Effective Factors for Optimizing Metallophthalocyanine-Based Optoelectronic Devices: Surface—Molecule Interactions
Abstract
1. Introduction
2. Influential Factors on Molecular Orientation
2.1. Internal Stimuli: Substrate Properties
2.1.1. Crystal Structure
2.1.2. Terminated Atoms
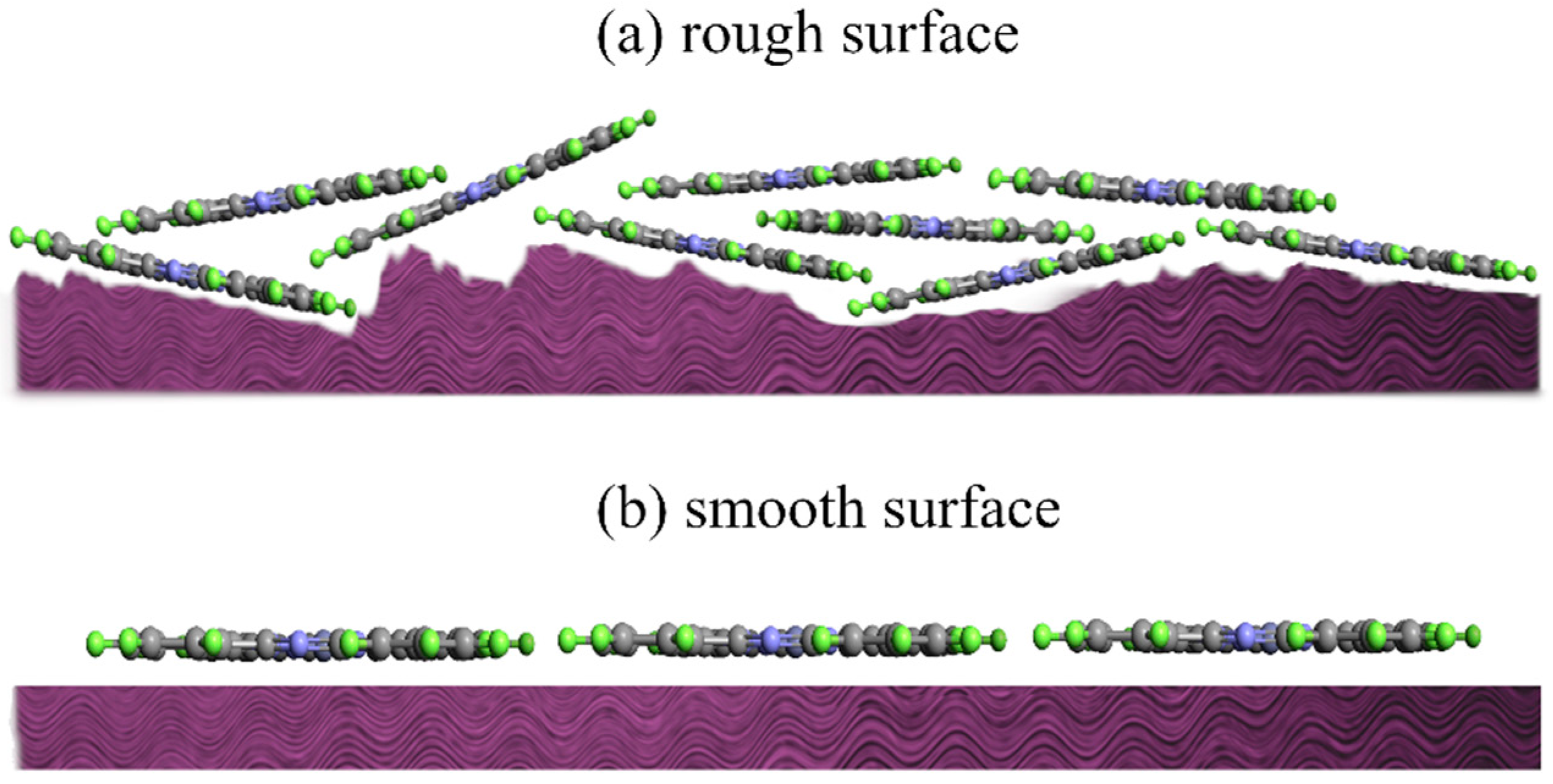
2.1.3. Substrate Roughness
2.2. External Stimuli: Thermal Processes and Post-Deposition Annealing
3. MPc–Substrate Interaction: MPc Materials on Gold, Silver, and Copper Substrates
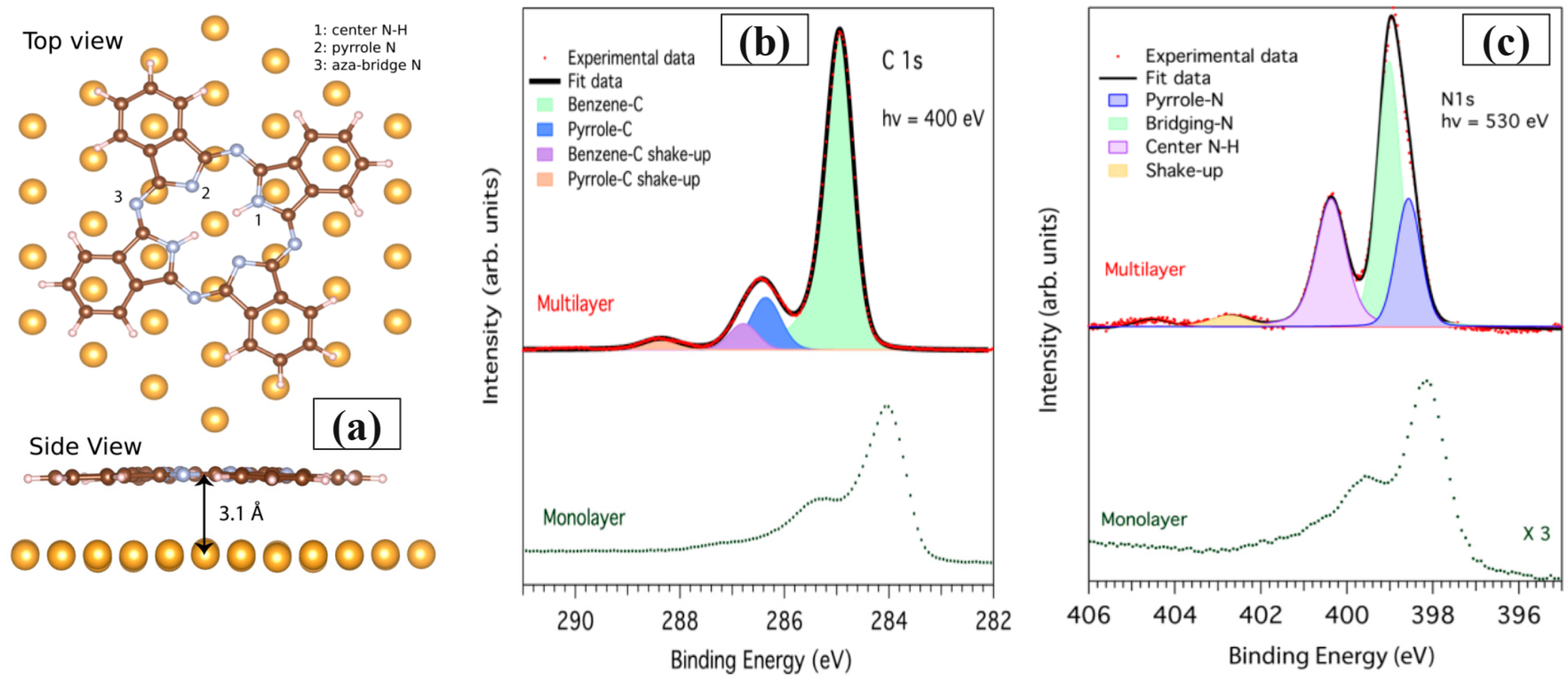
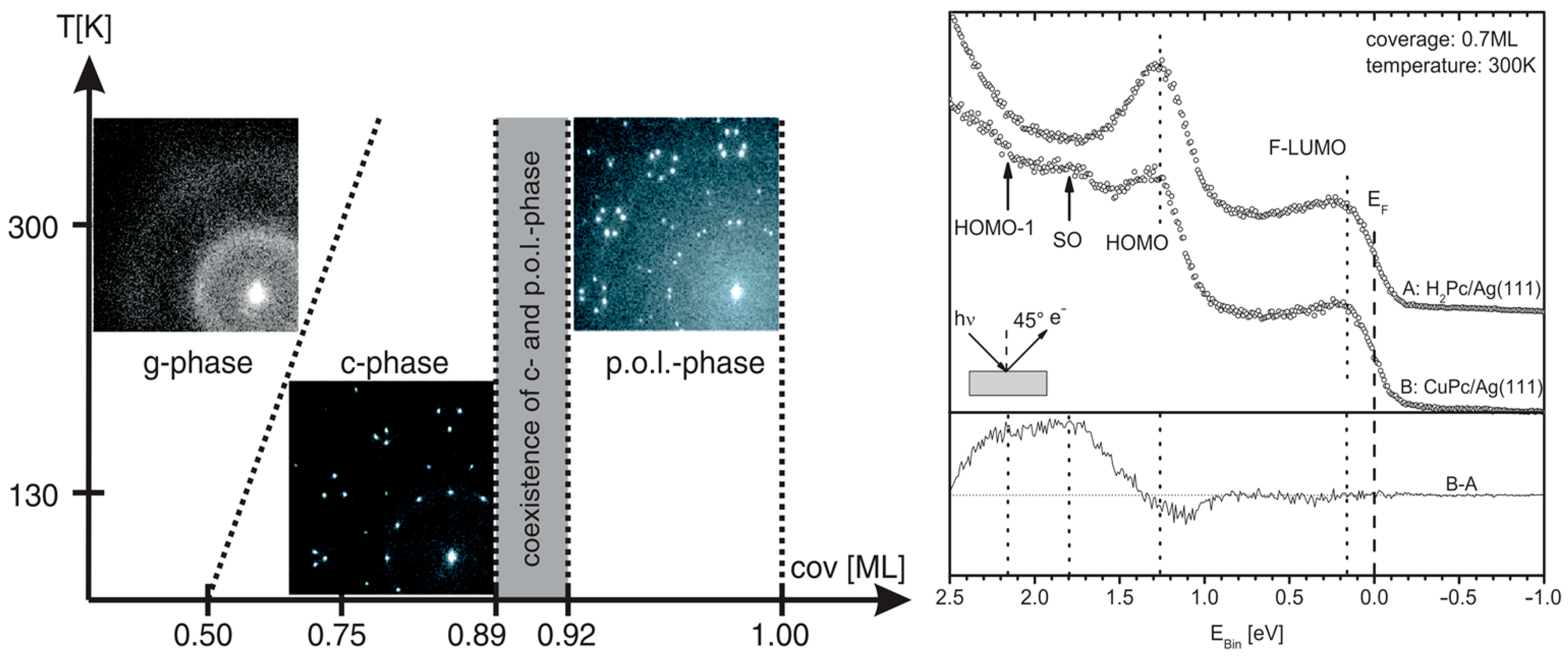
3.1. Free-Based Phthalocyanine (H2Pc)/(Au; Ag; Cu)
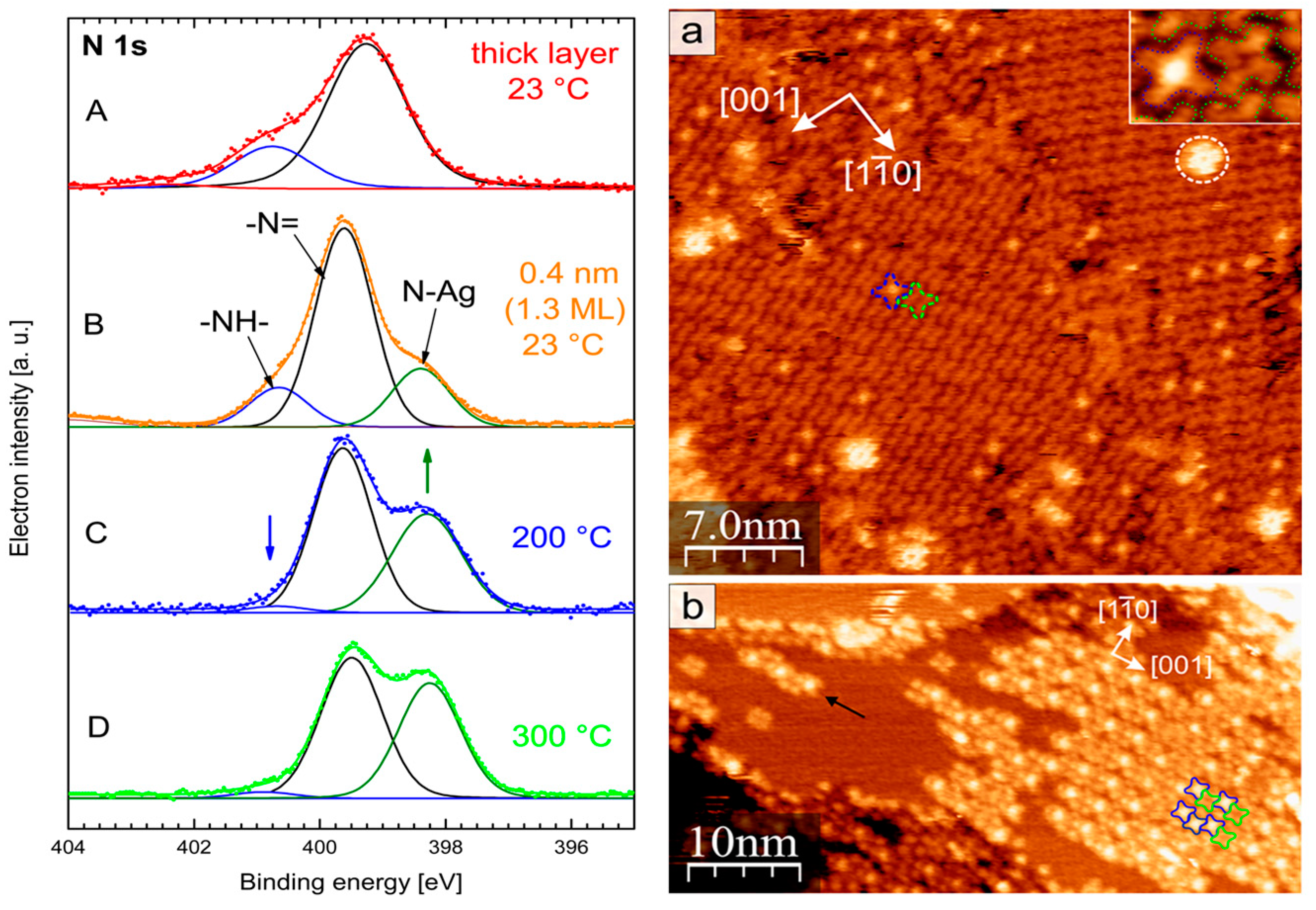
3.2. Copper Phthalocyanine (CuPc)/(Au; Ag; Cu)
3.3. Iron Phthalocyanine (FePc)/(Au; Ag; Cu)
3.4. Cobalt Phthalocyanine (CoPc)/(Au; Ag; Cu)
3.5. Vanadyl Phthalocyanine (VOPc)/(Au; Ag; Cu)
4. Metal–MPc Hybrid Layer’s Applications, Challenges, and Limitations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craciun, M.F.; Rogge, S.; Morpurgo, A.F. Correlation between molecular orbitals and doping dependence of the electrical conductivity in electron-doped metal-phthalocyanine compounds. J. Am. Chem. Soc. 2005, 127, 12210–12211. [Google Scholar] [CrossRef][Green Version]
- Papageorgiou, N.; Salomon, E.; Angot, T.; Layet, J.-M.; Giovanelli, L.; Le Lay, G. Physics of ultra-thin phthalocyanine films on semiconductors. Prog. Surf. Sci. 2004, 77, 139–170. [Google Scholar] [CrossRef]
- Joachim, C.; Gimzewski, J.K.; Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 2000, 408, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, F.; Ruocco, A.; Pasca, D.; Baldacchini, C.; Betti, M.G.; Corradini, V.; Mariani, C. Au (1 1 0) induced reconstruction by π conjugated molecules adsorption investigated by photoemission spectroscopy and low energy electron diffraction. Surf. Sci. 2004, 566, 79–83. [Google Scholar] [CrossRef]
- Aykanat, A.; Meng, Z.; Benedetto, G.; Mirica, K.A. Molecular engineering of multifunctional metallophthalocyanine-containing framework materials. Chem. Mater. 2020, 32, 5372–5409. [Google Scholar] [CrossRef]
- Rezaee, E.; Khan, D.; Cai, S.; Dong, L.; Xiao, H.; Silva, S.R.P.; Liu, X.; Xu, Z.-X. Phthalocyanine in perovskite solar cells: A review. Mater. Chem. Front. 2023, 7, 1704–1736. [Google Scholar] [CrossRef]
- Sánchez Vergara, M.E.; Canseco Juárez, M.J.; Ballinas Indili, R.; Carmona Reyes, G.; Álvarez Bada, J.R.; Álvarez Toledano, C. Studies on the structure, optical, and electrical properties of doped manganese (III) phthalocyanine chloride films for optoelectronic device applications. Coatings 2022, 12, 246. [Google Scholar] [CrossRef]
- Huang, X.; Ji, D.; Fuchs, H.; Hu, W.; Li, T. Recent progress in organic phototransistors: Semiconductor materials, device structures and optoelectronic applications. ChemPhotoChem 2020, 4, 9–38. [Google Scholar] [CrossRef]
- Zhong, C.; Yan, Y.; Peng, Q.; Zhang, Z.; Wang, T.; Chen, X.; Wang, J.; Wei, Y.; Yang, T.; Xie, L. Structure–property relationship of macrocycles in organic photoelectric devices: A comprehensive review. Nanomaterials 2023, 13, 1750. [Google Scholar] [CrossRef] [PubMed]
- Remiro Buenamanana, S. Contracted Phthalocyanine Macrocycles: Conjugation with Nanoparticles and the First Synthesis of Meso-Substituted Boron SubTriBenzoDiAzaPorphyrin Hybrids (SubTBDAPs); University of East Anglia: Norwich, UK, 2015. [Google Scholar]
- Allard, C.; Alvarez, L.; Bantignies, J.-L.; Bendiab, N.; Cambré, S.; Campidelli, S.; Fagan, J.A.; Flahaut, E.; Flavel, B.; Fossard, F.; et al. Advanced 1D heterostructures based on nanotube templates and molecules. Chem. Soc. Rev. 2024, 53, 8457–8512. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vergara, M.E.; Gómez-Gómez, M.; Hamui, L.; Álvarez-Bada, J.R.; Jiménez-Sandoval, O. Optoelectronic behaviour of zinc phthalocyanines doped with anthraquinone derivatives and their potential use in flexible devices. Mater. Technol. 2021, 36, 250–259. [Google Scholar] [CrossRef]
- Qian, C.; Sun, J.; Gao, Y. Transport of charge carriers and optoelectronic applications of highly ordered metal phthalocyanine heterojunction thin films. Phys. Chem. Chem. Phys. 2021, 23, 9631–9642. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, D.; Jones, T.S. Molecular template growth and its applications in organic electronics and optoelectronics. Chem. Rev. 2015, 115, 5570–5603. [Google Scholar] [CrossRef] [PubMed]
- Claessens, C.G.; Hahn, U.W.E.; Torres, T. Phthalocyanines: From outstanding electronic properties to emerging applications. Chem. Rec. 2008, 8, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wong, S.L.; Chen, W.; Wee, A.T.S. LT-STM studies on substrate-dependent self-assembly of small organic molecules. J. Phys. D Appl. Phys. 2011, 44, 464005. [Google Scholar] [CrossRef]
- Zhang, J.L.; Niu, T.C.; Wee, A.T.S.; Chen, W. Self-assembly of binary molecular nanostructure arrays on graphite. Phys. Chem. Chem. Phys. 2013, 15, 12414–12427. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, B.; Yang, J.; Hou, J.G. Single-molecule chemistry of metal phthalocyanine on noble metal surfaces. Acc. Chem. Res. 2010, 43, 954–962. [Google Scholar] [CrossRef]
