Determination of the Physico-Chemical Properties of the Feedstock (Coal Tar Coking Chemical Plants JSC “Shubarkol Komir” and “Qarmet”)
Table 1 and
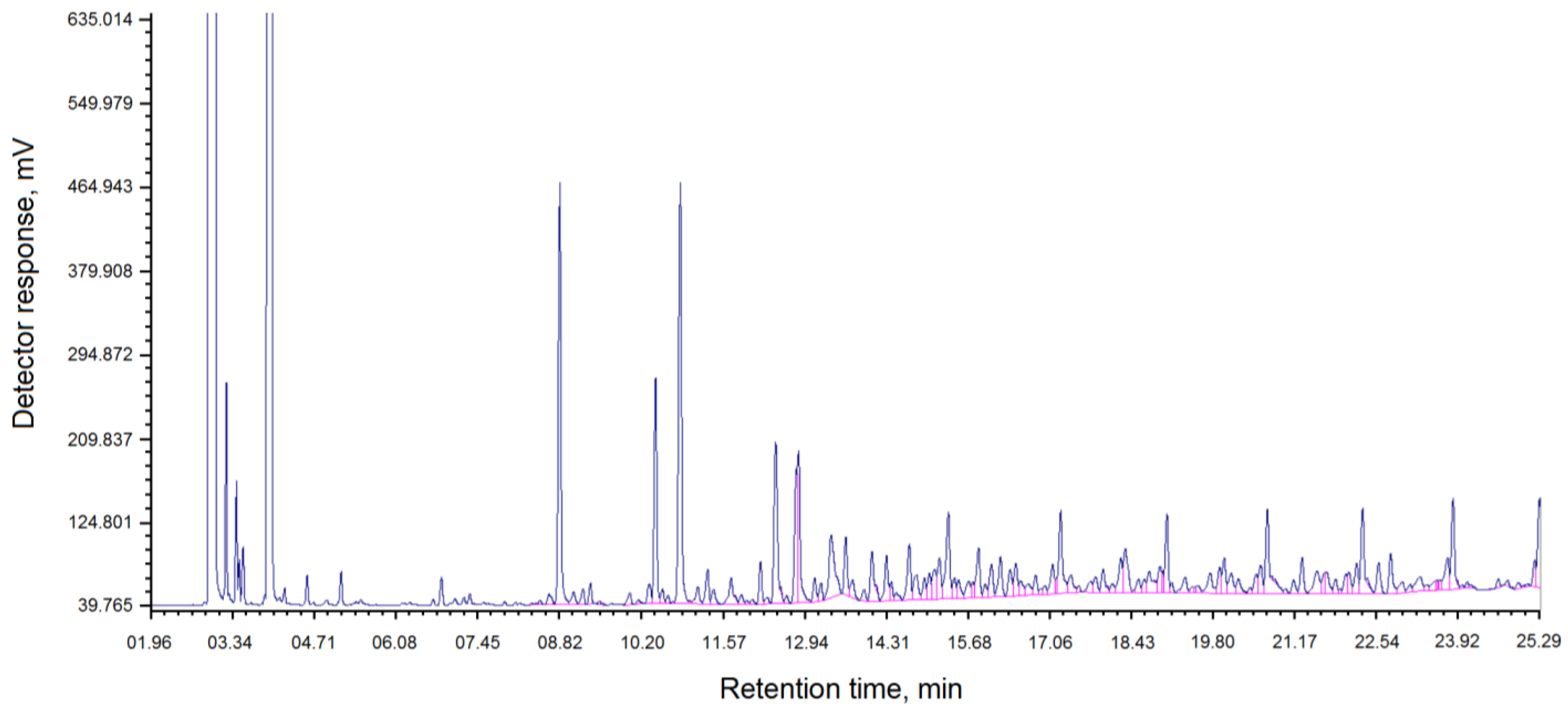
Figure 1 show the component composition of coal tar “Shubarkol Komir”.
The chromatogram of the component composition of the resin “Shubarkol Komir” is shown in
Figure 1.
As can be seen from
Table 1 and the chromatogram in
Figure 1, the content of phenol and its derivatives is 24.96% and paraffins are 24.96%.
Table 2 shows the main physico-chemical characteristics of pitch obtained from the coal tar of JSC Shubarkol Komir.
