Hydrogen Bonding in Chloro- and Hydroxy-7-Azaindoles: Insights from X-Ray, Vibrational Spectroscopy, and DFT Studies
Abstract
1. Introduction
2. Results and Discussion
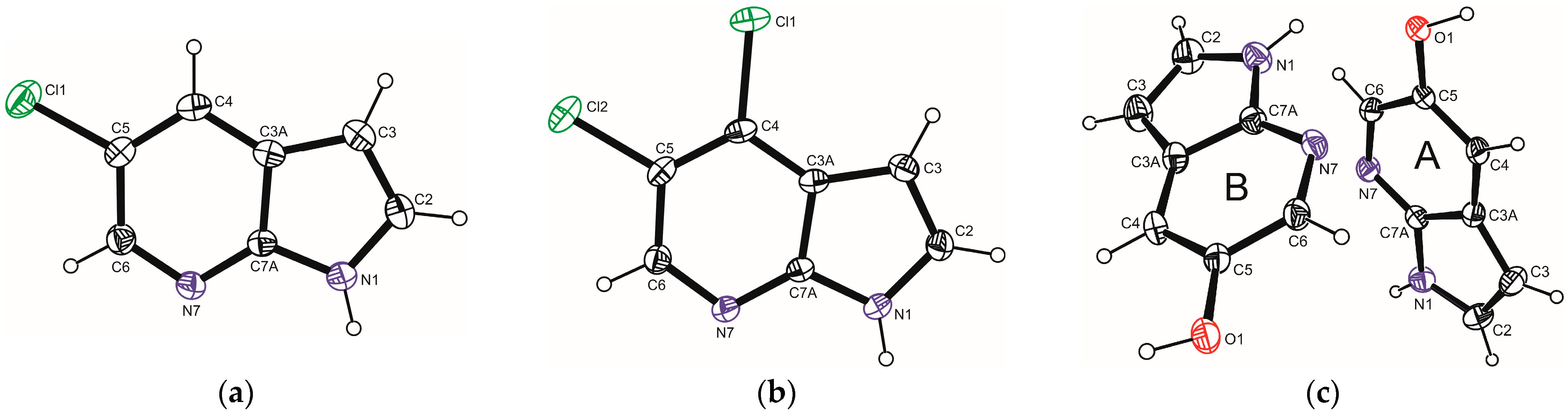
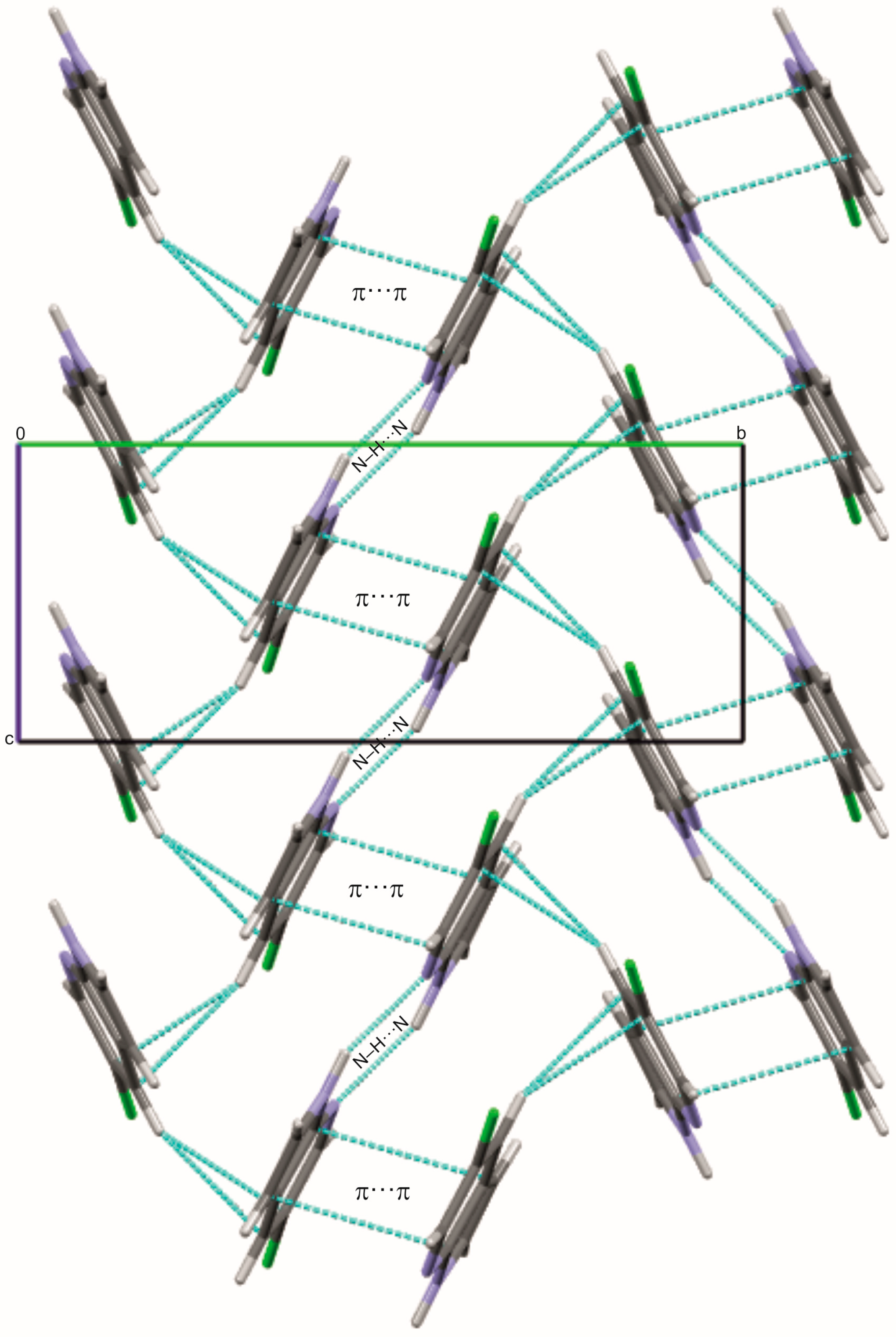
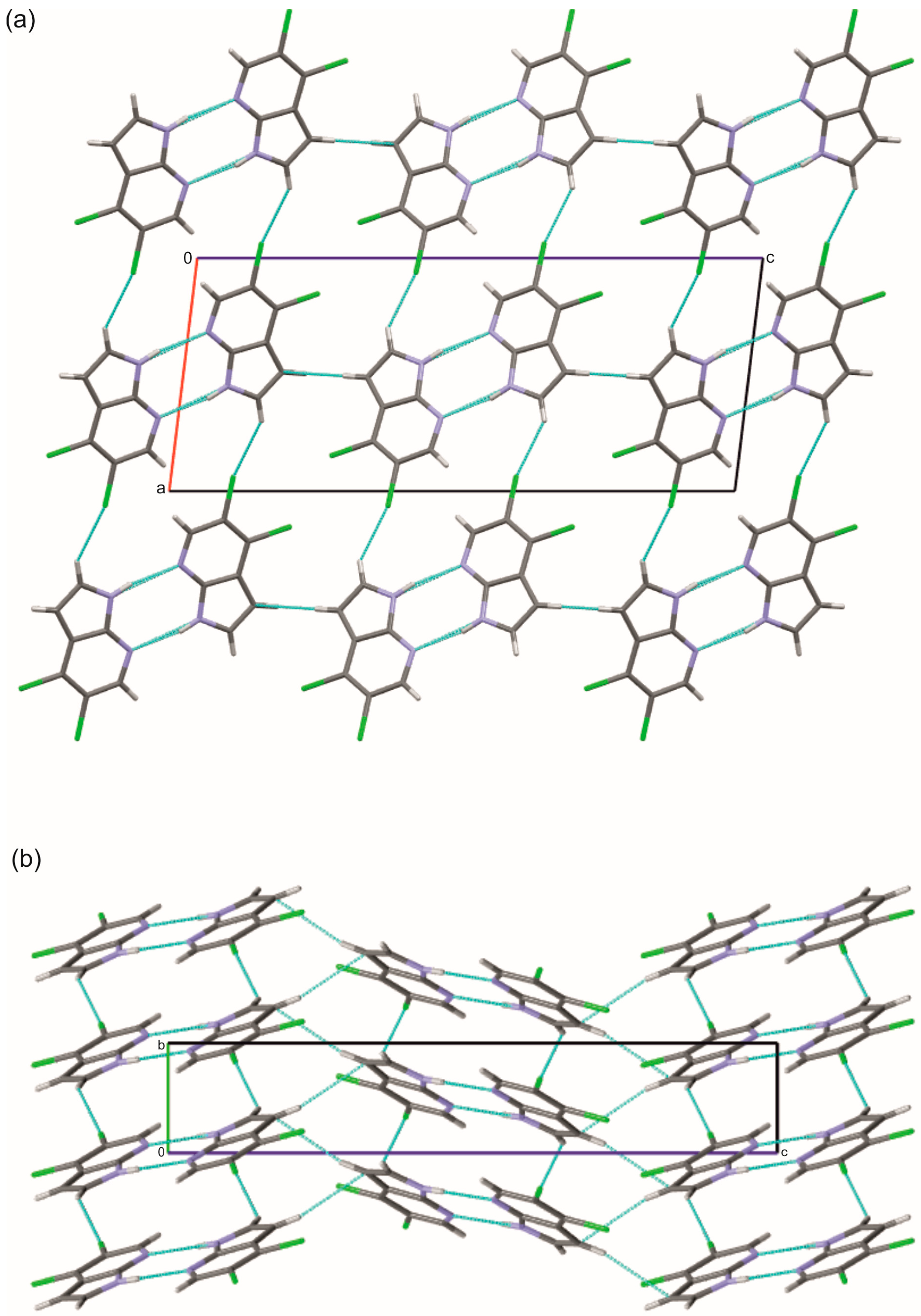
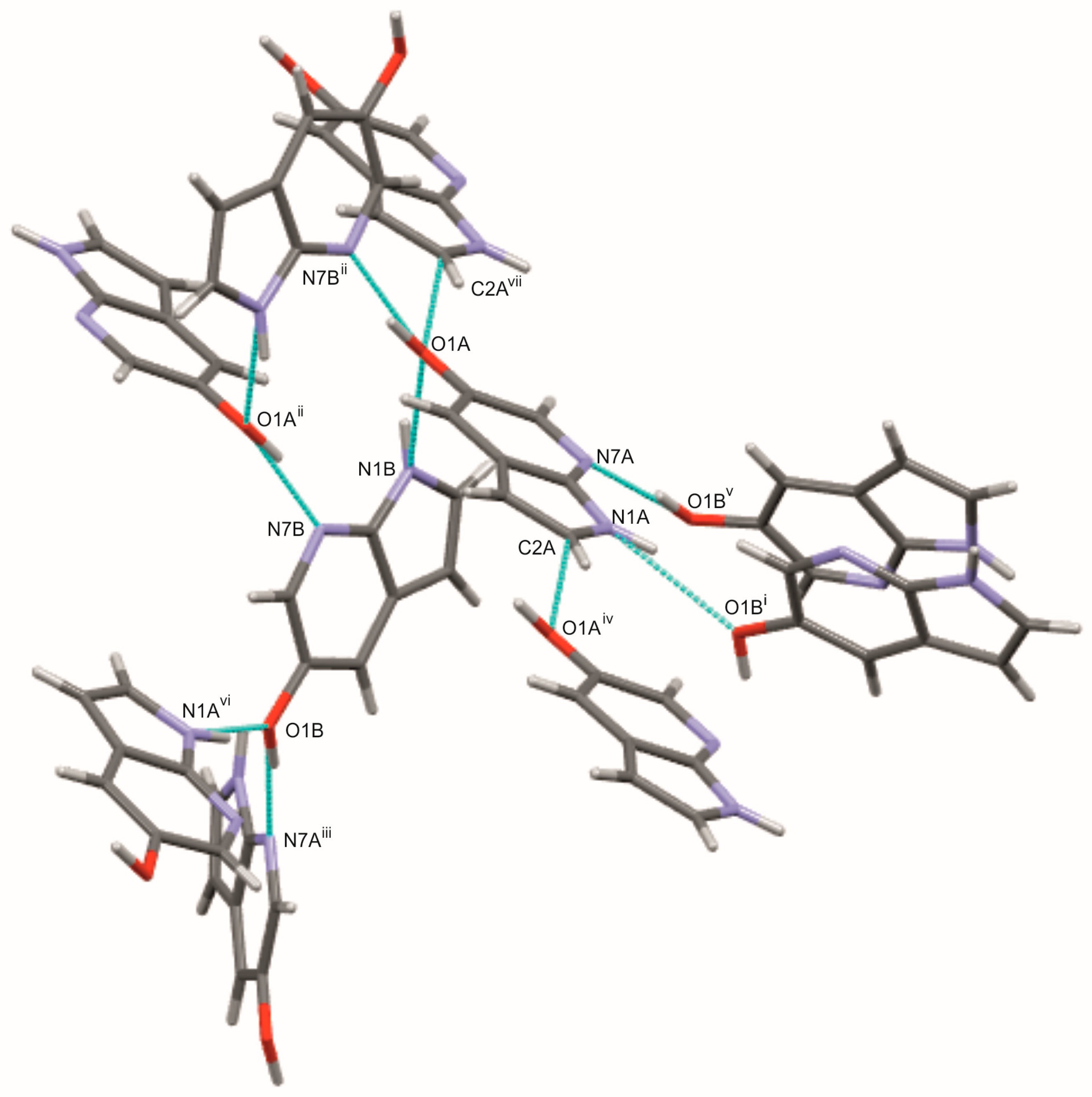
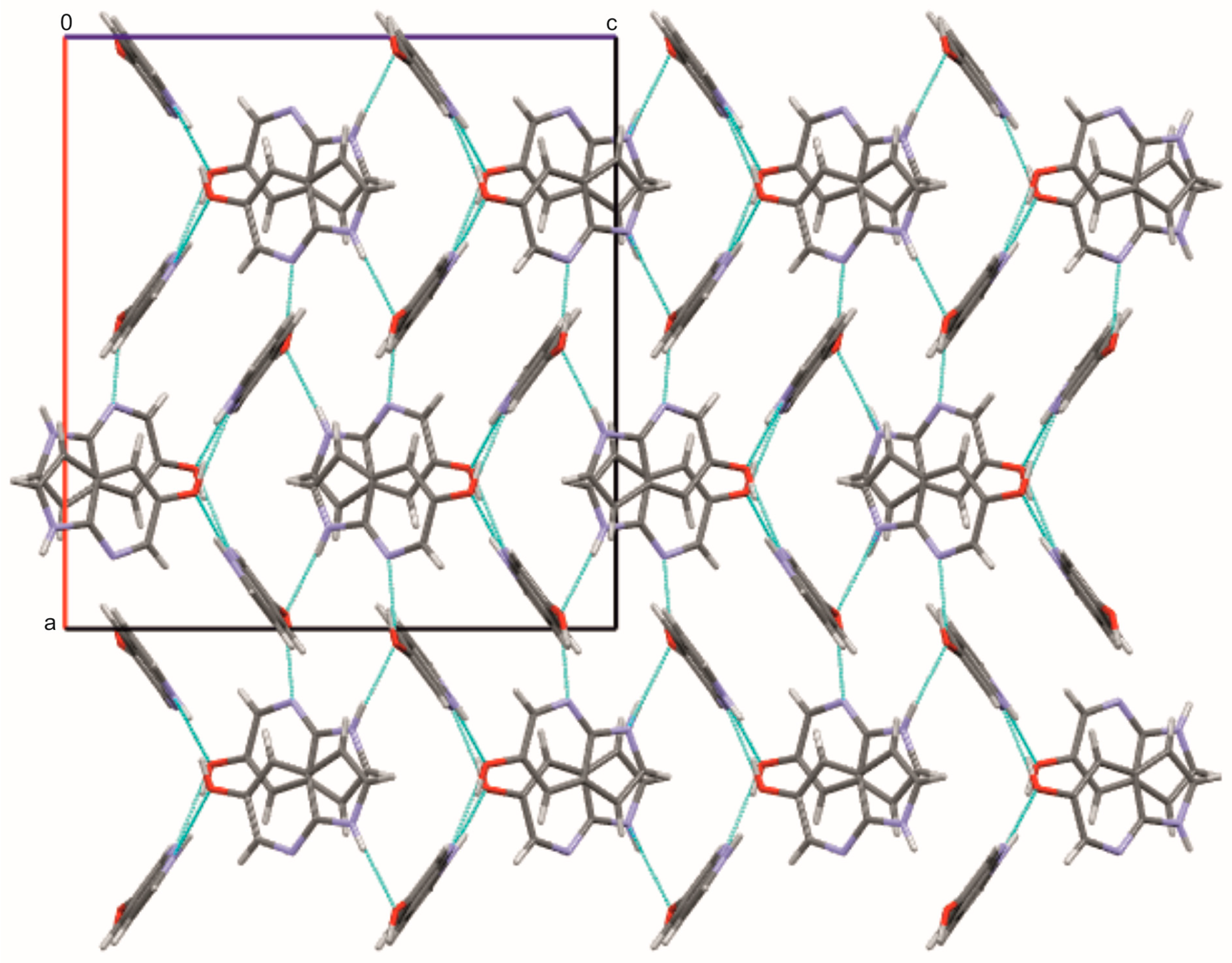
2.1. Crystal and Molecular Structures of 5Cl7AI, 4,5Cl7AI and 5OH7AI
2.2. DFT Calculations of Molecular Geometry and Intermolecular Interactions
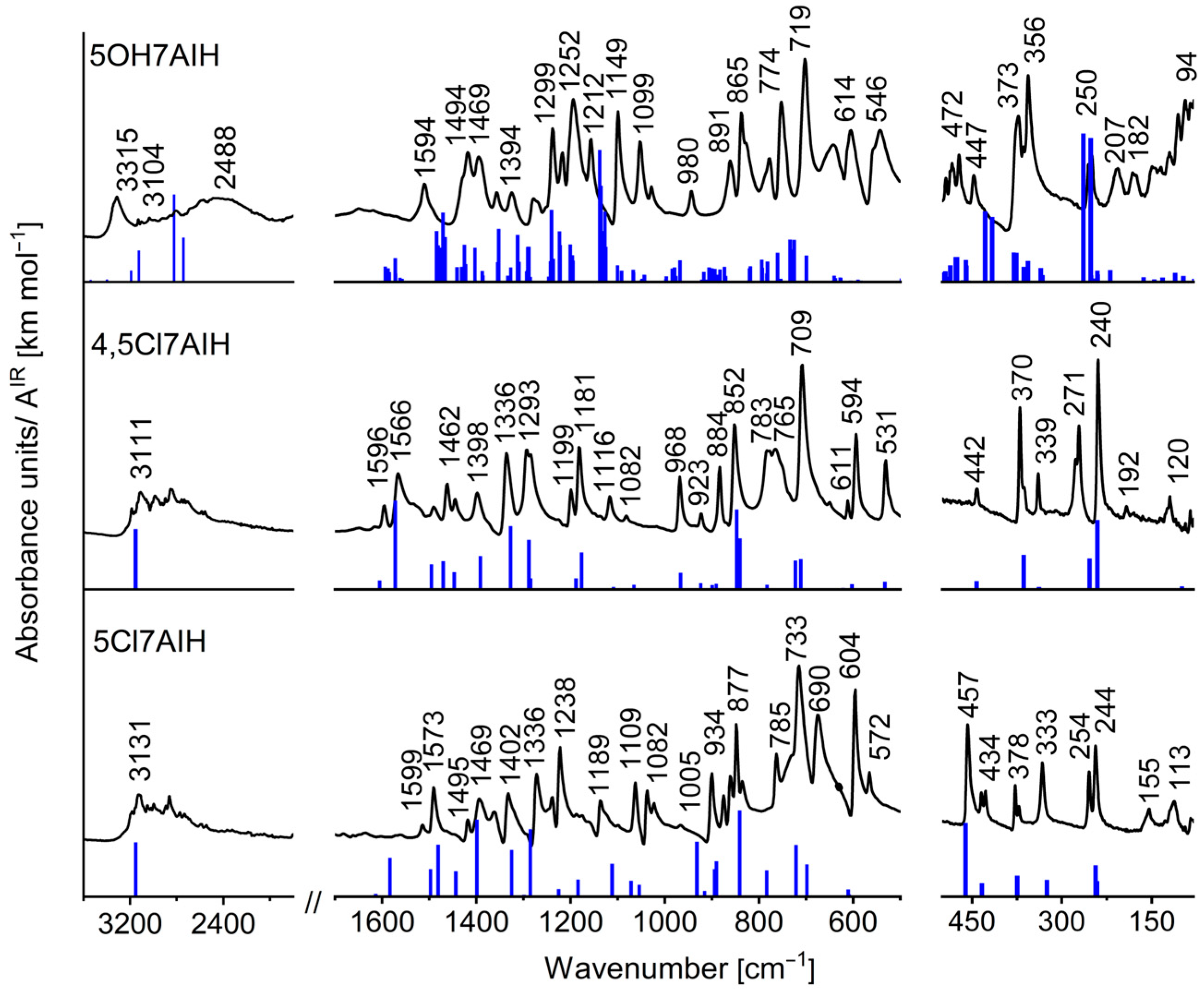
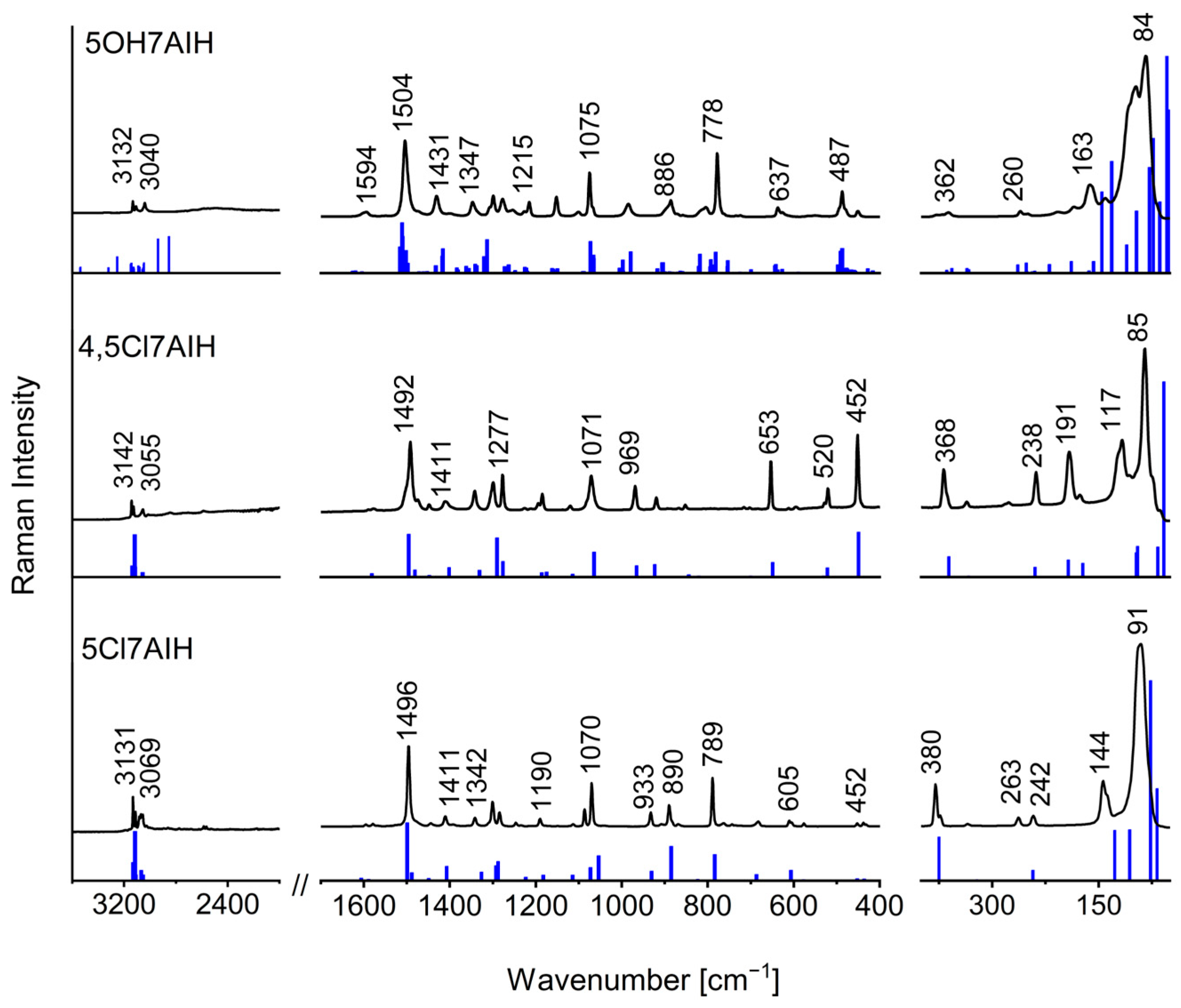
2.3. Influence of Intermolecular Interactions on Vibrational Spectra
3. Materials and Methods
3.1. Preparation of Crystals of 5Cl7AI, 4,5Cl7AI and 5OH7AI
3.2. X-Ray Diffraction Analysis
3.3. Spectroscopic Measurements
3.4. Theoretical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desiraju, G.R. The C−H···O Hydrogen Bond: Structural implications and supramolecular design. Acc. Chem. Res. 1996, 29, 441–449. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Lugger, S.J.D.; Houben, S.J.A.; Foelen, Y.; Debije, M.G.; Schenning, A.P.H.J.; Mulder, D.J. Hydrogen-bonded supramolecular liquid crystal polymers: Smart materials with stimuli-responsive, self-healing, and recyclable properties. Chem. Rev. 2022, 122, 4946–4975. [Google Scholar] [CrossRef]
