Impact of a Palladium(II)-tris(2-carboxyethyl)phosphine Complex on Normal Cells: Toxicity and Membrane Interaction
Abstract
1. Introduction
2. Results
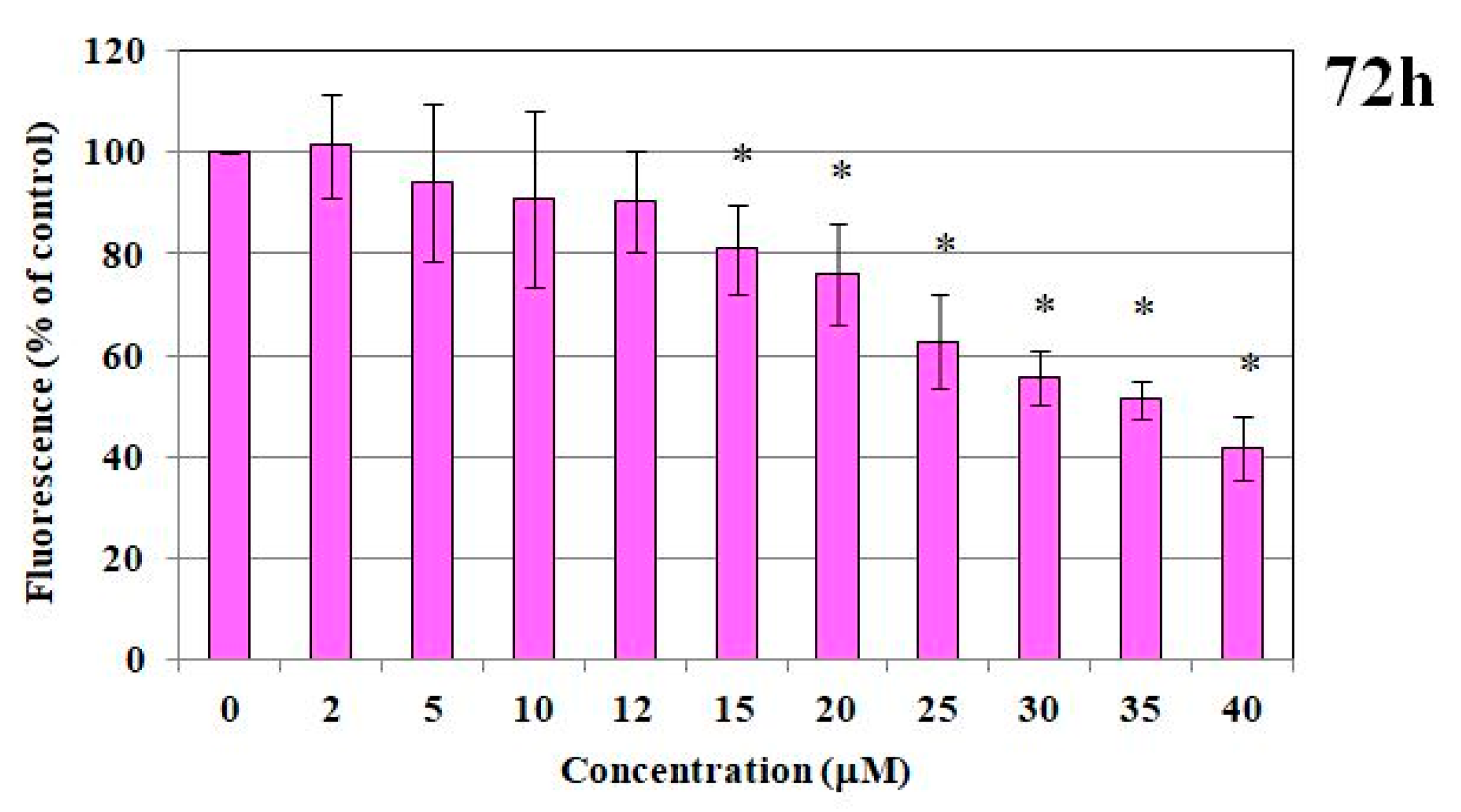
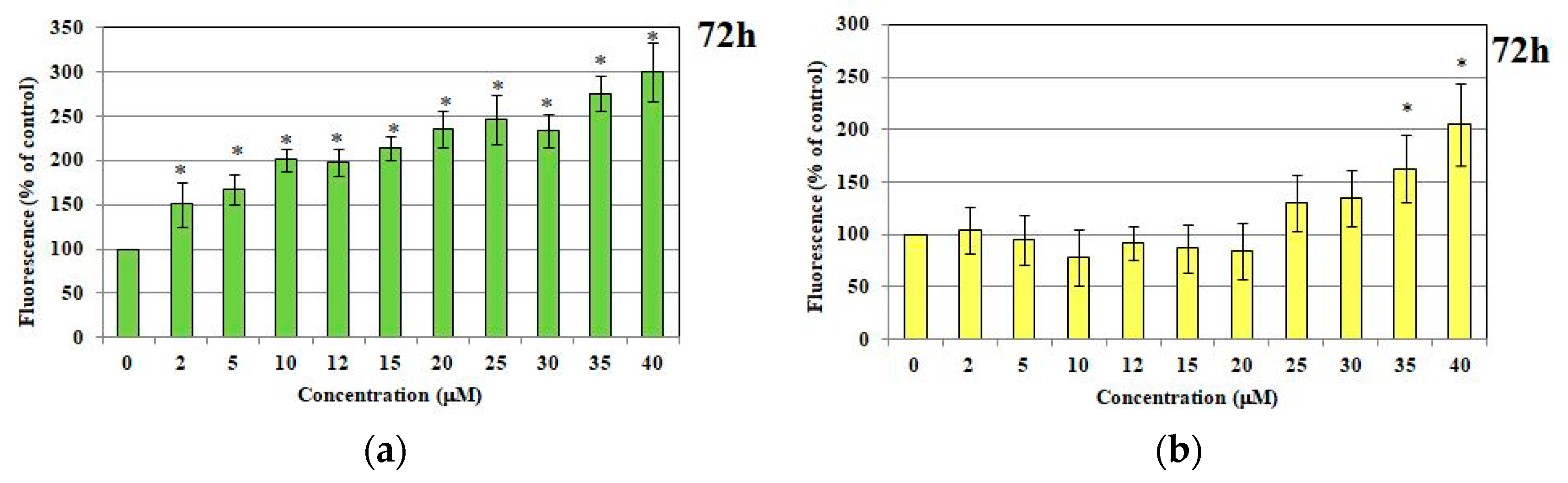
2.1. Immortalized Human Microvascular Endothelial Cells
2.1.1. MTT Test and DNA Content
2.1.2. ROS and RNS Production
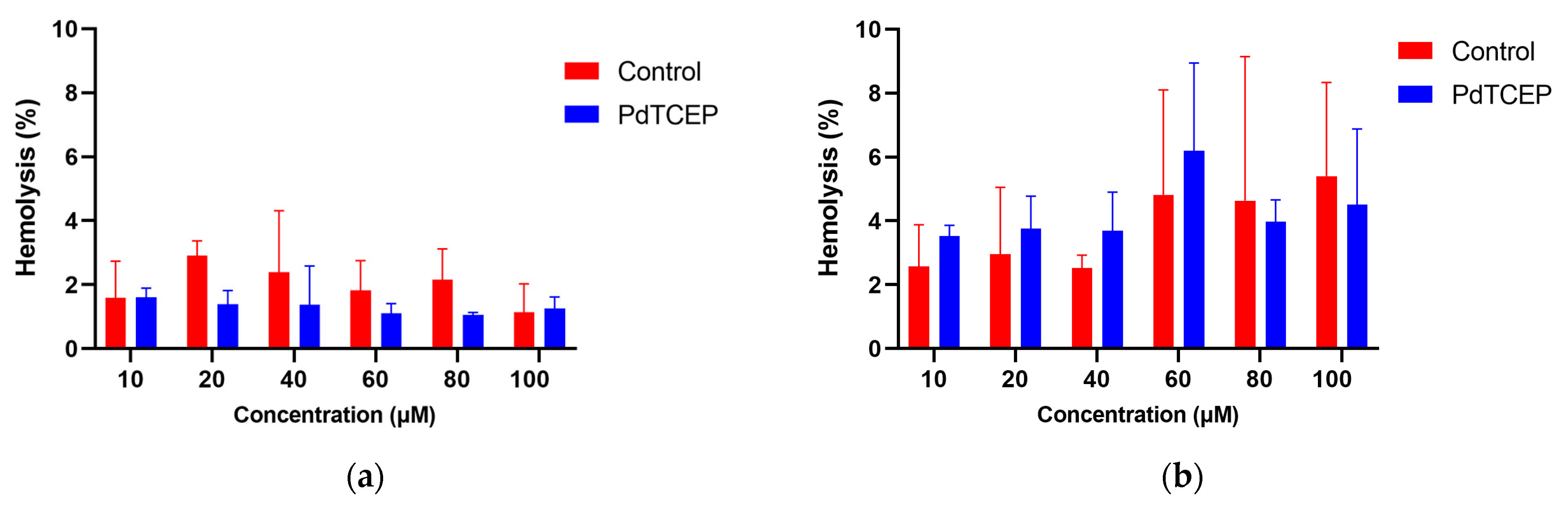
2.2. Red Blood Cells
2.2.1. Hemolysis Assay
2.2.2. Shape of Erythrocytes
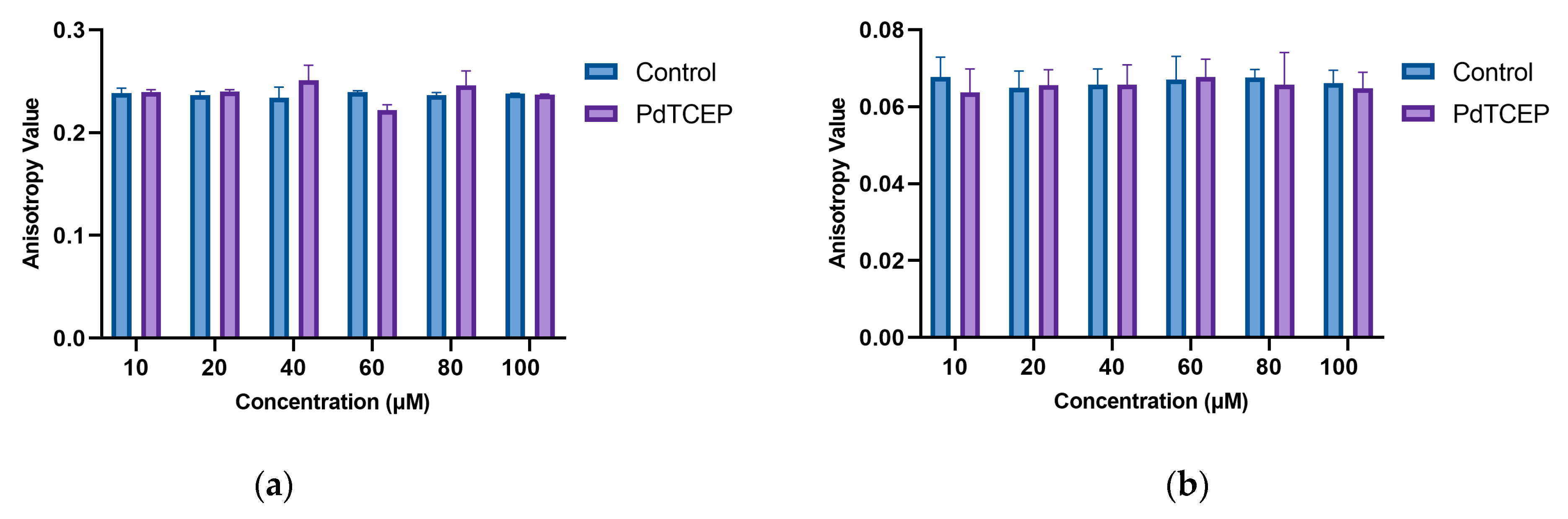
2.3. Influence of PdTCEP on the Selected Properties of the Membrane
2.3.1. Steady-State Fluorescence
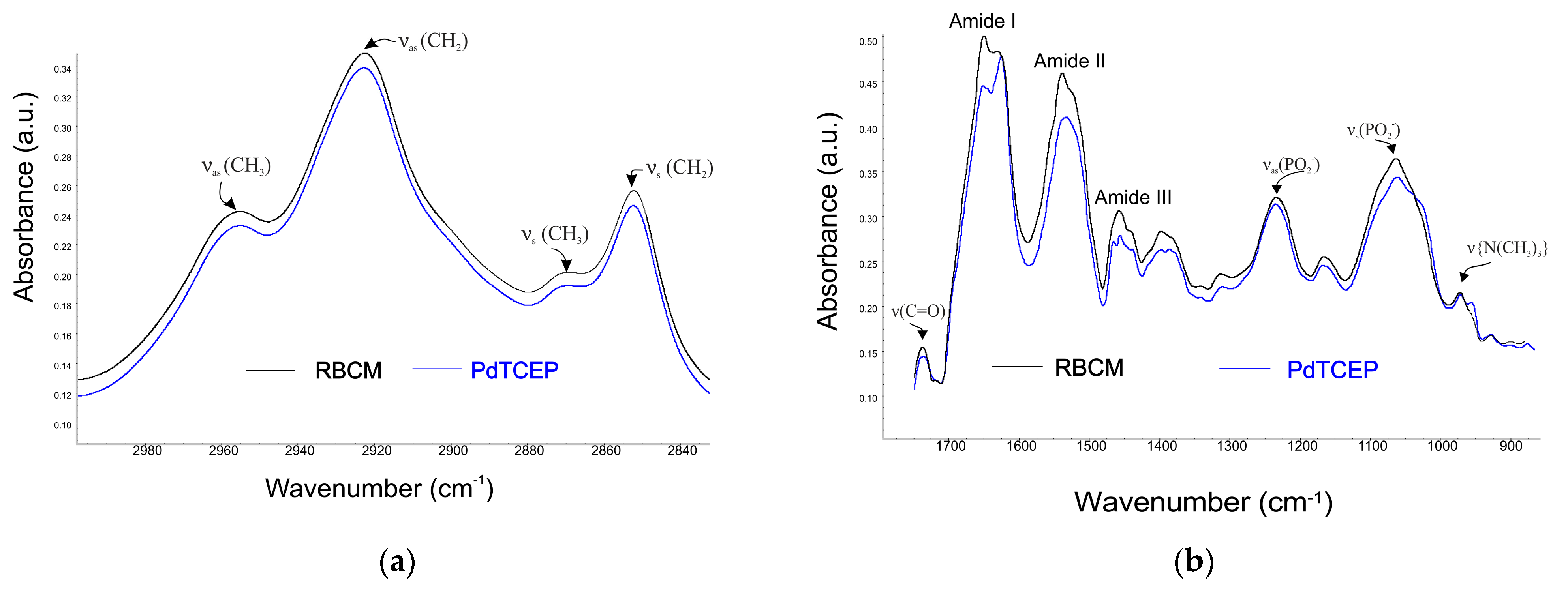
2.3.2. Attenuated Total Reflectance Infrared Spectroscopy Technique (ATR-FTIR)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Line
4.3. Cytotoxicity Test of Normal Cells
4.4. Assay of Cell Survival Based on DNA Content
4.5. Determination of ROS and RNS Production
4.6. Hemolysis of Erythrocytes
4.7. Shape of Erythrocytes
4.8. Influence of PdTCEP on the Selected Properties of the Membrane
4.8.1. Steady-State Fluorescence Spectroscopy
4.8.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef]
- World Health Organization. Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 1 February 2024).
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Devel. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Gielen, M.; Tiekink, E.R.T. Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Lapasam, A.; Hussain, O.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Synthesis, characterization and chemosensitivity studies of half-sandwich ruthenium, rhodium and iridium complexes containing к1(S) and к2(N,S) aroylthiourea ligands. J. Organomet. Chem. 2019, 880, 272–280. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Ge, X.; Wang, Q.; Xie, Y.; Hao, Y.; Zhang, Y.; Zhang, L.; Shang, W.; Liu, Z. Lysosome-Targeted iridium(iii) compounds with pyridine-Triphenylamine Schiff base ligands: Syntheses, antitumor applications and mechanisms. Inorg. Chem. Front. 2019, 7, 91–100. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Englinger, B.; Pirker, C.; Heffeter, P.; Terenzi, A.; Kowol, C.R.; Keppler, B.K.; Berger, W. Metal drugs and the anticancer immune response. Chem. Rev. 2019, 119, 1519–1624. [Google Scholar] [CrossRef]
- Ellahioui, Y.; Prashar, S.; Gómez-Ruiz, S. Anticancer applications and recent investigations of metallodrugs based on gallium, tin and titanium. Inorganics 2017, 5, 1–23. [Google Scholar] [CrossRef]
- Garcia, A.E.; Jalilehvand, F. Aerobic reactions of antitumor active dirhodium(II) tetraacetate Rh2(621 CH3COO)4 with glutathione. J. Biol. Inorg. Chem. 2018, 23, 231–239. [Google Scholar] [CrossRef]
- Wootton, C.A.; Millett, A.J.; Lopez-Clavijo, A.F.; Chiu, C.K.C.; Barrow, M.P.; Clarkson, G.J.; Sadler, P.J.; O’Connor, P.B. Structural analysis of peptides modified with organo-iridium complexes, opportunities from multi-mode fragmentation. Analyst 2019, 144, 1575–1581. [Google Scholar] [CrossRef]
- Pruchnik, H.; Lis, T.; Pruchnik, F.P. Platinum(II) complexes with tris(2-carboxyethyl)phosphine, X-ray structure and reactions with polar solvents and glutathione. J. Organomet. Chem. 2015, 791, 124–129. [Google Scholar] [CrossRef]