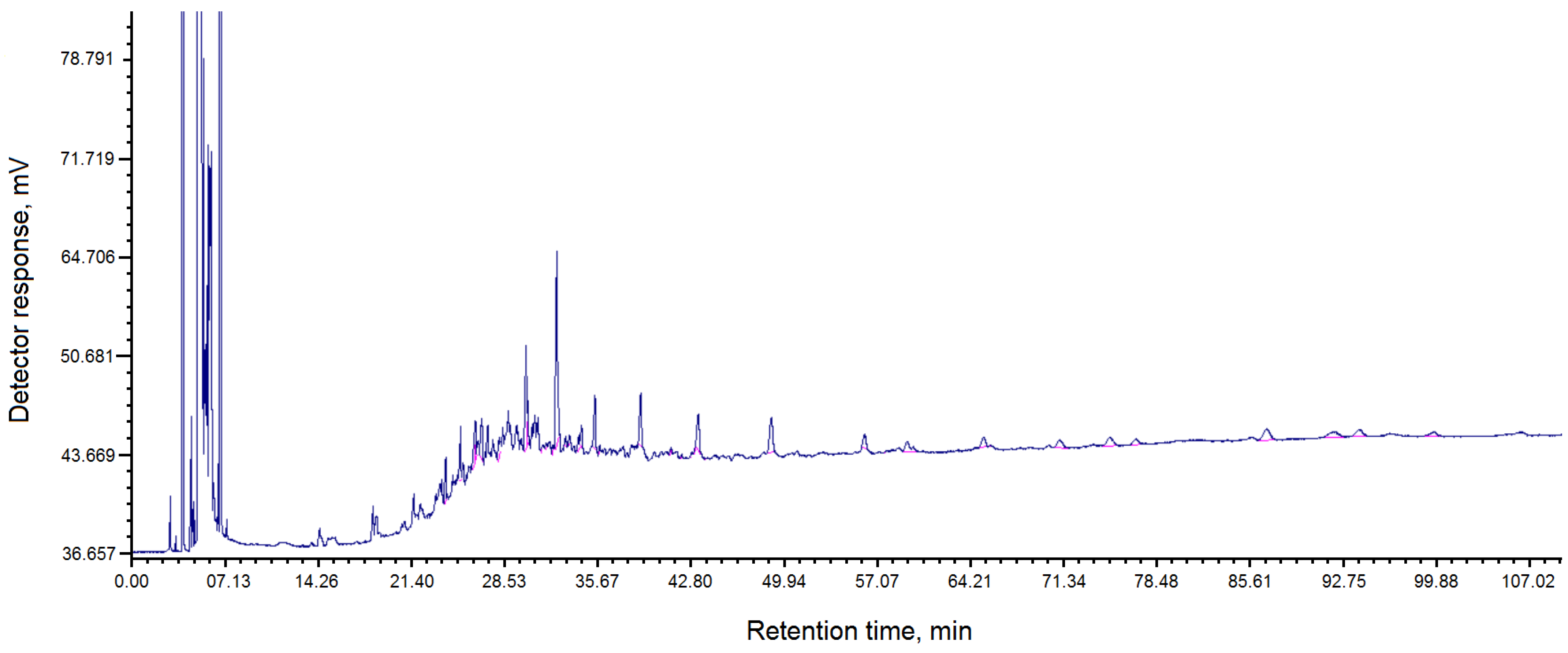
The composition of fractions of pitch “Shubarkol Komir”, soluble in toluene, is shown in
Table 3.
The chromatogram of the composition of fractions of pitch “Shubarkol Komir”, soluble in toluene, is shown in
Figure 2.