- Głowacki, E.D.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N.S. Hydrogen-bonds in molecular solids—From biological systems to organic electronics. J. Mater. Chem. B 2013, 1, 3742–3753. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Nian, Q.; Ren, X.; Tao, Z. Hydrogen-bond chemistry in rechargeable batteries. Joule 2023, 7, 2700–2731. [Google Scholar] [CrossRef]
- Derewenda, Z.S. C-H Groups as donors in hydrogen bonds: A historical overview and occurrence in proteins and nucleic acids. Int. J. Mol. Sci. 2023, 24, 13165. [Google Scholar] [CrossRef]
- Ivshin, K.; Metlushka, K.; Zinnatullin, R.; Nikitina, K.; Pashagin, A.; Zakharychev, D.V.; Efimova, A.; Kiiamov, A.; Latypov, S.; Kataeva, O. Competitive Hydrogen Bonding and Unprecedented Polymorphism in Selected Chiral Phosphorylated Thioureas. Cryst. Growth Des. 2021, 21, 5460–5471. [Google Scholar] [CrossRef]
- Wang, K.; Stringfellow, S.; Dong, S.; Jiao, Y.; Yu, H. Synthesis and fluorescence study of 7-azaindole in DNA oligonucleotides replacing a purine base. Spectrochim. Acta Part A 2002, 58, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.T.; Wei, C.Y.; Chang, C.P.; Kuo, M.S. Structure and thermodynamics of 7-azaindole hydrogen-bonded complexes. J. Phys. Chem. 1995, 99, 11994–12000. [Google Scholar] [CrossRef]