- Henklewska, M.; Pawlak, A.; Kutkowska, J.; Pruchnik, H.; Rapak, A. OMB. In vitro effects of the activity of novel platinum (II) complex in canine and human cell lines. Vet. Comp. Oncol. 2019, 17, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Brabec, V.; Kasparkova, J. Platinum-Based Drugs. In Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 489–506. [Google Scholar]
- Trynda-Lemiesz, L.; Łuczkowski, M. Human serum albumin: Spectroscopic studies of the paclitaxel binding and proximity relationships with cisplatin and adriamycin. J. Inorg. Biochem. 2004, 98, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Wiglusz, K.; Trynda-Lemiesz, L. Platinum drugs binding to human serum albumin: Effect of non-steroidal anti-inflammatory drugs. J. Photochem. Photobiol. A Chem. 2014, 289, 1–6. [Google Scholar] [CrossRef]
- Bourgaux, C.; Couvreur, P. Interactions of anticancer drugs with biomembranes: What can we learn from model membranes? J. Control Release 2014, 190, 127–138. [Google Scholar] [CrossRef]
- Pruchnik, H.; Kral, T.; Hof, M. Interaction of Newly Platinum(II) with Tris(2-carboxyethyl)phosphine Complex with DNA and Model Lipid Membrane. J. Membr. Biol. 2017, 250, 461–470. [Google Scholar] [CrossRef][Green Version]
- Pruchnik, H.; Lis, T.; Latocha, M.; Zielińska, A.; Pruchnik, F.P. Palladium(II) complexes with tris(2-carboxyethyl)phosphine, structure, reactions and cytostatic activity. J. Inorg. Biochem. 2016, 156, 14–21. [Google Scholar] [CrossRef]
- Pentak, D. Evaluation of the physicochemical properties of liposomes as potential carriers of anticancer drugs: Spectroscopic study. J. Nanopart. Res. 2016, 18, 126. [Google Scholar] [CrossRef]
