Abstract
Phytochemical investigation of Radula voluta, a liverwort species collected in the Ecuadorian Amazon, led to the isolation of four known bibenzyl derivatives: 2-prenyl-3,5-dihydroxy-bibenzyl (1), 2-geranyl-3,5-dihydroxybibenzyl (2), 2,2-dimethyl-5-phenethyl-2H-chromen-7-ol (3), and radulanin L (4). Structural elucidation was achieved through extensive NMR and MS analyses, supported by comparison with previously reported data. Compounds 1 and 4 are reported for the first time in R. voluta. The crude extract and isolated compounds were evaluated for their in vitro antiprotozoal activity against Trypanosoma cruzi, Leishmania amazonensis, Leishmania donovani, Naegleria fowleri, and Acanthamoeba castellanii Neff. Among the isolated compounds, bibenzyls 2 and 4 exhibited the most potent activity across multiple protozoan strains. Cytotoxicity was assessed against murine macrophages (J774A.1), obtaining moderate–low toxicities against compounds 1 and 3. These findings highlight the pharmacological value of liverwort-derived bibenzyls and support further research on R. voluta as a promising source of antiparasitic leads.
1. Introduction
Infectious diseases remain one of the leading causes of morbidity and mortality across the Americas, with a significant burden concentrated in South America [1]. Many parasitic infections either lack effective therapies or have current treatments that are highly toxic. Among these pathogens, kinetoplastids (such as Trypanosoma spp. and Leishmania spp.) and free-living amoebae, including Acanthamoeba spp. and the highly lethal Naegleria fowleri, represent urgent medical challenges. Human infections caused by these protozoa often lead to severe or fatal outcomes, and therapeutic options remain scarce, highlighting the critical need for new bioactive compounds with antiparasitic activity [2,3,4].
Ecuador, located in northwestern South America in the heart of the northern Andes, harbors exceptional botanical bryophyte diversity, with more than 720 liverwort species recorded [5,6]. Liverworts, in particular, represent a fascinating yet underexplored group of plants capable of producing a wide variety of biologically active metabolites. Despite their potential, fewer than 3% of liverwort species have been investigated for their chemical and pharmacological properties. These plants have already demonstrated antiparasitic potential: for example, marchantin A from Marchantia polymorpha L. exhibits inhibitory effects in Plasmodium falciparum, Trypanosoma brucei, Trypanosoma cruzi, and Leishmania donovani [7], while the essential oil of Plagiochila porelloides displays moderate activity against Trypanosoma brucei [8]. Despite these promising findings, the chemical composition of essential oils from Ecuadorian liverworts remains poorly studied [9,10,11,12].
Species of the genus Radula are leafy liverworts distributed worldwide, recognized for their structural diversity and production of unusual chemical scaffolds. They are particularly rich in aromatic derivatives such as bibenzyls, prenyl bibenzyls, and bis-bibenzyls, which are relatively rare in the plant kingdom [13]. These compounds often contain prenyl or geranyl chains that undergo intramolecular oxidations and cyclizations [14,15,16,17,18], giving rise to unique structures with diverse biological activities, ranging from antimicrobial and antifungal [19], antiviral [20], enzyme-inhibitory [21], vasopressin-inhibitory [22], antioxidant and nitric oxide-inhibitory [14], insect antifeedant [23], antitrypanosomal [24], antithrombin [25], and cytotoxicity [24].
Radula voluta is a liverwort that grows abundantly in montane forests and Ecuador páramos [5] between 450 and 4000 m, distributed across provinces such as Azuay, Carchi, Cotopaxi, Chimborazo, Galápagos, Loja, Morona Santiago, Napo, Pastaza, Pichincha, Sucumbíos, Tungurahua, and Zamora Chinchipe [6]. Previous phytochemical studies of this species carried out in Ecuador reported two known bibenzyls, 2-prenyl-3,5-dihydroxy-bibenzyl (1) and 2-geranyl-3,5-dihydroxy-bibenzyl (2) (Figure 1) [26]. Another chemical study of R. voluta carried out in Argentina reported the same compounds isolated in Ecuador [27]. With the aim of exploring the antiparasitic properties of endemic species of Ecuador, this paper reports on the isolation and characterization of four bibenzyl compounds, labeled 1–4, from R. voluta collected in the Ecuadorian Amazon and their biological activity against kinetoplastid parasites and free-living amoeba, highly pathogenic organisms that continue to challenge human health due to the lack of safe and effective treatments.
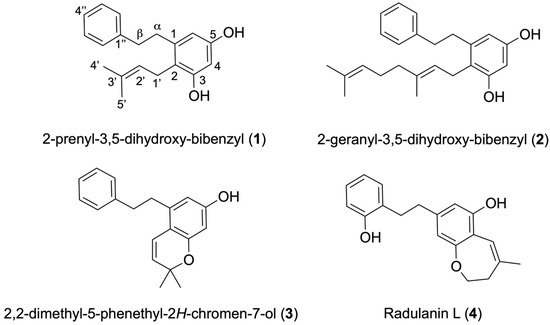
Figure 1.
Structure of compounds (1–4) isolated from Radula voluta.
2. Results
2.1. Isolation and Characterization
The ethanol extract of R. voluta was initially chromatographed on Sephadex LH-20, followed by purification on silica gel, which yielded four compounds (Figure 1). All molecules were characterized by spectroscopic techniques such as GC/MS, 1D and 2D NMR experiments, and further comparison with data from the literature.
The molecular formula of compound 1 was confirmed to be C19H22O2 by HR-ESIMS data, with a peak at m/z 281.1534 [M-H]¯ (calc. for C19H21O2 281.1542). The presence of a non-substituted benzyl group was confirmed by 1H NMR signals at [δ 7.29 (2H, m, H-3″-5″), 7.19 (3H, m, H-2″-4″-6″)]. In addition, the 1H NMR spectrum of compound 1 contained the signals of a 2,2-dimethylallyl group [δ 1.80 (3H, s, H-5′), 1.73 (3H, s, H-4′), 5.10 (1H, t, J= 6.0 Hz, H-2′), 3.30 (2H, d, J = 6.8 Hz, H-1′)], two meta coupled protons [δ 6.28 (1H, d, J = 2.6 Hz, H-6), 6.25 (1H, d, J = 2.6 Hz, H-4)] and two benzylic methylene [δ 2.84 (4H, s, H-α-β)]. Based on the above results and corroborated with the literature, the structure of 1 was elucidated to be 2-prenyl-3,5 dihydroxy-bibenzyl [28,29].
