Potential Transformation of Food Resveratrol: Mechanisms and Biological Impact
Abstract
1. Introduction
2. Food Sources
3. Resveratrol Chemical Transformations
3.1. Effect of pH
3.2. Effect of Light
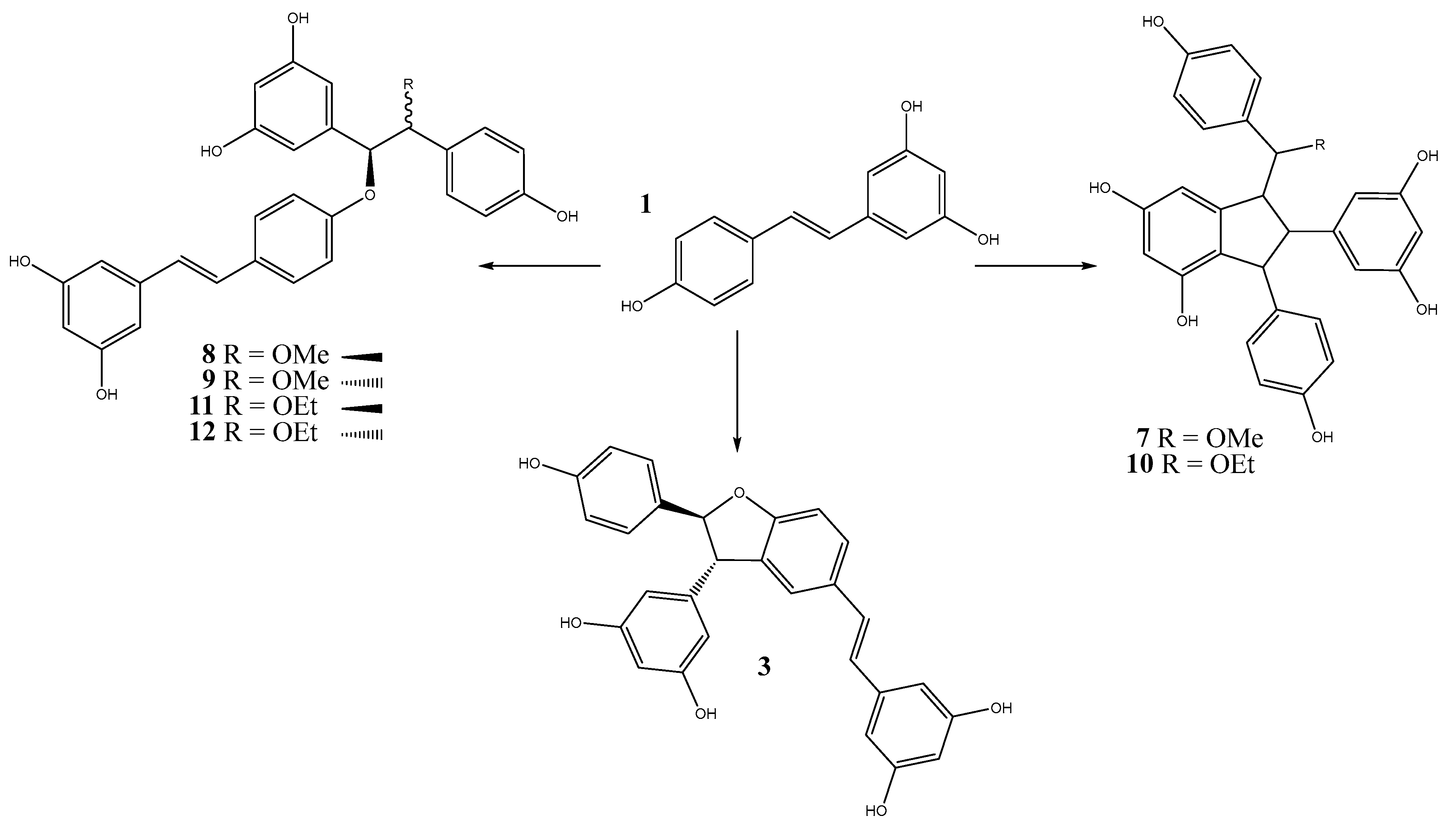
3.3. Oligomerization
4. Biological Properties of Resveratrol Products
4.1. Cis-Resveratrol
4.2. Other Compounds
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Takaoka, M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. J. Chem. Soc. Jpn. 1939, 60, 1090–1100. [Google Scholar]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Benbouguerra, N.; Hornedo-Ortega, R.; Garcia, F.; El Khawand, T.; Saucier, C.; Richard, T. Stilbenes in grape berries and wine and their potential role as anti-obesity agents: A review. Trends Food Sci. Technol. 2021, 112, 362–381. [Google Scholar] [CrossRef]
- Biagi, M.; Bertelli, A.A.E. Wine, alcohol and pills: What future for the French paradox? Life Sci. 2015, 131, 19–22. [Google Scholar] [CrossRef]
- Li, W.; Yuan, H.; Liu, Y.; Wang, B.; Xu, X.; Xu, X.; Hussain, D.; Ma, L.; Chen, D. Current analytical strategies for the determination of resveratrol in foods. Food Chem. 2024, 431, 137182. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Franco, F.N.; Caldeira, C.A.; de Araújo, G.R.; Vieira, A.; Chaves, M.M. Resveratrol has its antioxidant and anti-inflammatory protective mechanisms decreased in aging. Arch. Gerontol. Geriatr. 2023, 107, 104895. [Google Scholar] [CrossRef] [PubMed]
- Dikmetas, D.N.; Yenipazar, H.; Karaca, A.C. Recent advances in encapsulation of resveratrol for enhanced delivery. Food Chem. 2024, 460, 140475. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2019, 100, 1392–1404. [Google Scholar] [CrossRef]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol derivatives as potential treatments for Alzheimer’s and Parkinson’s disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Latruffe, N.; Vervandier-Fasseur, D. Strategic syntheses of vine and wine resveratrol derivatives to explore their effects on cell functions and dysfunctions. Diseases 2018, 6, 110. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, F.; Gao, Y.; Jia, S.; Ji, N.; Hua, E. Design, synthesis, and evaluation of methoxylated resveratrol derivatives as potential antitumor agents. Res. Chem. Intermed. 2015, 41, 2725–2738. [Google Scholar] [CrossRef]
- Wang, H.-L.; Gao, J.-P.; Han, Y.-L.; Xu, X.; Wu, R.; Gao, Y.; Cui, X.-H. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine 2015, 22, 553–559. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and its Human metabolites-effects on metabolic health and obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef]
- Trela, B.C.; Waterhouse, A.L. Resveratrol: isomeric molar absorptivities and stability. J. Agric. Food Chem. 1996, 44, 1253–1257. [Google Scholar] [CrossRef]