- Jensen, M.; Nerdal, W. Anticancer cisplatin interactions with bilayers of total lipid extract from pig brain: A 13C, 31P and 15N solid-state NMR study. Eur. J. Pharm. Sci. 2008, 34, 140–148. [Google Scholar] [CrossRef]
- Nierzwicki, L.; Wieczor, M.; Censi, V.; Baginski, M.; Calucci, L.; Samaritani, S.; Czub, J.; Forte, C. Interaction of cisplatin and two potential antitumoral platinum(II) complexes with a model lipid membrane: A combined NMR and MD study. Phys. Chem. Chem. Phys. 2015, 17, 1458–1468. [Google Scholar] [CrossRef]
- Alves, A.C.; Ribeiro, D.; Nunes, C.; Reis, S. Biophysics in cancer: The relevance of drug-membrane interaction studies. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2231–2244. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zeng, C.; Feng, Y.; Zhou, F.; Liao, F.; Liu, Y.; Feng, S.; Wang, X. The size-dependent effects of silica nanoparticles on endothelial cell apoptosis through activating the p53-caspase pathway. Environ. Pollut. 2018, 233, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Pruchnik, H.; Włoch, A.; Bonarska-Kujawa, D.; Kleszczyńska, H. An In Vitro Study of the Effect of Cytotoxic Triorganotin Dimethylaminophenylazobenzoate Complexes on Red Blood Cells. J. Membr. Biol. 2018, 251, 735–745. [Google Scholar] [CrossRef]
- Suwalsky, M.; Castillo, I.; Sánchez-Eguíab, B.N.; Gallardo, M.J.; Dukes, N.; Santiago-Osorio, E.; Aguińiga, I.; Rivera-Martínez, A.R. In vitro effects of benzimidazole/thioether-copper complexes with antitumor activity on human erythrocytes. J. Inorg. Biochem. 2018, 178, 87–93. [Google Scholar] [CrossRef]
- Suwalsky, M.; Duguet, J.; Speiscy, H. An In vitro study of the antioxidant and antihemolytic properties of Buddleja globosa (Matico). J. Membr. Biol. 2017, 250, 239–248. [Google Scholar] [CrossRef]
