Synthesis and Luminescent Properties of Dy3+-Activated Yellow Phosphors with Anomalous Thermal Quenching for w-LEDs
Abstract
1. Introduction
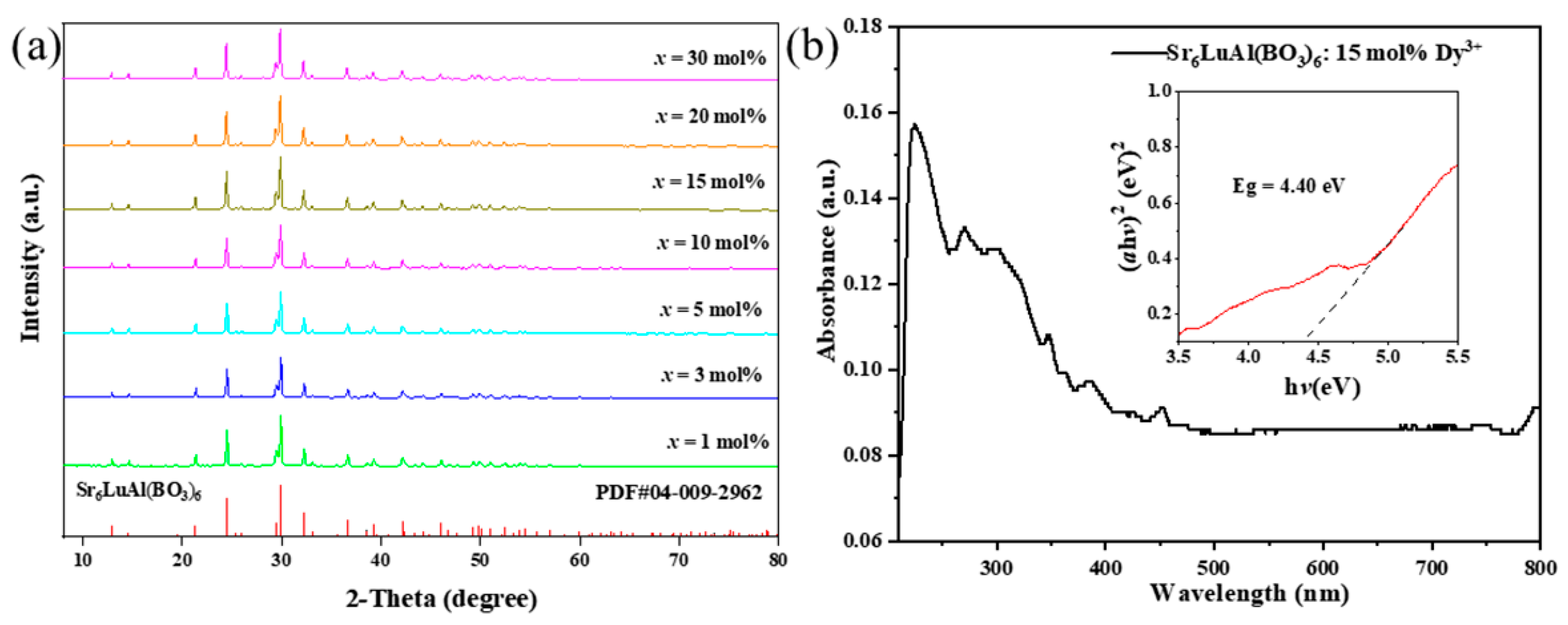
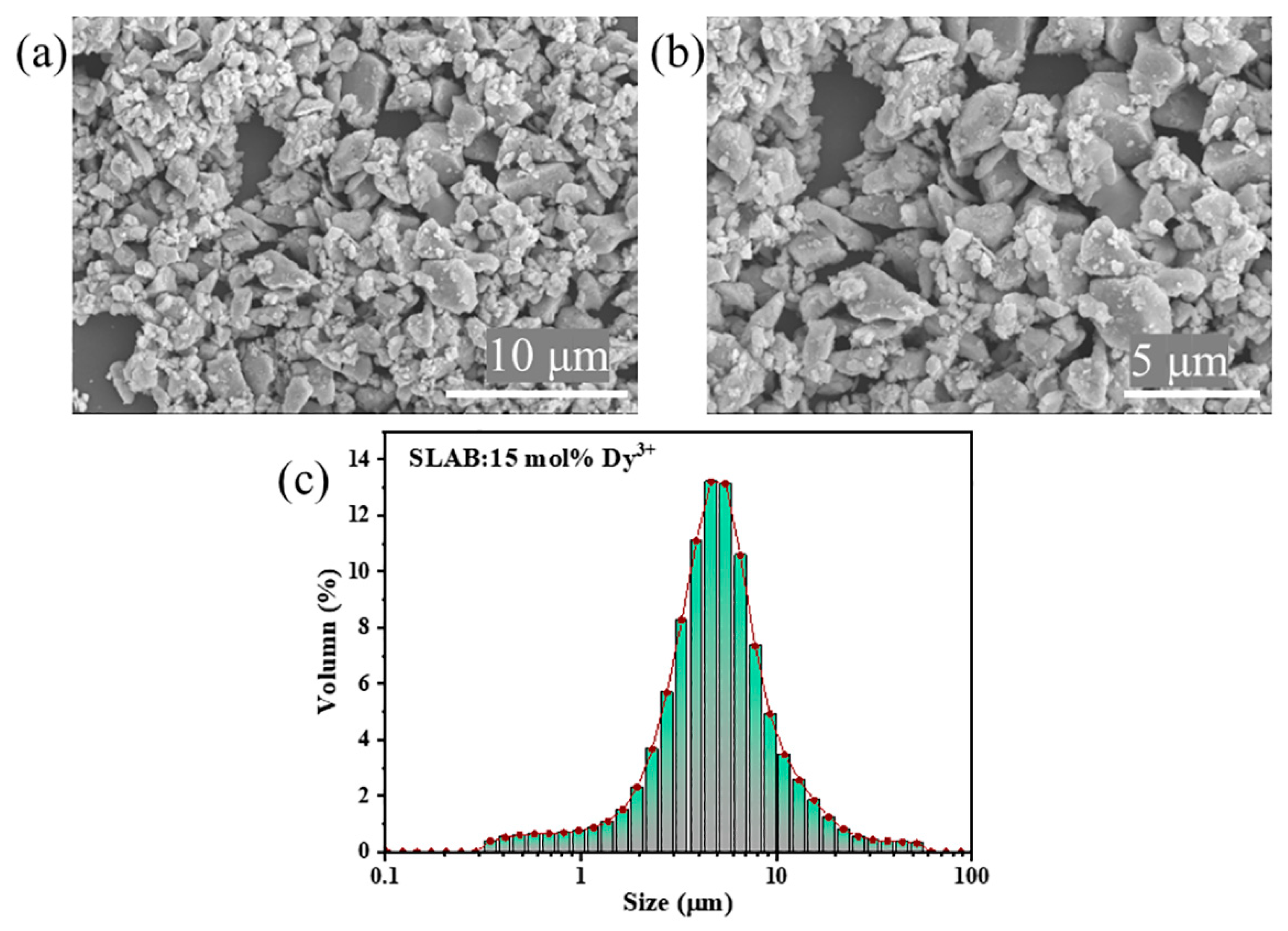
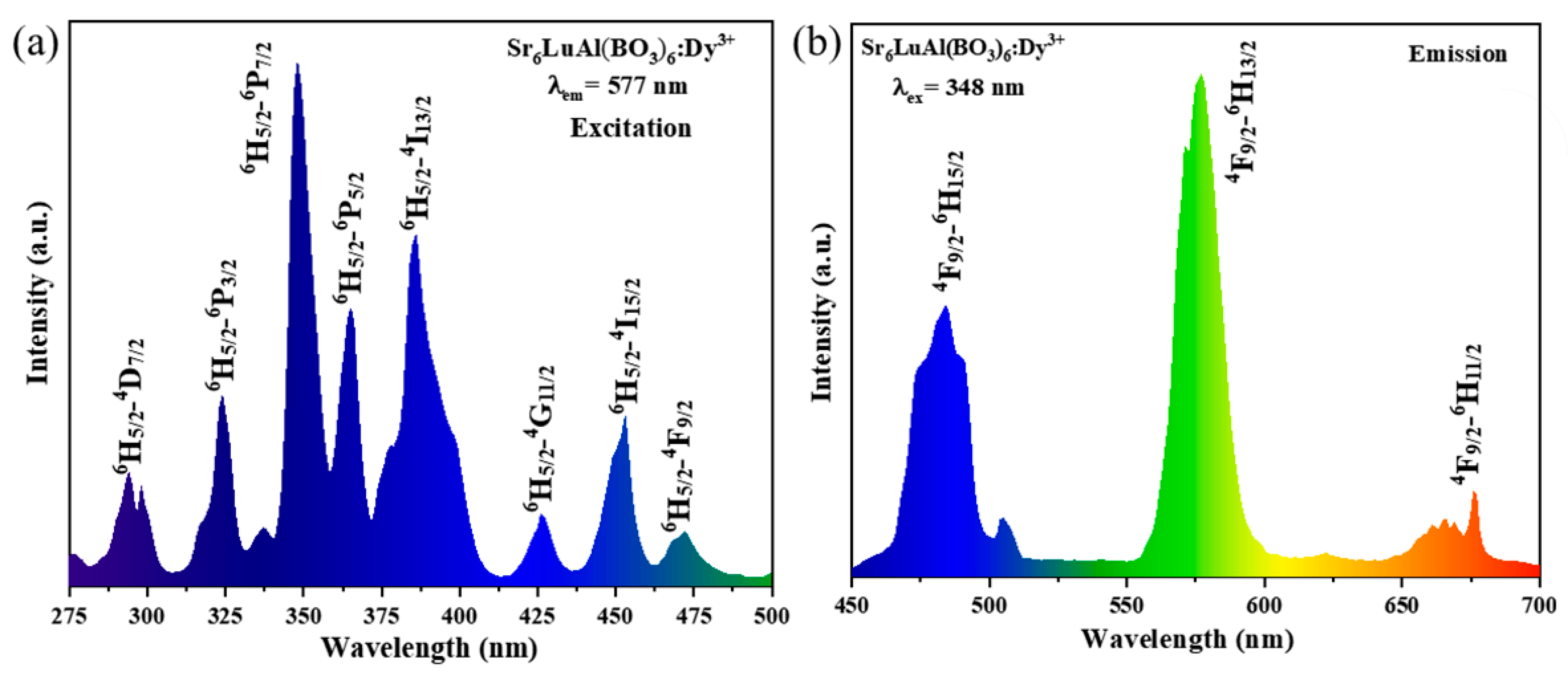
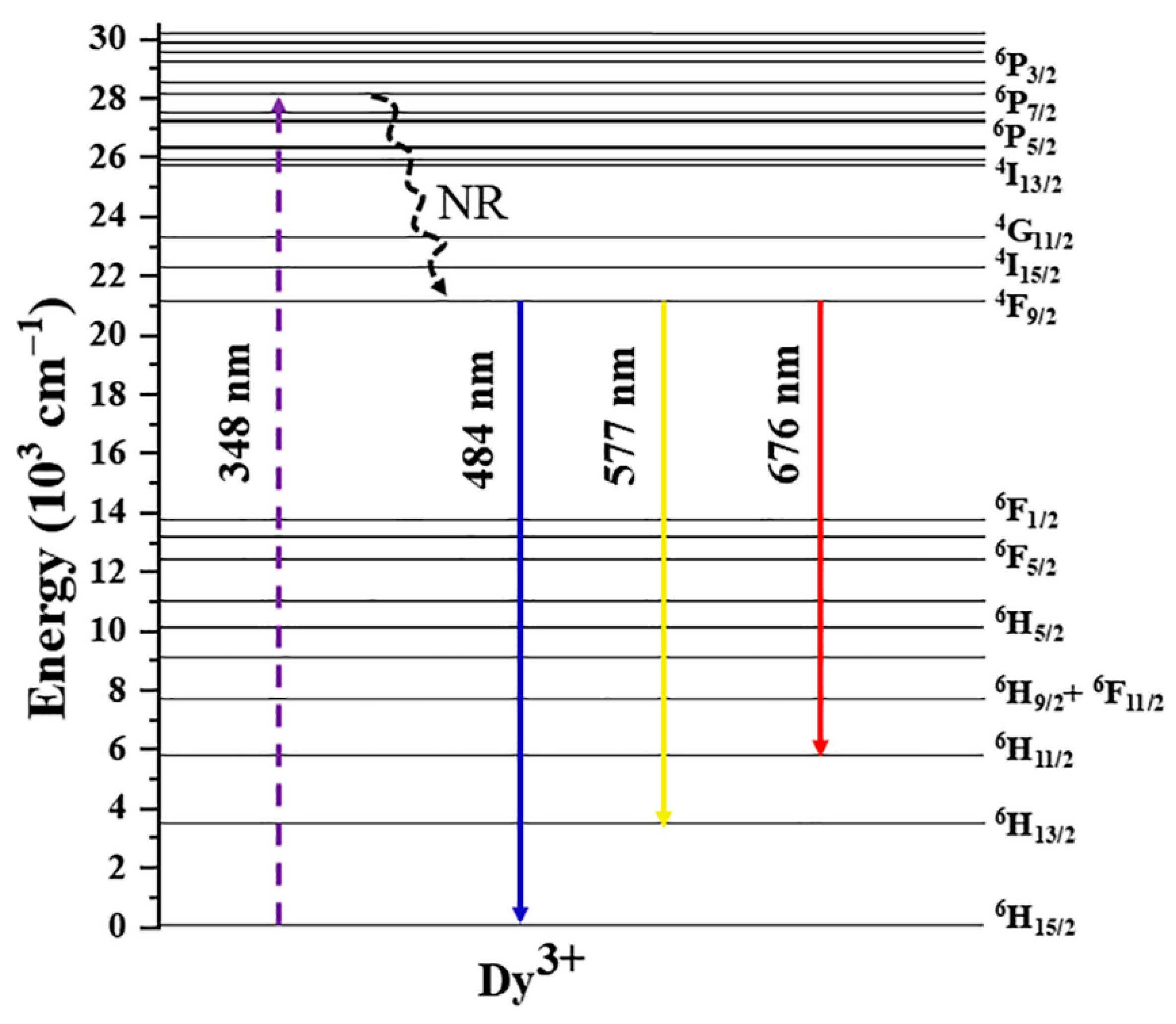
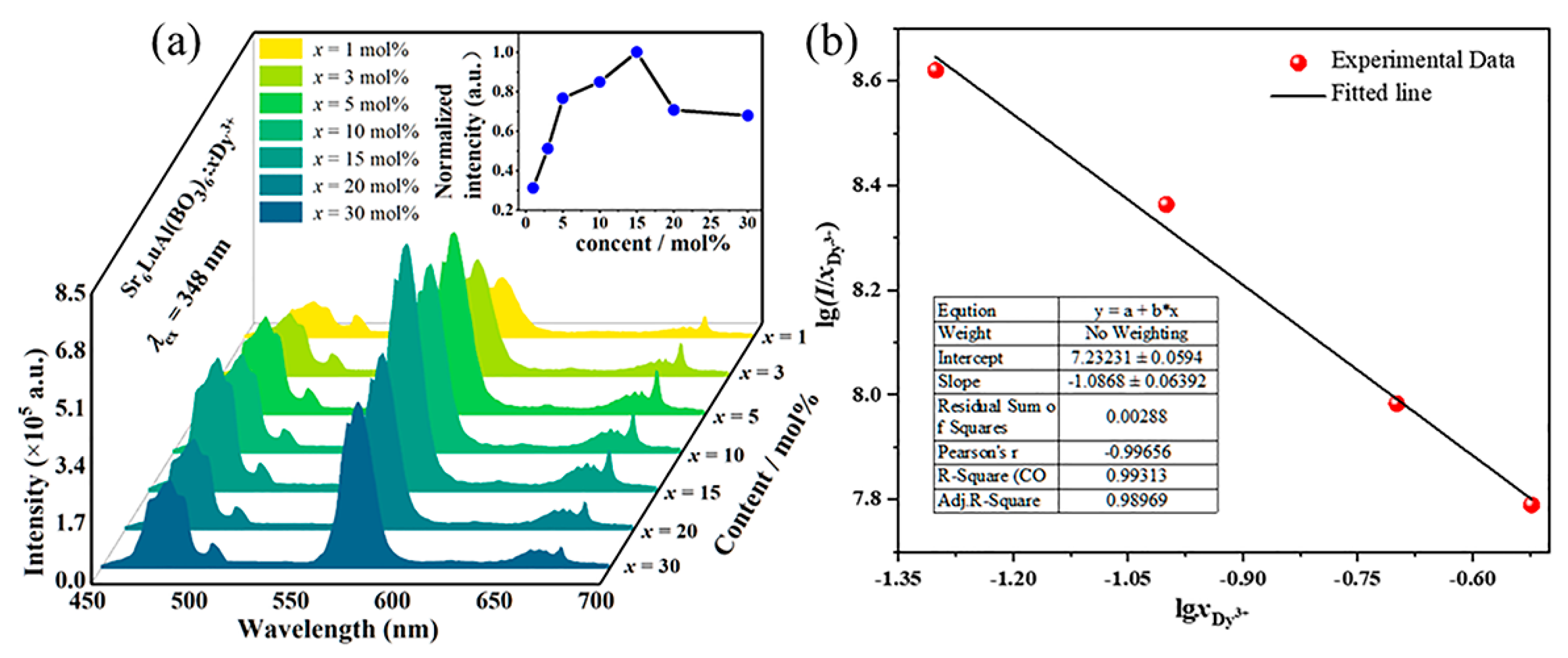
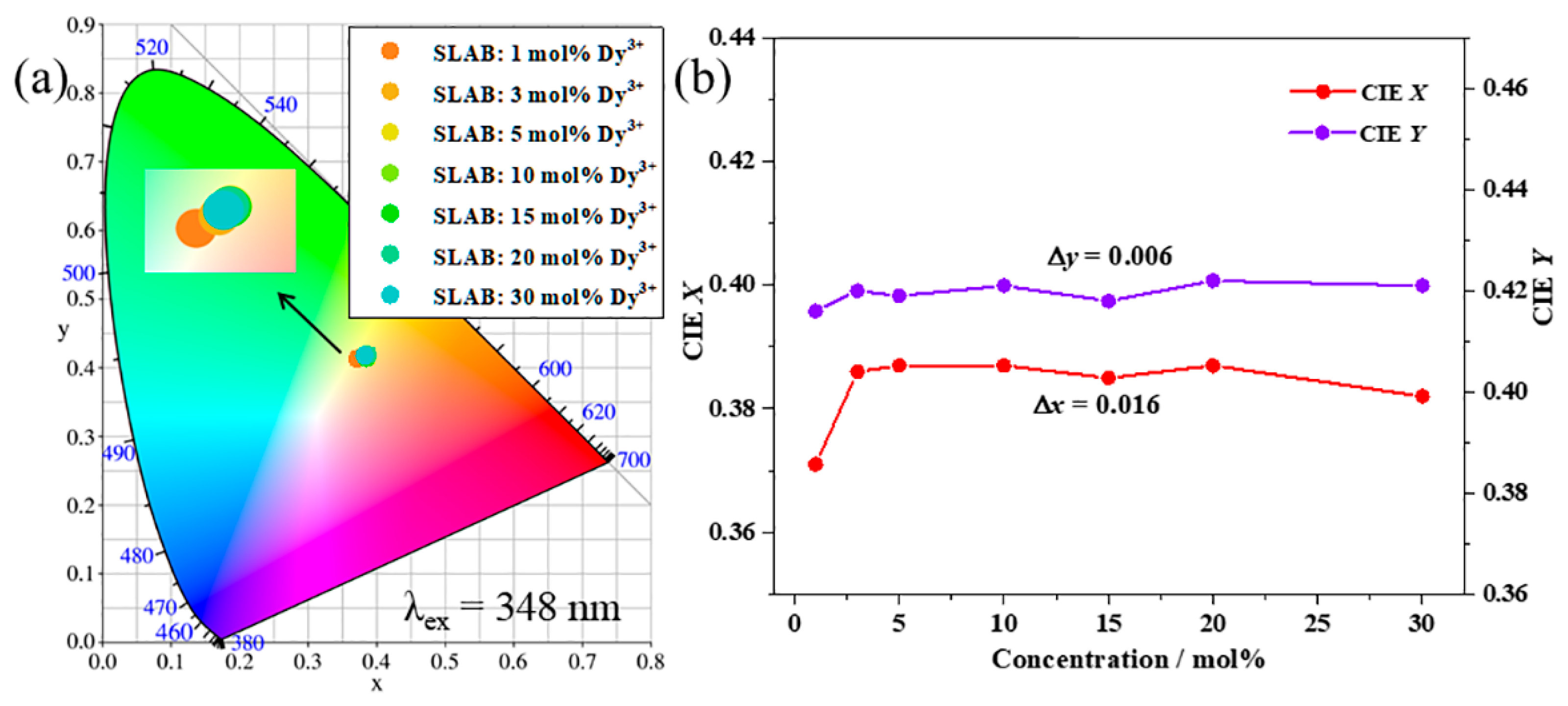
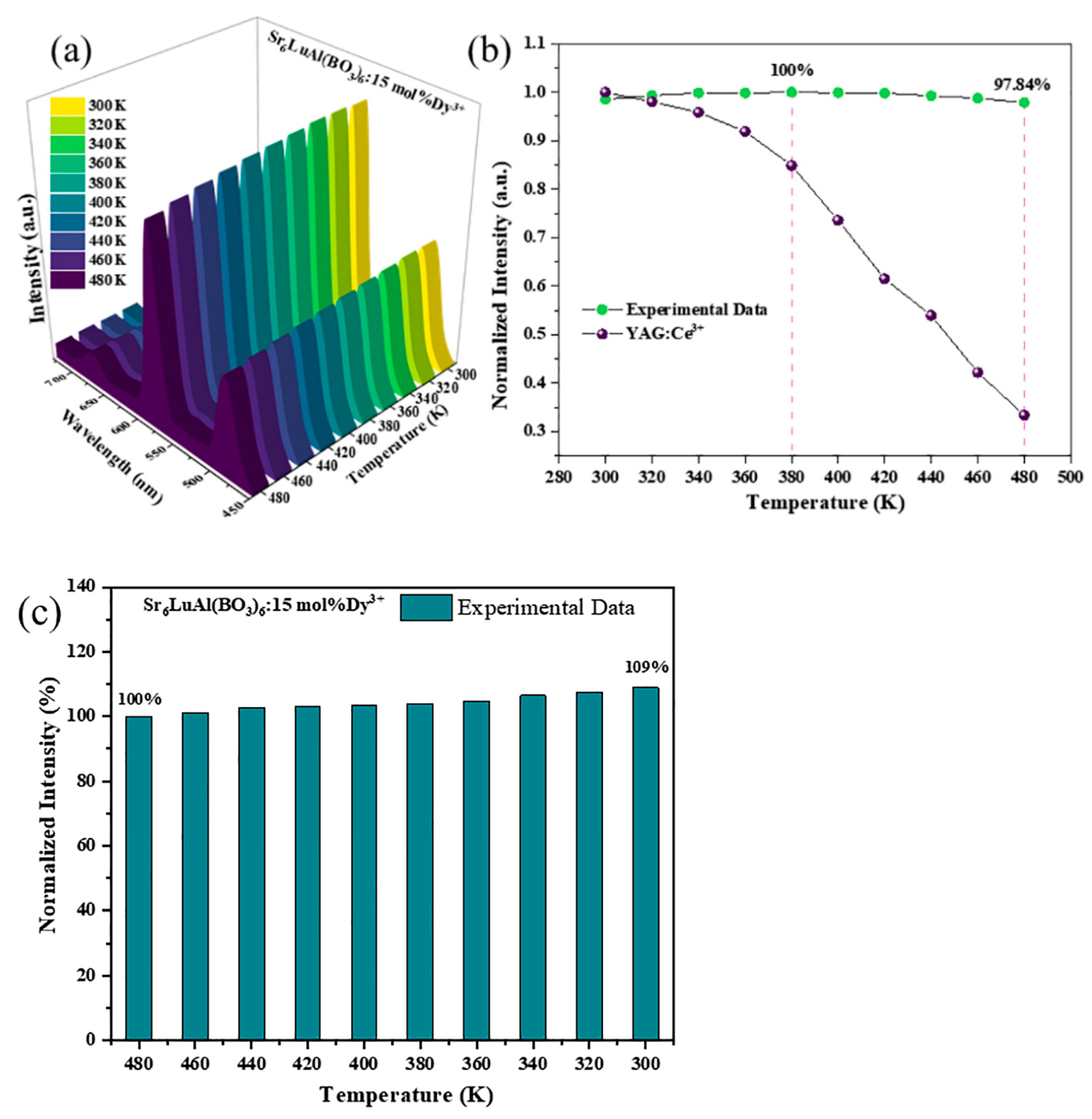
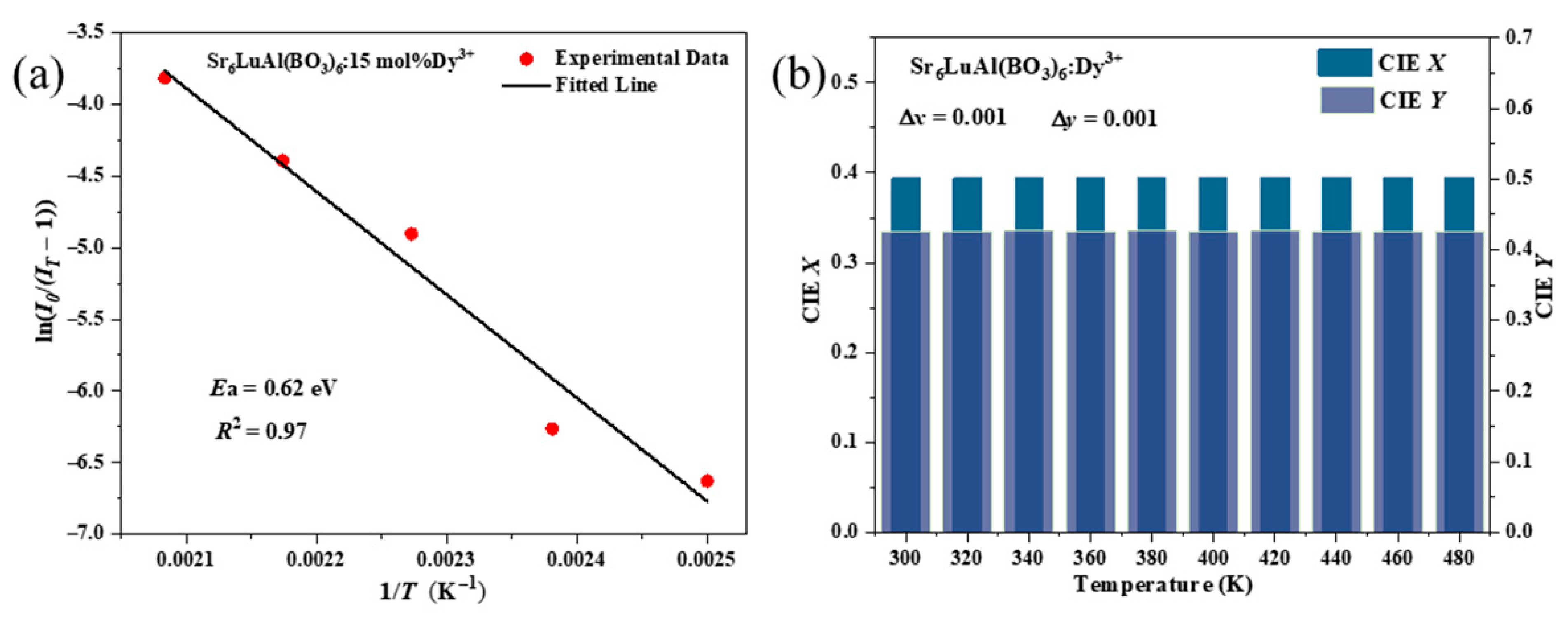
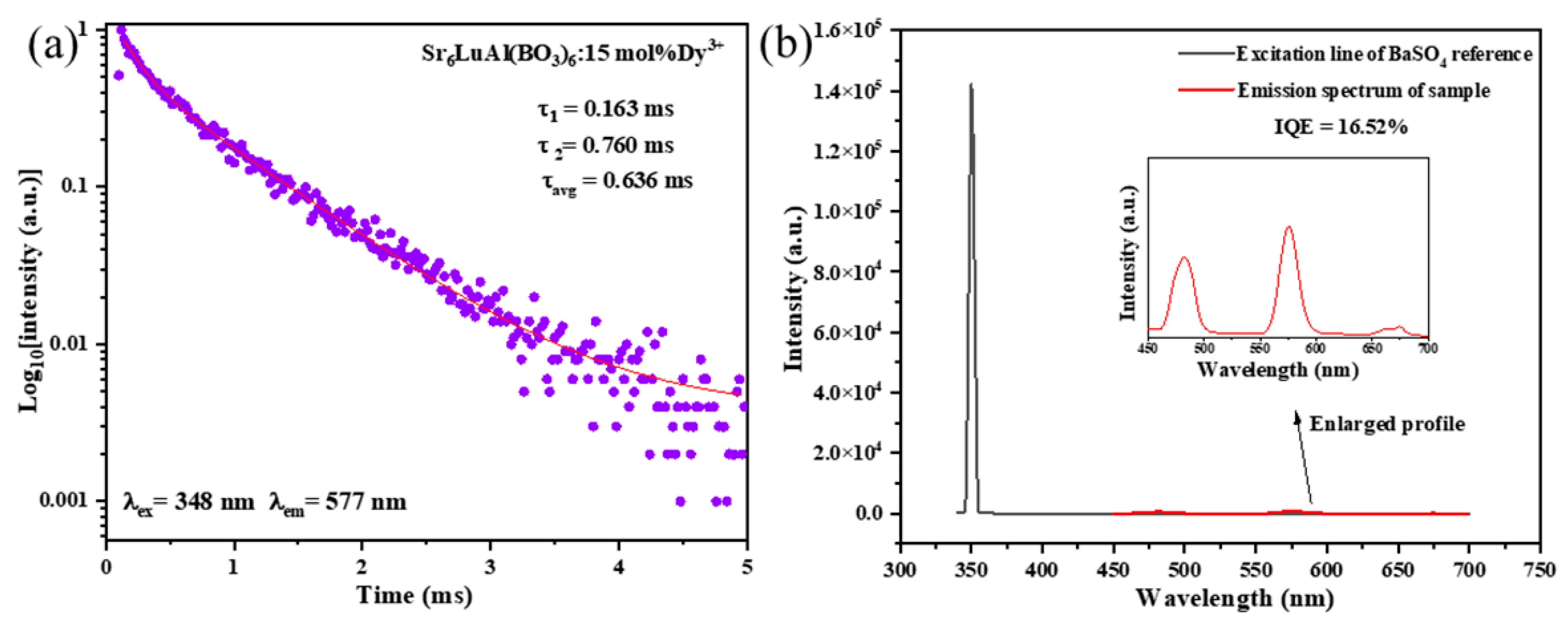
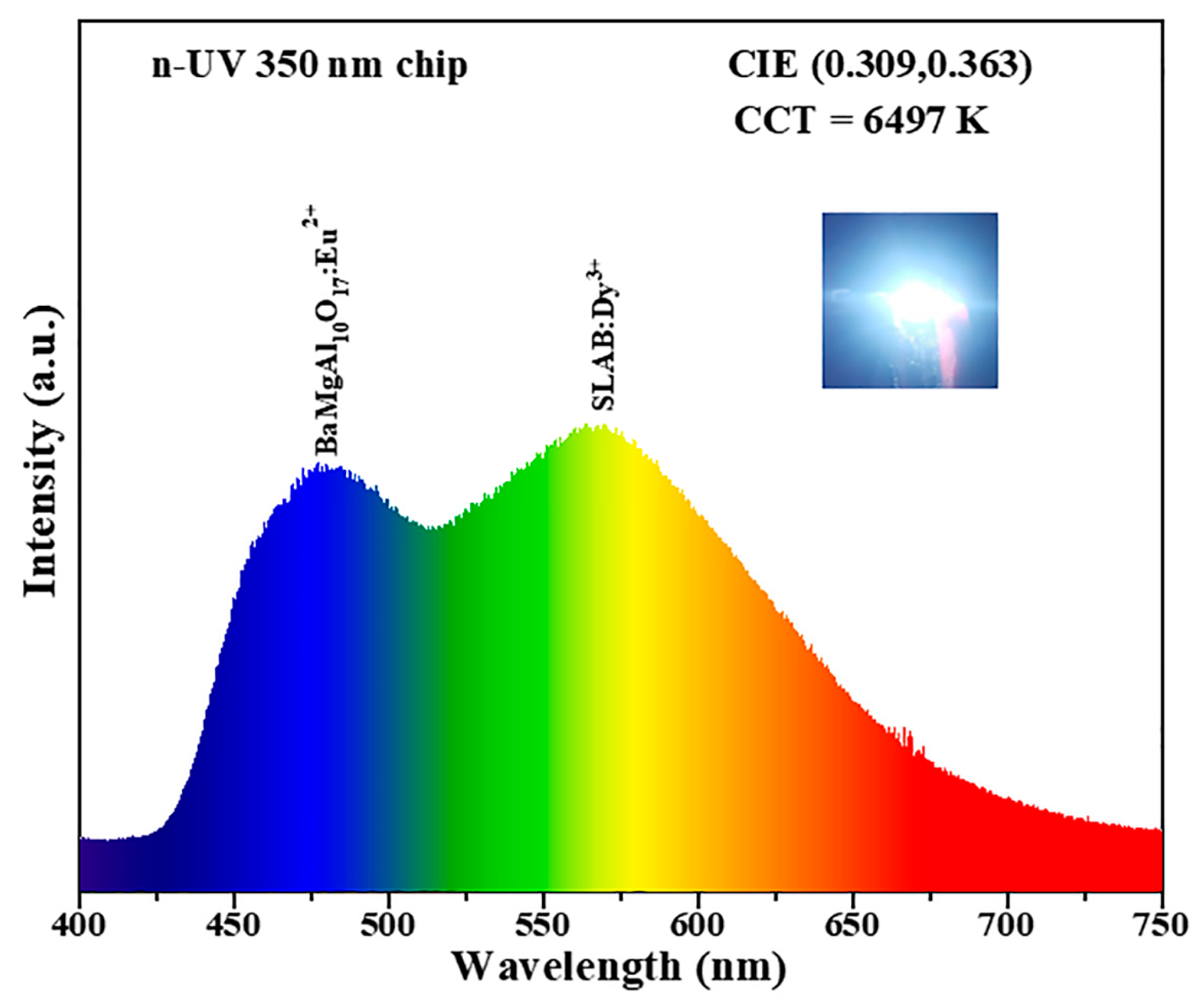
2. Results and Discussion
| Phosphors | Luminous Intensity at 420 K | References |
|---|---|---|
| SLAB:Dy3+ | 99.80% | This work |
| La5NbMo2O16:Dy3+ | 92.71% | [44] |
| La1.96LiNbO6:0.04Dy3+ | 83.00% | [30] |
| Na3La(PO4)2:Dy3+ | 52.00% | [45] |
| Ba2Gd5B5O17:Dy3+ | <52.20% | [46] |
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Wang, W.; Jin, P.; Sun, Z.; Zhan, Y.; Jiang, B. Reactive carbon dots/polysiloxane composites cross-linked silicone resin adhesives for stable white LED constructing. Compos. Commun. 2025, 53, 102172. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Pang, R.; Wang, W.; Li, D.; Jiang, L.; Zhang, S.; Zhang, H. Achieving chip-matched high purity red emission by Bi3+ sensitization in Ca3MgTiGe3O12:Pr3+. Mater. Res. Bull. 2023, 167, 112420. [Google Scholar] [CrossRef]
- Qiang, Y.; Cao, Y.; Ma, M.; Sun, L.; Zhang, M.; Liu, W.; Zhang, L.; You, W.; Qiu, G.; Zhao, H.; et al. BaO-Y2O3-Al2O3-SiO2/YAG:Ce: A novel YAG:Ce glass-ceramic with excellent fluorescent properties for phosphor-converted white LEDs. Ceram. Int. 2025, 51, 19199–19210. [Google Scholar] [CrossRef]
- Huang, X. Red phosphor converts white LEDs. Nat. Photonics 2014, 8, 748–749. [Google Scholar] [CrossRef]