- Francioso, A.; Boffi, A.; Villani, C.; Manzi, L.; D’Erme, M.; Macone, A.; Mosca, L. Isolation and identification of 2,4,6-trihydroxyphenanthrene as a byproduct of trans-resveratrol photochemical isomerization and electrocyclization. J. Org. Chem. 2014, 79, 9381–9384. [Google Scholar] [CrossRef] [PubMed]
- Jarosova, V.; Vesely, O.; Doskocil, I.; Tomisova, K.; Marsik, P.; Jaimes, J.D.; Smejkal, K.; Kloucek, P.; Havlik, J. Metabolism of cis- and trans-resveratrol and dihydroresveratrol in an intestinal epithelial model. Nutrients 2020, 12, 595. [Google Scholar] [CrossRef]
- Jhanji, M.; Rao, C.N.; Massey, J.C.; Hope, M.C.; Zhou, X.; Keene, C.D.; Ma, T.; Wyatt, M.D.; Stewart, J.A.; Sajish, M. Cis- and trans-resveratrol have opposite effects on histone serine-ADP-ribosylation and tyrosine induced neurodegeneration. Nat. Commun. 2022, 13, 3244. [Google Scholar] [CrossRef]
- Aumont, V.; Krisa, S.; Richard, T.; Mérillon, J.M.; Battaglia, E.; Netter, P.; Magdalou, J.; Sabolovic, N. Regioselective and stereospecific glucuronidation of trans- and cis-resveratrol in human. Arch. Biochem. Biophys. 2001, 393, 281–289. [Google Scholar] [CrossRef]
- Nonomura, S.; Kanagawa, H.; Makimoto, A. Chemical constituents of polygonaceous plants. I. Studies on the components of Ko-jô-Kon (Polygonum cunspidatum SIEB. et Zucc.). Yakugaku Zasshi 1963, 83, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Li, M.T.; Xu, S.; Ma, J.; Liu, M.Y.; Han, Y. Advances for pharmacological activities of Polygonum cuspidatum—A review. Pharm. Biol. 2023, 61, 177–188. [Google Scholar] [CrossRef]
- Silva, R.d.C.d.; Teixeira, J.A.; Nunes, W.D.G.; Zangaro, G.A.C.; Pivatto, M.; Caires, F.J.; Ionashiro, M. Resveratrol: A thermoanalytical study. Food Chem. 2017, 237, 561–565. [Google Scholar] [CrossRef]
- Francioso, A.; Laštovičková, L.; Mosca, L.; Boffi, A.; Bonamore, A.; Macone, A. Gas chromatographic-mass spectrometric method for the simultaneous determination of resveratrol isomers and 2,4,6-trihydroxyphenanthrene in red wines exposed to UV-light. J. Agric. Food Chem. 2019, 67, 11752–11757. [Google Scholar] [CrossRef] [PubMed]
- Takaya, Y.; Terashima, K.; Ito, J.; He, Y.-H.; Tateoka, M.; Yamaguchi, N.; Niwa, M. Biomimic transformation of resveratrol. Tetrahedron 2005, 61, 10285–10290. [Google Scholar] [CrossRef]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Mattivi, F.; Reniero, F.; Korhammer, S. Isolation, Characterization, and Evolution in Red Wine Vinification of Resveratrol Monomers. J. Agric. Food Chem. 1995, 43, 1820–1823. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, M.; Ye, J.-H.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R. Photo-induced chemical reaction of trans-resveratrol. Food Chem. 2015, 171, 137–143. [Google Scholar] [CrossRef]
