The Influence of pH on Long-Range Electron Transfer and Proton-Coupled Electron Transfer in Ruthenium-Modified Azurin
Abstract
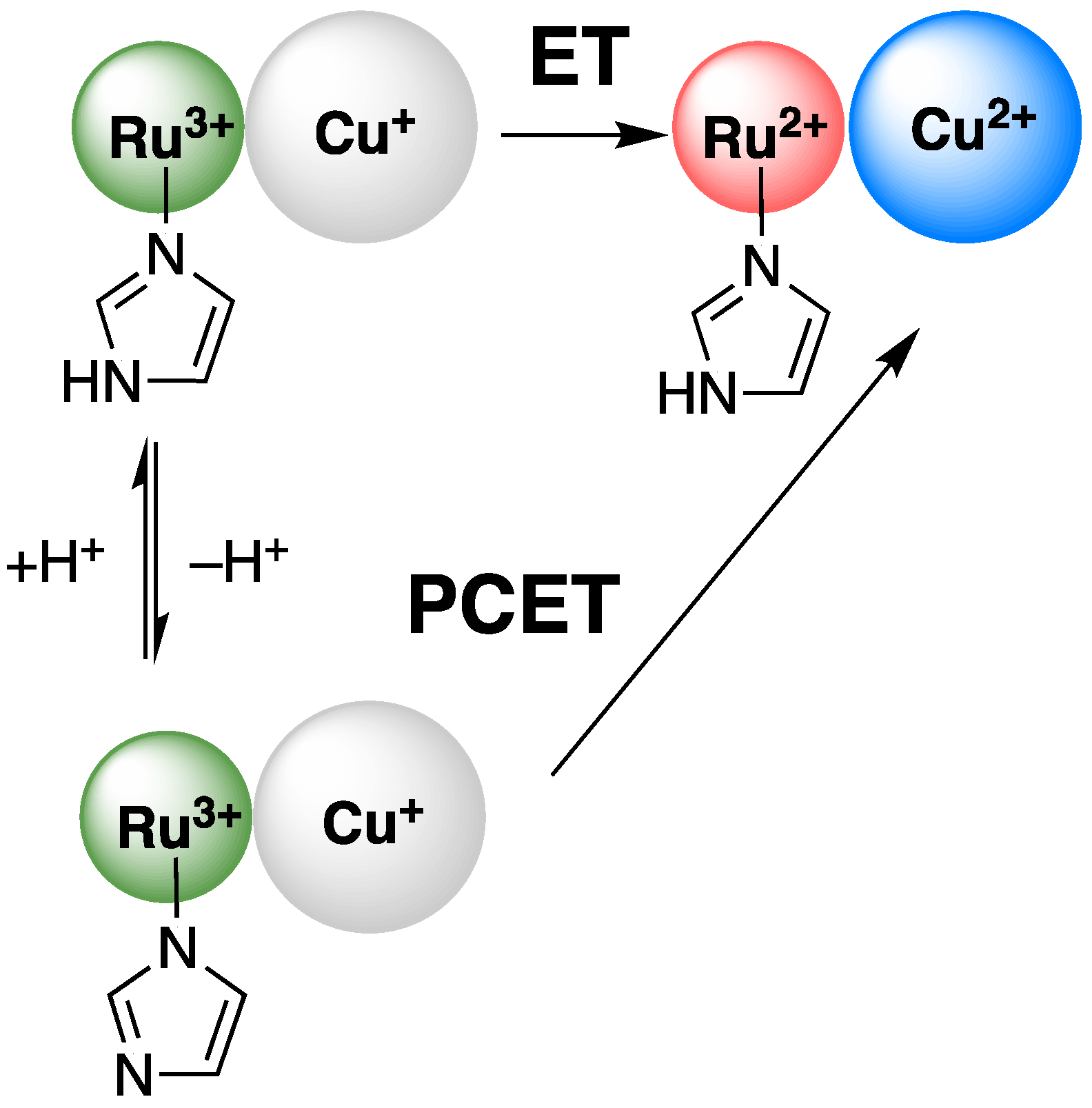
1. Introduction
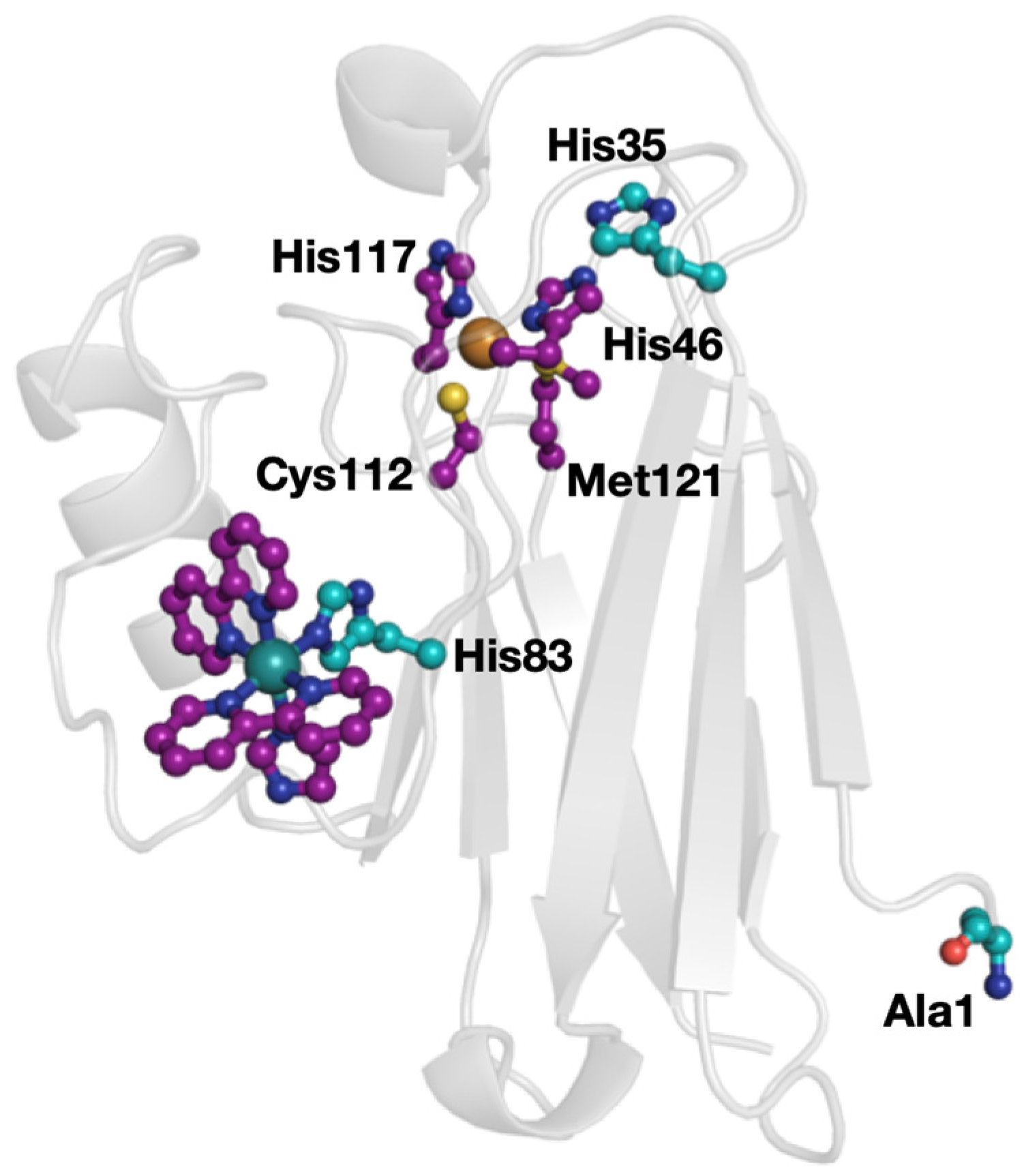
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Minnihan, E.C.; Nocera, D.G.; Stubbe, J. Reversible, Long-Range Radical Transfer in E. coli Class Ia Ribonucleotide Reductase. Acc. Chem. Res. 2013, 46, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.R.; Gray, H.B. Electron Flow through Metalloproteins. Chem. Rev. 2014, 114, 3369–3380, Correction in Chem. Rev. 2016, 116, 8313. [Google Scholar] [CrossRef]
- Wikström, M.; Krab, K.; Sharma, V. Oxygen Activation and Energy Conservation by Cytochrome c Oxidase. Chem. Rev. 2018, 118, 2469–2490. [Google Scholar] [CrossRef] [PubMed]
- Beratan, D.N. Why Are DNA and Protein Electron Transfer So Different? Annu. Rev. Phys. Chem. 2019, 70, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Blumberger, J. Electron Transfer and Transport through Multi-Heme Proteins: Recent Progress and Future Directions. Curr. Opin. Chem. Biol. 2018, 47, 24–31. [Google Scholar] [CrossRef]
- Tommos, C. Insights into the Thermodynamics and Kinetics of Amino-Acid Radicals in Proteins. Annu. Rev. Biophys. 2022, 51, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Cukier, R.I.; Nocera, D.G. Proton-Coupled Electron Transfer. Annu. Rev. Phys. Chem. 1998, 49, 337–369. [Google Scholar] [CrossRef]
- Dempsey, J.L.; Winkler, J.R.; Gray, H.B. Proton-Coupled Electron Flow in Protein Redox Machines. Chem. Rev. 2010, 110, 7024–7039. [Google Scholar] [CrossRef] [PubMed]
- Stubbe, J.; Nocera, D.G.; Yee, C.S.; Chang, M.C.Y. Radical Initiation in the Class I Ribonucleotide Reductase: Long-Range Proton-Coupled Electron Transfer? Chem. Rev. 2003, 103, 2167–2201. [Google Scholar] [CrossRef]