- Niu, T.; Zhang, J.; Chen, W. Molecular ordering and dipole alignment of vanadyl phthalocyanine monolayer on metals: The effects of interfacial interactions. J. Phys. Chem. C 2014, 118, 4151–4159. [Google Scholar] [CrossRef]
- Amsterdam, S.H.; Stanev, T.K.; Zhou, Q.; Lou, A.J.-T.; Bergeron, H.; Darancet, P.; Hersam, M.C.; Stern, N.P.; Marks, T.J. Electronic coupling in metallophthalocyanine–transition metal dichalcogenide mixed-dimensional heterojunctions. ACS Nano 2019, 13, 4183–4190. [Google Scholar] [CrossRef] [PubMed]
- Vergara, M.E.S.; Gallardo, D.M.; Estrada, I.L.V.; Sandoval, O.J. Optical absorption and electrical properties of MPc (M= Fe, Cu, Zn)-TCNQ interfaces for optoelectronic applications. J. Phys. Chem. Solids 2018, 115, 373–380. [Google Scholar] [CrossRef]
- Retamal, J.R.D.; Periyanagounder, D.; Ke, J.-J.; Tsai, M.-L.; He, J.-H. Charge carrier injection and transport engineering in two-dimensional transition metal dichalcogenides. Chem. Sci. 2018, 9, 7727–7745. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, K.; Ganguli, A.K. Interfacial charge transfer in photoelectrochemical processes. Adv. Mater. Interfaces 2017, 4, 1600981. [Google Scholar] [CrossRef]
- Kamat, P.V. Boosting the efficiency of quantum dot sensitized solar cells through modulation of interfacial charge transfer. Acc. Chem. Res. 2012, 45, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Annese, E.; Fujii, J.; Vobornik, I.; Rossi, G. Structure and electron states of Co-phthalocyanine interacting with the Cu (111) surface. J. Phys. Chem. C 2011, 115, 17409–17416. [Google Scholar] [CrossRef]
- Snezhkova, O.; Bischoff, F.; He, Y.; Wiengarten, A.; Chaudhary, S.; Johansson, N.; Schnadt, J. Iron phthalocyanine on Cu (111): Coverage-dependent assembly and symmetry breaking, temperature-induced homocoupling, and modification of the adsorbate-surface interaction by annealing. J. Chem. Phys. 2016, 144, 094702. [Google Scholar] [CrossRef]
- Javaid, S.; Lee, G. The impact of molecular orientation on carrier transfer characteristics at a phthalocyanine and halide perovskite interface. RSC Adv. 2021, 11, 31776–31782. [Google Scholar] [CrossRef]
- Lüder, J.; Sanyal, B.; Eriksson, O.; Puglia, C.; Brena, B. Comparison of van der Waals corrected and sparse-matter density functionals for the metal-free phthalocyanine/gold interface. Phys. Rev. B 2014, 89, 45416. [Google Scholar] [CrossRef]
- Komeda, T.; Isshiki, H.; Liu, J. Metal-free phthalocyanine (H2Pc) molecule adsorbed on the Au (111) surface: Formation of a wide domain along a single lattice direction. Sci. Technol. Adv. Mater. 2011, 11, 54602. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Liu, X.; Lu, Y.; Xiang, F.; Qiao, X.; Cai, Y.; Wang, Z.; Liu, S.; Wang, L. Positioning and switching phthalocyanine molecules on a Cu (100) surface at room temperature. ACS Nano 2014, 8, 12734–12740. [Google Scholar] [CrossRef]
- Shariati, M.N.; Luder, J.; Bidermane, I.; Ahmadi, S.; Gothelid, E.; Palmgren, P.; Sanyal, B.; Eriksson, O.; Piancastelli, M.N.; Brena, B.; et al. Photoelectron and absorption spectroscopy studies of metal-free phthalocyanine on Au (111): Experiment and theory. J. Phys. Chem. C 2013, 117, 7018–7025. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Nakashima, S.; Oiso, K.; Yamada, T.K. Recovery of nanomolecular electronic states from tunneling spectroscopy: LDOS of low-dimensional phthalocyanine molecular structures on Cu (111). Nanotechnology 2013, 24, 395704. [Google Scholar] [CrossRef] [PubMed]
- Greulich, K.; Belser, A.; Basova, T.; Chasse, T.; Peisert, H. Interfaces between different iron phthalocyanines and Au (111): Influence of the fluorination on structure and interfacial interactions. J. Phys. Chem. C 2021, 126, 716–727. [Google Scholar] [CrossRef]
- Petraki, F.; Peisert, H.; Biswas, I.; Chassé, T. Electronic structure of Co-phthalocyanine on gold investigated by photoexcited electron spectroscopies: Indication of Co ion−metal interaction. J. Phys. Chem. C 2010, 114, 17638–17643. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Gao, L.; Deng, Z.T.; Jiang, N.; Liu, Q.; Shi, D.X.; Du, S.X.; Guo, H.M.; Gao, H.-J. Adsorption behavior of iron phthalocyanine on Au (111) surface at submonolayer coverage. J. Phys. Chem. C 2007, 111, 9240–9244. [Google Scholar] [CrossRef]
- Rochford, L.A.; Ramadan, A.J.; Woodruff, D.P.; Heutz, S.; Jones, T.S. Ordered growth of vanadyl phthalocyanine (VOPc) on an iron phthalocyanine (FePc) monolayer. Phys. Chem. Chem. Phys. 2015, 17, 29747–29752. [Google Scholar] [CrossRef] [PubMed]
- Barlow, D.E.; Hipps, K.W. A scanning tunneling microscopy and spectroscopy study of vanadyl phthalocyanine on Au (111): The effect of oxygen binding and orbital mediated tunneling on the apparent corrugation. J. Phys. Chem. B 2000, 104, 5993–6000. [Google Scholar] [CrossRef]
- Kothe, M.; Witte, G. Orientational and Crystalline Order of Copper–Phthalocyanine Films on Gold: The Role of Substrate Roughness and Cleanliness. Langmuir 2019, 35, 13570–13577. [Google Scholar] [CrossRef]
- Struczyńska, M.; Firkowska-Boden, I.; Scheuer, K.; Jandt, K.D. Rutile facet-dependent fibrinogen conformation: Why crystallographic orientation matters. Colloids Surf. B Biointerfaces 2022, 215, 112506. [Google Scholar] [CrossRef]
