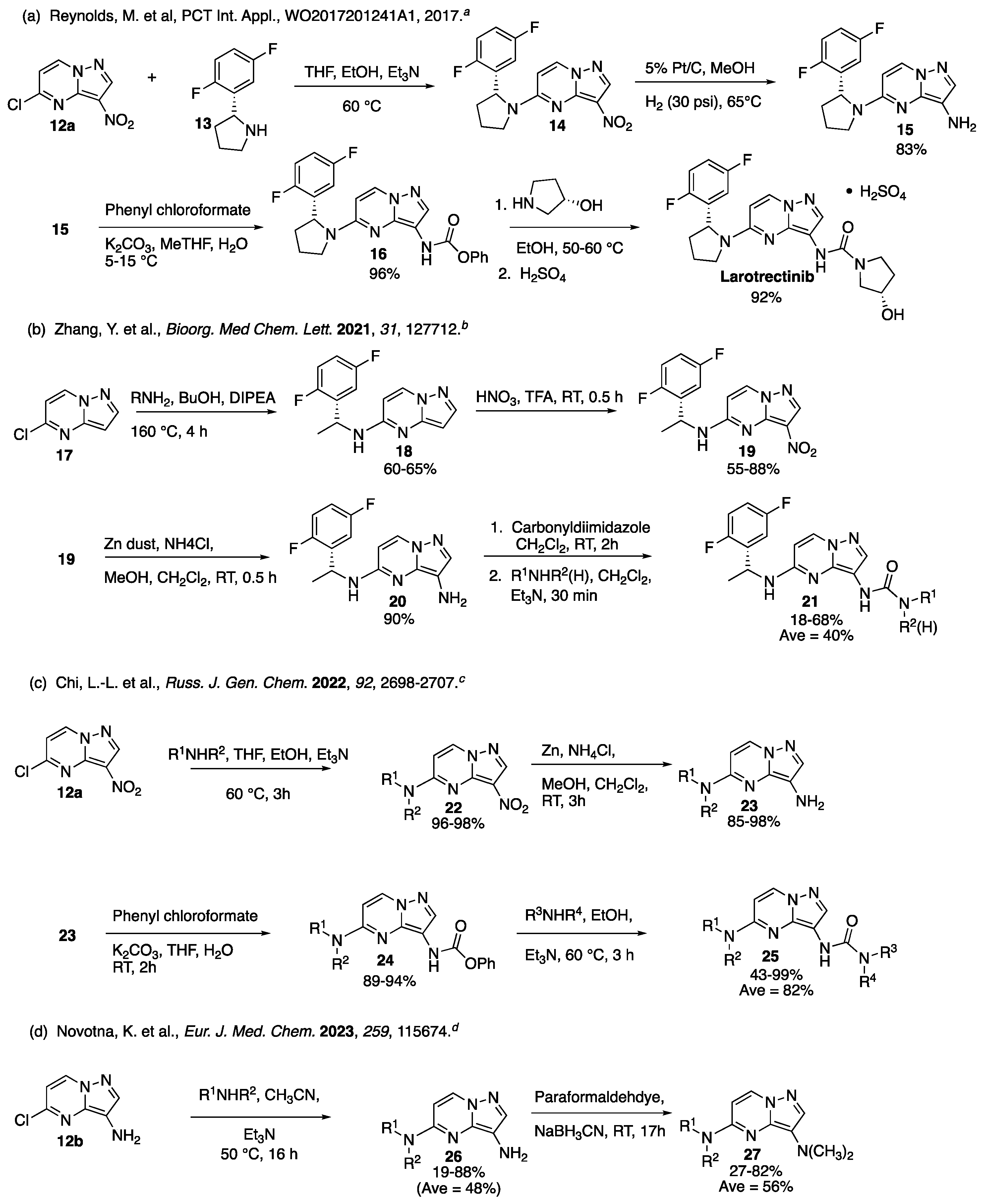
An Efficient Synthesis of 3,5-Bis-Aminated Pyrazolo[1,5-a]Pyrimidines: Microwave-Assisted Copper Catalyzed C-3 Amination of 5-Amino-3-Bromo-Substituted Precursors
Abstract
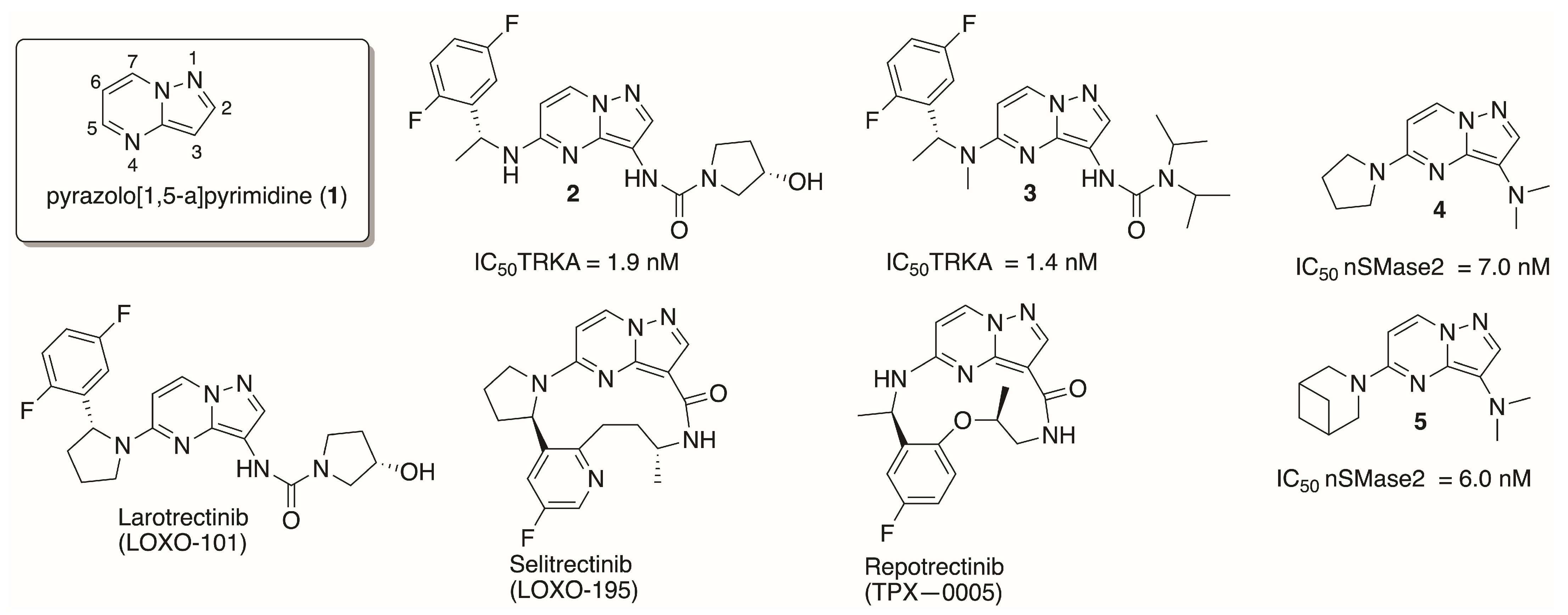
1. Introduction
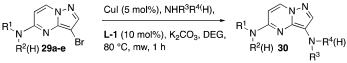
2. Results
2.1. Optimization
2.1.1. Temperature, Solvent, and Base
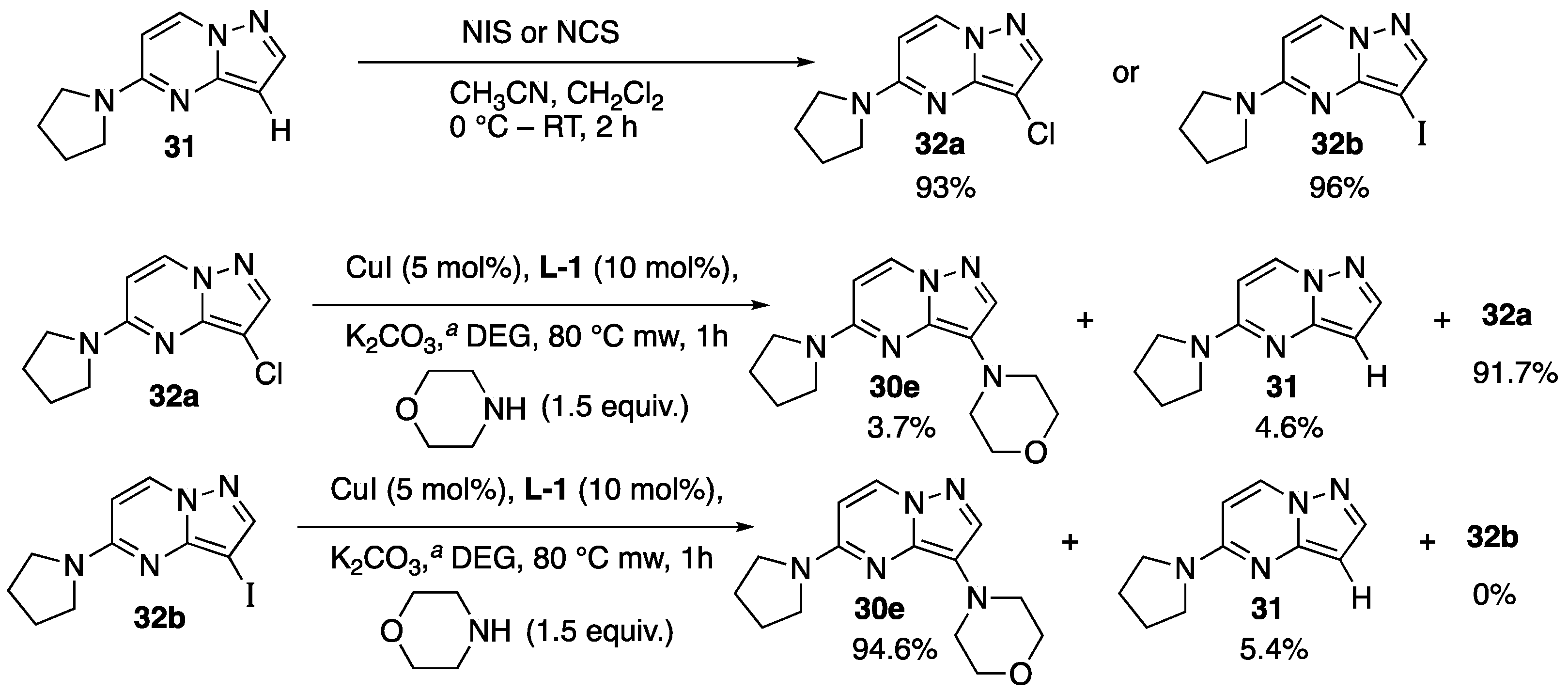
2.1.2. Ligand and C-3 Halogenated Precursor
3. Discussion
 |
|---|
 |
4. Materials and Methods
4.1. General Experimental
4.2. Ligand Synthesis
4.3. 5-Amino-3-Bromopyrazolo[1,5-a]Pyrimidines 29
- 3-Bromo-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (29a)
- 3-Bromo-5-(N-(isopropyl)amino)pyrazolo[1,5-a]pyrimidine (29b)
- 3-Bromo-5-(4-(methoxy)anilin-1-yl)pyrazolo[1,5-a]pyrimidine (29c)
- 3-Bromo-5-(4-(isopropyl)anilin-1-yl)pyrazolo[1,5-a]pyrimidine (29d)
- 3-Bomo-5-(4-(chloro)anilin-1-yl)-pyrazolo[1,5-a]pyrimidine (29e)
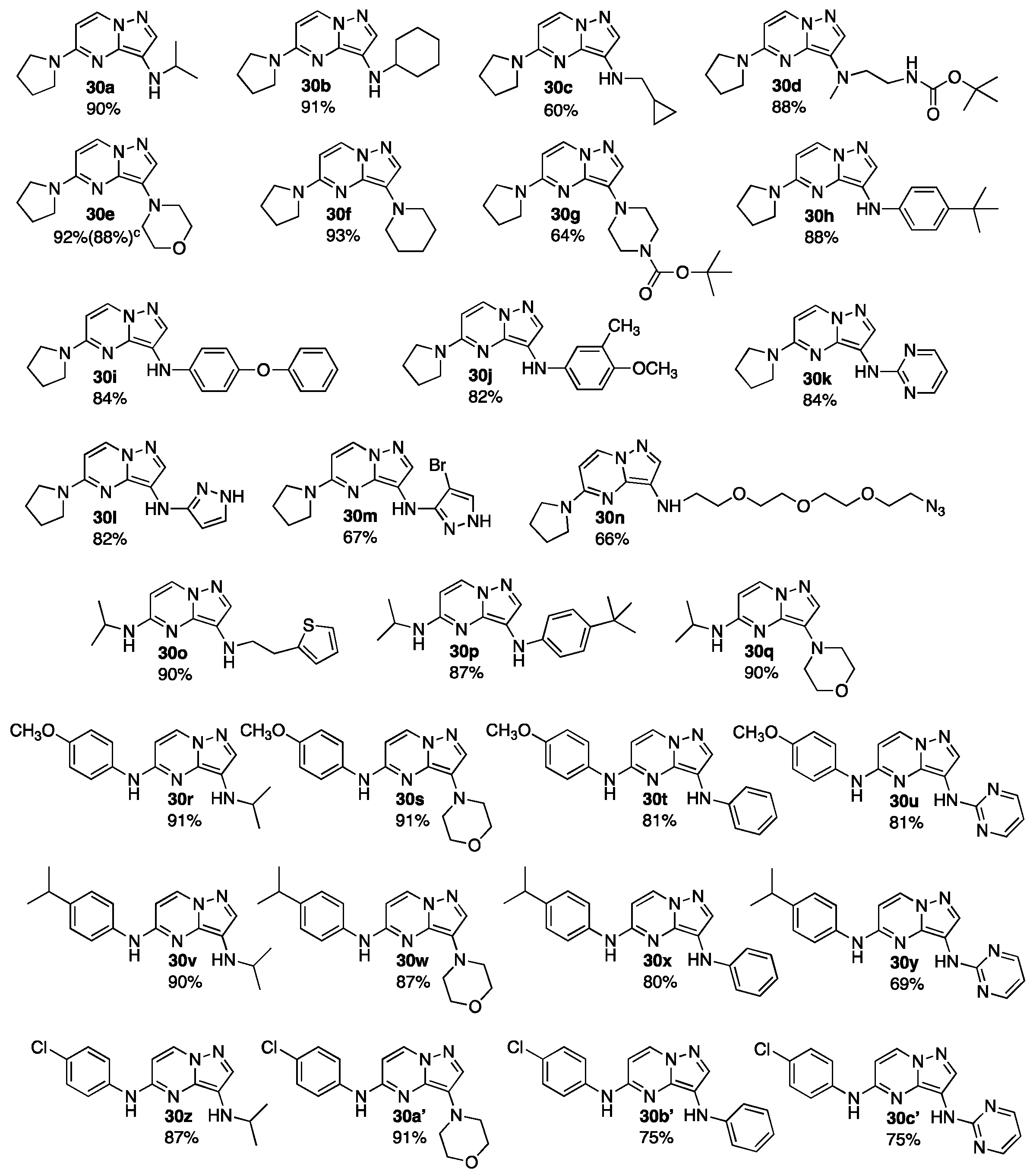
4.4. 3,5-Bis-Aminopyrazolo[1,5-a]Pyrimidines 30
- 3-(N-(Isopropyl)amino)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30a)
- 3-(N-(Cyclohexyl)amino)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30b)
- 3-(N-((Cyclopropyl)methyl)amino)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30c)
- 3-[2-(tert-Butyloxycarbonyl)amino)-1-N-methylamino)ethyl]-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30d)
- 3-(Morpholin-1-yl)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30e)
- 3-(Piperidin-1-yl)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30f)
- tert-Butyl 4-(5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidin-3-yl)piperazine-1-carboxylate (30g)
- 3-(4-(tert-Butyl)anilin-1-yl)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30h)
