2. Methods
As shown in
Figure 1, the system included an uncapped (6,6) armchair SWCNT embedded between two parallel graphene membranes along the
z-direction. To accommodate the nanotube, a circular pore was created at the center of each graphene sheet by removing carbon atoms. The radius of this pore was set to be the radius of the (6,6) SWCNT plus an additional 3 Å, ensuring no covalent bonds between the tube and the membrane. The system was centered at the coordinate origin. The ends of the nanotube were positioned to be co-planar with their respective graphene membranes (i.e., the distance between the tube end and the membrane plane is 0 Å). To simulate a fracture, the nanotube was modeled as two disjoint SWCNT segments separated by a gap of a specified distance. Their total length was equal to that of the intact nanotube before fracturing. The carbon atoms at the edges of the fracture were kept fixed in their positions throughout the simulation, and the dangling bonds of these edge carbon atoms were not passivated. MD simulations were conducted using NAMD3 with a 1 fs time step. All bonds involving hydrogen atoms were constrained using the SHAKE algorithm. A constant temperature of 300 K was maintained by Langevin dynamics (damping coefficient of 1 ps
−1) applied to all atoms. Periodic boundary conditions were applied in all directions; this ensures that water molecules exiting the downstream reservoir are reintroduced upstream, preventing any artificial density gradients or back pressure effects. Electrostatic interactions were managed using the particle mesh Ewald method. For non-bonded van der Waals and short-range electrostatic interactions, a real-space cutoff of 1.2 nm (12 Å) was used, with a potential switching function applied starting at 1.0 nm (10 Å) to ensure smooth truncation; no analytical long-range tail corrections were employed. The simulation was based on the CHARMM force field and TIP3P water model. To achieve directed flow, an external force of 1.345 pN was applied to water molecules within 15 Å of both ends in a water box containing a total of 1802 water molecules.
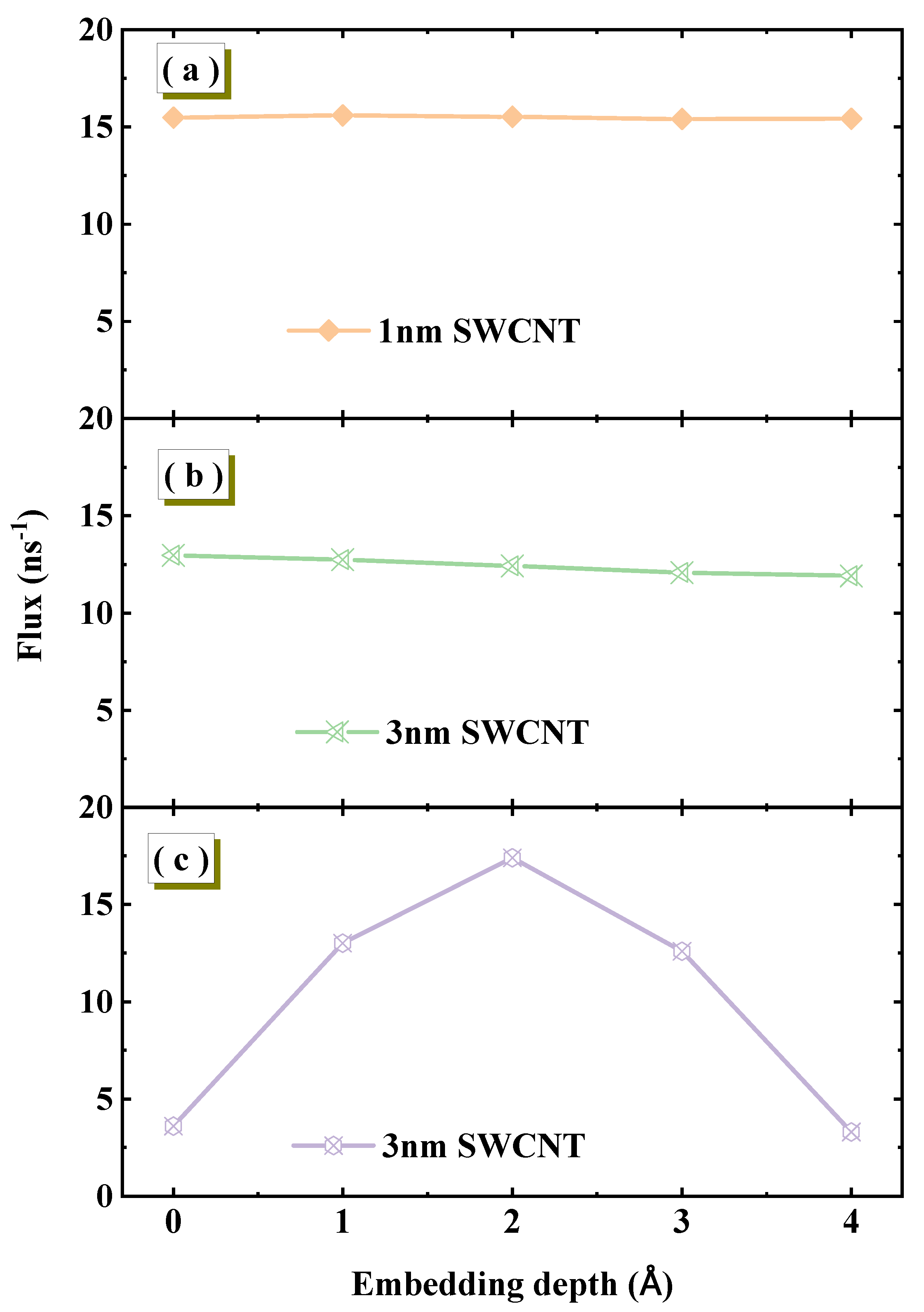
To ensure that the core conclusions of our study are not influenced by specific model geometric parameters, we first conducted a critical simulation aimed at investigating the sensitivity of water flux to the embedding depth of SWCNTs in graphene membranes. We designed three distinct systems. First, two systems fully equilibrated in the NPT ensemble were examined. For an SWCNT with a length of 1.34 nm, varying the embedding depth within the 0–4 Å range shows no significant dependence of water flux, which remains stable at approximately 16 ns
−1, as shown in
Figure 2a. Increasing the nanotube length to 3 nm yields a similar result, as shown in
Figure 2b. This indicates that under equilibrium conditions, nanotube length is not the primary factor controlling this sensitivity. To further validate the importance of equilibrium, we constructed a comparative system using the same 3 nm SWCNT but with insufficient equilibration in the NPT ensemble, and the results are shown in
Figure 2c. Under this non-equilibrium condition, flux exhibits high sensitivity to embedding depth, fluctuating dramatically with small depth changes. Therefore, whether the system is fully equilibrated is the decisive factor determining flux stability. Under equilibrium conditions, flux remains stable against embedding depth variations, while non-equilibrium systems display strong, irregular dependence. This sensitivity is directly related to the equilibrium state of the system. For systems that have been sufficiently equilibrated in the NPT ensemble, achieving physically realistic water densities, the water flux exhibits insensitivity to smaller changes in embedding depth. However, in systems that have not been sufficiently equilibrated, the water flux shows a strong, non-physical dependence on the embedding depth. Based on this conclusion, all simulations used for analysis in our study start from rigorously equilibrated states.
To quantify the water transport, two key metrics are employed. The flux is defined as the net number of water molecules translocating through the nanotube per unit time, calculated as , where and are the total counts of forward and backward translocation events, respectively, and is the total simulation time. The flow is defined as the total number of translocation events in both directions per unit time, given by . With these definitions, both flow and flux share the unit of ns−1. Consequently, the unidirectional transmission efficiency, , is a dimensionless ratio defined as .
A critical aspect of our methodology is the system equilibration, which addresses the determination of the simulation box size. All systems were constructed with a fixed number of 1802 water molecules. Before data collection, each system (corresponding to a specific nanotube length,
L) was rigorously equilibrated in the NPT ensemble (at 300 K and 1.01325 bar, using the Langevin piston method with a piston period of 200 fs and a decay time of 100 fs) until key properties, such as system temperature, density (approaching 1.0 g/cm
3), and total energy, reached a stable plateau (see
Figures S1–S3 in the Supplementary Materials for representative equilibration plots).
This NPT equilibration procedure allows the simulation box length in the z-direction () to dynamically adjust for each specific nanotube. Consequently, as the nanotube length (L) increases, the final box length () also increases proportionally. This robust approach ensures that the water reservoirs at both ends of the nanotube remain sufficient and consistent across all systems, thereby avoiding artificial finite-size effects from the simulation box.
Following this NPT equilibration, the system was switched to the NVT ensemble (300 K, using Langevin dynamics) for a 45 ns production run for data analysis. To ensure statistical reliability, this entire process was repeated four times for each case using different initial velocities, which were generated from a Maxwell–Boltzmann distribution at 300 K to ensure statistical independence of the trajectories. The results presented are the mean values from these independent runs, and the error bars represent the standard error of the mean.
To analyze the structural integrity of the water chain, a hydrogen bond is considered to exist if the oxygen–oxygen distance is less than 3.5 Å and the H-O⋯O angle is less than or equal to 30°. These geometric criteria are standard and have been widely adopted in molecular dynamics studies of water in nanoconfined systems [
24,
25].
The potential of mean force (PMF) for water molecules along the nanotube axis (z) was calculated using the Boltzmann inversion method, according to the relation . The density profile, , was obtained by taking the average over four independent, unbiased MD simulation trajectories. Each trajectory consisted of 45,000 frames, providing robust sampling of the system. In the calculation, is the Boltzmann constant, T is the temperature (300 K), the nanotube axis was divided into bins of 0.5 Å width, and is the bulk water number density (0.0334 molecules/Å3). To quantitatively characterize the PMF profiles, we performed a Fast Fourier Transform (FFT) analysis. After subtracting the mean value of each PMF curve to remove the DC component, we calculated the Power Spectral Density (PSD). To ensure a fair comparison of the intrinsic landscape features across different nanotube lengths, the analysis was grounded in Parseval’s theorem, which relates the total energy variance in the spatial domain to the integrated power in the frequency domain. The total power (), defined as the integral of the PSD over all wave numbers, reflects the overall roughness of the energy landscape. The peak power () corresponds to the power of the dominant periodic component and is proportional to the square of the primary energy barrier’s amplitude. The resulting unit of power from this analysis is (kcal/mol)2.