- Mänz, A.; Hauke, A.A.; Witte, G. Copper phthalocyanine as contact layers for pentacene films grown on coinage metals. J. Phys. Chem. C 2018, 122, 2165–2172. [Google Scholar] [CrossRef]
- Weng, Z.; Zhang, F.; Xu, C.; Zhou, J. The effect of incident energy, incident angle and substrate temperature on surface morphology and atomic distribution of NiTi films. Mater. Des. 2020, 187, 108350. [Google Scholar] [CrossRef]
- He, Y.; Kröger, J.; Wang, Y. Organic multilayer films studied by scanning tunneling microscopy. ChemPhysChem 2017, 18, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Min, B.K.; Alemozafar, A.R.; Biener, M.M.; Biener, J.; Friend, C.M. Reaction of Au (111) with sulfur and oxygen: Scanning tunneling microscopic study. Top. Catal. 2005, 36, 77–90. [Google Scholar] [CrossRef]
- Wang, Y.; Hush, N.S.; Reimers, J.R. Simulation of the Au (111)-(22× 3) surface reconstruction. Phys. Rev. B—Condens. Matter Mater. Phys. 2007, 75, 233416. [Google Scholar] [CrossRef]
- Barth, J.V. Molecular architectonic on metal surfaces. Annu. Rev. Phys. Chem. 2007, 58, 375–407. [Google Scholar] [CrossRef]
- Rochford, L.A. Structural, Electronic and Magnetic Properties of Metal Phthalocyanines; University of Warwick: Coventry, UK, 2013. [Google Scholar]
- Barth, J.V.; Brune, H.; Ertl, G.; Behm, R.J. Scanning tunneling microscopy observations on the reconstructed Au (111) surface: Atomic structure, long-range superstructure, rotational domains, and surface defects. Phys. Rev. B 1990, 42, 9307. [Google Scholar] [CrossRef]
- Woodruff, D.P. The interface structure of n-alkylthiolate self-assembled monolayers on coinage metal surfaces. Phys. Chem. Chem. Phys. 2008, 10, 7211–7221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bobaru, S.C.; Salomon, E.; Layet, J.-M.; Angot, T. Structural properties of iron phtalocyanines on Ag (111): From the submonolayer to monolayer range. J. Phys. Chem. C 2011, 115, 5875–5879. [Google Scholar] [CrossRef]
- Kumar, A.; Betti, M.G.; Mariani, C.; Kumar, M.; Gargiani, P.; Soncini, C.; Pedio, M. Intermixing of Unoccupied States of Metal Phthalocyanine Chains Assembled on Au (110). Nanomaterials 2024, 14, 158. [Google Scholar] [CrossRef]
- Stoodley, M.A.; Klein, B.P.; Clarke, M.; Williams, L.B.; Rochford, L.A.; Ferrer, P.; Grinter, D.C.; Saywell, A.; Duncan, D.A. Adsorption structure of iron phthalocyanine and titanyl phthalocyanine on Cu (1 1 1). Inorganica Chim. Acta 2023, 557, 121679. [Google Scholar] [CrossRef]
- Mattioli, G.; Contini, G.; Ronci, F.; Flammini, R.; Frezza, F.; Larciprete, R.; Raglione, V.; Alippi, P.; Filippone, F.; Bonapasta, A.A.; et al. Coverage-Dependent Modulation of Charge Density at the Interface between Ag (001) and Ruthenium Phthalocyanine. J. Phys. Chem. C 2023, 127, 3316–3329. [Google Scholar] [CrossRef]
- Rehman, R.; Cai, Y.-L.; Zhang, H.-J.; Wu, K.; Dou, W.-D.; Li, H.-Y.; He, P.-M.; Bao, S.-N. Differences in the adsorption of FePc on coinage metal surfaces. Chin. Phys. B 2013, 22, 63101. [Google Scholar] [CrossRef]
- Heinrich, B.W.; Iacovita, C.; Brumme, T.; Choi, D.-J.; Limot, L.; Rastei, M.V.; Hofer, W.A.; Kortus, J.; Bucher, J.-P. Direct observation of the tunneling channels of a chemisorbed molecule. J. Phys. Chem. Lett. 2010, 1, 1517–1523. [Google Scholar] [CrossRef]
- Chizhov, I.; Scoles, G.; Kahn, A. The influence of steps on the orientation of copper phthalocyanine monolayers on Au (111). Langmuir 2000, 16, 4358–4361. [Google Scholar] [CrossRef]
- Menozzi, C.; Corradini, V.; Cavallini, M.; Biscarini, F.; Betti, M.G.; Mariani, C. Pentacene self-aggregation at the Au (110)-(1× 2) surface: Growth morphology and interface electronic states. Thin Solid Film. 2003, 428, 227–231. [Google Scholar] [CrossRef]
- Haizmann, P.; Juriatti, E.; Klein, M.; Greulich, K.; Nagel, P.; Merz, M.; Schuppler, S.; Ghiami, A.; Ovsyannikov, R.; Giangrisostomi, E.; et al. Orientation of Cobalt-Phthalocyanines on Molybdenum Disulfide: Distinguishing between Single Crystals and Small Flakes. J. Phys. Chem. C 2024, 128, 2107–2115. [Google Scholar] [CrossRef]
- Peisert, H.; Biswas, I.; Zhang, L.; Knupfer, M.; Hanack, M.; Dini, D.; Batchelor, D.; Chassé, T. Molecular orientation of substituted phthalocyanines: Influence of the substrate roughness. Surf. Sci. 2006, 600, 4024–4029. [Google Scholar] [CrossRef]
- Popielarski, P.; Mosińska, L.; Skowronski, L.; Szczesny, R.; Figà, V.; Naparty, M.; Derkowska-Zielinska, B. Influence of heat treatment on surface, structural and optical properties of nickel and copper phthalocyanines thin films. Int. J. Mol. Sci. 2022, 23, 11055. [Google Scholar] [CrossRef]
- Brozek-Pluska, B.; Jarota, A.; Kania, R.; Abramczyk, H. Zinc phthalocyanine photochemistry by Raman imaging, fluorescence spectroscopy and femtosecond spectroscopy in normal and cancerous human colon tissues and single cells. Molecules 2020, 25, 2688. [Google Scholar] [CrossRef] [PubMed]
- Davia, F.G.; Johner, N.P.; Calvo, E.J.; Williams, F.J. Growth and electrochemical stability of a layer-by-layer thin film containing tetrasulfonated Fe phthalocyanine. J. Electroanal. Chem. 2020, 877, 114485. [Google Scholar] [CrossRef]
