The Effect of the Extraction Method on the Content of Bioactive Compounds and the Biological Activity of Nigella sativa Extracts
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Activity of N. sativa Extracts
2.1.1. Antibacterial Activity
2.1.2. Antifungal Activity
2.2. The Effect of N. sativa Extracts on Seed Germination
2.3. Characterization of Phenolic Composition and Antioxidant Properties of N. sativa Extracts
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Nigella Sativa Sample Extraction
4.2.1. Extraction Methods
4.2.2. Preparation of Extracts for Assays
4.3. Chemicals
4.4. Determination of Antimicrobial Activity of N. sativa Extracts
4.4.1. Indicator Microorganisms
4.4.2. Inoculum Preparation and Standardization
4.4.3. Minimal Inhibitory Concentration (MIC), Minimal Bactericidal Concentration (MBC), and Minimal Fungicidal Concentration (MFC) Determination
4.5. Phenolic Composition
4.6. Determination of Antioxidant Activity (TEAC) of N. sativa Extracts
4.7. The Effect of N. sativa Extracts on Wheat Seed Germination
4.8. Light and Fluorescence Microscopy
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Abd-Elghany, S.M.; Sallam, K.I. Rapid determination of total aflatoxins and ochratoxins A in meat products by immuno-affinity fluorimetry. Food Chem. 2015, 179, 253–256. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Gopane, R.E.; Mwanza, M. Occurrence of filamentous fungi in maize destined for human consumption in South Africa. Food Sci. Nutr. 2018, 6, 884–890. [Google Scholar] [CrossRef]
- Khan, R.; Anwar, F.; Ghazali, F.M. A comprehensive review of mycotoxins: Toxicology, detection, and effective mitigation approaches. Heliyon 2024, 10, e28361. [Google Scholar] [CrossRef] [PubMed]
- Mesterházy, Á.; Oláh, J.; Popp, J. Losses in the grain supply chain: Causes and solutions. Sustainability 2020, 12, 2342. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Foodborne Diseases. Available online: https://www.who.int/health-topics/foodborne-diseases (accessed on 25 October 2025).
- Ajayi, E.I. Food Preservation, Spoilage and Food Adulteration. In Nutrition and Diet in Health; CRC Press: Boca Raton, FL, USA, 2024; pp. 40–53. [Google Scholar]
- Schirone, M.; Visciano, P.; Tofalo, R.; Suzzi, G. Foodborne pathogens: Hygiene and safety. Front. Microbiol. 2019, 10, 1974. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Khan, M.K.I.; Fordos, S.; Hasan, A.; Khalid, S.; Naeem, M.Z.; Usman, A. Emerging foodborne pathogens: Challenges and strategies for ensuring food safety. Biol. Life Sci. Forum 2024, 31, 32. [Google Scholar]
- Ward, L.T.; Hladik, M.L.; Guzman, A.; Winsemius, S.; Bautista, A.; Kremen, C.; Mills, N.J. Pesticide exposure of wild bees and honey bees foraging from field border flowers in intensively managed agriculture areas. Sci. Total Environ. 2022, 831, 154697. [Google Scholar] [CrossRef]
- Silva, M.D.G.C.; Medeiros, A.O.; Converti, A.; Almeida, F.C.G.; Sarubbo, L.A. Biosurfactants: Promising biomolecules for agricultural applications. Sustainability 2024, 16, 449. [Google Scholar] [CrossRef]
- Gullberg, E.; Albrecht, L.M.; Karlsson, C.; Sandegren, L.; Andersson, D.I. Selection of a multidrug resistance plasmid by sublethal levels of antibiotics and heavy metals. mBio 2014, 5, e01918-14. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Zheng, J.S.; Burket, S.R.; Brooks, B.W. Select antibiotics in leachate from closed and active landfills exceed thresholds for antibiotic resistance development. Environ. Int. 2018, 115, 89–96. [Google Scholar] [CrossRef]
- Oulahal, N.; Degraeve, P. Phenolic-rich plant extracts with antimicrobial activity: An alternative to food preservatives and biocides? Front. Microbiol. 2022, 12, 753518. [Google Scholar] [CrossRef] [PubMed]
