The Effectiveness of Cerium Oxide Nanoparticle-Based Drugs in Wound Healing in Animal Models
Abstract
1. Introduction
2. Results
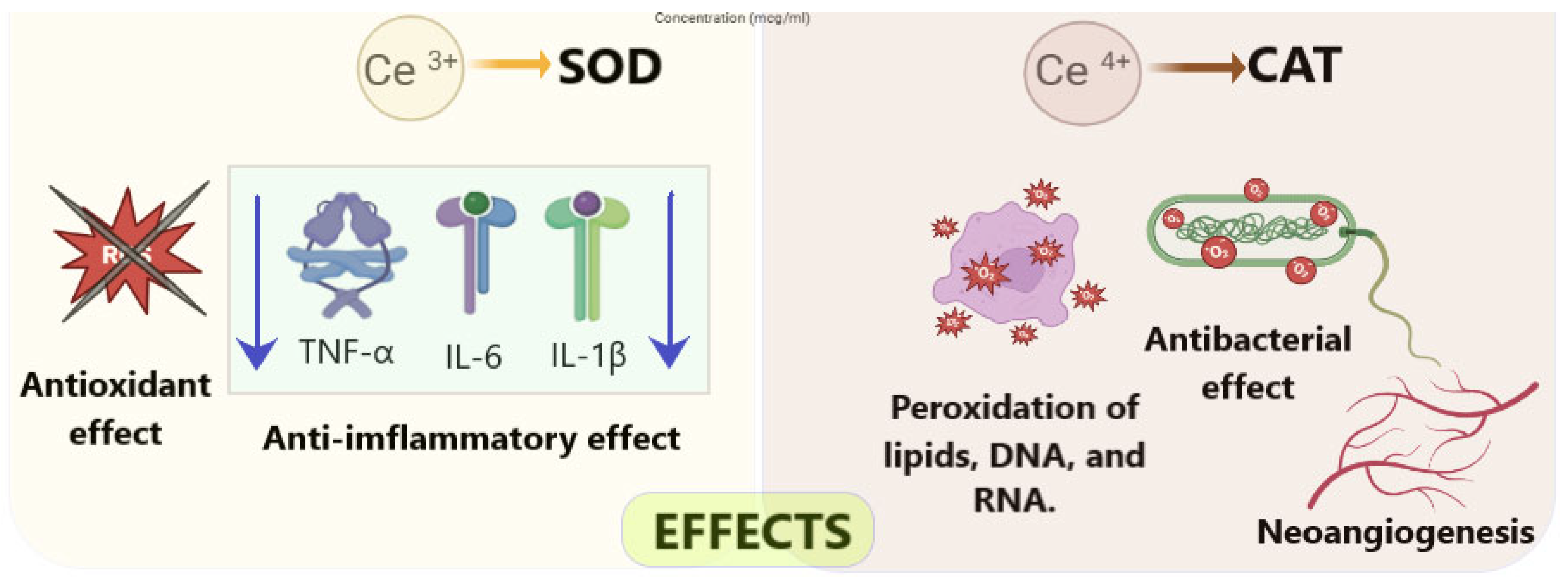
2.1. Mechanisms Underlying the Wound-Healing Effects of Cerium Dioxide Nanoparticles
2.2. Medical Devices and Pharmaceutical Formulations Based on Cerium Dioxide Nanoparticles
| # No. | Characteristics of the Obtained CeO2 NP Formulation | Synthesis Method | NP Size (nm) | Hydrodynamic Radius (nm) | Zeta Potential (mV) 1 | CeO2 NP Concentration | Excipients | Auxiliary Active Ingredients | References |
|---|---|---|---|---|---|---|---|---|---|
| Solutions | |||||||||
| 1 | Solution | Simple liquid-phase chemistry method [55] | 28.5± 0.8 | 3–5 | ND | 100 ng | PBS | miR146a | [75] |
| 2 | Solution | Simple liquid-phase chemistry method [55] | 3–5 | 15–20 | ND | ND | PBS | miR146a | [56] |
| 3 | Porous nanospheres in solution | Wet chemical method | 100–200 | 143.4 ± 4.3 | ND | ND | Distilled water | Copper | [83] |
| Suspensions | |||||||||
| 4 | Colloidal dispersion | Deposition method | 5.87 ± 1.27 | ND | ND | 0.0000156 wt% (1 µM) | Chitosan | Silver (5 or 7%) | [73] |
| 5 | Aqueous suspension | Hydrothermal method | 104.3 ± 13.1 | ND | 8.8 ± 1.1 mV—with silicon and −10.0 ± 1.3 mV—hollow | 10 mg mL−1 | No | L-arginine, silicon dioxide | [70] |
| 6 | Suspension | qa | 90 ± 6.4—hollow NP | ND | ND | 1 mg mL−1 | linker (N-Hydroxysuccinimide (NHS)-ester) i-motif DNA, MMP-cleavable stealth peptide | Graphene, arginine | [84] |
| Hydrogels | |||||||||
| 7 | Hydrogel | Oxidation method | 750–800 | ND | ND | 0.014 wt%. and 0.056 wt%. (0.1 and 0.4 mm, respectively) | Acrylamide, AMPS, MBA | Curcumin | [72] |
| 8 | Hydrogels | Oxidation method [58] | 3–5 | ND | ND | ND | Dextran, FITC, SBMA or CBMA, HEMA | miRNA146a | [57] |
| 9 | Hydrogel | ND | ND | ND | ND | ND | ND | ND | [85] |
| 10 | Hydrogel | ND | ND | ND | ND | ND | ND | ND | [86] |
| 11 | Hydrogel | Hydrothermal synthesis [87] | Rods: 9.6 ± 1.2 × (50–200) | ND | ND | PEI/PVP@CeO2 0.5 wt%. | PEI, PVP, F127/F127-CHO | No | [88] |
| 12 | Hydrogel | ND | ND | ND | ND | 1% | ND | ND | [77] |
| 13 | Gel | Hydrothermal method | 400–450 | ND | ND | ND | PHEM, Chitosan | No | [71] |
| 14 | Hydrogel | ND | ND | ND | ND | 500 µg/mL | Gellan gum, gelatin | Flurbiprofen | [79] |
| 15 | Hydrogel | Green synthesis | 18.8 ± 4.1 | ND | ND | 2 wt%. | Alginate | Curcumin | [68] |
| 16 | Hydrogel | Reverse micelle method [89] | 3.3 [66] | 18–30 [66] | ND | ND | ZIF-8, GelMA | Doxorubicin | [80] |
| Designed Products | |||||||||
| 17 | Lyophilized sponge | Hydrothermal method [89] | 2.5–6.5 | 195 ± 3 | 22.4 mV | 0.025 wt%. (250 µg/mL) | Gelatin, genipin, oleylamine coating (stabilized) | No | [90] |
| 18 | Patches | ND | ND | ND | ND | 1 wt%. | GelMA | No | [78] |
| 20 | Chitosan Hydrogel Membrane | Green synthesis | 35–40 | ND | ND | 1% and 5% of Chitosan wt | Chitosan, glycerol | No | [69] |
| 21 | Sprayable Hydrogel Dressing | Purchased from US Research Nanomaterials | 10–30 | ND | ND | 0.01 wt%. (100 µg/mL) | GelMA- dopamine | Antimicrobial peptides | [81] |
| 22 | Wound Dressing | Deposition method [91] | 2–3 | ND | −18.6 ± 2.59 mV | ND | PArg, DS, citric acid (stabilized) | Pirfenidone | [74] |
| Other | |||||||||
| 23 | ND | Oxidation method | 20 (190–CNP-miR146a) | 20 (190–CNP-miR146a) | 27 mV (−18mV–CNP-miR146a) | ND | Nanosilk | miR-146a | [82] |
| 24 | ND | Deposition method | 45 (CS-ZnO/CeO2) | ND | ND | ND | Chitosan | ZnO | [92] |
2.3. Evaluation of the Efficacy of Cerium Dioxide Nanoparticle-Based Medical Devices in Wound Regeneration
| # No. | Focus of the Research | Number of Subjects | Wound Type | Wound Manipulation | Drug Administration Method | Control Groups (Drug-Free) | Control Groups (Versus Comparator) | Frequency of Control | Research Methods | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Solutions | ||||||||||
| 1 | Female ICR mice | ND | Surgical wound (d = 5 mm) | Treatment 12 h after injury, irradiation with white light | Transdermal | PBS | CNP-miR146a (100 ng) | 0, 3, 7, 10, and 14 | Wound closure assessment, tissue histology (Masson’s Trichrome), analyzing the numbers of CD31-positive and CD45-positive cells | [75] |
| 2 | BKS.Cg-Dock7m+/+Leprdb/J, strain No. 000642 | ND | 8 mm surgical wound biopsy punch | Topical application of active substance, then dressed with a Tegaderm (3M), which was subsequently removed on post-operative day 2 | Transdermal | PBS (non-diabetics) | Diabetics: lenti-miRGFP (Control miR), lenti-miR146a, CNP-miR146a | every other day until wounds were fully closed | Wound closure assessment, tissue histology—immunohistochemistry | [56] |
| 3 | Male SD rats | ND | 10 mm surgical wound biopsy punch | a 10 μL suspension of E. coli (1.0 × 106 CFU/mL) was evenly applied to the wound surface. Then, the wounds were treated with 200 μL of PBS or 200 μL of PBS containing different concentrations of Cu2+, HMCe, and Cu-HMCe solutions | Transdermal | PBS | Cu-HMCe, HMCe, Cu | Daily before 14th day | Wound closure assessment, histology (H&E staining, Masson staining—14th day; immunohistochemical analysis TNF-α, IL-6, CD31—3 and 10 days); neovascularization ability—α-SMA and CD31 | [83] |
| Suspensions | ||||||||||
| 4 | albino mice | 12 | 4 mm surgical wound biopsy punch | Wound treatment every 24 h with removal of wound crusts | Transdermal | Off-dose | Ag–CeO–Chitosan (5 and 7%), Ag–Chitosan | 0, 30, 60, 90 days after the wound was inflicted | Wound closure assessment, collagen density assessment (Masson’s), wound microbial load assessment | [73] |
| 5 | Female BALB/c mice | ND | Surgical wound | Applying a drop to the wound and pressing the wound for 30 s | Transdermal | PBS | hCeO2 NPs, AhCeO2 NPS, AhCeO2 NPS + simulated sunlight irradiation | 0, 2, 4, 6, 8, 10 days after surgery | Wound closure assessment, histology (H & E) | [70] |
