Abstract
A series of 4-phenylurea chalcones (2a–2s) as VEGFR-2 inhibitors has been designed, synthesized, and evaluated for in vitro cytotoxic activity against K562, SiHa, and B16 cancer cells. Compared to sorafenib, the compounds exhibited strong cytotoxicity against K562, SiHa and B16 cells. Compounds 2r, 2o, and 2l exhibited remarkable cytotoxicity against K562, SiHa, and B16 cells, with IC50 values of 0.97 μM, 1.22 μM, and 1.39 μM, respectively. Moreover, compound 2l exhibited potent cytotoxicity against K562, SiHa, and B16, with IC50 values ranging from 1.25 μM to 1.39 μM. Compounds 2l and 2o also exhibited excellent inhibitory activity on VEGFR-2 kinase, with IC50 values of 0.42 ± 0.03 and 0.31 ± 0.02 μM, respectively. Molecular docking proved that the target compounds had strong binding interactions with VEGFR-2 proteins. Flow cytometry analysis showed that compound 2l induced apoptosis and arrested the cell cycle at the G1 and S phases.
1. Introduction
Cancer is a complex disease that affects various organs and systems in the body [1]. It is one of the deadliest diseases that seriously endanger human life [2]. Angiogenesis plays a crucial role in cancer cell reproduction and growth [3,4]. The vascular endothelial growth factor (VEGF) signaling pathway is crucial for regulating cancer angiogenesis [5,6]. Vascular endothelial growth factor receptor (VEGFR) is a member of the receptor tyrosine kinase family, which includes VEGFR-1, VEGFR-2, and VEGFR-3 subtypes [7,8]. VEGFR-2 binds to VEGF to regulate physiological responses in vivo and plays an important role in the proliferation, migration, and survival of cancer cells [9,10,11]. High expression of VEGFR-2 in cancers promotes the formation and growth of blood vessels supplying tumors [12,13]. Therefore, inhibition of VEGFR-2 has emerged as an attractive cancer therapeutic strategy [14,15].
Small molecule VEGFR-2 inhibitors, such as sorafenib and regorafenib, have been approved by the FDA for the treatment of various cancers [16,17,18]. The urea functional group in sorafenib processes both hydrogen bond donors and acceptors, which form hydrogen bonds with key amino acid residues Glu885 and Asp1046 in the DFG (Asp-Phe-Gly) motif [19]. In addition, urea is frequently used in the development of anticancer drugs [20,21,22].
Chalcones belong to the flavonoid family and are commonly found in traditional Chinese medicines, vegetables, fruits, and other plants [23,24]. Chalcones and their derivatives exhibit various biological activities, including anticancer, anti-inflammatory, and antibacterial properties [25,26,27]. The α, β-unsaturated ketone structure of chalcone is considered crucial to its biological activity [28]. Chalcone has been synthesized in high yield from aldehydes and ketones via the Claisen–Schmid condensation reaction [29]. Owing to their various biological activities and simple chemistry, chalcone fragments are commonly used in drug design [30,31]. Chalcone derivatives as VEGFR-2 inhibitors have been reported (see Figure 1), such as compounds I and II; these compounds exhibited inhibition against cancer in vitro [31,32,33].
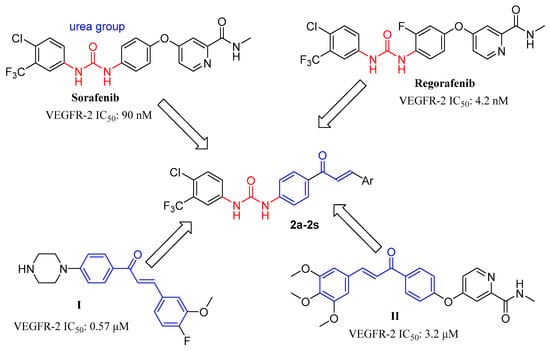
Figure 1.
Structures of sorafenib, regorafenib, chalcone derivatives, and target compounds (2a–2s).
2. Results and Discussion
2.1. Synthesis of Compounds 2a–2s
The synthetic approach for the preparation of compounds 2a–2s is illustrated in Scheme 1. 4-Aminoacetophenone was reacted with 4-chloro-3-(trifluoromethyl)phenyl isocyanate in the presence of triethylamine to form the intermediate 1a. Intermediate 1a was allowed to react with aromatic aldehydes to form chalcone derivatives 2a–2s using potassium hydroxide or thionyl chloride as catalysts [34,35].
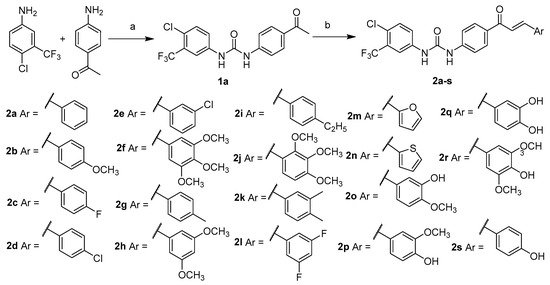
Scheme 1.
Synthesis of compounds (2a–2s). Reaction conditions: (a) triphosgene, Et3N, absolute DCM, N2, 0 °C, 2 h, 69.7%; (b) ArCHO, KOH, MeOH, r.t., overnight, 51.1–81.0%; or ArCHO, SOCl2, absolute EtOH, N2, 0 °C, 2 h, 46.3–69.8%.
2.2. Biological Evaluation
2.2.1. In Vitro Cytotoxic Activities Study
The CCK-8 assay was used to evaluate the cytotoxicity of compounds 2a–2s in human cervical squamous cell carcinoma cell line SiHa, human chronic myeloid leukemia cell line K562, mouse melanoma cell line B16, and human renal epithelial cell line HK-2. Sorafenib was used as the positive control. The cytotoxic activities of the tested compounds are summarized in Table 1. Data analysis revealed that the benzene ring demonstrated strong cytotoxicity, whereas heteroaromatic compounds 2m and 2n exhibited low cytotoxicity. Substituents on the benzene ring exhibited significant impacts on cytotoxicity. The para-substituted compounds 2b, 2c, 2d, 2g, 2i, and 2s increased cytotoxicity against K562 cells, while for B16 cells, cytotoxicity was enhanced, except for chlorine substitution compound 2d. However, only the fluorine-substituted compound 2c increased cytotoxicity against SiHa cells. The multi-substituted compounds significantly enhanced cytotoxicity against K562, B16, and SiHa.

Table 1.
Cytotoxicity of compounds 2a–2s against SiHa, K562, B16, and HK-2 Cells.
As illustrated in Table 1, compound 2c, containing a 4-fluorine substitution, exhibited significant cytotoxicity against K562, B16, and SiHa cells, with IC50 values of 2.05 ± 0.13, 5.02 ± 0.18, and 1.03 ± 0.02 μM, respectively, and represented 6.2-, 2.1-, and 4.3-fold increases in cytotoxicity compared to the unsubstituted compound 2a. Compared to compound 2c, 3,5-difluoro-substituted compound 2l increased cytotoxicity against K562 cells, with an IC50 value of 0.91 ± 0.07 μM, and enhanced inhibitory activity on B16, with an IC50 value of 1.39 ± 0.21 μM. The cytotoxicity of 3,5-dimethoxy-substituted compound 2h and 3,4,5-trimethoxy-substituted compound 2f against K562 and B16 cells was significantly higher than that of 4-methoxy-substituted compound 2b, with IC50 values ranging from 1.62 to 2.02 μM. Introducing hydroxyl (2q) or methoxy groups (2p) into the 4-hydroxy-substituted compound 2s increased its cytotoxicity against K562 cells, with IC50 values decreasing to 1.12 ± 0.10 and 3.42 ± 0.18 μM, respectively, from 6.47 ± 0.36 μM. Swapping the positions of the hydroxyl and methoxy groups in compound 2p significantly increased its cytotoxicity, with IC50 values decreasing from 4.25 μM to 1.22 μM for SiHa cells, from 3.42 μM to 1.51 μM for K562 cells, and from 4.37 μM to 3.06 μM for B16 cells.
