Comparison of Extraction, Isolation, Purification, Structural Characterization and Immunomodulatory Activity of Polysaccharides from Two Species of Cistanche
Abstract
1. Introduction
2. Results
2.1. Preparation of Glycans from CD and CT
2.2. Structure Characterization
2.2.1. Structure Characterization of CDP1-5-1
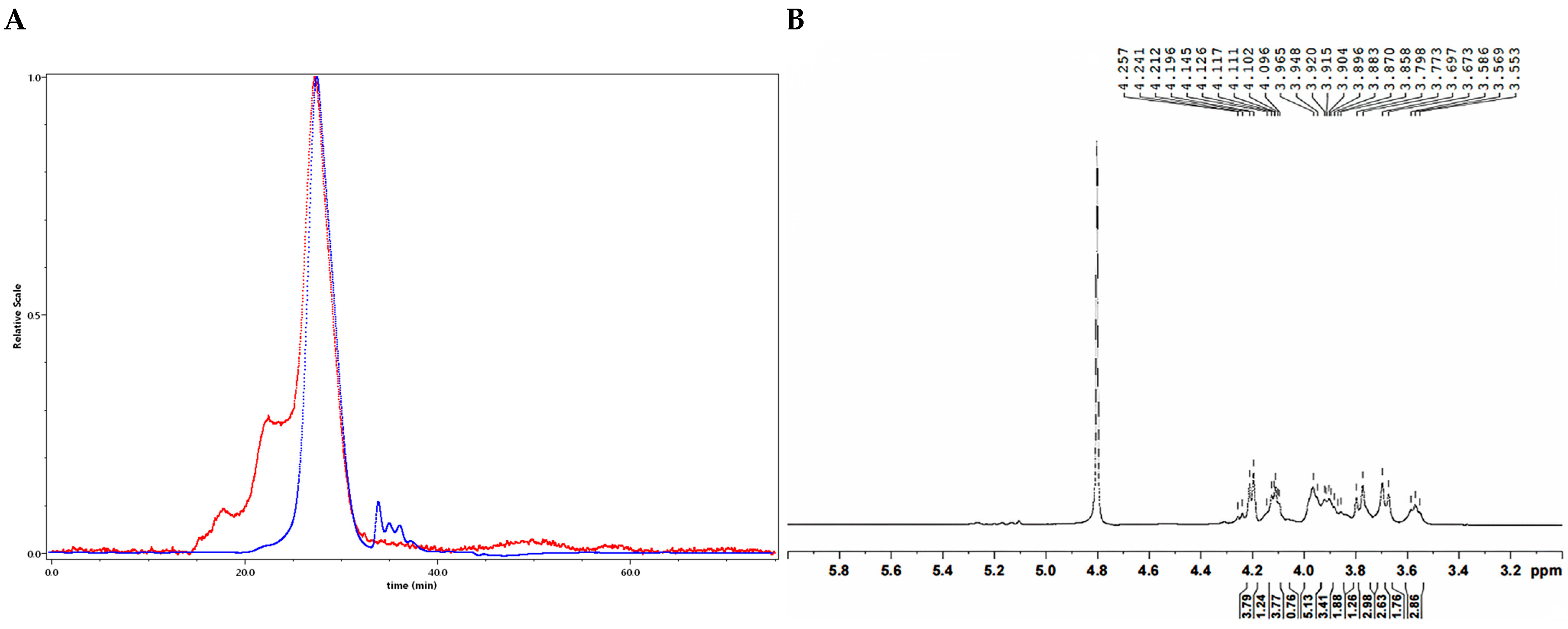
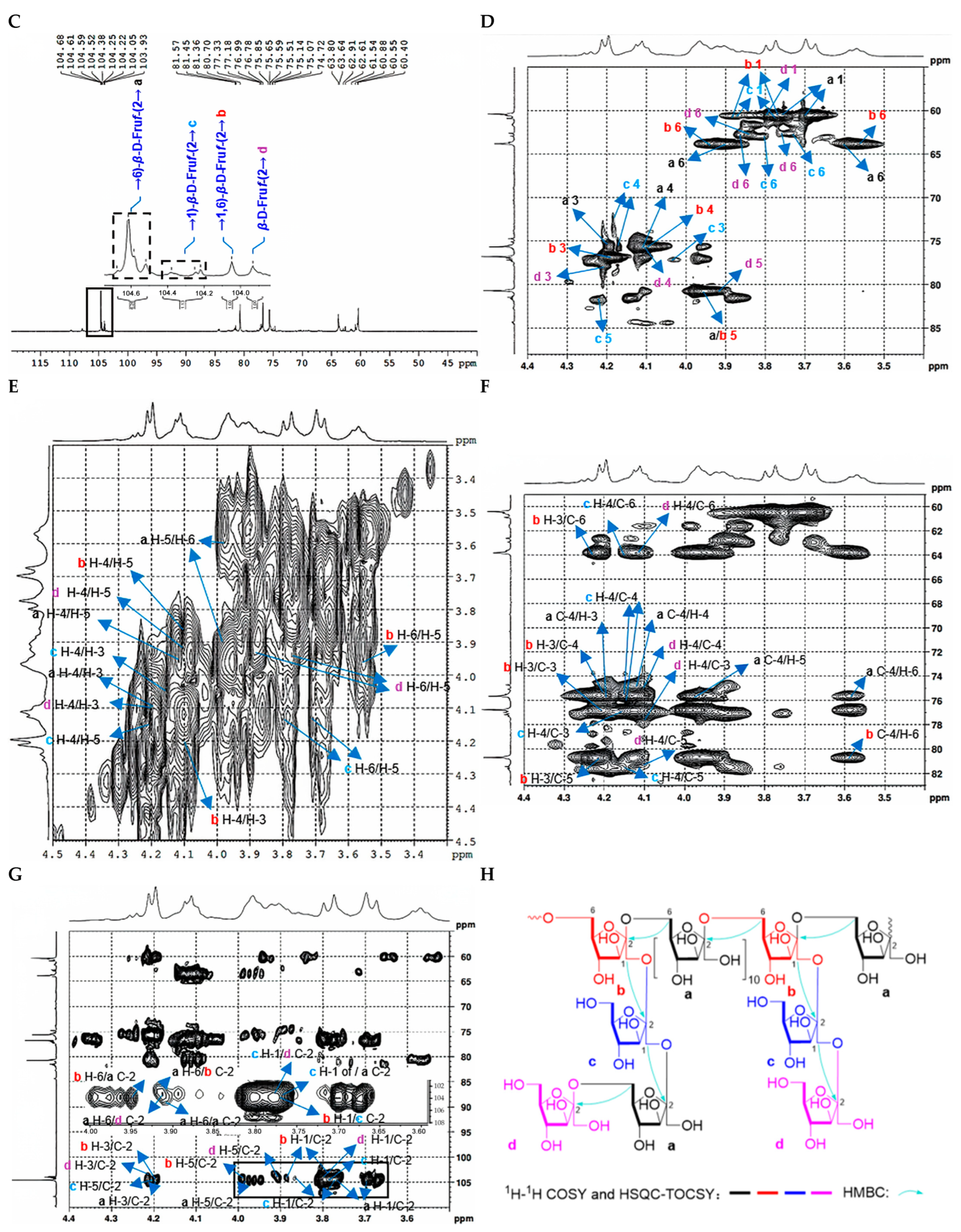
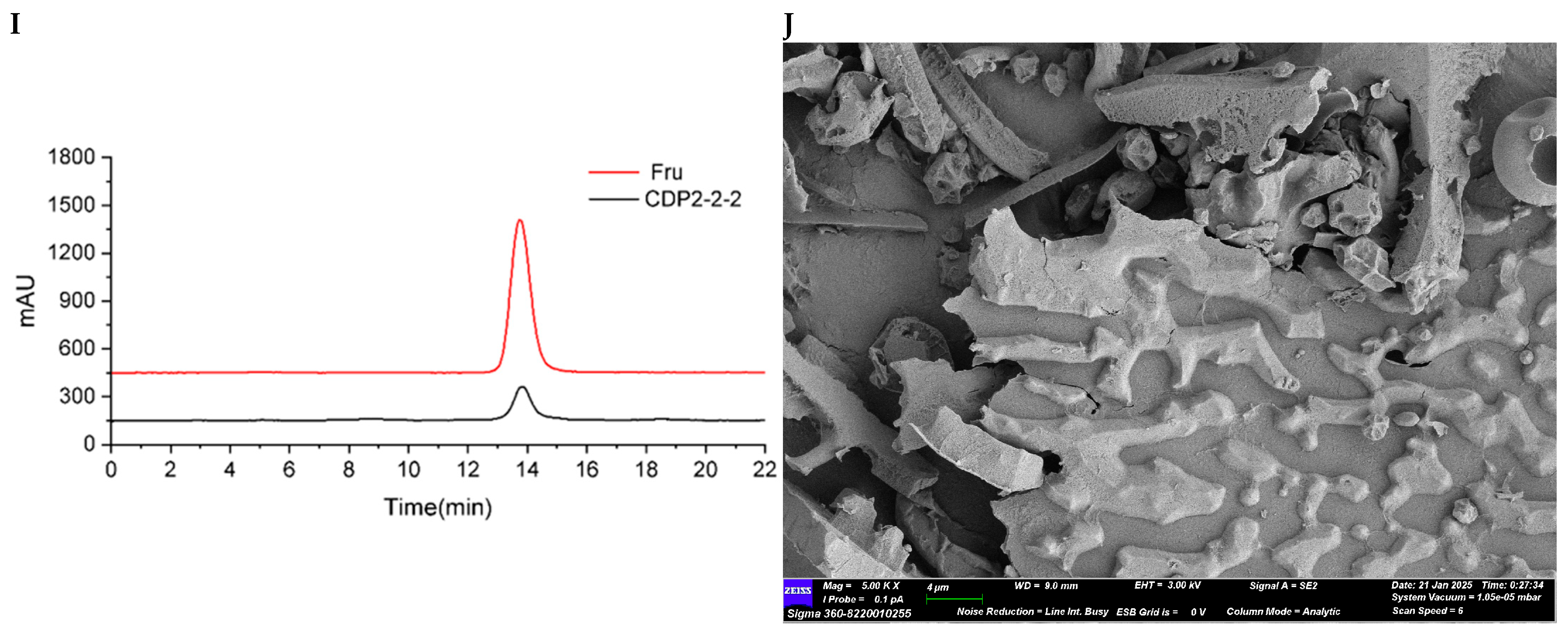
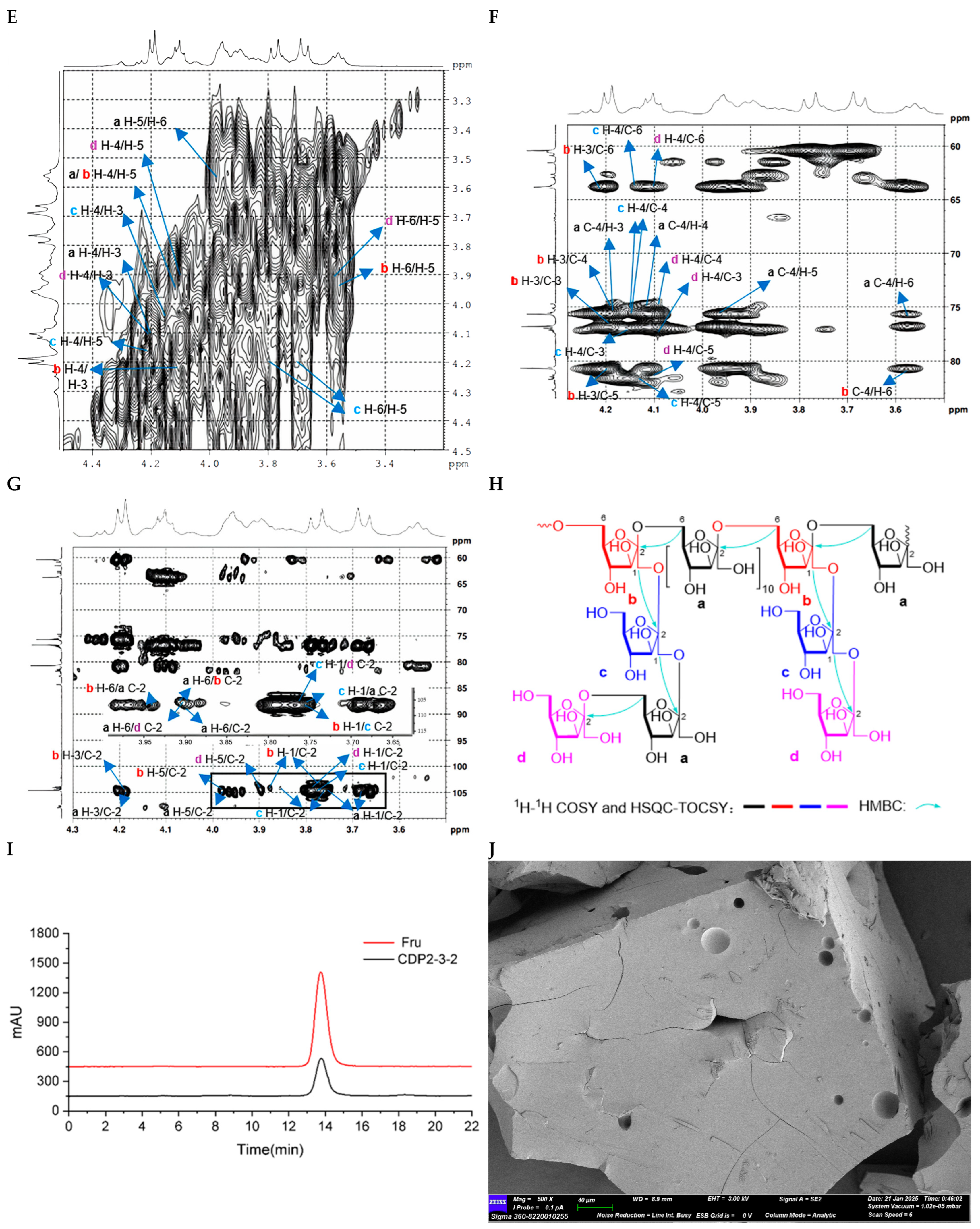
2.2.2. Structure Characterization of CDP2-2-2 and CDP2-3-2
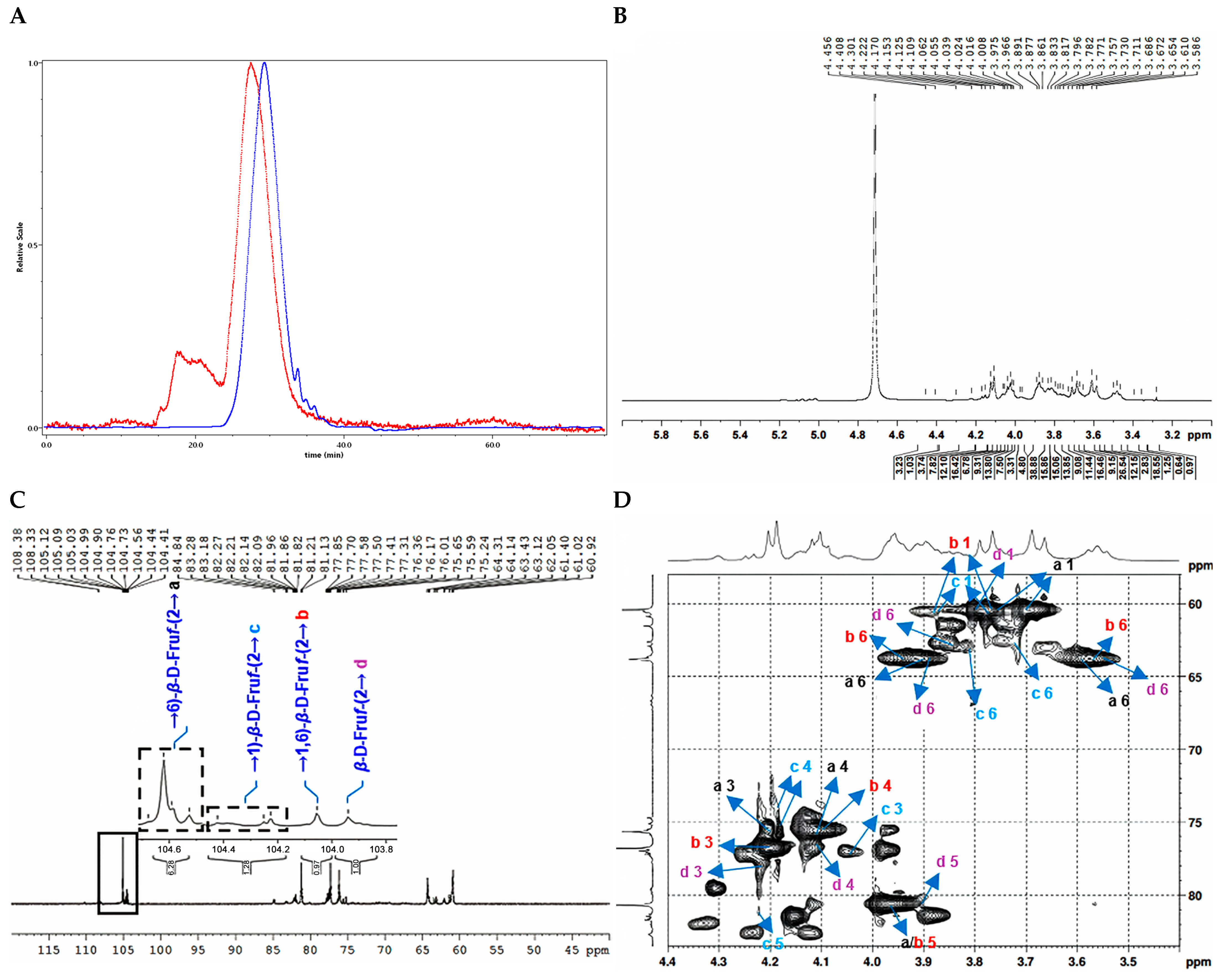
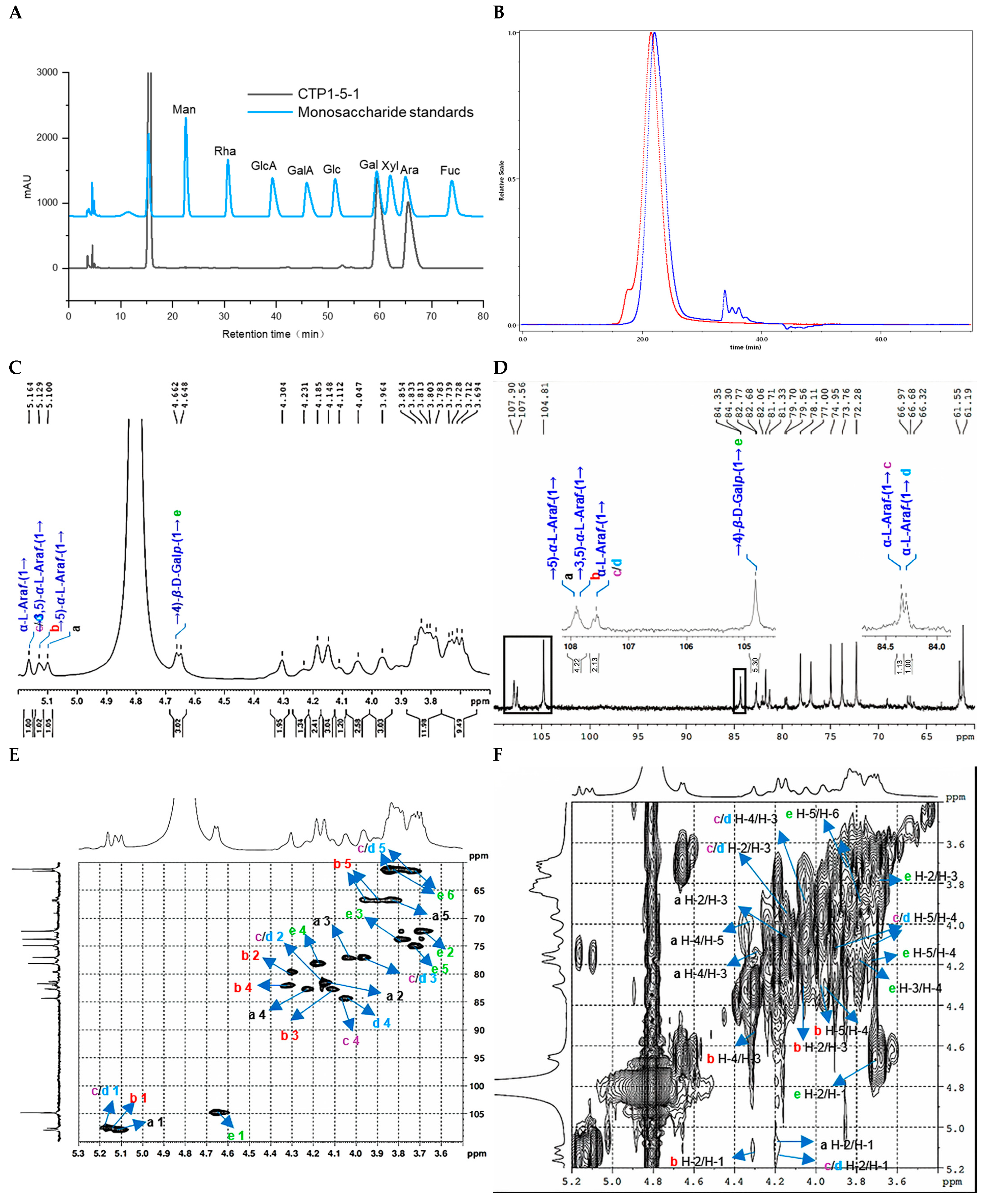
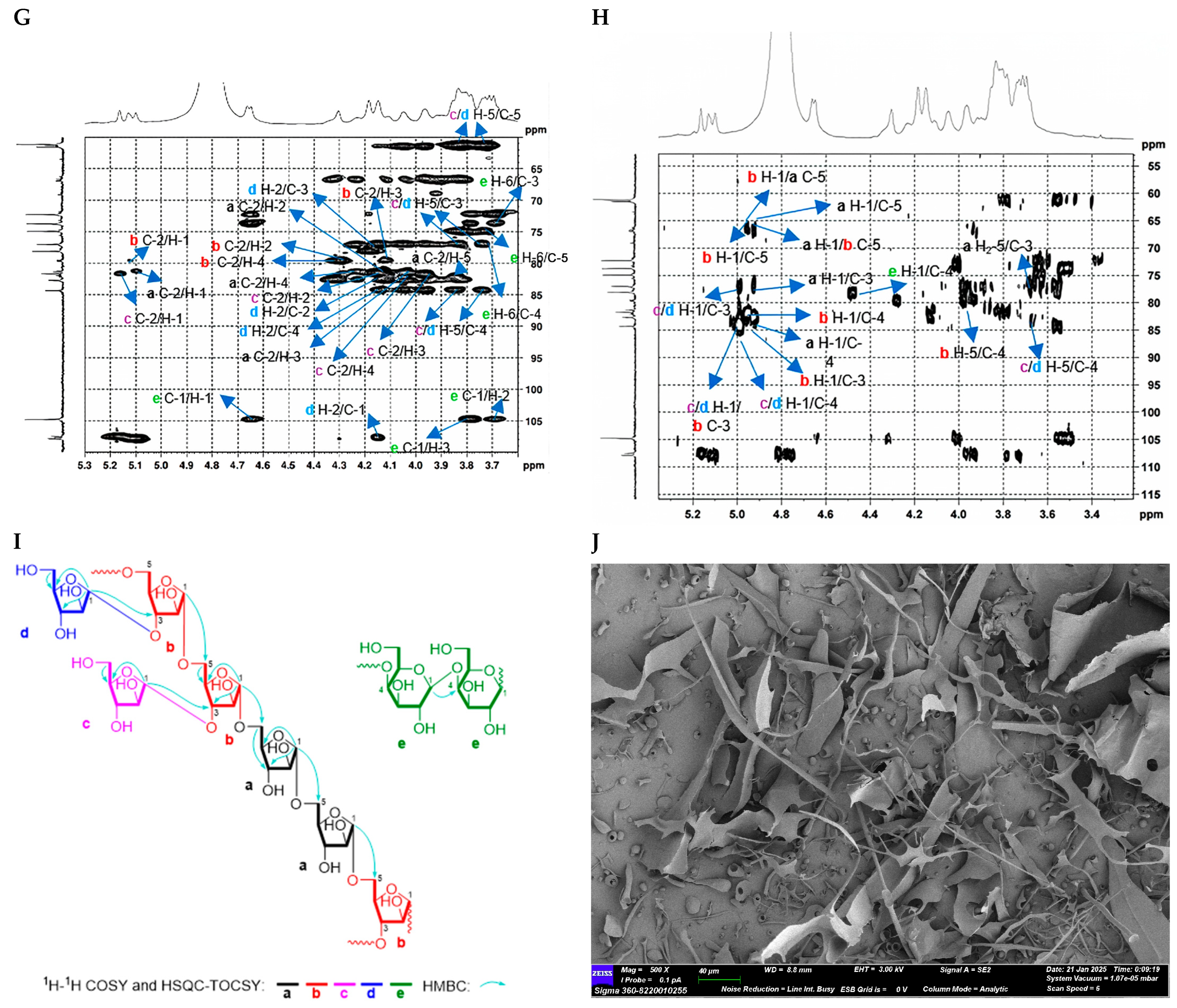
2.2.3. Structure Characterization of CTP1-5-1
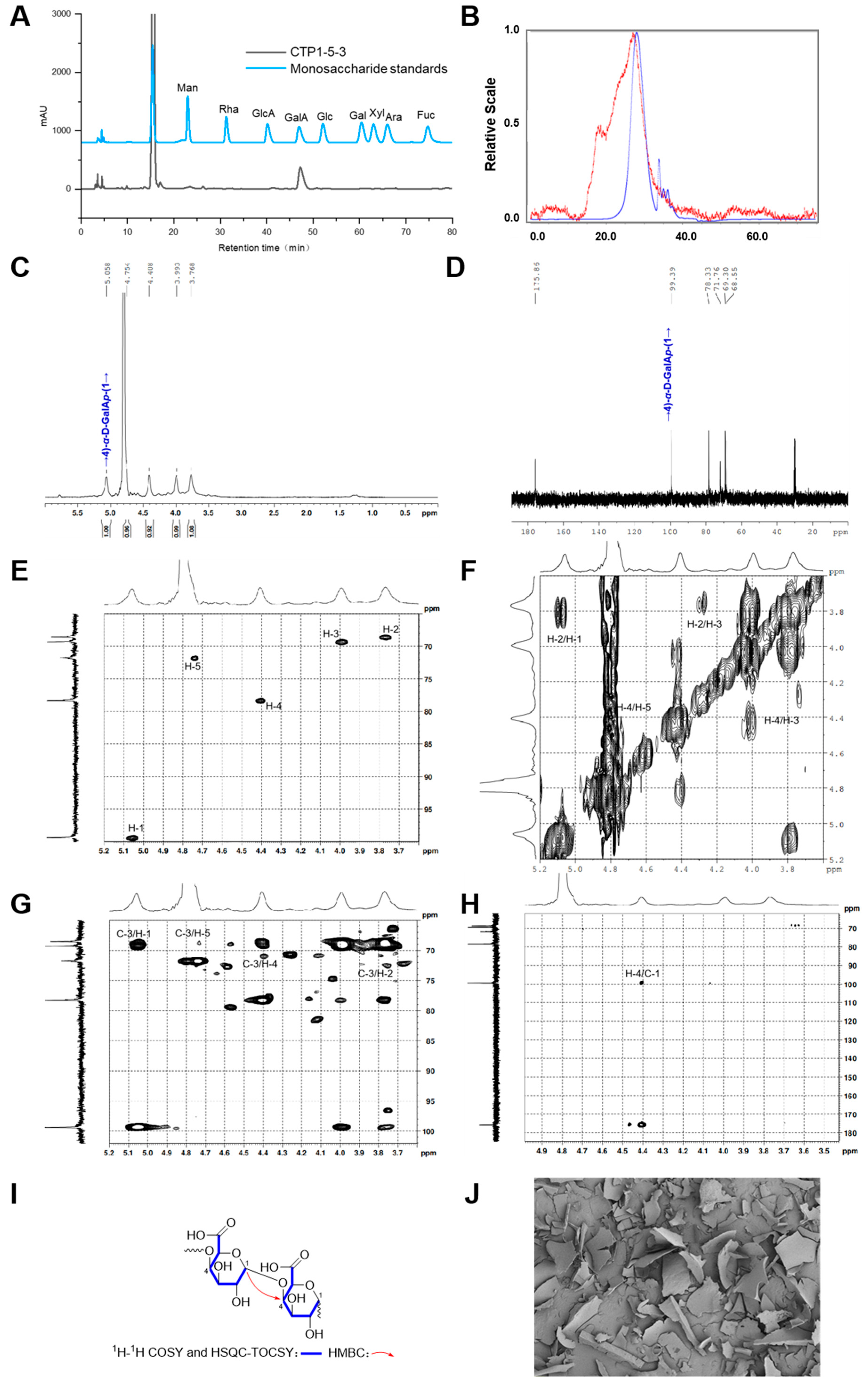
2.2.4. Structure Characterization of CTP1-5-3
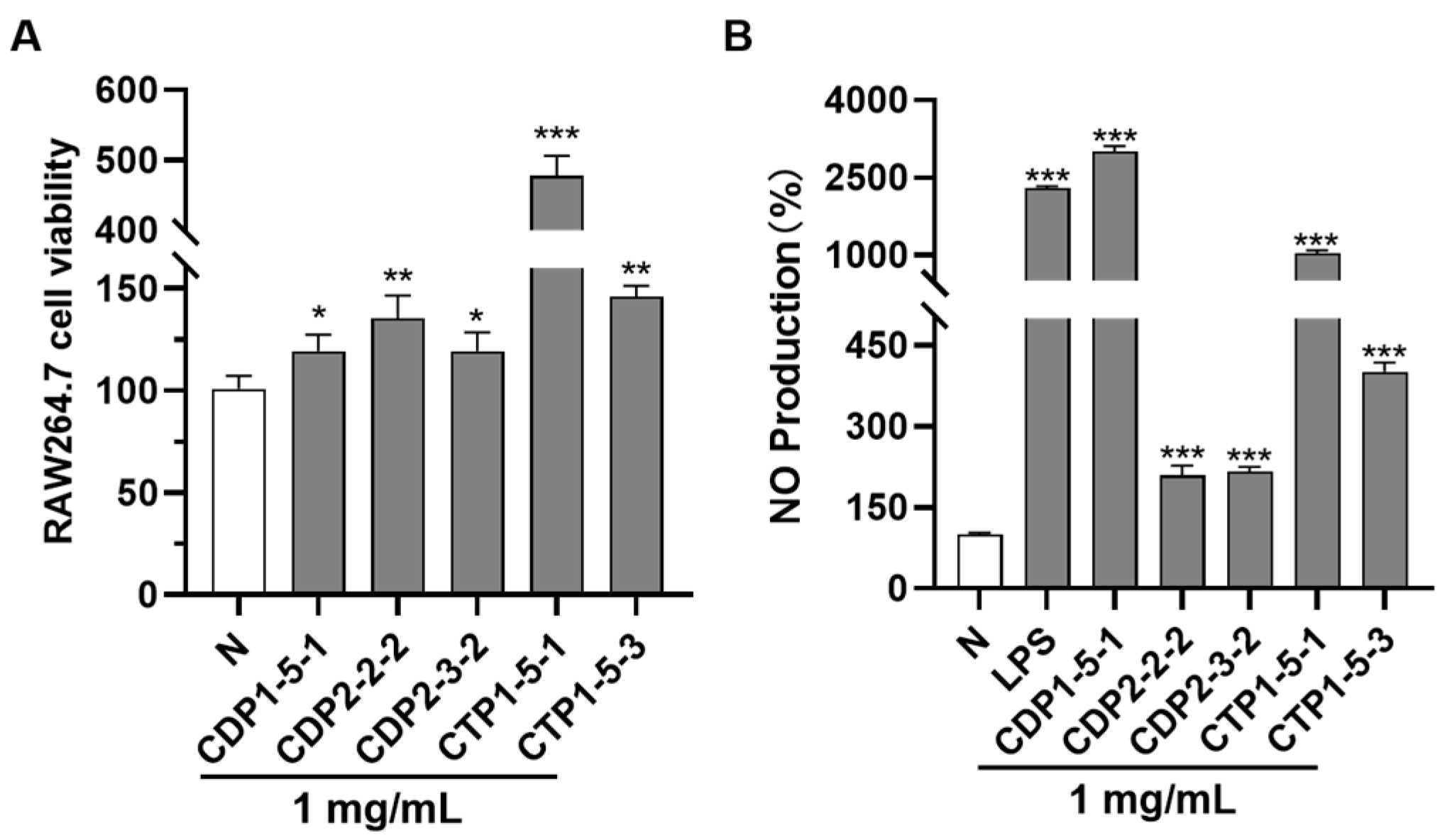
2.3. Evaluation of Immunomodulatory Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction and Purification of the Glycans from C. deserticola and C. tubulosa