- Recky, J.R.N.; Tosato, M.G.; Buglak, A.A.; Dántola, M.L.; Lorente, C. Photosensitized isomerization of resveratrol: Evaluation of energy and electron transfer pathways. Free Radic. Biol. Med. 2024, 216, 50–59. [Google Scholar] [CrossRef]
- Pébarthé-Courrouilh, A.; Jaa, A.; Valls-Fonayet, J.; Da Costa, G.; Palos-Pinto, A.; Richard, T.; Cluzet, S. UV-exposure decreases antimicrobial activities of a grapevine cane extract against Plasmopara viticola and Botrytis cinerea as a consequence of stilbene modifications—A kinetic study. Pest Manag. Sci. 2024, 80, 6389–6399. [Google Scholar] [CrossRef] [PubMed]
- Latva-Mäenpää, H.; Wufu, R.; Mulat, D.; Sarjala, T.; Saranpää, P.; Wähälä, K. Stability and photoisomerization of stilbenes Isolated from the bark of Norway spruce roots. Molecules 2021, 26, 1036. [Google Scholar] [CrossRef]
- Rodríguez-Cabo, T.; Rodríguez, I.; Ramil, M.; Cela, R. Comprehensive evaluation of the photo-transformation routes of trans-resveratrol. J. Chromatogr. A 2015, 1410, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Gensberger-Reigl, S.; Hoferer, L.; Abreu, V.L.R.G.; Graßl, F.; Fischer, O.; Heinrich, M.R. Identification and quantification of resveratrol and Its derivatives in Franconian wines by comprehensive liquid chromatography−Tandem mass spectrometry. ACS Food Sci. Technol. 2023, 3, 1057–1065. [Google Scholar]
- Quideau, S.; Deffieux, D.; Pouységu, L. Oxidative coupling of phenols and phenol ethers. In Comprehensive Organic Synthesis, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 3, pp. 656–740. [Google Scholar]
- Sursin, E.; Flourat, A.L.; Akissi, Z.L.E.; Martinez, A.; Borie, N.; Peyrot, C.; Courot, E.; Nuzillard, J.-M.; Renault, J.-H.; Voutquenne-Nazabadioko, L.; et al. Combining laccase-mediated dimerization of resveratrol and centrifugal partition chromatography: Optimization of E-labruscol production and identification of new resveratrol dimers. ACS Sustain. Chem. Eng. 2023, 11, 11559–11569. [Google Scholar] [CrossRef]
- Velu, S.S.; Buniyamin, I.; Ching, L.K.; Feroz, F.; Noorbatcha, I.; Gee, L.C.; Awang, K.; Wahab, I.A.; Weber, J.-F.F. Regio- and stereoselective biomimetic synthesis of oligostilbenoid dimers from resveratrol analogues: Influence of the solvent, oxidant, and substitution. Chem.–A Eur. J. 2008, 14, 11376–11384. [Google Scholar] [CrossRef] [PubMed]
- El Khawand, T.; Gabaston, J.; Taillis, D.; Iglesias, M.-L.; Pedrot, E.; Pinto, A.P.; Fonayet, J.V.; Merillon, J.M.; Decendit, A.; Cluzet, S.; et al. A dimeric stilbene extract produced by oxidative coupling of resveratrol active against Plasmopara viticola and Botrytis cinerea for vine treatments. OENO One 2020, 54, 157–164. [Google Scholar] [CrossRef]
- El Khawand, T.; Fonayet, J.V.; Da Costa, G.; Hornedo-Ortega, R.; Jourdes, M.; Franc, C.; de Revel, G.; Decendit, A.; Krisa, S.; Richard, T. Resveratrol transformation in red wine after heat treatment. Food Res. Int. 2020, 132, 109068. [Google Scholar] [CrossRef]
