From Lipid Regulation to Neuroprotection: Multitarget (Benzo)thiazine Derivatives as Promising Leads
Abstract
1. Introduction
2. Results and Discussion
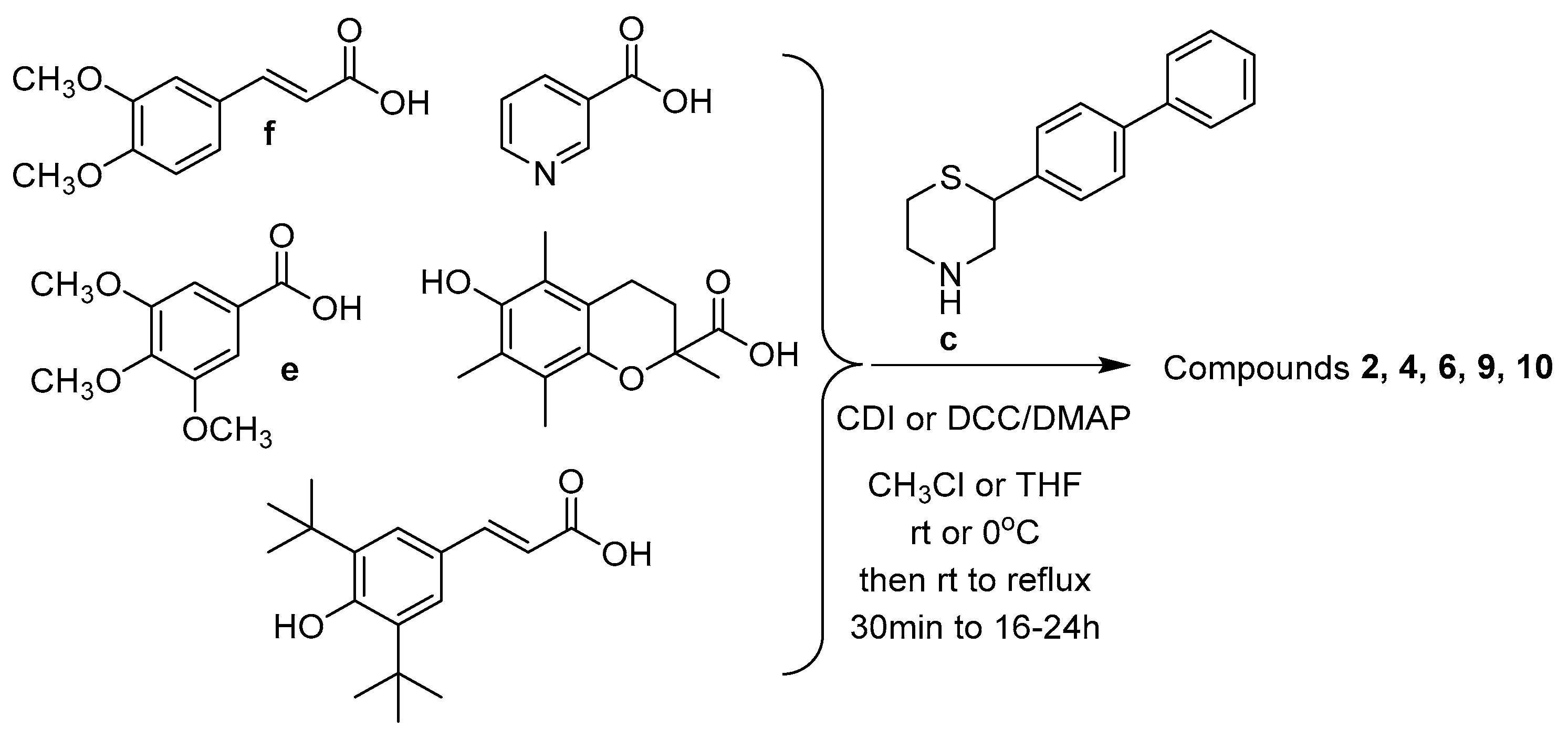
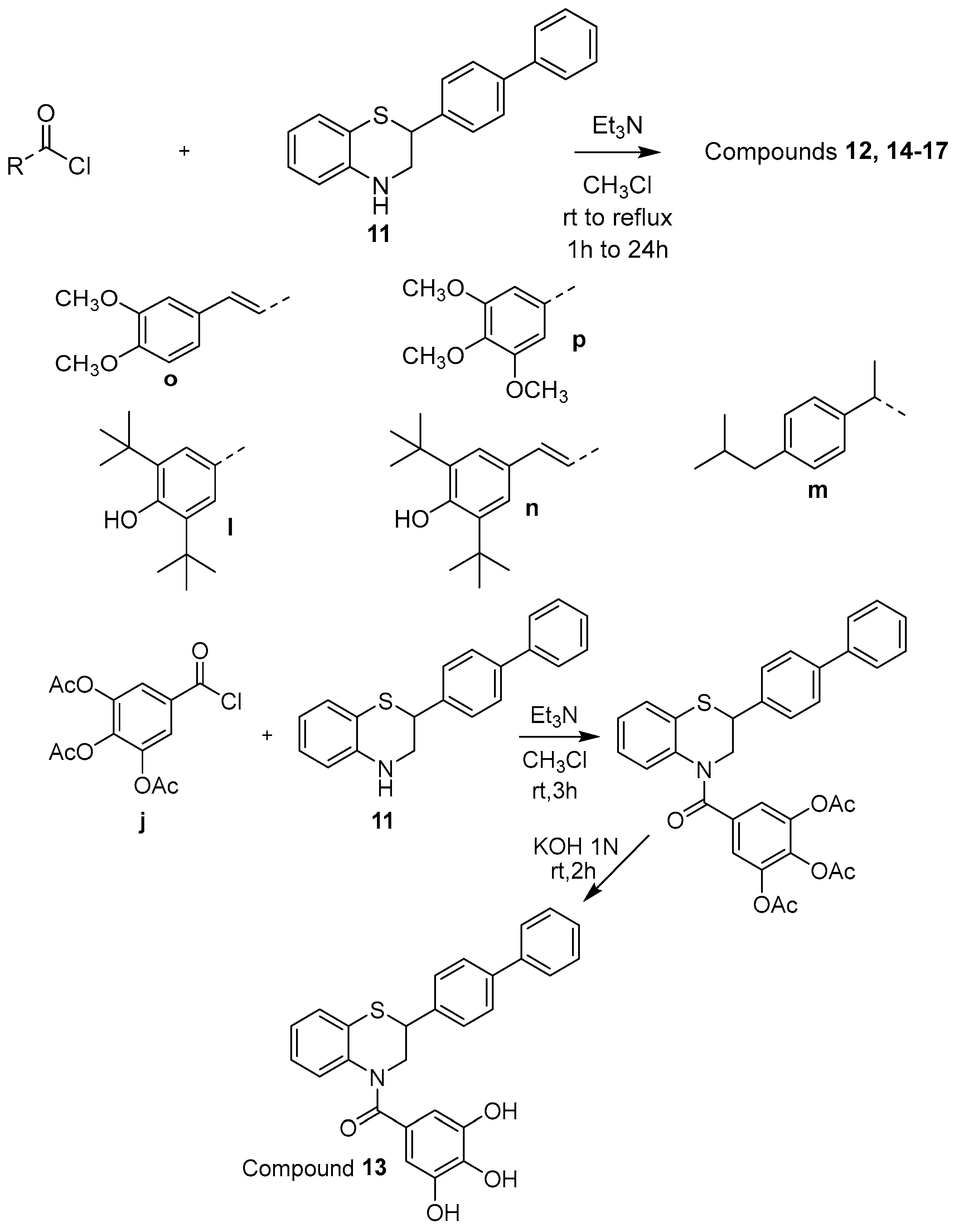
2.1. Synthesis
2.2. Evaluation of Pharmacological Activity of the Novel Compounds
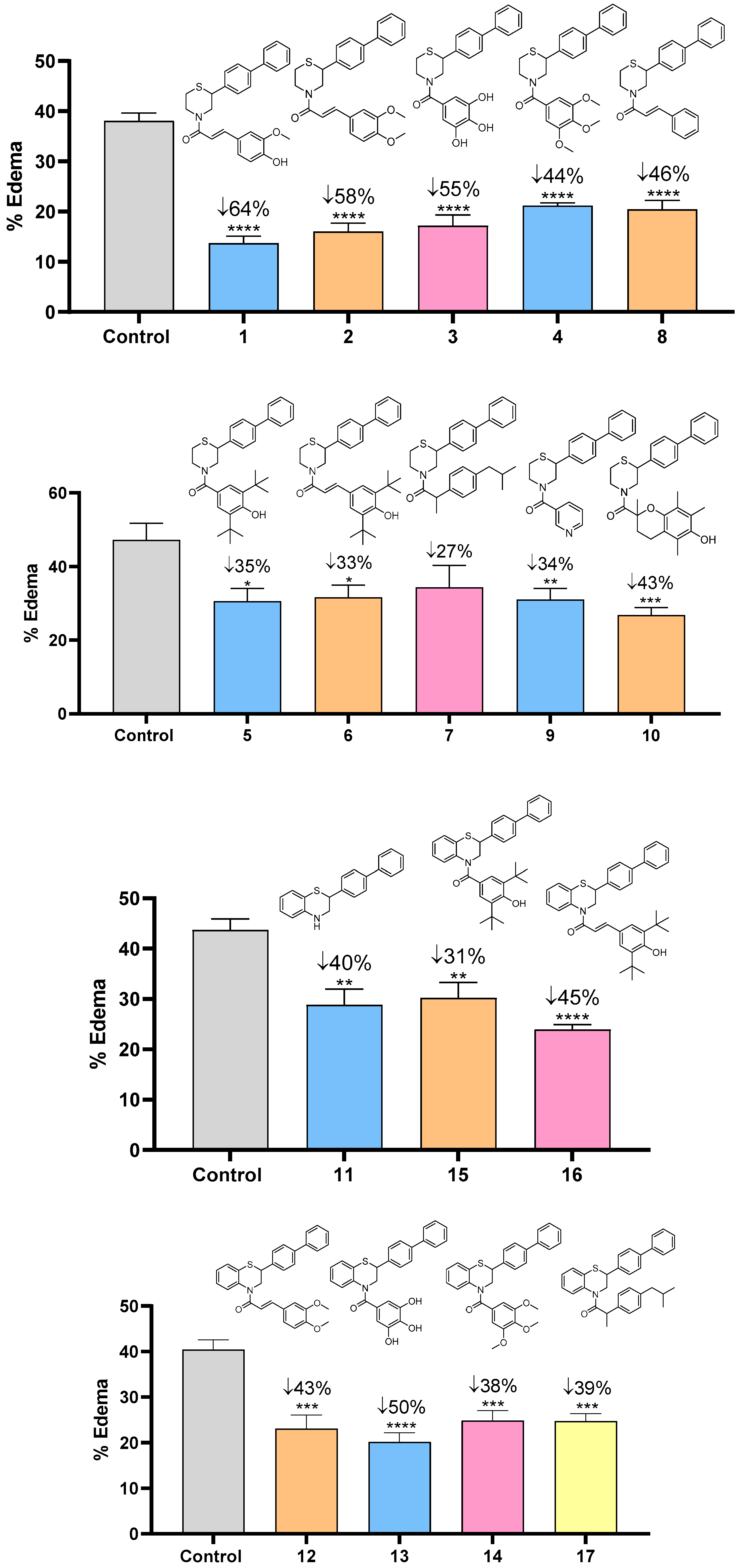
2.2.1. Anti-Inflammatory Profile
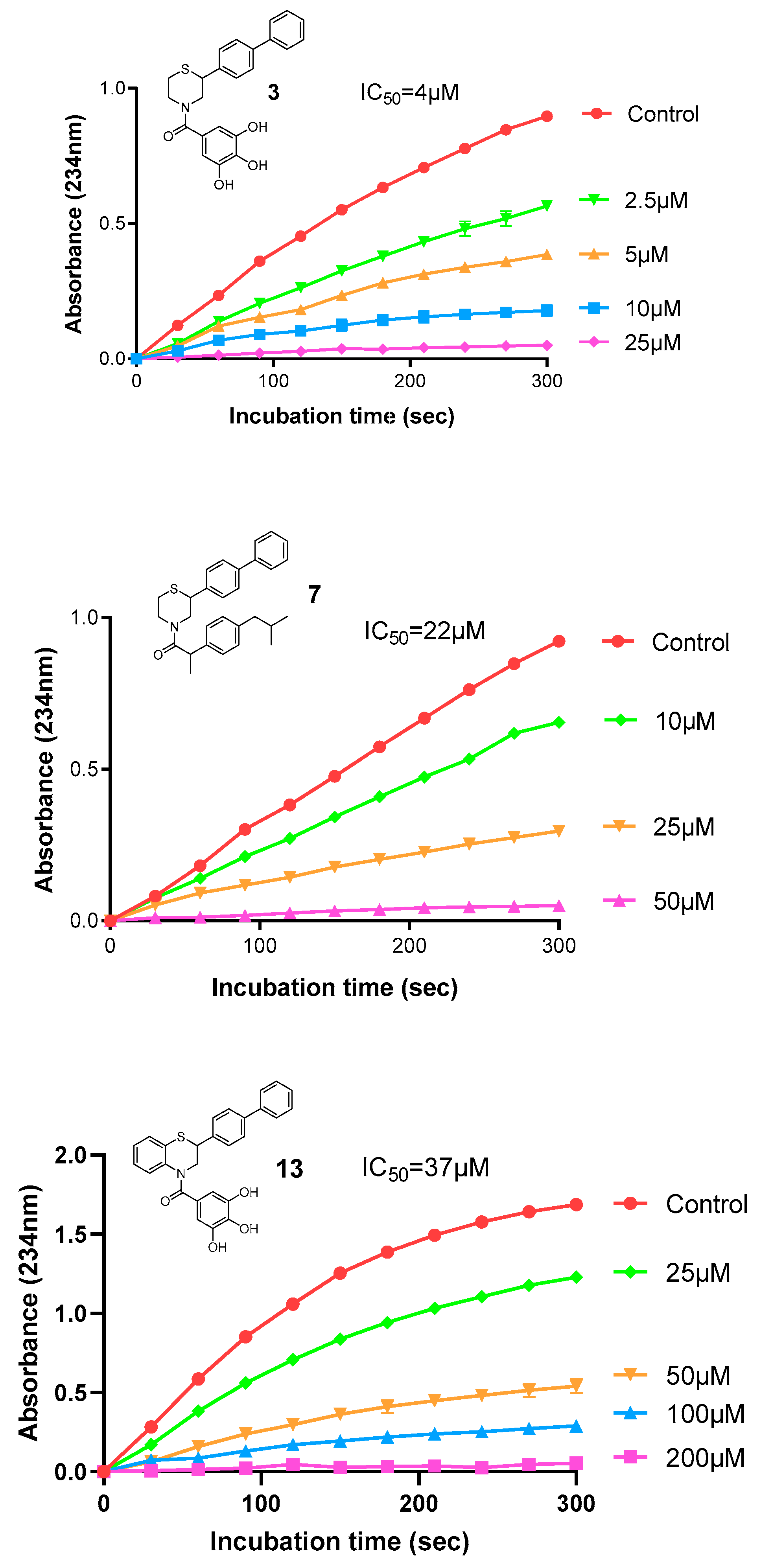
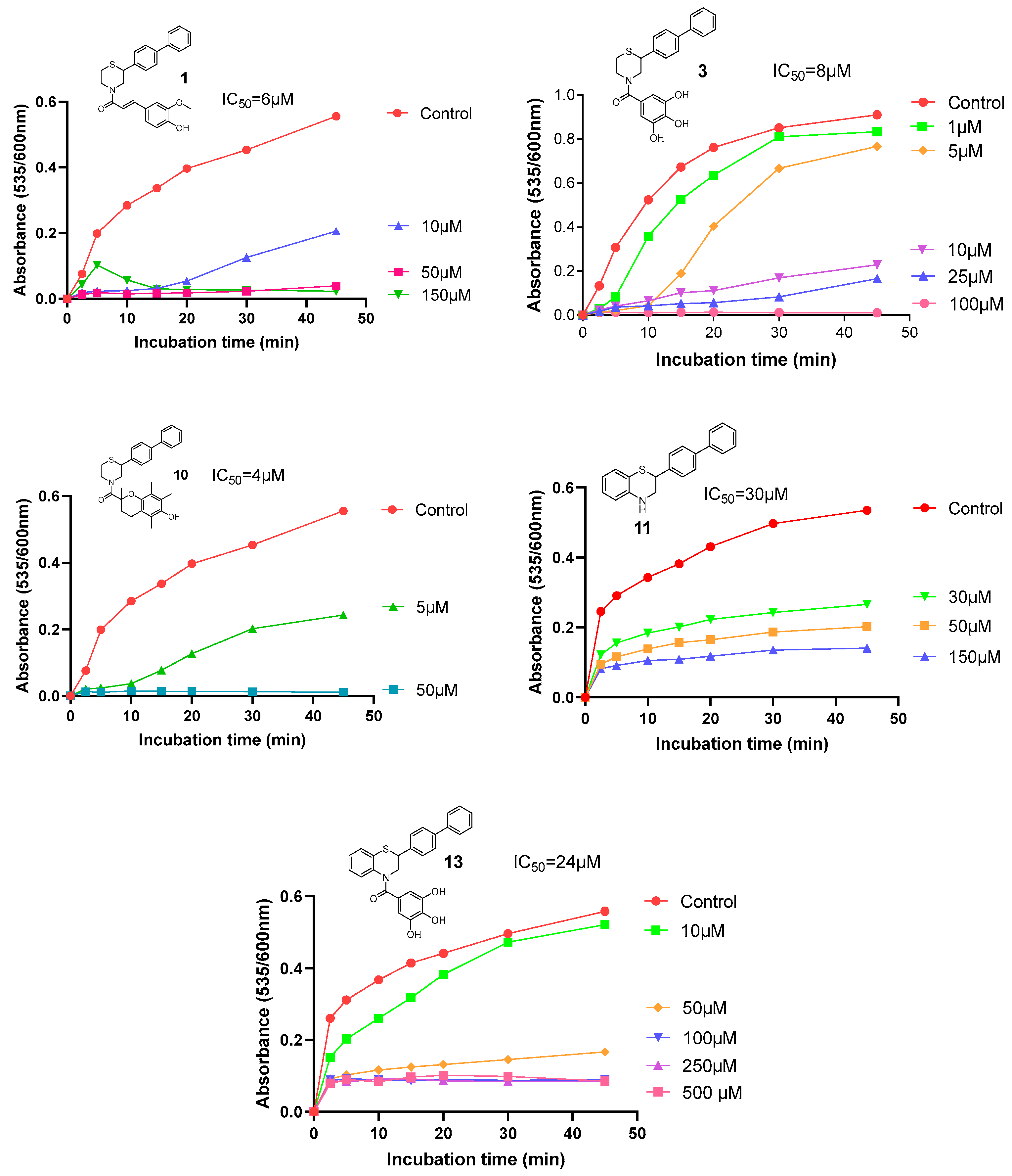
2.2.2. Antioxidant Profile
2.2.3. Inhibitory Activity Against Acetylcholinesterase (AChE)
2.2.4. Antihyperlipidaemic Profile of the Novel Compounds
2.3. Physicochemical Properties of the Novel Compounds
3. Materials and Methods
3.1. Chemical Synthesis
3.1.1. Ethyl 2-([1,1′-Biphenyl]-4-yl)-2-bromoacetate (a)
3.1.2. 2-([1,1′-Biphenyl]-4-yl)thiomorpholine-3-one (b)
3.1.3. 2-([1,1′-Biphenyl]-4-yl)thiomorpholine (c)
3.1.4. 2-([1,1′-Biphenyl]-4-yl)-2H-benzo[b][1,4]thiazin-3(4H)-one (d)
3.1.5. General Synthesis of Compounds e, f
3,4,5-trimethoxybenzoic Acid (e)
(E)-3-(3,4-dimethoxyphenyl)acrylic Acid (f)
3.1.6. (E)-3-(4-(Benzyloxy)-3-methoxyphenyl)acrylic Acid (g)
3.1.7. 3,4,5-Triacetoxybenzoic Acid (h)
3.1.8. General Synthesis of Compounds i–m
3.1.9. General Synthesis of Compounds 1, 3, 5, 7, and 8
(E)-1-(2-([1,1′-Biphenyl]-4-yl)thiomorpholino)-3-(4-hydroxy-3-methoxyphenyl)prop-2-en-1-one (1)
(2-([1,1′-Biphenyl]-4-yl)thiomorpholino)(3,4,5-trihydroxyphenyl)methanone (3)
(2-([1,1′-Biphenyl]-4-yl)thiomorpholino)(3,5-ditertbutyl-4-hydroxyphenyl)methanone (5)
1-(2-([1,1′-Biphenyl]-4-yl)thiomorpholino)-2-(4-isobutylphenyl)propan-1-one (7)
(E)-1-(2-([1,1′-Biphenyl]-4-yl)thiomorpholino)-3-phenylprop-2-en-1-one (8)
3.1.10. General Synthesis of Compounds 2, 4, 9, and 10
(E)-1-(2-([1,1′-Biphenyl]-4-yl)thiomorpholino)-3-(3,4-dimethoxyphenyl)prop-2-en-1-one (2)
(2-([1.1′-Biphenyl-4-yl]thiomorpholino)(3,4,5-trimethoxyphenyl)methanone (4)
(2-([1,1′-Biphenyl-4-yl]thiomorpholine)(pyridine-3-yl)methanone (9)
(2-([1,1′-Biphenyl-4-yl]thiomorpholino)(6-hydroxy-2,5,7,8-tetramethylchroman-2-yl)methanone (10)
3.1.11. Synthesis of Compound (E)-1-(2-([1.1′-Biphenyl]-4-yl)thiomorpholino)-3-(3,5-ditertbutyl-4-hydroxyphenyl)prop-2-en-1-one (6)
3.1.12. Synthesis of Compound 2-([1,1′-Biphenyl]-4-yl)-3,4-dihydro-2H-benzo[b][1,4]thiazine (11)
3.1.13. General Synthesis of Compounds 12, 14–17