3. Results and Discussion
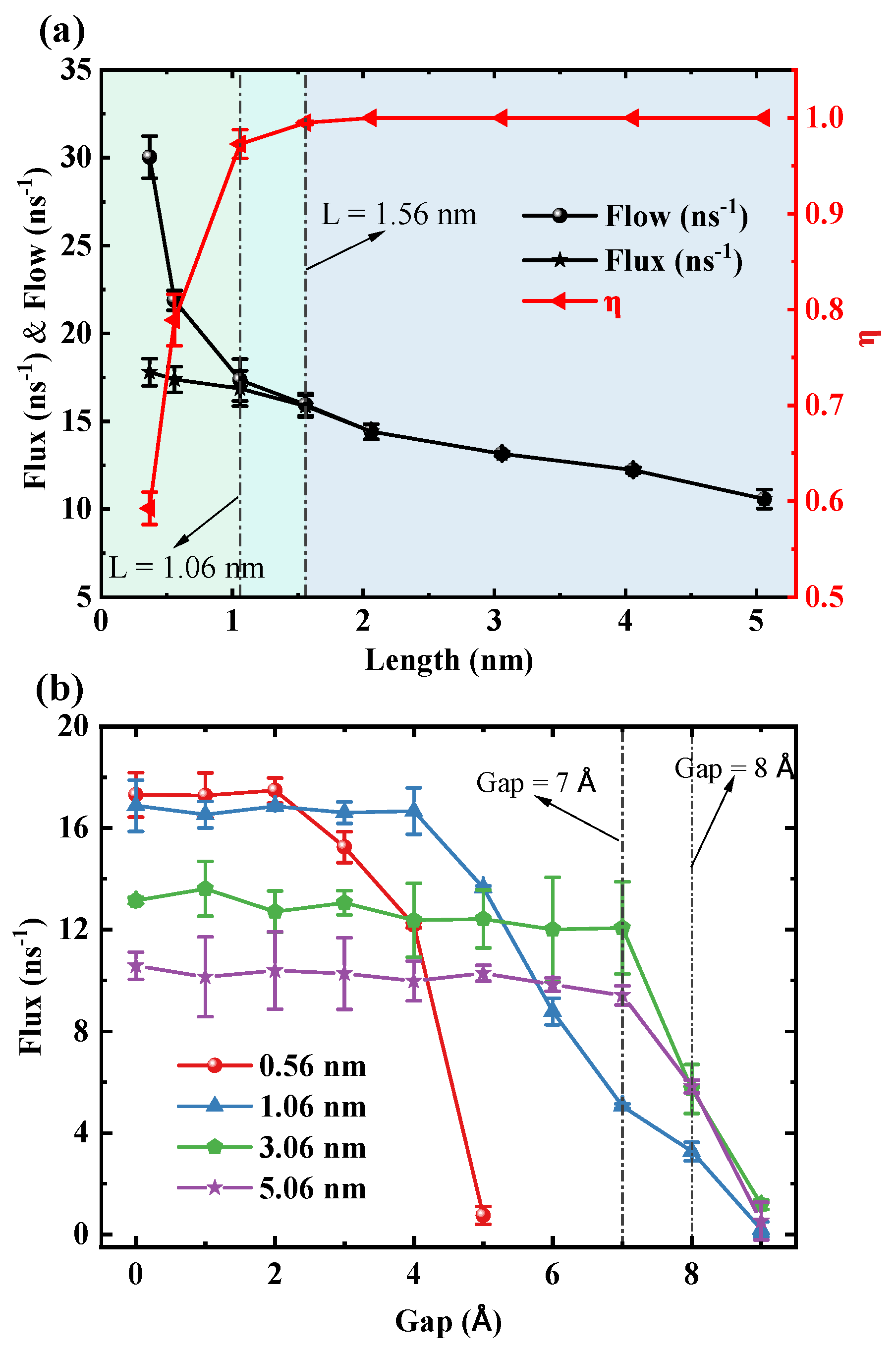
Figure 3a illustrates a significant trade-off between transmission rates (flow and flux) and unidirectional transmission efficiency (
). Specifically, both flow and flux decrease with increasing length of the SWCNT, with the most pronounced reduction in flow occurring in short tubes (
nm). Conversely,
increases rapidly with tube length, reaching 0.97 at 1.06 nm and approaching 1 in longer tubes, thereby achieving nearly perfect unidirectional transport. Thus, 1.06 nm represents a critical length at which the system maximizes transmission efficiency while sacrificing some transmission rate. To confirm that this transition is not an artifact of the TIP3P model, we performed comparative simulations using the SPC/E and TIP4P/2005 water models. These tests confirmed that the critical transition in transport efficiency at approximately 1.05 nm is a robust phenomenon, consistent across all three models (see
Figures S4 and S5 in the Supplementary Materials).
Figure 3b reveals that the ability of the water chain within the SWCNT to bridge the gap strongly depends on length. Driven by pressure, for short SWCNTs of 0.56 nm and 1.06 nm, the flux declines sharply at gaps of 3 Å and 5 Å, respectively. The gap value that causes the sharp decline in flux is defined as the critical gap. As the length increases to 3.06 nm and 5.06 nm, the system exhibits enhanced stability, with the critical gap delayed until 8 Å. Notably, beyond a length of 3 nm, the critical gap ceases to increase with further length extension, indicating a saturation effect. We also validated the robustness of this water-chain bridging mechanism. While the quantitative value of the critical gap is, as expected, model-dependent (5 Å for SPC/E and 3 Å for TIP4P/2005 compared to 7 Å for TIP3P), the fundamental physical phenomenon of a critical breakdown gap was validated in all cases (see
Figure S6 in the Supplementary Materials). This confirms that the mechanism is robust, although its precise range is modulated by the specific water–water interaction potential.
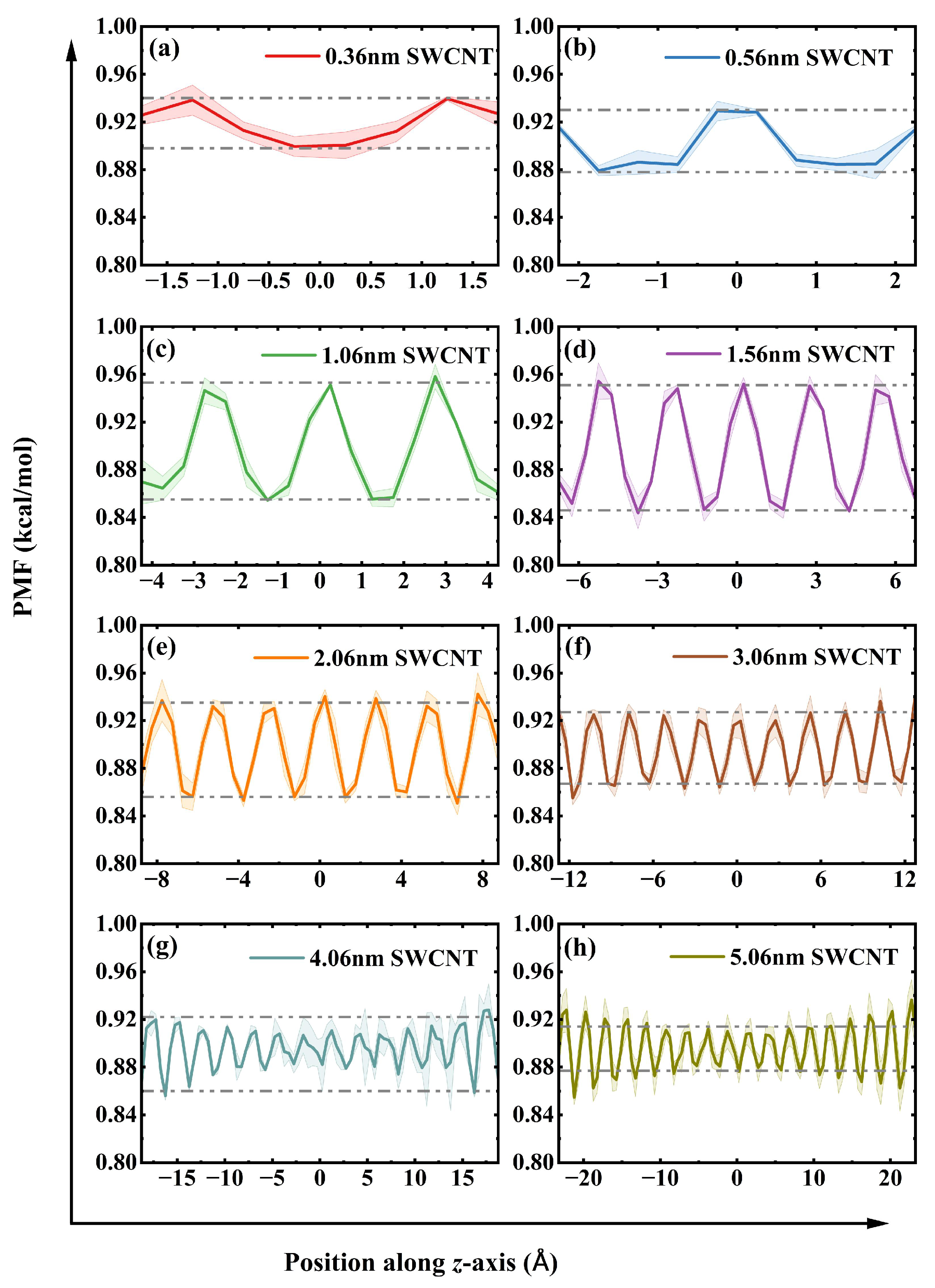
The trade-off between water transport rate, efficiency, and stability as a function of tube length can be explained by analyzing the potential of mean force (PMF) along the tube axis for water molecules. The PMF elucidates the effective free energy landscape experienced by water molecules within the nanoconfined channel, directly determining their dynamic behavior. We first confirm that all PMF curves exhibit thermodynamic symmetry along the nanotube axis (
z-direction). This symmetry is a direct consequence of the identical nature of the water reservoirs at both ends of the uncapped SWCNT, confirming that the channel itself possesses no inherent energetic bias for directional transport. As illustrated in
Figure 4, the PMF can be categorized into three stages. The first stage corresponds to a disordered and perturbation-sensitive shallow well. In short SWCNTs (
), the PMF curve is characterized by a flat profile with minimal energy barriers, indicating strong coupling between the water inside the tube and the reservoirs at both ends. Consequently, random thermal fluctuations at the tube’s openings are sufficient to reverse the effect of the externally applied pressure difference, thereby triggering significant backflow. This intense bidirectional motion results in extremely low