Compound 2 had been previously isolated from R. voluta [21,22] and other genus species of Radula [23,24]. The NMR spectroscopic data of compound 2 led to the assumption of a 2-geranyl-3,5-dihydroxy-bibenzyl, which was supported by data in the literature [17]. Its molecular formula was determined by HR-ESIMS to be m/z 349.2175 [M-H]¯ (calc. for C24H29O2 349.2168).
The structure of 2,2-dimethyl-5-phenethyl-2H-chromen-7-ol (3) was established by comparison of its spectral data with those bibenzyls previously reported in liverworts [12,23]. A peak at m/z 279.1387 [M-H]¯ (calc. for C19H19O2 279.1385) in the HR-ESIMS established the molecular formula. This structural transformation was corroborated by 1H NMR (δ 7.28, 2H, t; 7.18, 3H, m; 6.19, 2H, d; 2.84, 4H, br s) and by the 13C NMR spectrum. This is the first report of the presence of compound 3 in R. voluta.
The molecular formula of compound 4 was determined by HR-ESIMS to be m/z 295.13399 [M-H]¯ (calc. for C19H19O2 295.13397). The 1H NMR data displayed signals for a disubstituted aromatic ring [δ 7.08 (2H, m, H-4″-6″), 6.86 (1H, ddd, J = 7.5, 7.4, 1.2 Hz, H-5″), 6.75 (1H, dd, J = 8.4, 1.2 Hz, H-3″)], and signals for a tetrasubstituted aromatic ring [δ 6.55 (1H, d, J = 1.6 Hz, H-9), 6.39 (1H, d, J = 1.6 Hz, H-7)]. In addition, an olefinic methyl [δ 1.54 (3H, s, H-1′)] and two methylene group were observed [δ 4.40 (2H, br s, H-2), 3.40 (2H, d, J = 4.0 Hz, H-5)], which confirmed compound 4 as radulanin L, previously isolated from the following Radula species: R. complanate, R. appressa, R. constricta, and R. tokiensis [9]. This is the first report of the presence of compound 4 in R. voluta.
2.2. Antiprotozoal Effect and Cytotoxicity
The isolated compounds were evaluated for their in vitro antiprotozoal activity against the kinetoplastids Trypanosoma cruzi, Leishmania amazonensis, and Leishmania donovani, and the free-living amoebae Naegleria fowleri and Acanthamoeba castellanii Neff. Table 1 presents the results of the screening for each compound against all tested protozoa. Compounds 2 and 4 show promising antiparasitic activity, since they appear to be active against a broad range of parasites. In contrast, compounds 1 and 3 appear to be the compounds with the lowest activity against most parasites.

Table 1.
Activity of compounds 1–4 isolated from Radula voluta; the results demonstrate the activity of each compound at two concentrations, 25 and 50 µg/mL.
As shown in Table 1, Naegleria fowleri was the most sensitive protozoan strain, being affected by all the tested compounds. This suggests a potential general mechanism of action for the bibenzyl derivatives, as the parasites show sensitivity at 25 µg/mL. Despite the notable structural differences between compounds (2) and (4), Leishmania amazonensis and Trypanosoma cruzi were affected at the same dose level, 25 µg/mL. In contrast, Leishmania donovani, and Acanthamoeba castellanii were the least affected by the treatments, emerging as the most resistant strains. These findings indicate that the activity of bibenzyl derivatives cannot be clearly differentiated between kinetoplastids and free-living amoebae.
To assess the safety and therapeutic potential of the isolated bibenzyl derivatives, cytotoxicity was evaluated against murine macrophages (J774A.1) using the alamarBlue™ assay. The 50% cytotoxic concentration (CC50) effects are summarized in Table 2. Derivative 2 was the most toxic (CC50 = 14.32 µg/mL), followed by compound 4 (CC50 = 18.18 µg/mL). In contrast, compounds 1 and 3 were the least toxic, with CC50 values exceeding 50 µg/mL.

Table 2.
Cytotoxicity against murine macrophages of compounds 1–4 isolated from Radula voluta; the results are expressed as CC50 ± SD in µg/mL.
The reference drugs employed in this study are reported in Table 3. Miltefosine exhibited consistent activity against Leishmania genus parasites and moderate cytotoxicity. Benznidazole showed activity only against T. cruzi, while exhibiting low cytotoxicity. In contrast, chlorhexidine and voriconazole were highly effective against A. castellanii Neff, with moderate toxicities, even lower than those observed for the bibenzyl derivatives. In the case of N. fowleri, the standard treatment, amphotericin B, exhibits low toxicity and high efficacy.

Table 3.
Results of activity and toxicity of the commercial reference drugs; results were expressed as inhibitory concentration 50 (IC50) and cytotoxic concentration 50 (CC50) ± standard deviation (SD) [30,31,32].
3. Discussion
The present study reports for the first time the antiprotozoal activity and cytotoxicity profiles of four bibenzyl derivatives isolated from Radula voluta, a liverwort species from the Ecuadorian Amazon. Among these, two compounds, 3 and 4, are newly reported for this species, expanding the chemotaxonomic knowledge of the genus Radula. The biological evaluation targeted a broad spectrum of pathogenic protozoa, including kinetoplastids (T. cruzi, L. amazonensis, L. donovani) and free-living amoebae (N. fowleri, A. castellanii), which are recognized for their clinical relevance and limited therapeutic options [33,34,35].