- Chan, J.; Cao, L.; Xu, Z.; Huang, X. Cation substitution induced highly symmetric crystal structure in cyan-green-emitting Ca2La1−xLuxHf2Al3O12:Ce3+ solid-solution phosphors with enhanced photoluminescence emission and thermal stability: Toward full-visible-spectrum white LEDs. Mat. Today Phys. 2023, 35, 101130. [Google Scholar] [CrossRef]
- Huang, H.; Fu, Z.; Yin, S.; Chen, D.; Xie, A. Tunable Emission in Sb3+/Eu3+ Codoped Rare-Earth-Based Cs2NaTbCl6 Double Perovskite and Its LED Applications. Inorg. Chem. 2025, 64, 9287–9293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ding, X.; Wang, H.; Liu, B.; Wang, Y. A novel narrow-band blue-emitting phosphor with high efficiency and thermal stability for WLEDs and FEDs. J. Mater. Chem. C 2023, 11, 15366–15375. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, Y.; Fu, M.; Wang, C. Highly efficient rare-earth free vanadate phosphors for WLEDs. Dalton Trans. 2023, 52, 16819–16828. [Google Scholar] [CrossRef]
- Ma, N.; Luo, Z.; Liang, H.; He, L.; Yang, J.; Wu, T.; Lu, A. Near-pure red emission of highly thermally stable Eu3+ doped multi-component transparent oxyfluoride aluminosilicate glass-ceramic for red lasers and trichromatic WLEDs. Ceram. Int. 2024, 50, 45052–45063. [Google Scholar] [CrossRef]
- Génois, R.; Jobic, S.; Ouvrard, G.; Massuyeau, F.; Gautier, R. The crucial impact of cerium reduction on photoluminescence. Appl. Mater. Today 2020, 20, 100643. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, Z.; Gao, Y.; Feng, L.; Zhou, T.; Liu, M.; Zhao, Y.; Lai, X.; Bi, J.; Gao, D. Novel double perovskite Ca2Gd0.5Nb1-xW5x/6O6:0.5 Eu3+ red phosphors with excellent thermal stability and high color purity for white LEDs. Chem. Eng. J. 2023, 456, 140901. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Ding, N.; Yang, H.; Wang, L.; Zhang, Q. Dual-channel enhanced luminescence of double perovskite NaGdMgWO6:Eu3+ phosphor based on alternative excitation and delayed quenching. J. Alloys Compd. 2015, 642, 45–52. [Google Scholar] [CrossRef]