yet paradoxically yields a high flow due to the inclusion of numerous forward and backward movements. The low barrier landscape is too easily overcome by thermal noise, resulting in poor net efficiency. Consistent with this theoretical framework, we observe bidirectional, intermittent burst-like motion of water molecules inside the tube, aligning with previous research findings [
26]. This clear transition between distinct energy landscapes (
Figure 4) and their corresponding transport behaviors strongly supports the concept of multi-state diffusive mobility, where distinct fast and slow transport states co-exist in narrow nanochannels [
27]. To illustrate this behavior,
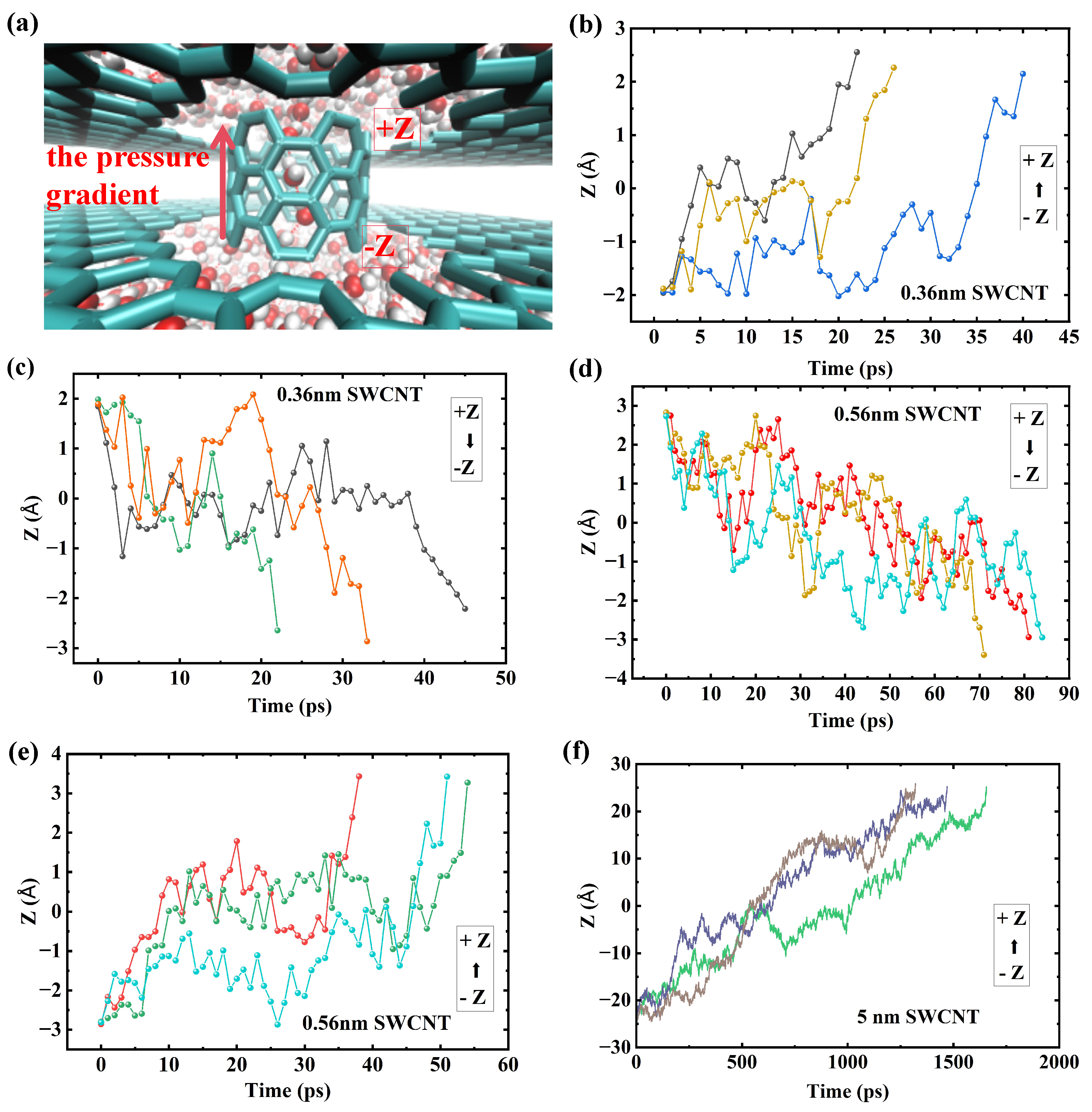
Figure 5 shows the trajectories of water molecules in SWCNTs of varying lengths under a pressure gradient. In short tubes (0.36 nm and 0.56 nm), the motion is characterized by bidirectional, burst-like jumps, with water molecules moving both along (from −Z to +Z) and against (from +Z to −Z) the pressure gradient. This reflects the dominance of thermal fluctuations, leading to low unidirectional efficiency. In contrast, for longer tubes (5.06 nm), the trajectories show primarily forward motion with occasional backward steps, but the overall trend is unidirectional, consistent with the formation of a stable, ordered water chain in the energy tunnel regime. This fluctuation-driven, burst-like motion observed in short tubes (
Figure 5b–e) is characteristic of the anomalous diffusion regimes commonly found in such single-file systems [
28]. Direct quantitative evidence for this ‘burst-like’ dynamic is provided in the
Supplementary Information (see Figure S7), which plots the instantaneous total flow for the
L = 0.56 nm system, showing its highly unstable and erratic magnitude.
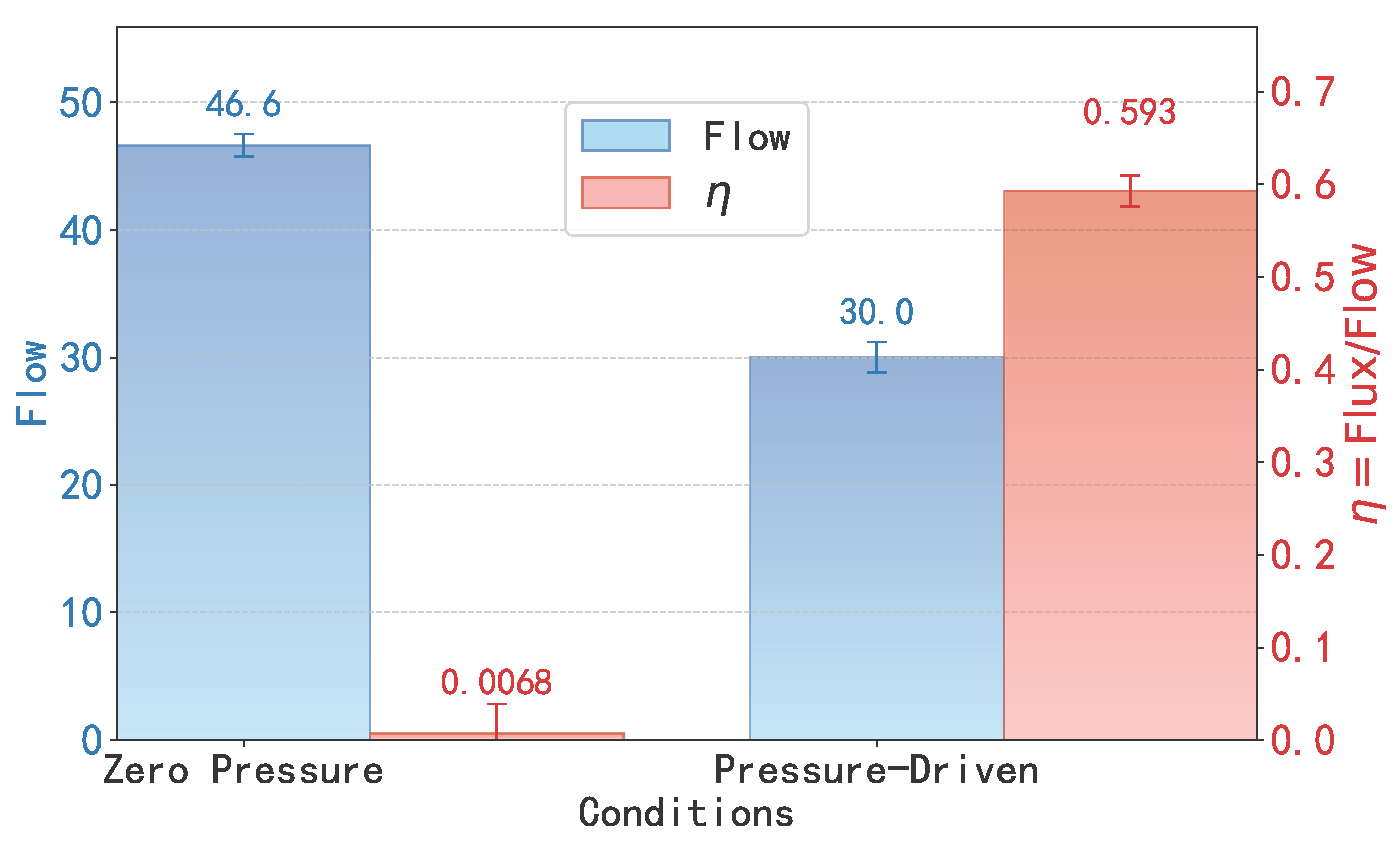
At the same time, this theoretical framework is further corroborated by direct comparison of flow under zero and pressure-driven conditions, as illustrated in
Figure 6. For a short SWCNT (
, well below the critical length of
), the flow under zero external pressure difference is notably higher than that under the applied pressure (1.345 pN). This counterintuitive behavior reveals that in the shallow-well regime, thermal fluctuations are the dominant driving mechanism of water transport. The complex, non-white (or ‘colored’) nature of this molecular-scale thermal noise, which is itself governed by the hydrogen bond network, is fundamental to understanding this regime [
29]. Crucially, the figure also visualizes the associated trade-off in efficiency (
, right axis). Under zero pressure, this high total flow is driven by chaotic, bidirectional thermal motion, resulting in a net efficiency
that is nearly zero. Once an external pressure is applied, it partially suppresses these random fluctuations, which reduces the total flow but establishes a net directional transport, causing
to rise significantly (from ≈0 to ≈0.59). These results provide direct evidence that in short SWCNTs, transport is governed by thermal noise rather than by external forces, in full agreement with the low-barrier PMF profile and the low values of
and
obtained from FFT analysis.
