Theoretical Investigation of Structural and Optical Peculiarities of Bikaverin Fungal Pigment in Chloroform Solution
Abstract
1. Introduction
2. Results and Discussion
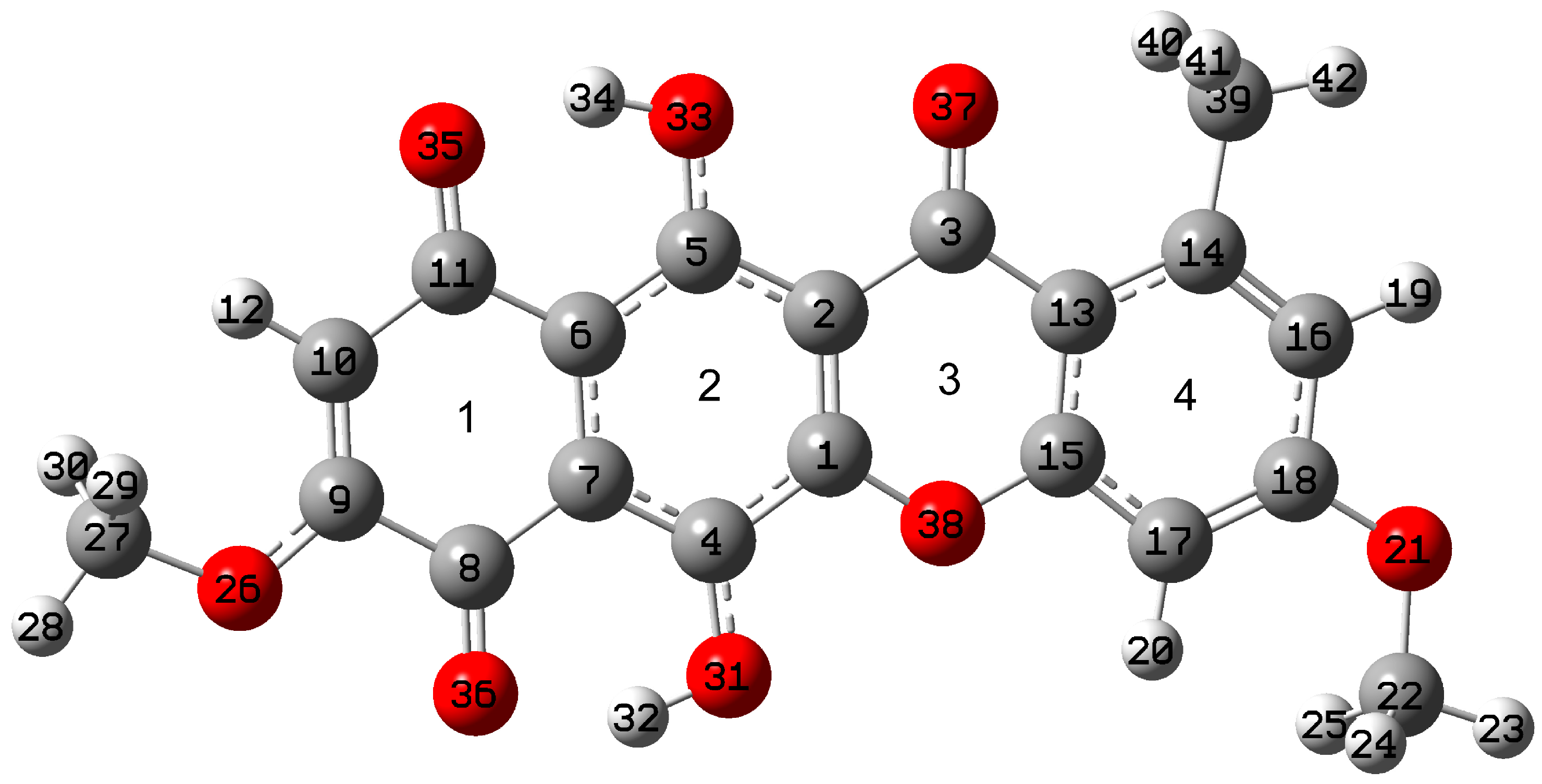
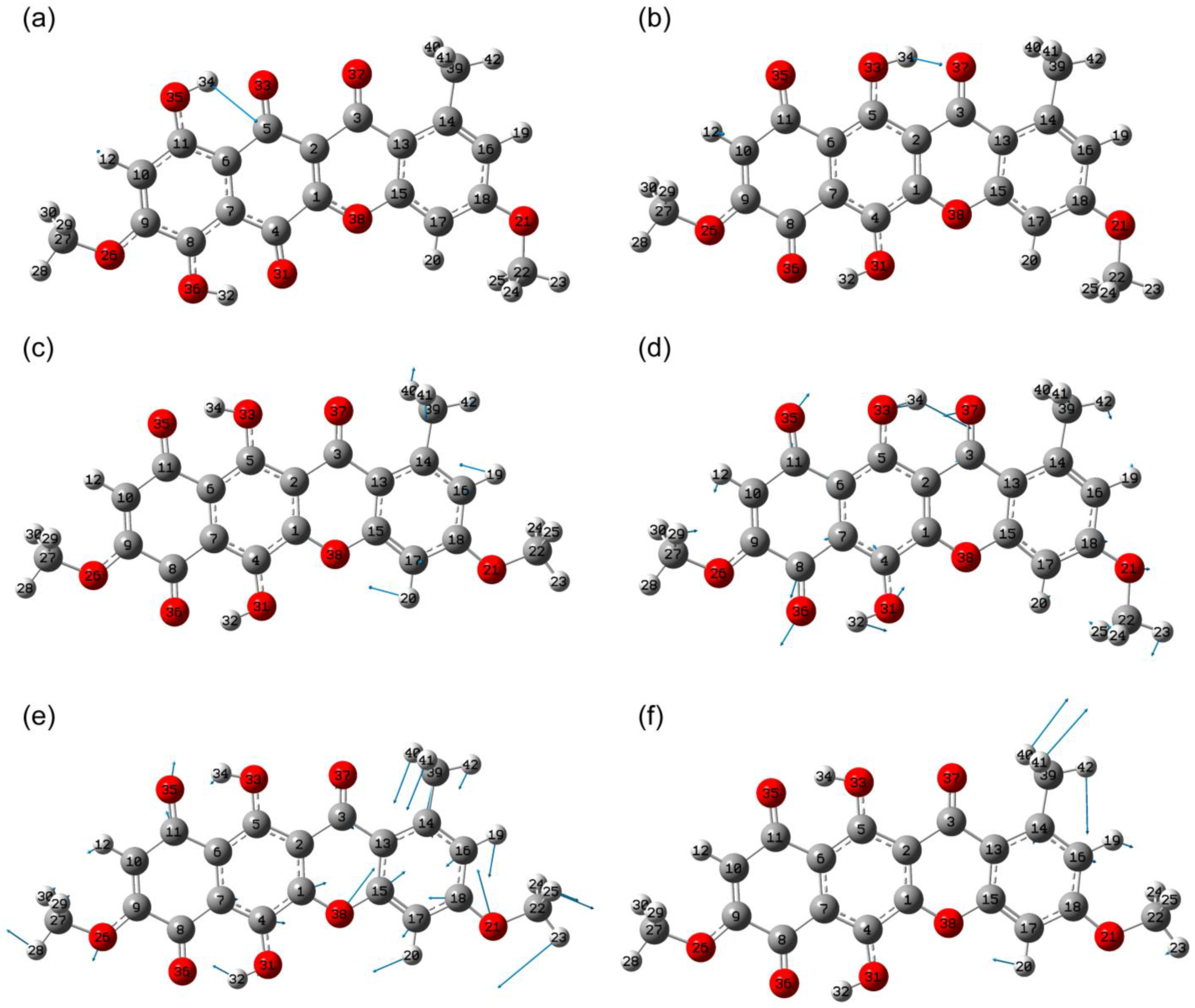
2.1. Structural Peculiarities
2.1.1. The Lowest Energy State
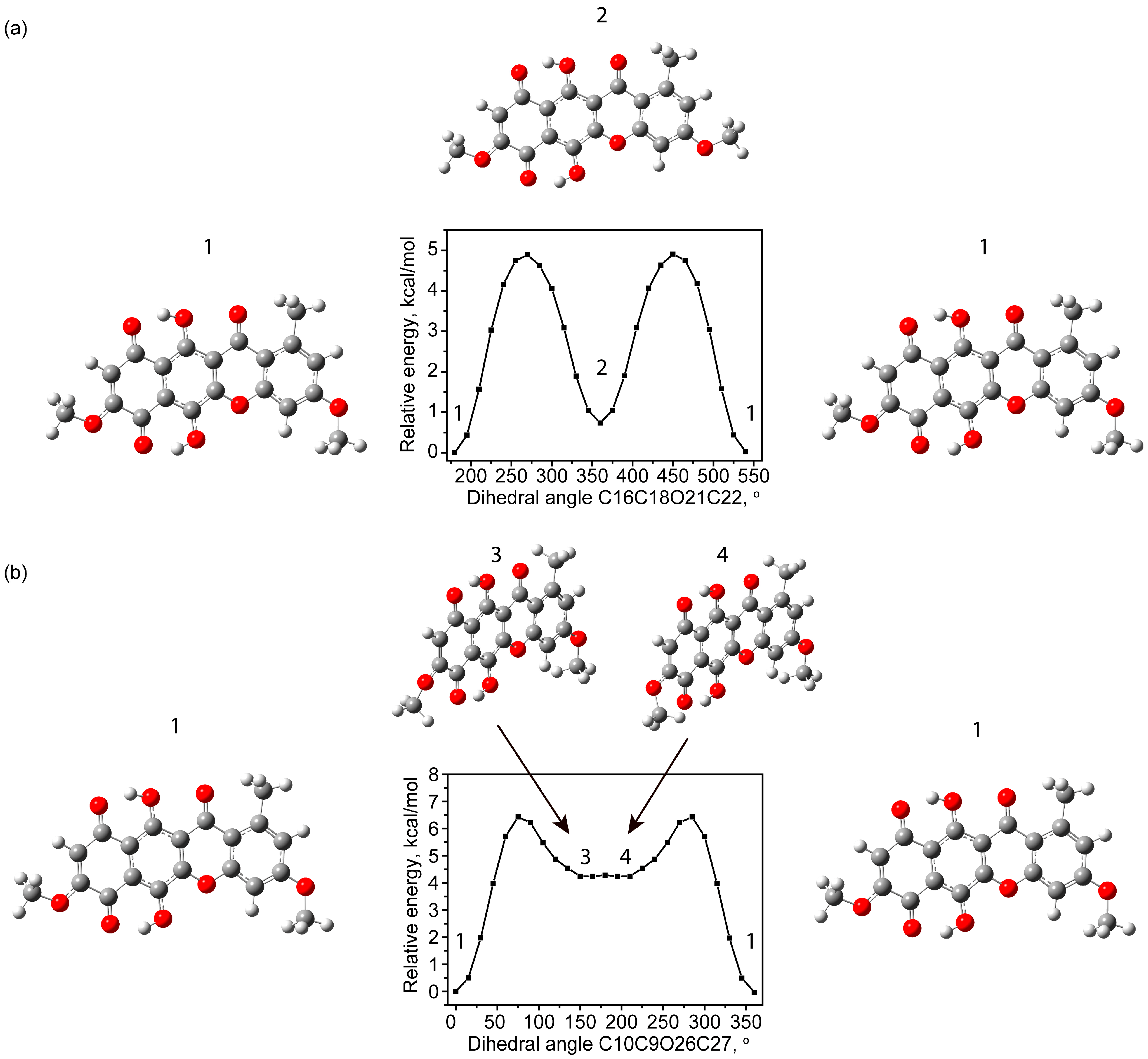
