Three New Aporphine Alkaloids with Glucose Consumption Increase Activity from Cassytha filiformis
Abstract
1. Introduction
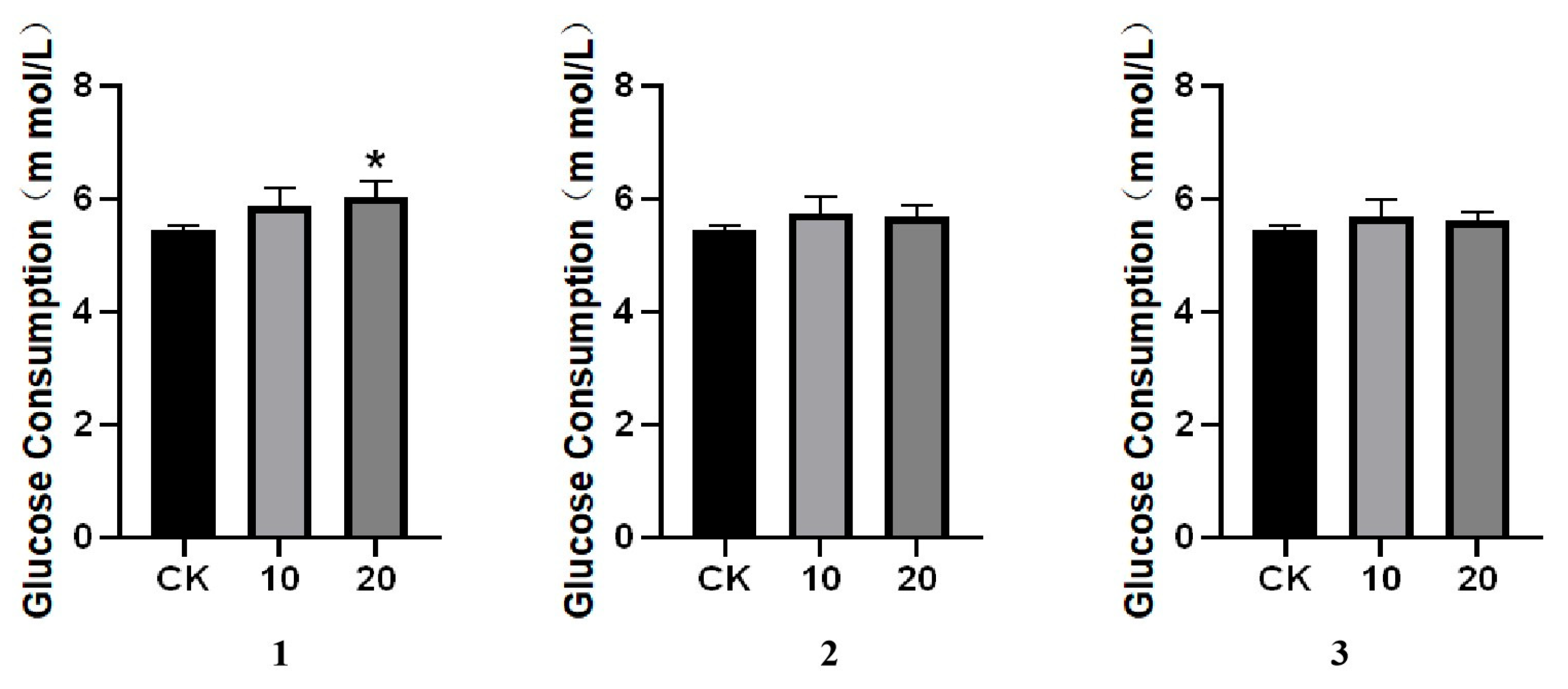
2. Results and Discussion
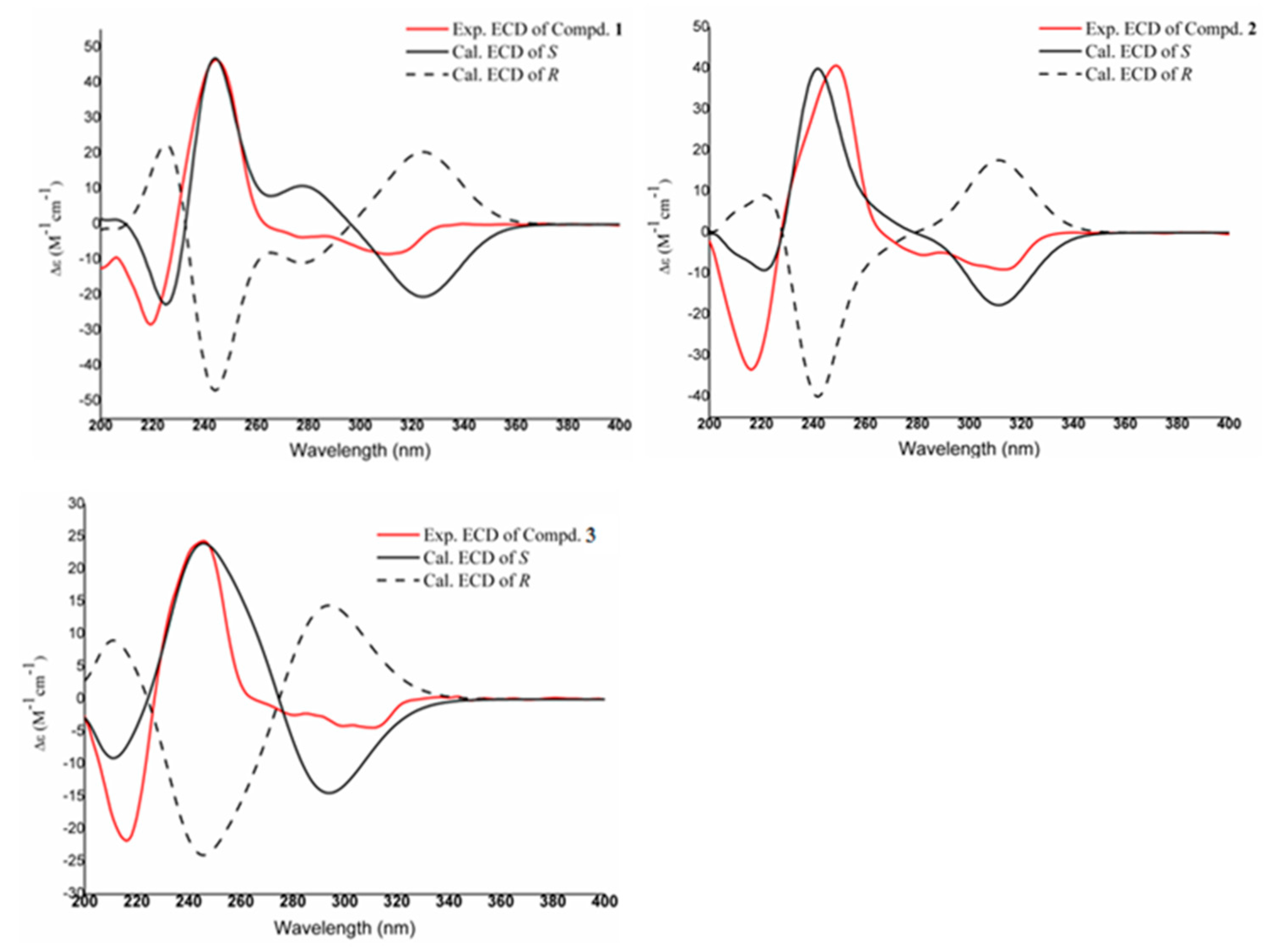
Structural Elucidation
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Glucose Consumption Assay
3.5. ECD Calculation
3.6. Spectroscopic Data of the New Compounds
3.6.1. 3-Demethylcassythine
3.6.2. 10-Demethylcassythine
3.6.3. N-Demethyllastourvilline
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosli, R.; Tennakoon, K.U.; Yaakub, M.Y.S.M.; Zainal Ariffin, N.A.H.; Metali, F. Host selectivity and distribution of Cassytha filiformis in the coastal bornean heath forests. Trop. Life Sci. Res. 2024, 35, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Liu, K.; Ren, H.; Jian, S.; Lu, H.; Cheng, Y.; Zou, Q.; Huang, Y. The invasion of Cassytha filiformis accelerated the litter decomposition of native plant communities in small tropical coral islands. BMC Plant Biol. 2025, 25, 504. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.L.; Law, C.Y.; Lee, H.C.H.; Tang, C.O.; Lam, Y.H.; Ng, S.W.; Chan, S.S.; Chow, T.C.; Pang, K.S.; Mak, T.W.L. Gelsemium poisoning mediated by the non-toxic plant Cassytha filiformis parasitizing Gelsemium elegans. Toxicon 2018, 154, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Agbodjento, E.; Klotoé, J.R.; Sacramento, T.I.; Dougnon, T.V.; Déguenon, E.; Agbankpé, J.; Fabiyi, K.; Assogba, P.; Hounkanrin, M.P.; Akotegnon, R.; et al. Larval cytotoxic and subacute toxicity of Gardenia ternifolia, Rourea coccinea, and Cassytha filiformis used in Traditional Medicine of Benin (West Africa). J. Toxicol. 2020, 2020, 8843575. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Sajadi, S.M. Green synthesis of a Cu/MgO nanocomposite by Cassytha filiformis L. extract and investigation of its catalytic activity in the reduction of methylene blue, congo red and nitro compounds in aqueous media. RSC Adv. 2018, 8, 3723–3735. [Google Scholar] [CrossRef]