(E)-1-(2-([1,1′-Biphenyl]-4-yl)-2,3-dihydro-4H-benzo[b][1,4]thiazin-4-yl)-3-(3,4-dimethoxyphenyl)prop-2-en-1-one (12)
(2-([1,1′-Biphenyl]-4-yl)-2,3-dihydro-4H-benzo[b][1,4]thiazin-4-yl)(3,4,5-trimethoxyphenyl)methanone (14)
(2-([1,1′-Biphenyl]-4-yl)-2,3-dihydro-4H-benzo[b][1,4]thiazin-4-yl)(3,5-ditertbutyl-4-hydroxyphenyl)methanone (15)
(E)-1-(2-([1,1′-Biphenyl]-4-yl)-2,3-dihydro-4H-benzo[b][1,4]thiazin-4-yl)-3-(3,5-ditertbutyl-4-hydroxyphenyl)prop-2-en-1-one (16)
1-(2-([1,1′-Biphenyl]-4-yl)-2,3-dihydro-4H-benzo[b][1,4]thiazin-4-yl)-2-(4-isobutylphenyl)propan-1-one (17)
3.1.14. Synthesis of Compound (2-([1,1′-Biphenyl]-4-yl)-2,3-dihydro-4H-benzo[b][1,4]thiazin-4-yl)(3,4,5-trihydroxyphenyl)methanone (13)
3.2. Pharmacological Evaluation
3.2.1. Lipoxygenase (LOX) Inhibition
3.2.2. Edema Reduction
3.2.3. Radical Scavenging of DPPH
3.2.4. Inhibition of Lipid Peroxidation
3.2.5. Hypolipidaemic Activity
3.2.6. Acetylcholinesterase (AChE) Inhibition
3.2.7. Iron Chelation (Ferrozine)
3.3. Physicochemical Properties and logBB Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| ALS | Amyotrophic lateral sclerosis |
| BBB | Blood–brain barrier |
| BHT | Butylated hydroxytoluene |
| CDI | N,N’-carbonyldiimidazole |
| CNS | Central nervous system |
| DCC | N/N’-dicyclohexylcarbodiimide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| LOX | Lipoxygenase |
| LP | Lipid peroxidation |
| MS | Multiple sclerosis |
| MW | Molecular weight |
| NSAID | Non-steroidal anti-inflammatory agent |
| ROS | Reactive Oxygen Species |
| TAC | Total antioxidant capacity |
| TC | Total cholesterol |
| TG | Triglycerides |
| TPSA | Topological polar surface area |
References
- Martin, L.J. Neurodegenerative Disorders. In Encyclopedia of the Human Brain; Elsevier Science: New York, NY, USA, 2002; Volume 3, pp. 441–463. [Google Scholar]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42–54. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative diseases. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Aborode, A.T.; Pustake, M.; Awuah, W.A.; Alwerdani, M.; Shah, P.; Yarlagadda, R.; Ahmad, S.; Silva Correia, I.F.; Chandra, A.; Nansubuga, E.P.; et al. Targeting oxidative stress mechanisms to treat Alzheimer’s and Parkinson’s disease: A critical review. Oxid. Med. Cell. Longev. 2022, 2022, 7934442. [Google Scholar] [CrossRef] [PubMed]