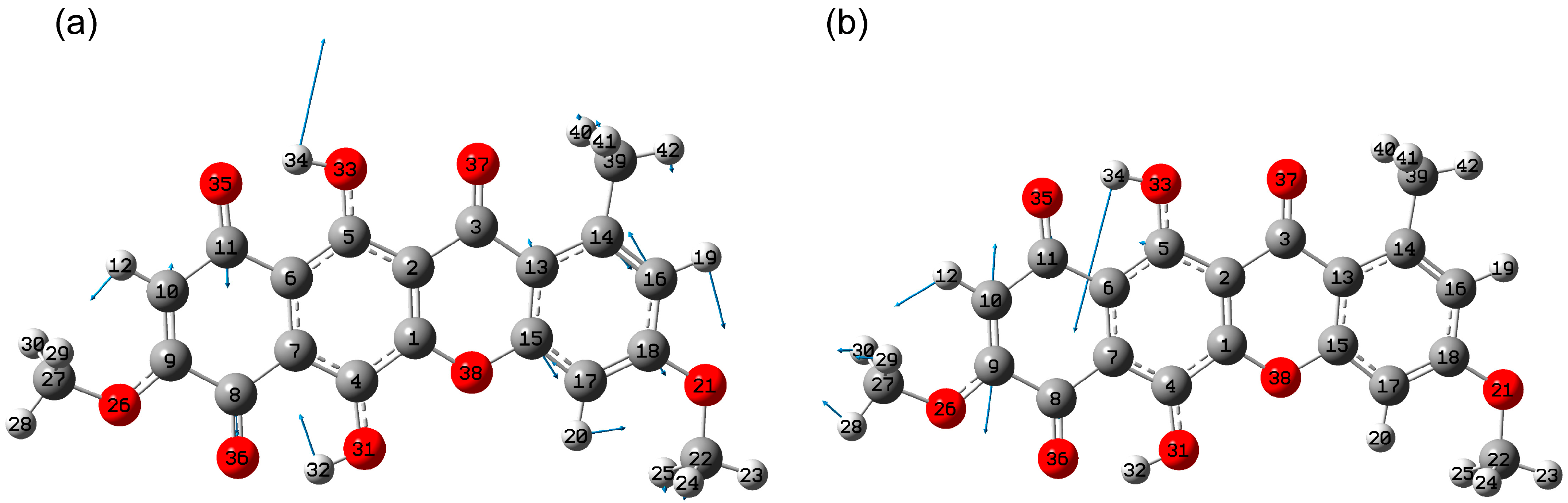
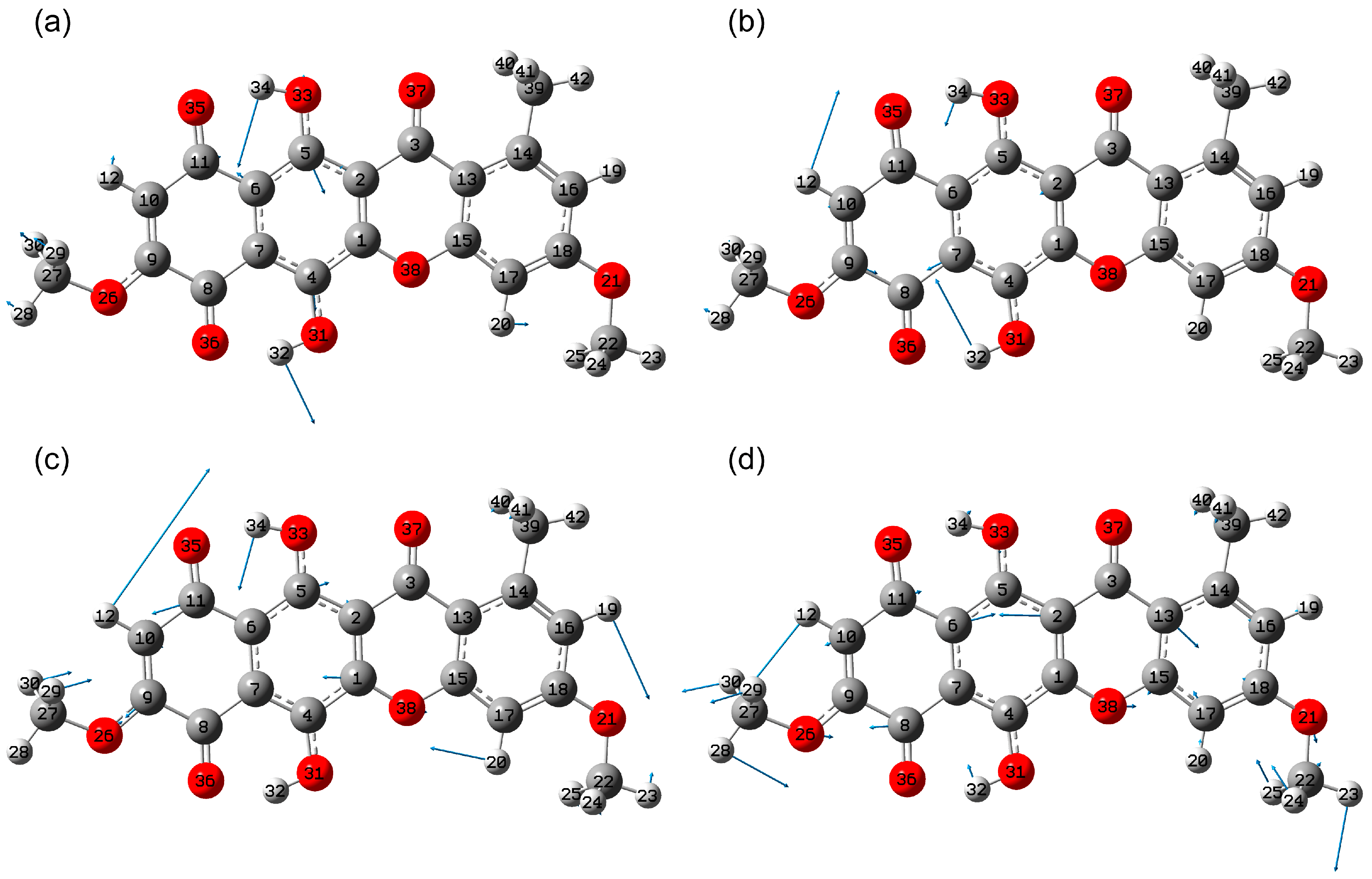
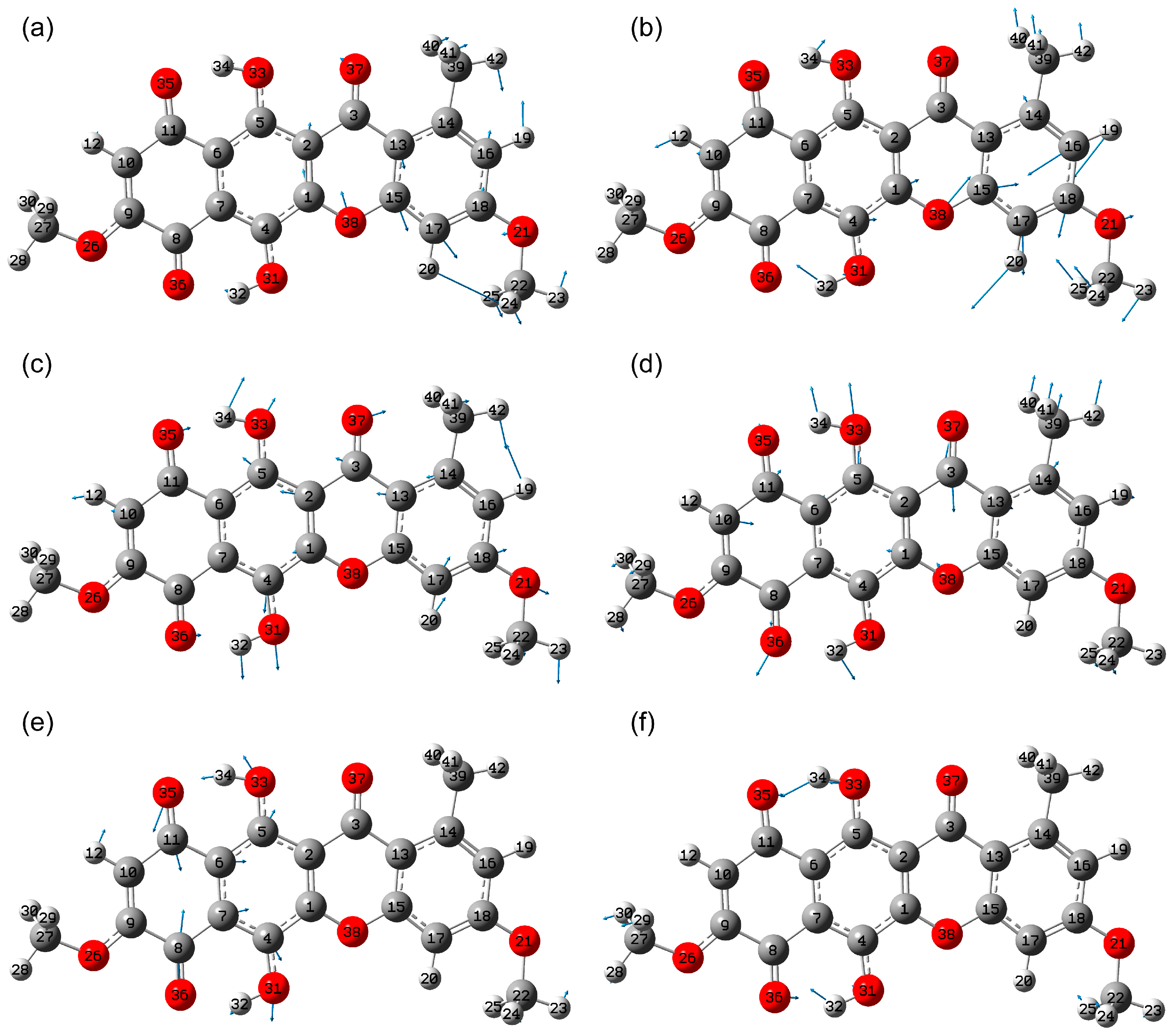
2.1.2. Conformational Isomers of Bikaverin
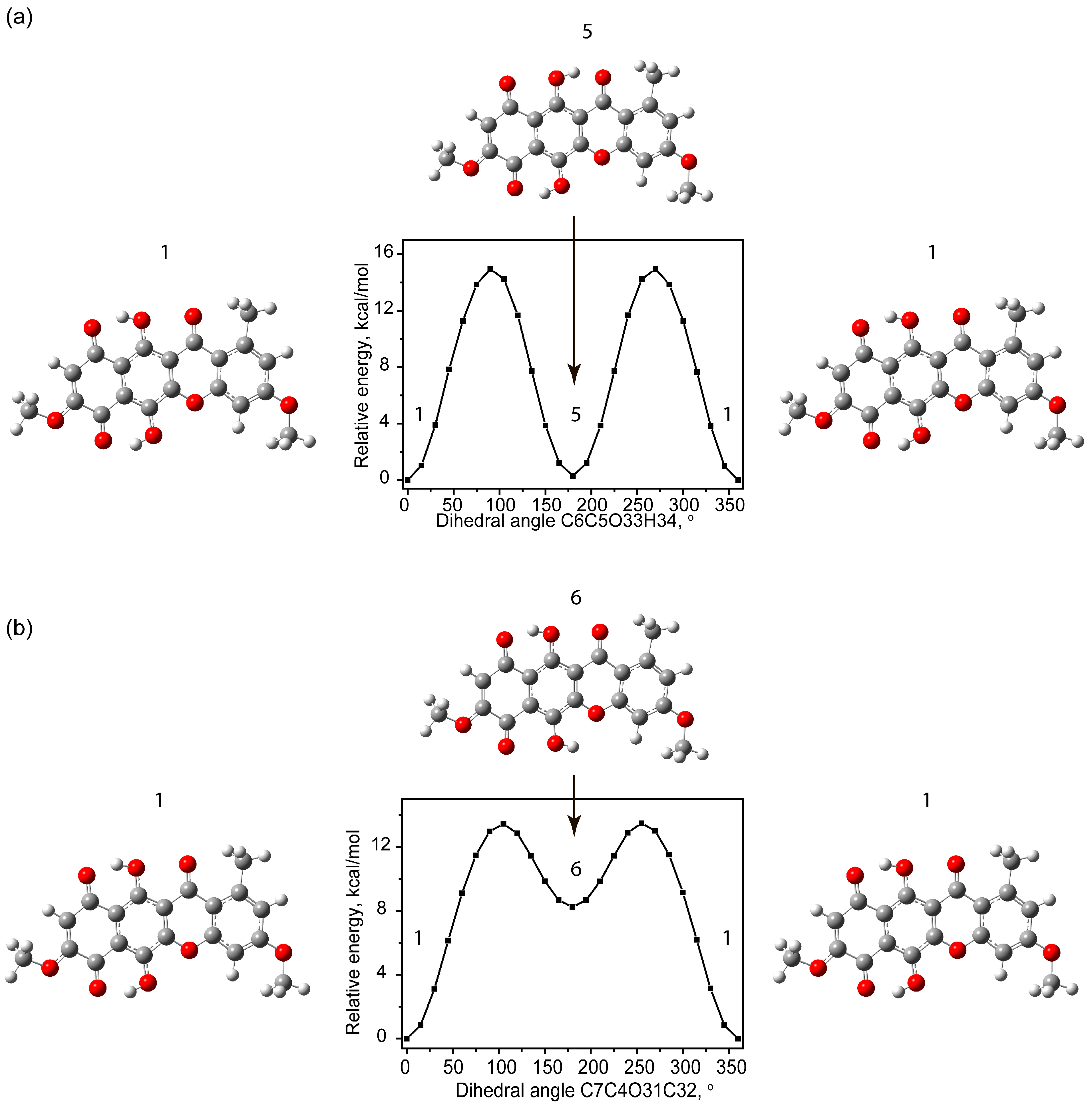
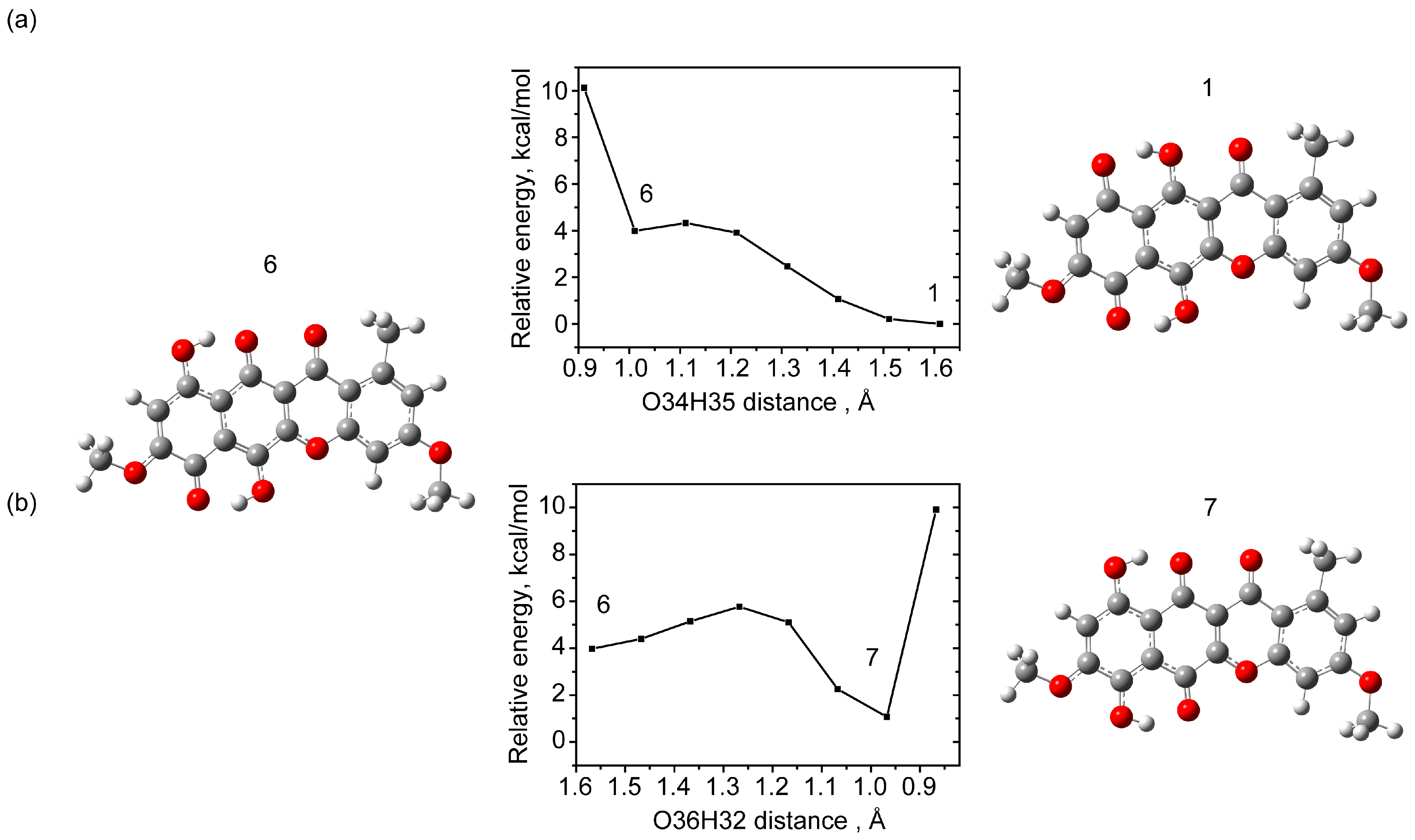
2.1.3. Tautomerism
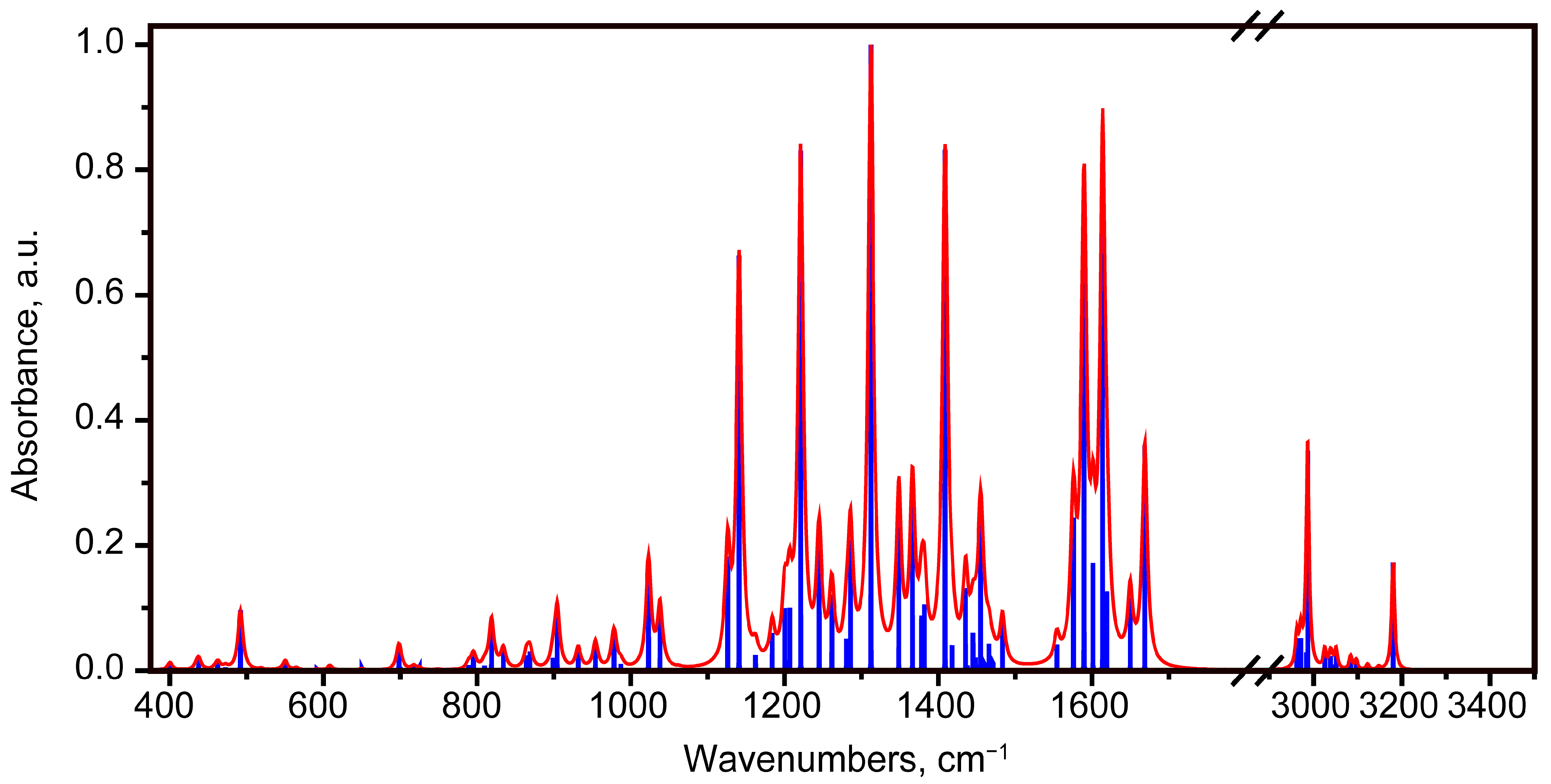