| 6 | Female ICR mice | ND | Surgical wound 0.5 cm × 0.5 cm | Application of 50 μL of NC CG or ACG suspension with a concentration of 1 mg/mL (equivalent to cerium) in PBS after 12 h | Transdermal | PBS | CG NCs, ACG NCs | 12, 24 h, 2nd–14th day, every other day | Wound closure assessment, tissue histology (H & E), collagen content assessment—hydroxyproline assay | [84] |
| Hydrogels | ||||||||||
| 7 | Male Wistar rats | 30 | Surgical wound (d = 10 mm) | Application of round hydrogel scaffolds followed by covering with a transparent gauze dressing (Medicare B.P.) and a sterile adhesive dressing (Medicare) | Transdermal | No | Medicare cotton wool, A; AC; AC’; ACC | 0, 7, 3 and 14 days | Wound closure assessment, collagen density assessment (Masson’s), stage evaluation (H & E) | [72] |
| 8 | 12-week Db/Db female mice | 10 | Surgical wound l = 8 mm | Single application of gel to the wound | Injection therapy | No | hydrogel, CNP-miR146a | In 1 day until 20th day | Wound closure assessment, biomechanical skin testing, gene expression | [57] |
| 9 | Male Wistar rats | 30 | Surgical wound non-sterile | Treatment after injury and on days of intermediate control, covering with a sterile plaster | Transdermal | Off-dose | Hydrogel | 0, 1, 2, 3, 4, 5, and 7; 14—euthanasia day | Wound closure assessment, wound histology (Masson’s) | [85] |
| 10 | Wistar rats | ND | 3rd-degree burn wound. | Application to a burn wound | Transdermal | Off-dose | Levomecol, intact gel, CNP-doped gel | 5, 25 | Wound closure assessment | [86] |
| 11 | Female mice | ND | 7 mm surgical wound biopsy punch | Treatment of the wound with the active substance | Injection therapy | Off-dose | 3M Tegaderm, FVEC-0, FVEC-1 (0.5%) | 0, 3, 8 and 14 days | Wound closure assessment, histology (H&E) | [88] |
| 12 | Male Wistar rats | 20 | Surgical wound line-like, l = 45 mm | The wound was sutured and the composition was applied once daily. The stitches were removed on the 10th day. | Transdermal | No | Hydrogel, hydrogel c CNP | Daily until 21st day | Wound closure assessment, mechanical characteristics of skin | [77] |
| 13 | Female SD rats | 8 | Surgical wound (d = 1 cm) | Single application to the wound | Transdermal | Off-dose | PHEM-CS gel, cerium-doped gel, PHEM-CS-CNP | 2, 6, 10, and 14 days | Wound closure assessment, tissue histology (H&E) | [71] |
| 14 | Rats | ND | ND | Application of the composition to the wound | Transdermal | Off-dose | Paraffin and material treated group (GG/Ge and GG/Ge/NC FLU) | 0, 3, 7, 11 and 14 | Wound closure assessment, tissue histology | [79] |
| 15 | Male SD rats | ND | 10 mm surgical wound biopsy punch | Application of the composition to the wound | Transdermal | Off-dose | Pure alginate hydrogel, Alg/CeO NPs 3%, Alg/CeO NPs 5%, Alg/CeO NPs 7% | 0, 14 | Wound closure assessment, tissue histology (H&E) | [68] |
| 16 | Male SD rats | 3 | 8 mm surgical wound biopsy punch | Application of the hydrogel to the wound | Transdermal | PBS | GelMA, and ZC@GelMA | 0, 3, 6, 9, 12 | Wound closure assessment, histology (H&E, Masson) | [80] |
| Designed products | ||||||||||
| 17 | Female Wistar rats | 24 | Surgical wound | Application of a sponge under the bandage | Transdermal | Off-dose | Gelatin with cerium oxide NP, gelatin | 0, 4, 8 and 12-th days after surgery | Wound closure assessment, collagen density assessment (Masson’s), lymphocytic infiltration assessment (H & E) | [90] |
| 18 | Diabetic rats | ND | ND | Application of the composition to the wound | Transdermal | PBS | Standard dressing (Puracol Plus-Ag+, Medline), GelMA gel, GelMA − CONP-1 patches | 0, 3, 7, 10, 30 + daily assessment | Wound closure assessment, histology—daily examination of the wound; tissue histology (H & E) | [78] |
| 19 | Male Swiss albino mice | 12 | Surgical wound (d = 2 cm) | Daily treatment of the wound with the active substance | Transdermal | No | Membrane, 1% CNP membrane, 5% CNP membrane | 4, 7, 11, 15 | Wound closure assessment | [69] |
| 20 | NS | ND | 8 mm surgical wound biopsy punch | S. aureus-induced wound infection | Transdermal | No | gel, gel + AMP, gel + CNP, gel + AMP + CNP | 0, 3, 7, 14 | Wound closure assessment | [81] |
| 21 | Male SD rats | 25 | 6 mm surgical wound biopsy punch | Application of dressings with various substances | Transdermal | Off-dose | PLA; PFD NCs + PLA; CeO2 NCs + PLA; PFD/CeO2 NCs + PLA | Every 2 days before 14th | Wound closure assessment, tissue histology | [74] |
| Other | ||||||||||
| 22 | BKS.Cg-Dock7m+/+Leprdb/J, strain No. 000642 | 12–15 | 8 mm surgical wound biopsy punch | Topical application of active substance, then dressed with a Tegaderm (3M), which was subsequently removed on post-operative day 2 | Transdermal | PBS | NS, NS-CNP-miR146a | 10, 12, 14, 16 | Wound closure assessment, tissue histology (Masson’s Trichrome), gene expression | [82] |
| 23 | Male Wister albino rats | 9 | Biopsy punch | Wound covering | Transdermal | Off-dose | CS-ZnO hybrid composite, CS-ZnO/CeO2 hybrid nanocomposite | Daily until 21st day | Wound closure assessment, mechanical skin characteristics | [92] |
- one burn wounds [86];
2.3.1. Assessment of Wound Healing Rate
2.3.2. Assessment of Regeneration Quality
2.3.3. Antibacterial Effect
3. Discussion
- (1)
- Component-wise control groups must be included to isolate and quantify the individual contribution of each formulation constituent to wound healing;
- (2)
- Species-specific limitations of rodent models, particularly the presence of a panniculus carnosus and dense hair follicle network, must be acknowledged and addressed using modified protocols to improve extrapolation to human wound healing;
- (3)
- Full-thickness excisional (punch biopsy) wounds should be adopted as a standardized, reproducible model for assessing regenerative outcomes;
- (4)
- Efficacy assessments should extend beyond sterile wound models to include wounds created under non-sterile conditions and those deliberately inoculated with pathogens to reflect real-world clinical scenarios;
- (5)