- 3-(4-(Phenoxy)anilin-1-yl)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30i)
- 3-((4-Methoxy-3-methyl)anilin-1-yl)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30j)
- 3-(N-(Pyrimidin-2-yl)amino)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30k)
- 3-(N-(Pyrazol-3-yl)amino)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30l)
- 3-(N-(5-Bromopyrazol-3-yl)amino)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (30m)
- N-(2-(2-(2-(2-Azidoethoxy)ethoxy)ethoxy)ethyl)-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidin-3-amine (30n)
- 5-(N-(Isopropyl)amino)-3-(2-N-(thiophen-2-yl)ethylamino)pyrazolo[1,5-a]pyrimidine (30o)
- 3-(4-(tert-Butyl)anilin-1-yl)-5-(N-(isopropyl)amino)pyrazolo[1,5-a]pyrimidine (30p)
- 5-(N-(Isopropyl)amino)-3-(morpholin-1-yl)pyrazolo[1,5-a]pyrimidine (30q)
- 3-(N-(Isopropyl)amino)-5-(4-(methoxy)anilin-1-yl)pyrazolo[1,5-a]pyrimidine (30r)
- 5-(4-(Methoxy)anilin-1-yl)-3-(morpholin-1-yl)pyrazolo[1,5-a]pyrimidine (30s)
- 5-(4-(Methoxy)anilin-1-yl)-3-(anilin-1-yl)pyrazolo[1,5-a]pyrimidine (30t)
- 5-(4-(Methoxy)anilin-1-yl)-3-(N-(pyrimidin-2-yl)amino)pyrazolo[1,5-a]pyrimidine (30u)
- 3-(N-(Isopropyl)amino)-5-(4-(isopropyl)anilin-1-yl)pyrazolo[1,5-a]pyrimidine (30v)
- 5-(4-(Isopropyl)anilin-1-yl)-3-(morpholin-1-yl)pyrazolo[1,5-a]pyrimidine (30w)
- 5-(4-(Isopropyl)anilin-1-yl)-3-(anilin-1-yl)pyrazolo[1,5-a]pyrimidine (30x)
- 5-(4-(Isopropyl)anilin-1-yl)-3-(N-(pyrimidin-2-yl)amino)pyrazolo[1,5-a]pyrimidine (30y)
- 5-(4-(Chloro)anilin-1-yl)-3-(N-isopropylamino)pyrazolo[1,5-a]pyrimidine (30z)
- 5-(4-(Chloro)anilin-1-yl)-3-(morpholin-1-yl)pyrazolo[1,5-a]pyrimidine (30a’)
- 5-(4-(Chloro)anilin-1-yl)-3-(anilin-1-yl)pyrazolo[1,5-a]pyrimidine (30b’)
- 5-(4-(Chloro)anilin-1-yl)-3-(N-(pyrimidin-2-yl)amino)pyrazolo[1,5-a]pyrimidine (30c’)