The cytotoxicity of synthesized compounds against HK-2 cells was also detected. As shown in Table 1, the target compound exhibited significantly lower cytotoxicity against HK-2 cells compared to SiHa, K562, and B16 cells. Compared to sorafenib, the compounds 2l, 2o, and 2q exhibited low cytotoxicity, with IC50 values of 25.36 ± 1.47, 19.14 ± 1.05, and 24.32 ± 1.71 μM, respectively. In addition, compound 2l also exhibited high selectivity toward SiHa, K562, and B16 cells, and represented 20.3-, 19.5, and 18.2-fold decreases in cytotoxicity, respectively.
2.2.2. VEGFR-2 Enzyme Inhibition Assay Results
Many studies have shown that abnormal VEGFR-2 signaling could induce proliferation of cervical squamous cell carcinoma cells, myeloid leukemia cancer cells, and melanoma cells [36,37,38]. Targeting VEGFR-2 with inhibitor molecules has been considered an effective method for treating cervical cancer, myeloid leukemia, and melanoma [39,40,41,42]. The inhibitory effects of the synthesized compounds on VEGFR-2 were evaluated, with sorafenib used as a reference drug. The results are shown in Table 2. Compounds 2f, 2l, and 2o exhibited excellent inhibitory activity on VEGFR-2 kinase, with IC50 values of 0.39 ± 0.02, 0.42 ± 0.03, and 0.31 ± 0.02 μM, respectively. Compound 2n showed low activity on VEGFR-2 kinase, with an IC50 value of 2.2 ± 0.1 μM. Although the activity of compound 2o on VEGFR-2 kinase was lower than sorafenib, it is higher than reported VEGFR-2 inhibitors I (0.57 μM) and II (3.2 μM) containing chalcone structures [31,32]. These results indicate that the inhibitory activity of VEGR-2 kinase is consistent with its cytotoxicity against cancer cells.

Table 2.
Activity of targeted compounds on VEGFR-2 kinase.
2.2.3. Compound 2l Induced K562 Apoptosis
Enhancing apoptotic cell death is a crucial way for anticancer drugs to eliminate cancer cells [43,44]. Compound 2l exhibited significant antiproliferative activity against K562, SiHa, and B16 cells. Therefore, compound 2l was selected for the apoptosis study. The apoptosis and necrosis patterns of K562 cells were studied after treatment with compound 2l at concentrations of 1.25, 2.5, and 5.0 μM. As shown in Figure 2, early apoptosis increased in a dose-dependent manner, reaching 5.80%, 8.34%, and 57.79% after treatment with 1.25, 2.5, and 5.0 μM of compound 2l, respectively. Late apoptosis increased in a dose-dependent manner. Almost no necrosis of the K562 cells was observed. These results demonstrated that compound 2l contributes to the induction of apoptosis in K562 cells.
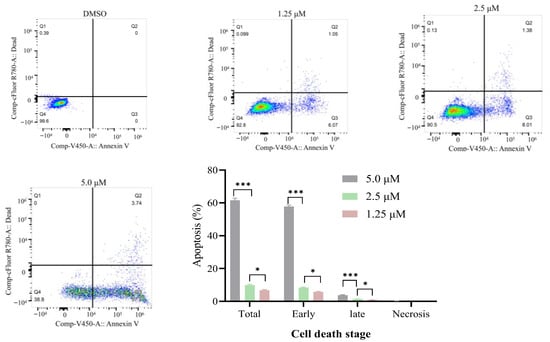
Figure 2.
Compound 2l induces apoptosis in K562 cells. Q1—necrotic cell content; Q2—late apoptotic; Q3—early apoptotic; Q4—viable cells. Values are expressed as an average of three independent replicates: Mean ± SD. * (p ≤ 0.05) and *** (p ≤ 0.001) indicate significant differences.
2.2.4. Compound 2l Induced K562 Cycle Analysis
Cytotoxic compounds often inhibit cell proliferation by arresting the cell cycle at a specific phase [44]. Flow cytometry was used to analyze the effect of compound 2l on the cell cycle of K562 cells at a concentration of 5 μM, and the results are shown in Figure 3. Compared with DMSO treatment, the percentage of K562 cells in the G1 phase was increased from 48.66% to 53.12%, and those in the G2/M phase dropped from 8.00% to 3.05% after treatment with compound 2l. On the other hand, the percentage of K562 cells in the S phase increased from 43.34% to 43.83%. These results showed that the K562 cell cycle was arrested in the G1 phase.
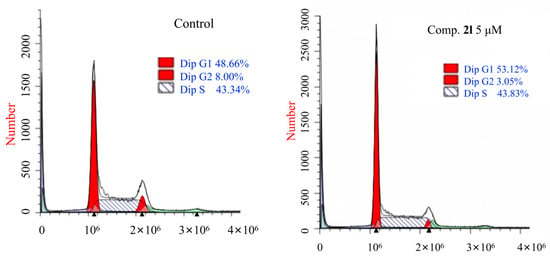
Figure 3.
Effect of compound 2l on the phases of the cell cycles of K562 cells.
2.3. Molecular Docking Results
Molecular docking is an important tool for studying the molecular interactions of ligand-binding sites [45]. MOE (2019) was used to assess the interaction behaviors of compounds 2a–2s with the active site of VEGFR-2 proteins, and sorafenib served as the control drug. Molecular docking was performed using a flexible ligand-docking protocol [46]. The binding free energies (ΔG) and interactions are presented in Table S1 and Figure 4. Sorafenib exhibited a binding affinity of −10.230 kcal/mol. In the sorafenib structure, the two N atoms, an O atom in the urea fragment, and an N atom in the pyridine fragment form hydrogen bonds with the amino acid residues Glu885, Asp1046, and Cys919, respectively. In addition, the amino acid residue Phe1047 forms hydrophobic interactions with the benzene ring. Compounds 2a–2s exhibited binding free energies from −8.983 kcal/mol to −10.178 kcal/mol, and both formed hydrogen bonds with amino acid residues Glu885 and Asp1046. Compounds 2a, 2c, 2g, 2h, 2j, 2l, and 2n–2q formed two hydrogen bonds with the amino acid residue Glu885. In additional, compound 2o formed a hydrogen bond interaction with Cys919, pi-stacking and hydrophobic interactions with Leu1019, and arene-H interactions with Leu840, Val848, and Lys868. Compound 2l formed arene-H interactions with Leu84o and Gly922. Compound 2f exhibited higher binding free energy, but its inhibitory activity on VEGFR-2 kinase was lower than that of compound 2o, which may be due to its lack of hydrogen bonds with amino acid residue Cys919, while 2o formed hydrogen bonds with cys919. Compared to the benzene ring compounds, heteroaromatic compounds 2m and 2n decreased binding free energies and cytotoxicity. The substitution of methoxy groups on the benzene ring, followed by the introduction of additional substituents on the benzene ring, significantly enhanced affinity and cytotoxicity towards VEGFR-2 (2b vs. 2f, 2h, 2o, 2p, and 2r). Compound 2c, with a single fluorine substitution, exhibits lower affinity and cytotoxicity towards VEGFR-2 compared to compound 2l, which has a double fluorine substitution.