- Isvoranu, C.; Wang, B.; Schulte, K.; Ataman, E.; Knudsen, J.; Andersen, J.N.; Bocquet, M.L.; Schnadt, J. Tuning the spin state of iron phthalocyanine by ligand adsorption. J. Phys. Condens. Matter 2010, 22, 472002. [Google Scholar] [CrossRef] [PubMed]
- Stadtmüller, B.; Kröger, I.; Reinert, F.; Kumpf, C. Submonolayer growth of CuPc on noble metal surfaces. Phys. Rev. B—Condens. Matter Mater. Phys. 2011, 83, 85416. [Google Scholar] [CrossRef]
- Manandhar, K.; Park, K.; Ma, S.; Hrbek, J. Heteroepitaxial thin film of iron phthalocyanine on Ag (111). Surf. Sci. 2009, 603, 636–640. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic–inorganic hybrid nanocomposite-based gas sensors for environmental monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.R.A.; Wiggins, B.; Hipps, K.W.; Mazur, U. Structural and electronic properties of columnar supramolecular assemblies formed from ionic metal-free phthalocyanine on Au (111). J. Phys. Chem. C 2011, 115, 16305–16314. [Google Scholar] [CrossRef]
- Baran, J.D.; Larsson, J.A. Theoretical insights into adsorption of cobalt phthalocyanine on Ag (111): A combination of chemical and van der waals bonding. J. Phys. Chem. C 2013, 117, 23887–23898. [Google Scholar] [CrossRef]
- Jabrane, M.; El Hafidi, M.; El Hafidi, M.Y.; Kara, A. Fe-Phthalocyanine on Cu (111) and Ag (111): A DFT+ vdWs investigation. Surf. Sci. 2022, 716, 121961. [Google Scholar] [CrossRef]
- Buimaga-Iarinca, L.; Morari, C. Translation of metal-phthalocyanines adsorbed on Au (111): From van der Waals interaction to strong electronic correlation. Sci. Rep. 2018, 8, 12728. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.G.A.; Miwa, R.H.; McLean, A.B. Adsorption of metal-phthalocyanine molecules onto the Si (111) surface passivated by δ doping: Ab initio calculations. Phys. Rev. B 2016, 93, 115301. [Google Scholar] [CrossRef]
- Hu, F.; Mao, H.; Zhang, H.; Wu, K.; Cai, Y.; He, P. Electronic and structural properties at the interface between iron-phthalocyanine and Cu (110). J. Chem. Phys. 2014, 140, 094704. [Google Scholar] [CrossRef]
- Brena, B.; Luo, Y.; Nyberg, M.; Carniato, S.; Nilson, K.; Alfredsson, Y.; Åhlund, J.; Mårtensson, N.; Siegbahn, H.; Puglia, C. Equivalent core-hole time-dependent density functional theory calculations of carbon 1 s shake-up states of phthalocyanine. Phys. Rev. B—Condens. Matter Mater. Phys. 2004, 70, 195214. [Google Scholar] [CrossRef]
- Alfredsson, Y.; Brena, B.; Nilson, K.; Åhlund, J.; Kjeldgaard, L.; Nyberg, M.; Luo, Y.; Mårtensson, N.; Sandell, A.; Puglia, C.; et al. Electronic structure of a vapor-deposited metal-free phthalocyanine thin film. J. Chem. Phys. 2005, 122, 214723. [Google Scholar] [CrossRef]
- Eguchi, K.; Nakagawa, T.; Takagi, Y.; Yokoyama, T. Direct synthesis of vanadium phthalocyanine and its electronic and magnetic states in monolayers and multilayers on Ag (111). J. Phys. Chem. C 2015, 119, 9805–9815. [Google Scholar] [CrossRef]
- Gottfried, M.; Marbach, H. Surface-confined coordination chemistry with porphyrins and phthalocyanines: Aspects of formation, electronic structure, and reactivity. Z. Für Phys. Chem. 2009, 223, 53–74. [Google Scholar] [CrossRef]
- Lukasczyk, T.; Flechtner, K.; Merte, L.R.; Jux, N.; Maier, F.; Gottfried, J.M.; Steinrück, H.-P. Interaction of cobalt (II) tetraarylporphyrins with a Ag (111) surface studied with photoelectron spectroscopy. J. Phys. Chem. C 2007, 111, 3090–3098. [Google Scholar] [CrossRef]
- Caplins, B.W.; Suich, D.E.; Shearer, A.J.; Harris, C.B. Metal/phthalocyanine hybrid interface states on Ag (111). J. Phys. Chem. Lett. 2014, 5, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Kröger, I.; Bayersdorfer, P.; Stadtmüller, B.; Kleimann, C.; Mercurio, G.; Reinert, F.; Kumpf, C. Submonolayer growth of H 2-phthalocyanine on Ag (111). Phys. Rev. B—Condens. Matter Mater. Phys. 2012, 86, 195412. [Google Scholar] [CrossRef]
- Morgenstern, K.; Lægsgaard, E.; Stensgaard, I.; Besenbacher, F. Transition from one-dimensional to two-dimensional island decay on an anisotropic surface. Phys. Rev. Lett. 1999, 83, 1613. [Google Scholar] [CrossRef]
- Smykalla, L.; Shukrynau, P.; Mende, C.; Rüffer, T.; Lang, H.; Hietschold, M. Interplay of hydrogen bonding and molecule–substrate interaction in self-assembled adlayer structures of a hydroxyphenyl-substituted porphyrin. Surf. Sci. 2014, 628, 132–140. [Google Scholar] [CrossRef]
- Toader, M.; Hietschold, M. Tuning the energy level alignment at the SnPc/Ag (111) interface using an STM tip. J. Phys. Chem. C 2011, 115, 3099–3105. [Google Scholar] [CrossRef]
- Gorgoi, M.; Zahn, D.R.T. Charge-transfer at silver/phthalocyanines interfaces. Appl. Surf. Sci. 2006, 252, 5453–5456. [Google Scholar] [CrossRef]
- Smykalla, L.; Shukrynau, P.; Zahn, D.R.T.; Hietschold, M. Self-metalation of phthalocyanine molecules with silver surface atoms by adsorption on Ag (110). J. Phys. Chem. C 2015, 119, 17228–17234. [Google Scholar] [CrossRef]
- Zaitsev, N.L.; Nechaev, I.A.; Echenique, P.M.; Chulkov, E.V. Transformation of the Ag (111) surface state due to molecule-surface interaction with ordered organic molecular monolayers. Phys. Rev. B—Condens. Matter Mater. Phys. 2012, 85, 115301. [Google Scholar] [CrossRef]