- Santos, L.M.; Silveira, N.J.F. Current fragment-to-lead approaches starting from the 7-azaindole: The pharmacological versatility of a privileged molecular fragment. Curr. Top. Med. Chem. 2023, 23, 2116–2130. [Google Scholar] [CrossRef]
- Sharma, N.; Anurag. 7-Azaindole analogues as bioactive agents and recent results. Mini Rev. Med. Chem. 2019, 19, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Urvashi; Kumar, J.B.S.; Das, P.; Tandon, V. Development of azaindole-based frameworks as potential antiviral agents and their future perspectives. J. Med. Chem. 2022, 65, 6454–6495. [Google Scholar] [CrossRef] [PubMed]
- Prudent, R.; Vassal-Stermann, É.; Nguyen, C.H.; Mollaret, M.; Viallet, J.; Desroches-Castan, A.; Martinez, A.; Barette, C.; Pillet, C.; Valdameri, G.; et al. Azaindole derivatives are inhibitors of microtubule dynamics, with anti-cancer and anti-angiogenic activities. Br. J. Pharmacol. 2013, 168, 673–685. [Google Scholar] [CrossRef]
- Mérour, J.Y.; Buron, F.; Plé, K.; Bonnet, P.; Routier, S. The azaindole framework in the design of kinase inhibitors. Molecules 2014, 19, 19935–19979. [Google Scholar] [CrossRef]