- Vitrac, X.; Bornet, A.; Vanderlinde, R.; Valls, J.; Richard, T.; Delaunay, J.C.; Merillon, J.M.; Teissedre, P.L. Determination of stilbenes (δ-viniferin, trans-astringin, trans-piceid, cis- and trans-resveratrol, ε-viniferin) in Brazilian wines. J. Agric. Food Chem. 2005, 53, 5664–5669. [Google Scholar] [CrossRef]
- Beaumont, P.; Courtois, A.; Atgié, C.; Richard, T.; Krisa, S. In the shadow of resveratrol: Biological activities of epsilon-viniferin. J. Physiol. Biochem. 2022, 78, 465–484. [Google Scholar] [CrossRef]
- Jeon, D.; Jo, M.; Lee, Y.; Park, S.-H.; Phan, H.T.L.; Nam, J.H.; Namkung, W. Inhibition of ANO1 by cis- and trans-resveratrol and their anticancer activity in Human prostate cancer PC-3 cells. Int. J. Mol. Sci. 2023, 24, 1186. [Google Scholar] [CrossRef] [PubMed]
- Leischner, C.; Burkard, M.; Michel, A.; Berchtold, S.; Niessner, H.; Marongiu, L.; Busch, C.; Frank, J.; Lauer, U.M.; Venturelli, S. Comparative analysis of the antitumor activity of cis- and trans-resveratrol in human cancer cells with different p53 status. Molecules 2021, 26, 5586. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Moreno, J.J. Resveratrol analogs with antioxidant activity inhibit intestinal epithelial cancer Caco-2 cell growth by modulating arachidonic acid cascade. J. Agric. Food Chem. 2019, 67, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-T.; Lai, H.-C.; Chen, Y.-B.; Chen, L.-G.; Wu, Y.-H.; Ko, Y.-F.; Lu, C.-C.; Chang, C.-J.; Wu, C.-Y.; Martel, J.; et al. cis-Resveratrol produces anti-inflammatory effects by inhibiting canonical and non-canonical inflammasomes in macrophages. Innate Immun. 2014, 20, 735–750. [Google Scholar] [CrossRef]
- Leiro, J.; Álvarez, E.; Arranz, J.A.; Laguna, R.; Uriarte, E.; Orallo, F. Effects of cis-resveratrol on inflammatory murine macrophages: Antioxidant activity and down-regulation of inflammatory genes. J. Leukoc. Biol. 2004, 75, 1156–1165. [Google Scholar] [CrossRef]
- Bertelli, A.A.; Giovannini, L.; Bernini, W.; Migliori, M.; Fregoni, M.; Bavaresco, L.; Bertelli, A. Antiplatelet activity of cis-resveratrol. Drugs Exp. Clin. Res. 1996, 22, 61–63. [Google Scholar]
- Kim, H.; Oh, S.J.; Liu, Y.; Lee, M.Y. Comparative study of the anti-platelet effects of cis- and trans-resveratrol. Biomol. Ther. 2011, 19, 201–205. [Google Scholar] [CrossRef]
- Cheng, H.; Fang, Z.; Wusigale; Bakry, A.M.; Chen, Y.; Liang, L. Complexation of trans- and cis-resveratrol with bovine serum albumin, β-lactoglobulin or α-lactalbumin. Food Hydrocoll. 2018, 81, 242–252. [Google Scholar] [CrossRef]
- Cheng, H.; Dong, H.; Liang, L. A comparison of β-casein complexes and micelles as vehicles for trans-/cis-resveratrol. Food Chem. 2020, 330, 127209. [Google Scholar] [CrossRef]