- Ezuruike, U.F.; Chieli, E.; Prieto, J.M. In vitro modulation of glibenclamide transport by P-glycoprotein inhibitory antidiabetic African plant extracts. Planta Med. 2019, 85, 987–996. [Google Scholar] [CrossRef]
- Huang, Z.; Cao, M.Y.; Wang, R.Q.; Zhang, Y.; Zhang, X.P.; Dong, L.; Zhang, C.Y. Two new aporphine alkaloids with glucose consumption increasing activity from Cassytha filiformis. Phyt. Lett. 2022, 51, 22–27. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, M.; Zhang, Y.; Zhang, X.; Dong, L.; Zhang, C. Analysis of aporphine alkaloids in Cassytha filiformis. J. Trop. Biol. 2023, 14, 251–258. [Google Scholar]
- Stévigny, C.; Block, S.; De Pauw-Gillet, M.C.; De Hoffmann, E.; Llabrès, G.; Adjakidjé, V.; Quetin-Leclercq, J. Cytotoxic aporphine alkaloids from Cassytha filiformis. Planta Med. 2002, 68, 1042–1044. [Google Scholar] [CrossRef]
- Jacqueline, E.R.; Jacques, B.; David, Z.S. Isolation of a new alkaloid from Artabotrys lastourvillensis. J. Nat. Prod. 1985, 48, 460–462. [Google Scholar] [CrossRef]
- Wang, S.; Wu, C.; Li, X.; Zhou, Y.; Zhang, Q.; Ma, F.; Wei, J.; Zhang, X.; Guo, P. Syringaresinol-4-O-β-d-glucoside alters lipid and glucose metabolism in HepG2 cells and C2C12 myotubes. Acta Pharm. Sin. B 2017, 7, 453–460. [Google Scholar] [CrossRef]
- Zhao, S.L.; Liu, D.; Ding, L.Q.; Liu, G.K.; Yao, T.; Wu, L.L.; Li, G.; Cao, S.J.; Qiu, F.; Kang, N. Schisandra chinensis lignans improve insulin resistance by targeting TLR4 and activating IRS-1/PI3K/AKT and NF-κB signaling pathways. Int. Immunopharmacol. 2024, 142, 113069. [Google Scholar] [CrossRef]
- Du, C.; Li, X.; Chen, J.; Luo, L.; Yuan, C.; Yang, J.; Hao, X.; Gu, W. Discovery of Coumarins from Zanthoxylum dimorphophyllum var. spinifoliumas and Their Potential against Rheumatoid Arthritis. Molecules 2024, 29, 4395. [Google Scholar]