4.3. Structural Characterization of CDP1-5-1, CDP2-2-2, CDP2-3-2, CTP1-5-1, and CTP1-5-3
4.3.1. Fourier Transform Infra-Red Spectrometer Analysis
4.3.2. Homogeneity and Molecular Weight Analysis
4.3.3. Monosaccharide Composition Analysis
4.3.4. Methylation Analysis
4.3.5. NMR Spectroscopy Analysis
4.3.6. Experimental Analysis of Congo Red
4.3.7. Scanning Electron Microscopy Analysis
4.4. Regulation of NO Production in Macrophage by CDP1-5-1, CDP2-2-2, CDP2-3-2, CTP1-5-1, and CTP1-5-3
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ara | Arabinose |
| CD | Residue of C. deserticola following 52% alcohol pretreatment |
| CDP | Crude polysaccharides of C. deserticola |
| CT | Residue of C. tubulosa following 52% alcohol pretreatment |
| CTP | Crude polysaccharides of C. Tubulosa |
| DMSO | Dimethyl sulfoxide |
| Fru | Fructose |
| FT-IR | Fourier-transform infrared |
| Gal | Galactose |
| GalA | Galacturonic acid |
| GC-MS | Gas chromatography–mass spectrometry |
| Glc | Glucose |
| GlcA | Glucuronic acid |
| HPAEC-MALLS-RID | High-performance anion-exchange chromatography coupled with multi-angle laser light scattering and refractive index detection |
| HPLC | High-performance liquid chromatography |
| IF | Immunofluorescence |
| Man | Mannose |
| Mn | Number-average molecular weight |
| Mw | Weight-average molecular weight |
| NMR | nuclear magnetic resonance |
| NO | Nitric oxide |
| PDI | Polydispersity index |
| PMAA | Partially methylated alditol acetate |
| PMP | 1-Phenyl-3-methyl-5-pyrazolone |
| Rha | Rhamnose |
| RMS | Root mean square |
| SAR | Structure–activity relationship |
| SEM | Scanning electron microscope |
| TCM | Traditional Chinese medicine |
| TFA | Trifluoroacetic acid |
| TNF-α | Tumor necrosis factor α |
| Xyl | Xylose |
References
- Zhao, W.X.; Wang, T.; Zhang, Y.N.; Chen, Q.; Wang, Y.; Xing, Y.Q.; Zheng, J.; Duan, C.C.; Chen, L.J.; Zhao, H.J.; et al. Molecular mechanism of polysaccharides extracted from Chinese medicine targeting gut microbiota for promoting health. Chin. J. Integr. Med. 2024, 30, 171–180. [Google Scholar] [CrossRef]
- Hou, Y.; Jin, C.; Wen, C.; Ding, K. Research progress on the mechanism of action and targeting of traditional Chinese medicine polysaccharides. Mod. Tradit. Chin. Med. Mater. Medica World Sci. Technol. 2024, 26, 1142–1161. [Google Scholar]
- Wang, W.; Zhao, B.; Zhang, Z.; Kikuchi, T.; Li, W.; Jantrawut, P.; Feng, F.; Liu, F.; Zhang, J. Natural polysaccharides and their derivatives targeting the tumor microenvironment: A review. Int. J. Biol. Macromol. 2024, 268, e131789. [Google Scholar] [CrossRef]
- Jiang, S.; Cui, Y.; Wang, B.; Fu, Z.; Dong, C. Acidic polysaccharides from Cistanche deserticola and their effects on the polarization of tumor-associated macrophages. Int. J. Biol. Macromol. 2024, 282, e137207. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Progr. Mol. Biol. Transl. 2019, 163, 423–444. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2020; Volume 1, p. 126.