- Huchchannanavar, S.; Yogesh, L.N.; Prashant, S.M. The black seed Nigella sativa: A wonder seed. Int. J. Chem. Stud. 2019, 7, 1320–1324. [Google Scholar]
- Cheikh-Rouhou, S.; Besbes, S.; Hentati, B.; Blecker, C.; Deroanne, C.; Attia, H. Nigella sativa L.: Chemical composition and physicochemical characteristics of lipid fraction. Food Chem. 2007, 101, 673–681. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Ali, M.A.; Sayeed, M.A.; Alam, M.S.; Yeasmin, M.S.; Khan, A.M.; Muhamad, I.I. Characteristics of oils and nutrient contents of Nigella sativa Linn. and Trigonella foenum-graecum seeds. Bull. Chem. Soc. Ethiop. 2012, 26, 55–64. [Google Scholar] [CrossRef]
- Albakry, Z.; Karrar, E.; Ahmed, I.A.M.; Oz, E.; Proestos, C.; El Sheikha, A.F.; Wang, X. Nutritional composition and volatile compounds of black cumin (Nigella sativa L.) seed, fatty acid composition and tocopherols, polyphenols, and antioxidant activity of its essential oil. Horticulturae 2022, 8, 575. [Google Scholar] [CrossRef]
- Tembhurne, S.V.; Feroz, S.; More, B.H.; Sakarkar, D.M. A review on therapeutic potential of Nigella sativa (kalonji) seeds. J. Med. Plants Res. 2014, 8, 167–177. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Leong, X.F.; Rais Mustafa, M.; Jaarin, K. Nigella sativa and its protective role in oxidative stress and hypertension. Evid.-Based Compl. Alt. Med. 2013, 2013, 120732. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; El Abhar, H.S.; Saleh, S.; Abdallah, D.M. The anti-inflammatory effects of Nigella sativa oil in models of pain and inflammation. J. Ethnopharmacol. 2022, 250, 112482. [Google Scholar]
- Forouzanfar, F.; Bazzaz, B.S.F.; Hosseinzadeh, H. Black cumin (Nigella sativa) and its constituent (thymoquinone): A review on antimicrobial effects. Iran. J. Basic Med. Sci. 2014, 17, 929–938. [Google Scholar]
- Hannan, A.; Saleem, S.; Chaudhary, S.; Barkaat, M.; Arshad, M.U. Anti bacterial activity of Nigella sativa against clinical isolates of methicillin resistant Staphylococcus aureus. J. Ayub Med. Coll. Abbottabad 2008, 20, 72–74. [Google Scholar]
- Dar, I.H.; Junaid, P.M.; Ahmad, S.; Shams, R.; Dash, K.K.; Shaikh, A.M.; Béla, K. Optimization of ultrasound-assisted extraction of Nigella sativa seed oil for enhancement of yield and antioxidant activity. Discov. Appl. Sci. 2024, 6, 104. [Google Scholar] [CrossRef]
- Khoddami, A.; Ghazali, H.M.; Yassoralipour, A.; Ramakrishnan, Y.; Ganjloo, A. Physicochemical characteristics of nigella seed (Nigella sativa L.) oil as affected by different extraction methods. J. Am. Oil Chem. Soc. 2011, 88, 533–540. [Google Scholar] [CrossRef]
- Chung, K.X.; Lee, Y.W.; Akowuah, G. Different extraction methods for thymoquinone from Nigella sativa L. seeds and antioxidant activity. Indian J. Nat. Prod. Resour. 2023, 14, 1134–1142. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Ahmed, M.M.; Rafea, M.A. Optimization of ultrasound assisted extraction of phenolic compounds from Nigella sativa seeds using response surface methodology. J. Food Process. Preserv. 2023, 47, e16945. [Google Scholar]
- Pan, Y.; Wang, K.; Huang, S.; Wang, H.; Mu, X.; He, C.; Huang, F. Antioxidant activity of microwave-assisted extract of longan (Dimocarpus longan Lour.) peel. Food Chem. 2008, 106, 1264–1270. [Google Scholar] [CrossRef]
- Gawron, G.; Krzyczkowski, W.; Łyżeń, R.; Kadziński, L.; Banecki, B. Influence of supercritical carbon dioxide extraction conditions on extraction yield and composition of Nigella sativa L. seed oil—Modelling, optimization and extraction kinetics regarding fatty acid and thymoquinone content. Molecules 2021, 26, 6419. [Google Scholar] [CrossRef] [PubMed]
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; European Commission: Brussels, Belgium, 2020; 23p. [Google Scholar]