- Mayer, J.M. Proton-Coupled Electron Transfer: A Reaction Chemist’s View. Annu. Rev. Phys. Chem. 2004, 55, 363–390. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.J.; Mayer, J.M. Moving Protons and Electrons in Biomimetic Systems. Biochemistry 2015, 54, 1863–1878. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.; Van, G. Primary Charge Separation in Photosystem II. Photosynth. Res. 2000, 63, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.; Alonso-Mori, R.; Tran, R.; Hattne, J.; Gildea, R.J.; Echols, N.; Gl√∂ckner, C.; Hellmich, J.; Laksmono, H.; Sierra, R.G.; et al. Simultaneous Femtosecond X-Ray Spectroscopy and Diffraction of Photosystem II at Room Temperature. Science 2013, 340, 491–495. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal Structure of Oxygen-Evolving Photosystem II at a Resolution of 1.9Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.J.; Herrera, N.; Hill, M.G.; Winkler, J.R.; Gray, H.B. Electron Flow through Nitrotyrosinate in Pseudomonas Aeruginosa Azurin. J. Am. Chem. Soc. 2013, 135, 11151–11158. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.J.; Shafaat, O.S.; Winkler, J.R.; Gray, H.B. Proton-Coupled Electron Hopping in Ru-Modified P. Aeruginosa Azurin. J. Biol. Inorg. Chem. 2016, 21, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Cordes, M.; Köttgen, A.; Jasper, C.; Jacques, O.; Boudebous, H.; Giese, B. Influence of Amino Acid Side Chains on Long-Distance Electron Transfer in Peptides: Electron Hopping via “Stepping Stones”. Angew. Chem. Int. Ed. 2008, 47, 3461–3463. [Google Scholar] [CrossRef] [PubMed]
- Cordes, M.; Giese, B. Electron Transfer in Peptides and Proteins. Chem. Soc. Rev. 2009, 38, 892–901. [Google Scholar] [CrossRef]
