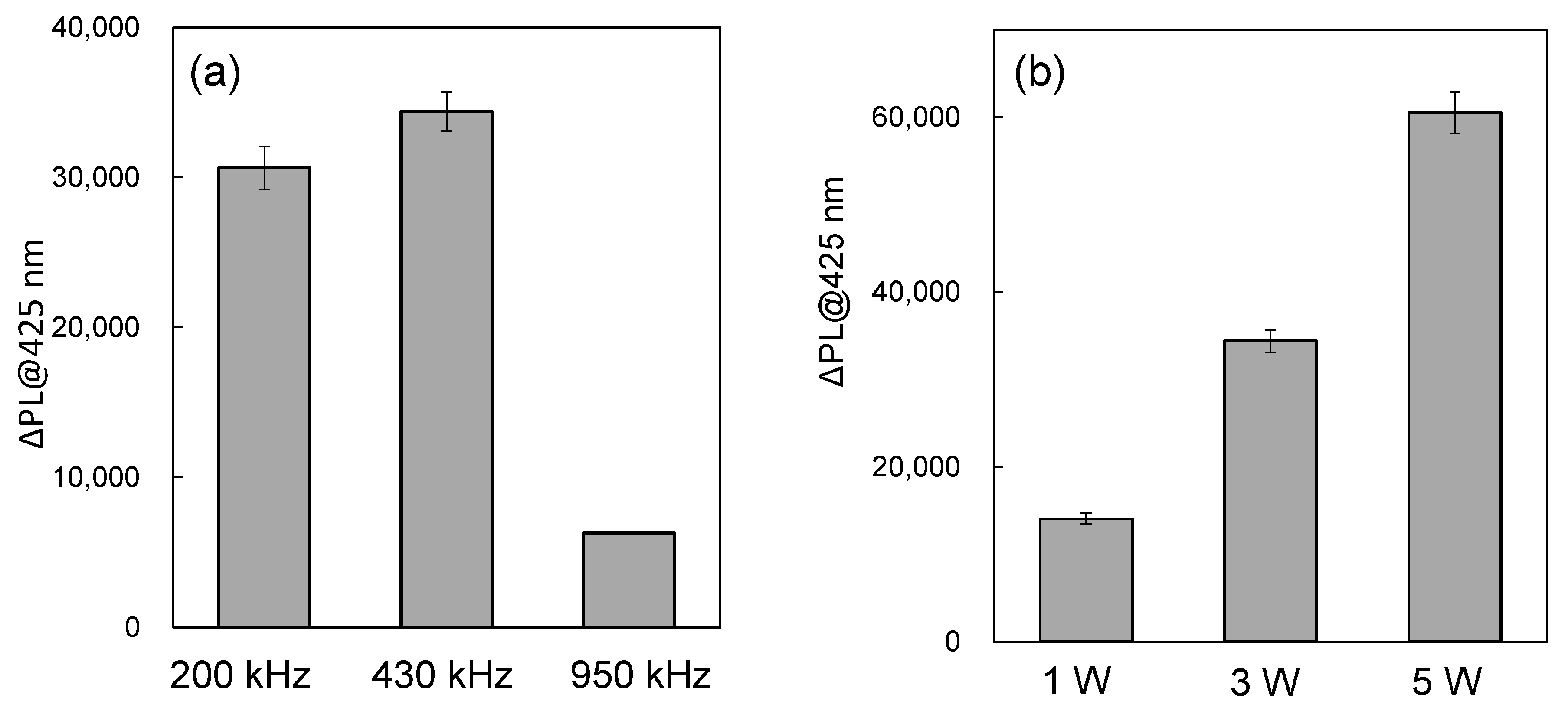
2.3. Ultrasound Frequency and Power Dependence
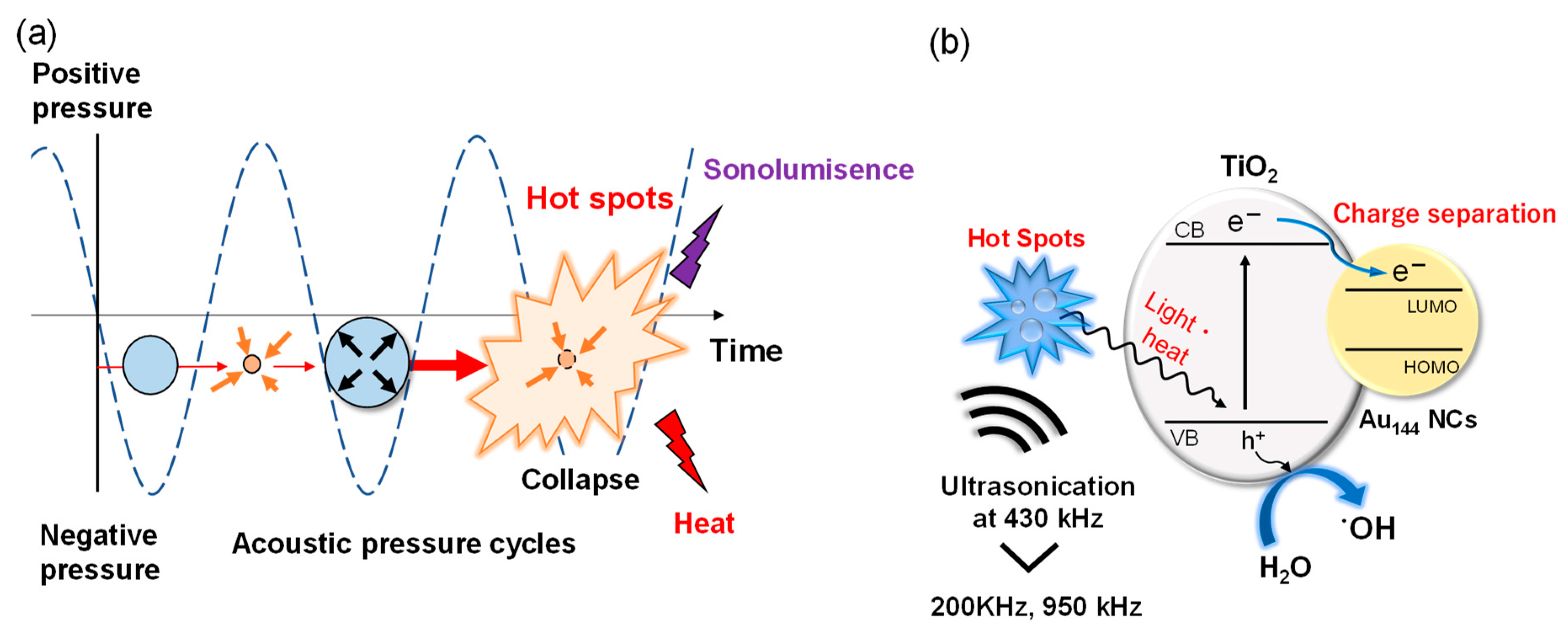
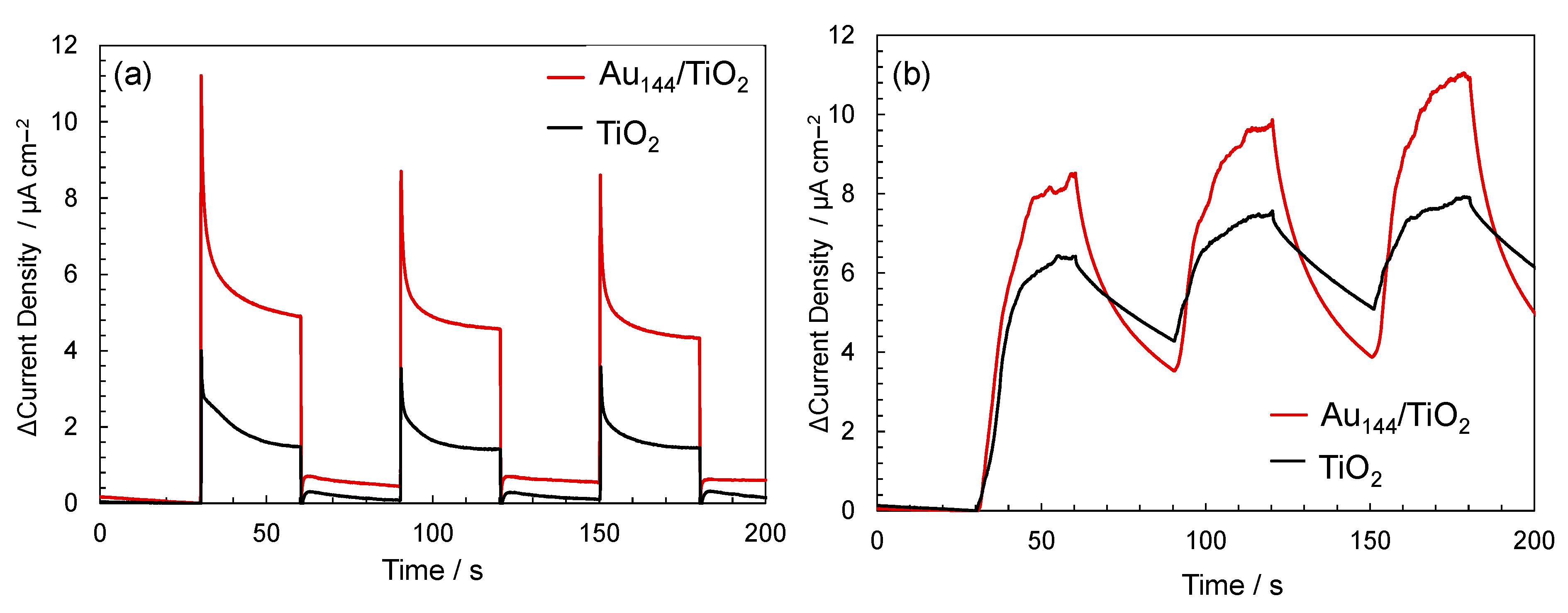
2.4. Mechanistic Insights into Enhanced Sonocatalytic Activity in Au144/TiO2
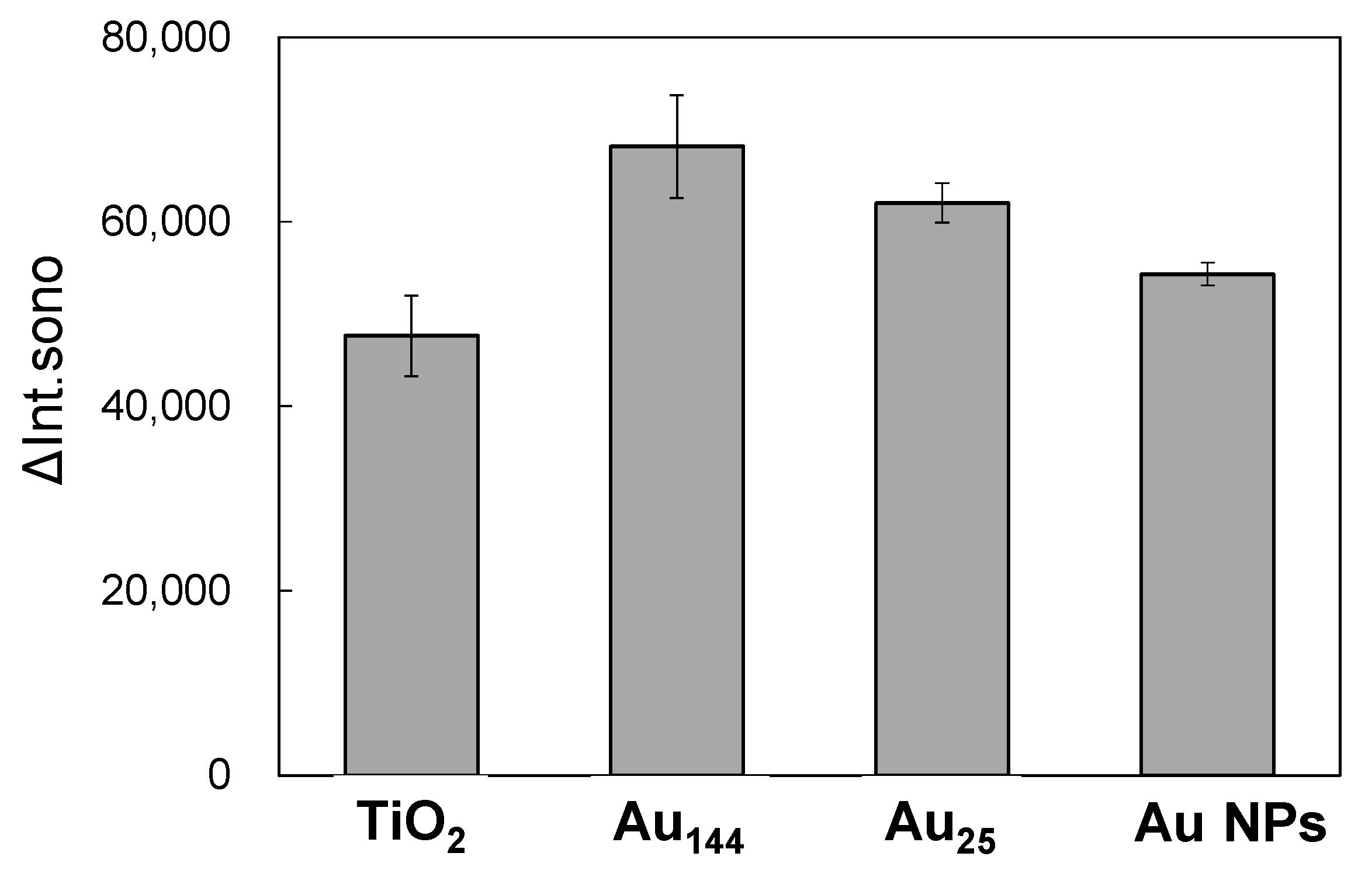
2.5. Size Dependence in Sonocatalytic Activity in Au/TiO2
3. Materials and Methods
3.1. Reagents
3.2. Instruments
3.3. Synthesis of Au144(pMBA)60
3.4. Synthesis of Au25(pMBA)18
3.5. Synthesis of Au Nanoparticles
3.6. Preparation of Au/TiO2 Composites
3.7. Evaluation of Sonocatalytic Activity
3.8. Transient Photocurrent/Sonocurrent Measuremetns in TiO2 and Au144 (3 wt%)/TiO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Mahbub, P.; Duke, M. Scalability of Advanced Oxidation Processes (AOPs) in Industrial Applications: A Review. J. Environ. Manag. 2023, 345, 118861. [Google Scholar] [CrossRef]
- Li, X.; Luo, R.; Liang, X.; Wu, Q.; Gong, C. Recent Advances in Enhancing Reactive Oxygen Species Based Chemodynamic Therapy. Chin. Chem. Lett. 2022, 33, 2213–2230. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Q.; Wang, W.; Shao, J.; Huang, W.; Dong, X. Type I Photosensitizers Revitalizing Photodynamic Oncotherapy. Small 2021, 17, 2006742. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Shah, M.U.H. Modification Strategies of TiO2-Based Photocatalysts for Enhanced Visible Light Activity and Energy Storage Ability: A Review. J. Environ. Chem. Eng. 2023, 11, 111532. [Google Scholar] [CrossRef]
- Abdurahman, M.H.; Abdullah, A.Z.; Shoparwe, N.F. A Comprehensive Review on Sonocatalytic, Photocatalytic, and Sonophotocatalytic Processes for the Degradation of Antibiotics in Water: Synergistic Mechanism and Degradation Pathway. Chem. Eng. J. 2021, 413, 127412. [Google Scholar] [CrossRef]