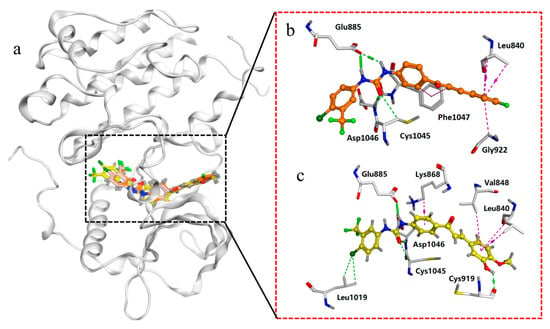
Figure 4.
(a) Binding mode of compounds 2l and 2o with VEGFR-2 (PDB: 4ASD). (b) Display interaction between compound 2l with amino acid residues. (c) Display interaction between compound 2o with amino acid residues. Note: Green dashed line—H-bond; purple dashed line—arene-H interaction.
3. Materials and Methods
3.1. Chemistry
All chemicals were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). 1H NMR and 13C NMR spectra were obtained using a Bruker 600 MHz NMR spectrometer (Karlsruhe, Germany) at the Instrumental Analysis Center of HFUT, Hefei University of Technology, Hefei, China. Dimethyl sulfoxide (DMSO-d6) was used as the solvent, and TMS was used as the internal reference. The melting point was measured using HMZ-2B Micro Processor Melting Point apparatus (HUAZHI Electronic Technology Co., Ltd., Putian, Fujian, China) and was uncorrected. Mass spectra were measured with LC-MS Agilent 6200 ESI-qTOF (Agilent Technologies, Santa Clara, CA, USA). Thin-layer chromatography (TLC) was used to monitor reactions on HSGF254 silica gel plates.
3.1.1. Procedure for Synthesis of Compound 1a
4-aminoacetophenone (1.35 g, 10.0 mmol) and triethylamine (1.21 g, 12 mmol) were added to absolute dichloromethane (DCM, 40 mL) under a nitrogen atmosphere. The reaction mixture was cooled to 0 °C and a dichloromethane solution containing 4-chloro-3-(trifluoromethyl)phenyl isocyanate (2.21 g, 10.0 mmol) was added dropwise to the reaction mixture. The reaction mixture proceeded at this temperature for 2 h, was warmed to 20–25 °C, and was then stirred for an additional 2 h. The precipitate was then filtered and washed with dichloromethane. The cake was dried to produce a 2.49 g off-white solid as the product, with 69.7% yield, and was used in the next reaction without further purification.
3.1.2. General Procedure for Preparation of 2a–2n
Compound 1a (0.178 g, 0.5 mmol), aldehyde (0.5 mmol), and potassium hydroxide (1.5 mmol) were added into methanol (5 mL). The reaction mixture was then stirred overnight at room temperature. Water (3 mL) was then added dropwise to the mixture and stirred for 1 h. The precipitate was filtered and recrystallized in ethanol/water to produce compounds 2a–2n.
3.1.3. General Procedure for Preparation of 2o–2s
Absolute ethanol (10 mL) was cooled to −5 °C under a nitrogen atmosphere, and thionyl chloride (0.5 mL) was added when below 0 °C. Then, compound 1a (0.178 g, 0.5 mmol) and aldehyde (0.55 mmol) were added, and the mixture was stirred at 0 °C for 2 h. The reaction was then quenched with water. The precipitate was filtered and recrystallized in ethanol/water to produce compounds 2o–2s.
3.1.4. Structural Characterization of Synthesized Compounds
1-(4-acetylphenyl)-3-(4-chloro-3-(trifluoromethyl)phenyl)urea (1a): Off-white solid, m.p. 247.2–248.2 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.28 (d, J = 4.6 Hz, 2H), 8.11 (d, J = 2.3 Hz, 1H), 7.91 (d, J = 8.7 Hz, 2H), 7.64 (dt, J = 16.6, 5.6 Hz, 2H), 7.60 (d, J = 8.7 Hz, 2H), 2.52 (s, 3H).
1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-cinnamoylphenyl)urea (2a): Yellow powder, yield 73.2%, m.p. 204.2–205.7 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.32 (s, 1H), 9.30 (s, 1H), 8.15 (d, J = 8.8 Hz, 2H,), 8.12 (d, J = 2.4 Hz, 1H), 7.94 (d, J = 15.6 Hz, 1H), 7.88 (dd, J = 7.3, 1.9 Hz, 2H), 7.72 (d, J = 15.6 Hz, 1H), 7.69–7.61 (m, 4H), 7.49–7.42 (m, 3H); 13C NMR (151MHz, DMSO-d6) δ 187.37, 152.14, 144.02, 143.31, 139.01, 134.83, 132.08, 131.39, 130.47, 130.10, 128.93, 128.82, 126.76 (q, J = 31.1 Hz), 123.38, 122.81 (q, J = 273.2 Hz), 122.75, 121.99, 117.71, 117.04 (q, J = 5.6 Hz). HR-MS (m/z), [M + H]+: 445.0925, found: 445.0931.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(4-methoxyphenyl)acryloyl)phenyl)urea (2b): Yellow powder, yield 81.0%, m.p. 220.6–222.5 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.35 (s, 2H), 8.15–8.12 (m, 3H), 7.84 (d, J = 8.7 Hz, 2H), 7.80 (d, J = 15.5 Hz, 1H), 7.70 (s, 1H), 7.68–7.58 (m, 4H), 7.01 (d, J = 8.7 Hz, 2H), 3.82 (s, 3H); 13C NMR (151MHz, DMSO-d6) 187.24, 161.22, 152.14, 143.81, 143.14, 139.02, 132.03, 131.65, 130.64, 129.89, 127.46, 126.64 (q, J = 30.5 Hz), 123.33, 122.79 (q, J = 273.3 Hz), 122.69, 119.46, 117.66, 117.01 (q, J = 5.7 Hz), 114.38, 51.36. HR-MS (m/z), [M-H]−: 473.0885, found: 473.0870.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(4-fluorophenyl)acryloyl)phenyl)urea (2c): Off-white powder, yield 62.7%, m.p. 211.6–212.6 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.37 (s, 2H), 8.14 (d, J = 8.7 Hz, 2H), 8.12 (d, J = 2.2 Hz, 1H), 7.96 (dd, J = 8.4, 5.8 Hz, 2H), 7.91 (d, J = 15.6 Hz, 1H), 7.72 (d, J = 15.6 Hz, 1H), 7.69–7.60 (m, 4H), 7.30 (t, J = 8.8 Hz, 2H); 13C NMR (151MHz, DMSO-d6) δ 187.27, 163.31 (d, J = 248.7 Hz), 152.14, 144.05, 141.92, 139.01, 133.03, 131.51(J = 3.1Hz), 131.35, 131.11 (d, J = 8.4Hz), 130.06, 126.74 (q, J = 30.5 Hz), 123.35, 122.79 (q, J = 273.3 Hz) 122.72,121.89, 117.67, 117.03 (q, J = 5.7 Hz), 115.90 (d, J = 21.6 Hz). HR-MS (m/z), [M-H]−: 461.0685, found: 461.0690.