- Hay, S.; Westerlund, K.; Tommos, C. Moving a Phenol Hydroxyl Group from the Surface to the Interior of a Protein: Effects on the Phenol Potential and pKA. Biochemistry 2005, 44, 11891–11902. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rivera, M.C.; Berry, B.W.; Valentine, K.G.; Westerlund, K.; Hay, S.; Tommos, C. Electrochemical and Structural Properties of a Protein System Designed To Generate Tyrosine Pourbaix Diagrams. J. Am. Chem. Soc. 2011, 133, 17786–17795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nilsen-Moe, A.; Reinhardt, C.R.; Huang, P.; Agarwala, H.; Lopes, R.; Lasagna, M.; Glover, S.; Hammes-Schiffer, S.; Tommos, C.; Hammarström, L. Switching the Proton-Coupled Electron Transfer Mechanism for Non-Canonical Tyrosine Residues in a de Novo Protein. Chem. Sci. 2024, 15, 3957–3970. [Google Scholar] [CrossRef]
- Fedoretz-Maxwell, B.P.; Shin, C.H.; MacNeil, G.A.; Worrall, L.J.; Park, R.; Strynadka, N.C.J.; Walsby, C.J.; Warren, J.J. The Impact of Second Coordination Sphere Methionine-Aromatic Interactions in Copper Proteins. Inorg. Chem. 2022, 61, 5563–5571. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.M.; Baker, M.H.; Reardon, N.J. Reduction Potential Variations in Azurin through Secondary Coordination Sphere Phenylalanine Incorporations. J. Inorg. Biochem. 2010, 104, 1071–1078. [Google Scholar] [CrossRef]
- Clair, C.S.S.; Ellis Jr, W.R.; Gray, H.B. Spectroelectrochemistry of Blue Copper Proteins: pH and Temperature Dependences of the Reduction Potentials of Five Azurins. Inorg. Chim. Acta 1992, 191, 149–155. [Google Scholar] [CrossRef]
- Magliozzo, R.S.; McIntosh, B.A.; Sweeney, W.V. Origin of the pH Dependence of the Midpoint Reduction Potential in Clostridium Pasteurianum Ferredoxin: Oxidation State-Dependent Hydrogen Ion Association. J. Biol. Chem. 1982, 257, 3506–3509. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Fee, J.A.; Hirst, J. Complete Thermodynamic Characterization of Reduction and Protonation of the Bc1-Type Rieske [2Fe-2S] Center of Thermus thermophilus. J. Am. Chem. Soc. 2001, 123, 9906–9907. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.S.; Taniguchi, V.T.; Gray, H.B.; Mauk, A.G. Oxidation-Reduction Equilibrium of Cytochrome B5. J. Am. Chem. Soc. 1982, 104, 7516–7519. [Google Scholar] [CrossRef]
- Reid, L.S.; Mauk, M.R.; Mauk, A.G. Role of Heme Propionate Groups in Cytochrome B5 Electron Transfer. J. Am. Chem. Soc. 1984, 106, 2182–2185. [Google Scholar] [CrossRef]
- Pettigrew, G.W.; Meyer, T.E.; Bartsch, R.G.; Kamen, M.D. pH Dependence of the Oxidation-Reduction Potential of Cytochrome c2. Biochim. Biophys. Acta—Bioenerg. 1976, 430, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.R.; Pettigrew, G.W.; Pitt, R.C.; Williams, R.J.P. pH Dependence of the Redox Potential of Pseudomonas Aeruginosa Cytochrome c-551. Biochim. Biophys. Acta—Bioenerg. 1980, 590, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Battistuzzi, G.; Borsari, M.; Sola, M. Redox Properties of Cytochrome c. Antioxid. Redox Signal. 2001, 3, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, R.T.; Ullmann, G.M. Coupling of Protonation, Reduction, and Conformational Change in Azurin from Pseudomonas Aeruginosa Investigated with Free Energy Measures of Cooperativity. J. Phys. Chem. B 2011, 115, 10346–10359. [Google Scholar] [CrossRef] [PubMed]
- Zahler, C.T.; Zhou, H.; Abdolvahabi, A.; Holden, R.L.; Rasouli, S.; Tao, P.; Shaw, B.F. Direct Measurement of Charge Regulation in Metalloprotein Electron Transfer. Angew. Chem. Int. Ed. 2018, 57, 5364–5368. [Google Scholar] [CrossRef]
- Zahler, C.T.; Shaw, B.F. What Are We Missing by Not Measuring the Net Charge of Proteins? Chem. Eur. J. 2019, 25, 7581–7590. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Koone, J.C.; Dashnaw, C.M.; Zahler, C.T.; Shaw, B.F. Complete Charge Regulation by a Redox Enzyme Upon Single Electron Transfer. Angew. Chem. Int. Ed. 2020, 59, 10989–10995. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.; Jönsson, B. On the Charge Regulation of Proteins. Biochemistry 2005, 44, 5722–5727. [Google Scholar] [CrossRef]