Bibenzyls 2 and 4 demonstrated the highest antiprotozoal activity, particularly against N. fowleri, T. cruzi, and L. amazonensis, with sensibility at 25 µg/mL. These values are comparable to or better than other liverwort-derived secondary metabolites previously reported [36,37,38,39]. However, the cytotoxicity profile revealed that compounds 2 and 4 had low CC50 values (14.32 and 18.18 µg/mL). Although some compounds exhibited biological activity, their very low toxicity suggests limited efficacy and renders them unsuitable as potential therapeutic agents, since they could lead to undesirable treatment effects. In contrast, compounds 1 and 3 displayed moderate toxicity (50.11 and 57.85 µg/mL) relative to the reference drugs, indicating a more favorable therapeutic window. Given their comparable activity but lower toxicity in mammalian cells, these compounds appear to pose a reduced risk of adverse effects during treatment.
Given that current treatments against these protozoa are not fully effective and often cause significant side effects, coupled with the limited treatment options, especially for N. fowleri [40], compounds demonstrating some activity against these parasites provide a strong impetus for the development of future therapies. This includes both the search for new bibenzyl derivatives that may improve upon those obtained in this study and the generation of novel semi-synthetic derivatives.
Structurally, the presence of the geranyl side chains on the bibenzyl core appears to enhance biological activity in compound 2 compared with the prenylated derivative 1. This effect is also evident in the toxicity values, with compound 2 showing the highest toxicity rates. Prenylation (or geranylation) of aromatic scaffolds such as the bibenzyl core has been repeatedly associated with enhanced biological activity due to increased lipophilicity and membrane-permeation potential. Therefore, lipophilicity was analyzed using the SwissADME platform [41,42]., which allowed us to predict the ADME parameters, pharmacokinetic properties, drug-like nature, and medicinal chemistry friendliness of compounds 1–4 (S21–S25, Supplementary Material) [41]. The calculated average Log P (n-octanol/water) value for 2-prenyl-3,5-dihydroxy-bibenzyl (1) (log P = 4.36) was significantly lower than that of 2-geranyl-3,5-dihydroxy-bibenzyl (2) (log P = 5.82), with compound 2 showing the best performance in the predictive model in terms of high passive absorption through the gastrointestinal tract (S25, Supplementary Material). This is consistent with previous reports on prenylated bibenzyls and other phenolic metabolites from liverworts, which have shown improved membrane permeability and biological interaction [43,44]. In contrast, radulanin L (4) showed lower values of lipophilicity (log P = 3.66) compared with 3 (log P = 4.11). The enhanced bioactivity of radulanin L (4) suggests that the additional hydroxyl group at position 2″ may play a role in improving its activity. This modification also confers favorable properties for crossing the blood–brain barrier (BBB), as indicated by the predictive model (S25, Supplementary Material).
Overall, the data support the antiprotozoal potential of bibenzyls derived from Radula voluta, with compounds 2 and 4 identified as the most effective candidates against all parasites. In contrast, compounds 1 and 3 exhibit the lowest cytotoxicity. These findings support the need for further investigation of the minor components of Radula voluta and structural optimization to improve their potential as antiparasitic leads, with future studies focusing on structure–activity relationship (SAR) modeling and semi-synthetic modification to enhance potency and selectivity.
4. Materials and Methods
4.1. General Information
NMR spectra were acquired on a Bruker AVANCE 500 MHz or 600 MHz (Bruker Biospin, Falländen, Switzerland) instrument spectrometer at 300 K when required. The Bruker AVANCE 600 MHz spectrometer was equipped with a 5 mm TCI inverse detection cryoprobe (Bruker Biospin, Falländen, Switzerland). Standard Bruker NMR pulse sequences were utilized. NMR spectra were obtained by dissolving samples in CDCl3 (99.9%). Electrospray ESI high-resolution mass spectra (HRMS) were recorded on a Waters VG-Micromass, model Zab 2F (Waters, Manchester, UK), and a Vion IMS Q-TOF (Waters, Manchester, UK). IR spectra were recorded between 450 and 4000 cm−1 on an Agilent Cary 630 FTIR Spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA). The GC-MS analyses were performed on an Agilent Technologies (Wilmington, DE, USA) 6890 N gas chromatograph coupled to an Agilent Technologies 5973 N mass spectrometer detector. Silica gel 60 (Merck KGaA, Darmstadt, Germany, from 0.063 to 0.200 mm) was used as stationary phase for column chromatography. Normal-phase Thin Layer Chromatography (TLC) plates, with fluorescence indicator at 254 nm, were purchased from Sigma-Aldrich. After exposure to UV light (254 and 366 nm), the plates were revealed with a mixture of sulfuric acid and vanillin. The organic solvents, used for CC and TLC, were purchased from Brenntag (Guayaquil, Ecuador) and carefully distilled before using. Reference commercial drugs were used as positive controls in both cytotoxicity and protozoan assays, including miltefosine (Cayman Chemical, Vitro SA, Madrid, Spain), benznidazole (Sigma-Aldrich, Madrid, Spain), chlorhexidine (chlorhexidine digluconate; Alfa Aesar), and voriconazole (Fluka; Sigma-Aldrich Chemistry Ltd., Madrid, Spain). The alamarBlueTM reagent (Life Technologies, Madrid, Spain) was employed as a metabolic indicator.
4.2. Plant Material
The samples of liverwort Radula voluta were collected in Morona Santiago province, Ecuador, Canton Morona, Sevilla Don Bosco Parish in May 2019, at 2500 m a.s.l. (coordinates: 2°39′37.2″ S., 77°42′39.4″ W). The sample collection was conducted in keeping with Ecuadorian law and authorized by the Ministry of Environment, Water, and Ecological Transition of Ecuador (MAATE) under permit code MAATE-DBI-CM-2022-0248. A voucher specimen (HUTPL AB-1338) has been deposited at the Herbarium HUTPL of the Universidad Técnica Particular de Loja. The identity of the plant material was confirmed by the curator of lichens and bryophytes at the mentioned herbarium. Plants samples that were free of impurities were placed in a dehydrator apparatus at 30 °C for 48 h before extraction.
