Comparative Analysis of Growth Traits and Metabolic Profiles in Camassia Cultivars ‘Alba’ and ‘Caerulea’ Under Varying Cultivation Conditions
Abstract
1. Introduction
2. Results
2.1. Morphological and Phenological Traits
2.2. Estimation of Soil Water Balance and Irrigation Regime
2.3. GC-MS Metabolite Profiles
2.4. Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Cultivation Conditions
4.2. Phenological and Biometric Observation
4.3. GC-MS Metabolite Profiling
4.4. Statistical and Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beckwith, B.R. The Queen Root of This Clime: Ethnoecological Investigations of Blue Camas (Camassia leichtlinii (Baker) Wats., C. quamash (Pursh) Greene; Liliaceae) and Its Landscapes on Southern Vancouver Island, British Columbia; University of Victoria: Victoria, BC, Canada, 2004. [Google Scholar]
- Turner, N.J. “That Was Our Candy!”: Sweet Foods in Indigenous Peoples’ Traditional Diets in Northwestern North America. J. Ethnobiol. 2020, 40, 305–327. [Google Scholar] [CrossRef]
- Draghia, L.; Ozarchevici, A.S.; Cantor, M.; Bernardis, R.R.; Chiruță, C.; Apostol, M. The Behavior of Great Camas (Camassia leichtlinii (Baker) S. Watson) in Different Culture Systems. Acta Hortic. 2025, 1417, 63–72. [Google Scholar] [CrossRef]
- Kephart, S. Camas. In The Oregon Encyclopedia; The Oregon Historical Society; Portland State University: Portland, Oregon, 2023. [Google Scholar]
- Lyons, N.; Ritchie, M. The archaeology of camas production and exchange on the Northwest Coast: With evidence from a Sts’ ailes (Chehalis) village on the Harrison River, British Columbia. J. Ethnobiol. 2017, 37, 346–367. [Google Scholar] [CrossRef]
- Matthews, K. Restoration Strategies for Propagation of Camassia quamash on the Weippe Prairie. Master’s Thesis, University of Idaho, Moscow, ID, USA, 2020. [Google Scholar]
- Anderson, M.K. From Tillage to Table: The Indigenous Cultivation of Geophytes for Food in California. J. Ethnobiol. 1997, 17, 149–169. [Google Scholar]
- Ozarchevici, A.S.; Apostol, M.; Chiruță, C.; Draghia, L. Preliminary Results Regarding the Behavior of Some Camassia Species in Iaşi Ecological Conditions (Northeastern Romania). Sci. Pap. Ser. B Hortic. 2023, 67, 468–478. [Google Scholar]
- Saunders, C.F. Useful Wild Plants of the United States and Canada; Robert, M., Ed.; McBride & Co.: New York, NY, USA, 1920. [Google Scholar]
- USDA–Natural Resources Conservation Service—Plants Database: Camassia leichtlinii (Baker) S. Watson. Available online: https://plants.usda.gov/ (accessed on 1 February 2025).
- Mathew, B. Genus Sorbus: Mountain Ash and Other Rowans; Royal Botanic Gardens, Kew: London, OH, USA, 2004. [Google Scholar]
- Proctor, K.Y. Renewing Central Coast Salish Camas (Camassia leichtlinii (Baker) Wats., C. quamash (Pursh) Greene; Liliaceae) Traditions Through Access to Protected Areas: An Ethnoecological Inquiry. Ph.D. Thesis, University of Victoria, Victoria, BC, Canada, 2013. [Google Scholar]
- Douglas, G.W.; Meidinger, D.; Pojar, J. Illustrated Flora of British Columbia: Volume 6—Monocotyledons (Acoraceae Through Najadaceae); Ministry of Environment, Lands and Parks; Ministry of Forests: Victoria, BC, Canada, 2001; Volume 6. [Google Scholar]
- Addai, I.K. Growth and Biochemistry of the Common Hyacinth (Hyacinthus orientalis L.) and the Lily (Lilium longiflorum L.); University of Sussex: East Sussex, England, 2010. [Google Scholar]
- Khodorova, N.V.; Boitel-Conti, M. The Role of Temperature in the Growth and Flowering of Geophytes. Plants 2013, 2, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Iqbal, M.; Vats, S. Cultivation of Some overlooked Bulbous Ornamentals—A review on its commercial viability. Rep. Opin. 2013, 5, 9–34. [Google Scholar]
- Addai, I.K.; Scott, P. Regulation of carbohydrates partitioning and metabolism of the common hyacinth. Agric. Biol. J. N. Am. 2011, 2, 279–297. [Google Scholar]
- Acuña, L.I. The economic contribution of root foods and other geophytes in prehistoric Texas. Master’s Thesis, Texas State University-San Marcos, San Marcos, TX, USA, 2006. [Google Scholar]
- Al-Tardeh, S.; Sawidis, T.; Dianneildis, B.E.; Delivopoulos, S. Water content and reserve allocation patterns within the bulb of the perennial geophyte red squill (Liliaceae) in relation to the Mediterranean climate. Botany 2008, 86, 291–299. [Google Scholar] [CrossRef]
- Klamath-Siskiyou Native Seeds. Growing Camas from Seed. 2020. Available online: https://klamathsiskiyouseeds.com/2020/03/01/growing-camas-from-seed/ (accessed on 20 January 2025).