- 5-(Pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (31)
4.5. 3-Halo-5-(Pyrrolidin-1-yl)Pyrazolo[1,5-a]Pyrimidines 32
- 3-Chloro-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (32a)
- 3-Iodo-5-(pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine (32b)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, J.; Deng, X.; Sun, R.; Tavallaie, M.S.; Wang, J.; Cai, Q.; Lam, C.; Lei, S.; Fu, L.; Jiang, F. Structural optimization of pyrazolo [1,5-a]pyrimidine derivatives as potent and highly selective DPP-4 inhibitors. Eur. J. Med. Chem. 2020, 208, 112850. [Google Scholar] [CrossRef]
- Arias-Gomez, A.; Godoy, A.; Portilla, J. Functional pyrazolo [1,5-a]pyrimidines: Current approaches in synthetic transformations and uses as an antitumor scaffold. Molecules 2021, 26, 2708. [Google Scholar] [CrossRef] [PubMed]
- Jismy, B.; Guillaumet, G.; Akssira, M.; Tikad, A.; Abarbri, M. Efficient microwave-assisted Suzuki–Miyaura cross-coupling reaction of 3-bromo pyrazolo [1,5-a]pyrimidin-5(4H)-one: Towards a new access to 3,5-diarylated 7-(trifluoromethyl)pyrazolo [1,5-a]pyrimidine derivatives. RSC Adv. 2021, 11, 1287–1302. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Sasaki, T.; Tokura, S.; Ogata, Y.; Kawaguchi, T.; Sugaya, Y.; Takahashi, R.; Iwakiri, K.; Abe-Kumasaka, T.; Yoshida, I.; Arikawa, K.; et al. Discovery of a potent dual inhibitor of wild-type and mutant respiratory syncytial virus fusion proteins. ACS Med. Chem. Lett. 2020, 11, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.M.; Abbas, H.-A.; Ahmed, E.H.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I. Synthesis, in vitro antimicrobial and cytotoxic activities of some new Pyrazolo [1,5-a]pyrimidine derivatives. Molecules 2019, 24, 1080. [Google Scholar] [CrossRef]