- Lindstrom, C.D.; Zhu, X.-Y. Photoinduced electron transfer at molecule− metal interfaces. Chem. Rev. 2006, 106, 4281–4300. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, N.L.; Nechaev, I.A.; Chulkov, E.V. Change in surface states of Ag (111) thin films upon adsorption of a monolayer of PTCDA organic molecules. J. Exp. Theor. Phys. 2010, 110, 114–120. [Google Scholar] [CrossRef]
- Nakashima, S.; Yamagishi, Y.; Oiso, K.; Yamada, T.K. How Contacting Electrodes Affect Single π-Conjugated Molecular Electronic States: Local Density of States of Phthalocyanine Nanomolecules on MgO (001), Cu (111), Ag (001), Fe (001), and Mn (001). Jpn J. Appl. Phys. 2013, 52, 110115. [Google Scholar] [CrossRef]
- Mao, H.-Q.; Li, N.; Chen, X.; Xue, Q.-K. Mechanical properties of H2Pc self-assembled monolayers at the single molecule level by noncontact atomic force microscopy. J. Phys. Condens. Matter 2012, 24, 84004. [Google Scholar] [CrossRef]
- Iacovita, C.; Rastei, M.V.; Heinrich, B.W.; Brumme, T.; Kortus, J.; Limot, L.; Bucher, J.P. Visualizing the spin of individual cobalt-phthalocyanine molecules. Phys. Rev. Lett. 2008, 101, 116602. [Google Scholar] [CrossRef] [PubMed]
- Scarfato, A.; Chang, S.H.; Kuck, S.; Brede, J.; Hoffmann, G.; Wiesendanger, R. Scanning tunneling microscope study of iron (II) phthlocyanine on metals and insulating surfaces. Surf. Sci. 2008, 602, 677–683. [Google Scholar] [CrossRef]
- Takács, A.F.; Witt, F.; Schmaus, S.; Balashov, T.; Bowen, M.; Beaurepaire, E.; Wulfhekel, W. Electron transport through single phthalocyanine molecules studied using scanning tunneling microscopy. Phys. Rev. B—Condens. Matter Mater. Phys. 2008, 78, 233404. [Google Scholar] [CrossRef]
- Chen, F.; Chen, X.; Liu, L.; Song, X.; Liu, S.; Liu, J.; Ouyang, H.; Cai, Y.; Liu, X.; Pan, H.; et al. Chiral recognition of zinc phthalocyanine on Cu (100) surface. Appl. Phys. Lett. 2012, 100, 081602. [Google Scholar] [CrossRef]
- Okuyama, H.; Kuwayama, S.; Nakazawa, Y.; Hatta, S.; Aruga, T. Structure and electronic states of strongly interacting metal-organic interfaces: CuPc on Cu (100) and Cu (110). Surf. Sci. 2022, 723, 122126. [Google Scholar] [CrossRef]
- Prato, S.; Floreano, L.; Cvetko, D.; De Renzi, V.; Morgante, A.; Modesti, S.; Biscarini, F.; Zamboni, R.; Taliani, C. Anisotropic ordered planar growth of α-sexithienyl thin films. J. Phys. Chem. B 1999, 103, 7788–7795. [Google Scholar] [CrossRef]
- Evangelista, F.; Ruocco, A.; Corradini, V.; Donzello, M.; Mariani, C.; Betti, M.G. CuPc molecules adsorbed on Au (1 1 0)-(1× 2): Growth morphology and evolution of valence band states. Surf Sci 2003, 531, 123–130. [Google Scholar] [CrossRef]
- Horn-von Hoegen, M. Growth of semiconductor layers studied by spot profile analysing low energy electron diffraction. Z. Für Krist. 1999, 214, 684–721. [Google Scholar]
- Mannsfeld, S.C.B.; Fritz, T. Understanding organic–inorganic heteroepitaxial growth of molecules on crystalline substrates: Experiment and theory. Phys. Rev. B—Condens. Matter Mater. Phys. 2005, 71, 235405. [Google Scholar] [CrossRef]
- Stadler, C.; Hansen, S.; Kröger, I.; Kumpf, C.; Umbach, E. Tuning intermolecular interaction in long-range-ordered submonolayer organic films. Nat. Phys. 2009, 5, 153–158. [Google Scholar] [CrossRef]
- Hauschild, A.; Karki, K.; Cowie, B.C.C.; Rohlfing, M.; Tautz, F.S.; Sokolowski, M. Molecular Distortions and Chemical Bonding of a Large π-Conjugated Molecule on a Metal Surface. Phys. Rev. Lett. 2005, 94, 36106. [Google Scholar] [CrossRef] [PubMed]
- Kilian, L.; Hauschild, A.; Temirov, R.; Soubatch, S.; Schöll, A.; Bendounan, A.; Umbach, E. Role of Intermolecular Interactions on the Electronic and Geometric Structure of a Large π-Conjugated Molecule Adsorbed on a Metal Surface. Phys. Rev. Lett. 2008, 100, 136103. [Google Scholar] [CrossRef] [PubMed]
- Kröger, I.; Stadtmüller, B.; Stadler, C.; Ziroff, J.; Kochler, M.; Stahl, A.; Pollinger, F.; Lee, T.-L.; Zegenhagen, J.; Reinert, F.; et al. Submonolayer growth of copper-phthalocyanine on Ag (111). New J. Phys. 2010, 12, 83038. [Google Scholar] [CrossRef]
- Stenzel, O.; Jähnig, R.; Wilbrandt, S. The optical properties of copper phthalocyanine ultrathin films deposited on silver: An ATR study. Phys. Status Solidi 2001, 187, 641–649. [Google Scholar] [CrossRef]
- Aristov, V.Y.; Molodtsova, O.V.; Zhilin, V.M.; Vyalikh, D.V.; Knupfer, M. Chemistry and electronic properties of a metal-organic semiconductor interface: In on CuPc. Phys. Rev. B—Condens. Matter Mater. Phys. 2005, 72, 165318. [Google Scholar] [CrossRef]
- Aristov, V.Y.; Molodtsova, O.V.; Zhilin, V.M.; Ossipyan, Y.A.; Vyalikh, D.V.; Doyle, B.P.; Nannarone, S.; Knupfer, M. Formation of sharp metal-organic semiconductor interfaces: Ag and Sn on CuPc. Eur. Phys. J. B 2007, 57, 379–384. [Google Scholar] [CrossRef]
- Molodtsova, O.V.; Zhilin, V.M.; Vyalikh, D.V.; Aristov, V.Y.; Knupfer, M. Electronic properties of potassium-doped CuPc. J. Appl. Phys. 2005, 98, 093702. [Google Scholar] [CrossRef]