- Kukric, Z.; Topalić-Trivunović, L. Antibacterial activity of cis- and trans-resveratrol isolated from Polygonum cuspidatum rhizome. Acta Period. Technol. 2006, 2006, 131–136. [Google Scholar] [CrossRef]
- Yin, R.; Zhang, Y.; Su, L.; Chen, D.; Lou, S.; Luo, X.; Wang, L.; Tang, R.; Zhang, L.; Tian, X. The mechanism of trans-δ-viniferin inhibiting the proliferation of lung cancer cells A549 by targeting the mitochondria. Front. Pharmacol. 2023, 14, 1190127. [Google Scholar] [CrossRef] [PubMed]
- Volpes, S.; Cruciata, I.; Ceraulo, F.; Schimmenti, C.; Naselli, F.; Pinna, C.; Mauro, M.; Picone, P.; Dallavalle, S.; Nuzzo, D.; et al. Nutritional epigenomic and DNA-damage modulation effect of natural stilbenoids. Sci. Rep. 2023, 13, 658. [Google Scholar] [CrossRef]
- Platella, C.; Mazzini, S.; Napolitano, E.; Mattio, L.M.; Beretta, G.L.; Zaffaroni, N.; Pinto, A.; Montesarchio, D.; Dallavalle, S. Plant-serived stilbenoids as DNA-binding agents: From monomers to dimers. Chem. Eur. J. 2021, 27, 8832–8845. [Google Scholar] [CrossRef] [PubMed]
- Shkryl, Y.; Tsydeneshieva, Z.; Menchinskaya, E.; Rusapetova, T.; Grishchenko, O.; Mironova, A.; Bulgakov, D.; Gorpenchenko, T.; Kazarin, V.; Tchernoded, G.; et al. Exosome-like nanoparticles, high in trans-δ-Viniferin derivatives, produced from grape cell cultures: Preparation, characterization, and anticancer properties. Biomedicines 2024, 12, 2142. [Google Scholar] [CrossRef]
- Giovannelli, L.; Innocenti, M.; Santamaria, A.R.; Bigagli, E.; Pasqua, G.; Mulinacci, N. Antitumoural activity of viniferin-enriched extracts from Vitis vinifera L. cell cultures. Nat. Prod. Res. 2014, 28, 2006–2016. [Google Scholar] [CrossRef]
- Choi, Y.S.; Yoon, D.H.; Kim, S.Y.; Kim, C.S.; Lee, K.R. Stilbene oligomers from the stems of Parthenocissus tricuspidata and their potential anti-neuroinflammatory and neuroprotective activity. Tetrahedron Lett. 2021, 71, 153027. [Google Scholar] [CrossRef]
- Nassra, M.; Krisa, S.; Papastamoulis, Y.; Kapche, G.D.; Bisson, J.; André, C.; Konsman, J.P.; Schmitter, J.M.; Mérillon, J.M.; Waffo-Téguo, P. Inhibitory activity of plant stilbenoids against nitric oxide production by lipopolysaccharide-activated microglia. Planta Medica 2013, 79, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, T.; Fan, B.; Yang, L.; Han, C.; Luo, J.; Kong, L. Protective effect of trans-δ-viniferin against high glucose-induced oxidative stress in human umbilical vein endothelial cells through the SIRT1 pathway. Free Radic. Res. 2016, 50, 68–83. [Google Scholar] [CrossRef]