4.3. Extraction and Isolation
Cleaned, air-dried material (200.1 g) was extracted with EtOH at room temperature for 8 days. Following this, ethanol extract was filtered, and the solvent evaporated under vacuum to give a green oil (13.1 g). The crude extract was chromatographed on a Sephadex LH-20 with MeOH (100%) in order remove impurities (chlorophyll) from the compounds of interest. A total of 5 fractions (RvF1–RvF5) were collected.
The RvFr3 (96.68 mg) was subjected to column chromatography on silica gel and eluted with a step gradient solvent system of n-hexane/EtOAc (90:10, 80:20, 70:30, v/v). Fractions with similar behavior on TLC were combined and evaporated, yielding 7 sub-fractions (RvF3-1 to RvF3-7). The sub-fraction RvF3-2 yielded the compound 2,2-dimethyl-7-hydroxy-5-(2-phenylethyl) chromene (3) (11.6 mg). The sub-fraction RvF3-4 yielded the compound 2-geranyl-3,5-dihydroxy bibenzyl (2) (10.9 mg). The fraction RvF3-5 contained the compound 3,5 dihydroxy-2-(3-methyl-2-butenyl) bibenzyl (1) (31.2 mg).
The fraction RvFr4 (31.3 mg) was submitted to silica column chromatography. The column was eluted with an isocratic system of n-hexane/EtOAc (70:30, v/v) to obtain four sub-fractions (RvF4-1 to RvF4-4). The RvF4-1 (3.0 mg) was purified by column chromatography on silica gel and eluted with an isocratic system of n-hexane/EtOAc (70:30, v/v) to obtain three sub-fractions (RvF4-1-1 to RvF4-1-3). The pure substance (RvF4-1-2) obtained in this purification was identified as radulanin L (4) (2.2 mg).
4.3.1. 3,5-Dihydroxy-2-(3-methyl-2-butenyl) Bibenzyl (1)
Compound (1) was obtained as a brown oil, FT-IR (ν, cm¯1) 3381, 2923, 1602, 1453, 1135, 699; 1H NMR (500 MHz, CDCl3) δ 7.29 (2H, m, H-3″-5″), 7.19 (3H, m, H-2″-4″-6″), 6.28 (1H, d, J = 2.6 Hz, H-6), 6.25 (1H, d, J = 2.6 Hz, H-4), 5.39 (1H, br s, OH), 5.18 (1H, br s, OH), 5.10 (1H, t, J= 6.0 Hz, H-2′) 3.30 (2H, d, J = 6.8 Hz, H-1′), 2.84 (4H, s, H-α-β), 1.80 (3H, s, H-5′), 1.73 (3H, s, H-4′); 13C NMR (126 MHz, CDCl3) δ 156.03 (C-3), 154.81(C-5), 142.51 (C-1″), 142.10 (C-1), 134.43 (C-3′) 128.78 (C-2″-3″-5″-6″), 126.38 (C-4″), 123.05 (C-2′), 118.02 (C-2), 109.27 (C-6), 101.80 (C-4), 37.92 (C-β), 36.00 (C-α), 26.11 (C-4′), 25.26 (C-1′), 18.32 (C-5′); HR-ESIMS m/z 281.1534 [M-H]¯ (calc. for C19H21O2 281.1542). One- and two-dimensional NMR spectra are included in the Supplementary Material.
4.3.2. 2-Geranyl-3,5-dihydroxy-bibenzyl (2)
Compound (2) was isolated as a brown oil, FT-IR (ν, cm¯1) 3381, 2924, 2856, 1597, 1456, 1134, 699; 1H NMR (600 MHz, CDCl3): δ 7.29 (2H, t, J = 7.5 Hz, H-3″-5″), 7.18 (3H, m, H-2″-4″-6″), 6.27 (1H, d, J = 2.2 Hz, H-6), 6.25 (1H, d, J = 2.8 Hz, H-4), 5.34 (1H, br s, OH), 5.11 (1H, t, J = 6.0 Hz, H-2′) 5.04 (1H, t, J = 6.6 Hz, H-6′), 3.29 (2H, d, J = 6.4 Hz, H-1′), 2.84 (4H, br s, H-α-β), 2.09 (2H, m, H-5′), 2.03 (2H, m, H-4′), 1.79 (3H, s, H-10′), 1.72 (3H, s, H-9′), 1.58 (3H, s, H-8′); 13C NMR (151 MHz, CDCl3): δ 155.88 (C-3), 154.66 (C-5), 142.22 (C-1), 141.86 (C-1″), 138.10 (C-3′), 134.20 (C-7′), 128.54 (C-2″-3″-5″-6″), 126.14 (C-4″), 123.95 (C-6′), 122.81 (C-2′), 117.70 (C-2), 108.98 (C-6), 101.54 (C-4), 39.78 (C-4′), 37.70 (C-β), 35.78 (C-α), 26.57 (C-5′), 25.87 (C-9′), 25.03 (C-1′), 18.08 (C-10′), 16.39 (C-8′). HR-ESIMS m/z 349.2175 [M-H]¯ (calc. for C24H29O2 349.2168). One- and two-dimensional NMR spectra are included in the Supplementary Material.
4.3.3. 2,2-Dimethyl-5-phenethyl-2H-chromen-7-ol (3)
Compound (3) yielded a dark-brown oil, FT-IR (ν, cm¯1) 2921, 2851, 1609, 1456, 1134, 699; 1H NMR (600 MHz, CDCl3): δ 7.28 (2H, m, H-3″-5″), 7.18 (1H, m, H-4″), 7.18 (2H, m, H-2″-6″), 6.44 (1H, d, J = 10.0 Hz, H-4), 6.19 (2H, d, J = 2.5 Hz, H-6-8), 5.50 (1H, d, J = 9.9 Hz, H-3), 2.84 (4H, br s, H-α-β), 1.40 (6H, s, H-1′-2′). 13C NMR (151 MHz, CDCl3) δ 156.09 (C-7), 154.78 (C-9), 141.71 (C-1″), 139.24 (C-5), 128.54 (C-2″-3″-5″-6″), 128.10 (C-3), 126.16 (C-4″), 118.87 (C-4), 113.06 (C-10), 108.81 (C-6), 102.15 (C-8), 75.77 (C-2), 37.54 (C-β), 34.53 (C-α), 27.84 (C-1′-2′). HR-ESIMS m/z 279.1387 [M-H]¯ (calc. for C19H19O2 279.1385). One- and two-dimensional NMR spectra are included in the Supplementary Material.