- Molodtsova, O.V.; Aristov, V.Y.; Zhilin, V.M.; Ossipyan, Y.A.; Vyalikh, D.V.; Doyle, B.P.; Knupfer, M. Silver on copper phthalocyanine: Abrupt and inert interfaces. Appl. Surf. Sci. 2007, 254, 99–102. [Google Scholar] [CrossRef]
- Karacuban, H.; Lange, M.; Schaffert, J.; Weingart, O.; Wagner, T.; Möller, R. Substrate-induced symmetry reduction of CuPc on Cu (111): An LT-STM study. Surf. Sci. 2009, 603, L39–L43. [Google Scholar] [CrossRef]
- Wagner, T. Organische Heteroschichten: Untersuchung der Wechselwirkung Organischer Moleküle auf Metallischen Oberflächen; University of Duisburg-Essen: Essen, Germany, 2006; Volume 2006. [Google Scholar]
- Takada, M.; Tada, H. Low temperature scanning tunneling microscopy of phthalocyanine multilayers on Au (111) surfaces. Chem. Phys. Lett. 2004, 392, 265–269. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Gao, L.; Deng, Z.T.; Liu, Q.; Jiang, N.; Lin, X.; He, X.B.; Du, S.X.; Gao, H.-J. Epitaxial growth of iron phthalocyanine at the initial stage on Au (111) surface. J. Phys. Chem. C 2007, 111, 2656–2660. [Google Scholar] [CrossRef]
- Isvoranu, C.; Knudsen, J.; Ataman, E.; Schulte, K.; Wang, B.; Bocquet, M.-L.; Andersen, J.N.; Schnadt, J. Adsorption of ammonia on multilayer iron phthalocyanine. J. Chem. Phys. 2011, 134, 114711. [Google Scholar] [CrossRef] [PubMed]
- Isvoranu, C.; Wang, B.; Ataman, E.; Schulte, K.; Knudsen, J.; Andersen, J.N.; Bocquet, M.-L.; Schnadt, J. Pyridine adsorption on single-layer iron phthalocyanine on Au (111). J. Phys. Chem. C 2011, 115, 20201–20208. [Google Scholar] [CrossRef]
- Wu, K.; Huang, Q.-H.; Zhang, H.-J.; Liao, Q.; He, P.-M. Adsorption behavior of iron phthalocyanine on a Ag (110) surface. Chin. Phys. B 2012, 21, 37202. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Du, S.X.; Gao, H.-J. Binding configuration, electronic structure, and magnetic properties of metal phthalocyanines on a Au (111) surface studied with ab initio calculations. Phys. Rev. B—Condens. Matter Mater. Phys. 2011, 84, 125446. [Google Scholar] [CrossRef]
- Peisert, H.; Biswas, I.; Knupfer, M.; Chassé, T. Orientation and electronic properties of phthalocyanines on polycrystalline substrates. Phys. Status Solidi 2009, 246, 1529–1545. [Google Scholar] [CrossRef]
- Rocco, M.L.M.; Frank, K.-H.; Yannoulis, P.; Koch, E.-E. Unoccupied electronic structure of phthalocyanine films. J. Chem. Phys. 1990, 93, 6859–6864. [Google Scholar] [CrossRef]
- Floreano, L.; Cossaro, A.; Gotter, R.; Verdini, A.; Bavdek, G.; Evangelista, F.; Ruocco, A.; Morgante, A.; Cvetko, D. Periodic arrays of Cu-phthalocyanine chains on Au (110). J. Phys. Chem. C 2008, 112, 10794–10802. [Google Scholar] [CrossRef]
- Peisert, H.; Biswas, I.; Aygül, U.; Vollmer, A.; Chassé, T. Electronic structure of cobalt phthalocyanine studied by resonant photoemission: Localization of Co-related valence band states. Chem. Phys. Lett. 2010, 493, 126–129. [Google Scholar] [CrossRef]
- Petraki, F.; Peisert, H.; Aygül, U.; Latteyer, F.; Uihlein, J.; Vollmer, A.; Chassé, T. Electronic structure of FePc and interface properties on Ag (111) and Au (100). J. Phys. Chem. C 2012, 116, 11110–11116. [Google Scholar] [CrossRef]
- Sinnokrot, M.O.; Valeev, E.F.; Sherrill, C.D. Estimates of the ab initio limit for π−π interactions: The benzene dimer. J. Am. Chem. Soc. 2002, 124, 10887–10893. [Google Scholar] [CrossRef]
- Sinnokrot, M.O.; Sherrill, C.D. Highly accurate coupled cluster potential energy curves for the benzene dimer: Sandwich, T-shaped, and parallel-displaced configurations. J. Phys. Chem. A 2004, 108, 10200–10207. [Google Scholar] [CrossRef]
- Åhlund, J.; Schnadt, J.; Nilson, K.; Göthelid, E.; Schiessling, J.; Besenbacher, F.; Mårtensson, N.; Puglia, C. The adsorption of iron phthalocyanine on graphite: A scanning tunnelling microscopy study. Surf. Sci. 2007, 601, 3661–3667. [Google Scholar] [CrossRef]
- Tsukahara, N.; Noto, K.-I.; Ohara, M.; Shiraki, S.; Takagi, N.; Takata, Y.; Miyawaki, J.; Taguchi, M.; Chainani, A.; Shin, S.; et al. Adsorption-induced switching of magnetic anisotropy in a single iron (II) phthalocyanine molecule on an oxidized Cu (110) surface. Phys. Rev. Lett. 2009, 102, 167203. [Google Scholar] [CrossRef]
- Snezhkova, O.; Lüder, J.; Wiengarten, A.; Burema, S.R.; Bischoff, F.; He, Y.; Rusz, J.; Knudsen, J.; Bocquet, M.-L.; Seufert, K.; et al. Nature of the bias-dependent symmetry reduction of iron phthalocyanine on Cu (111). Phys. Rev. B 2015, 92, 75428. [Google Scholar] [CrossRef]
- Chang, S.-H.; Kuck, S.; Brede, J.; Lichtenstein, L.; Hoffmann, G.; Wiesendanger, R. Symmetry reduction of metal phthalocyanines on metals. Phys. Rev. B—Condens. Matter Mater. Phys. 2008, 78, 233409. [Google Scholar] [CrossRef]
- Bai, Y.; Buchner, F.; Wendahl, M.T.; Kellner, I.; Bayer, A.; Steinrück, H.-P.; Marbach, H.; Gottfried, J.M. Direct metalation of a phthalocyanine monolayer on Ag (111) with coadsorbed iron atoms. J. Phys. Chem. C 2008, 112, 6087–6092. [Google Scholar] [CrossRef]
- Steiner, P.; Höchst, H.; Hüfner, S., II. XPS study of 3 d-metal ions dissolved in noble metals. Z. Für Phys. B Condens. Matter 1980, 38, 201–209. [Google Scholar]
- Belser, A.; Greulich, K.; Sättele, M.S.; Fingerle, M.; Ovsyannikov, R.; Giangrisostomi, E.; Chassé, T.; Peisert, H. Interface properties of CoPc on nanographene-covered Au (111) and the influence of annealing. Langmuir 2021, 37, 10750–10761. [Google Scholar] [CrossRef] [PubMed]