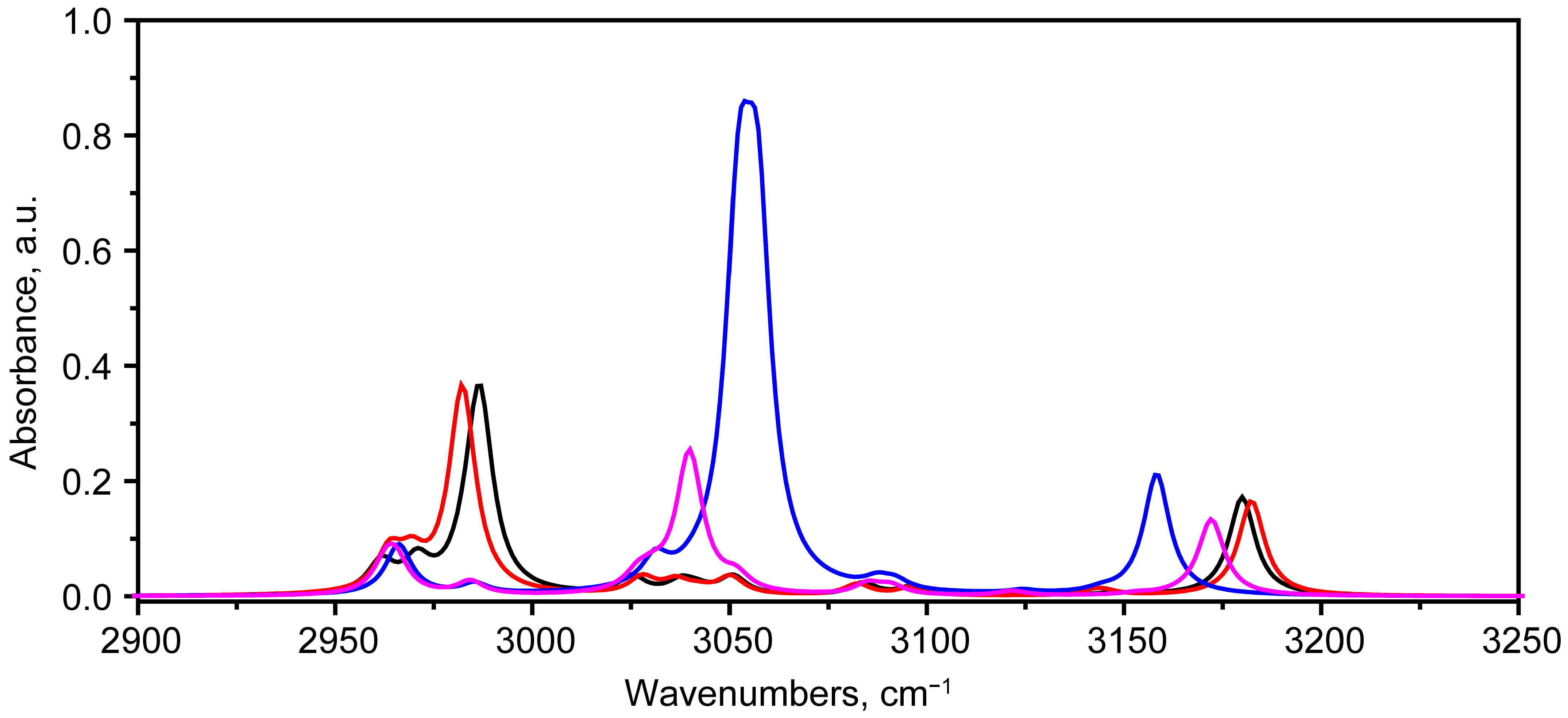
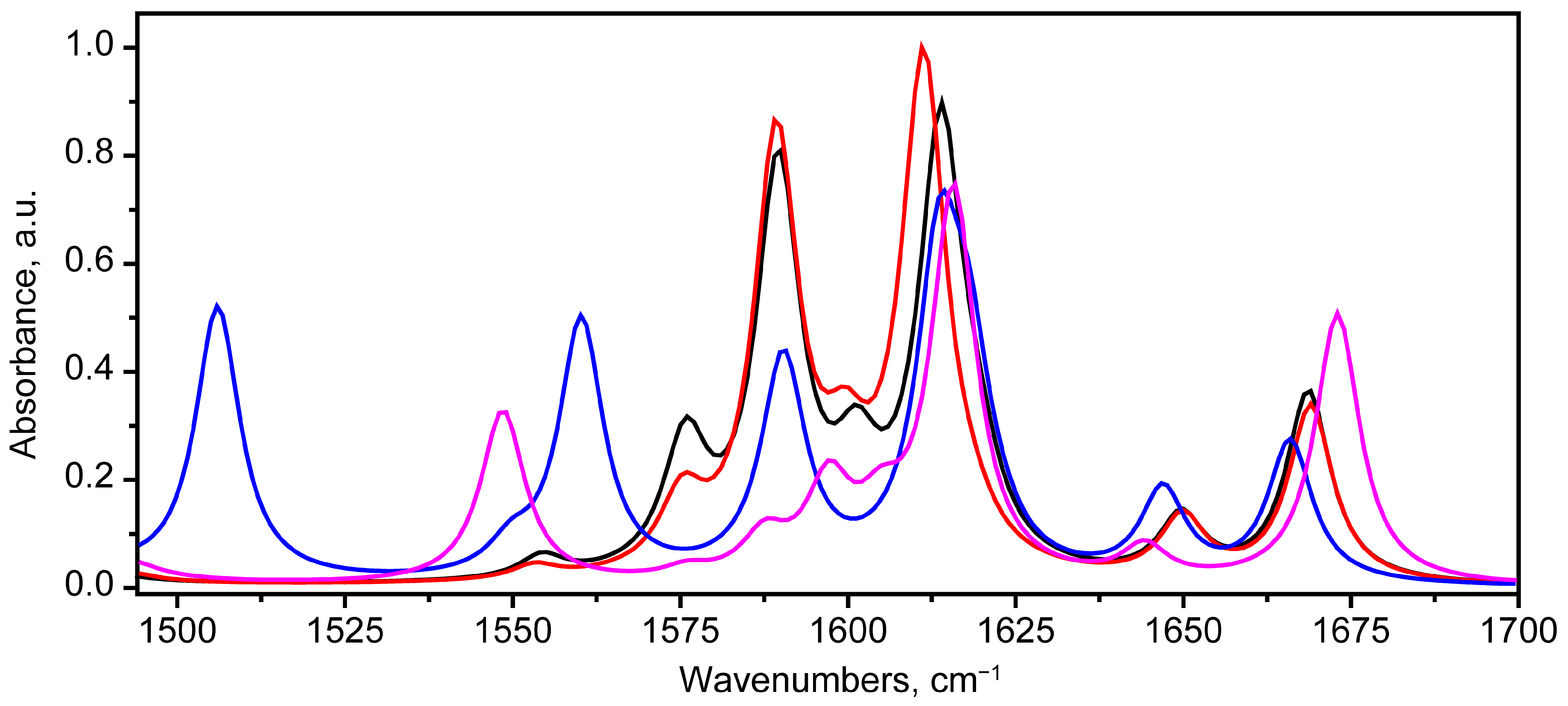
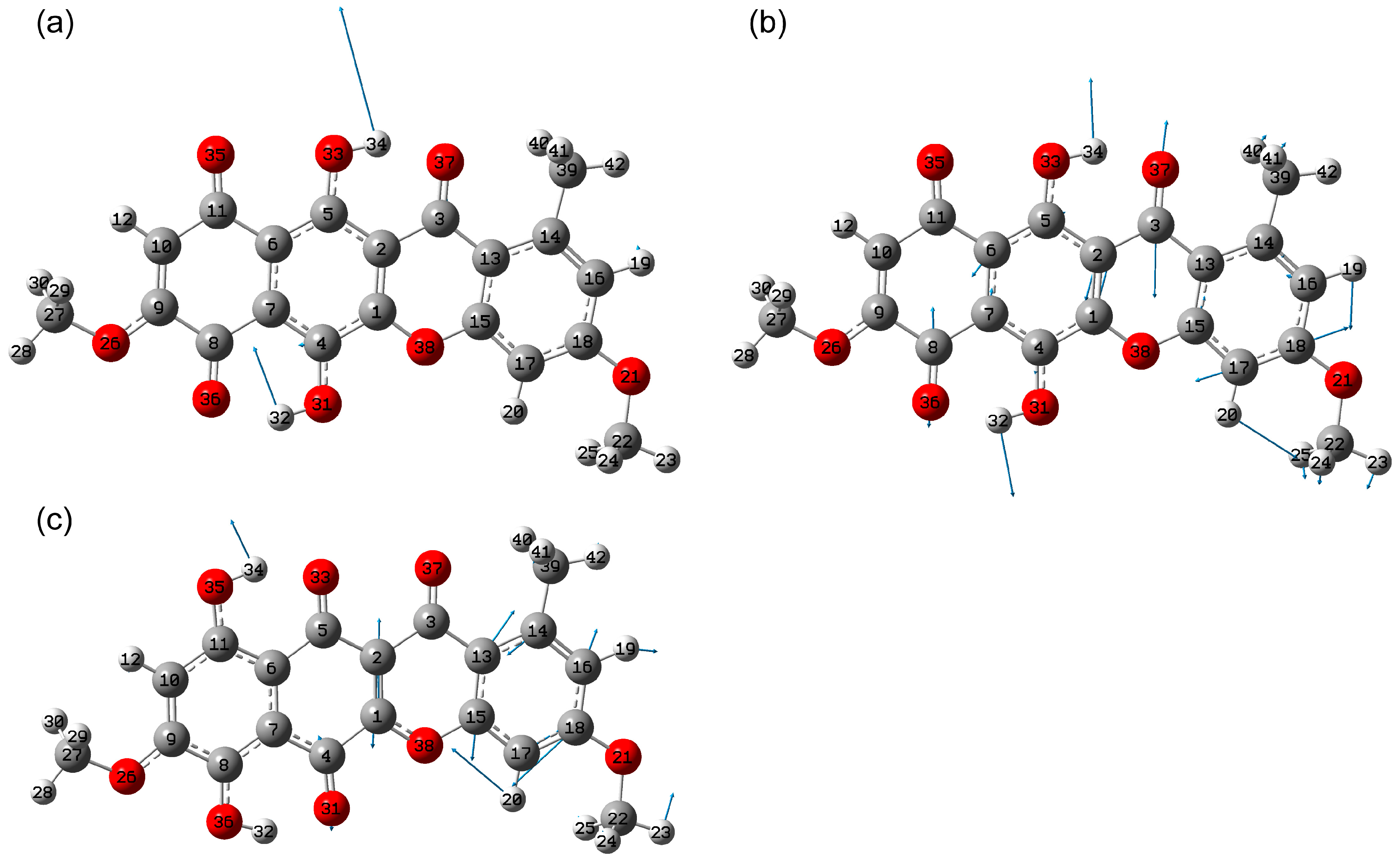
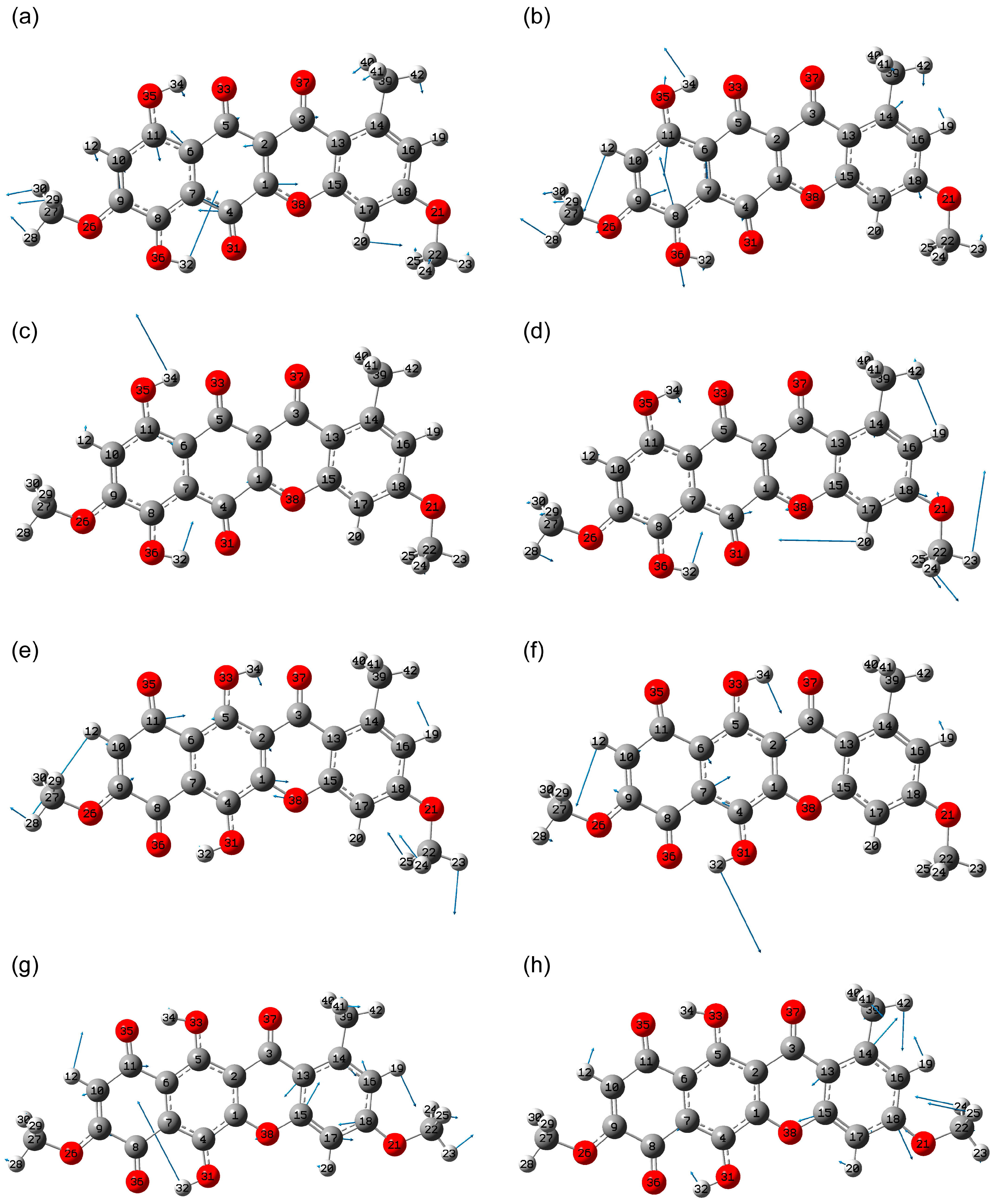
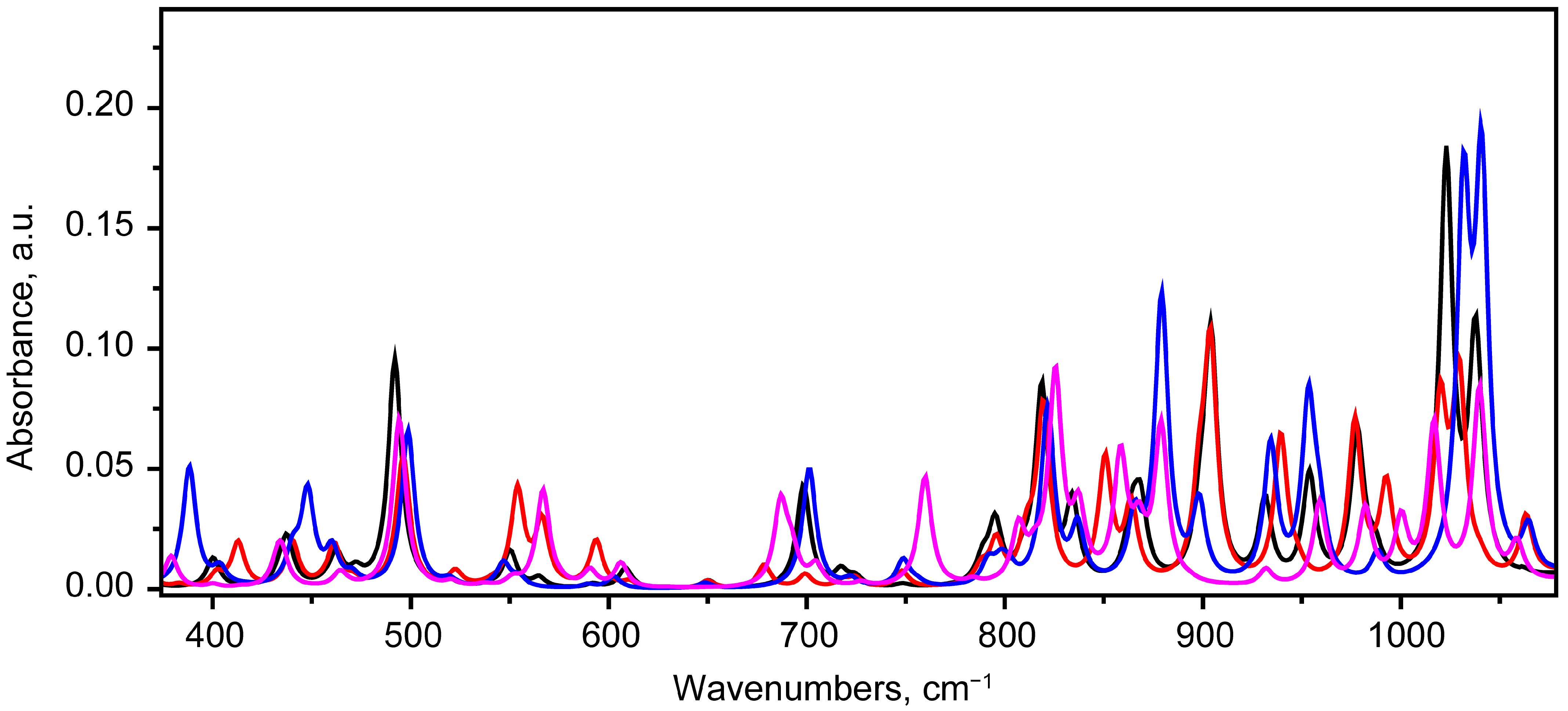
2.2. Vibrational Spectroscopy Analysis
2.2.1. IR Absorbance Spectrum of State 1
2.2.2. IR Absorbance Spectra Peculiarities of States 2, 5 and 7
Hydrogen Bond Stretching Region
The Region of 1494–1700 cm−1
The Region of 1100–1494 cm−1
- The presence of a significant contribution from deformation vibrations in δ(C11O35H34) and δ(C8O36H32);
- Carbon–carbon stretching vibrations in the rings of hydroquinone, whose environment in state 1 differs qualitatively from state 7, where hydroquinone is located at the edge and an MeO group is attached to it.
The Region of 375–1100 cm−1
- Mode 38 (567 cm−1), involving deformation of the hydroquinone moiety;
- Mode 44 (689 cm−1), involving δ(CCO) and rotation of the benzene ring of the hydroquinone moiety;
- Mode 49 (760 cm−1), involving δ(CCO) vibrations.