- Catalán, J.; Díaz, C.; Pérez, P.; de Paz, J.L.G. Excited state proton transfer in 3-methyl-7-azaindole dimer. Symmetry control. J. Phys. Chem. A 2006, 110, 9116–9122. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhao, J.F.; Li, Y.Q. The investigation of excited state proton transfer mechanism in water-bridged 7-azaindole. Spectrochim. Acta Part A 2016, 153, 147–151. [Google Scholar] [CrossRef]
- Tu, T.H.; Chen, Y.T.; Shen, J.Y.; Lin, T.C.; Chou, P.T. Excited-state proton transfer in 3-cyano-7-azaindole: From aqueous solution to ice. J. Phys. Chem. A 2018, 122, 2479–2484. [Google Scholar] [CrossRef] [PubMed]
- Baweja, S.; Kalal, B.; Mitra, P.K.; Maity, S. Competing excited-state hydrogen and proton-transfer processes in 6-azaindole-S3,4 and 2,6-diazaindole-S3,4 clusters (S = H2O, NH3). Chemphyschem 2023, 24, e202300270. [Google Scholar] [CrossRef]
- Waluk, J. Hydrogen-Bonding-Induced Phenomena in Bifunctional Heteroazaaromatics. J. Acc. Chem. Res. 2003, 36, 832–838. [Google Scholar] [CrossRef]
- Dufour, P.; Dartiguenave, Y.; Dartiguenave, M.; Dufour, N.; Lebuis, A.M.; Belanger-Gariepy, F.; Beauchamp, A.L. Crystal structures of 7-azaindole, an unusual hydrogen-bonded tetramer, and of two of its methylmercury(II) complexes. Can. J. Chem. 1990, 68, 193–201. [Google Scholar] [CrossRef]
- Chou, P.T.; Liao, J.H.; Wei, C.Y.; Yang, C.Y.; Yu, W.S.; Chou, Y.H. Excited-state double proton transfer on 3-iodo-7-azaindole dimer in a single crystal. J. Am. Chem. Soc. 2000, 122, 986–987. [Google Scholar] [CrossRef]