- Chronic wound models, particularly those involving genetically or chemically induced diabetes mellitus, should be prioritized to evaluate the therapeutic potential of nanoceria in impaired healing contexts.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| CeO2 NPs | Cerium oxide nanoparticles |
| PEI | Polyethyleneimine |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| ROS | Reactive Oxygen Species |
| VEGF | Vascular Endothelial Growth Factor |
References
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Sorg, C.G.G. Skin Wound Healing: Of Players, Patterns, and Processes. Eur. Surg. Res. 2023, 64, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Queen, D.; Harding, K. Estimating the cost of wounds both nationally and regionally within the top 10 highest spenders. Int. Wound J. 2024, 21, e14709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health. 2022, 19, 1338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Francesco, F.; Ogawa, R. From Time to Timer in Wound Healing Through the Regeneration. Adv. Exp. Med. Biol. 2024, 1470, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Fitridge, R.; Game, F.; Monteiro-Soares, M.; Senneville, E.; IWGDF Editorial Board. Practical guidelines on the prevention and management of diabetes—Related foot disease (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2024, 40, e3657. [Google Scholar] [CrossRef]
- Hines, A.; Kody, S.; Shakshouk, H.; Fett, N.; Alavi, A.; Ortega-Loayza, A.G. Inflammatory and vaso-occlusive ulcers: Part II—Management. J. Am. Acad. Dermatol. 2024, 91, 1051–1066. [Google Scholar] [CrossRef] [PubMed]
- Ronicke, M.; Berking, C.; Erfurt-Berge, C. Occlusive cutaneous vasculopathies as cause of chronic ulcers. J. Dtsch. Dermatol. Ges. 2024, 22, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Valerio, M.S.; Goldman, S.M.; Dearth, C.L. The Role of the Inflammatory Response in Mediating Functional Recovery Following Composite Tissue Injuries. Int. J. Mol. Sci. 2021, 22, 13552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The wound microbiota: Microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Swanson, T.; Ousey, K.; Haesler, E.; Bjarnsholt, T.; Carville, K.; Idensohn, P.; Kalan, L.; Keast, D.H.; Larsen, D.; Percival, S.; et al. IWII Wound Infection in Clinical Practice consensus document: 2022 update. J. Wound Care 2022, 31 (Suppl. 12), S10–S21. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.; Bluebelle Study Group. Developing outcome measures assessing wound management and patient experience: A mixed methods study. BMJ Open 2017, 7, e016155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, D.; Wang, B.; Zhang, X.; Zhang, J.; Zhang, H.; Liu, X.; Gao, Z.; Yu, Z. The spread of antibiotic resistance to humans and potential protection strategies. Ecotoxicol. Environ. Saf. 2023, 254, 114734. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Rani Raju, N.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.B.; Achar, R.R. Multifunctional and Smart Wound Dressings-A Review on Recent Research Advancements in Skin Regenerative Medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nosrati, H.; Heydari, M.; Khodaei, M. Cerium oxide nanoparticles: Synthesis methods and applications in wound healing. Mater. Today Bio 2023, 23, 100823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balint, A.; Ciontea, L.; Socaciu, C. Cerium Oxide Nanoparticles and Their Efficient Antibacterial Application In Vitro against Gram-Positive and Gram-Negative Pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abid, S.A.; Taha, A.A.; Ismail, R.A.; Mohsin, M.H. Antibacterial and cytotoxic activities of cerium oxide nanoparticles prepared by laser ablation in liquid. Environ. Sci. Pollut. Res. Int. 2020, 27, 30479–30489. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, Z.; Wang, T.; Ma, C.; Li, H.; Lei, H.; Yang, Y.; Wang, Y.; Pei, Z.; Liu, Z.; et al. Cerium oxide nanoparticles with antioxidative neurorestoration for ischemic stroke. Biomaterials 2022, 291, 121904, Erratum in Biomaterials 2024, 309, 122612. https://doi.org/10.1016/j.biomaterials.2024.122612. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hong, G.; Mazaleuskaya, L.; Hsu, J.C.; Rosario-Berrios, D.N.; Grosser, T.; Cho-Park, P.F.; Cormode, D.P. Ultrasmall Antioxidant Cerium Oxide Nanoparticles for Regulation of Acute Inflammation. ACS Appl. Mater. Interfaces 2021, 13, 60852–60864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silina, E.V.; Stupin, V.A.; Manturova, N.E.; Ivanova, O.S.; Popov, A.L.; Mysina, E.A.; Artyushkova, E.B.; Kryukov, A.A.; Dodonova, S.A.; Kruglova, M.P.; et al. Influence of the Synthesis Scheme of Nanocrystalline Cerium Oxide and Its Concentration on the Biological Activity of Cells Providing Wound Regeneration. Int. J. Mol. Sci. 2023, 24, 14501. [Google Scholar] [CrossRef] [PubMed]
- Titova, S.A.; Kruglova, M.P.; Stupin, V.A.; Manturova, N.E.; Achar, R.R.; Deshpande, G.; Parfenov, V.A.; Silina, E.V. Excipients for Cerium Dioxide Nanoparticle Stabilization in the Perspective of Biomedical Applications. Molecules 2025, 30, 1210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, F.; Neal, C.J.; Sakthivel, T.S.; Kean, T.; Seal, S.; Coathup, M.J. Multi-functional cerium oxide nanoparticles regulate inflammation and enhance osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112041. [Google Scholar] [CrossRef]
- Popov, A.L.; Popova, N.R.; Selezneva, I.I.; Akkizov, A.Y.; Ivanov, V.K. Cerium oxide nanoparticles stimulate proliferation of primary mouse embryonic fibroblasts in vitro. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 406–413. [Google Scholar] [CrossRef]