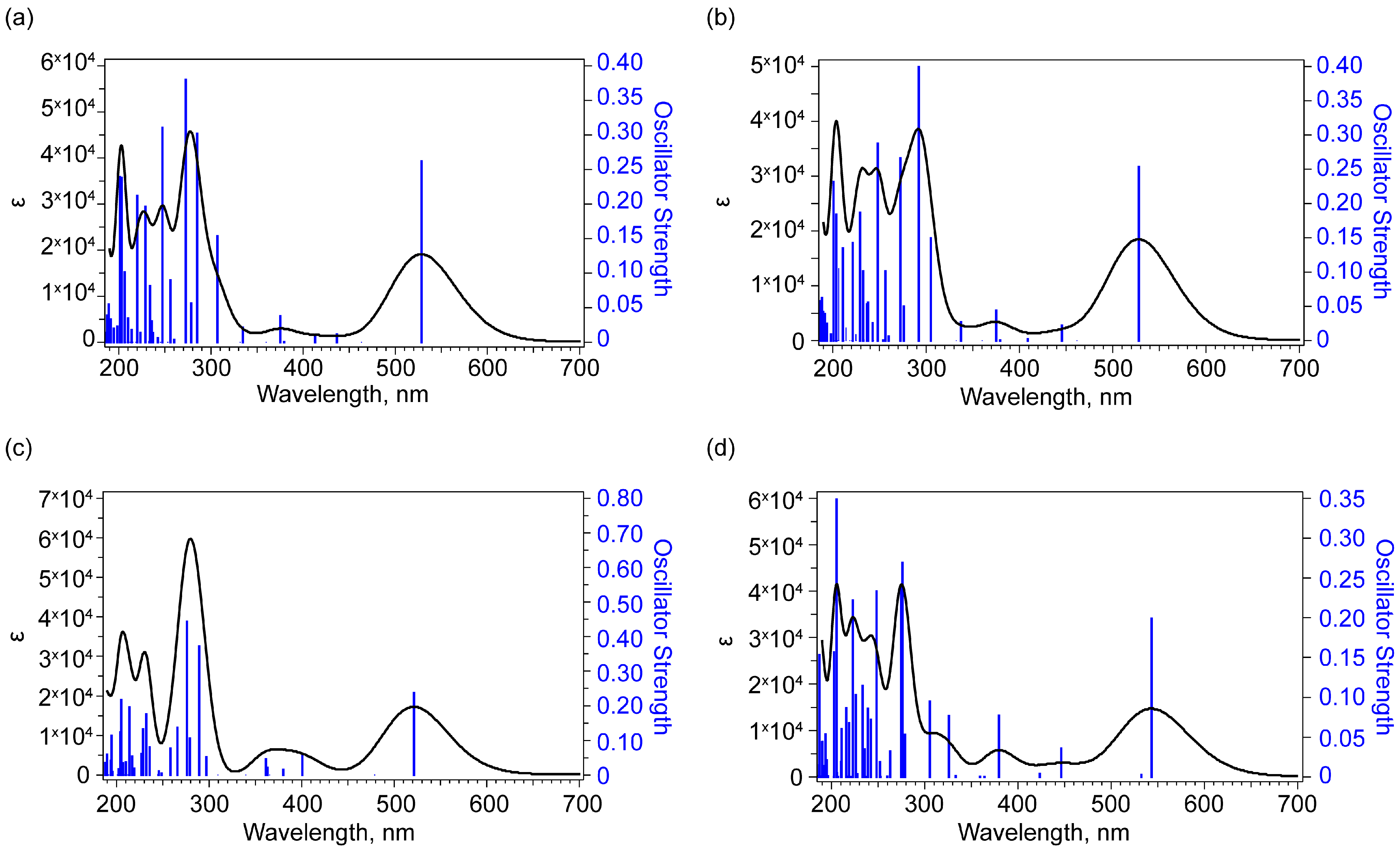
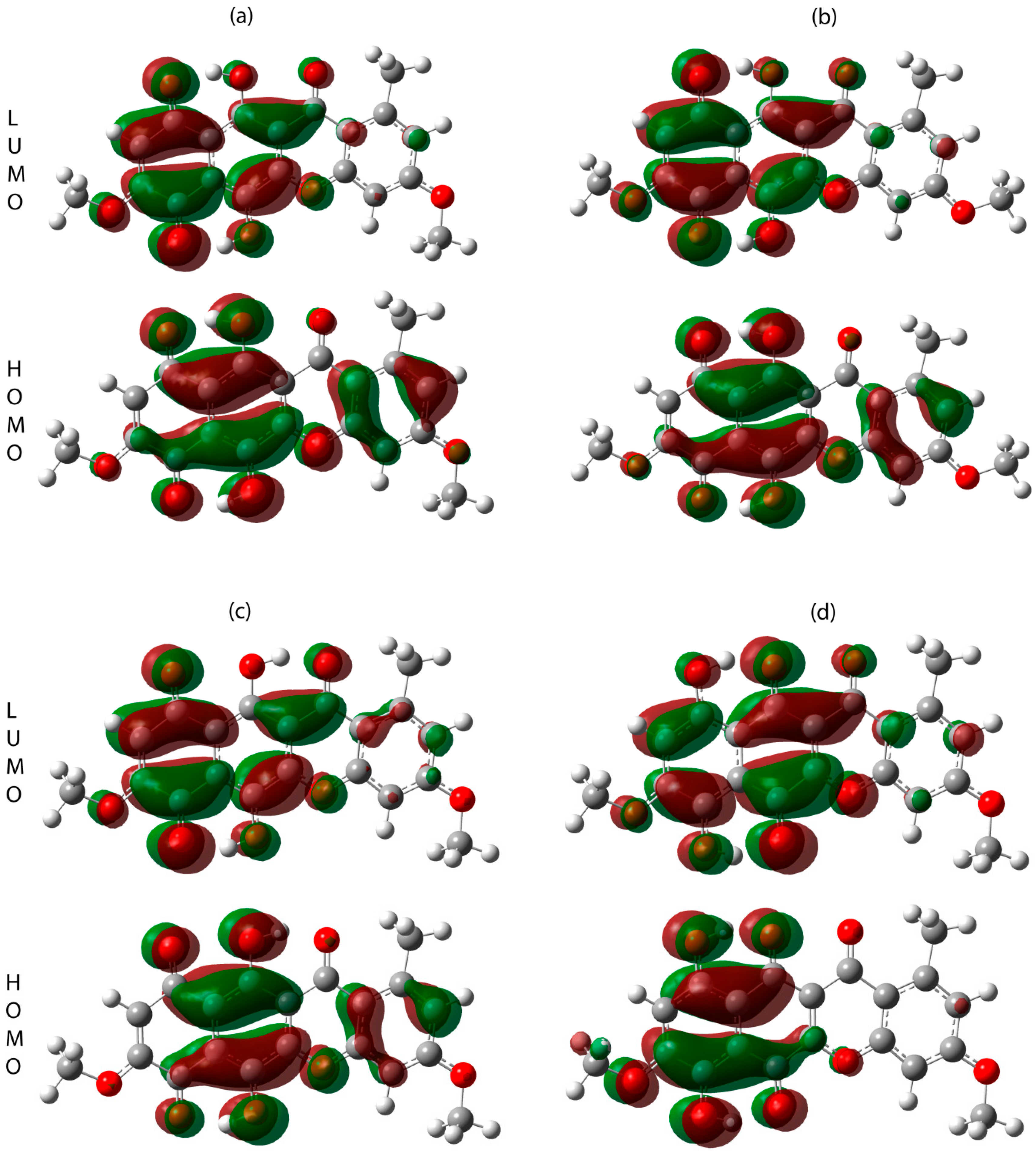
2.3. UV-Vis Absorption Spectroscopy
3. Theoretical Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Boer, J.J.; Bright, D.; Dallinga, G.; Hewitt, T.G. Crystal and Molecular Structure of the Chloroform Solvate of Bikaverin. J. Chem. Soc. C Org. 1971, 16, 2788. [Google Scholar] [CrossRef]
- Nakamura, Y.; Shinomura, T.; Ona, J. Nippon Nogei Kagaku. J. Chem. Soc. Jpn. Chem. Ind. Chem. 1957, 31, 669. [Google Scholar]
- Masters, K.S.; Bräse, S. Xanthones from Fungi, Lichens, and Bacteria: The Natural Products and Their Synthesis. Chem. Rev. 2012, 112, 3717–3776. [Google Scholar] [CrossRef]
- Lu, H.; Guo, S.; Yang, Y.; Zhao, Z.; Xie, Q.; Wu, Q.; Sun, C.; Luo, H.; An, B.; Wang, Q. Bikaverin as a Molecular Weapon: Enhancing Fusarium Oxysporum Pathogenicity in Bananas via Rhizosphere Microbiome Manipulation. Microbiome 2025, 13, 107. [Google Scholar] [CrossRef]
- Schumacher, J.; Gautier, A.; Morgant, G.; Studt, L.; Ducrot, P.H.; Le Pêcheur, P.; Azeddine, S.; Fillinger, S.; Leroux, P.; Tudzynski, B.; et al. A Functional Bikaverin Biosynthesis Gene Cluster in Rare Strains of Botrytis Cinerea Is Positively Controlled by VELVET. PLoS ONE 2013, 8, e53729. [Google Scholar] [CrossRef]
- Wiemann, P.; Willmann, A.; Straeten, M.; Kleigrewe, K.; Beyer, M.; Humpf, H.U.; Tudzynski, B. Biosynthesis of the Red Pigment Bikaverin in Fusarium Fujikuroi: Genes, Their Function and Regulation. Mol. Microbiol. 2009, 72, 931–946. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Y.; Yao, M.; Iqbal, H.; Hu, Q.; Liu, H.; Qiao, B.; Li, C.; Skovbjerg, C.A.S.; Nielsen, J.C.; et al. Pathway Engineering in Yeast for Synthesizing the Complex Polyketide Bikaverin. Nat. Commun. 2020, 11, 6167. [Google Scholar] [CrossRef]
- Lebeau, J.; Petit, T.; Dufossé, L.; Caro, Y. Putative Metabolic Pathway for the Bioproduction of Bikaverin and Intermediates Thereof in the Wild Fusarium Oxysporum LCP531 Strain. AMB Express 2019, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- de Reus, E.; Nielsen, M.R.; Frandsen, R.J.N. Metabolic and Regulatory Insights from the Experimental Horizontal Gene Transfer of the Aurofusarin and Bikaverin Gene Clusters to Aspergillus Nidulans. Mol. Microbiol. 2019, 112, 1684–1700. [Google Scholar] [CrossRef] [PubMed]
- Limón, M.C.; Rodríguez-Ortiz, R.; Avalos, J. Bikaverin Production and Applications. Appl. Microbiol. Biotechnol. 2010, 87, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Haidar, S.; Aichele, D.; Birus, R.; Hielscher, J.; Laitinen, T.; Poso, A.; Jose, J. In Vitro and In Silico Evaluation of Bikaverin as a Potent Inhibitor of Human Protein Kinase CK2. Molecules 2019, 24, 1380. [Google Scholar] [CrossRef]
- Hinojosa-Ventura, G.; Puebla-Pérez, A.M.; Gallegos-Arreola, M.P.; Chávez-Parga, M.D.C.; Romero-Estrada, A.; Delgado-Saucedo, J.I. Cytotoxic and Antitumoral Effects of Bikaverin Isolated from Gibberella Fujikuroi on L5178Y Lymphoma Murine Model. J. Mex. Chem. Soc. 2019, 63, 115–122. [Google Scholar] [CrossRef]
- Balan, J.; Fuska, J.; Kuhr, I.; Kuhrová, V. Bikaverin, an Antibiotic FromGibberella Fujikuroi, Effective AgainstLeishmania Brasiliensis. Folia Microbiol. 1970, 15, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Florez, H. Coronavirus Disease 2019 Drug Discovery through Molecular Docking. F1000Research 2020, 9, 502. [Google Scholar] [CrossRef]
- Kwon, H.-R.; Son, S.-W.; Han, H.-R.; Choi, G.-J.; Jang, K.-S.; Choi, Y.-H.; Lee, S.; Sung, N.-D.; Kim, J.-C. Nematicidal Activity of Bikaverin and Fusaric Acid Isolated from Fusarium Oxysporum against Pine Wood Nematode, Bursaphelenchus Xylophilus. Plant Pathol. J. 2007, 23, 318–321. [Google Scholar] [CrossRef]
- Son, S.W.; Kim, H.Y.; Choi, G.J.; Lim, H.K.; Jang, K.S.; Lee, S.O.; Lee, S.; Sung, N.D.; Kim, J.C. Bikaverin and Fusaric Acid from Fusarium Oxysporum Show Antioomycete Activity against Phytophthora Infestans. J. Appl. Microbiol. 2008, 104, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Busman, M.; Butchko, R.A.E.; Proctor, R.H. LC-MS/MS Method for the Determination of the Fungal Pigment Bikaverin in Maize Kernels as an Indicator of Ear Rot. Food Addit. Contam. Part A 2012, 29, 1736–1742. [Google Scholar] [CrossRef]
- Mendonça, M.; dos Santos, M.C.; Pereira, A.K.; Fill, T.P.; Forte, M.B.S.; Bicas, J.L. Recovery and Purification of Bikaverin Produced by Fusarium Oxysporum CCT7620. Food Chem. X 2021, 12, 100136. [Google Scholar] [CrossRef] [PubMed]
- Kjær, D.; Kjær, A.; Pedersen, C.; Bu’Lock, J.D.; Smith, J.R. Bikaverin and Norbikaverin, Benzoxanthentrione Pigments of Gibberella Fujikuroi. J. Chem. Soc. C Org. 1971, 2792–2797. [Google Scholar] [CrossRef]
- Kato, T.; Sato, M.; Katagiri, N.; Awaji, T.; Nakano, J. Chemical Reactions of Bikaverin. Chem. Pharm. Bull. 1978, 26, 209–214. [Google Scholar] [CrossRef]
- de Koning, C.B.; Giles, R.G.F.; Engelhardt, L.M.; Whitet, A.H. Convenient Syntheses of the Naturally Occurring Benzo[b]Xanthen-12-One Bikaverin. X-Ray Crystallographic Confirmation of the Product Regiochemistry. J. Chem. Soc. Perkin Trans. 1 1988, 3209–3216. [Google Scholar] [CrossRef]
- Cornforth, J.W.; Ryback, G.; Robinson, P.M.; Park, D. Isolation and Characterization of a Fungal Vacuolation Factor (Bikaverin). J. Chem. Soc. C Org. 1971, 2786. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Mathew, A.; Purohit, H.J. Characterization of Antibacterial Activity of Bikaverin from Fusarium Sp. HKF15. J. Biosci. Bioeng. 2014, 117, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Bhat, H.R.; Naqvi, T.; Rana, M.K.; Rizvi, M.A. Exploring Bikaverin as Metal Ion Biosensor: A Computational Approach. Acta Chim. Slov. 2019, 66, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Povolotckaia, A.; Pankin, D.; Novikov, V.; Borisov, E.; Kuznetsov, S.; Dorokhov, A.; Gulyaev, A.; Zavyalova, E.; Alieva, R.; Akulov, S.; et al. Investigation of Structural and Spectral Peculiarities of Fusarium Sp. Indicator Pigment Bostrycoidin. Molecules 2024, 29, 4765. [Google Scholar] [CrossRef]
- Pankin, D.; Povolotckaia, A.; Borisov, E.; Belyakov, M.; Borzenko, S.; Gulyaev, A.; Moskovskiy, M. Theoretical Modelling of Structure, Vibrational and UV–Vis Absorbance Spectra of Rubrofusarin Molecule. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 293, 122469. [Google Scholar] [CrossRef] [PubMed]