- Liu, X.; Yang, Z.; Han, M.; Zhang, Y.; Muhammad, H.; Zhong, H.; Guan, R. Bioactive components, pharmacological properties, and applications of Cistanche deserticola Y. C. Ma: A comprehensive review. Nutrients 2025, 17, 1501. [Google Scholar] [CrossRef]
- Wang, L.; Song, W.; Liu, X.; Qian, S.; Yan, W. Research progress on bioactive components and efficacy of Cistanche deserticola. Shipin Keji 2023, 48, 208–214. [Google Scholar]
- Wang, W.; Liu, C.; Zhao, Y.; Lu, S.; Liu, X.; Dai, X.; Zheng, M.; Cao, Y.; Xia, Q. Preparation, physicochemical properties of Cistanche deserticola polysaccharides and their bidirectional immunomodulatory activity analysis using Caco-2 cell models. Food Res. Int. 2025, 221, e117343. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, H.; Hao, H.; Rahman, F.U.; Zhang, Y. Research progress on polysaccharide components of Cistanche deserticola as potential pharmaceutical agents. Eur. J. Med. Chem. 2023, 245, 114892. [Google Scholar] [CrossRef]
- Mejia-Mendez, J.L.; Reza-Zaldívar, E.E.; Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Navarro-López, D.E.; Marcelo Lozano, L.; Armendariz-Borunda, J.; Tiwari, N.; Jacobo-Velázquez, D.A.; Sanchez-Ante, G.; et al. Exploring the cytotoxic and antioxidant properties of lanthanide-doped ZnO nanoparticles: A study with machine learning interpretation. J. Nanobiotechnol. 2024, 22, e687. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Ji, C.; Liu, X.; Gu, B.; Dong, T. Macrophage polarization in the tumor microenvironment: Emerging roles and therapeutic potentials. Biomed. Pharmacother. 2024, 177, e116930. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Schatz, V.; Hadrian, K.; Hos, D.; Holoborodko, B.; Jantsch, J.; Brigo, N. Macrophage variants in laboratory research: Most are well done, but some are RAW. Front. Cell. Infect. Microbiol. 2024, 14, e1457323. [Google Scholar] [CrossRef]
- Ma, G.F.; Zhao, B.B.; Tang, P.; Tian, D.; Zhao, Y.; Liu, Z.; Chen, L.L. Structural characterization and immunomodulatory activity of polysaccharides from the roots of Artemisia argyi. Carbohydr. Polym. 2025, 368, e124169. [Google Scholar] [CrossRef]
- Xue, H.; Liang, B.; Ji, L.; Li, X.; Wang, M.; Liao, X.; Tan, J. The structure-activity relationship of polysaccharides in fruits and vegetables and interaction between polysaccharides and anthocyanins/proteins: A review. Food Res. Int. 2025, 211, e116371. [Google Scholar]
- Shi, L.; He, Q.; Li, J.; Liu, Y.; Cao, Y.; Liu, Y.; Sun, C.; Pan, Y.; Li, X.; Zhao, X. Polysaccharides in fruits: Biological activities, structures, and structure-activity relationships and influencing factors-A review. Food Chem. 2024, 451, e139408. [Google Scholar]
- Li, Z.; Li, J.; Li, Y.; Guo, L.; Xu, P.; Du, H.; Lin, N.; Xu, Y. The role of Cistanches Herba and its ingredients in improving reproductive outcomes: A comprehensive review. Phytomedicine 2024, 129, e155681. [Google Scholar] [CrossRef]
- Makarova, E.N.; Shakhmatov, E.G. Characterization of pectin-xylan-glucan-arabinogalactan proteins complex from Siberian fir Abies sibirica Ledeb. Carbohydr. Polym. 2021, 260, 117825. [Google Scholar]
- Shao, X. Isolation, Purification, Structural Identification of a Novel Small Molecule Garlic Polysaccharide and Its Anti-Inflammatory Activity. Doctoral Dissertation, South China University of Technology, Guangzhou, China, 2021. [Google Scholar]