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(4-chlorophenyl)acryloyl)phenyl)urea (2d): Off-white powder, yield 80.3%, m.p. 230.1–231.5 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.38 (s, 2H), 8.15 (d, J = 8.7 Hz, 2H), 8.11 (d, J = 2.0 Hz, 1H), 7.96 (d, J = 15.6 Hz, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.70 (d, J = 15.8 Hz, 1H), 7.66–7.62 (m, 4H), 7.52 (d, J = 8.4 Hz, 2H); 13C NMR (151MHz, DMSO-d6) δ 187.22, 152.12, 144.12, 141.67, 139.00, 134.89, 133.81, 132.03, 131.25, 130.47, 130.10, 128.92, 126.74 (q, J = 30.5 Hz), 123.69, 123.34, 122.78 (J = 273.2 Hz), 122.74, 117.67, 117.02 (J = 5.7 Hz). HR-MS (m/z), [M + H]+: 479.0535, found, 479.0519.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(3-chlorophenyl)acryloyl)phenyl)urea (2e): Off-white powder, yield 51.1%, m.p. 245.1–246.7 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.33 (s, 1H), 9.31 (s, 1H), 8.17 (d, J = 8.8 Hz, 2H), 8.11 (d, J = 2.5 Hz, 1H), 8.07 (s, 1H), 8.03 (d, J = 15.6 Hz, 1H), 7.82~7.79 (m, 1H), 7.71–7.60 (m, 5H), 7.51–7.45 (m, 2H); 13C NMR (151MHz, DMSO-d6) δ 187.20, 152.10, 144.16, 141.45, 138.96, 137.11, 133.80, 132.05, 131.21, 130.66, 130.21, 129.96, 127.86, 127.81, 126.75 (q, J = 30.8 Hz), 123.53, 123.37, 122.80 (q, J = 273.2 Hz), 122.76, 117.67, 117.05 (q, J = 5.8Hz). HR-MS (m/z), [M − H]−: 477.0389, found: 477.0382.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(3,4,5-trimethoxyphenyl)acryloyl)phenyl)urea (2f): Off-white powder, yield 76.6%, m.p. 228.0–229.8 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.34 (s, 2H), 8.16 (d, J = 8.3 Hz, 2H), 8.11 (d, J = 2.1 Hz, 1H), 7.89 (d, J = 15.5 Hz, 1H), 7.72~7.60 (m, 5H), 7.22 (s, 2H), 3.87 (s, 6H3), 3.72 (s, 3H); 13C NMR (151MHz, DMSO-d6) δ 187.31, 153.10, 152.13, 143.96, 143.69, 139.60, 139.00, 132.05, 131.50, 130.38, 130.03, 126.75 (q, J = 30.6 Hz), 123.36, 122.79 (q, J = 273.6 Hz), 122.73, 121.16,117.65, 117.03 (q, J = 5.6 Hz), 106.44, 60.14, 56.13. HR-MS (m/z), [M − H]−:533.1096, found: 533.1099.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(p-tolyl)acryloyl)phenyl)urea (2g): Off-white powder, yield 69.9%, m.p. 201.8–202.6 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.36 (s, 2H), 8.13 (dd, J = 10.0, 5.6 Hz, 3H), 7.88 (d, J = 15.6 Hz, 1H), 7.77 (d, J = 8.0 Hz, 2H), 7.72–7.59 (m, 5H), 7.27 (d, J = 7.9 Hz, 2H), 2.35 (s, 3H); 13C NMR (151MHz, DMSO-d6) δ 187.32, 152.13, 143.94, 143.23, 140.45, 139.02, 132.10, 132.03, 131.48, 129.98, 129.52, 128.80, 126.74 (q, J = 30.4 Hz), 123.34, 122.79 (q, J = 273.5 Hz), 122.70, 120.91, 117.67, 117.02 (q, J = 5.6 Hz), 21.08. HR-MS (m/z), [M−H]−: 457.0936, found: 457.0940.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(3,5-dimethoxyphenyl)acryloyl)phenyl)urea (2h): Off-white powder, yield 73.3%, m.p. 244.7–246.2 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.37 (s, 2H), 8.16 (d, J = 8.5 Hz, 2H), 8.11 (d, J = 2.0 Hz, 1H), 7.94 (d, J = 15.5 Hz, 1H), 7.72–7.58 (m, 4H), 7.06 (s, 2H), 6.58 (s, 1H), 3.81 (s, 6H). 13C NMR (151MHz, DMSO-d6) δ 187.38, 160.72, 152.15, 144.10, 143.34, 139.02, 136.77, 132.06, 131.34, 130.15, 126.76 (q, J = 30.6 Hz),123.37, 122.81 (q, J = 273.3 Hz), 122.73, 122.46, 117.67, 117.04 (q, J = 5.5 Hz), 106.66, 102.72, 55.47, 55.45. HR-MS (m/z), [M + H]+: 505.1136, found: 505.1132.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(4-ethylphenyl)acryloyl)phenyl)urea (2i): Off-white powder, yield 61.4%, m.p. 242.5–244.2 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.31 (s, 2H), 8.14 (d, J = 8.8 Hz, 2H), 8.12 (d, J = 2.5 Hz, 1H), 7.88 (d, J = 15.6 Hz, 1H), 7.78 (d, J = 8.1 Hz, 2H), 7.70 (d, J = 15.6 Hz, 1H), 7.68–7.60 (m, 4H), 7.29 (d, J = 8.0 Hz, 2H), 2.64 (q, J = 7.6 Hz, 2H), 1.19 (t, J = 7.6 Hz, 3H); 13C NMR (151 MHz, DMSO-d6) δ 187.36, 152.13, 146.71, 143.94, 143.28, 139.00, 132.38, 132.05, 131.51, 130.01, 128.92, 128.36, 126.77 (q, J = 30.6 Hz), 123.36, 122.81 (q, J = 273.3Hz), 122.76, 120.99, 117.70, 117.04 (q, J = 5.5 Hz). HR-MS (m/z), [M + H]+: 473.1238, found: 473.1257.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(2,3,4-trimethoxyphenyl)acryloyl)phenyl)urea (2j): Off-white powder, yield 51.4%, m.p. 213.6–215.3 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.33 (s, 1H), 9.31 (s, 1H), 8.14–8.11 (m, 2H), 8.10 (s, 1H, 7.87–7.83 (m, 2H), 7.77 (d, J = 8.8 Hz, 1H), 7.69~7.61 (m, 4H), 6.92 (d, J = 8.9 Hz, 1H), 3.87 (s, 6H), 3.78 (s, 3H); 13C NMR (151MHz, DMSO-d6) δ 187.37, 155.62, 153.03, 152.13, 143.82, 141.78, 139.01, 137.63, 132.06, 131.67, 129.89, 126.75 (q, J = 30.5 Hz), 123.35, 122.80 (q, J = 273.3 Hz), 122.73, 121.15, 120.39, 117.70, 117.02 (q, J = 5.5 Hz), 108.47, 61.50, 60.47, 56.06. HR-MS (m/z), [M − H]−: 533.1096, found: 533.1099.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(3,4-dimethylphenyl)acryloyl)phenyl)urea (2k): Off-white powder, yield 64.5%, m.p. 253.8–255.6 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.32 (s, 1H), 9.30 (s, 1H), 8.14 (d, J = 8.6 Hz, 2H), 8.12 (d, J = 2.2 Hz, 1H), 7.87 (d, J = 15.5 Hz, 1H), 7.68–7.63 (m, 6H), 7.57 (d, J = 7.8 Hz, 1H), 7.22 (d, J = 7.8 Hz, 1H), 2.27 (s, 3H), 2.26 (s, 3H); 13C NMR (151MHz, DMSO-d6) δ 187.30, 152.12, 143.90, 143.46, 139.36, 138.99, 136.86, 132.41, 132.05, 130.01, 129.98, 129.61, 129.59, 126.74 (q, J = 32.8 Hz), 123.36, 122.80 (q, J = 273.5 Hz), 122.73, 120.67, 117.67, 117.53, 117.03 (q, J = 5.5 Hz), 19.46, 19.29. HR-MS (m/z), [M + H]+: 473.1238, found: 473.1240.