- Qiu, P.; Park, B.; Choi, J.; Thokchom, B.; Pandit, A.B.; Khim, J. A Review on Heterogeneous Sonocatalyst for Treatment of Organic Pollutants in Aqueous Phase Based on Catalytic Mechanism. Ultrason. Sonochem. 2018, 45, 29–49. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Gong, F.; Chao, Y.; Cheng, L. Newly Developed Strategies for Improving Sonodynamic Therapy. Mater. Horiz. 2020, 7, 2028–2046. [Google Scholar] [CrossRef]
- He, Z.; Du, J.; Miao, Y.; Li, Y. Recent Developments of Inorganic Nanosensitizers for Sonodynamic Therapy. Adv. Healthc. Mater. 2023, 12, 2300234. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Qian, H.; Cheng, L. Titanium-Based Sonosensitizers for Sonodynamic Cancer Therapy. Appl. Mater. Today 2021, 25, 101215. [Google Scholar] [CrossRef]
- Maleki, A.; Seyedhamzeh, M.; Yuan, M.; Agarwal, T.; Sharifi, I.; Mohammadi, A.; Kelicen-Uğur, P.; Hamidi, M.; Malaki, M.; Al Kheraif, A.A.; et al. Titanium-Based Nanoarchitectures for Sonodynamic Therapy-Involved Multimodal Treatments. Small 2023, 19, 22062. [Google Scholar] [CrossRef]
- Priya, M.H.; Madras, G. Kinetics of TiO2-Catalyzed Ultrasonic Degradation of Rhodamine Dyes. Ind. Eng. Chem. Res. 2006, 45, 913–921. [Google Scholar] [CrossRef]
- Khataee, A.; Sheydaei, M.; Hassani, A.; Taseidifar, M.; Karaca, S. Sonocatalytic Removal of an Organic Dye Using TiO2/Montmorillonite Nanocomposite. Ultrason. Sonochem. 2015, 22, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Kayan, B.; Gholami, P.; Kalderis, D.; Akay, S. Sonocatalytic Degradation of an Anthraquinone Dye Using TiO2-Biochar Nanocomposite. Ultrason. Sonochem. 