- Riyal, S.; Ranjan, A.K.; Gulati, A. Oxidative stress: A target to treat Alzheimer’ s disease and stroke. In Neurochemistry International; Elsevier Ltd.: Amsterdam, The Netherlands, 2023; Volume 165, p. 105509. [Google Scholar]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Birla, H.; Minocha, T.; Kumar, G.; Misra, A.; Singh, K.S. Role of oxidative stress and metal toxicity in the progression of Alzheimer’s disease. Cur. Neuropharmacol. 2020, 18, 552–562. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell Longev. 2013, 2013, 23983897. [Google Scholar] [CrossRef] [PubMed]
- Masaldan, S.; Bush, A.I.; Devos, D.; Rolland, A.S.; Moreau, C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 2019, 133, 221–233. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y. Tau and neuroinflammation in Alzheimer’s disease: Interplay mechanisms and clinical translation. J. Neuro inflamm. 2023, 20, 165–186. [Google Scholar] [CrossRef]
- Andronie-Cioara, F.L.; Ardelean, A.I.; Nistor-Cseppento, C.D.; Jurcau, A.; Jurcau, M.C.; Pascalau, N.; Marcu, F. Molecular mechanisms of neuroinflammation in ageing and Alzheimer’s disease progression. Int. J. Mol. Sci. 2023, 24, 1869. [Google Scholar] [CrossRef]
- Ardura-Fabregat, A.; Boddeke, E.W.G.M.; Boza-Serrano, A.; Brioschi, S.; Castro-Gomez, S.; Ceyzeriat, K.; Dansokho, C.; Dierkes, T.; Gelders, G.; Heneka, M.T.; et al. Targeting Neuroinflammation to treat Alzheimer’s disease. CNS Drugs 2017, 31, 1057–1082. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Cheng, J.; Hou, L. Drug development for Alzheimer’s disease: Microglia induced neuroinflammation as a target? Int. J. Mol. Sci. 2019, 20, 558. [Google Scholar] [CrossRef] [PubMed]
- Bolos, M.; Perea, J.R.; Avila, J. Alzheimer’s disease as an inflammatory disease. Biomol. Concepts 2017, 8, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.C.; Bellozi, P.M.Q.; Reis, H.J.; de Oliveira, A.C.P. Inflammation as a possible link between dyslipidemia and Alzheimer’s disease. Neuroscience 2018, 376, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.B.; Wang, H. The shared role of cholesterol in neuronal and peripheral inflammation. Pharmacol. Therap. 2023, 249, 108486. [Google Scholar] [CrossRef]
- Chrysselis, M.C.; Rekka, E.A.; Kourounakis, P.N. Hypocholesterolemic and hypolipidemic activity of some novel morpholine derivatives with antioxidant activity. J. Med. Chem. 2000, 43, 609–612. [Google Scholar] [CrossRef]