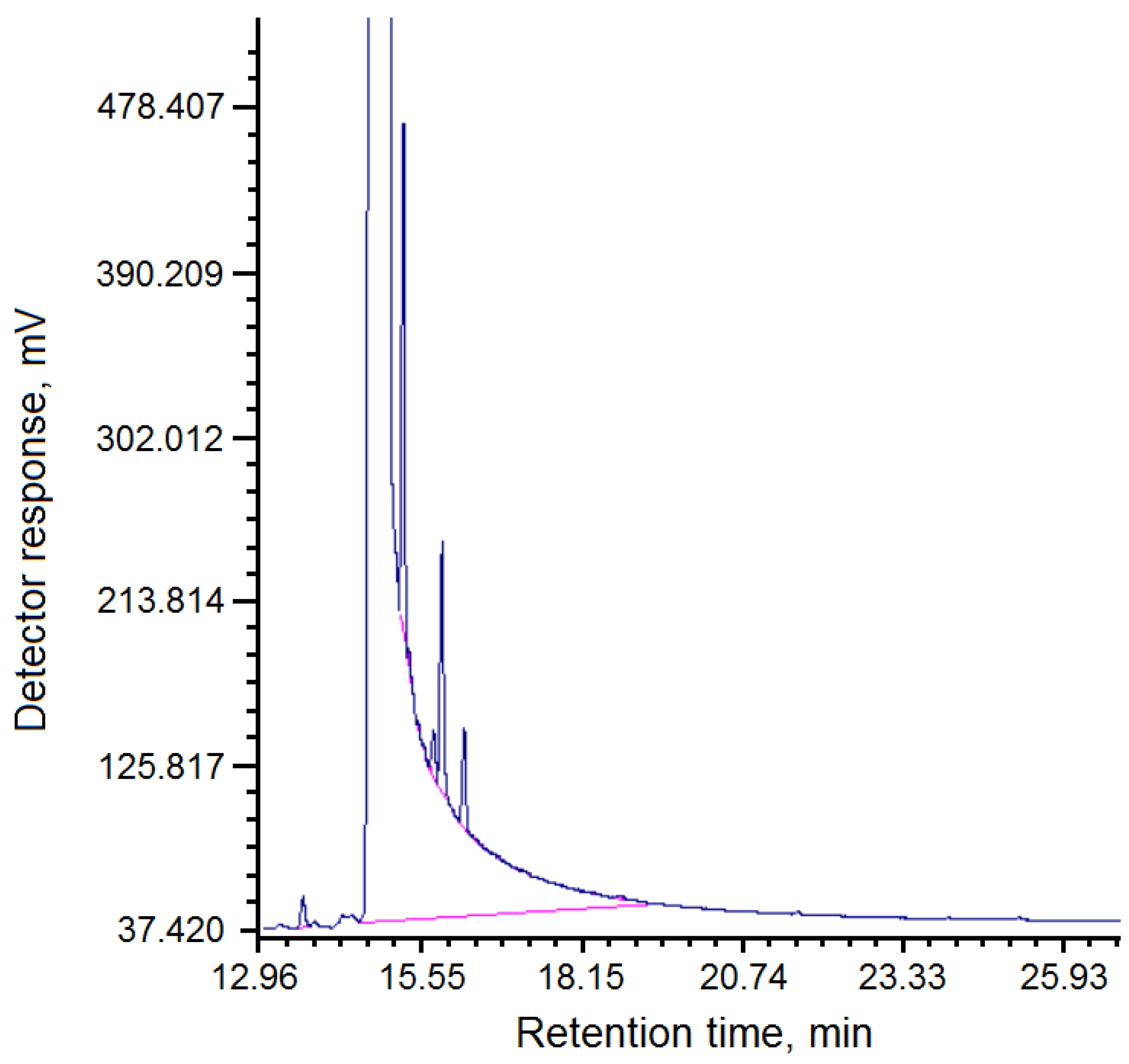
The composition of fractions of pitch “Shubarkol Komir”, soluble in quinoline, is presented in
Table 4.
The chromatogram of the composition of fractions of pitch “Shubarkol Komir”, soluble in quinoline, is shown in
Figure 3.
As can be seen from the results of studying the physico-chemical properties of the feedstock, the resulting pitch belongs to low-temperature (soft) pitches. Such pitches contain a large number of highly volatile and low-molecular-weight components, which reduce the temperature of transition to the plastic state [
4,
16].
As a result of the coking of the obtained composite mixtures under various conditions, samples of electrode coke were obtained, differing in ash content and contents of C, H, and S. The results of the coking of the obtained composite mixtures are presented in
Table 5.
An increase in the coking temperature from 800 to 1000 °C leads to a monotonous decrease in ash content by 70% and an increase in carbon content by ~1.7% (
Table 5), which may indicate increased dehydrogenation, the removal of volatile components, and an increase in the degree of aromatization of the carbon matrix. The decrease in ash content is probably due to the partial removal of mineral impurities in the form of volatile compounds or their sintering and incorporation into the carbon matrix, which reduces their share in the total mass of coke. The most pronounced effect was achieved with a pitch ratio of 1:2, which indicates the complementary effect of low-ash pitch “Shubarkol Komir” and, presumably, more coked pitch “Qarmet”.
The data in
Table 5 demonstrate a clear dependence of the elemental composition of coke on the heat treatment conditions: with an increase in temperature from 800 to 1000 °C and an increase in the holding time from 4 to 6 h, a monotonous increase in carbon content (from ~95% to 97.75%) and a decrease in concentrations of hydrogen and sulfur (H: from ~1.0% to 0.51%; S: from ~0.8% to 0.47%). These changes indicate an intensive thermal degradation of the initial baking matrix and the subsequent structural restructuring of the carbon deposit.