2017, 39, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, D.; Hong, S.; Khan, S.; Darya, B.; Lee, J.Y.; Chung, J.; Cho, S.H. Investigation of the Synergistic Effect of Sonolysis and Photocatalysis of Titanium Dioxide for Organic Dye Degradation. Catalysts 2020, 10, 500. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of Rhodamine B in Water by Ultrasound-Assisted TiO2 Photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Sheikhmohammadi, A.; Asgari, E.; Nourmoradi, H.; Fazli, M.M.; Yeganeh, M. Ultrasound-Assisted Decomposition of Metronidazole by Synthesized TiO2/Fe3O4 Nanocatalyst: Influencing Factors and Mechanisms. J. Environ. Chem. Eng. 2021, 9, 105844. [Google Scholar] [CrossRef]
- Jonnalagadda, U.S.; Su, X.; Kwan, J.J. Nanostructured TiO2 Cavitation Agents for Dual-Modal Sonophotocatalysis with Pulsed Ultrasound. Ultrason. Sonochem. 2021, 73, 105530. [Google Scholar] [CrossRef]
- Deepagan, V.G.; You, D.G.; Um, W.; Ko, H.; Kwon, S.; Choi, K.Y.; Yi, G.R.; Lee, J.Y.; Lee, D.S.; Kim, K.; et al. Long-Circulating Au-TiO2 Nanocomposite as a Sonosensitizer for ROS-Mediated Eradication of Cancer. Nano Lett. 2016, 16, 6257–6264. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, T.; Dai, W.; Dong, H.; Zhang, X. TiO2 Nanosheets with the Au Nanocrystal-Decorated Edge for Mitochondria-Targeting Enhanced Sonodynamic Therapy. Chem. Mater. 2019, 31, 9105–9114. [Google Scholar] [CrossRef]