- Mehta, A.; Scammon, B.; Shrake, K.; Bredikhin, M.; Gil, D.; Shekunova, T.; Baranchikov, A.; Ivanov, V.; Reukov, V. Nanoceria: Metabolic interactions and delivery through PLGA-encapsulation. Mater. Sci. Eng. C 2020, 114, 111003. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Bao, S.; Yao, L.; Fu, X.; Yu, Y.; Lyu, H.; Pang, H.; Guo, S.; Zhang, H.; et al. Cerium oxide nanoparticles in wound care: A review of mechanisms and therapeutic applications. Front. Bioeng. Biotechnol. 2024, 12, 1404651. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox. Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Bazhukova, I.N.; Myshkina, A.V.; Sokovnin, S.Y.; Ilves, V.G.; Kiryakov, A.N.; Bazhukov, S.I.; Vazirov, R.A.; Kasyanova, V.V.; Zvonareva, I.A. Modification of cerium oxide nanoparticles under irradiation with accelerated electrons. Phys. Solid State 2019, 61, 881–886. [Google Scholar] [CrossRef]
- Manturova, N.E.; Stupin, V.A.; Silina, E.V. Cerium oxide nanoparticles for surgery, plastic surgery and aesthetic medicine. Plast. Surg. Aesthet. Med. 2023, 3, 120–129. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Tang, M.; Xu, S.; Zhou, X.; Zhang, Z.; Yang, L.; Nüssler, A.K.; Liu, L.; Yang, W. Protective effects of bone marrow mesenchymal stem cell-derived exosomes loaded cerium dioxide nanoparticle against deoxynivalenol-induced liver damage. J. Nanobiotechnol. 2025, 23, 215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Selvaraj, V.; Nepal, N.; Rogers, S.; Manne, N.D.; Arvapalli, R.; Rice, K.M.; Asano, S.; Fankenhanel, E.; Ma, J.Y.; Shokuhfar, T.; et al. Lipopolysaccharide induced MAP kinase activation in RAW 264.7 cells attenuated by cerium oxide nanoparticles. Data Brief 2015, 4, 96–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdulaal, W.H.; Helmi, N.; Hamza, A.; Salem, N.D. Cerium oxide nanoparticles impact on sepsis-induced cerebral injury: Deciphering miRNA/NF-κB/TLR signalling pathway. Cell. Mol. Biol. 2024, 70, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Yang, F.; He, Y.; Wang, F.; Xia, D.; Liu, Y. Multifunctional Hydrogel with Photothermal ROS Scavenging and Antibacterial Activity Accelerates Diabetic Wound Healing. Adv. Healthc. Mater. 2025, 14, e2402236. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Pourbagher-Shahri, A.M.; Darroudi, M.; Sadeghi, M.; Vafaee, F.; Moghadam, O.F.; Mashhad, N.M.; Ghazavi, H.; Khorrami, M.B. Cerium Oxide Nanoparticles Ameliorate Oxidative Stress, Inflammation, and Pain Behavior in Neuropathic Rats. Curr. Neurovascular Res. 2023, 20, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, M.; Zheng, J.; Li, Z.; Zhang, Y.; Chen, Y.; Chen, Y.; Jiang, X.; Tang, J. A Multifunctional MXene@CeO2-Enhanced Hydrogel Dressing for Synergistic Photothermal Antibacterial and Antioxidative Therapy in Wound Healing. Adv. Healthc. Mater. 2025, 14, e2500656. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Seal, S.; McGinnis, J.F. Sustained inhibition of neovascularization in vldlr−/− mice following intravitreal injection of cerium oxide nanoparticles and the role of the ASK1-P38/JNK-NF-κB pathway. Biomaterials 2014, 35, 249–258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Babenko, L.P.; Zholobak, N.M.; Shcherbakov, A.B.; Voychuk, S.I.; Lazarenko, L.M.; Spivak, M.Y. Antibacterial activity of cerium colloids against opportunistic microorganisms in vitro. Mikrobiol. Z. 2012, 74, 54–62. [Google Scholar] [PubMed]
- Popova, N.; Andreeva, V.V.; Khohlov, N.V.; Popov, A.; Ivanov, V. Fabrication of CeO2 nanoparticles embedded in polysaccharide hydrogel and their application in skin wound healing. Nanosyst. Phys. Chem. Math. 2020, 11, 99–109. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Song, Y.; Lan, L.; Wang, J.; Xu, X.; Du, Y. Nano transdermal system combining mitochondria-targeting cerium oxide nanoparticles with all-trans retinoic acid for psoriasis. Asian J. Pharm. Sci. 2023, 18, 100846. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silina, E.V.; Stupin, V.A.; Manturova, N.E.; Chuvilina, E.L.; Gasanov, A.A.; Ostrovskaya, A.A.; Andreeva, O.I.; Tabachkova, N.Y.; Abakumov, M.A.; Nikitin, A.A.; et al. Development of Technology for the Synthesis of Nanocrystalline Cerium Oxide Under Production Conditions with the Best Regenerative Activity and Biocompatibility for Further Creation of Wound-Healing Agents. Pharmaceutics 2024, 16, 1365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silina, E.V.; Manturova, N.E.; Ivanova, O.S.; Baranchikov, A.E.; Artyushkova, E.B.; Medvedeva, O.A.; Kryukov, A.A.; Dodonova, S.A.; Gladchenko, M.P.; Vorsina, E.S.; et al. Cerium Dioxide-Dextran Nanocomposites in the Development of a Medical Product for Wound Healing: Physical, Chemical and Biomedical Characteristics. Molecules 2024, 29, 2853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, J.; Bishoff, B.; Mercer, R.R.; Barger, M.; Schwegler-Berry, D.; Castranova, V. Role of epithelial-mesenchymal transition (EMT) and fibroblast function in cerium oxide nanoparticles-induced lung fibrosis. Toxicol. Appl. Pharmacol. 2017, 323, 16–25. [Google Scholar] [CrossRef]
- Grillone, A.; Li, T.; Battaglini, M.; Scarpellini, A.; Prato, M.; Takeoka, S.; Ciofani, G. Preparation, Characterization, and Preliminary In Vitro Testing of Nanoceria-Loaded Liposomes. Nanomaterials 2017, 7, 276. [Google Scholar] [CrossRef]
- Fernández-Bertólez, N.; Martínez, L.; Ramos-Pan, L.; Touzani, A.; Costa, C.; Laffon, B.; Valdiglesias, V. In vitro and in vivo assessment of nanoceria biocompatibility for their safe use in nervous system applications. J. Hazard. Mater. 2025, 486, 137041. [Google Scholar] [CrossRef]
- Landsiedel, R.; Sauer, U.G.; Ma-Hock, L.; Schnekenburger, J.; Wiemann, M. Pulmonary toxicity of nanomaterials: A critical comparison of published in vitro assays and in vivo inhalation or instillation studies. Nanomedicine 2014, 9, 2557–2585. [Google Scholar] [CrossRef]
- Singh, S. Antioxidant nanozymes as next-generation therapeutics to free radical-mediated inflammatory diseases: A comprehensive review. Int. J. Biol. Macromol. 2024, 260 Pt 1, 129374. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Alagga, A.A.; Pellegrini, M.V.; Gupta, V. Drug Absorption. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557405/ (accessed on 18 November 2025).