- Ratnam, B.V.; Jayasimhadri, M.; Jang, K.; Sueb Lee, H.; Yi, S.S.; Jeong, J.H. White Light Emission from NaCaPO4:Dy3+ Phosphor for Ultraviolet-Based White Light-Emitting Diodes. J. Am. Ceram. Soc. 2010, 93, 3857–3861. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Liu, X.; Lin, L. Enhanced luminescence of novel Ca3B2O6:Dy3+ phosphors by Li+-codoping for LED applications. Ceram. Int. 2012, 38, 1065–1070. [Google Scholar] [CrossRef]
- Liu, S.; He, J.; Wu, Z.; Jeong, J.H.; Deng, B.; Yu, R. Preparation and study on the spectral properties of garnet-type Li3Gd3Te2O12:Dy3+ single-phase full-color phosphor. J. Lumin. 2018, 200, 164–168. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Ju, Z.; Zhao, S.; Pei, P.; Ma, X.; Liu, W. Novel white-emitting afterglow phosphor Na2CaSn2Ge3O12:Dy3+: Preparation, photoluminescence, and phosphorescence properties. J. Alloys Compd. 2021, 856, 157230. [Google Scholar] [CrossRef]
- Dewangan, P.; Bisen, D.P.; Brahme, N.; Sharma, S.; Tamrakar, R.K.; Sahu, I.P.; Upadhyay, K. Influence of Dy3+ concentration on spectroscopic behaviour of Sr3MgSi2O8:Dy3+ phosphors. J. Alloys Compd. 2020, 816, 152590. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, C.; Shi, W.; Kang, Y.; Wang, Y.; Zhang, W.; Wang, D. Enhanced novel white emission in Ca3(PO4)2:Dy3+ single-phase full-color phosphor by charge compensation. J. Mater. Sci. Mater. Electron. 2014, 26, 1923–1931. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, H.; Wang, J.; Xue, Y.; Li, S.; Li, X.; Wan, Z.; Luo, J.; Li, L.; Huang, H.; et al. A Novel Yellow Phosphor (Ba3Sc2(BO3)4: Eu2+) Suitable for Full-Spectrum Lighting, Featuring Tunable Photoluminescence and Enhanced Luminous Thermal Stability. Laser Photonics Rev. 2025, 2402304, 2402304. [Google Scholar] [CrossRef]