- Li, F.; Pan, Q.; Ling, Y.; Guo, J.; Huo, Y.; Xu, C.; Xiong, M.; Yuan, M.; Cheng, Z.; Liu, M.; et al. Gold–Titanium Dioxide Heterojunction for Enhanced Sonodynamic Mediated Biofilm Eradication and Peri-Implant Infection Treatment. Chem. Eng. J. 2021, 413, 127412. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Cao, Y.; Chai, O.J.H.; Xie, J. Ligand Design in Ligand-Protected Gold Nanoclusters. Small 2021, 17, 2004381. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Guo, Y.; Kawasaki, H. Review on Gold Nanoclusters for Cancer Phototherapy. ACS Appl. Bio Mater. 2023, 6, 4504–4517. [Google Scholar] [CrossRef]
- Albright, E.L.; Levchenko, T.I.; Kulkarni, V.K.; Sullivan, A.I.; DeJesus, J.F.; Malola, S.; Takano, S.; Nambo, M.; Stamplecoskie, K.; Häkkinen, H.; et al. Atomically Precise Gold Nanoclusters: Synthesis, Properties, and Applications. J. Am. Chem. Soc. 2024, 146, 5759–5780. [Google Scholar] [CrossRef] [PubMed]
- Kawawaki, T.; Negishi, Y.; Kawasaki, H. Photo/Electrocatalysis and Photosensitization Using Metal Nanoclusters for Green Energy and Medical Applications. Nanoscale Adv. 2020, 2, 17–36. [Google Scholar] [CrossRef]
- Hossain, S.; Hirayama, D.; Ikeda, A.; Ishimi, M.; Funaki, S.; Samanta, A.; Kawawaki, T.; Negishi, Y. Atomically Precise Thiolate-Protected Gold Nanoclusters: Current Status of Designability of the Structure and Physicochemical Properties. Aggregate 2023, 4, e255. [Google Scholar] [CrossRef]
- Li, S.; Du, X.; Liu, Z.; Li, Y.; Shao, Y.; Jin, R. Size Effects of Atomically Precise Gold Nanoclusters in Catalysis. Precis. Chem. 2023, 1, 14–28. [Google Scholar] [CrossRef]
- Kawamura, K.; Hikosou, D.; Inui, A.; Yamamoto, K.; Yagi, J.; Saita, S.; Kawasaki, H. Ultrasonic Activation of Water-Soluble Au25(SR)18 Nanoclusters for Singlet Oxygen Production. J. Phys. Chem. C 2019, 123, 26644–26652. [Google Scholar] [CrossRef]