The second stage is marked by an ordered locking phase with dramatically increased fluctuations. As the length extends to approximately 1.06 to 1.56 nm, the transport mechanism undergoes a fundamental transformation. Water molecules inside the tube organize into a structured, single-file water chain interconnected via a hydrogen bond network. This phenomenon, where interfacial confinement dictates the formation of non-bulk, ordered water structures at room temperature, is a fundamental effect observed not only in 1D channels but also as ordered bilayers on 2D surfaces [
30]. Such strong ordering inherently constrains the molecular motion, significantly limiting the rotational freedom of the water molecules [
31], a mechanism that has also been shown to govern other nanoscale phenomena like evaporation [
32]. This ordering is directly reflected in the PMF, which exhibits high-amplitude periodic fluctuations throughout the tube [
33]. This amplification of the energy barriers is a direct consequence of the water’s ordering; such ordered interfacial structures are known to possess an anomalously low dielectric constant, which reduces electrostatic shielding and thus magnifies the underlying potential landscape [
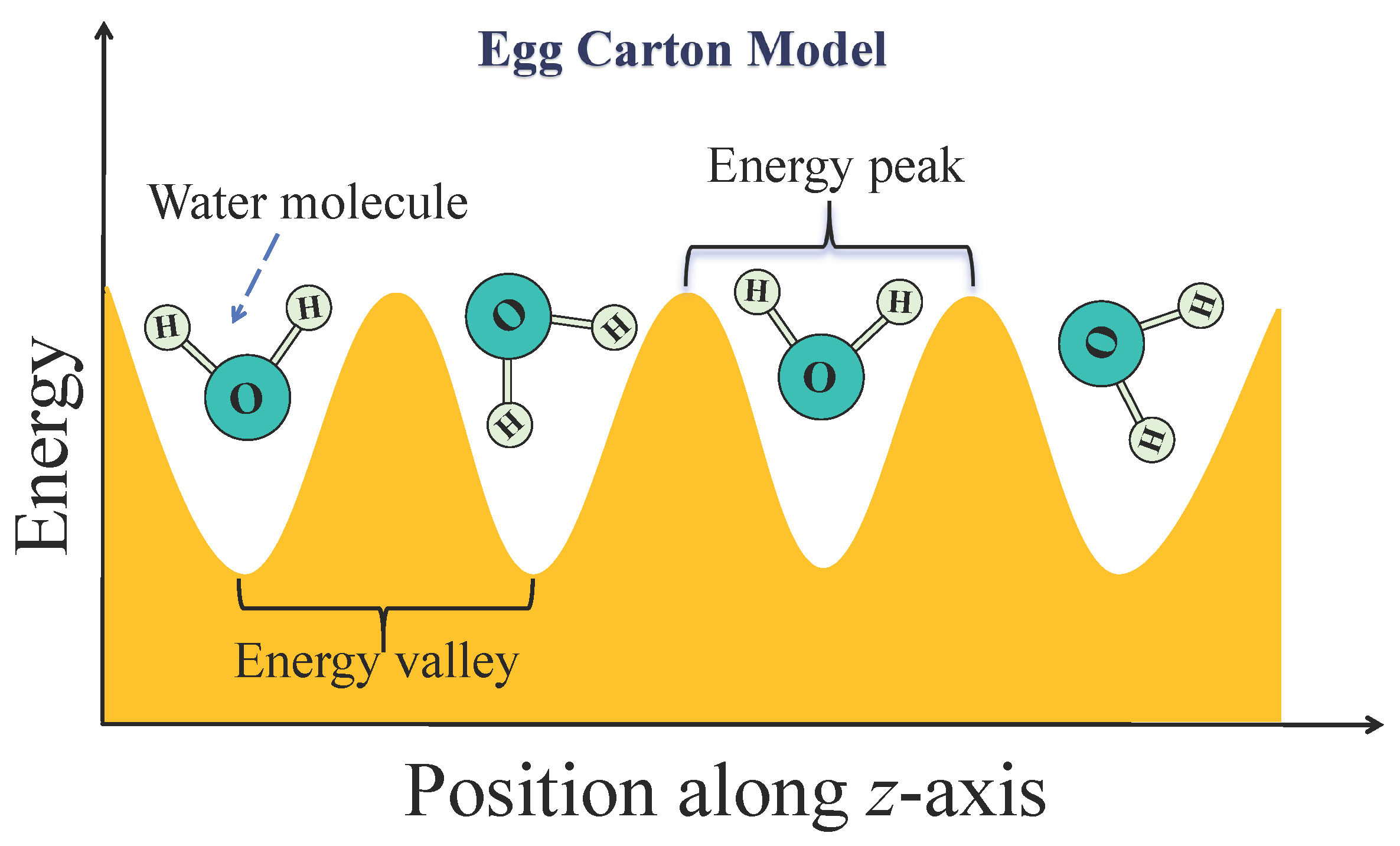
34]. Each peak represents a free energy barrier that a water molecule must surmount to transition from one stable position to the next. For the entire water chain, this resembles being firmly locked within the tube by an egg carton-like energy landscape. To visualize this,
Figure 7 presents a schematic of the egg carton model, where the axial position along the SWCNT corresponds to an alternating energy landscape of peaks and valleys. The troughs represent low-energy valleys, which are stable residence sites for water molecules, while the peaks are high-energy barriers that must be overcome for migration between stable positions. This model aids in understanding the PMF’s significant fluctuations with distinct amplitude and periodicity, corresponding to the energy differences between stable and transitional configurations of water molecules in the ordered locking phase. This rugged profile effectively suppresses backflow by requiring collective energy to surmount the barriers. Crucially, the energy provided by random, reverse thermal fluctuations at the tube openings is insufficient to collectively drive the water chain over this series of high internal energy barriers, thereby strongly suppressing the influence of thermal fluctuations and maximizing unidirectional efficiency (
). This is achieved despite the trade-off of slightly higher resistance to forward flow, which reduces the absolute rate compared to the shortest tubes.
The third stage occurs when the length exceeds 1.56 nm, gradually forming a stable tunnel transport mode, characterized by the transition of the water chain from a rigid short rod to a flexible long chain. These energy barriers govern the bridging capability of the fractured water chain. For short tubes of 0.56 nm, end effects hinder water molecules from bridging larger gaps. In nanotubes ranging from 1.06 nm to 1.56 nm, the PMF displays high-amplitude, drastic fluctuations across the entire domain. This implies that water molecules are confined in deep potential wells, and crossing a fracture requires overcoming a high energy cost. Consequently, it can only tolerate smaller fracture gaps. As the length increases to 3.06 nm, the overall fluctuation amplitude of the PMF decreases significantly, indicating that the energy barrier for the migration of water molecules is reduced, thereby empowering the water chain with astonishing bridging capabilities to autonomously span larger distances (up to 7 Å) in the absence of a pressure difference. In 5.06 nm tubes, the PMF adopts a high at both ends, low in the middle profile, with pronounced high-energy barrier regions near the entrance and exit due to end effects, while the extensive central region forms an energy tunnel with small fluctuation amplitude. This low-resistance characteristic is in excellent agreement with the mechanism that ordered water structures, such as monolayers, can serve as, as low-friction interfaces for water transport [
35,
36]. This chain-bridging behavior is further corroborated by simulations under zero external pressure difference. Under these conditions, the transport is driven purely by thermal fluctuations from the water reservoirs at both ends. This random thermal motion induces a high rate of bidirectional translocation, resulting in a stable total flow (
Figure 8a) that remains essentially unchanged until the critical gap. However, as shown in
Figure 8b, these forward and backward movements cancel each other out, leading to a net transmission efficiency
that is approximately zero across all gap sizes. This result explicitly clarifies that the high flow is due to chaotic thermal motion, not a net driving force. The fact that this flow (total motion) still decreases rapidly at the critical gap confirms the water chain’s breakdown and robustly underscores the intrinsic bridging capability.