- Katselou, M.G.; Matralis, A.N.; Kourounakis, A.P. Developing potential agents against atherosclerosis: Design, synthesis and pharmacological evaluation of novel dual inhibitors of oxidative stress and Squalene Synthase activity. Εur. J. Med. Chem. 2017, 138, 748–760. [Google Scholar] [CrossRef]
- Kourounakis, A.P.; Matralis, A.N.; Nikitakis, A. Design of more potent squalene synthase inhibitors with multiple activities. Bioorg. Med. Chem. 2010, 18, 7402–7412. [Google Scholar] [CrossRef]
- Ladopoulou, E.M.; Matralis, A.N.; Nikitakis, A.; Kourounakis, A.P. Antihyperlipidemic morpholine derivatives with antioxidant activity: An investigation of the aromatic substitution. Bioorg. Med. Chem. 2015, 23, 7015–7023. [Google Scholar] [CrossRef]
- Matralis, A.N.; Katselou, M.G.; Nikitakis, A.; Kourounakis, A.P. Novel benzoxazine and benzothiazine derivatives as multifunctional antihyperlipidemic agents. J. Med. Chem. 2011, 54, 5583–5591. [Google Scholar] [CrossRef] [PubMed]
- Matralis, A.N.; Kourounakis, A.P. Optimizing the pharmacological profile of new bifunctional antihyperlipidemic/antioxidant morpholine derivatives. ACS Med. Chem. Lett. 2019, 10, 98–104. [Google Scholar] [CrossRef]
- Silveira, A.C.; Dias, J.P.; Santos, V.M.; Oliveira, P.F.; Alves, M.G.; Rato, L.; Silva, B.M. The Action of Polyphenols in Diabetes Mellitus and Alzheimer’s Disease: A Common Agent for Overlapping Pathologies. Curr. Neuropharmacol. 2018, 17, 590–613. [Google Scholar] [CrossRef]
- Singh, Y.P.; Rai, H.; Singh, G.; Singh, G.K.; Mishra, S.; Kumar, S.; Srikrishna, S.; Modi, G. A review on ferulic acid and analogs based scaffolds for the management of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 215, 113278. [Google Scholar] [CrossRef]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef] [PubMed]
- Wuerch, E.; Urgoiti, G.R.; Yong, V.W. The Promise of Niacin in Neurology. Neurotherapeutics 2023, 20, 1037–1054. [Google Scholar] [CrossRef] [PubMed]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsuri, T.; Jyoyama, H.; Ono, T.; Yamada, K.; Kobayashi, M.; Hori, Y.; Arimura, A.; Yasui, K.; Ohno, K.; et al. Novel Antiarthritic Agents with 1,2-Isothiazolidine-1,1-dioxide (γ-Sultam) Skeleton: Cytokine Suppressive Dual Inhibitors of Cyclooxygenase-2 and 5-Lipoxygenase. J. Med. Chem. 2000, 43, 2040–2048. [Google Scholar] [CrossRef]