A decrease in the hydrogen content indicates the dehydrogenation and condensation of aromatic nuclei, accompanied by the loss of aliphatic and heteroatomic substituents, a process typical of the high-temperature coking of coal pitches [
4,
5]. A simultaneous decrease in sulfur (by ~44%) is possible due to the thermal decomposition of organic sulfur compounds (thiophenes, mercaptans, etc.) with the release of volatile products such as HS and carbon disulfide, which is consistent with data from thermogravimetric and mass spectrometric studies of pitches [
14,
15].
The purest and carbonized coke (C = 97.75%, ash content = 0.40%) was obtained at a pitch ratio of 1:2 at 1000 °C and 6 h, which confirms the effectiveness of the compositional approach: due to the complementary properties of the components, not only a reduction in mineral impurities is achieved, but also the formation of a more ordered aromatic structure. Such a material is characterized by signs of partial graphitization, which opens up the possibility of its use, not only as a structural (electrode), but also as a functional carbon material.
This sample was selected for an in-depth study of thermophysical and electrophysical properties. It has been established that the temperature dependence of the specific heat capacity shows a pronounced anomaly at 373 K—a sharp peak not accompanied by latent heat, which corresponds to a type II phase transition.
Table 6 shows the experimental values of the specific heat capacity of the Shubarkul–Qarmet electrode coke sample (1:2).
It can be seen from the experimental data that a non-monotonic increase in heat capacity is observed, and at a temperature of 373 K there is a type II phase transition.
It should be noted that an anomaly in the form of a peak on the curve of the temperature dependence of the specific heat at 373 K may indicate the presence of a phase or relaxation transition. Interestingly, in the same temperature range, there is a change in the nature of the dependence of electrical resistance on temperature: in the range of 293–373 K, resistance decreases with increasing temperature (behavior typical for semiconductors), whereas, in the range of 373–393 K, there is a short-term increase in resistance. This coincidence indicates a possible connection between the thermodynamic anomaly and the rearrangement of the electron structure or interfacial boundaries in the material. However, additional studies, including measurements of charge carrier density and mobility, are required to unambiguously interpret the nature of the transition.
Taking into account the temperature of the phase transition, the equations of the temperature dependence of the specific heat capacity [J/(g * K)] are derived:
For the temperature ranges under consideration, the value of the average random error was used to determine the coefficient error in the dependence equations ~ f(T).
The results of the electrophysical measurements of the obtained electrode coke material in the temperature range of 293–483 K and at an alternating electrical signal frequency of 1 kHz are presented in
Table 7.
Table 7,
Table 8 and
Table 9 also indicate the relative measurement errors of electrical capacity δ
C and electrical resistance δ
R obtained as a result of three parallel measurements (n = 3). The values of the dielectric constant (ε) shown in
Figure 4, as well as in
Table 7,
Table 8 and
Table 9, are the result of calculation using formula (10), which uses the average values of three parallel measurements (n = 3) of electrical capacity (C). The average values of electrical resistance (R) are also obtained as a result of three parallel measurements (n = 3).
The results of the electrophysical characteristics of the obtained electrode coke in the temperature range of 293–483 K and at an alternating electrical signal frequency of 5 kHz are presented in
Table 8.
The results of the measurements of the electrophysical characteristics of the obtained electrode coke in the temperature range of 293–483 K and at an alternating electrical signal frequency of 10 kHz are presented in
Table 9.