- Hassan, A.S.; Masoud, D.M.; Sroor, F.M.; Askar, A.A. Synthesis and biological evaluation of pyrazolo [1,5-a]pyrimidine-3-carboxamide as antimicrobial agents. Med. Chem. Res. 2017, 26, 2909–2919. [Google Scholar] [CrossRef]
- Al-Adiwish, W.M.; Tahir, M.I.M.; Siti-Noor-Adnalizawati, A.; Hashim, S.F.; Ibrahim, N.; Yaacob, W.A. Synthesis, antibacterial activity and cytotoxicity of new fused pyrazolo [1,5-a]pyrimidine and pyrazolo [5,1-c][1,2,4]triazine derivatives from new 5-aminopyrazoles. Eur. J. Med. Chem. 2013, 64, 464–476. [Google Scholar] [CrossRef]
- Novinson, T.; Bhooshan, B.; Okabe, T.; Revankar, G.R.; Robins, R.K.; Senga, K.; Wilson, H.R. Novel heterocyclic nitrofurfural hydrazones. In vivo antitrypanosomal activity. J. Med. Chem. 1976, 19, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.C.; Wang, I.J. Synthesis and solvatochromic properties of some disazo dyes derived from pyrazolo [1,5-a]pyrimidine derivatives. Dye. Pigm. 2005, 64, 259–264. [Google Scholar] [CrossRef]
- Tigreros, A.; Aranzazu, S.-L.; Bravo, N.F.; Zapata-Rivera, J.; Portilla, J. Pyrazolo [1,5-a]pyrimidines-based fluorophores: A comprehensive theoretical-experimental study. RSC Adv. 2020, 10, 39542–39552. [Google Scholar] [CrossRef]
- Tigreros, A.; Rosero, H.A.; Castillo, J.-C.; Portilla, J. Integrated pyrazolo [1,5-a]pyrimidine-hemicyanine system as a colorimetric and fluorometric chemosensor for cyanide recognition in water. Talanta 2019, 196, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; He, Y.; Xu, J.; Liu, H.; Wang, X.; Feng, M.; Qi, C.; Zhang, J.; Peng, C. Preparation and bioevaluation of 99mTc nitrido radiopharmaceuticals with pyrazolo [1,5-a]pyrimidine as tumor imaging agents. Med. Chem. Res. 2011, 21, 523–530. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhou, Y.; Zhang, Q.; Han, T.; Tang, C.; Fan, W. Pyrazolo [1,5-a]pyrimidine based trk inhibitors: Design, synthesis, biological activity evaluation. Bioorg. Med. Chem. Lett. 2021, 31, 127712. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, T.; Mitchell, D.R.; Fujino, A.; Imai, M.; Kambe, M.; Kobayashi, S.; Makino, H.; Matsueda, Y.; Oue, Y.; Komatsu, K.; et al. Mitogen-activated protein kinase-activated protein kinase 2 (mapkap-k2) as an antiinflammatory target: Discovery and in vivo activity of selective pyrazolo [1,5-a]pyrimidine inhibitors using a focused library and structure-based optimization approach. J. Med. Chem. 2012, 55, 6700–6715. [Google Scholar] [CrossRef]
- Loew, G.; Toll, L.; Lawson, J.; Uyeno, E.; Kaegi, H. Pyrazolo [1,5-a]pyrimidines: Receptor binding and anxiolytic behavioral studies. Pharmacol. Biochem. Behav. 1984, 20, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Selleri, S.; Bruni, F.; Costagli, C.; Costanzo, A.; Guerrini, G.; Ciciani, G.; Gratteri, P.; Besnard, F.; Costa, B.; Montali, M.; et al. A novel selective GABAAα1 receptor agonist displaying sedative and anxiolytic-like properties in rodents. J. Med. Chem. 2005, 48, 6756–6760. [Google Scholar] [CrossRef]
- Farago, A.F.; Demetri, G.D. Larotrectinib, a selective tropomyosin receptor kinase inhibitor for adult and pediatric tropomyosin receptor kinase fusion cancers. Future Oncol. 2020, 16, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Sava, J. FDA Grants Breakthrough Therapy Designation to Repotrectinib for ROS1+ Metastatic NSCLC. Available online: https://www.targetedonc.com/view/fda-grants-breakthrough-therapy-designation-to-repotrectinib-for-ros1-metastatic-nsclc (accessed on 20 January 2024).