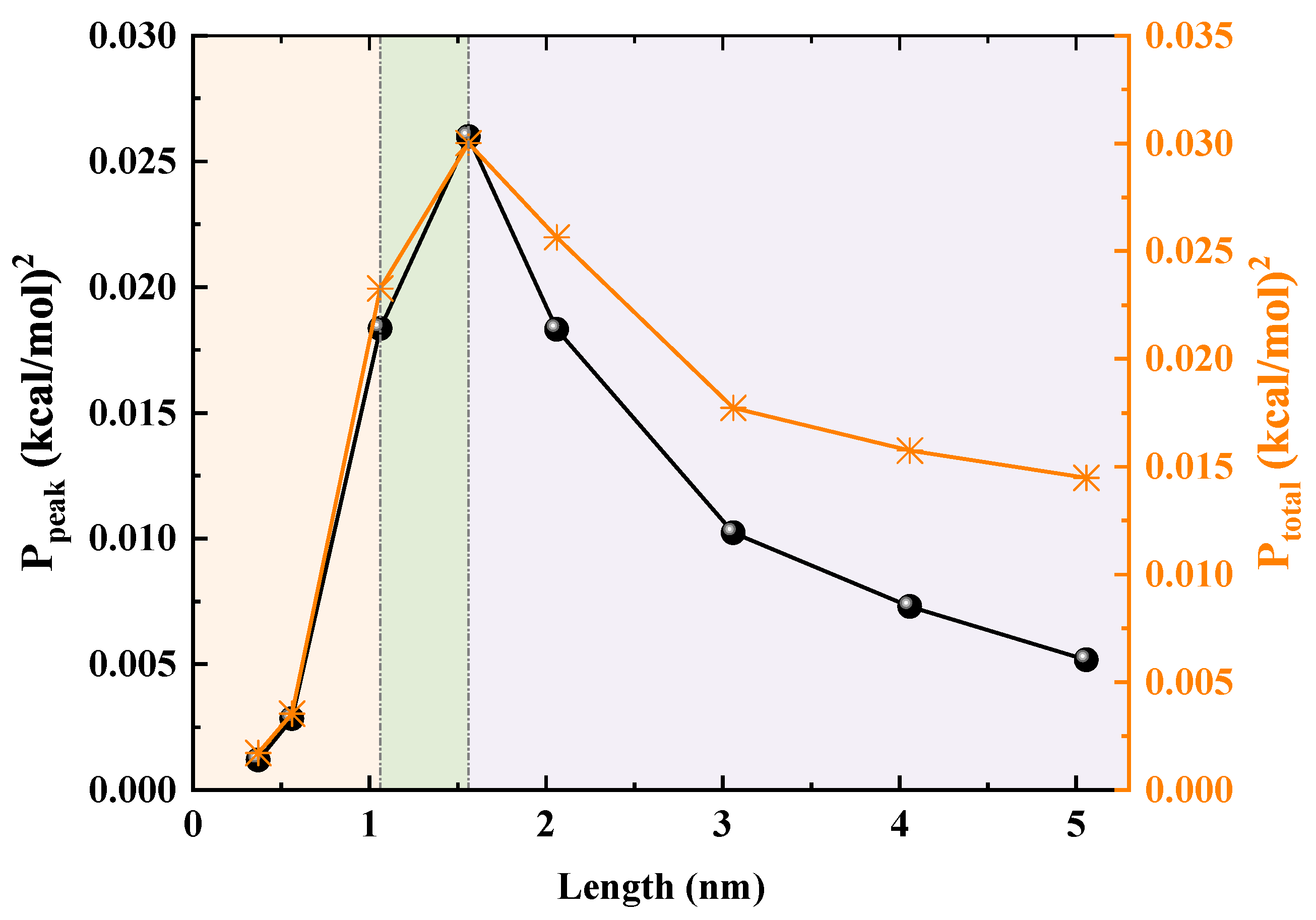
To transform the qualitative phenomenon into precise quantitative results, we apply FFT analysis to the PMF curves, as shown in
Figure 9. This method extracts essential physical parameters from complex PMF profiles. In analyzing the spectral characteristics of the free-energy sequence
, we first remove the DC component by computing
yielding a zero-mean signal
. We then apply the discrete Fourier transform
where
and
, with frequency resolution
and Nyquist frequency
. The two-sided power spectrum is
, from which the single-sided power spectral density is constructed as
We verify Parseval’s theorem via
Finally, letting
yields the index, frequency, and wavelength of the dominant component, and we characterize its strength and the total spectral energy by
and
We first identify the PMF’s primary wavelength (), which stabilizes at ∼ Å, closely matching the spacing between adjacent water molecules, thus providing a solid basis for further analysis. Next, the two key indicators are the peak frequency power (), which measures the height of the main energy barriers in the PMF, and the total power (), which reflects the energy landscape’s overall roughness. In short SWCNTs with lengths below 1.06 nm, and are low, indicating a flat, shallow-well PMF shape that lacks sufficient barriers to suppress thermal fluctuations at the tube openings. When the length reaches 1.06 nm, and surge and peak at 1.56 nm, marking a transition to a rugged egg carton PMF profile. The elevated barriers effectively suppress backflow and thermal disturbances, thereby enhancing transport efficiency, though they also increase resistance to forward flow, leading to a noticeable inflection point in the flow. For lengths exceeding 1.56 nm, and decline steadily, suggesting progressively lower energy barriers. Once these barriers fall below a certain threshold, the water chain’s ability to bridge gaps strengthens, enabling it to span larger structural gaps and significantly improving macroscopic stability.
To generalize the PMF evolution and length-driven trade-off observed in the FFT analysis, we propose a theoretical model treating the water chain as a flexible one-dimensional chain of
N particles (
,
nm) in an effective periodic potential [
26,
37]. We employ this model as a phenomenological description to link the static PMF landscape to dynamic transport kinetics. It is important to note that while the PMF is fundamentally a temperature-dependent free energy function (
), the effective potential
is written here solely in terms of spatial coordinates
z. In this approach, the critical parameter, the effective barrier height
, implicitly captures the effects of temperature as it is derived by fitting the PMF data calculated at
governed by overdamped Langevin dynamics [
38]
where
includes harmonic hydrogen bond interactions
k approximately 5 to 10
/Å
2. Here, the energy barrier
modulates the energy tunnel, with
fitted to the FFT data across all lengths. This form accurately captures the full curve. The power-law rise
reflects critical buildup from shallow wells to ordered locking, akin to order parameter scaling in Ising-like models near criticality [
39], peaking at
nm; the exponential decay
corresponds to low-damping tunnel formation in long tubes, with
as the correlation length matching confined fluid phase transitions [
40,
41]. Building on this foundation, the model further quantifies the chain-bridging phenomenon by considering the cumulative impact of ordering along the chain, which leads to an effective barrier form tailored for the critical gap
where
is a scaling parameter in units consistent with
in nm, fitted to MD trends. For this purpose, we refine
to modulate the energy tunnel based on integrated strength. This refined approach accurately captures the monotonic increase and saturation. The cumulative integral represents the total ordering strength accumulated along the chain, building critically via the power-law rise and saturating after the exponential decay.
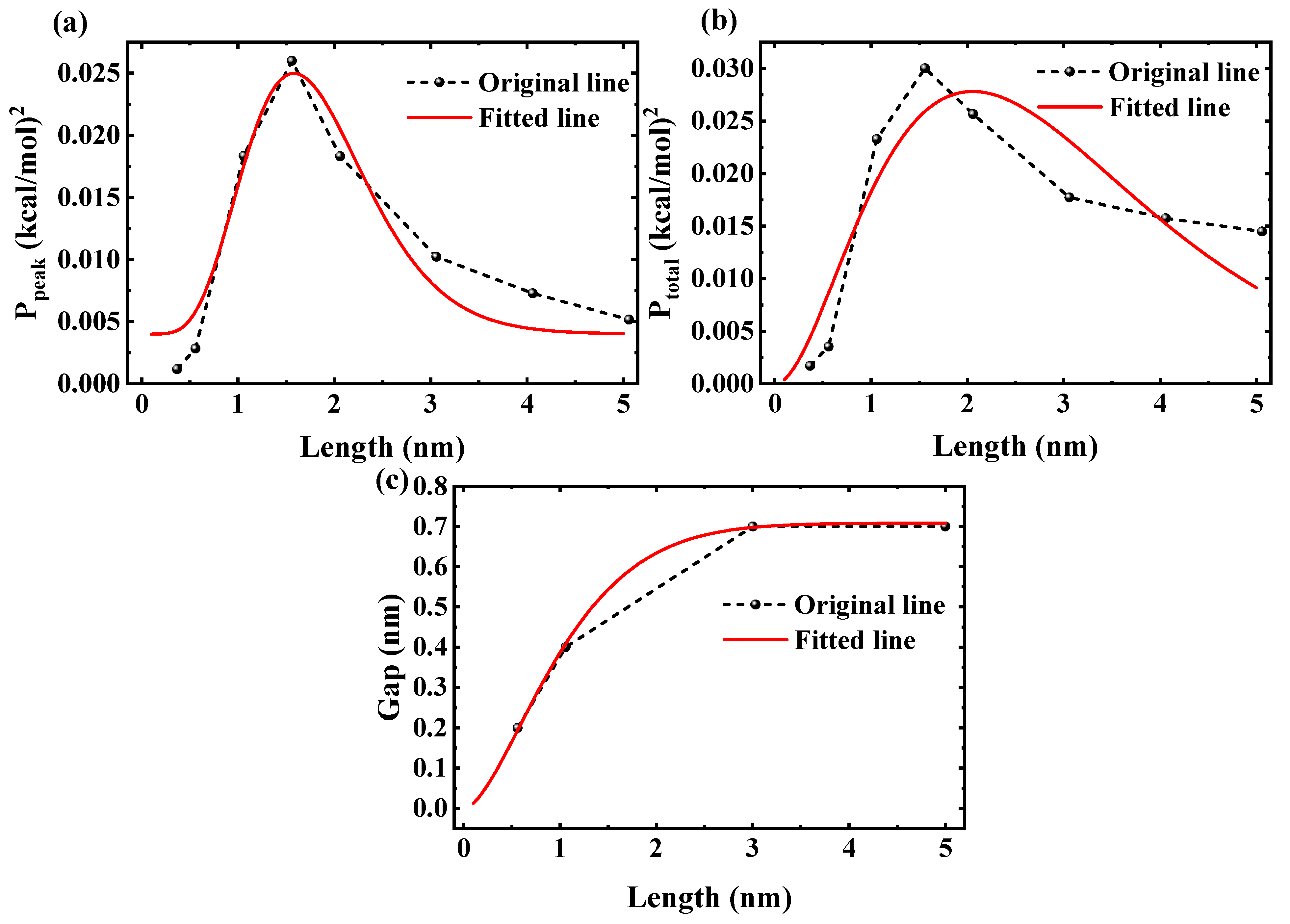
Figure 10a,b show the squared peak and total PMF amplitudes,
and
, as functions of nanotube length
L, with dashed lines from FFT data and solid lines representing fits via
yielding
and 0.814, respectively. The power-law rise
captures the critical ordering buildup and transition from shallow wells to locking, while the exponential decay
reflects damping in longer tubes, linking
to confined fluid phase transitions.
Figure 10c presents the predicted critical gap distance
for chain-bridging, with
fitted to MD trends and
. The integral accumulates total ordering strength, showing an increase from ∼0.2 nm at
nm to saturation at ∼0.7 nm for
nm.