- Hazra, D.K.; Mukherjee, A.K.; Helliwell, M.; Mukherjee, M. Topological features and electronic structure of 4-chloro-1H-pyrrolo [2,3-b]pyridine: Experimental charge density analysis and DFT studies. CrystEngComm 2012, 14, 993–1000. [Google Scholar] [CrossRef]
- Starha, P.; Travnícek, Z. 5-Bromo-1H-pyrrolo[2,3-b]pyridine. Acta Cryst. E 2013, 69, 381. [Google Scholar] [CrossRef] [PubMed]
- Morzyk-Ociepa, B.; Dysz, K.; Turowska-Tyrk, I.; Michalska, D. X-ray crystal structure, vibrational spectra and DFT calculations of 3-chloro-7-azaindole: A case of dual N–H⋯N hydrogen bonds in dimers. Spectrochim. Acta Part A 2015, 136, 405–415. [Google Scholar] [CrossRef]
- Morzyk-Ociepa, B.; Dysz, K.; Turowska-Tyrk, I.; Michalska, D. Reinvestigation of the crystal structure, vibrational spectroscopic studies and DFT calculations of 5-bromo-7-azaindole with dual N–H⋅⋅⋅N hydrogen bonds in dimers. J. Mol. Struct. 2015, 1101, 91–100. [Google Scholar] [CrossRef]
- Morzyk-Ociepa, B.; Dysz, K.; Turowska-Tyrk, I.; Michalska, D. Crystal structures, vibrational spectra and DFT calculations of five halogeno-derivatives of 7-azaindole (3Br7AI, 4Br7AI, 4Cl7AI, 3Br4Cl7AI and 5Br3Cl7AI): A comparative study. J. Mol. Struct. 2018, 1152, 386–398. [Google Scholar] [CrossRef]