- Nagumo, M.; Ninomiya, M.; Oshima, N.; Itoh, T.; Tanaka, K.; Nishina, A.; Koketsu, M. Comparative analysis of stilbene and benzofuran neolignan derivatives as acetylcholinesterase inhibitors with neuroprotective and anti-inflammatory activities. Bioorganic Med. Chem. Lett. 2019, 29, 2475–2479. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Bhan, N.; Masuko, S.; James, P.; Wood, J.; McCallum, S.; Linhardt, R.J.; Dordick, J.S.; Koffas, M.A.G. Antimicrobial mechanism of resveratrol-trans-dihydrodimer produced from peroxidase-catalyzed oxidation of resveratrol. Biotechnol. Bioeng. 2015, 112, 2417–2428. [Google Scholar] [CrossRef]
- Catinella, G.; Mattio, L.M.; Musso, L.; Arioli, S.; Mora, D.; Beretta, G.L.; Zaffaroni, N.; Pinto, A.; Dallavalle, S. Structural requirements of benzofuran derivatives dehydro-δ-and dehydro-ε-viniferin for antimicrobial activity against the foodborne pathogen listeria monocytogenes. Int. J. Mol. Sci. 2020, 21, 2168. [Google Scholar] [CrossRef]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Mosca, L.; Menéndez-Perdomo, I.M.; Fanelli, S.; Fontana, M.; D’Erme, M.; Fuentes-Leon, F.; Sanchez-Lamar, A. 2,4,6-Trihydroxyphenanthrene, a trans-resveratrol photoreaction byproduct: First evidences of genotoxic risk. Phytochem. Lett. 2019, 30, 362–366. [Google Scholar] [CrossRef]
- Jhanji, M.; Rao, C.N.; Sajish, M. Towards resolving the enigma of the dichotomy of resveratrol: Cis- and trans-resveratrol have opposite effects on TyrRS-regulated PARP1 activation. GeroScience 2021, 43, 1171–1200. [Google Scholar] [CrossRef]
- Hwangbo, K.; Zheng, M.S.; Kim, Y.J.; Im, J.Y.; Lee, C.S.; Woo, M.H.; Jahng, Y.; Chang, H.W.; Son, J.K. Inhibition of DNA topoisomerases i and II of compounds from Reynoutria japonica. Arch. Pharmacal Res. 2012, 35, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.H.; Li, H.; Sun, Z.; Wu, M.L.; Ma, J.X.; Wang, J.M.; Wang, Q.; Sun, Y.; Fu, Y.S.; Chen, X.Y.; et al. Identification of metabolic pattern and bioactive form of resveratrol in human medulloblastoma cells. Biochem. Pharmacol. 2010, 79, 1516–1525. [Google Scholar] [CrossRef]
- Morris, V.L.; Toseef, T.; Nazumudeen, F.B.; Rivoira, C.; Spatafora, C.; Tringali, C.; Rotenberg, S.A. Anti-tumor properties of cis-resveratrol methylated analogs in metastatic mouse melanoma cells. Mol. Cell. Biochem. 2015, 402, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Belleri, M.; Ribatti, D.; Savio, M.; Stivala, L.A.; Forti, L.; Tanghetti, E.; Alessi, P.; Coltrini, D.; Bugatti, A.; Mitola, S.; et al. αvβ3 Integrin-dependent antiangiogenic activity of resveratrol stereoisomers. Mol. Cancer Ther. 2008, 7, 3761–3770. [Google Scholar] [CrossRef]
- Sajish, M.; Schimmel, P. A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol. Nature 2015, 519, 370–373. [Google Scholar] [CrossRef]
- Fuloria, S.; Sekar, M.; Khattulanuar, F.S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Subramaniyan, V.; Jeyabalan, S.; Begum, M.Y.; Chidambaram, K.; et al. Chemistry, biosynthesis and pharmacology of viniferin: Potential resveratrol-derived molecules for new drug discovery, Development and therapy. Molecules 2022, 27, 5072. [Google Scholar] [CrossRef]