- Suwalsky, M.; Ramırez, P.; Avello, M.; Villena, F.; Gallardoc, M.J.; Barriga, A.; Manrique-Moreno, M. Morphological effects and antioxidant capacity of Solanum crispum (Natre) assayed on human erythrocytes. J. Membr. Biol. 2016, 249, 349–361. [Google Scholar] [CrossRef]
- Suwalsky, M.; Avello, M. Antioxidant capacity of Ugni molinae fruit extract on human erythrocytes: An in vitro study. J. Membr. Biol. 2014, 247, 703–712. [Google Scholar] [CrossRef]
- Suwalsky, M.; Oyarce, K.; Avello, M.; Villena, F.; Sotomayor, C.P. Human erythrocytes and molecular models are affected in vitro by Balbisia peduncularis (Amancay) extracts. Chem. Biol. Interact. 2009, 179, 413–418. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Z.; Zong, S.; Zhang, Y.; Chen, C.; Zhang, R.; Yun, B.; Cui, Y. Investigating the intracellular behaviors of liposomal nanohybrids via SERS: Insights into the influence of metal nanoparticles. Theranostics 2018, 8, 941–954. [Google Scholar] [CrossRef]
- Strugała, P.; Dudra, A.; Gabrielska, J. Interaction between mimic lipid membranes and acylated and nonacylated cyanidin and its bioactivity. J. Agric. Food Chem. 2016, 64, 7414–7422. [Google Scholar] [CrossRef]
- Dudek, A.; Szulc, N.; Pawlak, A.; Strugała-Danak, P.; Krawczyk-Łebek, A.; Perz, M.; Kostrzewa-Susłow, E.; Pruchnik, H. Structural investigation of interactions between halogenated flavonoids and the lipid membrane along with their role as cytotoxic agents. Sci. Rep. 2024, 14, 10561. [Google Scholar] [CrossRef] [PubMed]