- Kartsonakis, I.A.; Liatsi, P.; Daniilidis, I.; Kordas, G. Synthesis, characterization, and antibacterial action of hollow ceria nanospheres with/without a conductive polymer coating. J. Am. Ceram. Soc. 2008, 91, 372–378. [Google Scholar] [CrossRef]
- Cuahtecontzi-Delint, R.; Mendez-Rojas, M.A.; Bandala, E.R.; Quiroz, M.A.; Recillas, S.; Sanchez-Salas, J.L. Enhanced antibacterial activity of CeO2 nanoparticles by surfactants. Int. J. Chem. React. Eng. 2013, 11, 781–785. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Monteiro-Riviere, N.D.; Aggarwal, R.; Davis, J.P.; Narayan, R.J.; Self, W.T.; McGinnis, J.; Seal, S. Nanoceria as Antioxidant: Synthesis and Biomedical Applications. JOM 2008, 60, 33–37. [Google Scholar] [CrossRef]
- Dewberry, L.C.; Niemiec, S.M.; Hilton, S.A.; Louiselle, A.E.; Singh, S.; Sakthivel, T.S.; Hu, J.; Seal, S.; Liechty, K.W.; Zgheib, C. Cerium oxide nanoparticle conjugation to microRNA-146a mechanism of correction for impaired diabetic wound healing. Nanomed. NBM 2022, 40, 102483. [Google Scholar] [CrossRef]
- Sener, G.; Hilton, S.A.; Osmond, M.J.; Zgheib, C.; Newsom, J.P.; Dewberry, L.; Singh, S.; Sakthivel, T.S.; Seal, S.; Liechty, K.W.; et al. Injectable, self-healable zwitterionic cryogels with sustained microRNA—Cerium oxide nanoparticle release promote accelerated wound healing. Acta Biomater. 2020, 101, 262–272. [Google Scholar] [CrossRef]
- Singh, S.; Ly, A.; Das, S.; Sakthivel, T.S.; Barkam, S.; Seal, S. Cerium oxide nanoparticles at the nano-bio interface: Size-dependent cellular uptake. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S956–S963. [Google Scholar] [CrossRef]
- Thakur, N.; Manna, P.; Das, J. Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J. Nanobiotechnol. 2019, 17, 84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patil, S.N.; Paradeshi, J.S.; Chaudhari, P.B.; Mishra, S.J.; Chaudhari, B.L. Bio-therapeutic potential and cytotoxicity assessment of pectin-mediated synthesized nanostructured cerium oxide. Appl. Biochem. Biotechnol. 2016, 180, 638–654. [Google Scholar] [CrossRef]
- Kuang, Y.; He, X.; Zhang, Z.; Li, Y.; Zhang, H.; Ma, Y.; Wu, Z.; Chai, Z. Comparison study on the antibacterial activity of nano- or bulk-cerium oxide. J. Nanosci. Nanotechnol. 2011, 11, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Zhang, L.H.; Yun, K.; Kim, S.J. Surface chemistry of cerium oxide nanocubes: Toxicity against pathogenic bacteria and their mechanistic study. J. Ind. Eng. Chem. 2014, 20, 3513–3517. [Google Scholar] [CrossRef]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef]
- Burns, D.B.; Zydney, A.L. Effect of solution pH on protein transport through ultrafiltration membranes. Biotechnol. Bioeng. 1999, 64, 27–37. [Google Scholar] [CrossRef]
- Thill, A.; Zeyons, O.; Spalla, O.; Chauvat, F.; Rose, J.; Auffan, M.; Flank, A.M. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ. Sci. Technol. 2006, 40, 6151–6156. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Z.R.; Feraru, A.; Saszet, K.; Veréb, G.; Vodnar, D.C.; Todea, M.; Timar-Gabor, A.; Dave, A.K.; Sand, D.; Dreanca, A.; et al. Relation between shape-tailored CeO2 nanoparticles morphology and hemocompatibility and antimicrobial effect. Biomater. Adv. 2025, 171, 214229. [Google Scholar] [CrossRef] [PubMed]
- Oficerova, N.Y.; Bazhukova, I.L.; Myshkina, A.V. Mul’tifunkcional’nye nanozimy na osnove nanochastic oksida ceriya. Traektoriya Issled.—Chelovek Prir. Tekhnologii. 2023, pp. 104–119. Available online: https://restrajectory.ru/5-6.pdf (accessed on 18 November 2025).
- Zhao, R.; Zhao, C.; Wan, Y.; Majid, M.; Abbas, S.Q.; Wang, Y. In vitro and in vivo evaluation of alginate hydrogel-based wound dressing loaded with green chemistry cerium oxide nanoparticles. Front. Chem. 2023, 11, 1298808, Erratum in Front. Chem. 2024, 12, 1373205. https://doi.org/10.3389/fchem.2024.1373205. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.E.; Iqbal, Y.; Aziz, M.H.; Atif, M.; Batool, Z.; Hanif, A.; Yaqub, N.; Farooq, W.A.; Ahmad, S.; Fatehmulla, A.; et al. Green Synthesis of CeO2 Nanoparticles from the Abelmoschus esculentus Extract: Evaluation of Antioxidant, Anticancer, Antibacterial, and Wound-Healing Activities. Molecules 2021, 26, 4659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, X.; Cheng, Y.; Jian, H.; Feng, Y.; Chang, Y.; Zheng, R.; Wu, X.; Wang, L.; Li, X.; Zhang, H. Hollow, Rough, and Nitric Oxide-Releasing Cerium Oxide Nanoparticles for Promoting Multiple Stages of Wound Healing. Adv. Healthc. Mater. 2019, 8, e1900256. [Google Scholar] [CrossRef]
- Luo, J.; Liu, W.; Xie, Q.; He, J.; Jiang, L. Synthesis and characterisation of a novel poly(2-hydroxyethylmethacrylate)-chitosan hydrogels loaded cerium oxide nanocomposites dressing on cutaneous wound healing on nursing care of chronic wound. IET Nanobiotechnol. 2023, 17, 312–325. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Tiwari, R.; Bhatia, T.; Purohit, M.P.; Pal, A.; Jagdale, P.; Mudiam, M.K.R.; Chaudhari, B.P.; Shukla, Y.; Ansari, K.M.; et al. Accelerated and scarless wound repair by a multicomponent hydrogel through simultaneous activation of multiple pathways. Drug. Deliv. Transl. Res. 2019, 9, 1143–1158. [Google Scholar] [CrossRef]
- Es-Haghi, A.; Mashreghi, M.; Rezazade Bazaz, M.; Homayouni-Tabrizi, M.; Darroudi, M. Fabrication of biopolymer-based nanocomposite wound dressing: Evaluation of wound healing properties and wound microbial load. IET Nanobiotechnol. 2017, 11, 517–522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, J.; Meng, X.; Meng, C.; Zhao, J.; Chen, Y.; Zhang, Z.; Zhang, Y. Layer-by-Layer Pirfenidone/Cerium Oxide Nanocapsule Dressing Promotes Wound Repair and Prevents Scar Formation. Molecules 2022, 27, 1830. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, C.; Hilton, S.A.; Dewberry, L.C.; Hodges, M.M.; Ghatak, S.; Xu, J.; Singh, S.; Roy, S.; Sen, C.K.; Seal, S.; et al. Use of Cerium Oxide Nanoparticles Conjugated with MicroRNA-146a to Correct the Diabetic Wound Healing Impairment. J. Am. Coll. Surg. 2019, 228, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Legon’kova, O.A.; Ushakova, T.A.; Savchenkova, I.P.; Perova, N.V.; Belova, M.S.; Torkova, A.A.; Baranchikov, A.E.; Ivanova, O.S.; Korotaeva, A.I.; Ivanov, V.K. Experimental Study of the Effects of Nanodispersed Ceria on Wound Repair. Bull. Exp. Biol. Med. 2017, 162, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Galichenko, K.A.; Sukhov, A.V.; Timoshkin, S.P.; Alkhatatnekh, B.A.S.; Eldyreva, M.V.; Sorokvasha, I.N.; Blinova, E.V.; Mironov, M.M. Experimental study of topical application of cerium oxide nanoparticles on tissue regeneration. Pulse 2023, 25, 96–100. [Google Scholar] [CrossRef]