- Ullmann, G.M. The Coupling of Protonation and Reduction in Proteins with Multiple Redox Centers: Theory, Computational Method, and Application to Cytochrome C3. J. Phys. Chem. B 2000, 104, 6293–6301. [Google Scholar] [CrossRef]
- Ullmann, G.M.; Bombarda, E. pKa Values and Redox Potentials of Proteins. What Do They Mean? Biol. Chem. 2013, 394, 611–619. [Google Scholar] [CrossRef]
- Bombarda, E.; Ullmann, G.M. pH-Dependent pKa Values in Proteins—A Theoretical Analysis of Protonation Energies with Practical Consequences for Enzymatic Reactions. J. Phys. Chem. B 2010, 114, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Warburton, R.E.; Soudackov, A.V.; Hammes-Schiffer, S. Theoretical Modeling of Electrochemical Proton-Coupled Electron Transfer. Chem. Rev. 2022, 122, 10599–10650. [Google Scholar] [CrossRef] [PubMed]
- Faham, S.; Day, M.W.; Connick, W.B.; Crane, B.R.; Di Bilio, A.J.; Schaefer, W.P.; Rees, D.C.; Gray, H.B. Structures of Ruthenium-Modified Pseudomonas Aeruginosa Azurin and [Ru(2,2′-Bipyridine)2(Imidazole)2]SO4·10H2O. Acta Crystallogr. D Biol. Crystallogr. 1999, 55, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.V.; Brunschwig, B.S.; Creutz, C.; Sutin, N. Homogeneous Catalysis of the Photoreduction of Water. 6. Mediation by Polypyridine Complexes of Ruthenium(II) and Cobalt(II) in Alkaline Media. J. Am. Chem. Soc. 1985, 107, 2005–2015. [Google Scholar] [CrossRef]
- Long, C.; Vos, J.G. Acid-Base Chemistry of Some 1,2,4-Triazole and Imidazole Complexes of Ruthenium(II)Bis(2,2′-Bipyridyl). Inorganica Chim. Acta 1984, 89, 125–131. [Google Scholar] [CrossRef]