- Behera, M.; Panda, R.; Arun Kumar, R.; Wozny, P.; Mishra, N.K.; Kumar, K.; Runowski, M. Highly saturated red-emitting novel europium doped yttrium calcium borate (Eu3+: Y2CaB10O19) phosphor materials for eco-friendly solid-state lighting applications. J. Alloys Compd. 2024, 1006, 176294. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, Y.; Peng, Y.; Zhou, H.; Tang, W.; Jiang, J.; Wu, Y.; Cai, S.; Xie, L.; Deng, B. Synthesis of Sr6LuAl(BO3)6:Sm3+ Red Phosphor with Excellent Thermal Stability and Its Application in w-LEDs. Molecules 2024, 29, 5495. [Google Scholar] [CrossRef]
- Schaffers, K.I.; Thompson, P.D.; Alekel, T., III; Cox, J.R.; Keszler, D.A. StacK crystal chemistry. Chem. Mater. 1994, 6, 2014–2022. [Google Scholar] [CrossRef]
- Yang, D.; Fang, Z.Y.; Zheng, Y.K.; Xiang, Y.F.; Song, R.T.; Yang, T.S.; Song, J.L.; Zhu, J. Pink-emitting RbBaBP2O8:Sm phosphor with diamondoid structure and high thermostability. Ceram. Int. 2021, 47, 21828–21836. [Google Scholar] [CrossRef]
- Zheng, L.; Li, W.; Hu, W.; Deng, B. Synthesis and luminescence properties of yellow-emitting LiGd6(BO3)3O5:Dy3+ borate phosphors with good thermal stability for white LEDs. J. Mater. Sci. Mater. Electron. 2022, 33, 15904–15913. [Google Scholar] [CrossRef]
- Fan, F.Y.; Zhao, L.; Shang, Y.F.; Liu, J.; Chen, W.B.; Li, Y.Y. Thermally stable double-perovskite Ca3TeO6:Eu3+ red-emitting phosphors with high color purity. J. Lumin. 2019, 211, 14–19. [Google Scholar] [CrossRef]
- Yu, C.; He, H.; Zhou, W.; Liu, Z.; Wei, L. Novel rugby-ball-like Zn3(PO4)2@C3N4 photocatalyst with highly enhanced visible-light photocatalytic performance. Sep. Purif. Technol. 2019, 217, 137–146. [Google Scholar] [CrossRef]
- Li, J.; Wei, L.; Yu, C.; Fang, W.; Xie, Y.; Zhou, W.; Zhu, L. Preparation and characterization of graphene oxide/Ag2CO3 photocatalyst and its visible light photocatalytic activity. Appl. Surf. Sci. 2015, 358, 168–174. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Ji, H.; Wu, K.; Wang, Y. Preparation of Ba9Lu2Si6O24:Sm3+ red phosphor and its application in white LEDs. J. Alloys Compd. 