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(3,5-difluorophenyl)acryloyl)phenyl)urea (2l): Off-white powder, yield 45.8%, m.p. 267.7–269.4 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.33 (s, 1H), 9.31 (s, 1H), 8.17 (d, J = 8.7 Hz, 2H), 8.11 (d, J = 2.4 Hz, 1H), 8.06 (d, J = 15.6 Hz, 1H), 7.71 (d, J = 6.8 Hz, 2H), 7.69–7.61 (m, 5H), 7.31 (t, J = 9.1 Hz, 1H); 13C NMR (151MHz, DMSO-d6) δ 187.11, 162.65 (dd, J = 245.9, 13.3 Hz), 152.09, 144.28, 140.48, 138.95, 138.69 (t, J = 9.8 Hz), 132.05, 131.06, 130.27, 126.75 (d, J = 30.3 Hz), 124.71, 123.38, 122.79 (q, J = 273.5 Hz), 122.78, 117.67, 117.05 (d, J = 5.8 Hz), 111.68 (dd, J = 20.4, 5.2 Hz), 105.35 (t, J = 26.2 Hz). HR-MS (m/z), [M + H]+: 481.0737, found: 481.0744.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(furan-2-yl)acryloyl)phenyl)urea (2m): Yellow powder, yield 71.3%, m.p. 207.8–209.9 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.36 (s, 2H), 8.19 (s, 1H), 8.11 (d, J = 2.4 Hz, 1H), 8.09 (d, J = 8.7 Hz, 2H), 7.78 (s, 1H), 7.69–7.61 (m, 6H), 7.15 (d, J = 1.2 Hz, 1H); 13C NMR (151MHz, DMSO-d6) δ 187.23, 152.14, 145.56, 144.94, 143.92, 139.01, 132.75, 132.05, 131.36, 129.90, 126.76 (q, J = 30.3 Hz), 123.35, 123.30, 122.80 (q, J = 273.3 Hz),122.74, 121.62, 117.61, 117.02 (q, J = 5.9 Hz), 108.71. HR-MS (m/z), [M − H]−: 433.0572, found: 433.0577.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(thiophen-2-yl)acryloyl)phenyl)urea (2n): Off-white powder, yield 72.1%, m.p. 205.4–206.1 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.32 (s, 1H), 9.31 (s, 1H), 8.12 (dd, J = 5.7, 3.1 Hz, 3H), 8.08 (dd, J = 2.8, 1.0 Hz, 1H), 7.79–7.73 (m, 3H), 7.69–7.62 (m, 5H); 13C NMR (151 MHz, DMSO-d6) δ 187.59,152.13, 143.91, 138.99, 138.35, 137.05, 132.06, 131.48, 130.25, 129.95, 127.64, 126.76 (q, J = 30.5 Hz), 126.17, 123.36, 122.80 (q, J = 273.3 Hz), 122.74, 121.46, 117.68, 117.03 (q, J = 5.6 Hz). HR-MS (m/z), [M − H]−: 449.0343, found: 449.0336.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(3-hydroxy-4-methoxyphenyl)acryloyl)phenyl)urea (2o): Brown powder, yield 66.6%, m.p. 255.5–257.3 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.29 (s, 2H), 9.15 (s, 1H), 8.11 (d, J = 8.6 Hz, 3H), 7.71–7.58 (m, 6H), 7.32 (s, 1H), 7.28 (d, J = 8.4 Hz, 1H), 6.99 (d, J = 8.3 Hz, 1H), 3.84 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ 187.24, 152.13, 150.17, 146.66, 143.75, 143.68, 139.01, 132.06, 131.72, 129.88, 127.77, 126.76 (q, J = 30.5 Hz), 123.35, 122.81 (q, J = 273.3 Hz),122.75, 121.92, 119.33, 117.69, 117.03 (q, J = 5.8 Hz), 114.80, 119.91, 55.69. HR-MS (m/z), [M + H]+: 491.0980, found: 491.0967.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(4-hydroxy-3-methoxyphenyl)acryloyl)phenyl)urea (2p): Brown powder, yield 69.8%, m.p. 251.3–252.9 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.66 (s, 1H), 9.30 (s, 2H), 8.14 (d, J = 8.8 Hz, 2H), 8.12 (d, J = 2.4 Hz, 1H), 7.77 (d, J = 15.4 Hz, 1H), 7.70–7.62 (m, 5H), 7.51 (d, J = 1.7 Hz, 1H), 7.27 (dd, J = 8.2, 1.8 Hz, 1H), 6.84 (d, J = 8.1 Hz, 1H), 3.88 (s, 3H); 13C NMR (151MHz, DMSO-d6) δ 187.22, 152.13, 149.54, 147.98, 144.10, 143.70, 139.01, 132.05, 131.80, 129.87, 126.75 (q, J = 30.8 Hz), 126.41, 123.99, 123.35, 122.80 (q, J = 273.2 Hz), 122.71, 118.56, 117.64, 117.02 (q, J = 5.9 Hz), 115.57, 111.57, 55.82. HR-MS (m/z), [M − H]−: 489.0834, found: 489.0839.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(3,4-dihydroxyphenyl)acryloyl)phenyl)urea (2q): Brown powder, yield: 53.9%, m.p. 219.6–221.3 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.68 (s, 1H), 9.31 (s, 1H), 9.30 (s, 1H), 9.11 (s, 1H), 8.12 (d, J = 2.1 Hz, 1H), 8.09 (d, J = 8.6 Hz, 2H), 7.70–7.53 (m, 6H), 7.25 (d, J = 1.4 Hz, 1H), 7.20–7.15 (m, 1H), 6.81 (d, J = 8.1 Hz, 1H); 13C NMR (151MHz, DMSO-d6) δ 187.21, 152.14, 148.16, 145.59, 144.12, 143.65, 139.02, 132.06, 131.84, 129.79, 126.76 (q, J = 30.7 Hz), 126.42, 123.34, 122.80 (q, J = 270.0 Hz), 122.71, 122.02, 118.31, 117.68, 117.01 (d, J = 5.6 Hz), 115.74, 115.48. HR-MS (m/z), [M + H]+: 477.0823, found: 477.0808.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(4-hydroxy-3,5-dimethoxyphenyl)acryloyl)phenyl)urea (2r): Brown powder, yield 69.3%, m.p. 196.9–198.4 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.34 (s, 1H), 9.32 (s, 1H), 9.02 (s, 1H), 8.15 (d, J = 8.8 Hz, 2H), 8.11 (d, J = 2.5 Hz, 1H), 7.80 (d, J = 15.4 Hz, 1H), 7.69–7.62 (m, 5H), 7.19 (s, 2H), 3.85 (s, 6H); 13C NMR (151 MHz, DMSO-d6) δ 187.21, 152.17, 148.08, 144.51, 143.76, 139.04, 138.58, 132.07, 131.79, 129.93, 126.77 (q, J = 30.5 Hz),125.21, 123.37, 122.82 (q, J = 273.2 Hz), 122.72, 118.93, 117.65, 117.04 (q, J = 5.4 Hz), 106.84, 56.20. HR-MS (m/z), [M − H]−: 519.09403, found: 519.0933.