4.3.4. Radulanin L (4)
Compound (4) was recovered as a light-brown oily residue, FT-IR (ν, cm¯1) 2923, 2854, 1734, 1457; 1H NMR (500 MHz, CDCl3) δ 7.08 (2H, m, H-4″-6″), 6.86 (1H, ddd, J = 7.5, 7.4, 1.2 Hz, H-5″), 6.75 (1H, dd, J = 8.4, 1.2 Hz, H-3″), 6.55 (1H, d, J = 1.6 Hz, H-9), 6.39 (1H, d, J = 1.6 Hz, H-7), 5.61 (1H, t, J = 5.5 Hz, H-4), 4.87 (1H, br s, OH), 4.80 (1H, br s, OH), 4.40 (2H, br s, H-2), 3.40 (2H, d, J = 4.0 Hz, H-5), 2.83 (4H, m, H-α-β), 1.54 (3H, s, H-1′). 13C NMR (126 MHz, CDCl3) δ 159.85 (C-10), 153.64 (C-2″), 152.26 (C-6), 141.76 (C-8), 134.13 (C-3), 130.46 (C-6″), 127.5 (C-1″-4″), 121.03 (C-4-11), 120.85 (C-5″), 115.54 (C-3″), 113.91 (C-9), 111.70 (C-7), 74.41 (C-2), 35.84 (C-α), 32.15 (C-β), 21.84 (C-5), 20.24 (C-1′). HR-ESIMS m/z 295.13399 [M-H]¯ (calc. for C19H19O2 295.13397). One and two-dimensional NMR spectra are included in the Supplementary Material.
4.4. Cultures
To carry out the leishmanicidal and trypanocidal screening assays, promastigote forms of L. amazonensis (MHOM/BR/77/LTB0016) and L. donovani (MHOM/IN/90/GE1F8R) were grown at 26 °C in Schneider’s medium (SND, Sigma-Aldrich, Darmstadt, Germany), supplemented with 10% foetal bovine serum (FBS, VWR, Biowest, Nuaillé, France). Epimastigote forms of T. cruzi (Y strain) were cultured at 26 °C in Liver Infusion Tryptose (LIT) medium supplemented with 10% FBS. Trophozoites of Acanthamoeba castellanii Neff, genotype T4 (ATCC 30010), and N. fowleri (ATCC30808) were grown in Peptone yeast glucose (PYG) medium and Bactocasitone medium supplemented with 10% FBS, respectively. Murine macrophages from cell line J774A.1 (ATCC TIB-67) were cultured at 37 °C in a 5% CO2 atmosphere in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS. To develop the assays, the medium of the macrophage cells was changed to RPMI 1640 medium (Gibco, New York, MA, USA).
4.5. Preliminary Antiprotozoal Activity
4.5.1. In Vitro Activity Against Epimastigote of Trypanosoma cruzi and Promastigote of Leishmania spp
In 96-well plates, two concentration dilutions of each compound (25 and 50 µg/mL) were added with parasites at a concentration of 106 cells/mL in a final volume of 200 µL of LIT medium for T. cruzi and Schneider for Leishmania spp.
After 72 h of incubation at 26 °C, each plate was observed under a microscope to determine the concentration of compounds that allowed the parasites to survive. Observation of morphological changes, decreased parasite motility, or a reduction in parasite numbers indicated that the compound had activity against parasites [45].
4.5.2. In Vitro Activity Against Trophozoites of Acanthamoeba castellanii Neff and Naegleria fowleri
In 96-well plates, 50 µL of parasites were added to each plate (2.5 × 105 cells/mL of N. fowleri in Bactocasitone medium or 5 × 104 cells/mL of A. castellanii Neff in PYG medium). After trophozoite attachment, 50 μL of the dilutions (25 and 50 µg/mL) of each molecule were added to each well. After incubation (for 96 h at 26 °C for A. castellanii Neff. and 48 h at 37 °C for N. fowleri), the wells were examined, as in the kinetoplastid assay, to determine the potential activity of the compounds [46,47].
4.5.3. Results of the Screening
The results against each concentration were represented as (+) when compounds presented activity and the parasite was dead, or (−) when the compounds were not active and the parasites were alive. Each experiment was conducted in triplicate to ensure the result and its repeatability.
4.6. Cytotoxicity Assay
The cytotoxicity of the active compounds was assessed against murine macrophage cell line J774A.1 (ATCC® TIB-67). To determine the cytotoxic concentration fifty (CC50), the concentration of a compound that inhibits 50% of the cell population, a colorimetric method, based on the New York Cell Viability Reagent (Thermo Fisher Scientific, Waltham, MA, USA), was used.
In 96-well plates, 104 macrophages in 50 µL of RPMI medium were added to each well. Following complete adherence of the cells, serial dilutions of the compounds were added with 10% alamarBlueTM. The plates were incubated for 24 h at 37 °C in a 5% CO2 atmosphere. After 24 h of incubation, the plates were read using the EnSpire Multimode Plate Reader® at an excitation wavelength of 544 nm and an emission wavelength of 590 nm to determine the CC50.
Statistical Analysis
The inhibitory concentration fifty (CC50) was determined through the nonlinear regression analysis “[Inhibitor] vs. response—Variable slope (four parameters)”, using GraphPad Prism 10.1.1. Each experiment was conducted in triplicate, and average values were reported. The results were expressed as mean ± standard deviation. Statistical significance was evaluated using a paired two-tailed t-test, with p values below 0.05 considered statistically significant.