- Augustine, R.; Zahid, A.A.; Hasan, A.; Dalvi, Y.B.; Jacob, J. Cerium Oxide Nanoparticle-Loaded Gelatin Methacryloyl Hydrogel Wound-Healing Patch with Free Radical Scavenging Activity. ACS Biomater. Sci. Eng. 2021, 7, 279–290. [Google Scholar] [CrossRef]
- Singh, H.; Yadav, I.; Sheikh, W.M.; Dan, A.; Darban, Z.; Shah, S.A.; Mishra, N.C.; Shahabuddin, S.; Hassan, S.; Bashir, S.M.; et al. Dual cross-linked gellan gum/gelatin-based multifunctional nanocomposite hydrogel scaffold for full-thickness wound healing. Int. J. Biol. Macromol. 2023, 251, 126349. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Chen, H.; Sun, L.N.; Zhang, B.; Yue, D.S.; Wang, C.L.; Zhang, Z.F. Injectable hydrogel with doxorubicin-loaded ZIF-8 nanoparticles for tumor postoperative treatments and wound repair. Sci. Rep. 2024, 14, 9983. [Google Scholar] [CrossRef]
- Cheng, H.; Shi, Z.; Yue, K.; Huang, X.; Xu, Y.; Gao, C.; Yao, Z.; Zhang, Y.S.; Wang, J. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021, 124, 219–232. [Google Scholar] [CrossRef]
- Niemiec, S.M.; Louiselle, A.E.; Hilton, S.A.; Dewberry, L.C.; Zhang, L.; Azeltine, M.; Xu, J.; Singh, S.; Sakthivel, T.S.; Seal, S.; et al. Nanosilk Increases the Strength of Diabetic Skin and Delivers CNP-miR146a to Improve Wound Healing. Front. Immunol. 2020, 11, 590285. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, W.; Lu, C.; Yang, J.; Zeng, Z.; Li, W.; Liu, H.; Huang, N.; Chen, Y.; Liu, W. A Multifunctional Nanozyme Integrating Antioxidant, Antimicrobial and Pro-Vascularity for Skin Wound Management. Int. J. Nanomed. 2024, 19, 3217–3232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chang, Y.; Feng, Y.; Jian, H.; Wu, X.; Zheng, R.; Wang, L.; Ma, X.; Xu, K.; Song, P.; et al. Hierarchical Acceleration of Wound Healing through Intelligent Nanosystem to Promote Multiple Stages. ACS Appl. Mater. Interfaces 2019, 11, 33725–33733. [Google Scholar] [CrossRef] [PubMed]
- Silina, E.V.; Manturova, N.E.; Vasin, V.I.; Artyushkova, E.B.; Khokhlov, N.V.; Ivanov, A.V.; Stupin, V.A. Efficacy of a Novel Smart Polymeric Nanodrug in the Treatment of Experimental Wounds in Rats. Polymers 2020, 12, 1126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Popov, A.L.; Khohlov, N.V.; Popova, N.R.; Andreeva, V.V.; Kamenskikh, K.A.; Ermakov, A.M.; Ivanov, V.K. Composite cerium oxide nanoparticles-containing polysaccharide hydrogel as effective agent for burn wound healing. KEM 2021, 899, 493–505. [Google Scholar] [CrossRef]
- Mai, H.; Sun, L.; Zhang, Y.; Si, R.; Feng, W.; Zhang, H.; Liu, H.; Yan, C. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. A 2005, 109, 24380–24385. [Google Scholar] [CrossRef]
- Gong, X.; Luo, M.; Wang, M.; Niu, W.; Wang, Y.; Lei, B. Injectable self-healing ceria-based nanocomposite hydrogel with ROS-scavenging activity for skin wound repair. Regen. Biomater. 2021, 9, rbab074. [Google Scholar] [CrossRef]
- Lee, S.S.; Zhu, H.; Contreras, E.Q.; Prakash, A.; Puppala, H.L.; Colvin, V.L. High Temperature Decomposition of Cerium Precursors to Form Ceria Nanocrystal Libraries for Biological Applications. Chem. Mater. 2012, 24, 424–432. [Google Scholar] [CrossRef]
- Raja, I.S.; Fathima, N.N. Gelatin–Cerium Oxide Nanocomposite for Enhanced Excisional Wound Healing. ACS Appl. Bio. Mater. 2018, 1, 487–495. [Google Scholar] [CrossRef]
- Ivanova, O.S.; Shekunova, T.O.; Ivanov, V.K.; Shcherbakov, A.B.; Popov, A.L.; Davydova, G.A.; Selezneva, I.I.; Kopitsa, G.P.; Tret’yakov, Y.D. One-stage synthesis of ceria colloid solutions for biomedical use. Dokl. Chem. 2011, 437, 103–106. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Zhang, Y.; Li, J.; Zhang, H.; Li, J. Novel fabrication of bi-metal oxide hybrid nanocomposites for synergetic enhancement of in vivo healing and wound care after caesarean section surgery. Int. Wound J. 2022, 19, 1705–1716. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Maita, K.C.; Avila, F.R.; Torres-Guzman, R.A.; Garcia, J.P.; Eldaly, A.S.; Palmieri, L.; Emam, O.S.; Ho, O.; Forte, A.J. Local anti-inflammatory effect and immunomodulatory activity of chitosan-based dressing in skin wound healing: A systematic review. J. Clin. Transl. Res. 2022, 8, 488–498. [Google Scholar] [PubMed] [PubMed Central]
- Beck, B.; Yildirim Aksoy, M.; Shoemaker, C.; Fuller, A.; Peatman, E. Antimicrobial activity of the biopolymer chitosan against Streptococcus iniae. J. Fish. Dis. 2019, 42, 371–377. [Google Scholar] [CrossRef]
- Tamara, F.R.; Lin, C.; Mi, F.L.; Ho, Y.C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef]
- Yang, B.Y.; Ding, Q.; Montgomery, R. Heterogeneous components of chitosans. Biomacromolecules 2010, 11, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Fittolani, G.; Tyrikos-Ergas, T.; Vargová, D.; Chaube, M.A.; Delbianco, M. Progress and challenges in the synthesis of sequence controlled polysaccharides. Beilstein J. Org. Chem. 2021, 17, 1981–2025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shivakumar, P.; Gupta, M.S.; Jayakumar, R.; Gowda, D.V. Prospection of chitosan and its derivatives in wound healing: Proof of patent analysis (2010–2020). Int. J. Biol. Macromol. 2021, 184, 701–712. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O. A short review on chitosan and gelatin-based hydrogel composite polymers for wound healing. J. Biomater. Sci. Polym. Ed. 2022, 33, 1595–1622. [Google Scholar] [CrossRef]