(E)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-(3-(4-hydroxyphenyl)acryloyl)phenyl)urea (2s): Brown powder, yield 46.3%, m.p. 246.9–248.6 °C. 1H NMR (600 MHz, DMSO-d6) δ 10.06 (s, 1H), 9.31 (s, 1H), 9.30 (s, 1H), 8.13–8.09 (m, 3H), 7.72 (dd, J = 12.0, 3.3 Hz, 3H), 7.70–7.61 (m, 5H), 6.84 (d, J = 8.6 Hz, 2H); 13C NMR (151MHz, DMSO-d6) δ 187.24, 159.97, 152.13, 143.69, 143.66, 139.01, 132.05, 131.79, 130.87, 129.83, 126.76 (q, J = 30.7 Hz), 125.93, 123.34, 122.80 (q, J = 273.3 Hz), 122.72, 118.41, 117.67, 117.02 (q, J = 5.5 Hz), 115.81. HR-MS (m/z), [M − H]−: 459.07283, found: 459.0734.
3.2. In Vitro Cytotoxicity
The SiHa, K562, B16 and HK-2 cells were obtained from Wuhan Pricella Biotechnology Co., Ltd. (Wuhan, China). The in vitro cytotoxicity of compounds 2a–2s against SiHa, K562, B16, and HK-2 cells was detected by CCK-8 assay, following the method detailed in reference [47]. Briefly, cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum and 1% double antibody (penicillin–streptomycin) in a cell culture incubator at 37 °C and 5% CO2. 2–5 × 103 cells were added to each well of a 96-well plate and incubated for 12 h, followed by the addition of the tested compounds. After incubation for 48 h, 10 μL of the CCK-8 solution was added to each well and incubated for another 2 h. The absorbance of each well was measured at 450 nm using an enzyme-linked immunosorbent assay (ELISA) reader. The experiments were replicated three times. The inhibition rate of cancer cells was calculated using the Formula (1). The half-maximal inhibitory concentration (IC50) value was calculated using GraphPad Prism 8.0 software.
ODtest—absorbance of test sample; ODcontrol—absorbance of control.
3.3. VEGFR-2 Enzyme Inhibition Assay
A VEGFR-2 (KDR) Kinase Assay Kit (BPS Biosciences, San Diego, CA, USA) was used to conduct a VEGFR-2 inhibition assay following the manufacturer’s protocol. Briefly, kinase buffer and ATP and PTK substrate (Poly-Glu,Tyr 4:1) were diluted with distilled water to prepare a master mix solution. The master mix solution, test sample, and VEGFR-2 (KDR) protein kinase (2 ng/μL) were added to a 96-well plate and incubated at 30 °C for 45 min. After incubation, Kinase-Glo™ MAX (BPS Biosciences) reagent was added to each well, and incubated for 15 min at room temperature. Then it was immediately detected with an ELISA reader. The experiments were replicated three times.
3.4. Cell Apoptosis Assay
A RayBio Annexin V Apoptosis Detection Kit (RayBright, Violet 450, RayBiotech Co., Ltd., Guangzhou, China) was used to assess the apoptotic effect of compound 2l on K562 cells, following the methodology described in previous studies [48]. Briefly, cells were cultured in a 6-well plate for 12 h, and then compound 2l was added, followed by further culturing for 24 h. The sample was centrifuged to isolate the cells and the kit instructions were followed for subsequent processing. Finally, apoptosis was quantified using a flow cytometer (Cytek Northern Light 3 Laser 16V-14B-8R, Wuxi, China). The experiments were replicated three times.
3.5. Cell Cycle Analysis
The effect of compound 2l on the K562 cell cycle was assessed using propidium iodide (PI) staining on a flow cytometer according to a previously reported method [48]. Briefly, K562 cells were treated with compound 2l and cultured for 24 h. Cells were harvested and fixed with 75% ethanol at 4 °C overnight. The sample was then centrifuged (2000 rpm for 5 min), and the supernatant was aspirated. The fixed cells were resuspended in 0.5 mL of PI solution, cultured at 37 °C in the dark for 30 min, and then cultured at 4 °C in the dark for another 30 min Finally, the cell cycle sample was determined with a flow cytometer (Cytek Northern Light 3 laser 16V-14B-8R, Wuxi, China). The experiments were replicated three times.
3.6. Molecular Docking
Docking studies were conducted on compounds to explore their binding mode with VEGFR-2 proteins (PDB: 4ASD, resolution 2.03 Å) using MOE2019 software [49]. The proteins and compounds underwent energy minimization prior to molecular docking. Molecular docking was performed using a flexible docking approach, with the original ligand serving as the center. The London dG scoring function was employed for protein-binding affinity, with lower scores indicating stronger binding. Sorafenib was used as a control to verify the accuracy of the docking results.
3.7. Statistical Analysis
All the experiments were executed in triplicate, and experimental data were expressed as the mean ± standard deviation (SD), n = 3. The data were analyzed using analysis of variance (ANOVA), followed by suitable post-hoc tests, with p < 0.05 considered statistically significant. GraphPad Prism 8.0 was used to calculate IC50 values and create graphs.
4. Conclusions
A series of novel chalcone derivatives were designed and synthesized as VEGFR-2 inhibitors and their anti-proliferative activities against K562, SiHa, and B16 cells were evaluated. Most compounds, such as 2h, 2l, 2o, and 2r, exhibited strong antiproliferative activity compared with the reference drug sorafenib. Compound 2l exhibited the most potent inhibitory activity against SiHa, K562, and B16 cells, with IC50 values of 1.25 ± 0.13, 1.30 ± 0.07, and 1.39 ± 0.21 μM, respectively. Moreover, the flow cytometer analysis revealed that the compound 2l significantly promoted early and late apoptosis in k562 cells, and arrested the cell cycle of k562 cells at the G1 phase. Inhibition activity testing against VEGFR-2 kinase revealed that compounds 2l and 2o exhibited excellent inhibitory effects, with IC50 values of 0.42 ± 0.03 and 0.31 ± 0.02 μM, respectively. Furthermore, molecular docking studies also demonstrated that compounds 2l, 2o, and 2r formed strong binding interactions with VEGFR-2 kinase. These results indicate that introducing chalcone fragments into the design of VEGFR-2 inhibitors is viable.