- Gill, K.M.; Braje, T.J.; Smith, K.; Erlandson, J.M. Earliest Evidence for Geophyte Use in North America: 11,500-Year-Old Archaeobotanical Remains from California’s Santarosae Island. Am. Antiq. 2021, 86, 625–637. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci. Rep. 2016, 6, 30338. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB PLANTS 2018, 10, ply016. [Google Scholar] [CrossRef]
- Meza, S.L.R.; de Castro Tobaruela, E.; Pascoal, G.B.; Magalhães, H.C.R.; Massaretto, I.L.; Purgatto, E. Induction of Metabolic Changes in Amino Acid, Fatty Acid, Tocopherol, and Phytosterol Profiles by Exogenous Methyl Jasmonate Application in Tomato Fruits. Plants 2022, 11, 366. [Google Scholar] [CrossRef]
- Davis, M. Determining the Influence of Nutrition and Temperature on the Growth and Development of Camassia spp. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2018. [Google Scholar]
- Dincheva, I.; Badjakov, I.; Georgiev, V.; Semerdjieva, I.; Vrancheva, R.; Ivanov, I.; Pavlov, A. Comprehensive GC-MS Characterization and Histochemical Assessment of Various Parts of Three Colchicum Species from Bulgarian Flora. Plants 2025, 14, 270. [Google Scholar] [CrossRef]
- de la Fuente, B.; Luz, C.; Puchol, C.; Meca, G.; Barba, F.J. Evaluation of fermentation assisted by Lactobacillus brevis POM, and Lactobacillus plantarum (TR-7, TR-71, TR-14) on antioxidant compounds and organic acids of an orange juice-milk based beverage. Food Chem. 2021, 343, 128414. [Google Scholar] [CrossRef]
- Ferreira, M.I. Stress Coefficients for Soil Water Balance Combined with Water Stress Indicators for Irrigation Scheduling of Woody Crops. Horticulturae 2017, 3, 38. [Google Scholar] [CrossRef]
- Pereira, L.S.; Paredes, P.; Jovanovic, N. Soil water balance models for determining crop water and irrigation requirements and irrigation scheduling focusing on the FAO56 method and the dual Kc approach. Agric. Water Manag. 2020, 241, 106357. [Google Scholar] [CrossRef]
- Torres, A.P.; Lopez, R.G. Photoperiod and Temperature Influence Flowering Responses and Morphology of Tecoma stans. HortScience 2011, 46, 416–419. [Google Scholar] [CrossRef]