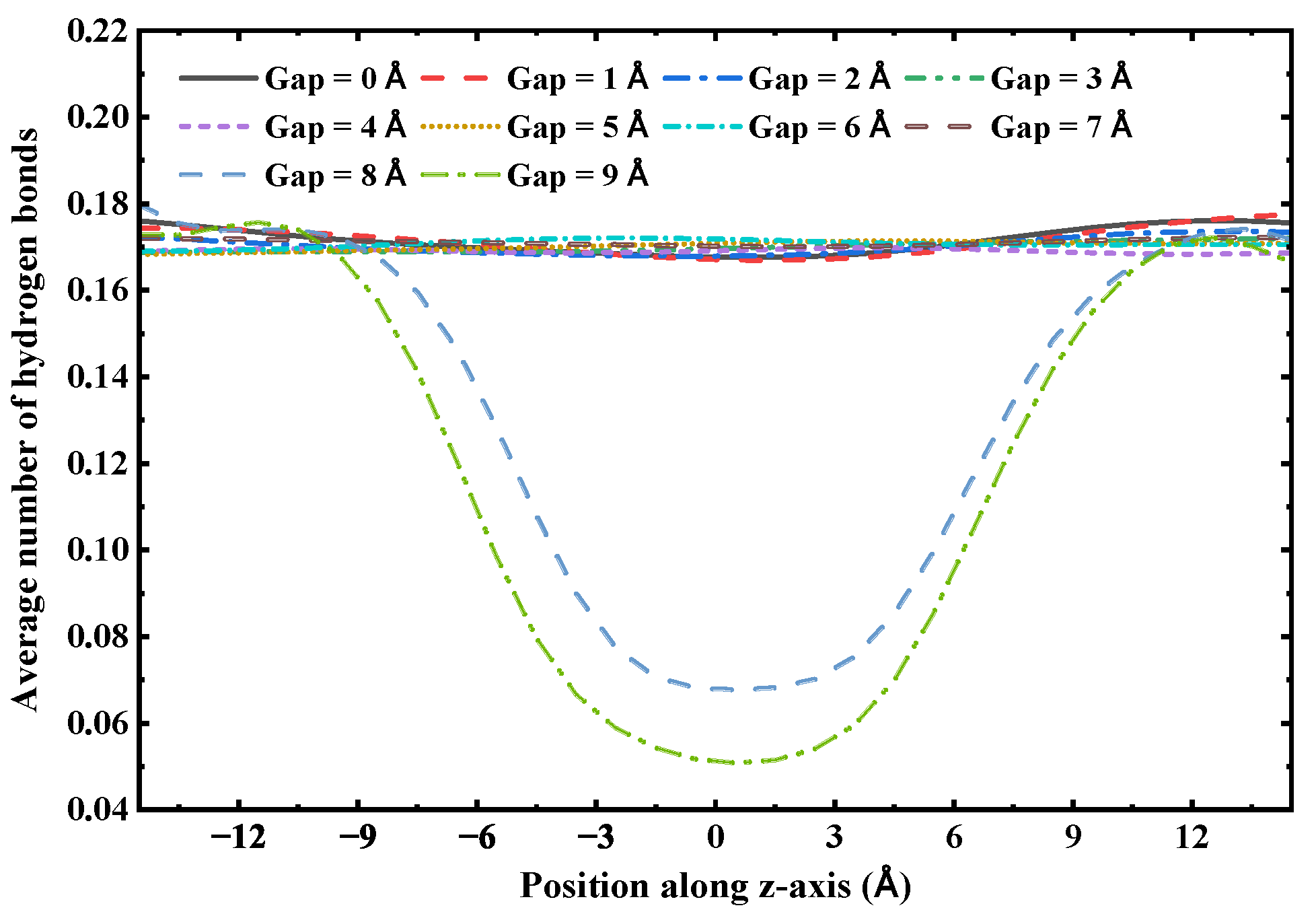
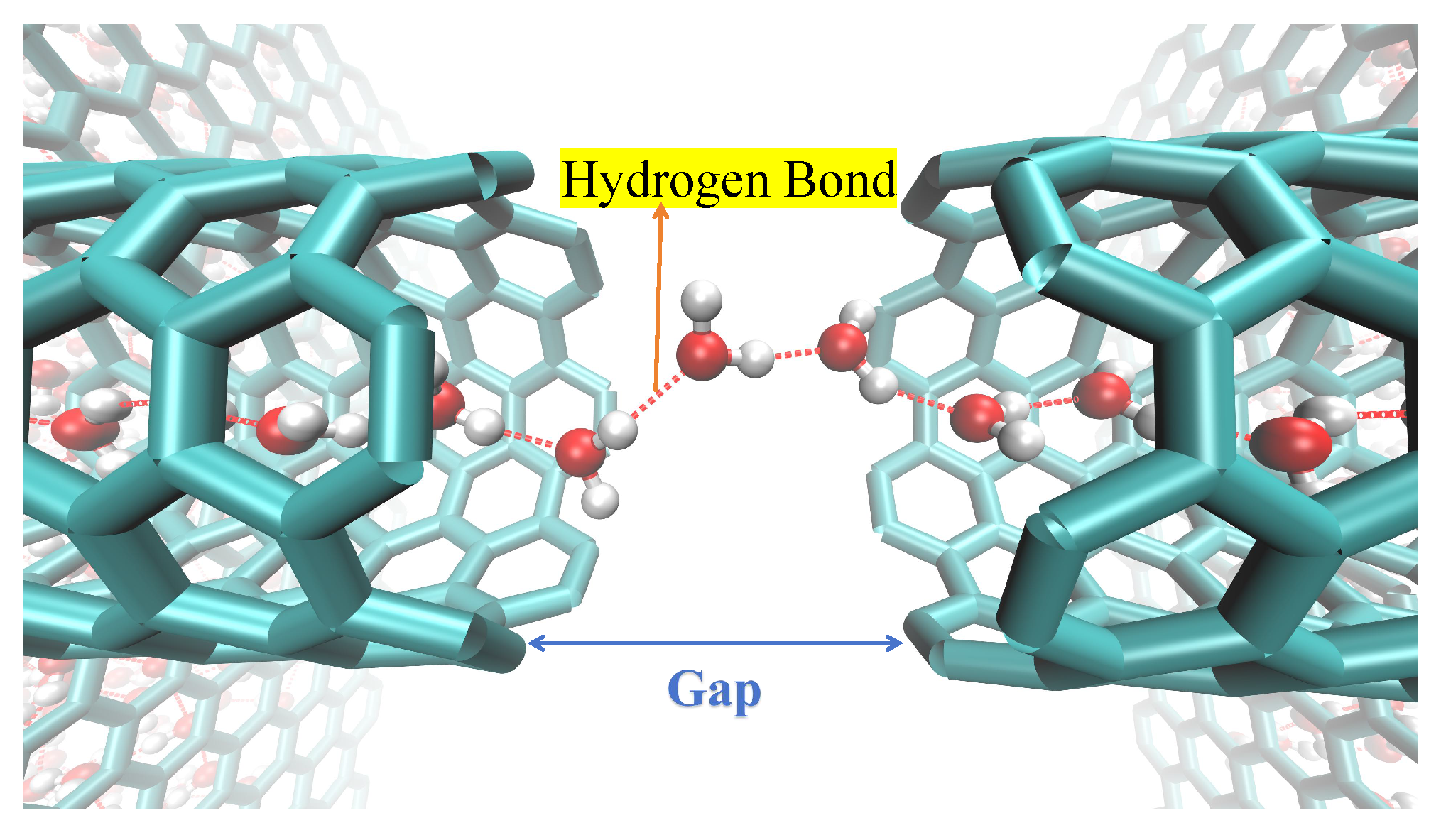
The axial distribution of hydrogen bonds indicates that the continuity of the hydrogen bond network is crucial for maintaining the structural integrity of the one-dimensional confined water chain, with its critical distance determined to be 7 Å. To illustrate this, we analyzed the distribution of the average number of hydrogen bonds along the tube axis in a 3 nm (6,6) SWCNT with varying gap sizes. As shown in
Figure 11, when the gap size ranges from 0 to 7 Å, the hydrogen bond distribution remains consistent across the entire nanotube, including the gap region. However, when the gap size increases to 8 Å or 9 Å, the number of hydrogen bonds within the gap region decreases substantially. Since the structural stability of the single-file water chain within the (6,6) SWCNT is primarily determined by the intermolecular hydrogen bond network, this indicates that beyond a gap size of 7 Å, the water chain is unable to bridge the gap and maintain a continuous hydrogen bond network, resulting in a loss of structural integrity and subsequent rupture of the chain.
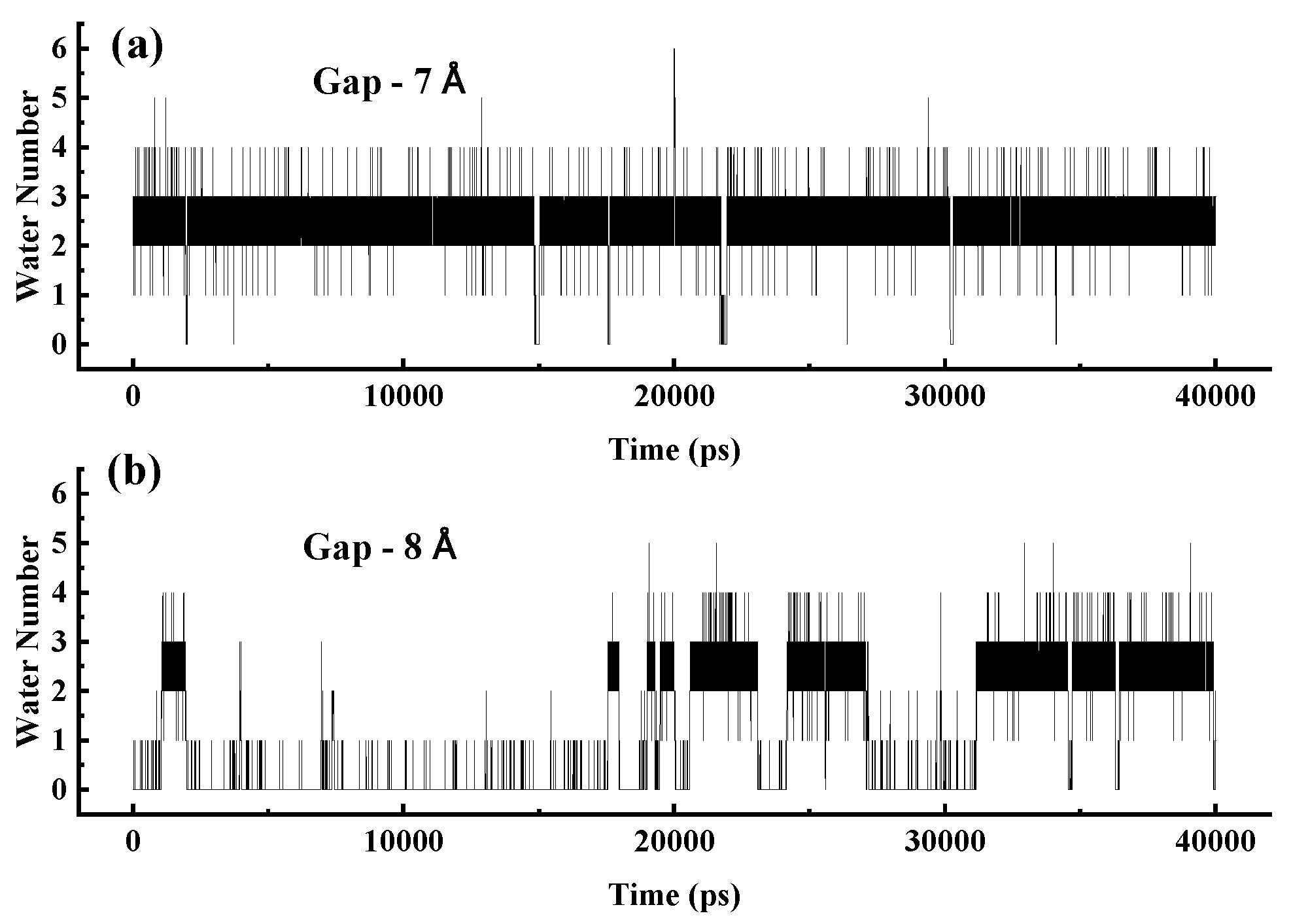
When the gap is less than 8 Å, two water molecules can effectively bridge the gap, connecting the water chains on both sides. To demonstrate this stability,
Figure 12 shows the variation in the number of water molecules within gaps in a 3 nm SWCNT over time. In panel (a), for a 7 Å gap, the number remains stable at 2–3, sufficient to establish a water bridge that restores the continuity of the broken water chain. In contrast, panel (b) shows that for an 8 Å gap, the number fluctuates significantly, often reaching zero, leading to the failure of the water-bridge mechanism and loss of structural and dynamic integrity in the water chain.