2025, 1021, 179727. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Wu, Y.; Sun, K.; Fang, Q.; Zhang, Y.; Zhang, Y. All-inorganic Sb3+-Ln3+ co-doped lead-free perovskites with dual-matrix advantage for efficient single-source white light emission. J. Alloys Compd. 2025, 1032, 181212. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Zhou, Q.; Chen, S.; Zhou, T.; Lai, X.; Bi, J.; Gao, D. White light emission and superior thermal stability in the single-phase La2LiNbO6:Dy3+,Eu3+ phosphors for WLEDs. Ceram. Int. 2025, 51, 37425–37433. [Google Scholar] [CrossRef]
- Jayachandiran, M.; Kennedy, S.M.M. Synthesis and optical properties of Ba3Bi2(PO4)4:Dy3+ phosphors for white light emitting diodes. J. Alloys Compd. 2019, 775, 353–359. [Google Scholar] [CrossRef]
- Boruc, Z.; Kaczkan, M.; Fetlinski, B.; Turczynski, S.; Malinowski, M. Blue emissions in Dy3+ doped Y4Al2O9 crystals for temperature sensing. Opt. Lett. 2012, 37, 5214. [Google Scholar] [CrossRef]
- Sun, P.; Liu, S.; Shu, S.; Ding, K.; Wang, Y.; Liu, Y.; Deng, B.; Yu, R. Highly thermal-stable yellow-emitting tantalate phosphor Ca2YTaO6:Dy3+ for white-LEDs. Opt. Mater. 2019, 96, 109300. [Google Scholar] [CrossRef]
- Manjunatha, C.; Sunitha, D.V.; Nagabhushana, H.; Nagabhushana, B.M.; Sharma, S.C.; Chakradhar, R.P.S. Combustion synthesis, structural characterization, thermo and photoluminescence studies of CdSiO3:Dy3+ nanophosphor. Spectrochim. Acta Part A 2012, 93, 140–148. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in Oxidic phosphors. Phys. Lett. 1968, 28A, 444–445. [Google Scholar] [CrossRef]
- Dexter, D.L.; Schulman, J.H. Theory of concentration qcuenching in inorganic phosphors. Adv. Chem. Phys. 1954, 22, 1063–1070. [Google Scholar] [CrossRef]
- Geng, X.; Xie, Y.; Chen, S.; Luo, J.; Li, S.; Wang, T.; Zhao, S.; Wang, H.; Deng, B.; Yu, R.; et al. Enhanced local symmetry achieved zero-thermal-quenching luminescence characteristic in the Ca2InSbO6:Sm3+ phosphors for w-LEDs. Chem. Eng. J. 2021, 410, 128396. [Google Scholar] [CrossRef]