- Bhujbal, S.P.; Keretsu, S.; Cho, S.J. Molecular modelling studies on pyrazole derivatives for the design of potent rearranged during transfection kinase inhibitors. Molecules 2021, 26, 691. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Stephens, P.J.; Harada, N. ECD cotton effect approximated by the Gaussian curve and other methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Neumiller, J.J.; Maruthur, N.M.; Wexler, D.J. Diagnosis and treatment of Type 2 diabetes in adults: A review. JAMA 2025, 334, 984–1002. [Google Scholar] [CrossRef]
- Harris, S. Nutrition and diet in type 2 diabetes management. Br. J. Nurs. 2025, 34, S11–S18. [Google Scholar] [CrossRef]
- Panwar, A.; Malik, S.O.; Adib, M.; Lopaschuk, G.D. Cardiac energy metabolism in diabetes: Emerging therapeutic targets and clinical implications. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H1089–H1112. [Google Scholar] [CrossRef]
- Gower, B.A.; Goss, A.M.; Yurchishin, M.L.; Deemer, S.E.; Sunil, B.; Garvey, W.T. Effects of a Carbohydrate-Restricted Diet on beta-Cell Response in Adults with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2025, 110, 1811–1817. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.Q.; Yang, Y.N.; Ma, N.; Zhou, Z.; Tan, Y.F.; Dong, L.; Li, Y.Y.; Lu, W.Y.; Wu, C.M.; et al. Laurolitsine ameliorates type 2 diabetes by regulating the hepatic LKB1-AMPK pathway and gut microbiota. Phytomedicine 2022, 106, 154423. [Google Scholar] [CrossRef]
- Amssayef, A.; Eddouks, M. Alkaloids as promising agents for the management of insulin resistance: A review. Curr. Pharm. Des. 2023, 29, 3123–3136. [Google Scholar] [CrossRef] [PubMed]