- Tzara, A.; Lambrinidis, G.; Kourounakis, A. Design of multifaceted antioxidants: Shifting towards anti-inflammatory and antihyperlipidemic activity. Molecules 2021, 26, 4928. [Google Scholar] [CrossRef]
- Islam, B.; Tabrez, S. Management of Alzheimer’s disease—An insight of the enzymatic and other novel potential targets. Int. J. Biol. Macrom. 2017, 97, 700–709. [Google Scholar] [CrossRef]
- Matralis, A.N.; Kourounakis, A.P. Design of Novel Potent Antihyperlipidemic Agents with Antioxidant/Anti-inflammatory Properties: Exploiting Phenothiazine’s Strong Antioxidant Activity. J. Med. Chem. 2014, 57, 2568–2581. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Zarguan, I.; Belayachi, L. Hydroxybenzoic acids as acetylcholinesterase inhibitors: Calorimetric and docking simulation studies. Nutrients 2022, 14, 2476. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic acid: A systematic review on the biological functions, mechanistic actions, and therapeutic potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Mugundhan, V.; Arthanari, A.; Parthasarathy, P.R.; Parameswari, R. Protective effect of ferulic acid on acetylcholinesterase and amyloid beta peptide plaque formation in Alzheimer’s disease: An in vitro study. Cureus 2024, 16, e5410. [Google Scholar] [CrossRef]
- Guenifi, N.E.; Kilani-Morakci, S.; Merabet-Khelassi, M.; Ferdenache, M.; Sifi, K.; Zeror, S. Promiscuous enzymatic synthesis of (S)-ibuprofen amides and assessment of their geroprotective and gastroprotective activities in Drosophila melanogaster. Process Biochem. 2025, 157, 93–100. [Google Scholar] [CrossRef]
- Reddy, M.V.K.; Rao, K.Y.; Anusha, G.; Kumar, G.M.; Damu, A.G.; Reddy, K.R.; Shetti, N.P.; Aminabhavi, T.M.; Reddy, P.V.G. In-vitro evaluation of antioxidant and anticholinesterase activities of novel pyridine, quinoxaline, and s-triazine derivatives. Environ. Res. 2021, 199, 111320. [Google Scholar] [CrossRef]
- Ullah, S.; Park, Y.; Ikram, M.; Lee, S.; Park, C.; Kang, D.; Yang, J.; Akter, J.; Yoon, S.; Chun, P.; et al. Design, synthesis and anti-melanogenic effect of cinnamamide derivatives. Bioorg. Med. Chem. 2018, 26, 5672–5681. [Google Scholar] [CrossRef]