- Moreira-Dantas, I.R.; Martínez-Zarzoso, I.; Torres-Munguía, J.A. Sustainable food chains to achieve SDG-12 in Europe: Perspectives from multi-stakeholders initiatives. In SDGs in the European Region; Springer International Publishing: Cham, Switzerland, 2023; pp. 315–340. [Google Scholar]
- Ncube, L.J. Strategies for Ensuring Food Protection From Farm to Fork. In Managing Food Safety and Safeguarding Consumer Health; IntechOpen: London, UK, 2025. [Google Scholar]
- Linke, B.G.; Casagrande, T.A.; Cardoso, L.A. Food additives and their health effects: A review on preservative sodium benzoate. Afr. J. Biotechnol. 2018, 17, 306–310. [Google Scholar] [CrossRef]
- Jayant, D.; Halami, P.M. Industrial perspective of food preservatives from microbial origin. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–261. [Google Scholar]
- Silva, V.; Yang, X.; Fleskens, L.; Ritsema, C.J.; Geissen, V. Environmental and human health at risk–Scenarios to achieve the Farm to Fork 50% pesticide reduction goals. Environ. Int. 2022, 165, 107296. [Google Scholar] [CrossRef]
- Haseena, S.; Aithal, M.; Das, K.K.; Saheb, S.H. Phytochemical analysis of Nigella sativa and its effect on reproductive system. J. Pharm. Sci. Res. 2015, 7, 514. [Google Scholar]
- Pop, R.M.; Vassilopoulou, E.; Jianu, M.E.; Roșian, Ș.H.; Taulescu, M.; Negru, M.; Buzoianu, A.D. Nigella sativa oil attenuates inflammation and oxidative stress in experimental myocardial infarction. BMC Complement. Med. Ther. 2024, 24, 362. [Google Scholar] [CrossRef]
- Hegazy, E.; Haggag, T.; Elmansy, M. The Protective role of Nigella sativa versus Lepidium sativum on the submandibular salivary gland in hypercholesterolemic albino rat (Histological and Immunohistochemical study). Egypt. Dent. J. 2021, 67, 3113–3125. [Google Scholar] [CrossRef]
- Abbas, M.; Gururani, M.A.; Ali, A.; Bajwa, S.; Hassan, R.; Batool, S.W.; Wei, D. Antimicrobial properties and therapeutic potential of bioactive compounds in Nigella sativa: A review. Molecules 2024, 29, 4914. [Google Scholar] [CrossRef]
- Ahmadian, S.; Kenari, R.E.; Amiri, Z.R.; Sohbatzadeh, F.; Khodaparast, M.H.H. Effect of ultrasound-assisted cold plasma pretreatment on cell wall polysaccharides distribution and extraction of phenolic compounds from hyssop (Hyssopus officinalis L.). Int. J. Biol. Macromol. 2023, 233, 123557. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Qin, L. The cause of germination increases the phenolic compound contents of Tartary buckwheat (Fagopyrum tataricum). J. Future Foods 2022, 2, 372–379. [Google Scholar] [CrossRef]
- Cañas, S.; Rebollo-Hernanz, M.; Braojos, C.; Benítez, V.; Ferreras-Charro, R.; Dueñas, M.; Aquilera, Y.; Martín-Cabrejas, M.A. Gastrointestinal fate of phenolic compounds and amino derivatives from the cocoa shell: An in vitro and in silico approach. Food Res. Int. 2022, 162, 112117. [Google Scholar] [CrossRef] [PubMed]
- Alshuniaber, M.A.; Krishnamoorthy, R.; AlQhtani, W.H. Antimicrobial activity of polyphenolic compounds from Spirulina against food-borne bacterial pathogens. Saudi J. Biol. Sci. 2021, 28, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Ulate, C.; Rodríguez-Sánchez, C.; Arias-Echandi, M.L.; Esquivel, P. Antimicrobial activities of phenolic extracts of coffee mucilage. NFS J. 2023, 31, 50–56. [Google Scholar] [CrossRef]
- Tian, Q.M.; Wei, S.; Su, H.R.; Zheng, S.M.; Xu, S.Y.; Liu, M.J.; Bo, R.N.; Li, J.G. Bactericidal activity of gallic acid against multi-drug resistance Escherichia coli. Microb. Pathog. 2022, 173, 10. [Google Scholar] [CrossRef]
- Zheng, X.; Al Naggar, Y.; Wu, Y.; Liu, D.; Hu, Y.; Wang, K.; Jin, X.; Peng, W. Untargeted metabolomics description of propolis’s in vitro antibacterial mechanisms against Clostridium perfringens. Food Chem. 2023, 406, 135061. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Macedo, N.S.; de Sousa Junior, D.L.; dos Santos, C.R.; Tintino, S.R.; da Hora, G.C.; da Cunha, F.A. Indirect inhibitory activity of pyrogallol against the Tet (K) efflux pump by a membrane effect: In vitro and in silico approach. Process Biochem. 2021, 107, 138–144. [Google Scholar] [CrossRef]