- Wang, J.; Peng, X.; Cheng, D.; Zheng, Z.; Guo, H. Tunable luminescence and energy transfer in Y2BaAl4SiO12:Tb3+,Eu3+ phosphors for solid-state lighting. J. Rare Earths 2021, 39, 284–290. [Google Scholar] [CrossRef]
- Santra, A.; Chakraborty, N.; Panigrahi, K.; Chattopadhyay, K.K.; Ghorai, U.K. SrTiO3: Sm3+, Na+-codoped orange-emitting nanophosphor for pc-WLEDs. J. Mater. Sci. Mater. Electron. 2021, 33, 1–15. [Google Scholar] [CrossRef]
- McCamy, C.S. Correlated color temperature as an explicit function of chromaticity coordinates. Color. Res. Appl. 1992, 17, 142–144. [Google Scholar] [CrossRef]
- Ma, N.; Li, W.; Devakumar, B.; Wang, S.; Sun, L.; Zhang, Z.; Huang, X. Bright red luminescence from Mn4+ ions doped Sr2LuTaO6 double-perovskite phosphors. J. Lumin. 2021, 233, 117901. [Google Scholar] [CrossRef]
- Liao, F.; Shen, B.; Wu, W.; Zhang, Y.; Hu, J. A Study on the Anti-thermal Dy3+/Eu3+ Co-doped BaLa4Si3O13 Red Phosphors for White-Light-Emitting Diodes and Optical Thermometry Applications. Ind. Eng. Chem. Res. 2021, 60, 2931–2943. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Z.; Wang, C.; Wang, X.; Zhou, F.; Gao, M.; Xin, S.; Wang, Y. Highly Eu3+ ions doped novel red emission solid solution phosphors Ca18Li3(Bi,Eu)(PO4)14: Structure design, characteristic luminescence and abnormal thermal quenching behavior investigation. Dalton Trans. 2019, 48, 1624–1632. [Google Scholar] [CrossRef]
- Deng, B.; Yang, Y.; Chen, W.S.; Xie, X.J.; Yang, R.Q.; Li, C.L.; Zou, Z.Q.; Ouyang, X.; Zhao, J.; Yu, R.J. Synthesis and characterization of thermostable Dy-doped La5NbMo2O16 yellow-emitting phosphors for w-LEDs. J. Mater. Sci. Mater. Electron. 2022, 33, 23042–23053. [Google Scholar] [CrossRef]