- Reddy, K.B.; Cho, M.P.; Wishart, J.F.; Emge, T.J.; Isied, S.S. cis-Bis(Bipyridine)Ruthenium Imidazole Derivatives: A Spectroscopic, Kinetic, and Structural Study. Inorg. Chem. 1996, 35, 7241–7245. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yang, H.; Malik, N.; Čolović, M.; Weber, D.S.; Wilson, D.; Bénard, F.; Martin, R.E.; Warren, J.J.; Schaffer, P.; et al. Electrostatic Effects Accelerate Decatungstate-Catalyzed C–H Fluorination Using [18F]- and [19F]NFSI in Small Molecules and Peptide Mimics. ACS Catal. 2019, 9, 8276–8284. [Google Scholar] [CrossRef]
- Gibbs, C.A.; Ghazi, N.; Tao, J.; Warren, J.J. An Investigation of the Influence of Tyrosine Local Interactions on Electron Hopping in a Model Protein. Molecules 2024, 29, 350. [Google Scholar] [CrossRef] [PubMed]
- Skov, L.K.; Pascher, T.; Winkler, J.R.; Gray, H.B. Rates of Intramolecular Electron Transfer in Ru(Bpy)2(Im)(His83)-Modified Azurin Increase below 220 K. J. Am. Chem. Soc. 1998, 120, 1102–1103. [Google Scholar] [CrossRef]
- Gray, H.B.; Winkler, J.R. Electron Tunneling through Proteins. Q. Rev. Biophys. 2003, 36, 341–372. [Google Scholar] [CrossRef] [PubMed]
- Malmström, B.G.; Wittung-Stafshede, P. Effects of Protein Folding on Metalloprotein Redox-Active Sites: Electron-Transfer Properties of Blue and Purple Copper Proteins. Coord. Chem. Rev. 1999, 185, 127–140. [Google Scholar] [CrossRef]
- Winkler, J.R.; Wittung-Stafshede, P.; Leckner, J.; Malmström, B.G.; Gray, H.B. Effects of Folding on Metalloprotein Active Sites. Proc. Natl. Acad. Sci. USA 1997, 94, 4246–4249. [Google Scholar] [CrossRef] [PubMed]
- Di Bilio, A.J.; Hill, M.G.; Bonander, N.; Karlsson, B.G.; Villahermosa, R.M.; Malmstroem, B.G.; Winkler, J.R.; Gray, H.B. Reorganization Energy of Blue Copper: Effects of Temperature and Driving Force on the Rates of Electron Transfer in Ruthenium- and Osmium-Modified Azurins. J. Am. Chem. Soc. 1997, 119, 9921–9922. [Google Scholar] [CrossRef]