- Zhao, P.; Zhou, H.; Zhao, C.; Li, X.; Wang, Y.; Wang, Y.; Huang, L.; Gao, W. Purification, characterization and immunomodulatory activity of fructans from Polygonatum odoratum and P. cyrtonema. Carbohydr. Polym. 2019, 214, 44–52. [Google Scholar] [CrossRef]
- Chandrashekar, P.M.; Prashanth, K.V.; Venkatesh, Y.P. Isolation, structural elucidation and immunomodulatory activity of fructans from aged garlic extract. Phytochemistry 2010, 72, 255–264. [Google Scholar]
- Mancilla-Margalli, N.A.; López, M.G. Water-soluble carbohydrates and fructan structure patterns from Agave and Dasylirion species. J. Agric. Food Chem. 2006, 54, 7832–7839. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cheong, K.L. Preparation, structural characterization, and bioactivities of fructans: A review. Molecules 2023, 28, e1613. [Google Scholar]
- Zhang, J.Y. Structures of Fructan and Galactan from Polygonatum cyrtonema and Their Utilization by Probiotic Bacteria. Master’s Thesis, Chengdu University of traditional Chinese medicine, Chengdu, China, 2021. [Google Scholar]
- Dong, C.X.; Zhang, L.J.; Xu, R.; Zhang, G.; Zhou, Y.B.; Han, X.Q.; Zhang, Y.; Sun, Y.X. Structural characterization and immunostimulating activity of a levan-type fructan from Curcuma kwangsiensis. Int. J. Biol. Macromol. 2015, 77, 99–104. [Google Scholar] [CrossRef]
- Carpita, N.C.; Housley, T.L.; Hendrix, J.E. New features of plant-fructan structure revealed by methylation analysis and carbon-13 NMR spectroscopy. Carbohydr. Res. 1991, 217, 127–136. [Google Scholar] [CrossRef]
- Liu, W.; Li, K.; Zhang, H.; Li, Y.; Lin, Z.; Xu, J.; Guo, Y. An antitumor arabinan from Glehnia littoralis activates immunity and inhibits angiogenesis. Int. J. Biol. Macromol. 2024, 263, e130242. [Google Scholar] [CrossRef]
- Humayun, S.; Rjabovs, V.; Justine, E.E.; Darko, C.N.S.; Howlader, M.M.; Reile, I.; Sim, J.H.; Kim, Y.J.; Tuvikene, R. Immunomodulatory activity of red algal galactans and their partially depolymerized derivatives in RAW264.7 macrophages. Carbohydr. Polym. 2025, 347, e122741. [Google Scholar] [CrossRef]
- Yao, J.; Wan, H.; Zhang, J.; Shen, W.; Wei, X.; Shi, C.; Ou, B.; Liu, D.; Ge, L.; Fei, J.; et al. Tubuloside B, a major constituent of Cistanche deserticola, inhibits migration of hepatocellular carcinoma by inhibiting Hippo-YAP pathway. Phytomedicine 2024, 129, e155552. [Google Scholar] [CrossRef]
- Tao, F.; Zhao, B.; Song, S.; Xu, Y.; Zhang, J. Extraction, purification, structure characterization of polysaccharides from Cistanche deserticola and their biological effects. Food Hydrocoll. Health 2025, 8, e100235. [Google Scholar] [CrossRef]
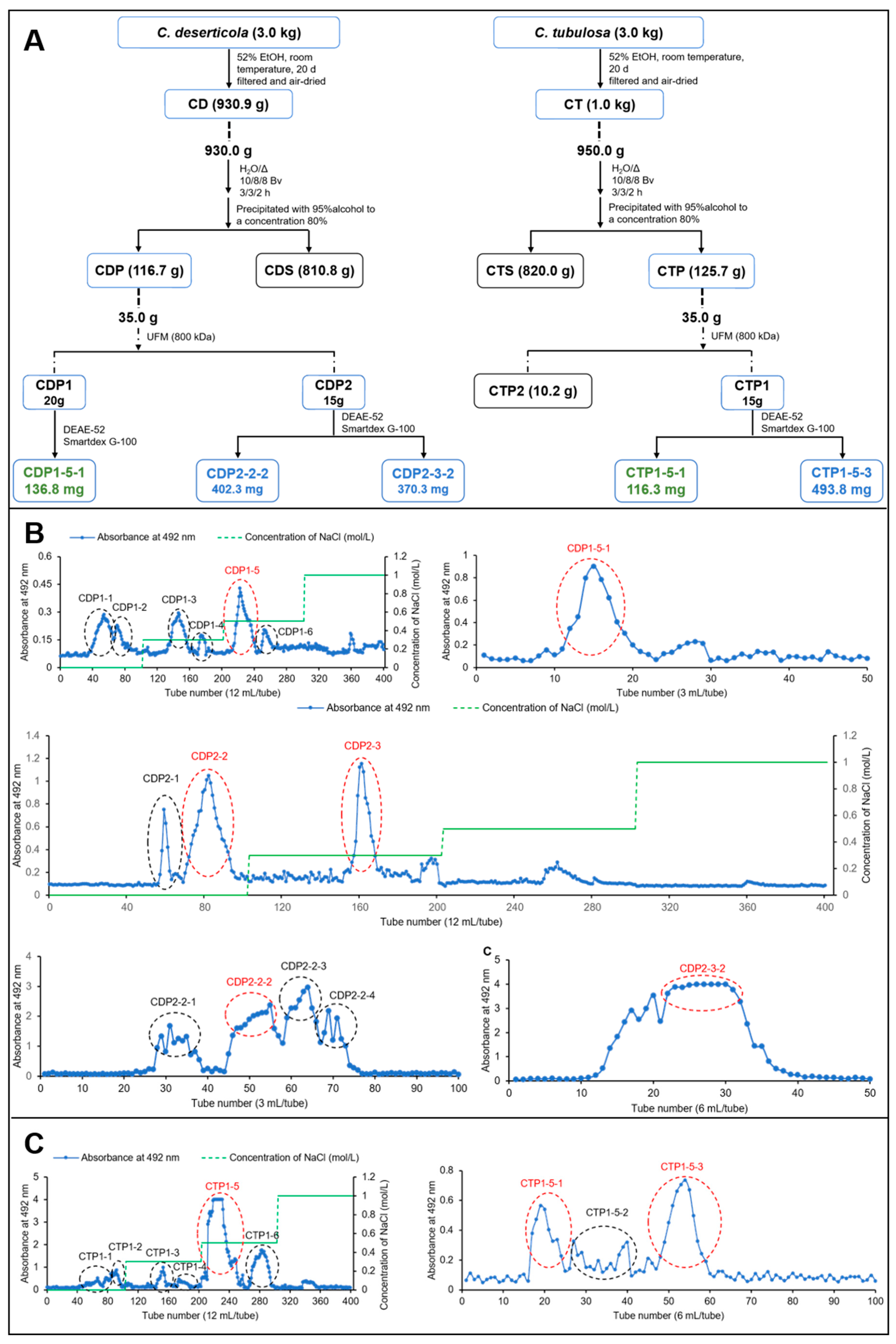
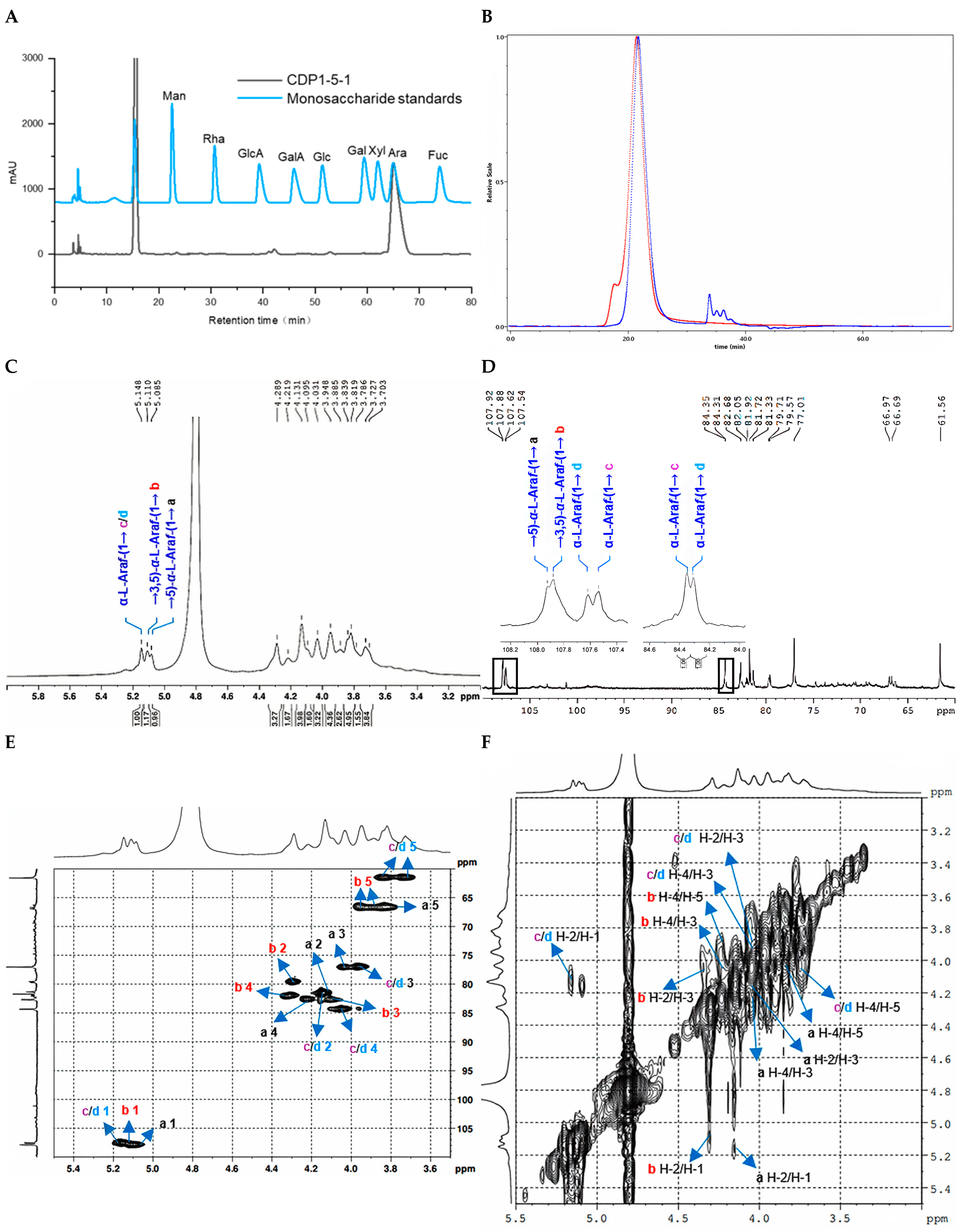
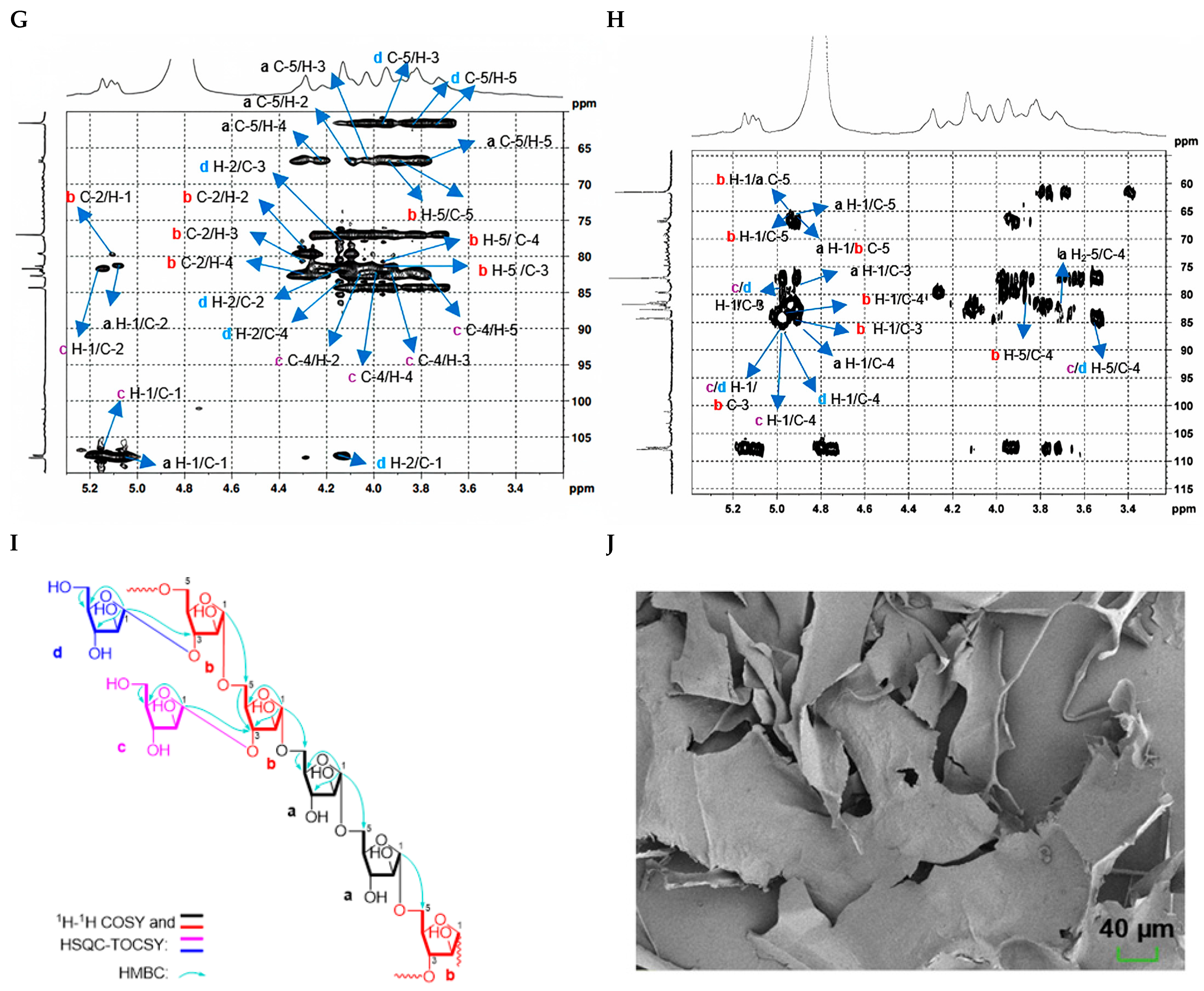
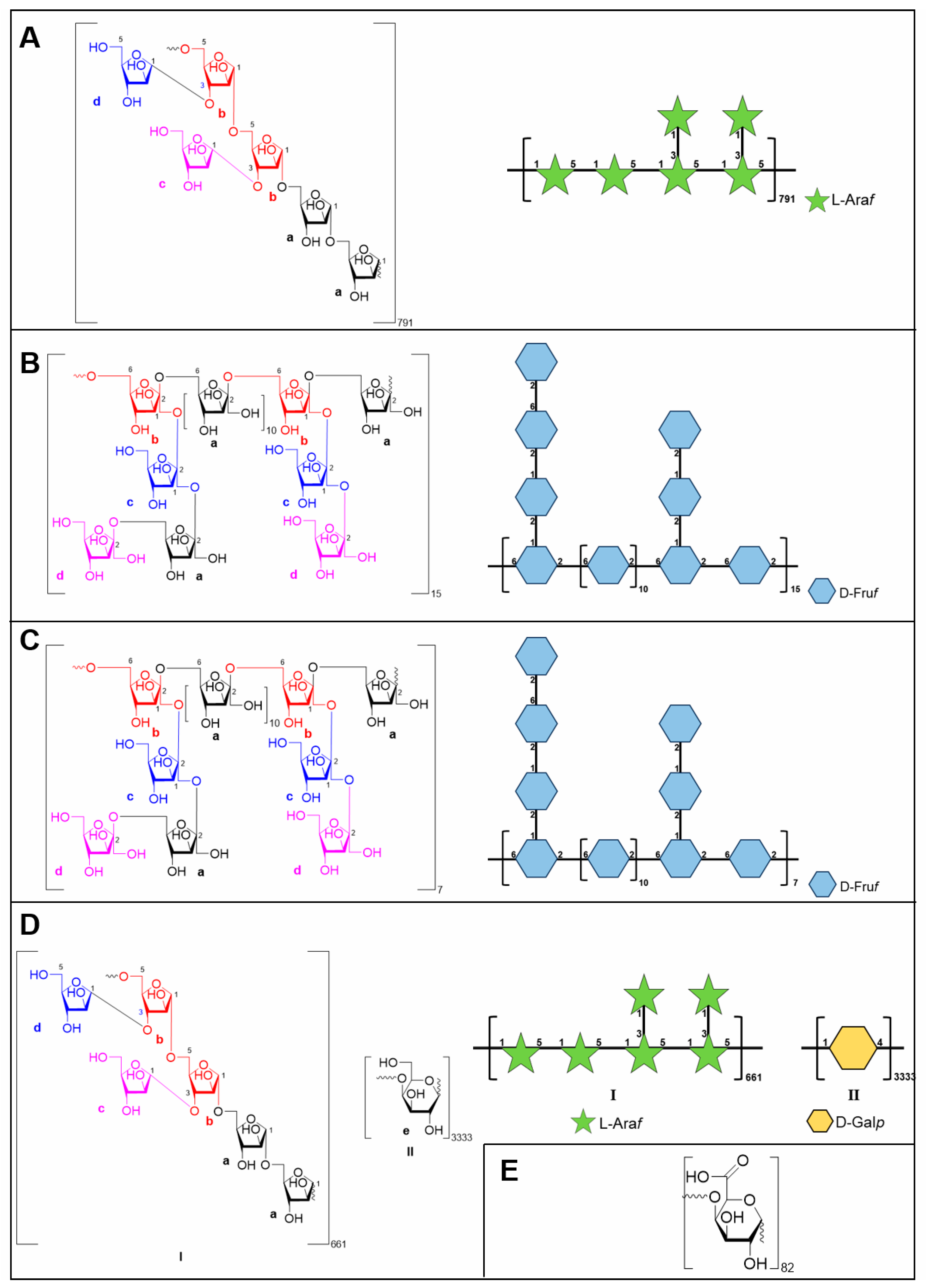
| Polysaccharides | Molecular Characteristics | Parameter | Detection Results |
|---|---|---|---|
| CDP1-5-1 | Polydispersity | Mn Mp Mw Mz | 766.2 kDa 773.5 kDa 852.9 kDa 1020.4 kDa |
| Molar mass moments (g/mol) | Mw/Mn Mz/Mn | 1.1 1.3 | |
| Rms radius moments (nm) | Rn Rw | 28.8 29.3 | |
| CDP2-2-2 | Polydispersity | Mn Mp Mw Mz | 29.7 kDa 27.5 kDa 39.2 kDa 119.2 kDa |
| Molar mass moments (g/mol) | Mw/Mn Mz/Mn | 1.3 4.0 | |
| Rms radius moments (nm) | Rn Rw | 8.1 9.2 | |
| CDP2-3-2 | Polydispersity | Mn Mp Mw Mz | 13.7 kDa 13.1 kDa 19.5 kDa 36.2 kDa |
| Molar mass moments (g/mol) | Mw/Mn Mz/Mn | 1.4 2.6 | |
| Rms radius moments (nm) | Rn Rw | 11.9 11.3 | |
| CTP1-5-1 | Polydispersity | Mn Mp Mw Mz | 640.7 kDa 754.7 kDa 797.1 kDa 1044.7 kDa |