- Teerapongpisan, P.; Suthiphasilp, V.; Kumboonma, P.; Maneerat, T.; Duangyod, T.; Charoensup, R.; Promnart, P.; Laphookhieo, S. Aporphine alkaloids and a naphthoquinone derivative from the leaves of Phaeanthus lucidus Oliv. and their alpha-glucosidase inhibitory activity. Phytochemistry 2024, 220, 114020. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.X.; Zhu, N.; Zhou, F.; Lin, D.X. Natural aporphine alkaloids with potential to impact metabolic syndrome. Molecules 2021, 26, 6117. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Xia, J.; Xu, J.F.; Chen, L.; Yang, Y.; Wu, J.J.; Tang, F.; Ao, H.; Peng, C. Nuciferine, an active ingredient derived from lotus leaf, lights up the way for the potential treatment of obesity and obesity-related diseases. Pharmacol. Res. 2022, 175, 106002. [Google Scholar] [CrossRef]
- Durmaz, L.; Kiziltas, H.; Guven, L.; Karagecili, H.; Alwasel, S.; Gulcin, İ. Antioxidant, antidiabetic, anticholinergic, and antiglaucoma effects of magnofluorine. Molecules 2022, 27, 5902. [Google Scholar] [CrossRef]
- Sun, J.; Zhan, X.; Wang, W.; Yang, X.; Liu, Y.; Yang, H.; Deng, J.; Yang, H. Natural aporphine alkaloids: A comprehensive review of phytochemistry, pharmacokinetics, anticancer activities, and clinical application. J. Adv. Res. 2024, 63, 231–253. [Google Scholar] [CrossRef]
- Zhu, R.; Jiang, G.; Tang, W.; Zhao, X.; Chen, F.; Zhang, X.; Ye, N. Aporphines: A privileged scaffold in CNS drug discovery. Eur. J. Med. Chem. 2023, 256, 115414. [Google Scholar] [CrossRef]

 ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of compounds 1–3.
) correlations of compounds 1–3.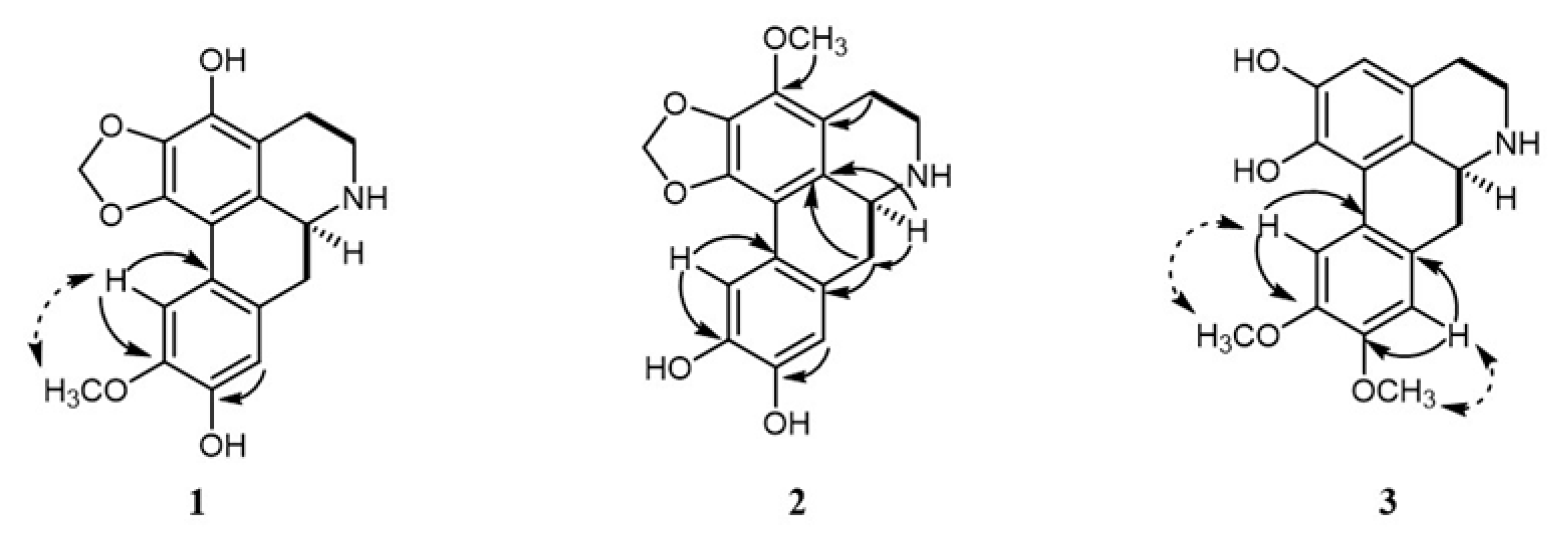
| No. | Compound 1 | Compound 2 | Compound 3 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 143.2 | 142.8 | 142.1 | |||
| 1a | 111.0 | 111.2 | 120.1 | |||
| 1b | 123.7 | 126.2 | 123.5 | |||
| 2 | 135.7 | 134.5 | 146.4 | |||
| 3 | 138.6 | 137.1 | 110.1 | |||
| 3a | 117.6 | 116.2 | 126.9 | |||
| 4 | 21.0 | 2.75, m | 21.5 | 2.76, m | 25.2 | 3.12, m; 2.83, m |