- Pandit, R.S.; Gaikwad, S.C.; Agarkar, G.A.; Gade, A.K.; Rai, M. Curcumin nanoparticles: Physico-chemical fabrication and its in vitro efficacy against human pathogens. 3 Biotech 2015, 5, 991–997. [Google Scholar] [CrossRef]
- Sadeghi, A.; Rajabiyan, A.; Nabizade, N.; Nezhad, N.M.; Zarei-Ahmady, A. Seaweed-derived phenolic compounds as diverse bioactive molecules: A review on identification, application, extraction and purification strategies. Int. J. Biol. Macromol. 2024, 266, 131147. [Google Scholar] [CrossRef] [PubMed]
- Saleh, F.A.; El-Darra, N.; Raafat, K.; El Ghazzawi, I. Phytochemical analysis of Nigella sativa L. Utilizing GC-MS exploring its antimicrobial effects against multidrug-resistant bacteria. Pharmacogn. J. 2018, 10, 99–105. [Google Scholar] [CrossRef]
- Shafodino, F.S.; Lusilao, J.M.; Mwapagha, L.M. Phytochemical characterization and antimicrobial activity of Nigella sativa seeds. PLoS ONE 2022, 17, e0272457. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 2003, 52, 750–763. [Google Scholar] [CrossRef]
- Naz, S.; Ahmad, S.; Rasool, S.A.; Sayeed, S.A.; Siddiqi, R. Antibacterial activity directed isolation of compounds from Onosma hispidum. Microbiol. Res. 2006, 161, 43–48. [Google Scholar] [CrossRef]
- Zouirech, O.; Alyousef, A.A.; El Barnossi, A.; El Moussaoui, A.; Bourhia, M.; Salamatullah, A.M.; Derwich, E. Phytochemical analysis and antioxidant, antibacterial, and antifungal effects of essential oil of black caraway (Nigella sativa L.) seeds against drug-resistant clinically pathogenic microorganisms. BioMed Res. Int. 2022, 2022, 5218950. [Google Scholar] [CrossRef]
- Khan, M.A.U.; Ashfaq, M.K.; Zuberi, H.S.; Mahmood, M.S.; Gilani, A.H. The in vivo antifungal activity of the aqueous extract from Nigella sativa seeds. Phytother. Res. 2003, 17, 183–186. [Google Scholar] [CrossRef]
- Egbe, N. Antifungal effects of Nigella sativa L. (Black cumin) seed extracts and seed oil on selected Candida albicans strains. J. Curr. Biomed. Res. 2023, 3, 993–1004. [Google Scholar] [CrossRef]
- Akansha Kaushal, S.; Arora, A.; Heena Sharma, P.; Jangra, R. Chemical composition and synergistic antifungal potential of Nigella sativa L. seeds and Syzygium aromaticum (L.) Merr. & LM Perry buds essential oils and their major compounds, and associated molecular docking studies. J. Essential Oil-Bear. Plants 2023, 26, 602–625. [Google Scholar]
- Aljabre, S.H.M.; Randhawa, M.A.; Akhtar, N.; Alakloby, O.M.; Alqurashi, A.M.; Aldossary, A. Antidermatophyte activity of ether extract of Nigella sativa and its active principle, thymoquinone. J. Ethnopharmacol. 2005, 101, 116–119. [Google Scholar] [CrossRef]
- Feroz, S.; Uddin, G. Phytochemical analysis, antimicrobial and antioxidant study of Nigella sativa L. Int. J. Pharm. Chem. 2016, 2, 39–43. [Google Scholar]
- Mohamed, M.S.N.; Jaikumar, K.; Babu, A.; Anand, D.; Saravanan, P. A study on the in vitro antifungal activity of Nigella sativa (Linn.) seed extract and it’s phytochemical screening using GC-MS analysis. World J. Pharm. Pharm. Sci. 2015, 4, 1003–1011. [Google Scholar]
- Muzolf-Panek, M.; Gliszczyńska-Świgło, A. Extraction optimization for the antioxidants from Nigella sativa seeds using response surface methodology. J. Food Meas. Charact. 2022, 16, 4741–4753. [Google Scholar] [CrossRef]
- Yasmeen, H.; Hassnain, S. Comparative analysis of different bioactivities of Curcuma longa, Nigella sativa seeds, and Camellia sinensis extracted by four different methods: A green way to reduce oxidative stress. Food Sci. Biotechnol. 2016, 25, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.K.; Abd Manap, M.Y.; Tan, C.P.; Muhialdin, B.J.; Alhelli, A.M.; Meor Hussin, A.S. The effects of different extraction methods on antioxidant properties, chemical composition, and thermal behavior of black seed (Nigella sativa L.) oil. Evid. Based Complement. Alternat. Med. 2016, 2016, 6273817. [Google Scholar] [CrossRef] [PubMed]