5. Patents
Mingjun Yu, Hui Zhu, and Xiaoqian Zhang, et al. Preparation method and application of a chalcone derivative-containing urea structure. 2025102951067, 13 March 2025.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30234526/s1.
Author Contributions
Conceptualization, M.Y.; methodology M.Y.; validation, X.Z. (Xin Zhang), H.Z., and X.Z. (Xiaoqian Zhang); resources, M.Y.; data curation, X.Z. (Xin Zhang); writing—original draft preparation, X.Z. (Xin Zhang); writing—review and editing, M.Y.; funding acquisition, M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Bozhou University Research Initiation Program, grant number BYKQ202417; Bozhou University Enterprise Cooperation Project, grant number BYH2025020; Bozhou University Teaching Reform Program, grant number 2022XJXM057; and Anhui Province University Science and Engineering Teachers’ Internship Program in Enterprises, grant number 2024jsqygz135.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| VEGFR-1 | Vascular endothelial growth factor receptor-1 |
| VEGFR-2 | Vascular endothelial growth factor receptor-2 |
| VEGFR-3 | Vascular endothelial growth factor receptor-3 |
| FDA | Food and Drug Administration |
| DFG | Dynamic form generator |
| Et3N | Triethylamine |
| DCM | Dichloromethane |
| CCK-8 | Cell Counting Kit 8 |
| MOE | Molecular Operating Environment |
| Glu | Glutamic acid |
| Asp | Aspartic acid |
| Cys | Cysteine |
| Phe | Phenylalanine |
| TLC | Thin-layer chromatography |
References
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Tomuleasa, C.; Tigu, A.B.; Munteanu, R.; Moldovan, C.S.; Kegyes, D.; Onaciu, A.; Gulei, D.; Ghiaur, G.; Einsele, H.; Croce, C.M. Therapeutic advances of targeting receptor tyrosine kinases in cancer. Signal Transduct. Target. Ther. 2024, 9, 201. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Wang, Y.; Lin, C.; Zhang, D.; Chen, J.; Ouyang, L.; Wu, F.; Zhang, J.; Chen, L. Recent progress on vascular endothelial growth factor receptor inhibitors with dual targeting capabilities for tumor therapy. J. Hematol. Oncol. 2022, 15, 89. [Google Scholar] [CrossRef]
- Kaufman, N.E.M.; Dhingra, S.; Jois, S.D.; Vicente, M.D.G.H. Molecular Targeting of Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor Receptor (VEGFR). Molecules 2021, 26, 1076. [Google Scholar] [CrossRef]
- Abdulkadir, S.; Li, C.; Jiang, W.; Zhao, X.; Sang, P.; Wei, L.; Hu, Y.; Li, Q.; Cai, J. Modulating Angiogenesis by Proteomimetics of Vascular Endothelial Growth Factor. J. Am. Chem. Soc. 2022, 144, 270–281. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, C.F.; Rao, G.W. Anti-angiogenic Agents: A Review on Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2) Inhibitors. Curr. Med. Chem. 2021, 28, 2540–2564. [Google Scholar] [CrossRef]
- Mabeta, P.; Steenkamp, V. The VEGF/VEGFR Axis Revisited: Implications for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 15585. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.H.; Nam, Y.S.; Bang, J.Y.; Hwang, I.S.; Kim, D.H.; Ki, M.; Lee, H.W. Targeting vascular endothelial growth receptor-2 (VEGFR-2): Structural biology, functional insights, and therapeutic resistance. Arch. Pharm. Res. 2025, 48, 404–425. [Google Scholar] [CrossRef]
- Zha, H.; Li, F.; Cai, L.; Liu, W.; Zhang, M.; Gu, S.; Feng, H.; Xia, Z.; Guo, C.; Wu, X.; et al. Design, synthesis and biological evaluation of indazole derivatives as VEGFR-2 kinase inhibitors with anti-angiogenic properties. Eur. J. Med. Chem. 2024, 279, 116889. [Google Scholar] [CrossRef]
- Shah, A.A.; Kamal, M.A.; Akhtar, S. Tumor Angiogenesis and VEGFR-2: Mechanism, Pathways and Current Biological Therapeutic Interventions. Curr. Drug Metab. 2021, 22, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Ryu, M.H.; Di Bartolomeo, M.; Chau, I.; Yoon, H.; Kim, J.G.; Lee, K.W.; Oh, S.C.; Takashima, A.; Kryzhanivska, A.; et al. Rivoceranib, a VEGFR-2 inhibitor, monotherapy in previously treated patients with advanced or metastatic gastric or gastroesophageal junction cancer (ANGEL study): An international, randomized, placebo-controlled, phase 3 trial. Gastric Cancer 2024, 27, 375–386. [Google Scholar] [CrossRef]
- Marques, C.S.; Brandão, P.; Burke, A.J. Targeting Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2): Latest Insights on Synthetic Strategies. Molecules 2024, 29, 5341. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Zhao, H.C.; Hou, S.J.; Zhang, H.J.; Cheng, L.; Yuan, S.; Zhang, L.R.; Song, J.; Zhang, S.Y.; Chen, S.W. Recent development of multi-target VEGFR-2 inhibitors for the cancer therapy. Bioorg. Chem. 2023, 133, 106425. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.J.; Nemade, A.R.; Shirkhedkar, A.A. Recent updates on potential of VEGFR-2 small-molecule inhibitors as anticancer agents. RSC Adv. 2024, 14, 33384–33417. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Al-Hasani, W.A.; Abdulwahab, H.G. An updated patent review of VEGFR-2 inhibitors (2017-present). Expert Opin. Ther. Patents 2021, 31, 989–1007. [Google Scholar] [CrossRef]
- Modi, S.J.; Kulkarni, V.M. Exploration of structural requirements for the inhibition of VEGFR-2 tyrosine kinase: Binding site analysis of type II, ‘DFG-out’ inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 5712–5727. [Google Scholar] [CrossRef]
- Sroor, F.M.; Othman, A.M.; Tantawy, M.A.; Mahrous, K.F.; El-Naggar, M.E. Synthesis, antimicrobial, anti-cancer and in silico studies of new urea derivatives. Bioorg. Chem. 2021, 112, 104953. [Google Scholar] [CrossRef]
- Listro, R.; Rossino, G.; Piaggi, F.; Sonekan, F.F.; Rossi, D.; Linciano, P.; Collina, S. Urea-based anticancer agents. Exploring 100-years of research with an eye to the future. Front. Chem. 2022, 10, 995351. [Google Scholar] [CrossRef]
- Martín-Beltrán, C.; Gil-Edo, R.; Hernández-Ribelles, G.; Agut, R.; Marí-Mezquita, P.; Carda, M.; Falomir, E. Aryl Urea based scaffolds for multitarget drug discovery in anticancer immunotherapies. Pharmaceuticals 2021, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.M.I.I.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef]
- Zhou, K.; Yang, S.; Li, S.M. Naturally occurring prenylated chalcones from plants: Structural diversity, distribution, activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 2236–2260. [Google Scholar] [CrossRef]
- Guazelli, C.F.S.; Fattori, V.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem. Biol. Interact. 2021, 333, 109315. [Google Scholar] [CrossRef]