- Pezet, R.; Gindro, K.; Viret, O.; Richter, H. Effects of resveratrol, viniferins and pterostilbene on Plasmopara viticola zoospore mobility and disease development. VITIS-J. Grapevine Res. 2004, 43, 145–148. [Google Scholar]
- Pezet, R.; Gindro, K.; Viret, O.; Spring, J.L. Glycosylation and oxidative dimerization of resveratrol are respectively associated to sensitivity and resistance of grapevine cultivars to downy mildew. Physiol. Mol. Plant Pathol. 2004, 65, 297–303. [Google Scholar] [CrossRef]
- Pezet, R.; Perret, C.; Jean-Denis, J.B.; Tabacchi, R.; Gindro, K.; Viret, O. δ-viniferin, a resveratrol dehydrodimer: One of the major stilbenes synthesized by stressed grapevine leaves. J. Agric. Food Chem. 2003, 51, 5488–5492. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Zanzotto, A.; de Rosso, M.; Lucchetta, G.; Vedova, A.D.; Bavaresco, L. Stilbene oligomer phytoalexins in grape as a response to Aspergillus carbonarius infection. Physiol. Mol. Plant Pathol. 2016, 93, 112–118. [Google Scholar] [CrossRef]
- Huber, R.; Marcourt, L.; Héritier, M.; Luscher, A.; Guebey, L.; Schnee, S.; Michellod, E.; Guerrier, S.; Wolfender, J.-L.; Scapozza, L.; et al. Generation of potent antibacterial compounds through enzymatic and chemical modifications of the trans-δ-viniferin scaffold. Sci. Rep. 2023, 13, 15986. [Google Scholar] [CrossRef]
- Ding, D.-J.; Cao, X.-Y.; Dai, F.; Li, X.-Z.; Liu, G.-Y.; Lin, D.; Fu, X.; Jin, X.-L.; Zhou, B. Synthesis and antioxidant activity of hydroxylated phenanthrenes as cis-restricted resveratrol analogues. Food Chem. 2012, 135, 1011–1019. [Google Scholar] [CrossRef]
- Zwygart, A.C.A.; Medaglia, C.; Huber, R.; Poli, R.; Marcourt, L.; Schnee, S.; Michellod, E.; Mazel-Sanchez, B.; Constant, S.; Huang, S.; et al. Antiviral properties of trans-δ-viniferin derivatives against enveloped viruses. Biomed. Pharmacother. 2023, 163, 114825. [Google Scholar] [CrossRef]

| Compounds | Biological Effects | Molecular Targets | Study Model | Dosis | Ref. |
|---|---|---|---|---|---|
| cis-resveratrol (2) | Anti-cancer | ↓ cell proliferation and migration ↓ mRNA, ANO1 expression ↑ ROS, caspase-3, PARP cleavage, sub G1 phase and apoptosis | Prostate cancer (PC-3) in vitro | 10–100 µM | [42] |
| ↓ cell proliferation | Hepatocellular/colon/pancreatic/renal carcinoma (HepG2, Hep3B, HCT-116), in vitro | [43] | |||
| ↓ COX-1, COX-2, 5-LOX, 12-LOX, 15-LOX, HODEs ↓ proliferation, ↑ATP release, ROS, apoptosis | Intestinal carcinoma cells (Caco-2) | [44] | |||
| Anti-inflammatory | ↓ ROS ↓ caspases-1 and -4 | Human macrophages in vitro | 1–100 µM | [45] | |
| ↓ IL-1β, pro-IL-1β ↓ mRNA, COX-2, NOS-2 | Rat macrophages in vitro | [46] | |||