- Degotte, G.; Pirotte, B.; Frederich, M.; Francotte, P. Polyhydroxybenzoic acid derivatives as potential new antimalarial agents. Arch. Der Pharm. 2021, 354, 2100190. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Li, Y.; Qiang, X.; Xiao, G.; Liu, Q.; Tan, Z.; Deng, Y. Multifunctional scutellarin-rivastigmine hybrids with cholinergic, antioxidant, biometal chelating and neuroprotective properties for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2015, 23, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Elsinghorst, P.W.; Cieslik, J.S.; Mohr, K.; Traenkle, C.; Guetschow, M. First Gallamine−Tacrine Hybrid: Design and Characterization at Cholinesterases and the M2 Muscarinic Receptor. J. Med. Chem. 2007, 50, 5685–5695. [Google Scholar] [CrossRef] [PubMed]
- Rekka, E.; Chrysellis, M.; Siskou, I.; Kourounakis, A. Synthesis of new azulene derivatives and study of their effect on lipid peroxidation and lipoxygenase activity. Chem. Pharm. Bull. 2002, 50, 904–907. [Google Scholar] [CrossRef]
- Kourounakis, A.P.; Galanakis, D.; Tsiakitzis, K.; Rekka, E.A.; Kourounakis, P.N. Synthesis and pharmacological evaluation of novel derivatives of anti-inflammatory drugs with increased antioxidant and anti-inflammatory activities. Drug Dev. Res. 1999, 47, 9–16. [Google Scholar] [CrossRef]
- Nabavi, S.; Ebrahimzadeh, M.; Nabavi, S.; Jafari, M. Free radical scavenging activity and antioxidant capacity of Eryngium caucasicum trautv and Froripia subpinnata. Pharmacol. Online 2008, 3, 19–25. [Google Scholar]
- Vilar, S.; Chakrabarti, M.; Costanzi, S. Prediction of passive blood-brain partitioning: Straightforward and effective classification models based on in silico derived physicochemical descriptors. J. Mol. Graph. Model. 2010, 28, 899–903. [Google Scholar] [CrossRef] [PubMed]
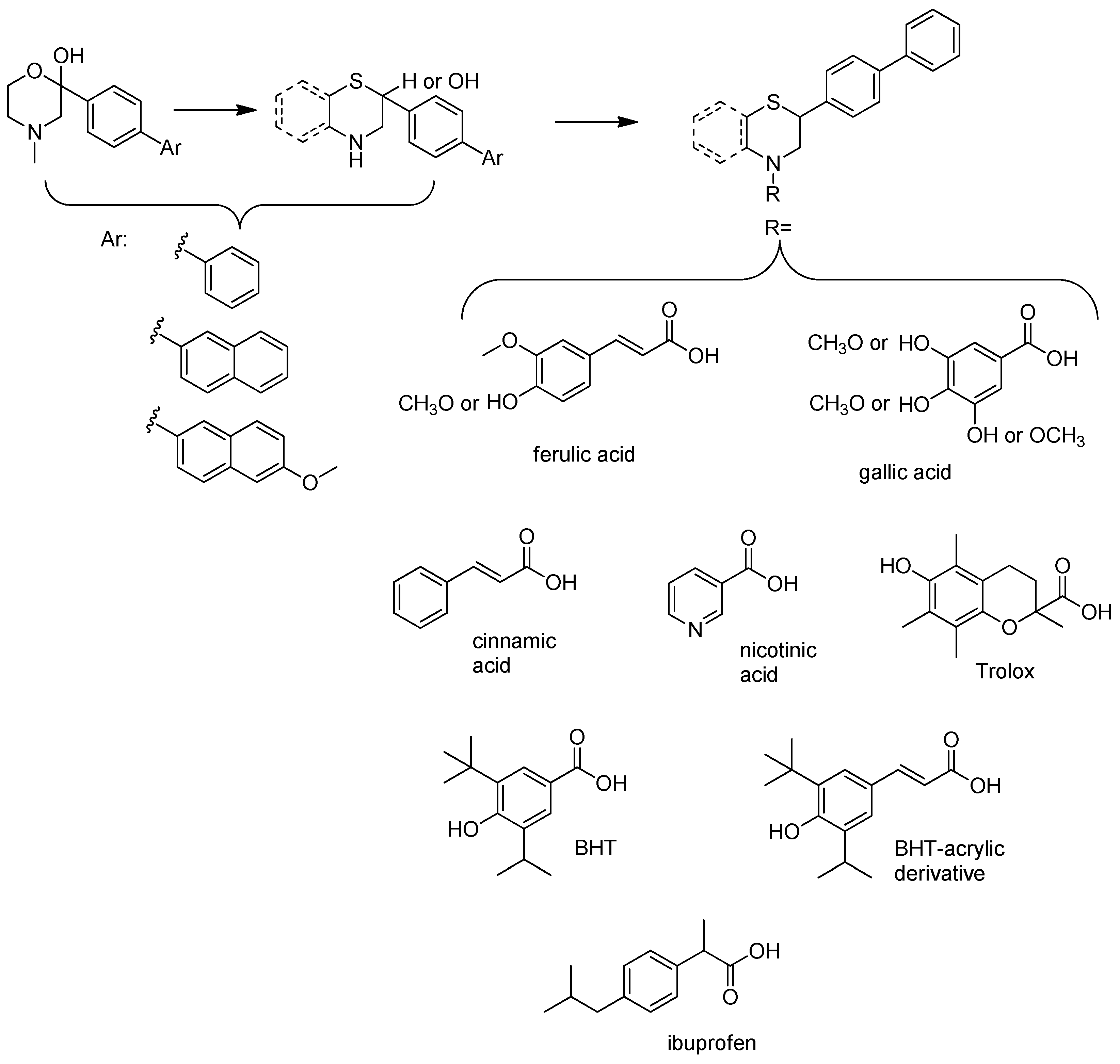
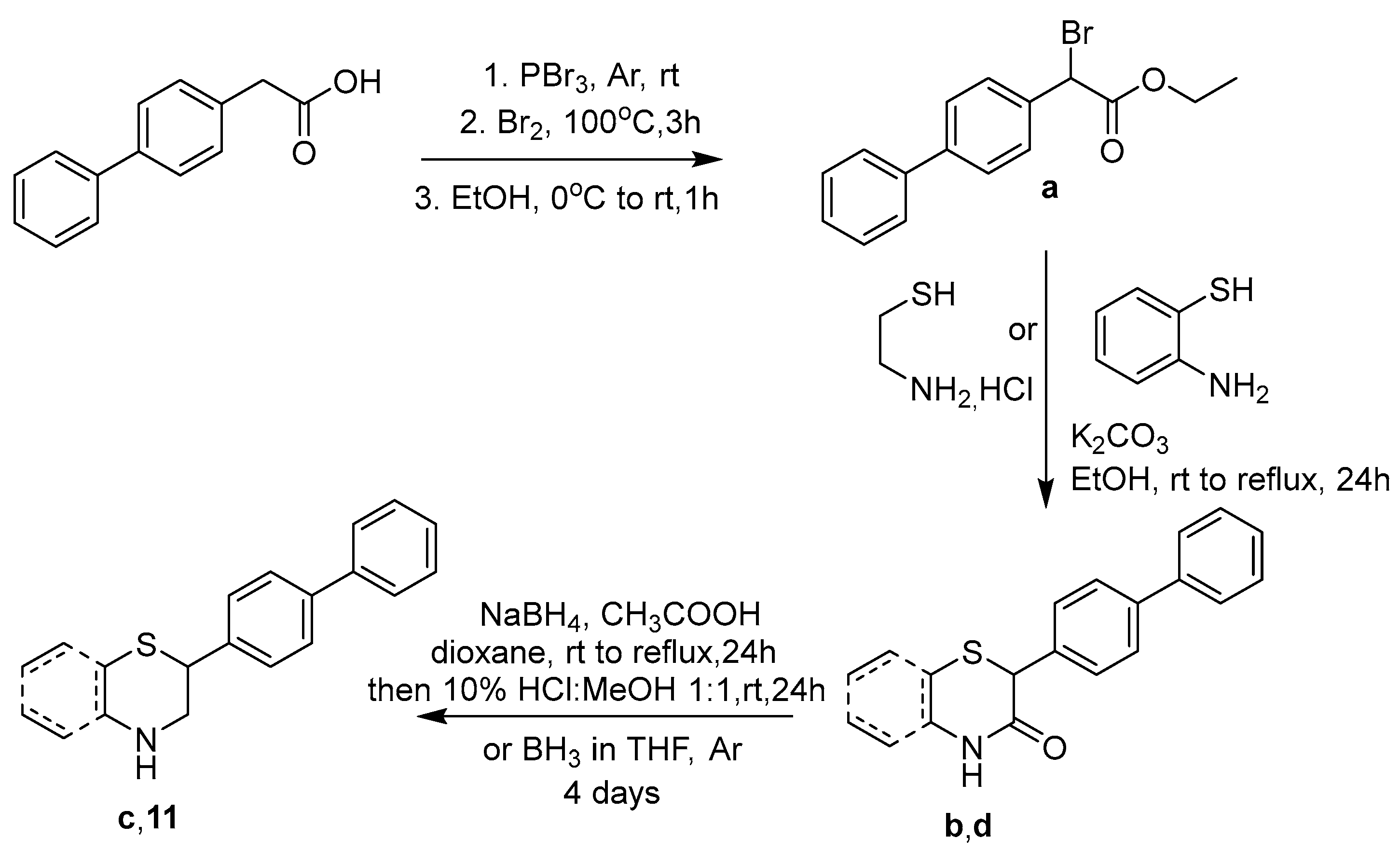
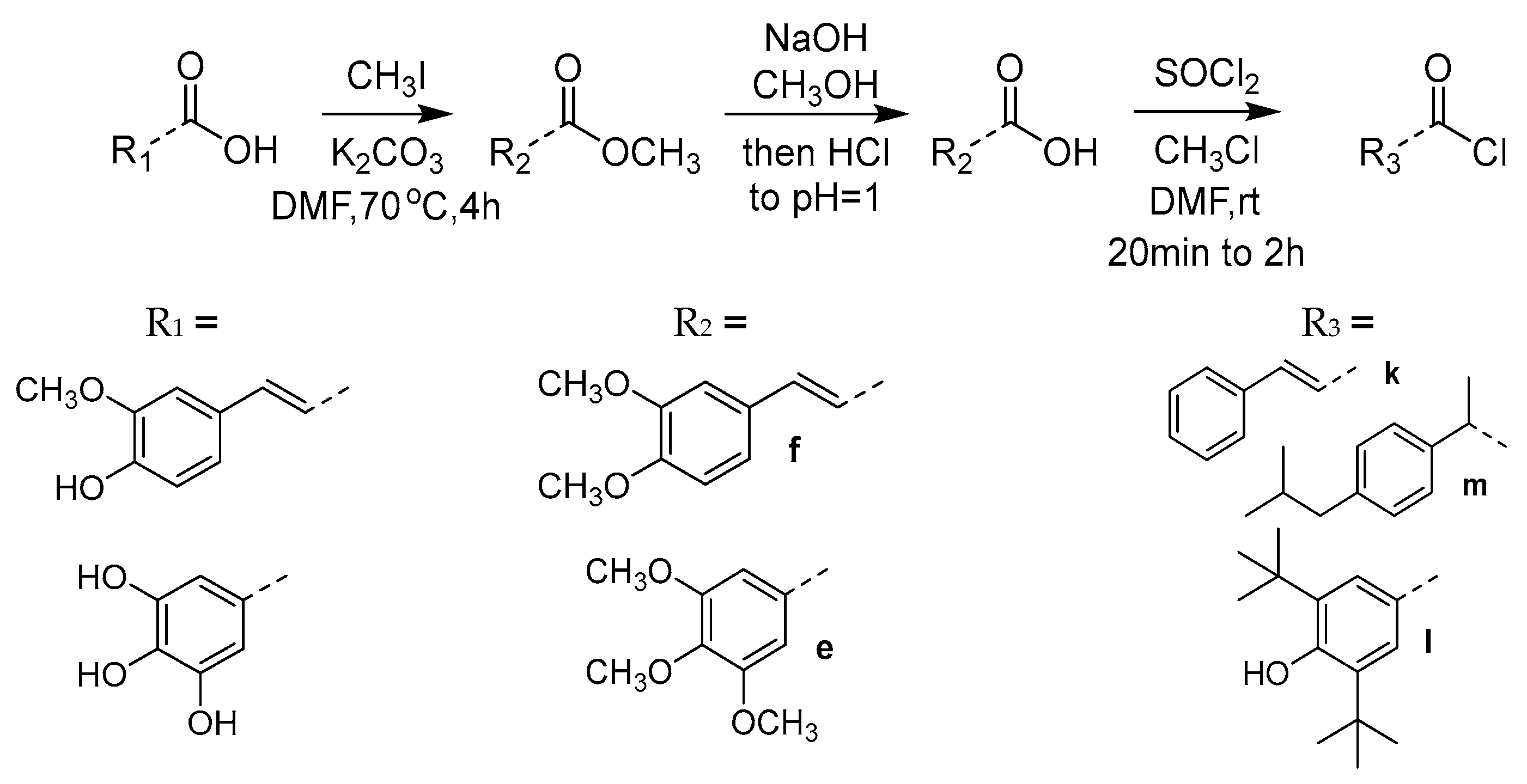
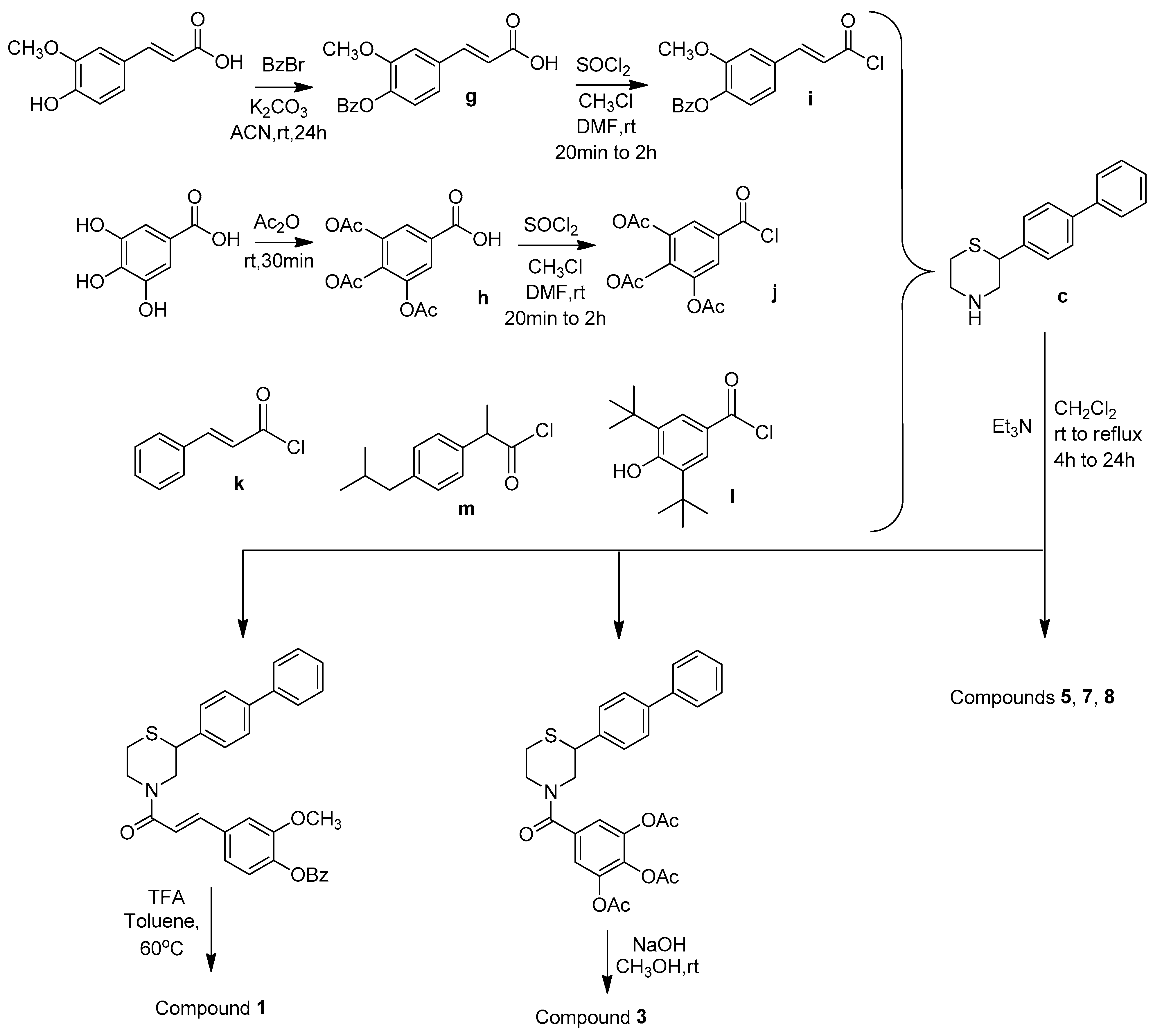
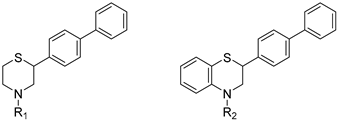 | |||
| Compound | R1 = | Compound | R1 = |
| 1 | 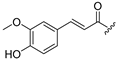 | 10 | 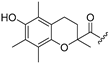 |
| 2 | 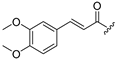 | R2 = | |
| 3 | 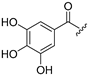 | 11 | H |
| 4 | 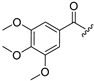 | 12 |  |
| 5 | 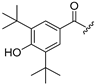 | 13 | 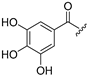 |
| 6 | 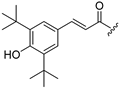 | 14 | 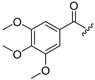 |