- Salomon, E.; Amsalem, P.; Marom, N.; Vondracek, M.; Kronik, L.; Koch, N.; Angot, T. Electronic structure of CoPc adsorbed on Ag (100): Evidence for molecule-substrate interaction mediated by Co 3 d orbitals. Phys. Rev. B—Condens. Matter Mater. Phys. 2013, 87, 75407. [Google Scholar] [CrossRef]
- Caplins, B.W.; Shearer, A.J.; Suich, D.E.; Muller, E.A.; Harris, C.B. Measuring the electronic corrugation at the metal/organic interface. Phys. Rev. B 2014, 89, 155422. [Google Scholar] [CrossRef]
- Toader, M.; Shukrynau, P.; Knupfer, M.; Zahn, D.R.T.; Hietschold, M. Site-dependent donation/backdonation charge transfer at the CoPc/Ag (111) interface. Langmuir 2012, 28, 13325–13330. [Google Scholar] [CrossRef]
- Mugarza, A.; Robles, R.; Krull, C.; Korytár, R.; Lorente, N.; Gambardella, P. Electronic and magnetic properties of molecule-metal interfaces: Transition-metal phthalocyanines adsorbed on Ag (100). Phys. Rev. B—Condens. Matter Mater. Phys. 2012, 85, 155437. [Google Scholar] [CrossRef]
- Salomon, E.; Beato-Medina, D.; Verdini, A.; Cossaro, A.; Cvetko, D.; Kladnik, G.; Floreano, L.; Angot, T. Correlation between charge transfer and adsorption site in CoPc overlayers adsorbed on Ag (100). J. Phys. Chem. C 2015, 119, 23422–23429. [Google Scholar] [CrossRef]
- Petraki, F.; Peisert, H.; Latteyer, F.; Aygül, U.; Vollmer, A.; Chassé, T. Impact of the 3d electronic states of cobalt and manganese phthalocyanines on the electronic structure at the interface to Ag (111). J. Phys. Chem. C 2011, 115, 21334–21340. [Google Scholar] [CrossRef]
- Baran, J.D.; Larsson, J.A.; Woolley, R.A.J.; Cong, Y.; Moriarty, P.J.; Cafolla, A.A.; Dhanak, V.R. Theoretical and experimental comparison of SnPc, PbPc, and CoPc adsorption on Ag (111). Phys. Rev. B—Condens. Matter Mater. Phys. 2010, 81, 75413. [Google Scholar] [CrossRef]
- Moeck, P.; Straton, J.; Toader, M.; Hietschold, M. Crystallographic processing of scanning tunneling microscopy images of cobalt phthalocyanines on silver and graphite. MRS Online Proc. Libr. 2011, 1318, mrsf10-1318. [Google Scholar] [CrossRef]
- Sabik, A.; Gołek, F.; Antczak, G. Thermal desorption and stability of cobalt phthalocyanine on Ag (100). Appl. Surf. Sci. 2018, 435, 894–902. [Google Scholar] [CrossRef]
- Sabik, A.; Mazur, P.; Gołek, F.; Trembulowicz, A.; Antczak, G. Phthalocyanine arrangements on Ag (100): From pure overlayers of CoPc and F16CuPc to bimolecular heterostructure. J. Chem. Phys. 2018, 149, 144702. [Google Scholar] [CrossRef]
- Kroll, T.; Aristov, V.Y.; Molodtsova, O.V.; Ossipyan, Y.A.; Vyalikh, D.V.; Büchner, B.; Knupfer, M. Spin and orbital ground state of Co in cobalt phthalocyanine. J. Phys. Chem. A 2009, 113, 8917–8922. [Google Scholar] [CrossRef]
- Shen, K.; Narsu, B.; Ji, G.; Sun, H.; Hu, J.; Liang, Z.; Gao, X.; Li, H.; Li, Z.; Song, B.; et al. On-surface manipulation of atom substitution between cobalt phthalocyanine and the Cu (111) substrate. RSC Adv. 2017, 7, 13827–13835. [Google Scholar] [CrossRef]
- Gargiani, P.; Angelucci, M.; Mariani, C.; Betti, M.G. Metal-phthalocyanine chains on the Au (110) surface: Interaction states versus d-metal states occupancy. Phys. Rev. B 2010, 81, 85412. [Google Scholar] [CrossRef]
- Wang, H.; Song, D.; Yang, J.; Yu, B.; Geng, Y.; Yan, D. High mobility vanadyl-phthalocyanine polycrystalline films for organic field-effect transistors. Appl. Phys. Lett. 2007, 90, 253510. [Google Scholar] [CrossRef]
- Ohta, H.; Kambayashi, T.; Hirano, M.; Hoshi, H.; Ishikawa, K.; Takezoe, H.; Hosono, H. Application of a transparent conductive substrate with an atomically flat and stepped surface to lateral growth of an organic molecule: Vanadyl phthalocyanine. Adv. Mater. 2003, 15, 1258–1262. [Google Scholar] [CrossRef]
- Duncan, D.A.; Unterberger, W.; Hogan, K.A.; Lerotholi, T.J.; Lamont, C.L.A.; Woodruff, D.P. A photoelectron diffraction investigation of vanadyl phthalocyanine on Au (1 1 1). Surf. Sci. 2010, 604, 47–53. [Google Scholar] [CrossRef]
- Sautet, P. Atomic adsorbate identification with the STM: A theoretical approach. Surf. Sci. 1997, 374, 406–417. [Google Scholar] [CrossRef]
- Fernandez-Torrente, I.; Monturet, S.; Franke, K.J.; Fraxedas, J.; Lorente, N.; Pascual, J.I. Long-Range Repulsive Interaction between Molecules on a Metal Surface Induced by Charge Transfer. Phys. Rev. Lett. 2007, 99, 176103. [Google Scholar] [CrossRef]
- Yokoyama, T.; Takahashi, T.; Shinozaki, K. Surface diffusion of Ir (ppy) 3 on Cu (111). Phys. Rev. B—Condens. Matter Mater. Phys. 2010, 82, 155414. [Google Scholar] [CrossRef]
- Blowey, P.J.; Maurer, R.J.; Rochford, L.A.; Duncan, D.A.; Kang, J.-H.; Warr, D.A.; Woodruff, D.P. The structure of VOPc on Cu (111): Does V═ O point up, or down, or both? J. Phys. Chem. C 2018, 123, 8101–8111. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, G.; Filippone, F.; Bonapasta, A.A. Controlling the magnetic properties of a single phthalocyanine molecule through its strong coupling with the GaAs surface. J. Phys. Chem. Lett. 2010, 1, 2757–2762. [Google Scholar] [CrossRef]