| Molar mass moments (g/mol) | Mw/Mn Mz/Mn | 1.2 1.6 | |
| Rms radius moments (nm) | Rn Rw | 24.9 26.1 | |
| CTP1-5-3 | Polydispersity | Mn Mp Mw Mz | 15.9 kDa 13.1 kDa 20.7 kDa 39.6 kDa |
| Molar mass moments (g/mol) | Mw/Mn Mz/Mn | 1.3 4.0 | |
| Rms radius moments (nm) | Rn Rw | 43.1 43.9 |
| Polysaccharides | Linkage Type | Methylated Sugars | Molar Ratio (%) | tR (min) | Mass Fragments (m/z) |
|---|---|---|---|---|---|
| CDP1-5-1 | t-L-Araf | 1,4-Di-O-acetyl-1-deuterio-2,3,5-tri-O-methyl-D-arabinitol | 1.0 | 25.8 | 59, 71, 87, 102, 118, 129, 145, 161, 162 |
| → 5)-L-Araf-(1 → | 1,4,5-Tri-O-acetyl-1-deuterio-2,3-di-O-methyl-D-arabinitol | 1.2 | 29.3 | 59, 80, 102, 118, 129, 162, 189 | |
| → 3,5)-L-Araf-(1 → | 1,3,4,5-Tetra-O-acetyl-1-deuterio-2-O-methyl-D-arabinitol | 1.3 | 31.1 | 59, 73, 99, 118, 127, 159, 201, 261 | |
| CDP2-2-2 | D-Fruf-(2 → | 2,5-Di-O-acetyl-2-deuterio-1,3,4,6-tetra-O-methyl-D-mannitol | – | 28.8 | 71, 87,101, 129, 145, 161, 162, 186, 205 |
| → 6)-D-Fruf-(2 → | 2,5,6-Tri-O-acetyl-2-deuterio-1,3,4-tri-O-methyl-D-mannitol | – | 32.2 | 57, 87, 129, 146, 162, 173, 189, 206 | |
| → 1)-D-Fruf-(2 → | 1,2,5-Tri-O-acetyl-2-deuterio-3,4,6-tri-O-methyl-D-mannitol | – | 32.3 | 57, 87, 118, 129, 146, 161, 189, 203 | |
| → 1,6)-D-Fruf-(2 → | 1,2,5,6-Tetra-O-acetyl-2-deuterio-3,4-di-O-methyl-D-mannitol | – | 34.5 | 60, 73, 87, 99, 115, 129, 143, 157, 171, 191, 199 | |
| CDP2-3-2 | D-Fruf-(2 → | 2,5-Di-O-acetyl-2-deuterio-1,3,4,6-tetra-O-methyl-D-mannitol | – | 28.8 | 71, 87,101, 129, 145, 161, 162, 186, 205 |
| → 6)-D-Fruf-(2 → | 2,5,6-Tri-O-acetyl-2-deuterio-1,3,4-tri-O-methyl-D-mannitol | – | 32.2 | 57, 87, 129, 146, 162, 173, 189, 206 | |
| → 1)-D-Fruf-(2 → | 1,2,5-Tri-O-acetyl-2-deuterio-3,4,6-tri-O-methyl-D-mannitol | – | 32.3 | 57, 87, 118, 129, 146, 161, 189, 203 | |
| → 1,6)-D-Fruf-(2 → | 1,2,5,6-Tetra-O-acetyl-2-deuterio-3,4-di-O-methyl-D-mannitol | – | 34.5 | 60, 73, 87, 99, 115, 129, 143, 157, 191, 199 | |
| CTP1-5-1 | t-L-Araf | 1,4-Di-O-acetyl-1-deuterio-2,3,5-tri-O-methyl-D-arabinitol | 2.0 | 25.8 | 59, 71, 87, 102, 118, 129, 145, 161, 162 |
| → 5)-L-Araf-(1 → | 1,4,5-Tri-O-acetyl-1-deuterio-2,3-di-O-methyl-D-arabinitol | 2.1 | 29.3 | 59, 80, 102, 118, 129, 162, 189 | |
| → 3,5)-L-Araf-(1 → | 1,3,4,5-Tetra-O-acetyl-1-deuterio-2-O-methyl-D-arabinitol | 2.0 | 31.1 | 59, 73, 99, 118, 127, 159, 201, 261 | |
| → 4)-D-Galp-(1 → | 1,4,5-Tri-O-acetyl-1-deuterio-2,3,6-tri-O-methyl-D-galactitol | 7.9 | 32.4 | 71, 87, 102, 118, 131, 142, 173, 203, 233 | |
| CTP1-5-3 | → 4)-D-Galp-(1 → | 1,4,5-Tri-O-acetyl-1-deuterio-2,3,6-tri-O-methyl-D-galactitol | – | 32.8 | 71, 87, 102, 118, 131, 142, 173, 203, 233 |
| Sugar Residues | Chemical Shifts (δ ppm) | ||||
|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | |
| C-1 | C-2 | C-3 | C-4 | C-5 | |
| → 5)-α-L-Araf-(1 → a | 5.09 | 4.12 | 4.03 | 4.22 | 3.82 |
| 107.9 | 81.3 | 77.0 | 82.8 | 66.7 | |
| → 3,5)-α-L-Araf-(1 → b | 5.11 | 4.29 | 4.10 | 4.30 | 3.95/3.89 |
| 107.9 | 79.6/79.7 | 82.7 | 82.1 | 66.3/67.0 | |
| α-L-Araf-(1 → c | 5.15 | 4.13 | 3.95 | 4.03 | 3.73, 3.84 |
| 107.5 | 81.7 | 77.0 | 84.4 | 61.6 | |
| α-L-Araf-(1 → d | 5.15 | 4.13 | 3.95 | 4.03 | 3.73, 3.84 |
| 107.6 | 81.7 | 77.0 | 84.3 | 61.6 | |
| Sugar Residues | Chemical Shifts (δ ppm) | |||||
|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | |
| C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | |
| → 6)-β-D-Fruf-(2 → a | 3.70, 3.77 | — | 4.20 | 4.11 | 3.97 | 3.59, 3.90 |