- 103 Gaspar-Pintiliescu, A.; Stanciuc, A.M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Costa, D.; Yamashita, F.; Martelli, S.M.; Jesus, R.C.; Alganer, K.; Collares-Queiroz, F.P.; Innocentini-Mei, L.H. Comparative study of processing methods for starch/gelatin films. Carbohydr. Polym. 2013, 95, 681–689. [Google Scholar] [CrossRef]
- Yu, N.; Luo, Z.; Ma, F.; Li, J.; Yang, P.; Li, G.; Li, J. Cationic Gelatin Cross-Linked with Transglutaminase and Its Electrospinning in Aqueous Solution. Langmuir 2023, 39, 3668–3677. [Google Scholar] [CrossRef] [PubMed]
- Davis, Z.G.; Hussain, A.F.; Fisher, M.B. Processing variables of direct-write, near-field electrospinning impact size and morphology of gelatin fibers. Biomed. Mater. 2021, 16, 045017. [Google Scholar] [CrossRef] [PubMed]
- Navaei, T.; Milan, P.B.; Samadikuchaksaraei, A.; Davari, H.R.; Hardy, J.G.; Mozafari, M. Design and fabrication of polycaprolactone/gelatin composite scaffolds for diaphragmatic muscle reconstruction. J. Tissue Eng. Regen. Med. 2021, 15, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Chen, J.; Yang, X.; Chen, Y.; Feng, Y.; Pan, J.; Shi, C. Expandable, biodegradable, bioactive quaternized gelatin sponges for rapidly controlling incompressible hemorrhage and promoting wound healing. Biomater. Adv. 2022, 136, 212776. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, M.; Peng, Y.; He, S.; Zhu, X.; Hu, C.; Xia, G.; Zuo, T.; Zhang, X.; Yun, Y.; et al. A physicochemical double-cross-linked gelatin hydrogel with enhanced antibacterial and anti-inflammatory capabilities for improving wound healing. J. Nanobiotechnol. 2022, 20, 426, Erratum in J. Nanobiotechnol. 2023, 21, 262. https://doi.org/10.1186/s12951-023-02029-4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bates, N.M.; Puy, C.; Jurney, P.L.; McCarty, O.J.T.; Hinds, M.T. Evaluation of the Effect of Crosslinking Method of Poly(Vinyl Alcohol) Hydrogels on Thrombogenicity. Cardiovasc. Eng. Technol. 2020, 11, 448–455. [Google Scholar] [CrossRef]
- Rizwana, N.; Maslekar, N.; Chatterjee, K.; Yao, Y.; Agarwal, V.; Nune, M. Dual Crosslinked Antioxidant Mixture of Poly(vinyl alcohol) and Cerium Oxide Nanoparticles as a Bioink for 3D Bioprinting. ACS Appl. Nano Mater. 2023, 7, 18177–18188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Guo, S.; Ren, Y.; Chang, R.; He, Y.; Zhang, D.; Guan, F.; Yao, M. Injectable Self-Healing Adhesive Chitosan Hydrogel with Antioxidative, Antibacterial, and Hemostatic Activities for Rapid Hemostasis and Skin Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 34455–34469. [Google Scholar] [CrossRef]
- Ban, E.; Jeong, S.; Park, M.; Kwon, H.; Park, J.; Song, E.J.; Kim, A. Accelerated wound healing in diabetic mice by miRNA-497 and its anti-inflammatory activity. Biomed. Pharmacother. 2020, 121, 109613. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, Y.; Chen, L.; Wang, M. Clinical Significance of microRNA-146a in Patients with Ulcerative Colitis. Ann. Clin. Lab. Sci. 2020, 50, 463–467. [Google Scholar] [PubMed]
- Gilyazova, I.; Asadullina, D.; Kagirova, E.; Sikka, R.; Mustafin, A.; Ivanova, E.; Bakhtiyarova, K.; Gilyazova, G.; Gupta, S.; Khusnutdinova, E.; et al. MiRNA-146a-A Key Player in Immunity and Diseases. Int. J. Mol. Sci. 2023, 24, 12767. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, G.; Guha Ray, P.; Byram, P.K.; Kaushal, M.; Dhara, S.; Das, S. Tailorable hydrogel of gelatin with silk fibroin and its activation/crosslinking for enhanced proliferation of fibroblast cells. Int. J. Biol. Macromol. 2020, 164, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Lu, W.; Hu, Y. Review on chitosan-based antibacterial hydrogels: Preparation, mechanisms, and applications. Int. J. Biol. Macromol. 2024, 255, 128080. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Kim, T.; Choi, I.Y.; Soh, M.; Kim, D.; Kim, Y.J.; Jang, H.; Yang, H.S.; Kim, J.Y.; Park, H.K. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem. Int. Ed. 2012, 51, 11039–11043. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius Red Staining: A Useful Tool to Appraise Collagen Networks in Normal and Pathological Tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- Serebrennikova, S.N.; Seminsky, I.Z.h.; Guzovskaiia, E.V.; Gutsol, L.O. Inflammation as a fundamental pathological process: Lecture 2 (cellular component). Baikal Med. J. 2023, 2, 65–76. [Google Scholar] [CrossRef]
- Chen, P.; Vilorio, N.C.; Dhatariya, K.; Jeffcoate, W.; Lobmann, R.; McIntosh, C.; Piaggesi, A.; Steinberg, J.; Vas, P.; Viswanathan, V.; et al. Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2024, 40, e3644. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, e2100477. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.S.; Ge, F.J.; Zhang, B.; Wang, Y.; Silvestris, N.; Liu, L.J.; Zhao, C.H.; Lin, L.; Brunetti, A.E.; Fu, Y.L.; et al. Expression and prognostic value of VEGFR-2, PDGFR-β, and c-Met in advanced hepatocellular carcinoma. J. Exp. Clin. Cancer. Res. 2013, 32, 16. [Google Scholar] [CrossRef] [PubMed]
- Boshtam, M.; Asgary, S.; Kouhpayeh, S.; Shariati, L.; Khanahmad, H. Aptamers Against Pro- and Anti-Inflammatory Cytokines: A Review. Inflammation 2017, 40, 340–349. [Google Scholar] [CrossRef]
- Lee, D.U.; Kim, S.C.; Choi, D.Y.; Jung, W.K.; Moon, M.J. Basic amino acid-mediated cationic amphiphilic surfaces for antimicrobial pH monitoring sensor with wound healing effects. Biomater. Res. 2023, 27, 14. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, R.; Han, F.; Zheng, Z.; Liu, Y.; Zhou, X.; Li, Q.; Zhai, X.; Wu, J.; Pan, X.; et al. A soft intelligent dressing with pH and temperature sensors for early detection of wound infection. RSC Adv. 2022, 12, 3243–3252. [Google Scholar] [CrossRef]
- Kızılkonca, E.; Torlak, E.; Erim, F.B. Preparation and characterization of antibacterial nano cerium oxide/chitosan/hydroxyethylcellulose/polyethylene glycol composite films. Int. J. Biol. Macromol. 2021, 177, 351–359. [Google Scholar] [CrossRef]