| 5α | 40.6 | 3.45, brd, 12.0 | 41.2 | 3.47, brd, 16.4 | 41.0 | 3.58, m |
| 5β | 40.6 | 2.97, m | 41.2 | 2.98, m | 41.0 | 3.16, m |
| 6a | 52.3 | 4.00, dd,14.0, 4.4 | 52.7 | 3.99, m | 52.6 | 4.14, d, 12.0 |
| 7α | 32.7 | 2.64, t, 14.0 | 33.7 | 2.67, d, 12.4 | 32.9 | 2.74, t, 12.0 |
| 7β | 32.7 | 2.92, overlapped | 33.7 | 2.98, d, 12.4 | 32.9 | 2.92, d, 12.0 |
| 7a | 121.0 | 122.1 | 121.8 | |||
| 8 | 115.7 | 6.70, s | 116.0 | 6.78, s | 115.6 | |
| 9 | 144.3 | 146.3 | 146.5 | |||
| 10 | 145.1 | 147.0 | 148.4 | |||
| 11 | 114.1 | 7.37, s | 108.8 | 7.46, s | 114.2 | 8.04, s |
| 11a | 124.3 | 124.0 | 121.5 | |||
| 3-OMe | 59.4 | 3.93, s | ||||
| 8-OMe | 56.4 | 3.84, s | ||||
| 10-OMe | 56.4 | 3.76, s | ||||
| 11-OMe | 56.5 | 3.76, s | ||||
| O-CH2-O | 101.1 | 6.12, s; 6.00, s | 101.1 | 6.13, s; 6.00, s | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Lin, Y.; Xie, L.; Wang, Y.; Xie, Z.; Dong, L.; Fu, Y. Three New Aporphine Alkaloids with Glucose Consumption Increase Activity from Cassytha filiformis. Molecules 2025, 30, 4544. https://doi.org/10.3390/molecules30234544
Zhang C, Lin Y, Xie L, Wang Y, Xie Z, Dong L, Fu Y. Three New Aporphine Alkaloids with Glucose Consumption Increase Activity from Cassytha filiformis. Molecules. 2025; 30(23):4544. https://doi.org/10.3390/molecules30234544
Chicago/Turabian StyleZhang, Caiyun, Yongrui Lin, Licui Xie, Yiru Wang, Zhiren Xie, Lin Dong, and Yanhui Fu. 2025. "Three New Aporphine Alkaloids with Glucose Consumption Increase Activity from Cassytha filiformis" Molecules 30, no. 23: 4544. https://doi.org/10.3390/molecules30234544
APA StyleZhang, C., Lin, Y., Xie, L., Wang, Y., Xie, Z., Dong, L., & Fu, Y. (2025). Three New Aporphine Alkaloids with Glucose Consumption Increase Activity from Cassytha filiformis. Molecules, 30(23), 4544. https://doi.org/10.3390/molecules30234544