- A Study to Test the Safety of the Investigational Drug Selitrectinib in Children and Adults That May Treat Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03215511 (accessed on 20 January 2024).
- Novotna, K.; Thomas, A.G.; Stepanek, O.; Murphy, B.; Hin, N.; Skacel, J.; Mueller, L.; Tenora, L.; Pal, A.; Alt, J.; et al. Neutral sphingomyelinase 2 inhibitors based on the pyrazolo [1,5-a]pyrimidin-3-amine scaffold. Eur. J. Med. Chem. 2023, 259, 115674. [Google Scholar] [CrossRef] [PubMed]
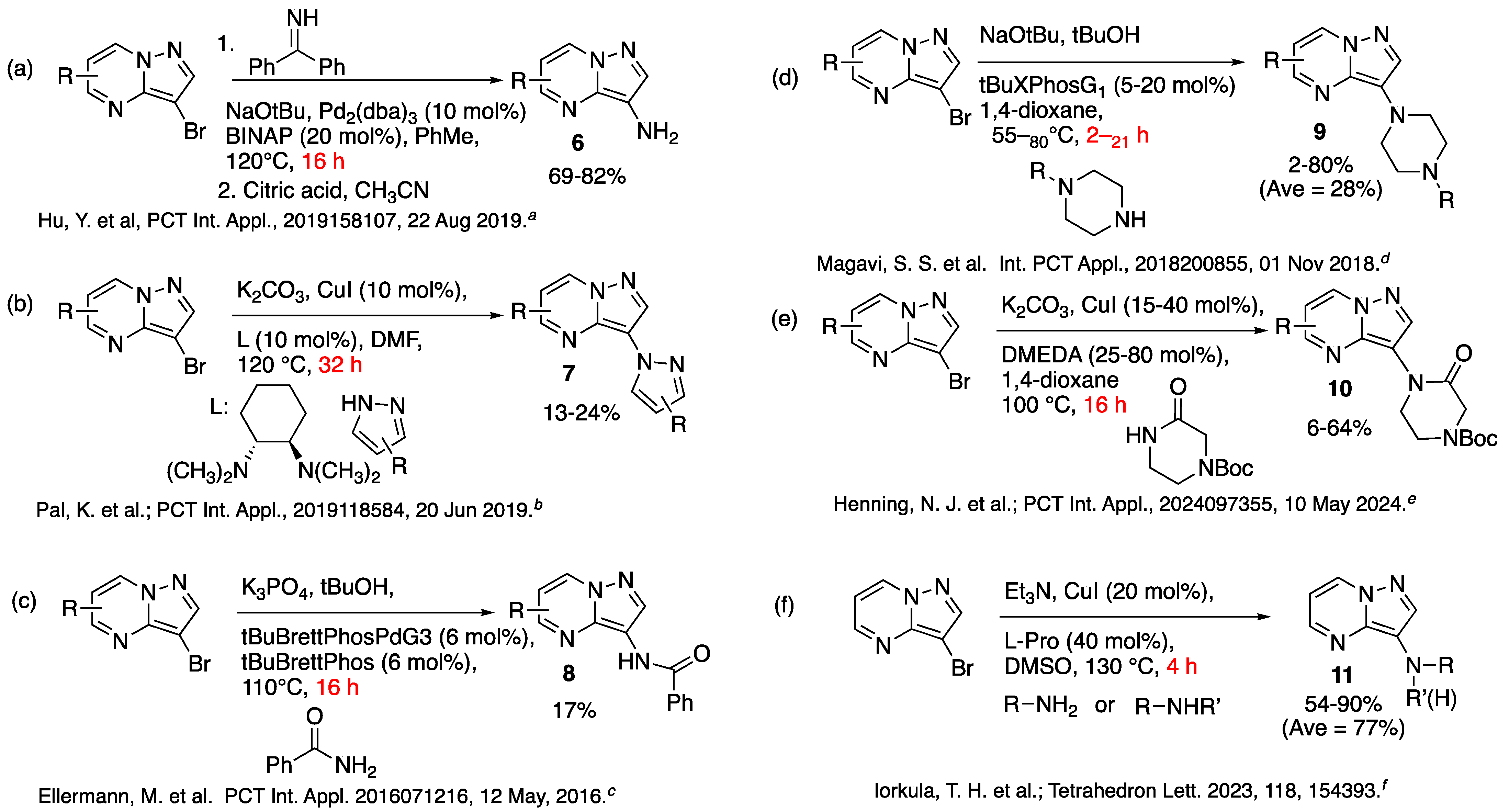
- Hu, Y.; Wu, D.; Peng, W.; Li, X.; Hu, F.; Huang, B.; Zhu, J.; Wu, Y. Heterocyclic compound, application thereof and pharmaceutical composition comprising same. PCT Int. Appl. 2019, 2019, 158107. [Google Scholar]
- Pal, K.; Ciblat, S.; Albert, V.; Bruneau-Latour, N.; Boudreault, J. Preparation of 5-(2-(2,5-difluorophenyl)pyrrolidin-1-yl)-3-(1H-pyrazol-1-yl)pyrazolo [1,5-a]pyrimidine derivatives and related compounds as Trk kinase inhibitors for treating cancer. PCT Int. Appl. 2019, 2019, 118584. [Google Scholar]
- Ellermann, M.; Valot, G.; Cancho Grande, Y.; Hassfeld, J.; Kinzel, T.; Koebberling, J.; Beyer, K.; Roehrig, S.; Sperzel, M.; Stampfuss, J.; et al. Piperidinylpyrazolopyrimidinones as plasminogen inhibitors and their preparation. PCT Int. Appl. 2016, 2016, 071216. [Google Scholar]
- Magavi, S.S.; Parks, D.J.; Tait, B.D.; Cho, J.; Agrawal, R.; Shaw, P.R. Preparation of substituted pyrazolopyridines as platelet-derived growth factor receptor (PDGFR) alpha inhibitors and uses thereof. PCT Int. Appl. 2023, 2023, 081923. [Google Scholar]
- Henning, N.J.; Nomura, D.K.; Boike, L.; Marquess, D.; Keitz, P. Preparation of bifunctional compounds as protein-stabilizing deubiquitinase-targeting chimeras useful in treatment of cystic fibrosis and other diseases. PCT Int. Appl. 2024, 2024, 097355. [Google Scholar]
- Iorkula, T.H.; Tolman, B.A.; Singleton, J.D.; Peterson, M.A. An efficient microwave assisted copper catalyzed C-3 amination of 3-bromopyrazolo [1,5-a]pyrimidine. Tetrahedron Lett. 2023, 118, 154393. [Google Scholar] [CrossRef]
- Reynolds, M.; Eary, C.T.; Spencer, S.; Juengst, D.; Hache, B.; Jiang, Y.; Haas, J.; Andrews, S.W. Preparation of (S)-N-(5-((R)-2-(2,5-Difluorophenyl)pyrrolidin-1-yl)pyrazolo [1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide. WO, 201241, 2017. U.S. Patent 11,214,571, 4 January 2022. [Google Scholar]