5. Conclusions
Our findings highlight the chemical and biological significance of the four bibenzyl derivatives isolated from Radula voluta. Compounds 3 and 4 are reported here for the first time from this species, expanding the known chemical diversity within the genus. The overall antiprotozoal profile of these metabolites reinforces the potential of bibenzyl scaffolds as promising antiparasitic templates. Among the isolates, compounds 2 and 4 showed the highest activity, likely influenced by the presence of isoprenoid side chains and free phenolic hydroxyl groups, which enhance lipophilicity and membrane interaction. Compound 1 exhibited the best selectivity index, suggesting that subtle structural variations within this class can markedly affect biological performance. Together, these results support the notion that R. voluta is a valuable natural source of structurally diverse bibenzyls and provide a foundation for future semi-synthetic modification aimed at optimizing potency and selectivity against protozoan pathogens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30234543/s1, Table S1. 1H NMR and 13C NMR data of compounds (1–4) in CDCl3; Figure S1. 1H NMR data of 2-prenyl-3,5 dihydroxy-bibenzyl (1) in CDCl3; Figure S2. 13C NMR data of 2-prenyl-3,5 dihydroxy-bibenzyl (1) in CDCl3; Figure S3. HSQC of 2-prenyl-3,5 dihydroxy-bibenzyl (1) in CDCl3; Figure S4. HMBC of 2-prenyl-3,5 dihydroxy-bibenzyl (1) in CDCl3; Figure S5. 1H NMR data of 2-geranyl-3,5-dihydroxy-bibenzyl (2) in CDCl3; Figure S6. 13C NMR data of 2-geranyl-3,5-dihydroxy-bibenzyl (2) in CDCl3; Figure S7. HSQC of 2-geranyl-3,5-dihydroxy-bibenzyl (2) in CDCl3; Figure S8. HMBC of 2-geranyl-3,5-dihydroxy-bibenzyl (2) in CDCl3; Figure S9. 1H NMR data of 2,2-dimethyl-5-phenethyl-2H-chromen-7-ol (3) in CDCl3; Figure S10. 13C NMR data of 2,2-dimethyl-5-phenethyl-2H-chromen-7-ol (3) in CDCl3; Figure S11. HSQC of 2,2-dimethyl-5-phenethyl-2H-chromen-7-ol (3) in CDCl3; Figure S12. HMBC of 2,2-dimethyl-5-phenethyl-2H-chromen-7-ol (3) in CDCl3; Figure S13. 1H NMR data of radulanin L (4) in CDCl3; Figure S14. 13C NMR data of radulanin L (4) in CDCl3 (126 MHz); Figure S15. HSQC of radulanin L (4) in CDCl3; Figure S16. HMBC of radulanin L (4) in CDCl3; S21: SwissADME analysis of 2-prenyl-3,5 dihydroxy-bibenzyl (1); S22: SwissADME analysis of 2-geranyl-3,5-dihydroxy-bibenzyl (2); S23: SwissADME analysis of 2,2-dimethyl-5-phenethyl-2H-chromen-7-ol (3); S24: SwissADME analysis of radulanin L (4): S25: BOILED-Egg Model of compounds 1–4. Refs. [41,42] are cited in the Supplementary Materials.
Author Contributions
Conceptualization: J.M.A., L.C., and V.M.; Methodology: J.M.A., L.C., and J.J.F.; Investigation: J.M.A., L.C., Á.B., C.J.B.-E., and R.L.R.-E.; Data curation: L.C. and Á.B.; Spectroscopic analysis and compound identification: L.C., J.M.A., A.R.D.-M., and J.J.F.; Biological assays and analysis: C.J.B.-E., R.L.R.-E., J.C.-P., J.E.P., and J.L.-M.; Writing—original draft preparation: L.C. and J.M.A.; Writing—review and editing: C.J.B.-E., A.R.D.-M., J.L.-M., and J.J.F.; Supervision: A.R.D.-M., J.L.-M., J.E.P. and J.J.F.; Project administration: A.R.D.-M., J.L.-M., J.E.P. and J.J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia e Innovación, Spain (Project No. PID2019-109476RB-C21, BIOALGRI); Gobierno de Canarias: PROMOTUR Turismo de Canarias (Acciones para la gestión inteligente y creación del producto de Turismo Azul), specifically the project “Islas Canarias, naturaleza marina singular: Salud y bienestar”, within the framework of the Recovery, Transformation and Resilience Plan (Next Generation EU Funds); and ProID2024010033 (BIMICMAR), co-funded by EU; Plan Propio de Investigación 2025, Universidad de La Laguna, 2025/0002183; Consorcio Centro de Investigación Biomédica (CIBER) de Enfermedades Infecciosas (CIBERINFEC); the Instituto de Salud Carlos III, 28006 Madrid, Spain (CB21/13/00100); project CC20230222, CABILDO.23 funded by Cabildo Insular de Tenerife 2023–2028 and the Ministerio de Sanidad, Spain. This research was also supported by Personalized and precision medicine grant (MePRAM Project, PMP22/00092), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación. Funded by Next Generation EU funds from the European Union that finance the actions of the The Resilience and Recovery Facility. C.J.B.-E., R.L.R.-E. and J.C.-P. contracts (CC20230222, CABILDO.23) were funded by Cabildo Insular de Tenerife 2023–2028 and Ministerio de Sanidad, Spain; PROMETEO Program, Programa de Ayudas para Grupos de Investigación de Excelencia (CIPROM/2024/88), Generalitat Valenciana, Valencia, Spain.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon reasonable request.