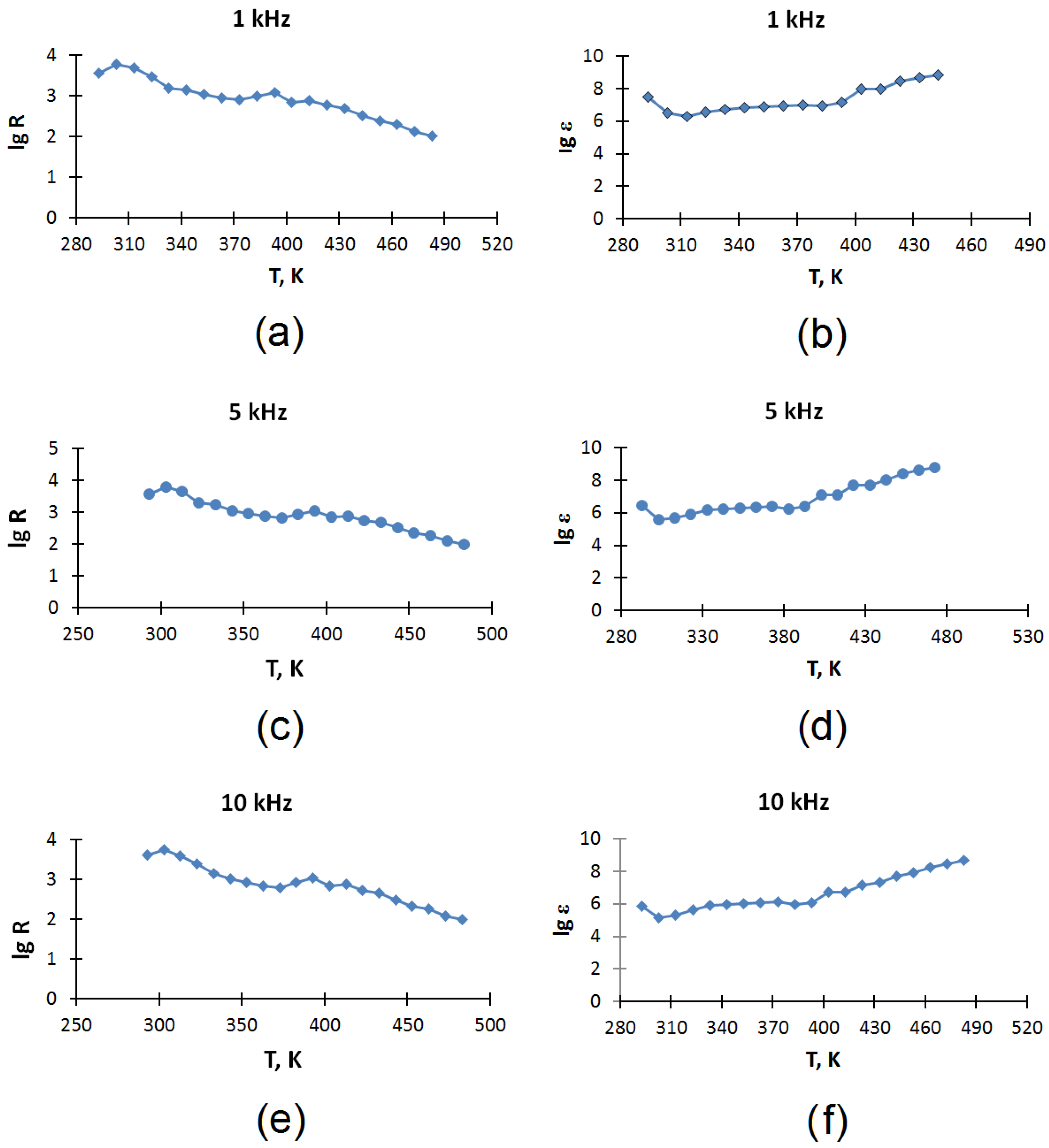
The temperature dependences of the dielectric constant (ε) and electrical resistance (R) of an electrode coke sample from a Shubarkol–Qarmet mixture in the temperature range of 293–483 K at frequencies of 1, 5, and 10 kHz are shown in
Figure 4.
Analysis of the temperature dependence of conductivity and dielectric properties.
It can be seen from the experimental data that the material (electrode coke obtained from a mixture of Shubarkol and Qarmet pitches in a ratio of 1:2) demonstrates temperature-dependent conductivity. According to the nature of the change in resistance with temperature, three sections can be distinguished:
293–373 K—a decrease in resistance is observed with increasing temperature, which is typical for the semiconductor type of conductivity;
373–393 K—resistance increases, which corresponds to metallic behavior;
393–483 K—the resistance decreases again with increasing temperature, which indicates a return to semiconductor behavior.
Calculation of the band gap (ΔE) in the range of 293–373 K. Readings at 293
K—3.56
lg R, 373
K—2.90
lg R:In this range, the band gap is 0.67 eV and can be attributed to narrow-probe semiconductors.
Calculation of the band gap (ΔE) in the range of 393–483 K. The reading at 393 K is 3.09
lg R, 483 K is 2.01
lg R:
In this range, the band gap is 0.55 eV and can be attributed to narrow-probe semiconductors.
The material (electrode coke obtained from a mixture of Shubarkol and Qarmet pitches in a ratio of 1:2) also has very high values of dielectric constant (ε).
In addition to the electrical characteristics, the material exhibits very high values of dielectric constant (ε). Values of ε reach the following levels:
More than 107 at low temperatures (293–373 K);
108–109 when the temperature rises above 393 K.
Ferroelectrics or materials with a gigantic permittivity value will be stunned by similar values.