| 7 | 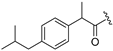 | 15 | 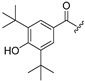 |
| 8 | 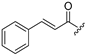 | 16 | 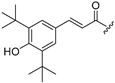 |
| 9 |  | 17 | 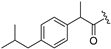 |
| Compound | IC50 Value (μM) | Compound | IC50 Value (μM) |
|---|---|---|---|
| 1 | 68 | 11 | 134 |
| 2 | >300 | 12 | 74 |
| 3 | 4 | 13 | 37 |
| 4 | 71 | 14 | 71 |
| 5 | 150 | 15 | 83 |
| 6 | 252 | 16 | 58 |
| 7 | 22 | 17 | 104 |
| 8 | >200 a | cinnamic acid | >300 |
| 9 | 192 | ferulic acid | 132 |
| 10 | 140 | ibuprofen | >300 |
| naproxen | 25 |
| Compound | DPPH IC50 Value (μM) | LP IC50 Value (μM) or % Inhibition at 100 μΜ | % of Fe(II) Chelation (at 100 μM) |
|---|---|---|---|
| 1 | 166 | 6 | 19 |
| 2 | >400 | >1000 | 18 |
| 3 | 31 | 8 | 89 (56% at 10 μM) |
| 4 | >400 | >500 | 4 |
| 5 | 300 | 250 | 0 |
| 6 | 288 | >500 | 0 |
| 7 | >400 | 299 | 3 |
| 8 | >400 | >1000 | 5 |
| 9 | >400 | >1000 | 2 |
| 10 | 78 | 4 | 0 |
| 11 | >400 | 30 | 10 |
| 12 | >400 | >1000 | 17 |
| 13 | 36 | 24 | 91 (12% at 10 μM) |
| 14 | >400 | >1000 | 6 |
| 15 | >400 | >500 | 8 |
| 16 | 141 | >1000 | 9 |
| 17 | >400 | >1000 | 29 |
| Trolox | 33 | 43% | 29 |
| BHT | 31 | 0% | 0 |
| gallic acid | 14 | 73% | 98 |
| ascorbic acid | 42 | 30 | n.t |
| Compound | Total Antioxidant Capacity (TAC) Increase (%) | Compound | Total Antioxidant Capacity (TAC) Increase (%) |
|---|---|---|---|
| 1 | 329 ** | 11 | 199 *** |
| 2 | 43 * | 12 | 203 *** |
| 3 | 780 *** | 13 | 60 |
| 4 | 87 | 14 | 144 * |
| 5 | 0 | 15 | 131 *** |
| 6 | 808 *** | 16 | 262 *** |
| 7 | 0 | 17 | 139 ** |
| 8 | 104 *** | ||
| 9 | 60 * | ||
| 10 | 296 * |
| Compound | AChE Inhibition (%) | Compound | AChE Inhibition (%) |
|---|---|---|---|
| 1 | 22 | 11 | n.a. |
| 2 | 26 | 12 | 17 |
| 3 | 10 | 13 | 43 |
| 4 | 30 | 14 | 47 |
| 5 | 24 | 15 | 23 |
| 6 | 43 | 16 | 24 |
| 7 | 27 | 17 | 13 |
| 8 | 19 | donepezil | 100 |
| 9 | 33 | tacrine | 100 |
| 10 | 45 | galanthamine | 100 |
| Compound | % Total Cholesterol (TC) Reduction | % Triglycerides (TG) Reduction | % HDL Increase | % LDL Decrease | % HDL/LDL Increase |
|---|---|---|---|---|---|
| 1 | 40 * | 83 **** | 84 ** | 59 ** | 242 **,1,2 |
| 2 | 30 * | 60 ** | 44 ** | 53 ** | 122 *** |
| 3 | 51 *** | 86 **** | 49 *** | 79 *** | 682 *** |
| 4 | 10 | 35 * | 95 ** | 5 | n.a. |
| 5 | 55 * | 50 | n.t. | n.t. | n.t. |
| 6 | 36 ** | 65 **** | 23 **,2 | 49 ** | 43 * |