- Yagi, J.; Ikeda, A.; Wang, L.-C.; Yeh, C.-S.; Kawasaki, H. Singlet Oxygen Generation Using Thiolated Gold Nanoclusters under Photo- and Ultrasonic Excitation: Size and Ligand Effect. J. Phys. Chem. C 2022, 126, 19693–19703. [Google Scholar] [CrossRef]
- Kawamura, K.; Ikeda, A.; Inui, A.; Yamamoto, K.; Kawasaki, H. TiO2-Supported Au144 Nanoclusters for Enhanced Sonocatalytic Performance. J. Chem. Phys. 2021, 155, 124702. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Xia, N.; Liao, L.; Zhu, M.; Jin, F.; Jin, R.; Wu, Z. Unraveling the Long-Pursued Au144 Structure by X-Ray Crysta lography. Sci. Adv. 2018, 4, eaat7259. [Google Scholar] [CrossRef]
- Fang, X.; Mark, G.; von Sonntag, C. OH Radical Formation by Ultrasound in Aqueous Solutions. Part I: The Chemistry Underlying the Terephthalate Dosimeter. Ultrason. Sonochem. 1996, 3, 57–63. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, T.; Tang, A.; Yang, H. Modulating Coal-Derived Carbon toward Electrocatalytic Generation of Hydroxyl Radi cals for Organic Contaminant Removal. J. Mater. Chem. A 2024, 12, 7227–7236. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, X.; Ruan, W.; Zhao, B.; Xu, W.; Lombardi, J.R. Adsorption Study of 4-MBA on TiO2 Nanoparticles by Surface-Enhanced Raman Spectroscopy. J. Raman Spectrosc. 2009, 40, 2000–2005. [Google Scholar] [CrossRef]
- Compeán-González, C.L.; Thomas, A.G.; Syres, K.L.; Cole, J.; Li, Z. 4-Mercaptobenzoic Acid Adsorption on TiO2 Anatase (101) and TiO2 Rutile (110) Surfaces. Surfaces 2022, 5, 238–250. [Google Scholar] [CrossRef]