- Bedyal, A.K.; Kunti, A.K.; Kumar, V.; Swart, H.C. Effects of cationic substitution on the luminescence behavior of Dy3+ doped orthophosphate phosphor. J. Alloys Compd. 2019, 806, 1127–1137. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Huang, X. Synthesis and photoluminescence properties of novel yellow-emitting Ba2Gd5−xDyxB5O17 phosphors. J. Mater. Sci. Mater. Electron. 2018, 29, 15022–15028. [Google Scholar] [CrossRef]
- Xu, O.; Peng, H.; Wei, Q.; Kong, L.; Wang, X.; Zhang, H.; Zhao, J.; Zou, B. Large-scale preparation of Sb3+-activated hybrid metal halides with efficient tunable emission from visible to near-infrared regions for advanced photonic applications. Mater. Horiz. 2025, 12, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Gao, Y.; Chen, J.; Lu, X.; Zhu, B.; Huang, L.; Qiu, J. A Nickel-doped, lanthanum gallium germanate-based phosphor as an ultra-broadband short-wavelength infrared region emitter for optical imaging. Ceram. Int. 2024, 50, 23685–23693. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, L.; Liu, H.; Xiong, G.; Wu, H.; Hu, Y.; Cheng, X.; Jin, Y. Multi-site occupation of Cr3+ toward developing broadband near-infrared phosphors. Ceram. Int. 2021, 47, 23558–23563. [Google Scholar] [CrossRef]
- Wu, J.; Liu, C.; Jia, H.; Qi, Y.; Liu, Z.; Hu, Y.; Feng, F. Optical properties, energy transfer and thermal stability of spherical nano-phosphor YPO4:Eu3+:Sm3+. J. Lumin. 2022, 245, 118791. [Google Scholar] [CrossRef]
- Ji, W.; Zhu, F.; Gao, Y.; Qiu, J. Near-Infrared Luminescence of Cr3+-Doped Ba2NaNb5O15 Embedded in Transparent Glass-Ceramics as Solid-State Illumination for Night-Vision Imaging. ACS Appl. Mat. Interfaces 2025, 17, 28366–28373. [Google Scholar] [CrossRef]