| 60.4 | 104.5/104.6 | 76.8 | 75.7 | 80.7 | 63.8 | |
| → 1,6)-β-D-Fruf-(2 → b | 3.77, 3.87 | — | 4.21 | 4.10 | 3.97 | 3.57, 3.95 |
| 60.9 | 104.1 | 77.0 | 75.4 | 80.7 | 63.6 | |
| → 1)-β-D-Fruf-(2 → c | 3.76, 3.86 | — | 4.06 | 4.15 | 4.21 | 3.72, 3.80 |
| 61.5 | 104.2/104.4 | 77.2/77.3 | 74.7/75.1 | 81.4/81.5/81.6 | 62.9/63.0 | |
| β-D-Fruf-(2 → d | 3.77, 3.80 | — | 4.21 | 4.10 | 3.92 | 3.77, 3.87 |
| 60.9 | 103.9 | 77.8 | 75.6 | 81.5 | 62.6 | |
| Sugar Residues | Chemical Shifts (δ ppm) | |||||
|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | |
| C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | |
| → 6)-β-D-Fruf-(2 → a | 3.69, 3.77 | — | 4.19 | 4.10 | 3.96 | 3.58, 3.90 |
| 60.4 | 104.5/104.6/104.7 | 76.8 | 75.8 | 80.7 | 63.8 | |
| → 1,6)-β-D-Fruf-(2 → b | 3.77, 3.87 | — | 4.20 | 4.10 | 3.96 | 3.56, 3.94 |
| 60.5 | 104.1 | 77.0 | 75.5 | 80.7 | 63.7 | |
| → 1)-β-D-Fruf-(2 → c | 3.75, 3.85 | — | 4.05 | 4.12 | 4.19 | 3.71, 3.81 |
| 61.5 | 104.2/104.3/104.4 | 77.0/77.2 | 74.7/75.1 | 81.3/81.5/81.6 | 62.9 | |
| β-D-Fruf-(2 → d | 3.73, 3.84 | — | 4.19 | 4.10 | 3.90 | 3.54, 3.89 |
| 60.9 | 103.9 | 77.8 | 75.7 | 81.6 | 62.6 | |
| Sugar Residues | Chemical Shifts (δ ppm) | |||||
|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | |
| C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | |
| → 5)-α-L-Araf-(1 → a | 5.10 | 4.15 | 4.05 | 4.23 | 3.83 | |
| 107.9 | 81.3 | 77.0 | 82.9 | 66.7 | ||
| → 3,5)-α-L-Araf-(1 → b | 5.13 | 4.30 | 4.11 | 4.31 | 3.96/3.88 | |
| 107.9 | 79.6/79.7 | 82.9 | 82.6 | 66.3/67.0 | ||
| α-L-Araf-(1 → c | 5.16 | 4.15 | 3.96 | 4.05 | 3.74, 3.85 | |
| 107.6 | 81.7 | 77.0 | 84.3 | 61.6 | ||
| α-L-Araf-(1 → d | 5.16 | 4.15 | 3.96 | 4.05 | 3.74, 3.85 | |
| 107.6 | 81.7 | 77.0 | 84.3 | 61.6 | ||
| → 4)-β-D-Galp-(1 → e | 4.66 | 3.69 | 3.78 | 4.19 | 3.73 | 3.71, 3.83 |
| 104.8 | 72.3 | 73.8 | 78.1 | 75.0 | 61.2 | |
| Sugar Residues | Chemical Shifts (δ ppm) | |||||
|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | |
| C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | |
| → 4)-α-D-GalpA-(1 → | 5.06 | 3.77 | 3.99 | 4.41 | 4.75 | — |
| 99.4 | 68.6 | 69.3 | 78.3 | 71.8 | 175.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, J.; Zhang, J.; Yu, L.; Zhang, P.; Chen, A.; Wang, D.; Zhang, Y.; Wang, T. Comparison of Extraction, Isolation, Purification, Structural Characterization and Immunomodulatory Activity of Polysaccharides from Two Species of Cistanche. Molecules 2025, 30, 4754. https://doi.org/10.3390/molecules30244754
Ruan J, Zhang J, Yu L, Zhang P, Chen A, Wang D, Zhang Y, Wang T. Comparison of Extraction, Isolation, Purification, Structural Characterization and Immunomodulatory Activity of Polysaccharides from Two Species of Cistanche. Molecules. 2025; 30(24):4754. https://doi.org/10.3390/molecules30244754
Chicago/Turabian StyleRuan, Jingya, Juan Zhang, Lequan Yu, Ping Zhang, Anxin Chen, Dongmei Wang, Yi Zhang, and Tao Wang. 2025. "Comparison of Extraction, Isolation, Purification, Structural Characterization and Immunomodulatory Activity of Polysaccharides from Two Species of Cistanche" Molecules 30, no. 24: 4754. https://doi.org/10.3390/molecules30244754
APA StyleRuan, J., Zhang, J., Yu, L., Zhang, P., Chen, A., Wang, D., Zhang, Y., & Wang, T. (2025). Comparison of Extraction, Isolation, Purification, Structural Characterization and Immunomodulatory Activity of Polysaccharides from Two Species of Cistanche. Molecules, 30(24), 4754. https://doi.org/10.3390/molecules30244754