- Bakhshabadi, H.; Mirzaei, H.; Ghodsvali, A.; Jafari, S.M.; Ziaiifar, A.M. The influence of pulsed electric fields and microwave pretreatments on some selected physicochemical properties of oil extracted from black cumin seed. Food Sci. Nutr. 2018, 6, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Sanket, S.; Sharma, P.K.; Mani, I.; Nain, L.; Satheesh, N. Optimization of ohmic parameters in enzyme assisted aqueous extraction for better physico-chemical properties of the black cumin seed oil. Ind. Crops Prod. 2024, 208, 117892. [Google Scholar] [CrossRef]
- Soleimanifar, M.; Niazmand, R.; Jafari, S.M. Evaluation of oxidative stability, fatty acid profile, and antioxidant properties of black cumin seed oil and extract. J. Food Meas. Character. 2019, 13, 383–389. [Google Scholar] [CrossRef]
- Hameed, S.; Imran, A.; Nisa, M.U.; Arshad, M.S.; Saeed, F.; Arshad, M.U.; Asif Khan, M. Characterization of extracted phenolics from black cumin (Nigella sativa linn), coriander seed (Coriandrum sativum L.), and fenugreek seed (Trigonella foenum-graecum). Int. J. Food Prop. 2019, 22, 714–726. [Google Scholar] [CrossRef]
- Krimer Malešević, V.; Vaštag, Ž.; Popović, L.; Popović, S.; Peričin-Starčevič, I. Characterisation of black cumin, pomegranate and flaxseed meals as sources of phenolic acids. Int. J. Food Sci. Technol. 2014, 49, 210–216. [Google Scholar] [CrossRef]
- Feng, Y.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF/MS characterization of bioactive compounds from black spices and their potential antioxidant activities. J. Food Sci. Technol. 2020, 57, 4671–4687. [Google Scholar] [CrossRef]
- Sun, L.; Ren, J.; Feng, X.; Li, S.; Wang, Y.; Jiang, Y.; Zheng, C. Caffeic Acid Markedly Induced Apoptosis of Human Multiple Myeloma Cells through the Caspase-dependent Pathway. Pharmacogn. Mag. 2023, 19, 720–726. [Google Scholar] [CrossRef]
- Cheng, A.W.; Tan, X.; Sun, J.Y.; Gu, C.M.; Liu, C.; Guo, X. Catechin attenuates TNF-α induced inflammatory response via AMPK-SIRT1 pathway in 3T3-L1 adipocytes. PLoS ONE 2019, 14, e0217090. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Sarkaki, A.; Rashno, M.; Farbood, Y. Memory deficits and hippocampal inflammation in cerebral hypoperfusion and reperfusion in male rats: Neuroprotective role of vanillic acid. Life Sci. 2018, 211, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Hossen, J.; Ali, M.A.; Reza, S. Theoretical investigations on the antioxidant potential of a non-phenolic compound thymoquinone: A DFT approach. J. Mol. Model. 2021, 27, 173. [Google Scholar] [CrossRef]
- Kassab, R.B.; El-Hennamy, R.E. The role of thymoquinone as a potent antioxidant in ameliorating the neurotoxic effect of sodium arsenate in female rat. Egypt. J. Basic Appl. Sci. 2017, 4, 160–167. [Google Scholar] [CrossRef]
- Erdoğan, Ü.; Erbaş, S.; Muhammed, M.T.; Onem, E.; Soyocak, A.; Ak, A. Isolation and characterization of thymoquinone from Nigella sativa essential oil: Antioxidant and antibacterial activities, molecular modeling studies, and cytotoxic effects on lung cancer A549 cells. J. Essent. Oil Bear. Plants 2024, 27, 787–803. [Google Scholar] [CrossRef]
- Khan, B.A.; Mahmood, T.; Menaa, F.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; Ray, S.D. New perspectives on the efficacy of gallic acid in cosmetics & nanocosmeceuticals. Curr. Pharm. Des. 2018, 24, 5181–5187. [Google Scholar]