These molecules form a stable configuration and establish hydrogen bonds with the adjacent chains that, though stretched, remain sufficiently strong. This water-bridge mechanism, even in the absence of an external pressure difference, can effectively bridge breaks in the water chain, thereby endowing the water chain inside the tube with a strong ability to maintain its continuity. This mechanism is critically supported by the diffusive properties of water within the nanoscale bridge itself [
42], as shown in
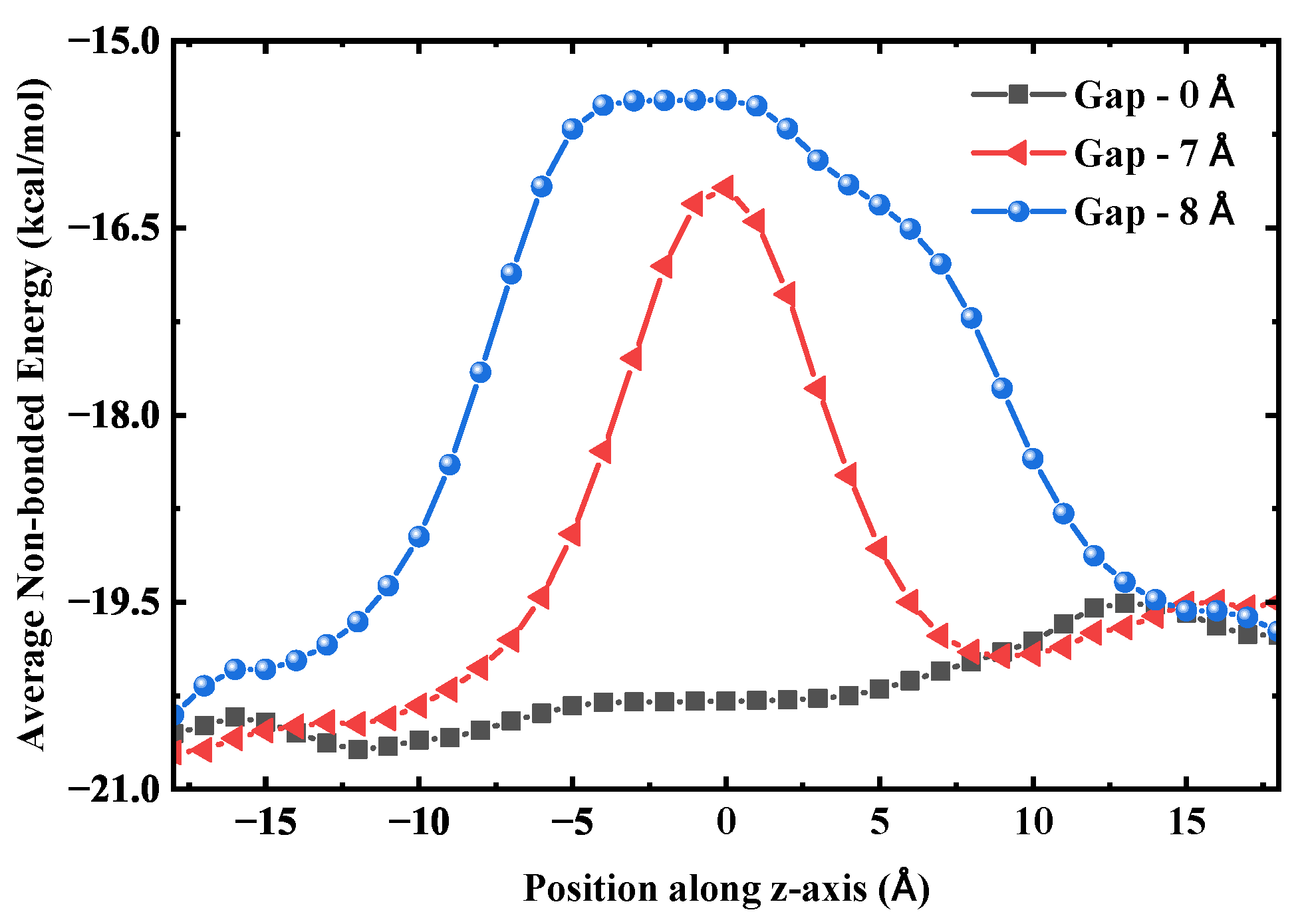
Figure 13. This ensures the continuity of its structure, thereby maintaining a stable water flux. The hydrogen bond length is approximately 2.8 Å [
43], and two molecules can span a distance of 5.6∼7.0 Å via stretched bonds. The 8 Å distance exceeds the limit, causing the hydrogen bonds to become overstretched and their strength to sharply decline, resulting in a high transport energy barrier. To examine this from an energetic perspective, we calculated the average non-bonded interaction energy of water molecules with their surrounding environment along the axial direction for three SWCNT configurations: an intact tube, a fractured tube with a 7 Å gap, and one with an 8 Å gap. As shown in
Figure 14, the energy profile for the intact SWCNT is relatively flat, allowing smooth traversal without substantial barriers. For the 7 Å gap, a distinct energy barrier emerges. At 8 Å, both the height and width of this barrier increase, expanding the energetically unfavorable region and further hindering water transport, which correlates with the observed reduction in flux for larger gaps. This leads to a significant reduction in the average number of hydrogen bonds at the gap, signaling the rupture of the water chain and a substantial decrease in water flux.
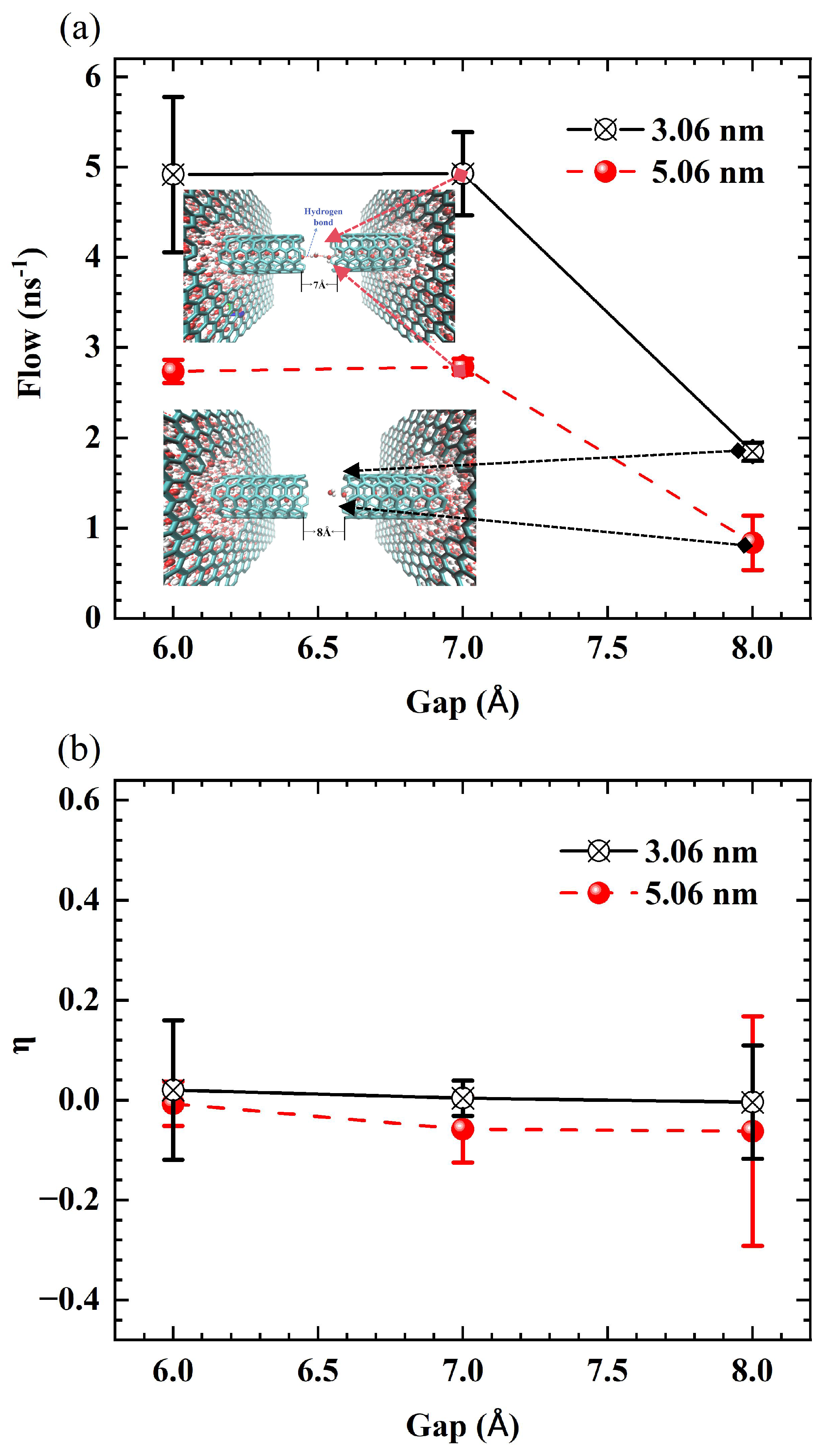
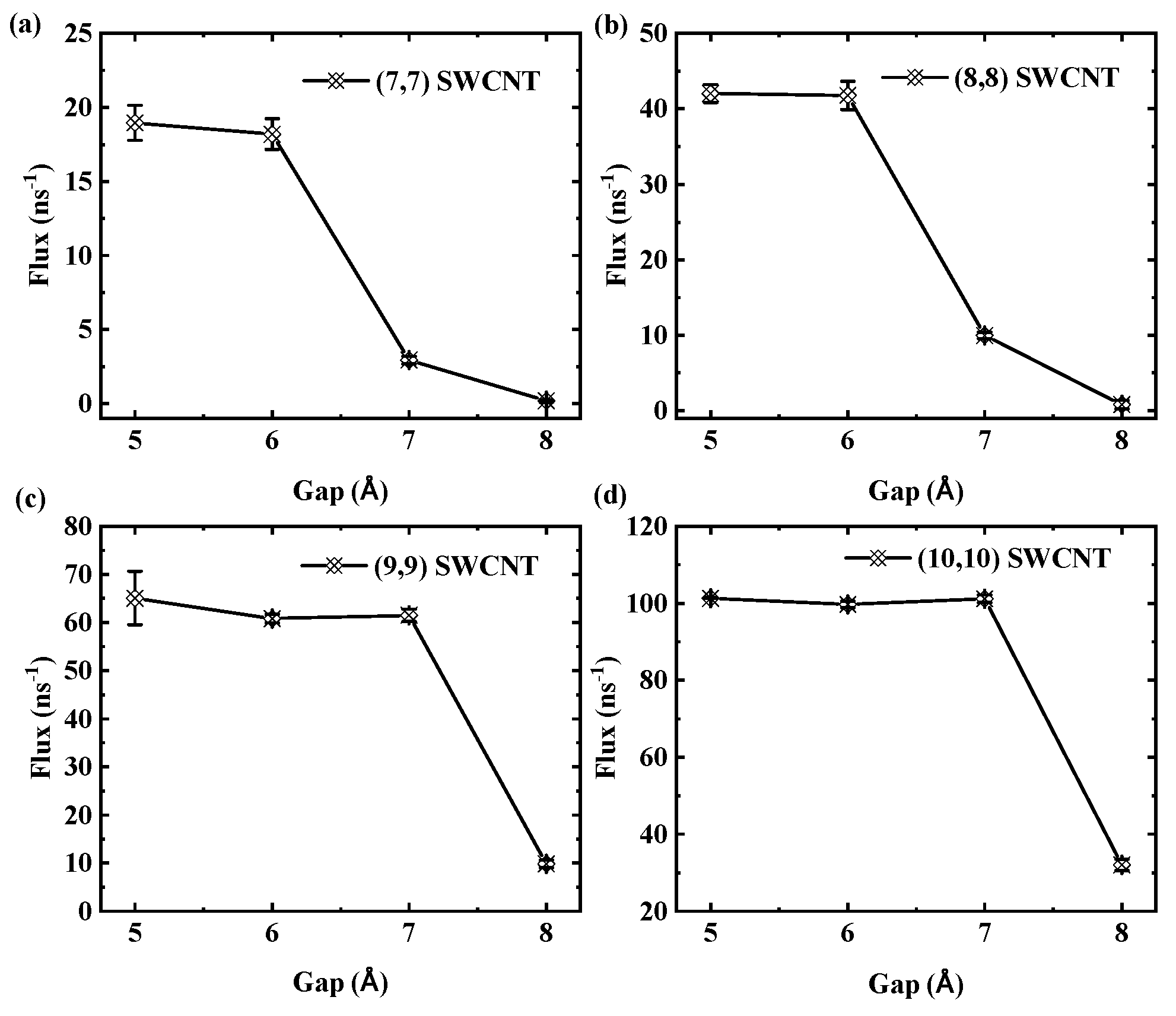
We further investigate the effects of tube diameter and thermodynamic parameters on the critical gap in SWCNTs. The results indicate a slight dependence of this value on diameter. Specifically, for 3 nm long tubes, the critical gap is 7 Å for (7,7) and (8,8) SWCNTs, while it is 8 Å for (9,9) and (10,10) SWCNTs. To illustrate this diameter dependence,