- Flick, A.C.; Leverett, C.A.; Ding, H.X.; McInturff, E.; Fink, S.J.; Helal, C.J.; DeForest, J.C.; Morse, P.D.; Mahapatra, S.; O’Donnell, C.J. Synthetic approaches to new drugs approved during 2018. J. Med. Chem. 2020, 63, 10652–10704. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.-L.; Hao, L.-L.; Cai, Z.-Q.; Kong, D.-L.; Wang, Y.-N.; Qin, W.-T.; Gao, Y.; Qu, Z.-Z. Design, synthesis, and biological evaluation of novel pyrazolo [1,5-a]pyrimidine and 1,3-Benzodiazine derivatives as potent antitumor agents. Russ. J. Gen. Chem. 2022, 92, 2698–2707. [Google Scholar] [CrossRef]
- Hong, P.; Zhu, X.; Lai, X.; Gong, Z.; Huang, M.; Wan, Y. Room-temperature CuI-catalyzed N-arylation of cyclopropylamine. J. Org. Chem. 2024, 89, 57–67. [Google Scholar] [CrossRef]
- Hong, P.; Zhu, X.; Chen, F.; Huang, M.; Wan, Y. CuSO4/N-(9H-carbazol-9-yl)picolinamide-catalyzed C-O coupling of (hetero)aryl chlorides with phenols on water. Org Lett. 2024, 26, 7202–7206. [Google Scholar] [CrossRef]
- FrØyen, P. Formation of acyl bromides from carboxylic acids and N-bromosuccinimide; some reactions of bromocyanotriphenylphosphorane. Phosphorus Sulfur Silicon Relat. Elem. 1995, 102, 253–259. [Google Scholar] [CrossRef]
- Wangngae, S.; Duangkamol, C.; Pattarawarapana, M.; Phakhodee, W. Significance of reagent addition sequence in the amidation of carboxylic acids mediated by PPh3 and I2. RSC Adv. 2015, 5, 25789–25793. [Google Scholar] [CrossRef]
- Cui, H.; Wang, L.; Bai, G.; Li, D.; Lin, P. Synthesis of N-Amino-Carbazole. Yingyong Huagong 2006, 35, 295–297. [Google Scholar] [CrossRef]
- Iorkula, T.H.; Tolman, B.A.; Burt, S.R.; Peterson, M.A. An Efficient synthesis of C-6 aminated 3-bromoimidazo [1,2-b]pyridazines. Synth. Comm. 2024, 54, 121–132. [Google Scholar] [CrossRef]

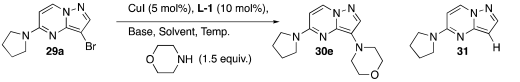 | ||||||
|---|---|---|---|---|---|---|
| Entry | Temp. (°C) | Ligand | Time | Solvent | Base c | Products d |
| 1 | RT | L-1 | 7 days | DEG | K2CO3 | 30e (---) 31 (---) e 29a (100%) |
| 2 | 50 a | L-1 | 2 days | DEG | K2CO3 | 30e (7.7%) 31 (---) e 29a (92.3%) |
| 3 | 70 a | L-1 | 24 h | DEG | K2CO3 | 30e (27.3%) 31 (2.8%); 29a (69.9%) |
| 4 | 80 a | L-1 | 2 days | DEG | K2CO3 | 30e (82.4%) 31 (17.6%); 29a (---) |
| 5 | 70 b | L-1 | 30 min | DEG | K2CO3 | 30e (61.4%) 31 (1.8%); 29a (36.8%) |
| 6 | 70 b | L-1 | 1 h | DEG | K2CO3 | 30e (78.7%) 31 (2.4%); 29a (18.9%) |
| 7 | 70 b | L-1 | 1.5 h | DEG | K2CO3 | 30e (87.2%) 31 (2.1%); 29a (10.7%) |
| 8 | 70 b | L-1 | 2 h | DEG | K2CO3 | 30e (96.7%) 31 (2.5%); 29a (0.8%) |
| 9 | 70 b | L-1 | 3 h | DEG | K2CO3 | 30e (97.3%) 31 (2.7%); 29a (---) |