- Zahrani, N.A.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Minich, A.; Levarski, Z.; Mikulášová, M.; Straka, M.; Liptáková, A.; Stuchlík, S. Complex analysis of vanillin and syringic acid as natural antimicrobial agents against Staphylococcus epidermidis biofilms. Int. J. Mol. Sci. 2022, 23, 1816. [Google Scholar] [CrossRef] [PubMed]
- Stojković, D.S.; Živković, J.; Soković, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Janković, T.; Maksimović, Z. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013, 55, 209–213. [Google Scholar] [CrossRef]
- Fifere, A.; Turin-Moleavin, I.A.; Rosca, I. Does protocatechuic acid affect the activity of commonly used antibiotics and antifungals? Life 2022, 12, 1010. [Google Scholar] [CrossRef]
- Xu, H.; Wang, G.; Zhang, J.; Zhang, M.; Fu, M.; Xiang, K.; Zhang, M.; Chen, X. Identification of phenolic compounds and active antifungal ingredients of walnut in response to anthracnose (Colletotrichum gloeosporioides). Postharvest Biol. Technol. 2022, 192, 112019. [Google Scholar] [CrossRef]
- Liu, X.; Ji, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. P-Coumaric Acid Induces Antioxidant Capacity and Defense Responses of Sweet Cherry Fruit to Fungal Pathogens. Postharvest Biol. Technol. 2020, 169, 111297. [Google Scholar] [CrossRef]
- Chong, K.P.; Atong, M.; Rossall, S. The role of syringic acid in the interaction between oil palm and Ganoderma boninense, the causal agent of basal stem rot. Plant Pathol. 2012, 61, 953–963. [Google Scholar] [CrossRef]
- El-Nagar, A.; Elzaawely, A.A.; Taha, N.A.; Nehela, Y. The antifungal activity of gallic acid and its derivatives against Alternaria solani, the causal agent of tomato early blight. Agronomy 2020, 10, 1402. [Google Scholar] [CrossRef]
- Jaiswal, N.; Kumar, A. Identification, quantification, and bioactivity of Vitex negundo phenolic acids as efficacious anti-candidal and antibiofilm agents targeting Candida albicans. J. Med. Mycol. 2025, 35, 101550. [Google Scholar] [CrossRef] [PubMed]
- Ponts, N.; Pinson-Gadais, L.; Boutigny, A.L.; Barreau, C.; Richard-Forget, F. Cinnamic-derived acids significantly affect Fusarium graminearum growth and in vitro synthesis of type B trichothecenes. Phytopathology 2011, 101, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Halawani, E. Antibacterial activity of thymoquinone and thymohydroquinone of Nigella sativa L. and their interaction with some antibiotics. Advan. Biol. Res. 2009, 3, 148–152. [Google Scholar]
- Miao, X.; Liu, H.; Zheng, Y.; Guo, D.; Shi, C.; Xu, Y.; Xia, X. Inhibitory effect of thymoquinone on Listeria monocytogenes ATCC 19115 biofilm formation and virulence attributes critical for human infection. Front. Cell. Infect. Microbiol. 2019, 9, 304. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.; Zhao, P.; Yong, Q.; Huang, Q.; Al-Asmari, F.; Sameeh, M.Y.; Yang, B.; Zhang, C.; Wang, X.; et al. Thymoquinone as a potent antimicrobial agent against Yersinia enterocolitica: Mechanisms of action and potential food safety applications. Int. J. Food Microbiol. 2025, 431, 111071. [Google Scholar] [CrossRef]
- Johnston, M.D.; Hanlon, G.W.; Denyer, S.P.; Lambert, R.J.W. Membrane damage to bacteria caused by single and combined biocides. J. Appl. Microbiol. 2003, 94, 1015–1023. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Tóth, I.V.; Rangel, A.O.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef]
- Toma, C.C.; Olah, N.K.; Vlase, L.; Mogoșan, C.; Mocan, A. Comparative studies on polyphenolic composition, antioxidant and diuretic effects of Nigella sativa L. (black cumin) and Nigella damascena L. (lady-in-a-mist) seeds. Molecules 2015, 20, 9560–9574. [Google Scholar] [CrossRef]
- Zwolan, A.; Pietrzak, D.; Adamczak, L.; Chmiel, M.; Kalisz, S.; Wirkowska-Wojdyła, M.; Oszmiański, J. Effects of Nigella sativa L. seed extracts on lipid oxidation and color of chicken meatballs during refrigerated storage. LWT 2020, 130, 109718. [Google Scholar] [CrossRef]