| 7 | 27 * | 70 ** | n.t. | n.t. | n.t. |
| 8 | 45 ** | 53 ** | 9 | 57 * | 91 * |
| 9 | 35 * | 33 *,1 | n.a. | 44 * | 105 * |
| 10 | 13 | 52 * | 32 | 23 | 77 |
| 11 | 23 * | 59 ** | 101 * | 48 *** | 232 ** |
| 12 | 27 * | 29 | 63 **** | 59 *** | 282 *** |
| 13 | 47 **** | 53 * | 25 * | 62 **** | 442 ** |
| 14 | 51 **** | 50 * | 13 | 73 **** | 320 **** |
| 15 | 55 **** | 35 | 79 ** | 74 **** | 714 **** |
| 16 | 45 **** | 67 ** | 131 **** | 66 **** | 604 **** |
| 17 | 45 **** | 58 * | 43 ** | 68 **** | 321 *** |
| Compound | MW (g/mol) | ClogP | H-Bond Donors | H-Bond Acceptors | TPSA | logBB |
|---|---|---|---|---|---|---|
| 1 | 431.55 | 5.13 | 1 | 3 | 49.77 | 0.9 |
| 2 | 445.58 | 5.60 | 0 | 4 | 38.77 | 1.5 |
| 3 | 407.48 | 3.77 | 3 | 2 | 81.00 | −0.6 |
| 4 | 449.57 | 4.46 | 0 | 5 | 48.00 | 0.6 |
| 5 | 487.70 | 8.28 | 1 | 2 | 40.54 | 2.8 |
| 6 | 513.74 | 8.73 | 1 | 2 | 40.54 | 3.0 |
| 7 | 443.65 | 7.69 | 0 | 2 | 20.31 | 3.1 |
| 8 | 385.53 | 5.95 | 0 | 2 | 20.31 | 2.2 |
| 9 | 360.48 | 3.85 | 0 | 3 | 32.67 | 0.7 |
| 10 | 487.66 | 7.38 | 1 | 3 | 49.77 | 2.1 |
| 11 | 303.42 | 5.56 | 1 | 1 | 12.03 | 2.2 |
| 12 | 493.62 | 7.92 | 0 | 4 | 38.77 | 2.7 |
| 13 | 455.53 | 5.24 | 3 | 2 | 81.00 | 0.1 |
| 14 | 497.61 | 5.92 | 0 | 5 | 48.00 | 1.4 |
| 15 | 535.75 | 9.74 | 1 | 2 | 40.54 | 3.6 |
| 16 | 561.78 | 11.05 | 1 | 2 | 40.54 | 4.2 |
| 17 | 491.69 | 9.18 | 0 | 2 | 20.31 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzara, A.; Andreou, A.; Kourounakis, A.P. From Lipid Regulation to Neuroprotection: Multitarget (Benzo)thiazine Derivatives as Promising Leads. Molecules 2025, 30, 4542. https://doi.org/10.3390/molecules30234542
Tzara A, Andreou A, Kourounakis AP. From Lipid Regulation to Neuroprotection: Multitarget (Benzo)thiazine Derivatives as Promising Leads. Molecules. 2025; 30(23):4542. https://doi.org/10.3390/molecules30234542
Chicago/Turabian StyleTzara, Ariadni, Andrea Andreou, and Angeliki P. Kourounakis. 2025. "From Lipid Regulation to Neuroprotection: Multitarget (Benzo)thiazine Derivatives as Promising Leads" Molecules 30, no. 23: 4542. https://doi.org/10.3390/molecules30234542
APA StyleTzara, A., Andreou, A., & Kourounakis, A. P. (2025). From Lipid Regulation to Neuroprotection: Multitarget (Benzo)thiazine Derivatives as Promising Leads. Molecules, 30(23), 4542. https://doi.org/10.3390/molecules30234542