- Pankin, D.; Povolotckaia, A.; Smirnov, M.; Borisov, E.; Gulyaev, A.; Dorochov, A.; Novikov, V.; Kuznetsov, S.; Noy, O.; Belousov, S.; et al. Theoretical Investigation of Anhydrofusarubin: Structural and Optical Properties. Crystals 2023, 13, 1556. [Google Scholar] [CrossRef]
- Pankin, D.; Smirnov, M.; Povolotckaia, A.; Povolotskiy, A.; Borisov, E.; Moskovskiy, M.; Gulyaev, A.; Gerasimenko, S.; Aksenov, A.; Litvinov, M.; et al. DFT Modelling of Molecular Structure, Vibrational and UV-Vis Absorption Spectra of T-2 Toxin and 3-Deacetylcalonectrin. Materials 2022, 15, 649. [Google Scholar] [CrossRef] [PubMed]
- Mayo, D.W.; Miller, F.A.; Hannah, R.W. Course Notes on the Interpretation of Infrared and Raman Spectra; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; Wiley: Hoboken, NJ, USA, 2004; ISBN 9780470093078. [Google Scholar]
- Zhan, C.-G.; Nichols, J.A.; Dixon, D.A. Ionization Potential, Electron Affinity, Electronegativity, Hardness, and Electron Excitation Energy: Molecular Properties from Density Functional Theory Orbital Energies. J. Phys. Chem. A 2003, 107, 4184–4195. [Google Scholar] [CrossRef]
- Senet, P. Chemical Hardnesses of Atoms and Molecules from Frontier Orbitals. Chem. Phys. Lett. 1997, 275, 527–532. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; ScienceOpen, Inc.: Lexington, MA, USA, 2010. [Google Scholar]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results Obtained with the Correlation Energy Density Functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian Basis Sets for Molecular Calculations. I. Second Row Atoms, Z. = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Kopbalina, K.; Pankin, D.; Smirnov, M.; Ibrayev, N.; Turdybekov, D. Arrangement of Azidomethyl Group in Lupinine Azide: Structural and Spectroscopic Properties. Molecules 2025, 30, 582. [Google Scholar] [CrossRef]
- Kishkentayeva, A.; Kopbalina, K.; Shaimerdenova, Z.; Shults, E.; Gatilov, Y.; Pankin, D.; Smirnov, M.; Povolotckaia, A.; Turdybekov, D.; Mazhenov, N. Investigation of N-(2-Oxo-2H-Chromen-3-Carbonyl)Cytisine’s Crystal Structure and Optical Properties. Materials 2025, 18, 3153. [Google Scholar] [CrossRef]
- Dev, P.; Agrawal, S.; English, N.J. Determining the Appropriate Exchange-Correlation Functional for Time-Dependent Density Functional Theory Studies of Charge-Transfer Excitations in Organic Dyes. J. Chem. Phys. 2012, 136, 224301. [Google Scholar] [CrossRef]
- Peach, M.J.G.; Benfield, P.; Helgaker, T.; Tozer, D.J. Excitation Energies in Density Functional Theory: An Evaluation and a Diagnostic Test. J. Chem. Phys. 2008, 128, 044118. [Google Scholar] [CrossRef]
- Silla, E.; Tuñón, I.; Pascual-Ahuir, J.L. GEPOL: An Improved Description of Molecular Surfaces II. Computing the Molecular Area and Volume. J. Comput. Chem. 1991, 12, 1077–1088. [Google Scholar] [CrossRef]
| Theory | Experiment | |||||
|---|---|---|---|---|---|---|
| State 1 | State 2 | State 5 | State 7 | |||
| Bond label | Bond length, Å | |||||
| Ring 1 | C7C8 | 1.459 | 1.459 | 1.467 | 1.397 | 1.44(2) |
| C8C9 | 1.492 | 1.492 | 1.485 | 1.433 | 1.51(2) | |
| C9C10 | 1.353 | 1.353 | 1.347 | 1.376 | 1.33(2) | |
| C10C11 | 1.454 | 1.454 | 1.469 | 1.412 | 1.44(2) | |
| C11C6 | 1.457 | 1.456 | 1.481 | 1.401 | 1.47(2) | |
| C8O36 | 1.236 | 1.236 | 1.236 | 1.331 | 1.23(2) | |
| C9O26 | 1.331 | 1.331 | 1.337 | 1.341 | 1.32(2) | |
| O26C27 | 1.433 | 1.433 | 1.431 | 1.431 | 1.49(2) | |
| C11O35 | 1.250 | 1.250 | 1.226 | 1.334 | 1.25(2) | |
| Ring 2 | C1C4 | 1.425 | 1.426 | 1.414 | 1.490 | 1.41(2) |
| C4C7 | 1.391 | 1.391 | 1.398 | 1.445 | 1.40(2) | |
| C7C6 | 1.421 | 1.421 | 1.428 | 1.423 | 1.44(2) | |
| C6C5 | 1.402 | 1.403 | 1.399 | 1.456 | 1.38(2) | |
| C5C2 | 1.435 | 1.435 | 1.436 | 1.485 | 1.45(2) | |
| C4O31 | 1.333 | 1.333 | 1.336 | 1.239 | 1.34(2) | |
| C5O33 | 1.325 | 1.325 | 1.329 | 1.243 | 1.35(2) | |
| Ring 3 | C15O38 | 1.368 | 1.369 | 1.364 | 1.371 | 1.38(2) |
| O38C1 | 1.345 | 1.344 | 1.348 | 1.334 | 1.37(2) | |
| C1C2 | 1.386 | 1.386 | 1.383 | 1.361 | 1.36(2) | |
| C2C3 | 1.491 | 1.492 | 1.470 | 1.492 | 1.47(2) | |
| C3C13 | 1.473 | 1.474 | 1.455 | 1.475 | 1.47(2) | |
| C3O37 | 1.222 | 1.223 | 1.245 | 1.221 | 1.22(2) | |
| Ring 4 | C15C13 | 1.399 | 1.404 | 1.405 | 1.398 | 1.39(2) |
| C13C14 | 1.427 | 1.420 | 1.429 | 1.427 | 1.39(2) | |
| C14C16 | 1.380 | 1.388 | 1.377 | 1.380 | 1.45(2) | |
| C16C18 | 1.406 | 1.402 | 1.408 | 1.406 | 1.43(2) | |
| C18C17 | 1.386 | 1.389 | 1.386 | 1.386 | 1.37(2) | |
| C17C15 | 1.390 | 1.382 | 1.389 | 1.390 | 1.41(2) | |
| C14C39 | 1.507 | 1.507 | 1.506 | 1.507 | 1.54(2) | |
| C18O21 | 1.349 | 1.350 | 1.346 | 1.348 | 1.41(2) | |
| O21C22 | 1.429 | 1.429 | 1.431 | 1.430 | 1.49(2) | |
| Angle label | Angle, degree | |||||
| Ring 1 | C7C8C9 | 117.50 | 117.49 | 118.16 | 119.16 | 118.3(15) |
| C8C9C10 | 120.78 | 120.76 | 120.33 | 119.64 | 120.6(15) | |
| C9C10C11 | 122.16 | 122.18 | 123.40 | 121.09 | 121.9(15) | |
| C10C11C6 | 119.00 | 118.99 | 117.8 | 120.47 | 120.5(16) | |
| C11C6C7 | 119.48 | 119.51 | 118.98 | 118.42 | 117.8(15) | |
| C6C11O35 | 121.69 | 121.71 | 123.63 | 122.27 | 117.8(15) | |
| C10C11O35 | 119.31 | 119.29 | 118.49 | 117.26 | 121.7(15) | |
| C7C8O36 | 122.52 | 122.54 | 122.34 | 123.10 | 122.4(14) | |
| C9C8O36 | 119.98 | 119.97 | 119.5 | 117.74 | 119.2(15) | |
| C8C9O26 | 112.37 | 112.40 | 112.49 | 114.66 | 111.5(14) | |
| C9O26C27 | 118.13 | 118.15 | 117.73 | 118.31 | 115.7(12) | |
| Ring 2 | C1C4C7 | 118.32 | 118.36 | 118.10 | 116.73 | 118.1(15) |
| C4C7C6 | 120.36 | 120.34 | 121.60 | 120.13 | 119.6(15) | |
| C7C6C5 | 120.50 | 120.49 | 119.09 | 121.77 | 120.2(14) | |
| C6C5C2 | 119.89 | 119.93 | 119.68 | 118.38 | 120.3(15) | |
| C1C4O31 | 117.84 | 117.79 | 117.57 | 119.6 | 118.3(13) | |
| C4C7C8 | 118.56 | 118.59 | 117.16 | 118.65 | 119.8(14) | |
| C7C4O31 | 123.85 | 123.84 | 124.33 | 123.64 | 123.5(15) | |
| C2C5O33 | 119.62 | 119.61 | 118.72 | 121.23 | 120.0(14) | |
| C6C5O33 | 120.49 | 120.45 | 121.60 | 120.39 | 119.7(13) | |
| C5C6C11 | 120.01 | 120.00 | 121.93 | 119.81 | 122.0(15) | |
| Ring 3 | C15O38C1 | 120.65 | 120.67 | 120.44 | 120.37 | 118.8(12) |
| O38C1C4 | 113.71 | 113.66 | 115.41 | 111.43 | 112.7(13) | |