- O’Connor, C.J.; Laraia, L.; Spring, D.R. Chemical biology of indoles and azaindoles: Bioisosterism and medicinal potential. Chem. Soc. Rev. 2011, 40, 4332–4345. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Deng, Z.; Chen, L.; Wu, X.; Gao, Y.; Hu, Y.; Li, M.; Wang, H. Hydrogen bonding induced highperformance stretchable organic semiconductors: A review. Front. Chem. 2023, 11, 1200644. [Google Scholar] [CrossRef] [PubMed]
- Forgan, R.S.; Roach, B.D.; Wood, P.A.; White, F.J.; Campbell, J.; Henderson, D.K.; Kamenetzky, E.; McAllister, F.E.; Parsons, S.; Pidcock, E.; et al. Using the outer coordination sphere to tune the strength of metal extractants. Inorg. Chem. 2011, 50, 4515–4522. [Google Scholar] [CrossRef]
- Dittrich, B.; Breznikar, R.; Santarossa, G.; Whitfield, P.; Moebitz, H. Benchmarking quantum chemical methods with X-ray structures via structure-specific restraints. IUCrJ 2025, 12, 472–487. [Google Scholar] [CrossRef]
- Karthikeyan, B. Density functional calculations on the structure, vibrational frequencies and normal modes of 7-Azaindole. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 64, 1083–1087. [Google Scholar] [CrossRef]
- Dreyer, J. Unraveling the structure of hydrogen bond stretching mode infrared absorption bands: An anharmonic density functional theory study on 7-azaindole dimers. J. Chem. Phys. 2007, 127, 54309. [Google Scholar] [CrossRef] [PubMed]