- Zhou, W.; Wen, Z.; Gao, P. Less is more: Dopant-free hole transporting materials for high-efficiency perovskite solar cells. Adv. Energy Mater. 2018, 8, 1702512. [Google Scholar] [CrossRef]
- Kumar, C.V.; Sfyri, G.; Raptis, D.; Stathatos, E.; Lianos, P. Perovskite solar cell with low cost Cu-phthalocyanine as hole transporting material. RSC Adv. 2015, 5, 3786–3791. [Google Scholar] [CrossRef]
- Ke, W.; Zhao, D.; Grice, C.R.; Cimaroli, A.J.; Fang, G.; Yan, Y. Efficient fully-vacuum-processed perovskite solar cells using copper phthalocyanine as hole selective layers. J. Mater. Chem. A 2015, 3, 23888–23894. [Google Scholar] [CrossRef]
- Yuan, Y.Y.; Han, S.; Grozea, D.; Lu, Z.H. Fullerene-organic nanocomposite: A flexible material platform for organic light-emitting diodes. Appl. Phys. Lett. 2006, 88, 093503. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, X.; Cheng, M.; Wang, W.; Sun, L. Boosting the efficiency and the stability of low cost perovskite solar cells by using CuPc nanorods as hole transport material and carbon as counter electrode. Nano Energy 2016, 20, 108–116. [Google Scholar] [CrossRef]
- Torabi, N.; Rahnamanic, A.; Amrollahi, H.; Mirjalili, F.; Sadeghzade, M.; Behjat, A. Performance enhancement of perovskite solar cell by controlling deposition temperature of copper phthalocyanine as a dopant-free hole transporting layer. Org. Electron. 2017, 48, 211–216. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, L.; Mu, X.; Chen, C.; Wu, Y.; Cao, J.; Tang, Y. Intramolecular electric field construction in metal phthalocyanine as dopant-free hole transporting material for stable perovskite solar cells with >21% efficiency. Angew. Chem. Int. Ed. 2021, 60, 6294–6299. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Ohashi, N.; Oku, T.; Tachikawa, T.; Hasegawa, T.; Fukunishi, S. Effects of Metal Phthalocyanine and Naphthalocyanine on Perovskite Solar Cells. J. Electron. Mater. 2024, 53, 6049–6063. [Google Scholar] [CrossRef]
- Reecht, G.; Krane, N.; Lotze, C.; Franke, K.J. π-radical formation by pyrrolic H abstraction of phthalocyanine molecules on molybdenum disulfide. ACS Nano 2019, 13, 7031–7035. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, W.; Antczak, G.; Morgenstern, K. Bistable H2Pc molecular conductance switch on Ag (100). J. Phys. Chem. C 2022, 126, 16767–16776. [Google Scholar] [CrossRef]
- Schönauer, K.M. Structural and Electronic Investigations on Homo-and Hetero-Organic Layers Involving CuPc on Silver Single Crystal Surfaces. Ph.D. Dissertation, RWTH Aachen, Aachen, Germany, 2015. [Google Scholar]
- Bidermane, I.; Brumboiu, I.E.; Totani, R.; Grazioli, C.; Shariati-Nilsson, M.N.; Herper, H.C.; Eriksson, O.; Sanyal, B.; Ressel, B.; de Simone, M.; et al. Atomic contributions to the valence band photoelectron spectra of metal-free, iron and manganese phthalocyanines. J. Electron. Spectros. Relat. Phenom. 2015, 205, 92–97. [Google Scholar] [CrossRef]
- Massimi, L.; Angelucci, M.; Gargiani, P.; Betti, M.G.; Montoro, S.; Mariani, C. Metal-phthalocyanine ordered layers on Au (110): Metal-dependent adsorption energy. J. Chem. Phys. 2014, 140, 244704. [Google Scholar] [CrossRef] [PubMed]

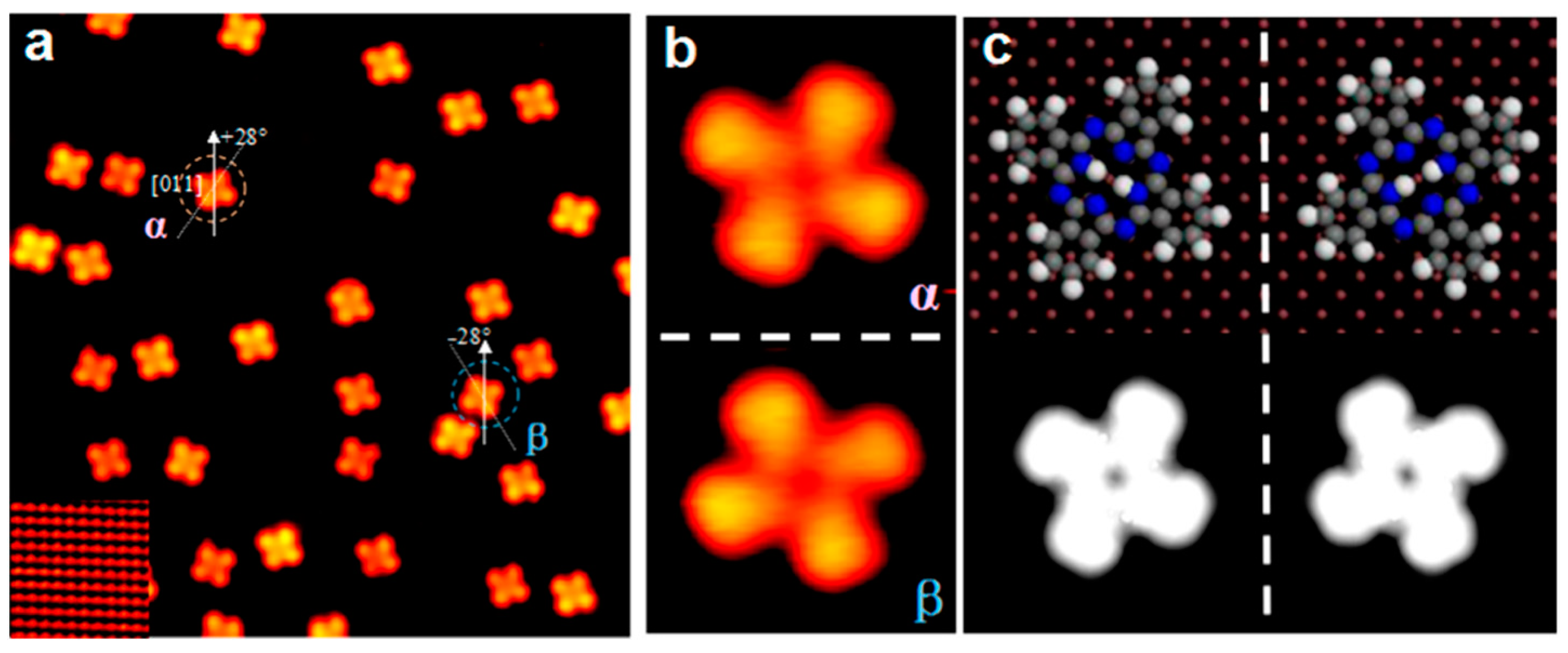
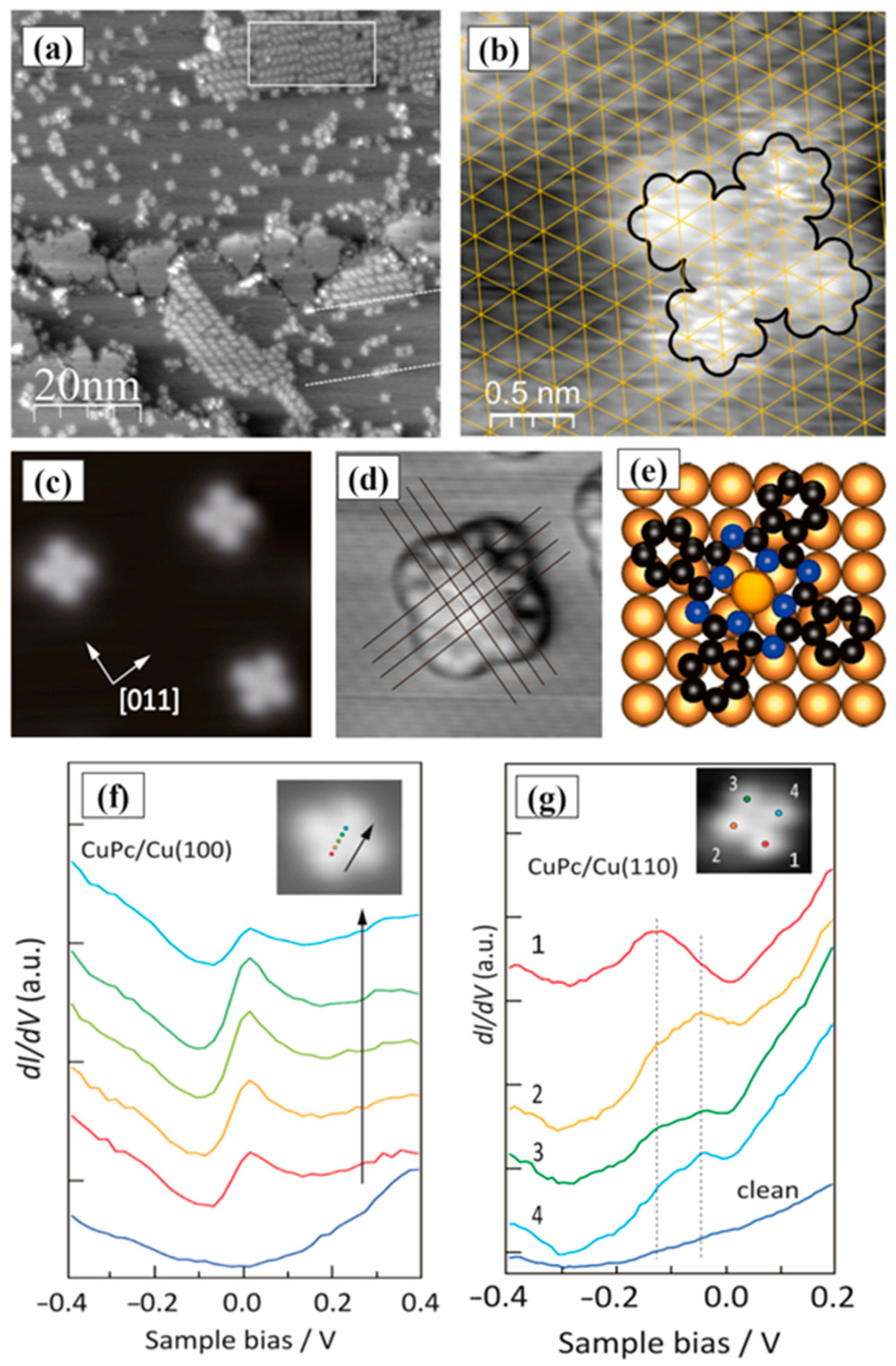
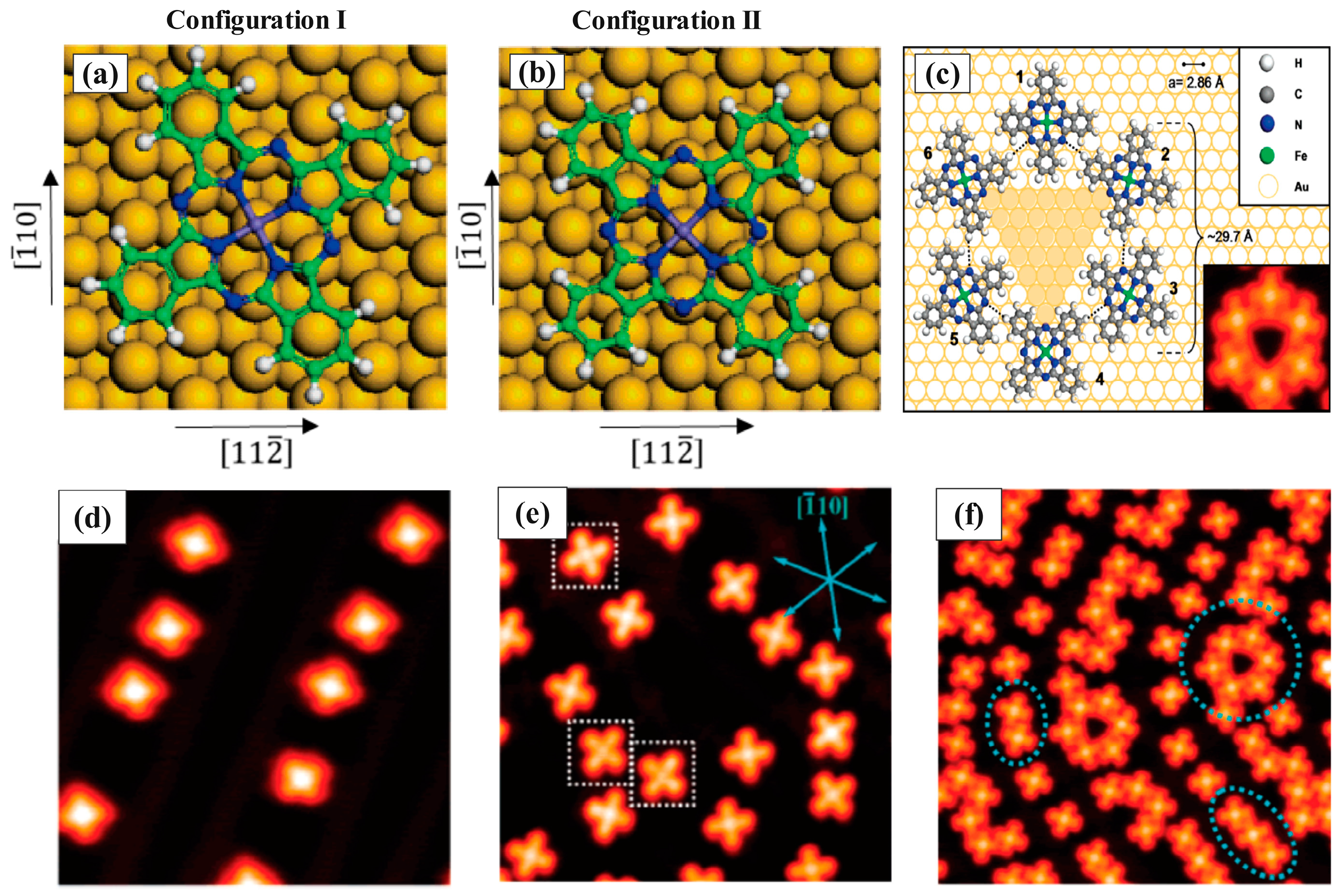
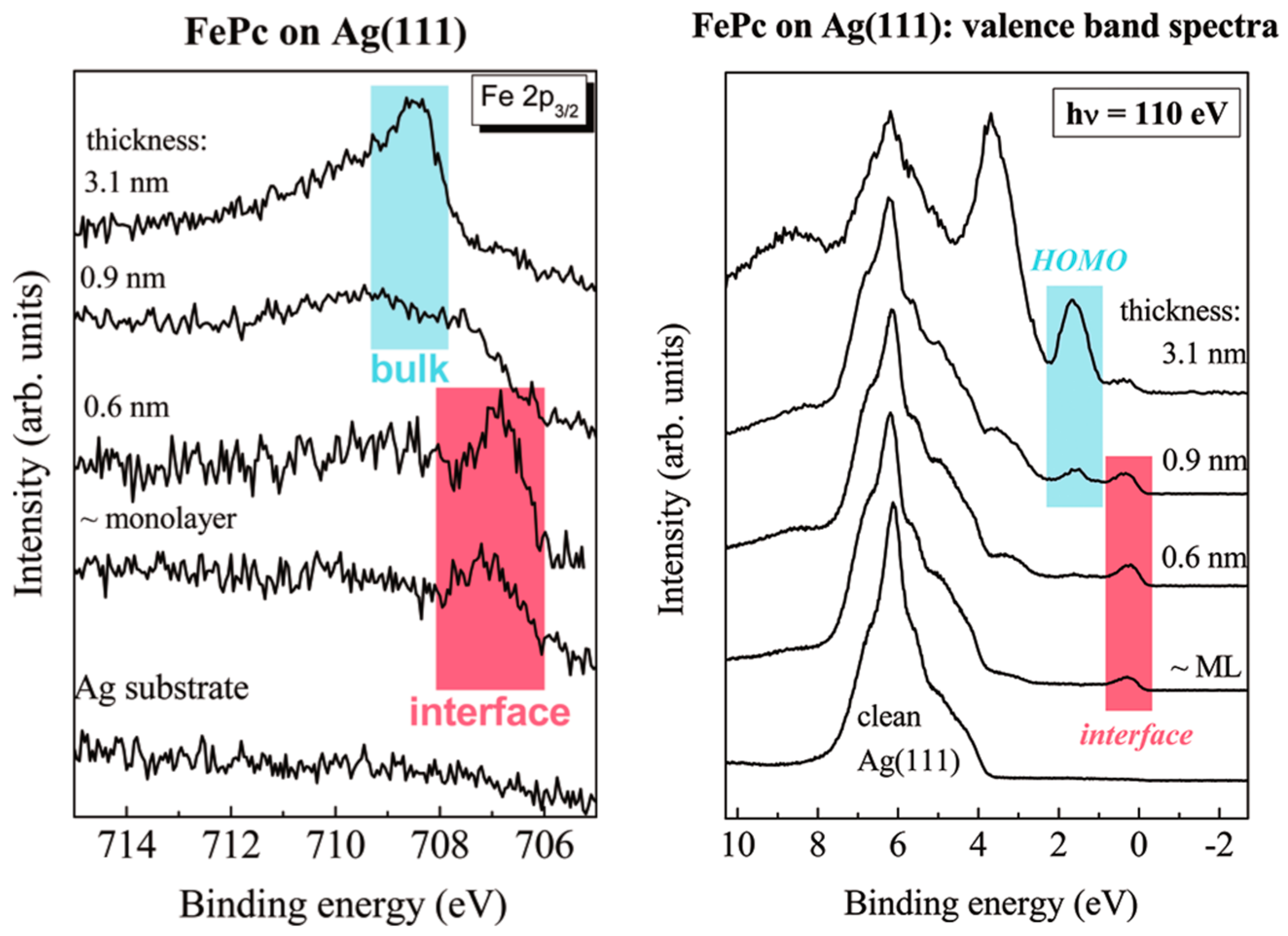
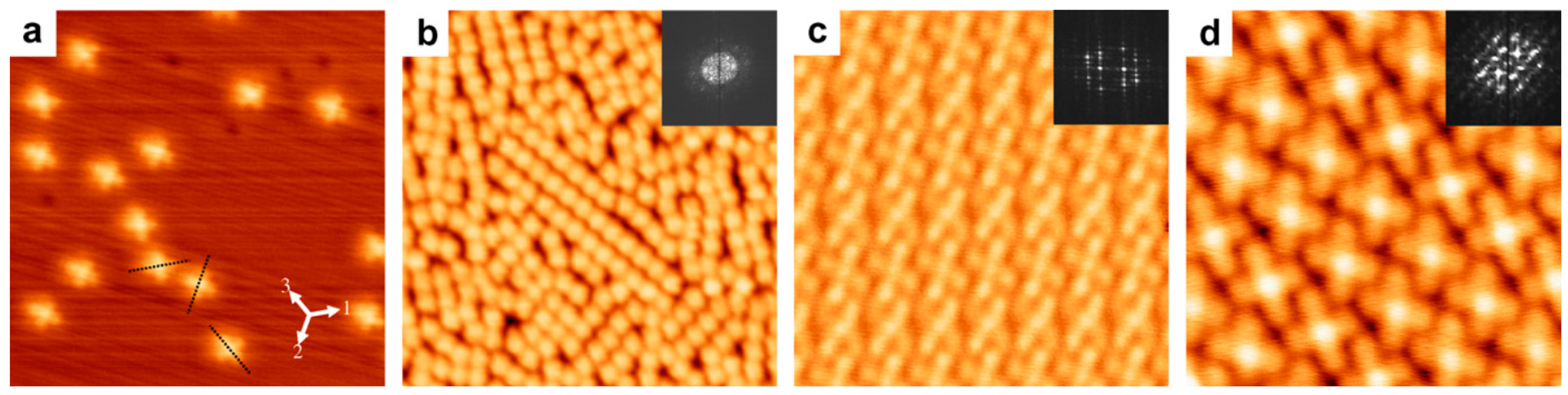
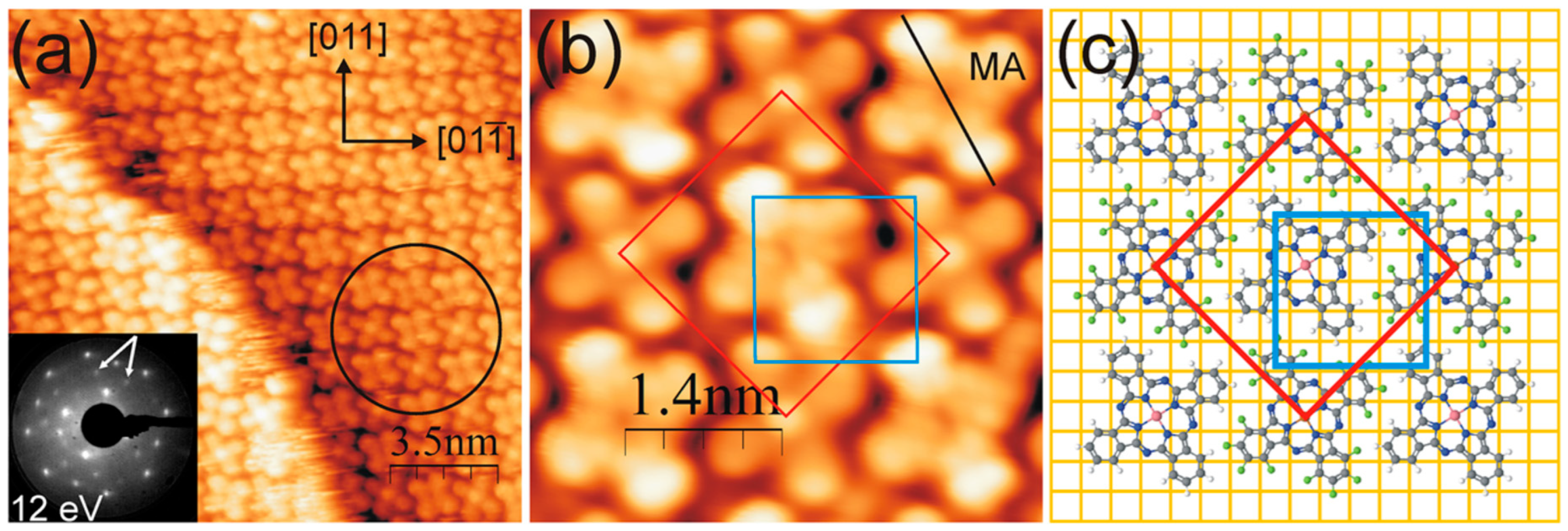


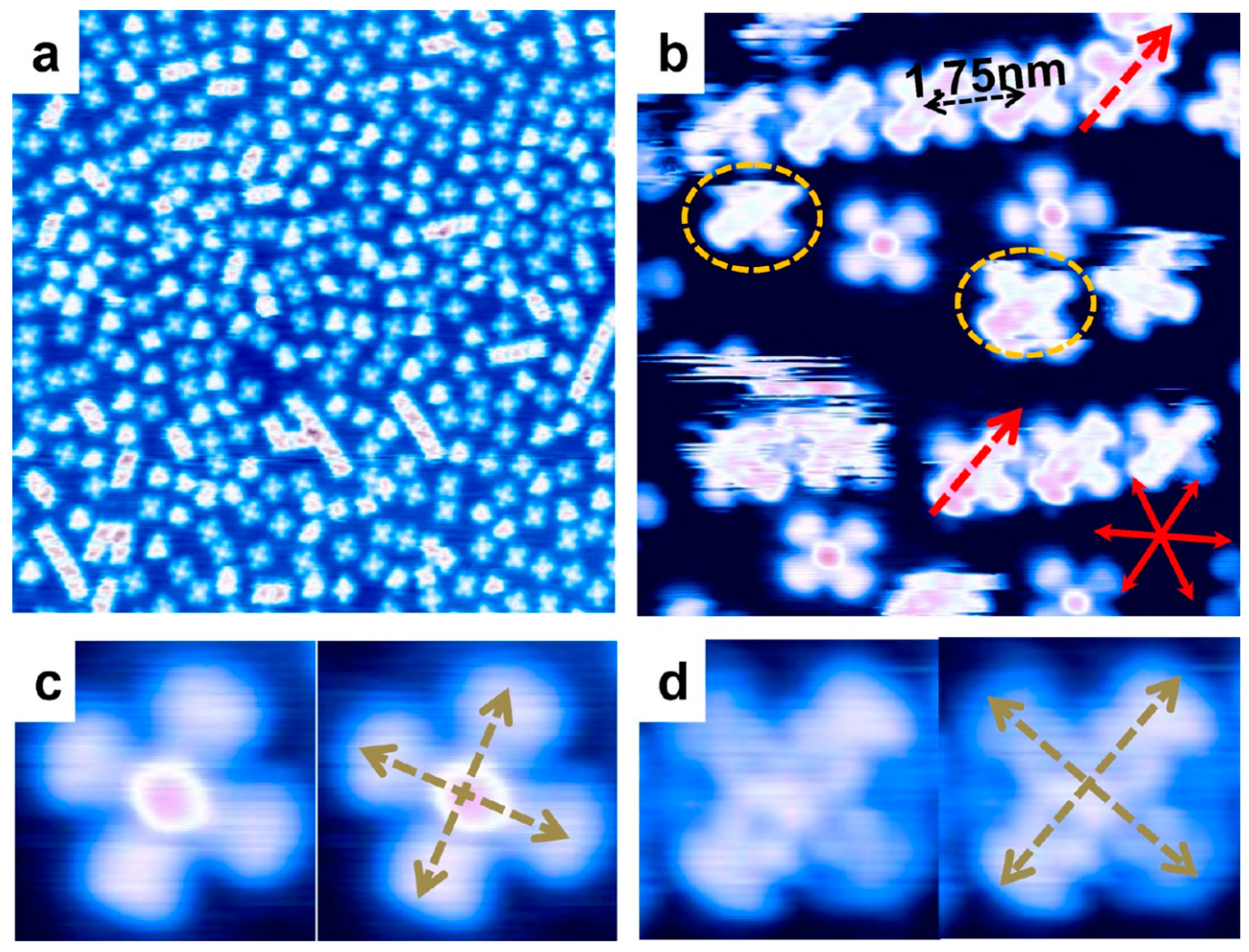
| Symmetry | H2Pc | CuPc | FePc | CoPc | VOPc |
|---|---|---|---|---|---|
| Au | Four-fold [159] | Four-fold [115] | - | - | Four-fold [46] |
| Ag | Two-fold [160] | Two-fold [161] | Four-fold [69] | Two-fold [134] | Four-fold [19] |
| Cu | Four-fold [40] | Four-fold and two-fold [102,116] | Two-fold and one-fold [26,131,132] | Two-fold [126] | One-fold and two-fold [19] |
| HOMO (eV) | H2Pc | CuPc | FePc | CoPc | VOPc |
| Au | 1.6 [162] | 0.71–0.82 [63] | 1.4 [163] | 1.25 (onset = 0.75) [34] | 0.9 1 [46] |
| Ag | 1.5 [160] | 1.25 [102] | 1.4 [120] | 1.2 2 [46] | |
| Cu | - | 1.4–1.46 [63,141] | - | 1.37–1.4 [25,141] | 0.8 3 [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbari Nia, S.; Tomaszowska, A.; Powroźnik, P.; Krzywiecki, M. Effective Factors for Optimizing Metallophthalocyanine-Based Optoelectronic Devices: Surface—Molecule Interactions. Molecules 2025, 30, 471. https://doi.org/10.3390/molecules30030471
Akbari Nia S, Tomaszowska A, Powroźnik P, Krzywiecki M. Effective Factors for Optimizing Metallophthalocyanine-Based Optoelectronic Devices: Surface—Molecule Interactions. Molecules. 2025; 30(3):471. https://doi.org/10.3390/molecules30030471
Chicago/Turabian StyleAkbari Nia, Sakineh, Aleksandra Tomaszowska, Paulina Powroźnik, and Maciej Krzywiecki. 2025. "Effective Factors for Optimizing Metallophthalocyanine-Based Optoelectronic Devices: Surface—Molecule Interactions" Molecules 30, no. 3: 471. https://doi.org/10.3390/molecules30030471
APA StyleAkbari Nia, S., Tomaszowska, A., Powroźnik, P., & Krzywiecki, M. (2025). Effective Factors for Optimizing Metallophthalocyanine-Based Optoelectronic Devices: Surface—Molecule Interactions. Molecules, 30(3), 471. https://doi.org/10.3390/molecules30030471