- Yefimova, S.; Klochkov, V.; Kavok, N.; Tkachenko, A.; Onishchenko, A.; Chumachenko, T.; Dizge, N.; Özdemir, S.; Gonca, S.; Ocakoglu, K. Antimicrobial activity and cytotoxicity study of cerium oxide nanoparticles with two different sizes. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 872–880. [Google Scholar] [CrossRef]
- Chatzimentor, I.; Tsamesidis, I.; Ioannou, M.E.; Pouroutzidou, G.K.; Beketova, A.; Giourieva, V.; Papi, R.; Kontonasaki, E. Study of Biological Behavior and Antimicrobial Properties of Cerium Oxide Nanoparticles. Pharmaceutics 2023, 15, 2509. [Google Scholar] [CrossRef]
- Bellio, P.; Luzi, C.; Mancini, A.; Cracchiolo, S.; Passacantando, M.; Di Pietro, L.; Perilli, M.; Amicosante, G.; Santucci, S.; Celenza, G. Cerium oxide nanoparticles as potential antibiotic adjuvant. Effects of CeO2 nanoparticles on bacterial outer membrane permeability. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2428–2435. [Google Scholar] [CrossRef]
- Zamani, K.; Allah-Bakhshi, N.; Akhavan, F.; Yousefi, M.; Golmoradi, R.; Ramezani, M.; Bach, H.; Razavi, S.; Irajian, G.-R.; Gerami, M.; et al. Antibacterial effect of cerium oxide nanoparticle against Pseudomonas aeruginosa. BMC Biotechnol. 2021, 21, 68. [Google Scholar] [CrossRef]
- Shah, V.; Shah, S.; Shah, H.; Rispoli, F.J.; McDonnell, K.T.; Workeneh, S.; Karakoti, A.; Kumar, A.; Seal, S. Antibacterial activity of polymer coated cerium oxide nanoparticles. PLoS ONE 2012, 7, e47827. [Google Scholar] [CrossRef]
| No. | Active Ingredient | Day | % Wound Closure | References |
|---|---|---|---|---|
| 2 | CeONPs | 12 | 100% | [90] |
| 3 | CeONPs | 10 | 88–100% (depending on NP form) | [70] |
| 4 | CeONPs | 14 | 89% | [72] |
| 5 | CeONPs | 14 | 79.1 ± 0.6% | [84] |
| 6 | CeONPs | 14 | 94.7% | [85] |
| 7 | CeONPs | 15 | 75% | [78] |
| 20 | 97% | |||
| 9 | CeONPs | 15 | 5%CeO—95% | [69] |
| 1%CeO—62% | ||||
| 10 | CeONPs | 14 | 87.5% | [81] |
| 11 | CeONPs | 14 | 90% | [74] |
| 12 | CeONPs | 6 ± 2 | 100% | [77] |
| 13 | CeONPs | 14 | 86.7% | [79] |
| 14 | CeONPs | 14 | 80% | [83] |
| 15 | CeONPs | 25 | 100% | [86] |
| Multicomponent active substances | ||||
| 16 | 12GEL ZIF-8 CeO2-loaded GelMA | 12 | 90% | [80] |
| 17 | PHEM-CS/CeONPs | 14 | 98.5 ± 4.95% | [71] |
| 18 | CS-ZnO/CeO2 | 12 | 100% | [92] |
| 19 | 0.5%PEI/PVP CeO2 | 14 | 100% | [88] |
| 20 | CNP-miR146a | 14 | 100% | [56] |
| 21 | CNP-miR146a | 14 | 97% | [82] |
| 16 | 100% | |||
| 22 | CNP-miR146a | 14 | 60% | [75] |
| 23 | CNP-miR146a | 14 | 100% | [57] |
| # No. | Active Ingredient | Day | Exudation | Intact Epidermis | Collagen | Hair Follicle | Neoangiogenesis | References |
|---|---|---|---|---|---|---|---|---|
| 1 | CeONPs | 14 | ND | + | + immature | + | + | [74] |
| 2 | CeONPs | 14 | ND | + | ± | ± | ++ | [57] |
| 3 | CeONPs | 18 | ++ | ± | ND | ND | ± | [72] |
| 24 | − | + | ND | ND | ++ | |||
| 5 | CeONPs | 10 | ND | ± | + | ND | ND | [90] |
| 6 | CeONPs | 14 | ND | ± | ± | − | ND | [70] |
| 7 | CeONPs | 14 | ND | ± | + | ± | ND | [73] |
| 8 | CNP-miR146a | 14 | ND | ± | ND | ND | ++ | [84] |
| 9 | CeONPs | 3 | + | ± | + | ND | ND | [85] |
| 7 | ± | ± | + | + | + | |||
| 14 | − | + | + (immature) | ++ | ||||
| 10 | CeONPs | 20 | − | + | ND | + | + | [78] |
| 11 | 0.5%PEI/PVP CeO2 | 14 | − | + | ND | + | ND | [88] |
| 12 | CS-ZnO/CeO2 | 6 | ND | ND | + | ND | ND | [92] |
| 12 | ND | + | + | ND | ND | |||
| 13 | PHEM-CS/CeONPs | 14 | ND | + | ND | + | ND | [71] |
| 14 | CeONPs | 7 | + | ND | ND | ND | + | [79] |
| 14 | − | ± | ++ | + | + | |||
| 15 | CeONPs | 14 | ND | ± | ± | ND | ND | [83] |
| # No. | Active Ingredient | Il6 | Il8 | Il10 | TGF-β | VEGFR | MCP-1 | CD45 | CD31 | CXCL2 | Col1a2 | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CeONPs | ↑ | ND | ↑ | ↑↑ | ↑↑ | ↓ | ND | ND | ND | ND | [72] |
| 2 | CNP-miR146a | ND | ND | ND | ND | ND | ND | ↑ | ↑↑ | ND | ND | [84] |
| 3 | CNP-miR146a | ↓↓ | ND | ND | ND | ND | ND | ND | ND | ↓↓ | ↑↑ | [57] |
| 4 | CNP-miR146a | ↓↓ | ↓↓ | ND | ↑↑ | ND | ND | ↓ | ND | ND | ↑↑ | [82] |
| 5 | CNP-miR146a | ↑↓ | ND | ND | ND | ↑↑ | ND | ↓ | ↑ | ND | ND | [56] |
| 6 | CeONPs | ND | ND | ↑ | ↑ | ↑↑ | ND | ND | ND | ND | ND | [79] |
| 7 | CeONPs | ↓ | ND | ND | ↓ | ND | ND | ND | ↑ | ND | ND | [83] |
| # No. | Active Ingredient | Day | Tensile Strength of Skin, MPa | Young Modulus, MPa | % of the Norm | References | |
|---|---|---|---|---|---|---|---|
| 1 | CeONPs | 24 | 6.09 ± 0.23 | 7.85 ± 0.12 | ND | 77.6% | [90] |
| 2 | CNP-miR146a | 14 | ND | ND | 8.5–13.7 | ND | [84] |
| 3 | CNP-miR146a | 14 | 2.5 | 4 | 22 | 55% | [57] |
| 4 | CNP-miR146a | 18 | ND | ND | 99.67 ± 3.316 | ND | [82] |
| 5 | CeONPs | 12 | 4.18 | 5.109 | ND | 81,8% | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erokhina, A.G.; Kruglova, M.P.; Stupin, V.A.; Tsaregorodtsev, A.V.; Parfenov, V.A.; Manturova, N.E.; Silina, E.V. The Effectiveness of Cerium Oxide Nanoparticle-Based Drugs in Wound Healing in Animal Models. Molecules 2025, 30, 4536. https://doi.org/10.3390/molecules30234536
Erokhina AG, Kruglova MP, Stupin VA, Tsaregorodtsev AV, Parfenov VA, Manturova NE, Silina EV. The Effectiveness of Cerium Oxide Nanoparticle-Based Drugs in Wound Healing in Animal Models. Molecules. 2025; 30(23):4536. https://doi.org/10.3390/molecules30234536
Chicago/Turabian StyleErokhina, Anna G., Maria P. Kruglova, Victor A. Stupin, Anton V. Tsaregorodtsev, Vladimir A. Parfenov, Natalia E. Manturova, and Ekaterina V. Silina. 2025. "The Effectiveness of Cerium Oxide Nanoparticle-Based Drugs in Wound Healing in Animal Models" Molecules 30, no. 23: 4536. https://doi.org/10.3390/molecules30234536
APA StyleErokhina, A. G., Kruglova, M. P., Stupin, V. A., Tsaregorodtsev, A. V., Parfenov, V. A., Manturova, N. E., & Silina, E. V. (2025). The Effectiveness of Cerium Oxide Nanoparticle-Based Drugs in Wound Healing in Animal Models. Molecules, 30(23), 4536. https://doi.org/10.3390/molecules30234536