| O38C1C2 | 123.34 | 123.46 | 122.72 | 124.22 | 123.1(14) | |
| C3C2C1 | 119.01 | 119.06 | 119.27 | 119.24 | 120.8(14) | |
| C2C3C13 | 115.36 | 115.34 | 116.62 | 114.86 | 114.7(14) | |
| C2C3O37 | 121.87 | 121.89 | 120.24 | 122.37 | 123.7(15) | |
| Ring 4 | C13C14C16 | 118.93 | 119.37 | 118.79 | 118.85 | 121.2(15) |
| C39C14C13 | 122.82 | 122.76 | 122.66 | 122.78 | 124.2(14) | |
| C14C16C18 | 122.19 | 121.64 | 122.17 | 122.32 | 115.4(15) | |
| C16C18O21 | 115.59 | 124.37 | 115.45 | 115.60 | 125.4(15) | |
| C18C17C15 | 117.83 | 118.45 | 117.96 | 117.66 | 115.1(15) | |
| C17C15C13 | 123.98 | 123.58 | 123.52 | 124.24 | 124.7(15) | |
| C17C15O38 | 113.93 | 114.47 | 114.11 | 113.99 | 112.6(14) | |
| Hydrogen bonds and contacts label | Hydrogen bonds and contacts length, Å | |||||
| O33H34 | 1.003 | 1.003 | 0.999 | 1.613 | -- | |
| H34O35 | 1.594 | 1.593 | -- | 1.001 | -- | |
| O37H40 | 2.532 | 2.523 | 2.544 | 2.537 | -- | |
| O37H41 | 2.532 | 2.523 | 2.544 | 2.537 | -- | |
| O31H32 | 0.993 | 0.993 | 0.994 | 1.667 | -- | |
| H32O36 | 1.665 | 1.668 | 1.633 | 0.993 | -- | |
| H34O37 | -- | -- | 1.600 | -- | -- | |
| Theory | ||||
|---|---|---|---|---|
| State | Excited State № | Orbitals with > 14% Contribution (Percent) | Oscillator Strength | Wavelength, nm (Energy, eV) |
| 1 | 1 | 99HOMO -> 100LUMO (98) | 0.2620 | 528.48 (2.3461) |
| 6 | 95HOMO-4 -> 100 LUMO (88) | 0.0375 | 375.26 (3.3040) | |
| 10 | 93HOMO-6 -> 100 LUMO (88) | 0.1532 | 306.98 (4.0388) | |
| 11 | 98 HOMO-1 -> 101 LUMO+1 (58) 96 HOMO-3 -> 101 LUMO+1 (28) | 0.3014 | 284.92 (4.3515) | |
| 13 | 99 HOMO -> 102 LUMO+2 (82) | 0.3796 | 272.63 (4.5477) | |
| 17 | 95 HOMO-4 -> 101 LUMO+1 (58) 91 HOMO-8 -> 100 LUMO (17) 99 HOMO -> 103 LUMO+2 (14) | 0.3104 | 247.34 (5.0127) | |
| 24 | 93 HOMO-6 -> 101 LUMO+1 (78) | 0.1960 | 228.71 (5.4210) | |
| 28 | 98 HOMO-1 -> 103 LUMO+3 (61) | 0.2118 | 219.95 (5.6368) | |
| 37 | 96 HOMO-3 -> 104 LUMO+4 (52) 95 HOMO-4 -> 103 LUMO+3 (17) | 0.2380 | 203.07 (6.1056) | |
| 39 | 95HOMO-4 -> 103 LUMO+3 (63) 96 HOMO-3 -> 104 LUMO+4 (18) | 0.2391 | 201.16 (6.1635) | |
| 2 | 1 | 99 HOMO -> 100 LUMO (98) | 0.2532 | 528.05 (2.3480) |
| 6 | 95 HOMO-4 -> 100 LUMO (88) | 0.0436 | 375.32 (3.3034) | |
| 10 | 93 HOMO-6 -> 100 LUMO (89) | 0.1493 | 305.10 (4.0637) | |
| 11 | 98 HOMO-1 -> 101 LUMO+1 (82) | 0.3990 | 292.09 (4.2447) | |
| 13 | 99 HOMO -> 102 LUMO+2 (83) | 0.2659 | 272.37 (4.5520) | |
| 17 | 95 HOMO-4 -> 101 LUMO+1 (58) 91 HOMO-8 -> 100 LUMO (22) | 0.2873 | 248.15 (4.9962) | |
| 24 | 93 HOMO-6 -> 101 LUMO+1 (69) 98 HOMO-1 -> 103 LUMO+3 (15) | 0.1865 | 229.34 (5.4062) | |
| 37 | 96 HOMO-3 -> 104 LUMO+4 (62) 93 HOMO6 -> 102 LUMO+2 (16) | 0.1838 | 203.79 (6.0840) | |
| 39 | 95 HOMO-4 -> 103 LUMO+2 (80) | 0.2313 | 200.84 (6.1732) | |
| 5 | 1 | 99 HOMO -> 100 LUMO (99) | 0.2367 | 521.28 (2.3784) |
| 3 | 97 HOMO-2 -> 100 LUMO (59) 96 HOMO-3 -> 100 LUMO (30) | 0.0572 | 400.84 (3.0931) | |
| 7 | 99 HOMO -> 101 LUMO+1 57 | 0.0453 | 361.40 (3.4307) | |
| 11 | 97 HOMO-2 -> 101 LUMO+1 (57) 93 HOMO-6 -> 100 LUMO (14) | 0.3715 | 289.30 (4.2856) | |
| 14 | 99 HOMO -> 102 LUMO+2 (36) 97 HOMO-2 -> 101 LUMO+1 (20) | 0.4427 | 276.00 (4.4922) | |
| 22 | 97 HOMO-2 -> 102 LUMO+2 (58) 93 HOMO-6 -> 101 LUMO+1 (22) | 0.1756 | 232.11 (5.3416) | |
| 24 | 90 HOMO-9 -> 100 LUMO (68) 96 HOMO-3 -> 102 LUMO+2 (25) | 0.1320 | 228.58 (5.4240) | |
| 29 | 97 HOMO-2 -> 103 LUMO+3 (51) 96 HOMO-3 -> 103 LUMO+3 (14) | 0.1956 | 214.03 (5.7928) | |
| 35 | 95 HOMO-4 -> 103 LUMO+3 (40) 91 HOMO-8 -> 101 LUMO+1 (26) | 0.2172 | 204.93 (6.0501) | |
| 7 | 1 | 99 HOMO -> 100 LUMO (97) | 0.1989 | 543.42 (2.2815) |
| 3 | 97 HOMO-2 -> 100 LUMO (94) | 0.0357 | 446.38 (2.7776) | |
| 5 | 95 HOMO-4 -> 100 LUMO (92) | 0.0771 | 379.60 (3.2662) | |
| 9 | 99 HOMO -> 101 LUMO+1 (89) | 0.0764 | 325.86 (3.8049) | |
| 10 | 93 HOMO-6 -> 100 LUMO (80) | 0.0948 | 305.59 (4.0572) | |
| 12 | 96 HOMO-3 -> 101 LUMO+1 (79) | 0.2689 | 275.96 (4.4929) | |
| 18 | 95 HOMO-4 -> 101 LUMO+1 (78) | 0.2328 | 248.34 (4.9925) | |
| 26 | 97 HOMO-4 -> 103 LUMO+3 (37) 93 HOMO-6 -> 101 LUMO+1 (26) | 0.2214 | 222.98 (5.5603) | |
| 37 | 95 HOMO-4 -> 103 LUMO+3 (72) | 0.3487 | 205.53 (6.0324) | |
| State | 1 | 2 | 5 | 7 |
|---|---|---|---|---|
| Energy of HOMO, Ha (eV) | −0.22583 (−6.145) | −0.2258 (−6.144) | −0.22271 (−6.060) | −0.22457 (−6.111) |
| Energy of LUMO, Ha (eV) | −0.12547 (−3.414) | −0.12508 (−3.404) | −0.12000 (−3.265) | −0.12632 (−3.437) |
| HOMO-LUMO gap, Ha (eV) | 0.10036 (2.731) | 0.1005 (2.735) | 0.10271 (2.795) | 0.09825 (2.674) |
| Ionization potential, eV | 6.145 | 6.144 | 6.060 | 6.111 |
| Electron affinity, eV | 3.414 | 3.404 | 3.265 | 3.437 |
| Electronegativity, eV | 4.780 | 4.774 | 4.663 | 4.774 |
| Chemical hardness, eV | 1.366 | 1.370 | 1.398 | 1.337 |
| Electrophilicity, eV | 8.365 | 8.318 | 7.778 | 8.523 |
| Chemical softness, eV | 0.366 | 0.365 | 0.358 | 0.374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Povolotckaia, A.; Pankin, D.; Belousov, S.; Boyko, A.; Akulov, S.; Borisov, E.; Gulyaev, A.; Gudkov, S.; Izmailov, A.; Moskovskiy, M. Theoretical Investigation of Structural and Optical Peculiarities of Bikaverin Fungal Pigment in Chloroform Solution. Molecules 2025, 30, 4634. https://doi.org/10.3390/molecules30234634
Povolotckaia A, Pankin D, Belousov S, Boyko A, Akulov S, Borisov E, Gulyaev A, Gudkov S, Izmailov A, Moskovskiy M. Theoretical Investigation of Structural and Optical Peculiarities of Bikaverin Fungal Pigment in Chloroform Solution. Molecules. 2025; 30(23):4634. https://doi.org/10.3390/molecules30234634
Chicago/Turabian StylePovolotckaia, Anastasia, Dmitrii Pankin, Sergey Belousov, Andrey Boyko, Sergey Akulov, Evgenii Borisov, Anatoliy Gulyaev, Sergey Gudkov, Andrey Izmailov, and Maxim Moskovskiy. 2025. "Theoretical Investigation of Structural and Optical Peculiarities of Bikaverin Fungal Pigment in Chloroform Solution" Molecules 30, no. 23: 4634. https://doi.org/10.3390/molecules30234634
APA StylePovolotckaia, A., Pankin, D., Belousov, S., Boyko, A., Akulov, S., Borisov, E., Gulyaev, A., Gudkov, S., Izmailov, A., & Moskovskiy, M. (2025). Theoretical Investigation of Structural and Optical Peculiarities of Bikaverin Fungal Pigment in Chloroform Solution. Molecules, 30(23), 4634. https://doi.org/10.3390/molecules30234634