- Topcagic, A.; Zeljkovic, S.C.; Karalija, E.; Galijasevic, S.; Sofic, E. Evaluation of phenolic profile, enzyme inhibitory and antimicrobial activities of Nigella sativa L. seed extracts. Bosn. J. Basic Med. Sci. 2017, 17, 286. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, A.; Doncheva, N.; Vlasheva, M.; Katsarova, M.; Gardjeva, P.; Dimitrova, S.; Kostadinov, I. Investigation of the Immunomodulatory and Neuroprotective Properties of Nigella sativa Oil in Experimental Systemic and Neuroinflammation. Int. J. Mol. Sci. 2025, 26, 2235. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.S.; Ahmad, A.; Pandey, B. Solvent based optimization for extraction and stability of thymoquinone from Nigella sativa Linn. and its quantification using RP-HPLC. Physiol. Mol. Biol. Plants 2018, 24, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Emam-Djomeh, Z.; Mirzaee, H.A.; Khomeiri, M.; Mahoonak, A.S.; Aydani, E. Optimization of microwave assisted extraction (MAE) and soxhlet extraction of phenolic compound from licorice root. J. Food Sci. Technol. 2015, 52, 3242–3253. [Google Scholar] [CrossRef]
- Hossain, M.B.; Brunton, N.P.; Patras, A.; Tiwari, B.; O’donnell, C.P.; Martin-Diana, A.B.; BarryRyan, C. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanum majorana L.) using response surface methodology. Ultrason. Sonochem. 2012, 19, 582–590. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Gramza-Michałowska, A.; Bryła, M.; Waśkiewicz, A. Antioxidant activity and bioactive compounds of Lamium album flower extracts obtained by supercritical fluid extraction. Appl. Sci. 2021, 11, 7419. [Google Scholar] [CrossRef]
- Gwiazdowska, D.; Uwineza, P.A.; Frąk, S.; Juś, K.; Marchwińska, K.; Gwiazdowski, R.; Waśkiewicz, A. Antioxidant, antimicrobial and antibiofilm properties of Glechoma hederacea extracts obtained by supercritical fluid extraction, using different extraction conditions. Appl. Sci. 2022, 12, 3572. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: Towards a total phenolic index using high-performance liquid chromatography. J. Chromatogr. A 2003, 1018, 29–40. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- ISTA. Chapter 5: The Germination Test. In International Rules for Seed Testing; ISTA: Zurich, Switzerland, 2021. [Google Scholar]
- Wang, Y.; Li, J.; Chen, Q.; Zhou, J.; Xu, J.; Zhao, T.; Liu, D. The role of antifungal activity of ethyl acetate extract from Artemisia argyi on Verticillium dahliae. J. Appl. Microbiol. 2022, 132, 1343–1356. [Google Scholar] [CrossRef]
- Gwiazdowska, D.; Marchwińska, K.; Juś, K.; Uwineza, P.A.; Gwiazdowski, R.; Waśkiewicz, A.; Kierzek, R. The concentration-dependent effects of essential oils on the growth of Fusarium graminearum and mycotoxins biosynthesis in wheat and maize grain. Appl. Sci. 2022, 12, 473. [Google Scholar] [CrossRef]
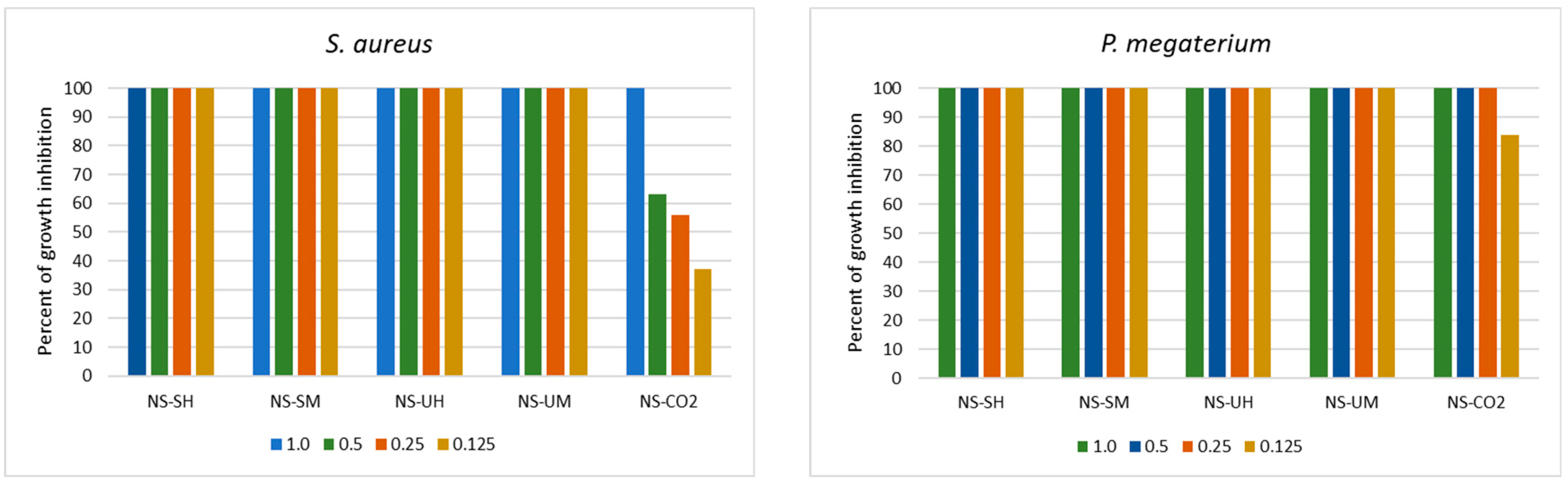

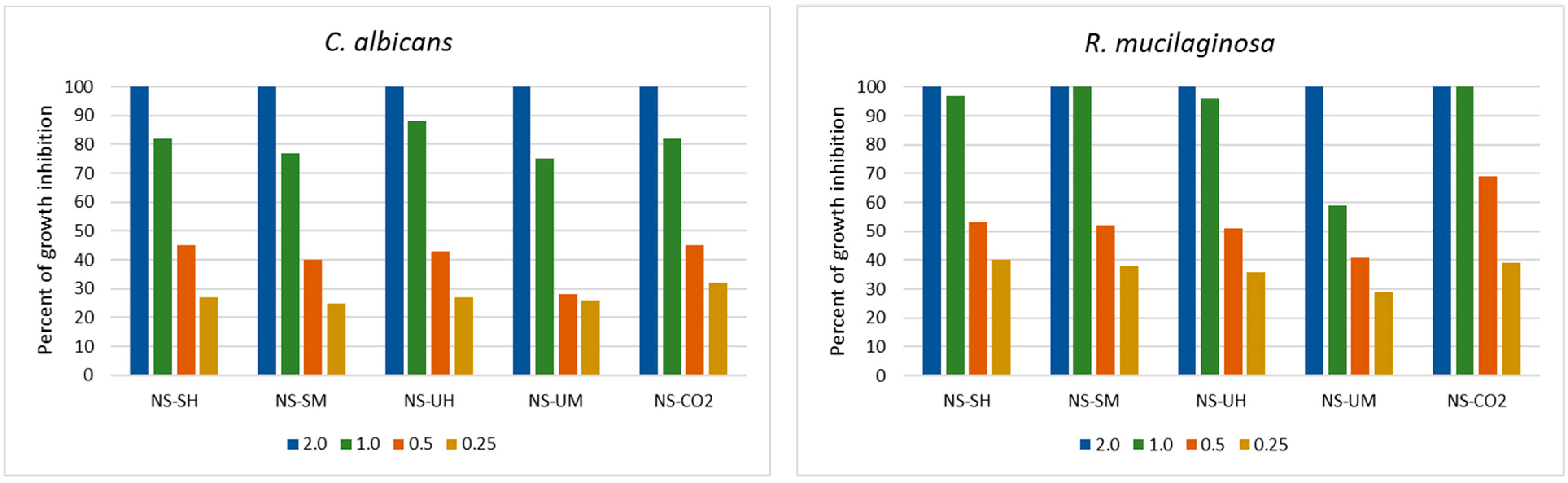
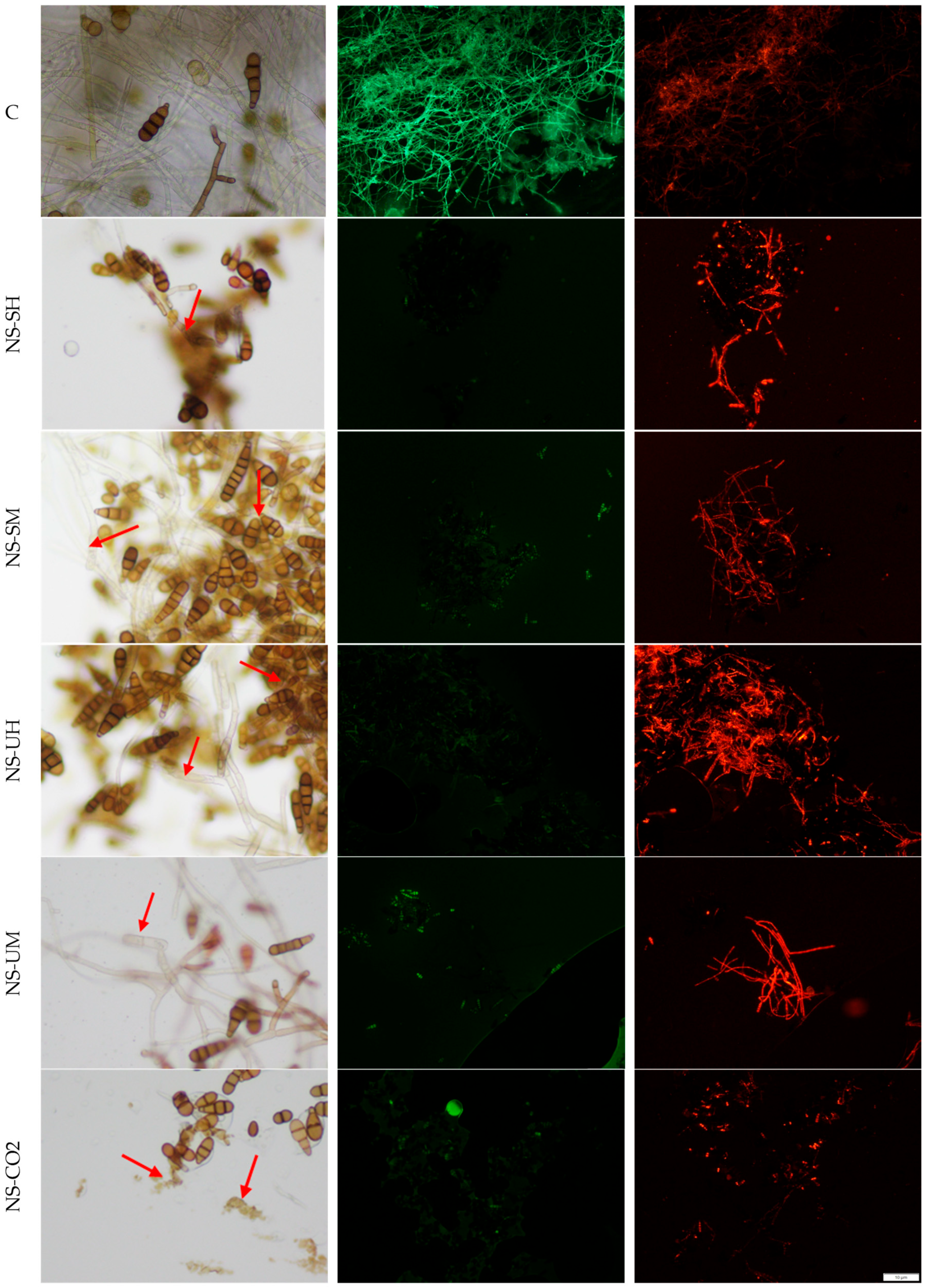
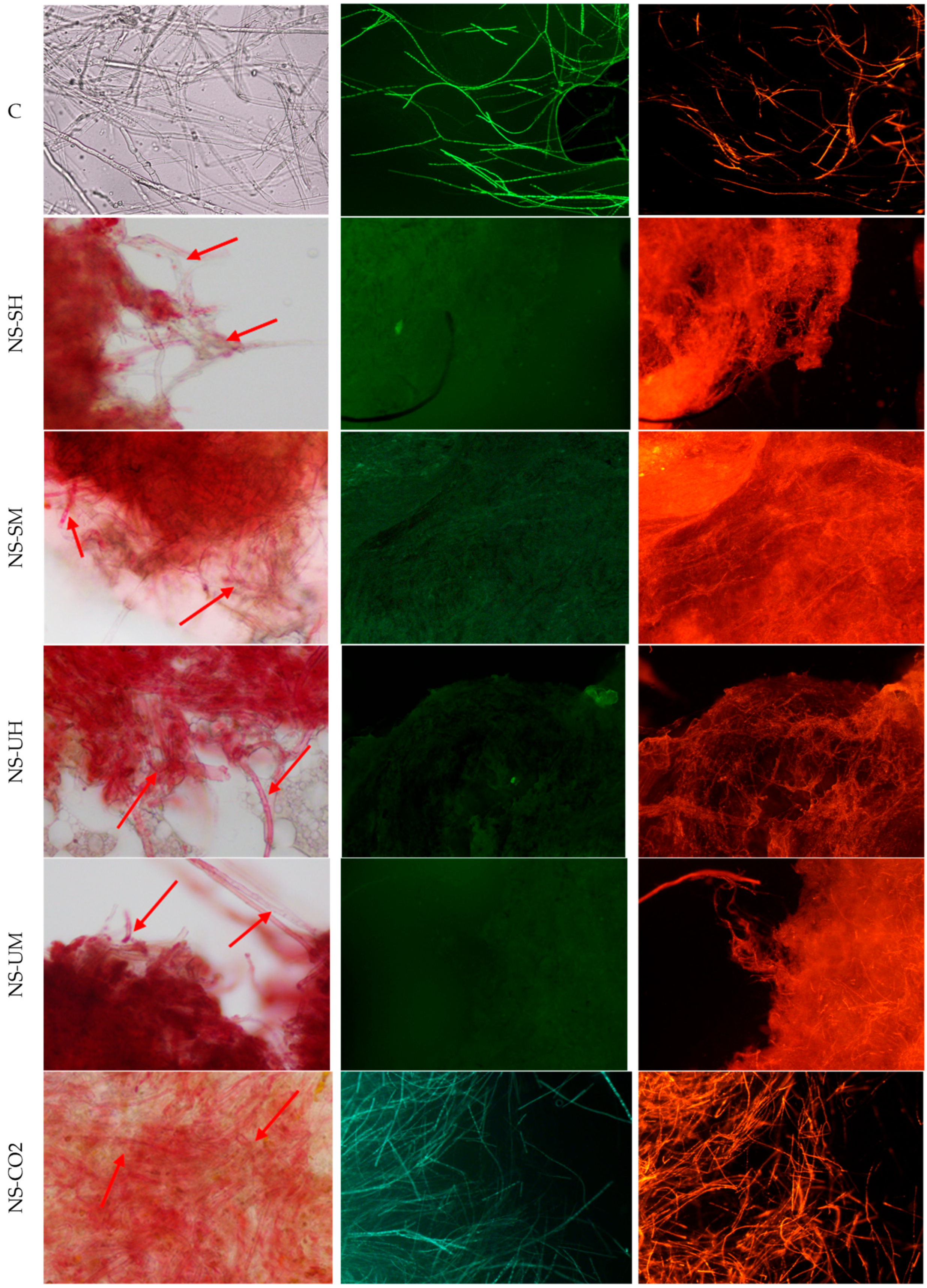
| Indicator Bacteria | MIC and MBC of N. sativa Extracts * [mg/mL] | |||||
|---|---|---|---|---|---|---|
| NS-SH | NS-SM | NS-UH | NS-UM | NS-CO2 | ||
| Gram-positive bacteria | ||||||
| S. aureus | MIC | <0.125 | <0.125 | <0.125 | <0.125 | 1 |
| MBC | <0.125 | <0.125 | <0.125 | <0.125 | 1 | |
| P. megaterium | MIC | <0.125 | <0.125 | <0.125 | <0.125 | 0.125 |
| MBC | <0.125 | <0.125 | <0.125 | <0.125 | 0.25 | |
| L. monocytogenes | MIC | 0.125 | 0.125 | 0.25 | 0.25 | 0.125 |
| MBC | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | |
| Gram-negative bacteria | ||||||
| E. coli | MIC | 1 | 1 | 1 | 1 | 1 |
| MBC | 2 | 2 | 2 | 2 | 2 | |
| P. aeruginosa | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 1 |
| MBC | 1 | 0.5 | 0.5 | 0.5 | 1 | |
| S. enteritidis | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| MBC | 0.5 | 1 | 0.5 | 0.5 | 0.5 | |