Acknowledgments
All the authors would like to thank the Universidad Técnica Particular de Loja (UTPL) for supporting this open-access publication. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Q.; Liu, M.; Liang, W.; Li, X.; Jing, W.; Chen, Z.; Liu, J. Global distribution and health impact of infectious disease outbreaks, 1996–2023: A worldwide retrospective analysis of World Health Organization emergency event reports. J. Glob. Health 2025, 15, 04151. [Google Scholar] [CrossRef]
- Sangenito, L.S.; da Silva Santos, V.; d’Avila-Levy, C.M.; Branquinha, M.H.; Souza Dos Santos, A.L.; de Oliveira, S.S.C. Leishmaniasis and Chagas Disease—Neglected Tropical Diseases: Treatment Updates. Curr. Top. Med. Chem. 2019, 19, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Pana, A.; Vijayan, V.; Anilkumar, A.C. Amebic Meningoencephalitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Raghavan, A.; Rammohan, R. Acanthamoeba keratitis—A review. Indian J. Ophthalmol. 2024, 72, 473–482. [Google Scholar] [PubMed]
- Gradstein, S.R. The Liverworts and Hornworts of Colombia and Ecuador, 1st ed.; William, R., Ed.; Buck: Bronx, NY, USA, 2021; p. 121. [Google Scholar]
- Gradstein, S.R. Checklist of the Liverworts and hornworts of Ecuador. Frahmia 2020, 17, 1–40. [Google Scholar]
- Jensen, S.; Omarsdottir, S.; Bwalya, A.G.; Nielsen, M.A.; Tasdemir, D.; Olafsdottir, E.S. Marchantin A, a macrocyclic bisbibenzyl ether, isolated from the liverwort Marchantia polymorpha, inhibits protozoal growth in vitro. Phytomedicine 2012, 19, 1191–1195. [Google Scholar] [CrossRef]
- Pannequin, A.; Quetin-Leclercq, J.; Costa, J.; Tintaru, A.; Muselli, A. First phytochemical profiling and in-vitro antiprotozoal activity of essential oil and extract of Plagiochila porelloides. Molecules 2023, 28, 616. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Nagashima, F.; Gradstein, R.S.; Asakawa, Y. Volatile components from selected Mexican, Ecuadorian, Greek, German and Japanese liverworts. Nat. Prod. Commun. 2008, 3, 133–140. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F.; Toyota, M.; Hashimoto, T.; Tori, M.; Harinantenaina, L. Bryophytes: Bio-and chemical diversity, bioactivity and chemosystematics. Heterocycles 2009, 77, 99–150. [Google Scholar] [CrossRef]
- Valarezo, E.; Tandazo, O.; Galán, K.; Rosales, J.; Benítez, Á. Volatile metabolites in Liverworts of Ecuador. Metabolites 2020, 10, 92. [Google Scholar] [CrossRef]
- Morocho, V.; Benitez, Á.; Carrión, B.; Cartuche, L. Novel study on chemical characterization and antimicrobial, antioxidant, and anticholinesterase activity of essential oil from Ecuadorian bryophyte Syzygiella rubricaulis (Nees) Stephani. Plants 2024, 13, 935. [Google Scholar] [CrossRef]
- Toyota, M.; Kinugawa, T.; Asakawa, Y. Bibenzyl cannabinoid and bisbibenzyl derivative from the liverwort Radula perrottetii. Phytochemistry 1994, 37, 859–862. [Google Scholar] [CrossRef]
- Asakawa, Y.; Nagashima, F.; Ludwiczuk, A. Distribution of bibenzyls, prenyl bibenzyls, bis-bibenzyls, and terpenoids in the liverwort genus Radula. J. Nat. Prod. 2020, 83, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Toyota, M.; Nakaishi, E.; Tada, Y. Distribution of terpenoids and aromatic compounds in New Zealand liverworts. J. Hattori Bot. Lab. 1996, 80, 271–295. [Google Scholar]
- Zhang, C.Y.; Gao, Y.; Zhu, R.X.; Qiao, Y.N.; Zhou, J.C.; Zhang, J.Z.; Li, Y.; Li, S.W.; Fan, S.H.; Lou, H.X. Prenylated bibenzyls from the Chinese Liverwort Radula constricta and their mitochondria-derived paraptotic cytotoxic activities. J. Nat. Prod. 2019, 82, 741–1751. [Google Scholar] [CrossRef]
- Kinghorn, A.; Falk, O.H.; Kobayashi, L.J. Chemical Constituents of Bryophyta. In Chemical Constituents of Bryophytes. Bio- and Chemical Diversity, Biological Activity, and Chemosystematics; Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 2013; Volume 95, pp. 593–605. [Google Scholar]
- Mues, R.; Zinsmeister, H.D. The chemotaxonomy of phenolic compounds in bryophytes. J. Hattori Bot. Lab. 1988, 64, 109–141. [Google Scholar]
- Lorimer, S.D.; Perry, N.B.; Tangney, R.S. An antifungal bibenzyl from the New Zealand liverwort, Plagiochzla stephensonzana. Synthesis, and analysis Bioactivity-directed isolation. J. Nat. Prod. 1993, 56, 1444–1450. [Google Scholar] [CrossRef]
- Iwai, Y.; Murakami, K.; Gomi, Y.; Hashimoto, T.; Asakawa, Y.; Okuno, Y.; Ishikawa, T.; Hatakeyama, D.; Echigo, N.; Kusuhara, T. Anti-influenza activity of marchantins, macrocyclic bisbibenzyls contained in liverworts. PLoS ONE 2011, 6, e19825. [Google Scholar] [CrossRef]
- Nandy, S.; Dey, A. Bibenzyls and bisbibenzyls of bryophytic origin as promising source of novel therapeutics: Pharmacology, synthesis and structure-activity. J. Pharm. Sci. 2020, 28, 701–734. [Google Scholar]
- Asakawa, Y.; Hashimoto, T.; Takikawa, K.; Tori, M.; Ogawa, S. Prenyl bibenzyls from the liverworts Radula perrottetii and Radula complanata. Phytochemistry 1991, 30, 235–251. [Google Scholar] [CrossRef]
- Labbé, C.; Faini, F.; Villagrán, C.; Coll, J.; Rycroft, D.S. Bioactive polychlorinated bibenzyls from the liverwort Riccardia polyclada. J. Nat. Prod. 2007, 70, 2019–2021. [Google Scholar] [CrossRef]