| 10 | 80 b | L-1 | 30 min | DEG | K2CO3 | 30e (93.7%) 31 (1.9%); 29a (4.4%) |
| 11 | 80 b | L-1 | 1 h | DEG | K2CO3 | 30e (97.1%) 31 (2.9%); 29a (---) |
| 12 | 80 b | L-1 | 1.5 h | DEG | K2CO3 | 30e (97.0%) 31 (3.0%); 29a (---) |
| 13 | 80 b | L-1 | 1 h | Ethylene Glycol | K2CO3 | 30e (90.1%) 31 (9.9%); 29a (---) |
| 14 | 80 b | L-1 | 1 h | DMSO | K2CO3 | 30e (10.2%) 31 (5.1%); 29a (84.7%) |
| 15 | 80 b | L-1 | 1 h | n Butanol | K2CO3 | 30e (3.6%) 31 (6.3%); 29a (90.1%) |
| 16 | 80 b | L-1 | 1 h | Dioxane | K2CO3 | 30e (---) 31 (7.2%); 29a (92.8%) |
| 17 | 80 b | L-1 | 1 h | NMP | K2CO3 | 30e (78%) 31 (19%); 29a (3%) |
| 18 | 80 b | L-1 | 1 h | 1,2-Propanediol | K2CO3 | 30e (94.3%) 31 (5.7%); 29a (---) |
| 19 | 80 b | L-1 | 1 h | DEG | tBuOK | 30e (95.2%) 31 (4.8%); 29a (---) |
| 20 | 80 b | L-1 | 1 h | DEG | TMSONa | 30e (97.1%) 31 (2.9%); 29a (---) |
| 21 | 80 b | L-1 | 1 h | DEG | K3PO4 | 30e (94.3%) 31 (5.7%); 29a (---) |
| 22 | 80 b | L-1 | 1 h | DEG | DBU | 30e (48.8%) 31 (1.4%); 29a (49.8%) |
| 23 | 80 b | L-2 | 1 h | DEG | K2CO3 | 30e (21%) 31 (39%); 29a (40%) |
| 24 | 80 b | L-3 | 1 h | DEG | K2CO3 | 30e (17.4%) 31 (0.7%); 29a (81.9%) |
| 25 | 80 b | L-4 | 1 h | DEG | K2CO3 | 30e (12.3%) 31 (---); 29a (87.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iorkula, T.H.; Tolman, B.A.; Ganiyu, L.O.; Peterson, M.A. An Efficient Synthesis of 3,5-Bis-Aminated Pyrazolo[1,5-a]Pyrimidines: Microwave-Assisted Copper Catalyzed C-3 Amination of 5-Amino-3-Bromo-Substituted Precursors. Molecules 2025, 30, 458. https://doi.org/10.3390/molecules30030458
Iorkula TH, Tolman BA, Ganiyu LO, Peterson MA. An Efficient Synthesis of 3,5-Bis-Aminated Pyrazolo[1,5-a]Pyrimidines: Microwave-Assisted Copper Catalyzed C-3 Amination of 5-Amino-3-Bromo-Substituted Precursors. Molecules. 2025; 30(3):458. https://doi.org/10.3390/molecules30030458
Chicago/Turabian StyleIorkula, Terungwa H., Bryce A. Tolman, Latifat O. Ganiyu, and Matt A. Peterson. 2025. "An Efficient Synthesis of 3,5-Bis-Aminated Pyrazolo[1,5-a]Pyrimidines: Microwave-Assisted Copper Catalyzed C-3 Amination of 5-Amino-3-Bromo-Substituted Precursors" Molecules 30, no. 3: 458. https://doi.org/10.3390/molecules30030458
APA StyleIorkula, T. H., Tolman, B. A., Ganiyu, L. O., & Peterson, M. A. (2025). An Efficient Synthesis of 3,5-Bis-Aminated Pyrazolo[1,5-a]Pyrimidines: Microwave-Assisted Copper Catalyzed C-3 Amination of 5-Amino-3-Bromo-Substituted Precursors. Molecules, 30(3), 458. https://doi.org/10.3390/molecules30030458