- Pan, Q.; Yang, H.; Du, Z.; Ni, Z.; Zhu, Q.; Tu, S.; Zhao, Y.; Ye, F. Synthesis, characterization, and anticancer activity of syringaldehyde-derived chalcones against female cancers. Med. Chem. Res. 2024, 33, 532–547. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological properties of chalcones: A review of preclinical including molecular mechanisms and clinical evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef]
- Elkanzi, N.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of chalcones derivatives and their biological activities: A review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef]
- Alswah, M.; Bayoumi, A.; Elgamal, K.; Elmorsy, A.; Ihmaid, S.; Ahmed, H.E.A. Design, synthesis and cytotoxic evaluation of novel chalcone derivatives bearing triazolo[4,3-a]-quinoxaline moieties as potent anticancer agents with dual EGFR kinase and tubulin polymerization inhibitory effects. Molecules 2017, 23, 48. [Google Scholar] [CrossRef]
- Emam, S.H.; Sonousi, A.; Osman, E.O.; Hwang, D.; Kim, G.D.; Hassan, R.A. Design and synthesis of methoxyphenyl- and coumarin-based chalcone derivatives as anti-inflammatory agents by inhibition of NO production and down-regulation of NF-κB in LPS-induced RAW264.7 macrophage cells. Bioorg. Chem. 2021, 107, 104630. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, S.; Wu, C.; Liu, X.; Tao, H.; Huang, Y.; Liu, Y.; Zheng, P.; Zhu, W. Design, synthesis and activity of novel sorafenib analogues bearing chalcone unit. Bioorg. Med. Chem. Lett. 2016, 26, 5450–5454. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Santali, E.Y.; El-Haggar, R. Novel piperazine–chalcone hybrids and related pyrazoline analogues targeting VEGFR-2 kinase; design, synthesis, molecular docking studies, and anticancer evaluation. J. Enzym. Inhib. Med. Chem. 2020, 36, 308–319. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Rizk, S.A.; Fahim, A.M. Synthesis, reactions and application of chalcones: A systematic review. Org. Biomol. Chem. 2023, 21, 5317–5346. [Google Scholar] [CrossRef]
- Dutta, U.; Das, J.; Goswami, M.J.; Bhardwaj, S.; Verma, A.K.; Kakati, D. Design and synthesis of O-glycoside derivatives with promising antidiabetic and anticancer potential. Med. Chem. Res. 2025, 34, 1025–1039. [Google Scholar] [CrossRef]
- Othman, E.M.; Fayed, E.A.; Husseiny, E.M.; Abulkhair, H.S. The effect of novel synthetic semicarbazone-and thiosemicarbazone-linked 1, 2, 3-triazoles on the apoptotic markers, VEGFR-2, and cell cycle of myeloid leukemia. Bioorg. Chem. 2022, 127, 105968. [Google Scholar] [CrossRef]
- Shirogane, Y.; Usami, Y.; Okumura, M.; Hirose, K.; Naniwa, K.; Ikebe, K.; Toyosawa, S. Anti-VEGFR2 neutralising antibody slows the progression of multistep oral carcinogenesis. J. Pathol. 2024, 4, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Padró, T.; Bieker, R.; Ruiz, S.; Steins, M.; Retzlaff, S.; Bürger, H.; Büchner, T.; Kessler, T.; Herrera, F.; Kienast, J.; et al. Overexpression of vascular endothelial growth factor (VEGF) and its cellular receptor KDR (VEGFR-2) in the bone marrow of patients with acute myeloid leukemia. Leukemia 2002, 7, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Molhoek, K.R.; Erdag, G.; Rasamny, J.K.; Murphy, C.; Deacon, D.; Patterson, J.W.; Slingluff, C.L., Jr.; Brautigan, D.L. VEGFR-2 expression in human melanoma: Revised assessment. Int. J. Cancer 2011, 12, 2807–2815. [Google Scholar] [CrossRef]
- Sano, D.; Fooshee, D.R.; Zhao, M.; Andrews, G.A.; Frederick, M.J.; Galer, C.; Milas, Z.L.; Morrow, P.K.; Myers, J.N. Targeted molecular therapy of head and neck squamous cell carcinoma with the tyrosine kinase inhibitor vandetanib in a mouse model. Head Neck 2011, 3, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Li, G.B.; Ma, S.; Zou, C.; Zhou, S.; Sun, Q.Z.; Chen, C.; Wang, L.J.; Feng, S.; Li, L.L.; et al. Structure-activity relationship studies of pyrazolo[3,4-d]pyrimidine derivatives leading to the discovery of a novel multikinase inhibitor that potently inhibits FLT3 and VEGFR2 and evaluation of its activity against acute myeloid leukemia in vitro and in vivo. J. Med. Chem. 2013, 4, 1641–1655. [Google Scholar] [CrossRef]
- Sabt, A.; Khedr, M.A.; Eldehna, W.M.; Elshamy, A.I.; Abdelhameed, M.F.; Allam, R.M.; Batran, R.Z. New pyrazolylindolin-2-one based coumarin derivatives as anti-melanoma agents: Design, synthesis, dual BRAFV600E/VEGFR-2 inhibition, and computational studies. RSC Adv. 2024, 9, 5907–5925. [Google Scholar] [CrossRef]
- Moradi, M.; Mousavi, A.; Emamgholipour, Z.; Giovannini, J.; Moghimi, S.; Peytam, F.; Honarmand, A.; Bach, S.; Foroumadi, A. Quinazoline-based VEGFR-2 inhibitors as potential anti-angiogenic agents: A contemporary perspective of SAR and molecular docking studies. Eur. J. Med. Chem. 2023, 259, 115626. [Google Scholar] [CrossRef]
- Chaudhry, G.E.; Md Akim, A.; Sung, Y.Y.; Sifzizul, T.M.T. Cancer and apoptosis: The apoptotic activity of plant and marine natural products and their potential as targeted cancer therapeutics. Front. Pharmacol. 2022, 13, 842376. [Google Scholar] [CrossRef]
- Ma, C.; Gurkan-Cavusoglu, E. A comprehensive review of computational cell cycle models in guiding cancer treatment strategies. NPJ Syst. Biol. Appl. 2024, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Lim, J.H.; Jang, J.H.; Lee, J.Y. The use of machine learning modeling, virtual screening, molecular docking, and molecular dynamics simulations to identify potential VEGFR2 kinase inhibitors. Sci. Rep. 2022, 12, 18825. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huang, T.; Wang, Y.; Wang, L.; Feng, S.; Cheng, W.; Yang, L.; Duan, Y. Angiogenesis and anti-leukaemia activity of novel indole derivatives as potent colchicine binding site inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Abouzid, K.A. New fluorinated diarylureas linked to pyrrolo[2,3-d] pyrimidine scaffold as VEGFR-2 inhibitors: Molecular docking and biological evaluation. Bioorg. Chem. 2022, 127, 106006. [Google Scholar] [CrossRef]
- Brestoff, J.R. Full spectrum flow cytometry in the clinical laboratory. Int. J. Lab. Hematol. 2023, 45, 44–49. [Google Scholar] [CrossRef]
- Fouad, M.A.; Osman, A.A.; Abdelhamid, N.M.; Rashad, M.W.; Nabawy, A.Y.; El Kerdawy, A.M. Discovery of dual kinase inhibitors targeting VEGFR2 and FAK: Structure-based pharmacophore modeling, virtual screening, and molecular docking studies. BMC Chem. 2024, 18, 29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).