- Otoguro, K.; Ishiyama, A.; Iwatsuki, M.; Namatame, M.; Tukashima, A.N.; Kiyohara, H.; Hashimoto, T.; Asakawa, Y.; Omura, S.; Yamada, H. In vitro antitrypanosomal activity of bis(bibenzyls) and bibenzyls from liverworts against Trypanosoma brucei. J. Nat. Med. 2012, 66, 377–382. [Google Scholar] [CrossRef]
- Nagashima, F.; Momosaki, S.; Watanabe, Y.; Toyota, M.; Huneck, S.; Asakawat, Y. Terpenoids and aromatic compounds from six liverworts. Phytochemistry 1996, 41, 207–211. [Google Scholar] [CrossRef]
- Kraut, L.; Must, R.; Dietmar, Z.H. Prenylated bibenzyl derivatives from Lethocolea glossophylla and Radula voluta. Phytochemistry 1997, 45, 1249–1255. [Google Scholar] [CrossRef]
- Nagashima, F.; Asakawa, Y. Terpenoids and bibenzyls from three Argentine liverworts. Molecules 2011, 16, 10471–10478. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Kondo, K.; Tori, M. Cyclopropanochroman derivatives from the liverwort Radula javanica. Phytochemistry 1991, 30, 325–328. [Google Scholar] [CrossRef]
- Asakawa, Y.; Kondo, K.; Takikawa, E.K.; Tori, M.; Hashimoto, T.; Ogawa, S. Prenyl bibenzyls from the liverworts Radula kojana. Phytochemistry 1991, 30, 219–234. [Google Scholar] [CrossRef]
- Bethencourt-Estrella, C.J.; López-Arencibia, A.; Lorenzo-Morales, J.; Piñero, J.E. Global Health Priority Box: Discovering Flucofuron as a Promising Antikinetoplastid Compound. Pharmaceuticals 2024, 17, 554. [Google Scholar] [CrossRef]
- Rodríguez-Expósito, R.L.; Nicolás-Hernández, D.S.; Sifaoui, I.; Cuadrado, C.; Salazar-Villatoro, L.; Reyes-Batlle, M.; Hernández-Daranas, A.; Omaña-Molina, M.; Fernández, J.J.; Díaz-Marrero, A.R.; et al. Gongolarones as antiamoeboid chemical scaffold. Biomed. Pharmacother. 2023, 158, 114185. [Google Scholar] [CrossRef]
- Chao-Pellicer, J.; Arberas-Jiménez, I.; Delgado-Hernández, S.; Sifaoui, I.; Tejedor, D.; García-Tellado, F.; Piñero, J.E.; Lorenzo-Morales, J. Cyanomethyl Vinyl Ethers Against Naegleria fowleri. ACS Chem. Neurosci. 2023, 14, 2123–2133. [Google Scholar] [CrossRef]
- Stuart, K.; Brun, R.; Croft, S.; Fairlamb, A.; Gütteridge, W.; McKerrow, J.; Reed, S.; Tarleton, R. Kinetoplastids: Related protozoan pathogens, different diseases. J. Clin. Investig. 2008, 118, 1301–1310. [Google Scholar] [CrossRef]
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous manifestations of human and murine leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296. [Google Scholar] [CrossRef]
- Grace, E.; Asbill, S.; Virga, K. Naegleria fowleri: Pathogenesis, diagnosis, and treatment options. Antimicrob. Agents Chemother. 2015, 59, 6677–6881. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y. Biologically active compounds from bryophytes. Pure Appl. Chem. 2007, 79, 557–580. [Google Scholar] [CrossRef]
- Rosa, L.H.; Furtado, G.P.; Barata, L.E.S. Antimicrobial activity of compounds isolated from liverworts: A review. Phytomedicine 2011, 18, 1135–1140. [Google Scholar]
- Roldos, V.; Nakayama, H.; Rolón, M.; Montero-Torres, A.; Truccu, F.; Torres, S.; Vega, C.; Marrero-Ponce, Y.; Heguaburu, V.; Yaluff, G.; et al. Activity of a hydroxybibenzyl bryophyte constituent against Leishamania spp. and Trypanosoma cruzi: In silico, in vitro and in vivo activity studies. Eur. J. Med. Chem. 2008, 43, 1797–1807. [Google Scholar] [CrossRef]
- Cos, P.; Maes, L.; Vlietinck, A.J.; Berghe, D.V. Plant-derived leading compounds for chemotherapy of human protozoan infections. Planta Med. 2004, 70, 501–518. [Google Scholar]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Naegleria fowleri. Acta Trop. 2014, 132, 173–177. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokineZcs, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict GastrointesZnal AbsorpZon and Brain PenetraZon of Small Molecules. ChemMedChem 2016, 11, 1117. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Biologically Active Compounds of the Marchantiophyta and Bryophyta. In Chemical Constituents of Bryophytes: Bio- and Chemical Diversity, Biological Activity, and Chemosystematics; Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 2013; Volume 95, pp. 619–638. [Google Scholar]
- Zhang, X.; Liu, J.; Liang, J.; Wang, Z.; Wang, C. Structure-activity relationships of prenylated natural products with antiprotozoal activity. Molecules 2021, 26, 2537. [Google Scholar]
- Cartuche, L.; Sifaoui, I.; López-Arencibia, A.; Bethencourt-Estrella, C.J.; San Nicolás-Hernández, D.; Lorenzo-Morales, J.; Piñero, J.E.; Díaz-Marrero, A.R.; Fernández, J.J. Antikinetoplastid Activity of Indolocarbazoles from Streptomyces sanyensis. Biomolecules 2020, 10, 657. [Google Scholar] [CrossRef]
- Cartuche, L.; Reyes-Batlle, M.; Sifaoui, I.; Arberas-Jiménez, I.; Piñero, J.E.; Fernández, J.J.; Lorenzo-Morales, J.; Díaz-Marrero, A.R. Antiamoebic Activities of Indolocarbazole Metabolites Isolated from Streptomyces sanyensis Cultures. Mar. Drugs 2019, 17, 588. [Google Scholar] [CrossRef]
- Rizo-Liendo, A.; Sifaoui, I.; Reyes-Batlle, M.; Chiboub, O.; Rodríguez-Expósito, R.L.; Bethencourt-Estrella, C.J.; San Nicolás-Hernández, D.; Hendiger, E.B.; López-Arencibia, A.; Rocha-Cabrera, P.; et al. In Vitro Activity of Statins against Naegleria fowleri. Pathogens 2019, 8, 122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).