- Didenko, Y.T.; Pugach, S.P. Spectra of Water Sonoluminescence. J. Phys. Chem. 1994, 98, 9742–9749. [Google Scholar] [CrossRef]
- Yasui, K. Production of O Radicals from Cavitation Bubbles under Ultrasound. Molecules 2022, 27, 4788. [Google Scholar] [CrossRef] [PubMed]
- Beckett, M.A.; Hua, I. Impact of Ultrasonic Frequency on Aqueous Sonoluminescence and Sonochemistry. J. Phys. Chem. A 2001, 105, 3796–3802. [Google Scholar] [CrossRef]
- Zhou, M.; Mohd Yusof, N.S.; Ashokkumar, M. Correlation between Sonochemistry and Sonoluminescence at Various Frequencies. RSC Adv. 2013, 3, 9319–9324. [Google Scholar] [CrossRef]
- Ji, R.; Pflieger, R.; Virot, M.; Nikitenko, S.I. Multibubble Sonochemistry and Sonoluminescence at 100 kHz: The Missing Link between Low- and High-Frequency Ultrasound. J. Phys. Chem. B 2018, 122, 6989–6994. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K. Multibubble Sonoluminescence from a Theoretical Perspective. Molecules 2021, 26, 4624. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; He, Q.; Wu, M.; Xu, Y.; Sun, P.; Dong, X. Piezoelectric Polarization Promoted Spatial Separation of Photoexcited Electrons and Holes in Two-Dimensional g-C3N4 Nanosheets for Efficient Elimination of Chlorophenols. J. Hazard. Mater. 2022, 421, 126696. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wierzbicka, E.; Springer, A.; Pinna, N.; Wang, Y. Influence of the Electronic Properties of the Ligand on the Photoelectrochemical Behavior of Au25 Nanocluster-Sensitized TiO2 Photoanode. J. Phys. Chem. C 2022, 126, 1778–1784. [Google Scholar] [CrossRef]