| Skin protection | Isomerization trans/cis | in vitro, in silico | – | [30] | |
| Antiplatelet | ↓ platelet aggregation | Human plasma in vitro | 1–10 µM | [47] | |
| ↓ platelet aggregation thrombin, collagen and ADP | Rat plasma in vitro | [48] | |||
| Protein-ligand interaction | ↑ BSA | in vitro | 0–20 µM | [49] | |
| ↓ β-LG, α-LA, β-casein | [50] | ||||
| Antibacterial | Escherichia coli Staphylococcus sp. | in vitro | – | [51] | |
| δ-viniferin (3) | Anti-cancer | ↓ proliferation, ΔΨm, GR, GSH, PI3K/Akt/mTOR pathway ↑ ATP release, ROS, apoptosis | Lung Cancer A549 in vitro | 0–100 µM | [52] |
| ↓ proliferation | Caco-2, HepG-2 cells | [53] | |||
| ↓ proliferation ↑ DNA damage ↑ epigenotoxic and cyto-genotoxic effects | A375, H460, PC3, WS1 cells | 0–200 µM | [54] | ||
| ↓ proliferation Cell cycle arrest | Breast cancer MDA-MB-231 In vitro | [55] | |||
| ↑ S and G2/M arrest, apoptosis | Vitis vinifera extract HCC-1500, HCC-1954, MCF-7, HepG2 in vitro | 0–125 µM | [56] | ||
| Anti-inflammatory | ↓ NO | Murine microglial BV2 cells | 5–40 μM | [57,58] | |
| Cardiovascular | ↓ Cytotoxicity and apoptosis ↓ ROS, Caspase-3, -7 and -9 ↑ MMP, SIRT1 | Endothelial HUVECs cells | 0.5–5 μM | [59] | |
| Neuroprotective | ↓ Cytotoxicity ↓ NO | Murine macrophage RAW264.7, PC12 Cells | 3–100 μM | [60] | |
| Antibacterial | Bacillus cereus Staphylococcus aureus Listeria monocytogenes Membrane disruption ↓ DNA gyrase activity | in vitro | 1–200 μM | [61] | |
| Listeria monocytogenes | 1–200 μg/mL | [62] | |||
| Staphylococcus aureus, Pseudomonas aeruginosa Escherichia coli, Proteus Hauser, Listeria monocytogenes ↑ β-galactosidase activity, DNA damage | 1–512 μg/mL | [63] | |||
| 2,4,6-trihydroxy-phenanthrene (4) | Antibacterial | ↑ β-galactosidase activity, DNA damage | Caulobacter crescentus | 10 µM | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaa, A.; de Moura, P.H.B.; Ruiz-Larrea, M.B.; Ruiz Sanz, J.I.; Richard, T. Potential Transformation of Food Resveratrol: Mechanisms and Biological Impact. Molecules 2025, 30, 536. https://doi.org/10.3390/molecules30030536
Jaa A, de Moura PHB, Ruiz-Larrea MB, Ruiz Sanz JI, Richard T. Potential Transformation of Food Resveratrol: Mechanisms and Biological Impact. Molecules. 2025; 30(3):536. https://doi.org/10.3390/molecules30030536
Chicago/Turabian StyleJaa, Ayoub, Patricia Homobono Brito de Moura, María Begoña Ruiz-Larrea, José Ignacio Ruiz Sanz, and Tristan Richard. 2025. "Potential Transformation of Food Resveratrol: Mechanisms and Biological Impact" Molecules 30, no. 3: 536. https://doi.org/10.3390/molecules30030536
APA StyleJaa, A., de Moura, P. H. B., Ruiz-Larrea, M. B., Ruiz Sanz, J. I., & Richard, T. (2025). Potential Transformation of Food Resveratrol: Mechanisms and Biological Impact. Molecules, 30(3), 536. https://doi.org/10.3390/molecules30030536