- Xu, Z.; Li, L.; Feng, L.; Wu, D.; Wang, X.; Shang, M. Trace Bi3+ doped single-phase Ca3WO6 phosphor with tunable cool-to-warm white light emission. Ceram. Int. 2025, 51, 28745–28752. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, Q.; Cao, Y.; Li, Y.; Wang, Y. Eu2+-doped ultra-broadband VIS-NIR emitting phosphor. Chem. Eng. J. 2020, 388, 124231. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, M. Photoluminescence and Energy Transfer of Ce3+, Tb3+, and Eu3+ Doped KBaY(BO3)2 as Near-Ultraviolet-Excited Color-Tunable Phosphors. Ind. Eng. Chem. Res. 2015, 54, 7632–7639. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, L.; Devakumar, B.; Liang, J.; Wang, S.; Sun, Q.; Dhoble, S.J.; Huang, X. Novel highly luminescent double-perovskite Ca2GdSbO6:Eu3+ red phosphors with high color purity for white LEDs: Synthesis, crystal structure, and photoluminescence properties. J. Lumin. 2020, 221, 117105. [Google Scholar] [CrossRef]
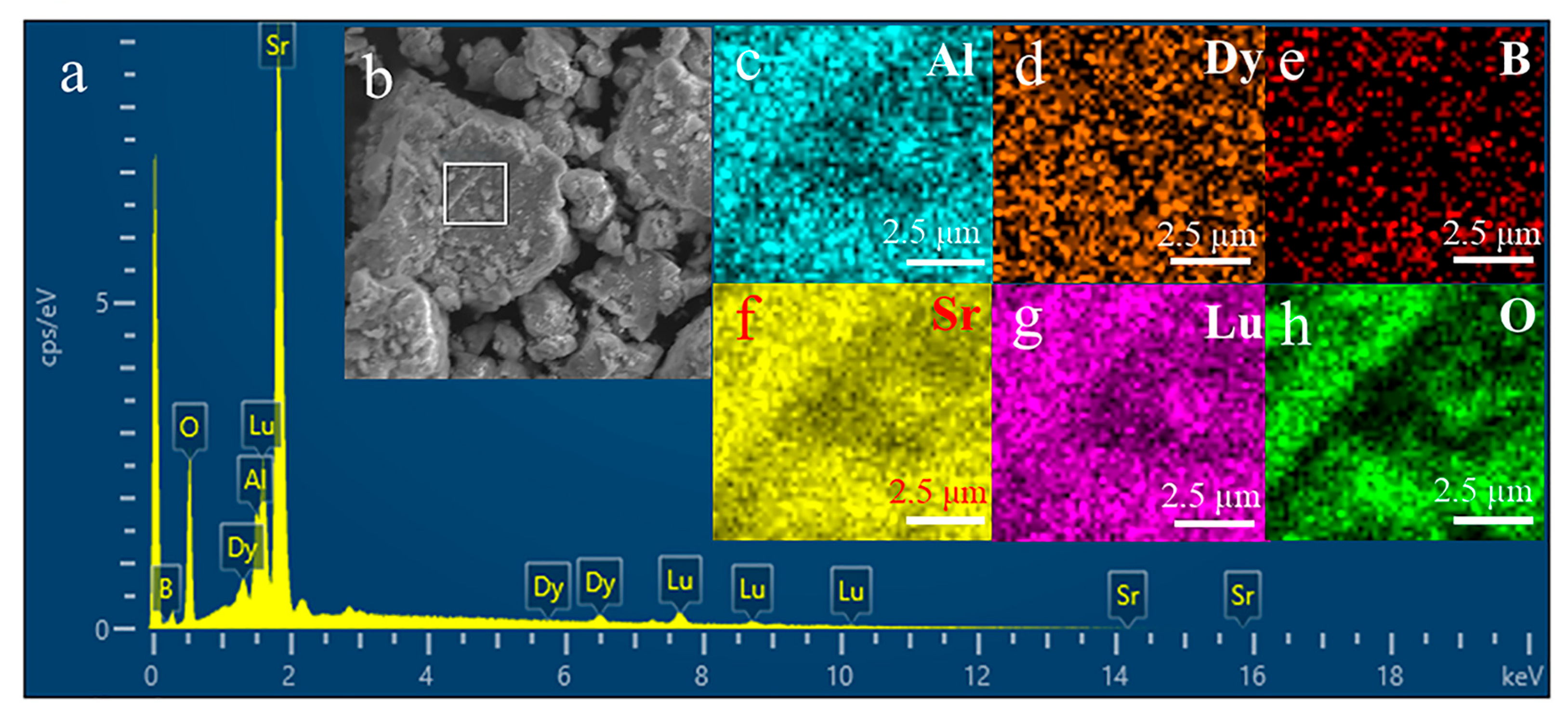
| Elements | Weight (%) | Atomic (%) |
|---|---|---|
| O | 25.53 | 52.30 |
| B | 13.40 | 25.45 |
| Al | 2.55 | 3.18 |
| Lu | 8.53 | 2.80 |
| Sr | 44.88 | 15.85 |
| Dy | 5.12 | 0.42 |
| Sum | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Sun, H.; Li, X.; Deng, B. Synthesis and Luminescent Properties of Dy3+-Activated Yellow Phosphors with Anomalous Thermal Quenching for w-LEDs. Molecules 2025, 30, 4562. https://doi.org/10.3390/molecules30234562
Zhang A, Sun H, Li X, Deng B. Synthesis and Luminescent Properties of Dy3+-Activated Yellow Phosphors with Anomalous Thermal Quenching for w-LEDs. Molecules. 2025; 30(23):4562. https://doi.org/10.3390/molecules30234562
Chicago/Turabian StyleZhang, Anlin, Huapeng Sun, Xiang Li, and Bin Deng. 2025. "Synthesis and Luminescent Properties of Dy3+-Activated Yellow Phosphors with Anomalous Thermal Quenching for w-LEDs" Molecules 30, no. 23: 4562. https://doi.org/10.3390/molecules30234562
APA StyleZhang, A., Sun, H., Li, X., & Deng, B. (2025). Synthesis and Luminescent Properties of Dy3+-Activated Yellow Phosphors with Anomalous Thermal Quenching for w-LEDs. Molecules, 30(23), 4562. https://doi.org/10.3390/molecules30234562