- Bagatolli, L.A. LAURDAN Fluorescence Properties in Membranes: A Journey from the Fluorometer to the Microscope. In Fluorescent Methods to Study Biological Membranes; Springer: Berlin/Heidelberg, Germany, 2012; Volume 13, pp. 3–35. [Google Scholar] [CrossRef]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.; Gratton, E. Laurdan and Prodan as Polarity-Sensitive Fluorescent Membrane Probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Maddy, A.H.; Dunn, M.J.; Kelly, P.G. The characterization of membrane proteins by centrifugation and gel electrophoresis: A com parison of proteins prepared by different methods. Biochim. Biophys. Acta 1972, 288, 263–276. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 589–602. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruchnik, H.; Solarska-Ściuk, K.; Dudek, A.; Włoch, A. Impact of a Palladium(II)-tris(2-carboxyethyl)phosphine Complex on Normal Cells: Toxicity and Membrane Interaction. Molecules 2025, 30, 476. https://doi.org/10.3390/molecules30030476
Pruchnik H, Solarska-Ściuk K, Dudek A, Włoch A. Impact of a Palladium(II)-tris(2-carboxyethyl)phosphine Complex on Normal Cells: Toxicity and Membrane Interaction. Molecules. 2025; 30(3):476. https://doi.org/10.3390/molecules30030476
Chicago/Turabian StylePruchnik, Hanna, Katarzyna Solarska-Ściuk, Anita Dudek, and Aleksandra Włoch. 2025. "Impact of a Palladium(II)-tris(2-carboxyethyl)phosphine Complex on Normal Cells: Toxicity and Membrane Interaction" Molecules 30, no. 3: 476. https://doi.org/10.3390/molecules30030476
APA StylePruchnik, H., Solarska-Ściuk, K., Dudek, A., & Włoch, A. (2025). Impact of a Palladium(II)-tris(2-carboxyethyl)phosphine Complex on Normal Cells: Toxicity and Membrane Interaction. Molecules, 30(3), 476. https://doi.org/10.3390/molecules30030476