- Gray, H.B.; Winkler, J.R. Long-Range Electron Transfer. Proc. Natl. Acad. Sci. USA 2005, 102, 3534–3539. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.G.; Coste, S.C.; Groff, B.D.; Heuer, A.M.; Noh, H.; Parada, G.A.; Wise, C.F.; Nichols, E.M.; Warren, J.J.; Mayer, J.M. Free Energies of Proton-Coupled Electron Transfer Reagents and Their Applications. Chem. Rev. 2022, 122, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.J.; Tronic, T.A.; Mayer, J.M. Thermochemistry of Proton-Coupled Electron Transfer Reagents and Its Implications. Chem. Rev. 2010, 110, 6961–7001. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, D.R.; Gagliardi, C.J.; Hull, J.F.; Murphy, C.F.; Kent, C.A.; Westlake, B.C.; Paul, A.; Ess, D.H.; McCafferty, D.G.; Meyer, T.J. Proton-Coupled Electron Transfer. Chem. Rev. 2012, 112, 4016–4093. [Google Scholar] [CrossRef]
- Roth, J.P.; Lovell, S.; Mayer, J.M. Intrinsic Barriers for Electron and Hydrogen Atom Transfer Reactions of Biomimetic Iron Complexes. J. Am. Chem. Soc. 2000, 122, 5486–5498. [Google Scholar] [CrossRef]
- Kessinger, M.; Soudackov, A.V.; Schneider, J.; Bangle, R.E.; Hammes-Schiffer, S.; Meyer, G.J. Reorganization Energies for Interfacial Proton-Coupled Electron Transfer to a Water Oxidation Catalyst. J. Am. Chem. Soc. 2022, 144, 20514–20524. [Google Scholar] [CrossRef]
- Farver, O.; Bonander, N.; Skov, L.K.; Pecht, I. The pH Dependence of Intramolecular Electron Transfer in Azurins. Inorganica Chim. Acta 1996, 243, 127–133. [Google Scholar] [CrossRef]
- Hamann, T.W.; Gstrein, F.; Brunschwig, B.S.; Lewis, N.S. Measurement of the Dependence of Interfacial Charge-Transfer Rate Constants on the Reorganization Energy of Redox Species at n-ZnO/H2O Interfaces. J. Am. Chem. Soc. 2005, 127, 13949–13954. [Google Scholar] [CrossRef]