- Beć, K.B.; Grabska, J.; Huck, C.W.; Mazurek, S.; Czarnecki, M.A. Anharmonicity and spectra–structure correlations in MIR and NIR spectra of crystalline menadione (vitamin K3). Molecules 2021, 26, 6779. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysAlis PRO; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2015. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Michalska, D.; Wysokiński, R. The prediction of Raman spectra of platinum(II) anticancer drugs by density functional theory. Chem. Phys. Lett. 2005, 403, 211–217. [Google Scholar] [CrossRef]
- Zapata Trujillo, J.C.; McKemmish, L.K. Model chemistry recommendations for scaled harmonic frequency calculations: A benchmark study. J. Phys. Chem. A 2023, 127, 1715–1735. [Google Scholar] [CrossRef]
- Legler, C.R.; Brown, N.R.; Dunbar, R.A.; Harness, M.D.; Nguyen, K.; Oyewole, O.; Collier, W.B. Scaled quantum mechanical scale factors for vibrational calculations using alternate polarized and augmented basis sets with the B3LYP density functional calculation model. Spectrochim. Acta Part A 2015, 145, 15–24. [Google Scholar] [CrossRef]
- Chemcraft. Graphical Software for Visualization of Quantum Chemistry Computations. Version 1.8, Build 682. Available online: https://www.chemcraftprog.com (accessed on 7 May 2025).