Figure 15 shows the flux as a function of gap size for SWCNTs of different diameters with a fixed length of 3 nm. Panels (a) and (b) indicate that for (7,7) and (8,8) SWCNTs, the flux rapidly declines at a gap of 7 Å. In contrast, panels (c) and (d) show that for the larger-diameter (9,9) and (10,10) SWCNTs, the critical gap occurs at 8 Å, highlighting a subtle shift in transport thresholds with increasing diameter.
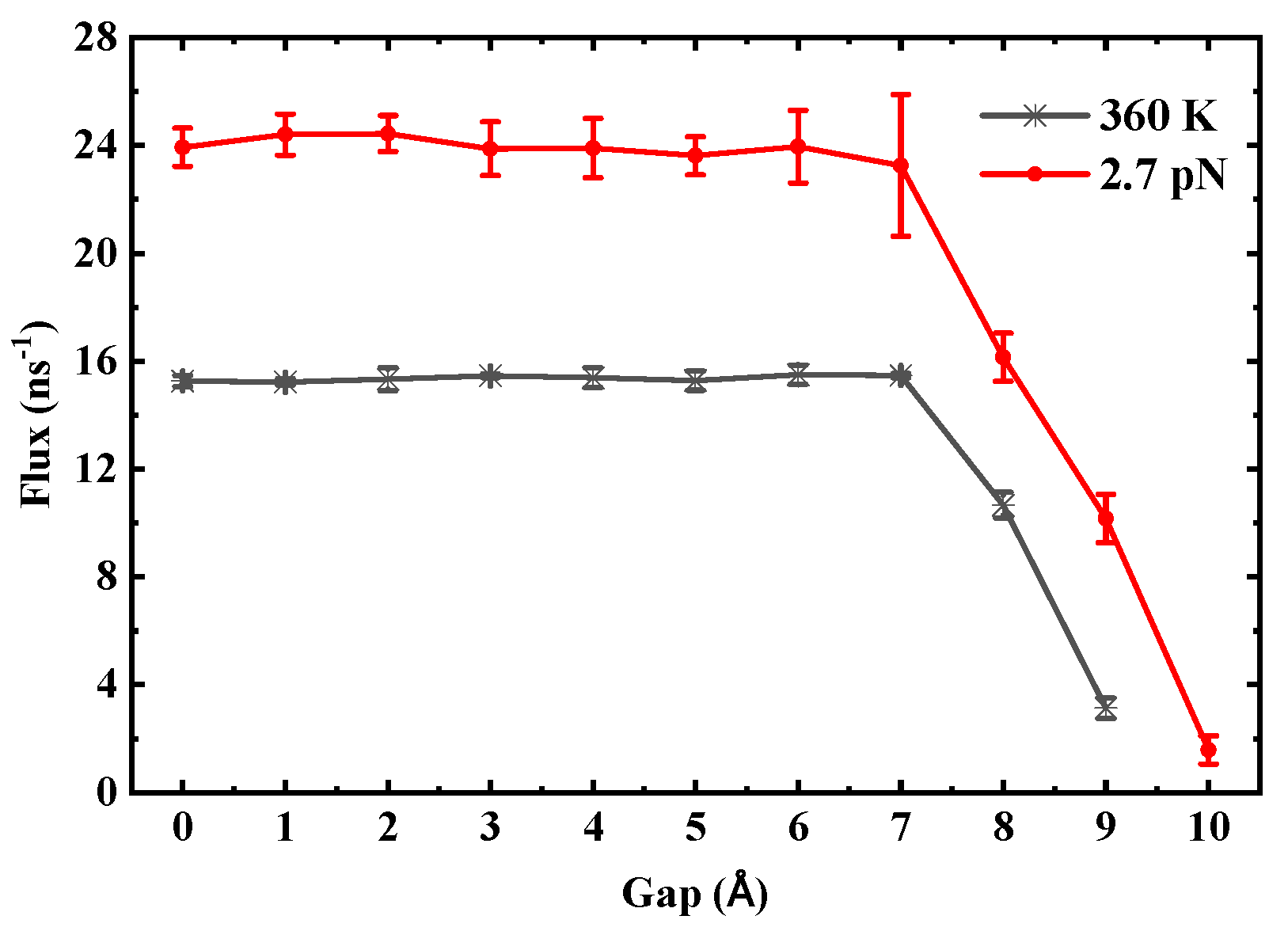
In contrast, the critical gap exhibits stability against thermodynamic perturbations. For (6,6) SWCNTs, even under conditions such as a temperature increase to 360 K or a doubled driving pressure difference, the critical gap remains consistently at 8 Å. To illustrate this robustness,
Figure 16 shows the flux as a function of gap in SWCNTs with a length of 3 nm under varied conditions: temperature increased to 360 K (black line) and doubled driving pressure difference (red line). The critical gap remains stable at 8 Å in both cases, indicating minimal sensitivity to these external factors and underscoring the dominant role of nanotube geometry in determining transport thresholds.
Finally, we also considered the effect of the gap’s axial placement, as a defect is unlikely to be perfectly centered in a real-world system. Our preliminary investigations (from a related study) confirm that the water transport stability is indeed highly sensitive to the gap’s position. For a constant-length SWCNT (e.g.,
L = 5.06 nm), we found that moving the gap from the center towards either the entrance or the exit significantly reduces the critical gap at which transport fails. This observation is fully consistent with our PMF analysis (
Figure 4), which shows high-energy barriers near the tube openings due to ‘end effects’. A gap located in these high-barrier regions compounds the total energy barrier for translocation, thus destabilizing the water chain’s continuity more easily than a centered gap.