- Johnson, E.C.; Sullivan, B.P.; Salmon, D.J.; Adeyemi, S.A.; Meyer, T.J. Synthesis and Properties of the Chloro-Bridged Dimer [(Bpy)2RuCl]22+ and Its Transient 3+ Mixed-Valence Ion. Inorg. Chem. 1978, 17, 2211–2215. [Google Scholar] [CrossRef]
- Meyer, T.J.; Taube, H. Electron-Transfer Reactions of Ruthenium Ammines. Inorg. Chem. 1968, 7, 2369–2379. [Google Scholar] [CrossRef]
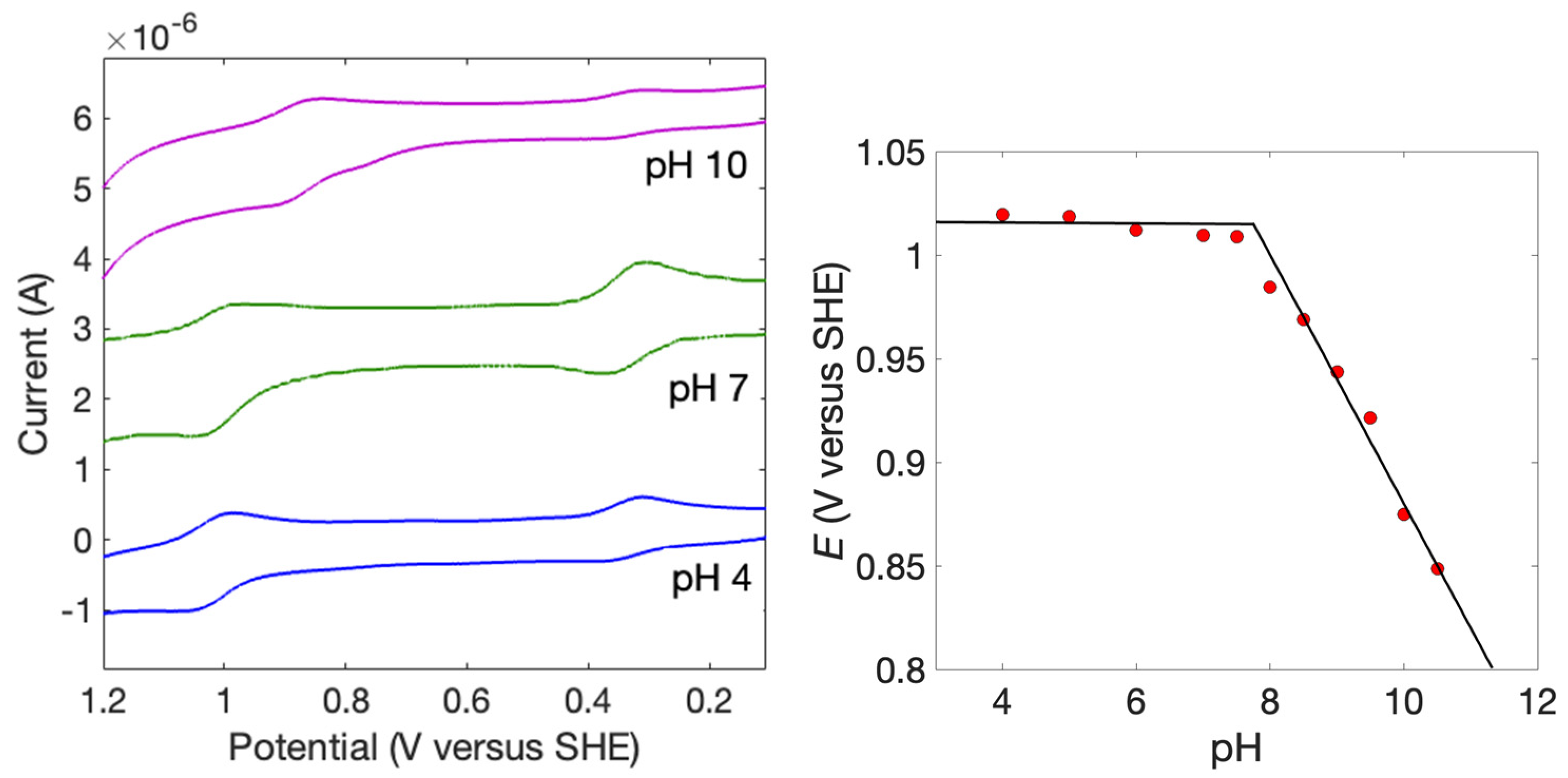
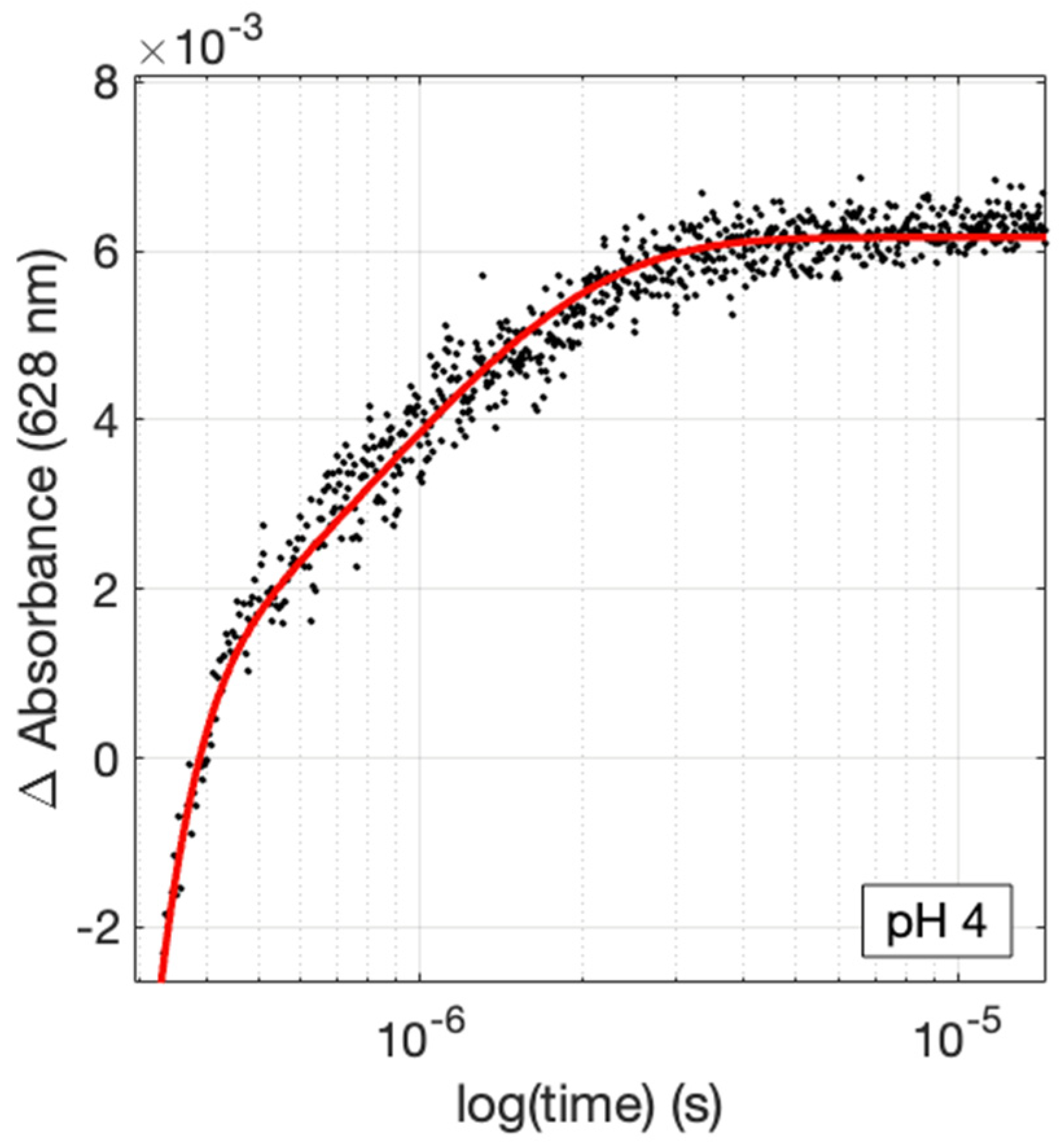
| pH | −∆G° 1 | kET (×10−6) 2 | kET,calc (×10−6) 3 |
|---|---|---|---|
| 4 | −0.671 | 1.2 ± 0.1 | 2.18 |
| 5 | −0.671 | 1.4 ± 0.1 | 2.18 |
| 6 | −0.673 | 1.3 ± 0.1 | 2.18 |
| 7 | −0.698 | 1.3 ± 0.1 | 2.20 |
| 8 | −0.693 | 1.3 ± 0.1 | 2.20 |
| 9 | −0.655 | 0.3 ± 0.1 | 2.14 |
| 9.5 | −0.634 | 0.4 ± 0.1 | 2.07 |
| pH | λ 1 |
|---|---|
| 4 | 0.88 |
| 5 | 0.85 |
| 6 | 0.87 |
| 7 | 0.89 |
| 8 | 0.85 |
| 9 | 1.10 |
| 9.5 | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazi, N.; Warren, J.J. The Influence of pH on Long-Range Electron Transfer and Proton-Coupled Electron Transfer in Ruthenium-Modified Azurin. Molecules 2025, 30, 472. https://doi.org/10.3390/molecules30030472
Ghazi N, Warren JJ. The Influence of pH on Long-Range Electron Transfer and Proton-Coupled Electron Transfer in Ruthenium-Modified Azurin. Molecules. 2025; 30(3):472. https://doi.org/10.3390/molecules30030472
Chicago/Turabian StyleGhazi, Nikta, and Jeffrey J. Warren. 2025. "The Influence of pH on Long-Range Electron Transfer and Proton-Coupled Electron Transfer in Ruthenium-Modified Azurin" Molecules 30, no. 3: 472. https://doi.org/10.3390/molecules30030472
APA StyleGhazi, N., & Warren, J. J. (2025). The Influence of pH on Long-Range Electron Transfer and Proton-Coupled Electron Transfer in Ruthenium-Modified Azurin. Molecules, 30(3), 472. https://doi.org/10.3390/molecules30030472