- Zhou, M.; Zeng, C.; Chen, Y.; Jin, R. Evolution from the Plasmon to Exciton State in Ligand-Protected Atomically Precise Gold Nanoparticles. Nat. Commun. 2016, 7, 13240. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Naveen, M.H.; Abbas, M.A.; Lee, J.; Kim, H.; Bang, J.H. Photoelectrochemistry of Au Nanocluster-Sensitized TiO2: Intricacy Arising from the Light-Induced Transformation of Nanoclusters into Nanoparticles. ACS Energy Lett. 2021, 6, 24–32. [Google Scholar] [CrossRef]
- Shaltiel, L.; Shemesh, A.; Raviv, U.; Barenholz, Y.; Levi-Kalisman, Y. Synthesis and Characterization of Thiolate-Protected Gold Nanoparticles of Controlled Diameter. J. Phys. Chem. C 2019, 123, 28486–28493. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsurunishi, T.; Furui, Y.; Kawasaki, H. Ultrasonic Activation of Au Nanoclusters/TiO2: Tuning Hydroxyl Radical Production Through Frequency and Nanocluster Size. Molecules 2025, 30, 541. https://doi.org/10.3390/molecules30030541
Tsurunishi T, Furui Y, Kawasaki H. Ultrasonic Activation of Au Nanoclusters/TiO2: Tuning Hydroxyl Radical Production Through Frequency and Nanocluster Size. Molecules. 2025; 30(3):541. https://doi.org/10.3390/molecules30030541
Chicago/Turabian StyleTsurunishi, Takaaki, Yuzuki Furui, and Hideya Kawasaki. 2025. "Ultrasonic Activation of Au Nanoclusters/TiO2: Tuning Hydroxyl Radical Production Through Frequency and Nanocluster Size" Molecules 30, no. 3: 541. https://doi.org/10.3390/molecules30030541
APA StyleTsurunishi, T., Furui, Y., & Kawasaki, H. (2025). Ultrasonic Activation of Au Nanoclusters/TiO2: Tuning Hydroxyl Radical Production Through Frequency and Nanocluster Size. Molecules, 30(3), 541. https://doi.org/10.3390/molecules30030541