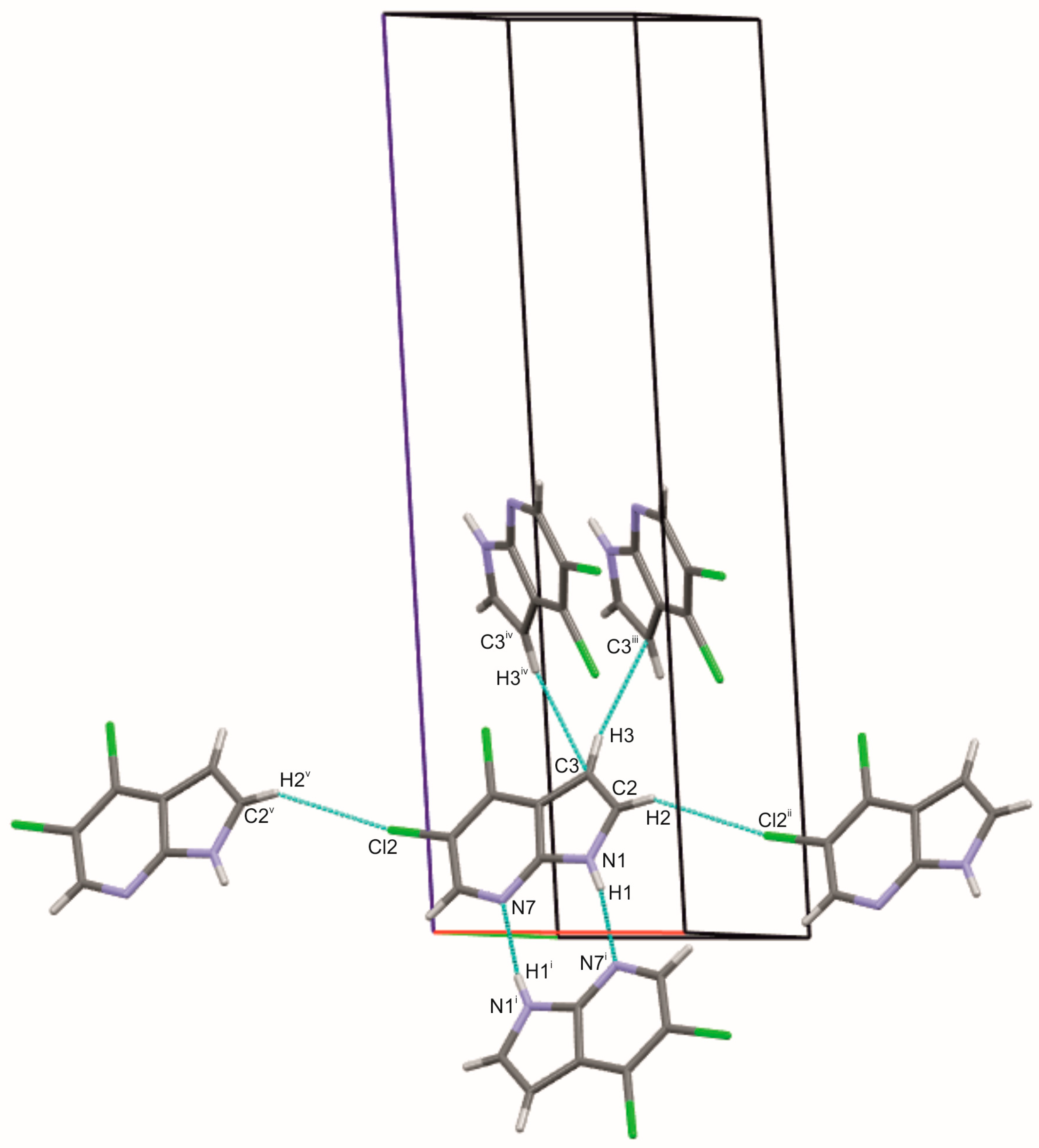
| Compound | H-Bond Type | H···A (Å) | D···A (Å) | D···A (Å) | D–H···A (°) | ν(N–H/O–H) (cm−1) | Network |
|---|---|---|---|---|---|---|---|
| 5Cl7AI | N1–H1···N7 | 0.86 | 2.16 | 2.983 (2) | 160 | 3300–2500 | Dimer |
| 4,5Cl7AI | N1–H1···N7 | 0.86 | 2.09 | 2.939 (2) | 169 | 3300–2500 | Layer |
| 5OH7AI (A) | N1A–H1A···O1B | 0.86 | 2.15 | 2.938 (2) | 152 | 3315 | 3D |
| 5OH7AI (B) | N1B–H1B···O1A | 0.86 | 2.23 | 2.941 (2) | 141 | 3315 | 3D |
| 5OH7AI (A) | O1A–H11A···N7B | 0.93 (2) | 1.73 (2) | 2.643 (2) | 166 | 2800–2000 | 3D |
| 5OH7AI (B) | O1B–H11B···N7A | 0.88 (3) | 1.79 (3) | 2.663 (2) | 172 | 2800–2000 | 3D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dysz, K.; Bąkowicz, J.; Szmigiel-Bakalarz, K.; Rydz, M.; Morzyk-Ociepa, B. Hydrogen Bonding in Chloro- and Hydroxy-7-Azaindoles: Insights from X-Ray, Vibrational Spectroscopy, and DFT Studies. Molecules 2025, 30, 4525. https://doi.org/10.3390/molecules30234525
Dysz K, Bąkowicz J, Szmigiel-Bakalarz K, Rydz M, Morzyk-Ociepa B. Hydrogen Bonding in Chloro- and Hydroxy-7-Azaindoles: Insights from X-Ray, Vibrational Spectroscopy, and DFT Studies. Molecules. 2025; 30(23):4525. https://doi.org/10.3390/molecules30234525
Chicago/Turabian StyleDysz, Karolina, Julia Bąkowicz, Ksenia Szmigiel-Bakalarz, Magdalena Rydz, and Barbara Morzyk-Ociepa. 2025. "Hydrogen Bonding in Chloro- and Hydroxy-7-Azaindoles: Insights from X-Ray, Vibrational Spectroscopy, and DFT Studies" Molecules 30, no. 23: 4525. https://doi.org/10.3390/molecules30234525
APA StyleDysz, K., Bąkowicz, J., Szmigiel-Bakalarz, K., Rydz, M., & Morzyk-Ociepa, B. (2025). Hydrogen Bonding in Chloro- and Hydroxy-7-Azaindoles: Insights from X-Ray, Vibrational Spectroscopy, and DFT Studies. Molecules, 30(23), 4525. https://doi.org/10.3390/molecules30234525