| Indicator Fungi | MIC and MFC of N. sativa Extracts * [mg/mL] | |||||
|---|---|---|---|---|---|---|
| NS-SH | NS-SM | NS-UH | NS-UM | NS-CO2 | ||
| Yeasts | ||||||
| C. albicans | MIC | 2 | 2 | 2 | 2 | 2 |
| MFC | 2 | 2 | 2 | 2 | 2 | |
| R. mucilaginosa | MIC | 1 | 1 | 1 | 2 | 1 |
| MFC | 2 | 1 | 2 | 2 | 1 | |
| Filamentous fungi | ||||||
| A. brassicicola | MIC | 4 | 4 | 4 | 4 | 4 |
| MFC | 4 | 4 | 4 | 4 | 4 | |
| F. culmorum | MIC | 4 | 8 | 4 | 8 | 8 |
| MFC | 4 | 8 | 4 | 8 | 8 | |
| F. graminearum | MIC | 2 | 4 | 4 | 4 | 4 |
| MFC | 2 | 4 | 4 | 4 | 4 | |
| Pythium spp. | MIC | 8 | 8 | 8 | 8 | 8 |
| MFC | 8 | 8 | 8 | 8 | 8 | |
| N. sativa Extracts | Grain Germinability [%] | |
|---|---|---|
| After 4 Days | After 8 Days | |
| NS-SH | 85.3 a ± 2.1 | 96.0 a ± 1.4 |
| NS-SM | 91.3 ab ± 2.4 | 98.3 a ± 2.9 |
| NS-UH | 93.0 b ± 1.4 | 98.5 a ± 0.6 |
| NS-UM | 93.8 b ± 3.5 | 98.3 a ± 1.7 |
| NS-CO2 | 96.0 b ± 0.8 | 98.3 a ± 1.5 |
| PC | 90.8 ab ± 3.8 | 99.0 a ± 0.8 |
| NC | 93.5 b ± 3.9 | 97.5 a ±1.7 |
| N. sativa Extracts | Derivatives of Hydroxybenzoic Acid | Derivatives of Hydroxycinnamic Acid | Flavanols | Flavonols | NI | TPC *** | TQ | Antioxidant Activity μmol/g **** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA | VA * | HA * | SA * | PTA * | P-CA * | CFA * | SP * | CA ** | ECA * | MI | LT | AP | |||||
| NS-SH | - | 0.072 | 3.313 | 0.375 | 0.938 | 0.344 | - | - | 1.250 | 0.938 | - | - | - | 0.14 | 11.605 | 60.687 | 21.63 b ± 1.18 |
| NS-SM | 1.125 | 0.438 | 2.781 | 4.125 | 5.938 | 2.344 | 8.438 | - | 2.656 | 1.406 | 5.000 | 0.188 | 0.375 | 0.136 | 39.064 | 47.313 | 44.91 c ± 0.97 |
| NS-UH | - | 0.063 | 2.813 | 0.313 | 0.156 | 0.250 | - | - | 1.375 | 0.844 | - | - | - | 0.13 | 9.877 | 63.125 | 21.00 b ± 1.15 |
| NS-UM | 1.094 | 0.438 | 2.781 | 4.156 | 5.938 | 1.125 | 0.469 | 5.625 | 2.656 | 1.406 | 6.688 | 0.625 | 0.313 | 0.136 | 37.564 | 34.696 | 52.19 d ± 1.19 |
| NS-CO2 | - | 0.313 | 18.125 | 9.063 | 0.125 | 0.125 | 0.250 | - | 1.094 | 0.125 | - | - | - | - | 29.220 | 8.344 | 10.17 a ± 0.71 |
| Extract | Extraction Solvent | Yield of Extraction (%) | Dissolving Solvent | Extract Concentration (mg/mL) |
|---|---|---|---|---|
| Soxhlet extraction | ||||
| NS-SH | hexane | 16.10 | ethanol | 32 |
| NS-SM | methanol | 15.30 | ethanol | 32 |
| ultrasound-assisted extraction | ||||
| NS-UH | hexane | 16.30 | ethanol | 32 |
| NS-UM | methanol | 15.50 | ethanol | 32 |
| supercritical CO2 extraction | ||||
| NS-CO2 | methanol | 26.85 | ethanol | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwiazdowski, R.; Juś, K.; Kubiak, K.; Biegańska-Marecik, R.; Waśkiewicz, A.; Gwiazdowska, D. The Effect of the Extraction Method on the Content of Bioactive Compounds and the Biological Activity of Nigella sativa Extracts. Molecules 2025, 30, 4736. https://doi.org/10.3390/molecules30244736
Gwiazdowski R, Juś K, Kubiak K, Biegańska-Marecik R, Waśkiewicz A, Gwiazdowska D. The Effect of the Extraction Method on the Content of Bioactive Compounds and the Biological Activity of Nigella sativa Extracts. Molecules. 2025; 30(24):4736. https://doi.org/10.3390/molecules30244736
Chicago/Turabian StyleGwiazdowski, Romuald, Krzysztof Juś, Krzysztof Kubiak, Róża Biegańska-Marecik, Agnieszka Waśkiewicz, and Daniela Gwiazdowska. 2025. "The Effect of the Extraction Method on the Content of Bioactive Compounds and the Biological Activity of Nigella sativa Extracts" Molecules 30, no. 24: 4736. https://doi.org/10.3390/molecules30244736
APA StyleGwiazdowski, R., Juś, K., Kubiak, K., Biegańska-Marecik, R., Waśkiewicz, A., & Gwiazdowska, D. (2025). The Effect of the Extraction Method on the Content of Bioactive Compounds and the Biological Activity of Nigella sativa Extracts. Molecules, 30(24), 4736. https://doi.org/10.3390/molecules30244736