- Lee, D.G.; Park, S.; Kim, C.W.; Kim, J.H.; Lee, S.Y. Flowering Responses to Vernalization and Photoperiod in Minuartia laricina (L.) Mattf., a Perennial Herb in the Korean Peninsula. Horticulturae 2025, 11, 188. [Google Scholar] [CrossRef]
- Turner, N.J.; Turner, K.L. “Where our women used to get the food”: Cumulative effects and loss of ethnobotanical knowledge and practice; case study from coastal British Columbia. Botany 2008, 86, 103–115. [Google Scholar] [CrossRef]
- Ranwala, A.P.; Miller, W.B. Analysis of nonstructural carbohydrates in storage organs of 30 ornamental geophytes by high-performance anion-exchange chromatography with pulsed amperometric detection. New Phytol. 2008, 180, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Lazare, S.; Burgos, A.; Brotman, Y.; Zaccai, M. The metabolic (under)groundwork of the lily bulb toward sprouting. Physiol. Plant. 2018, 163, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, T.J.; Tushingham, S. Geophyte field processing, storage, and women’s decision-making in hunter-gatherer societies: An archaeological case study from western North America. J. Anthropol. Archaeol. 2021, 62, 101299. [Google Scholar] [CrossRef]
- Kuhnlein, H.V. Nutrient values in indigenous wild plant greens and roots used by the Nuxalk people of Bella Coola, British Columbia. J. Food Compos. Anal. 1990, 3, 38–46. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Technical advance: Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 2000, 23, 131–142. [Google Scholar] [CrossRef]
- Ivanova, T.; Dincheva, I.; Badjakov, I.; Iantcheva, A. Transcriptional and Metabolic Profiling of Arabidopsis thaliana Transgenic Plants Expressing Histone Acetyltransferase HAC1 upon the Application of Abiotic Stress-Salt and Low Temperature. Metabolites 2023, 13, 994. [Google Scholar] [CrossRef]
- Hummel, J.; Strehmel, N.; Bölling, C.; Schmidt, S.; Walther, D.; Kopka, J. Mass Spectral Search and Analysis Using the Golm Metabolome Database. In The Handbook of Plant Metabolomics; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 321–343. [Google Scholar]
- Taylor, B. The International System of Units (SI), 2008 Edition; Special Publication (NIST SP); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
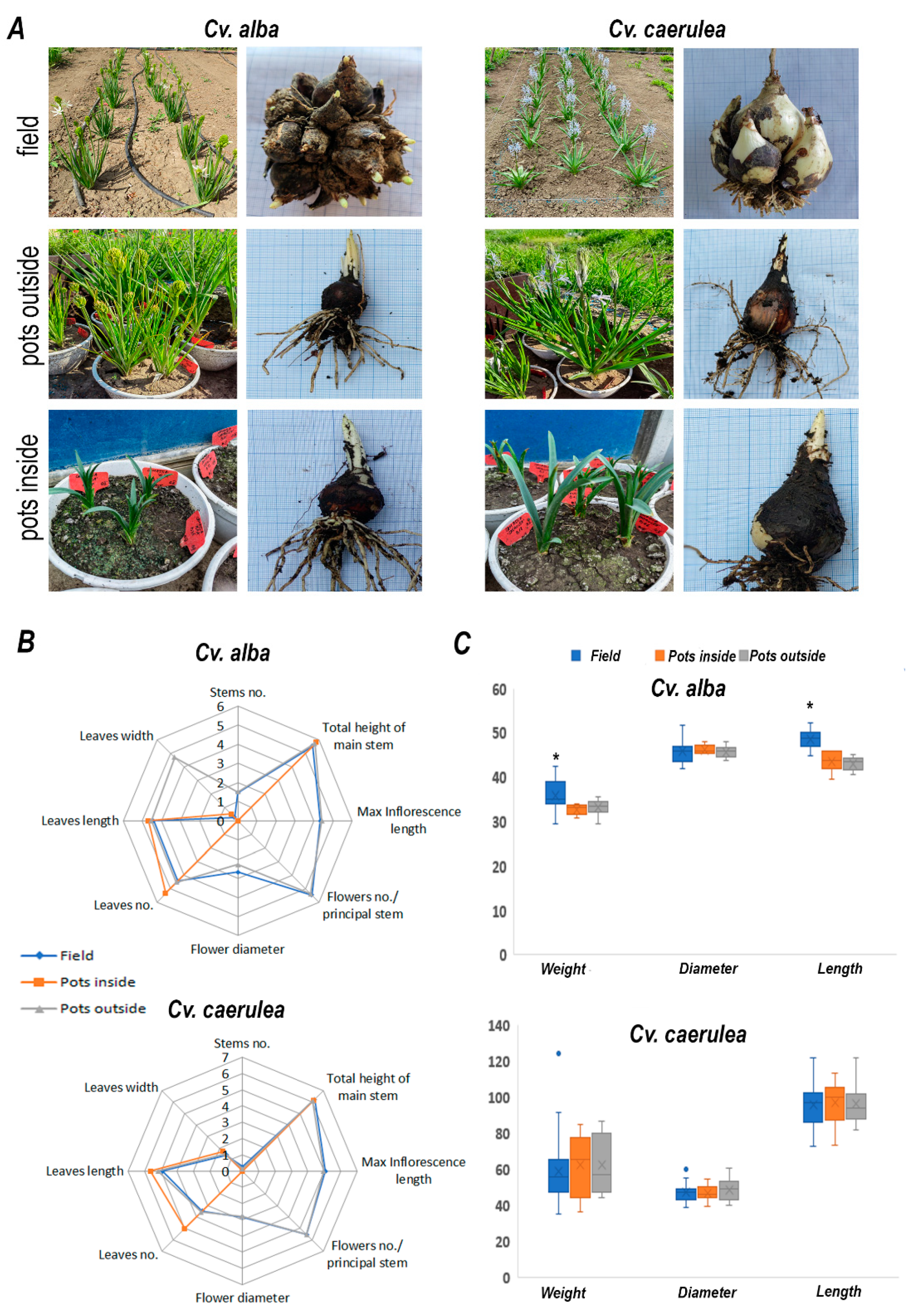


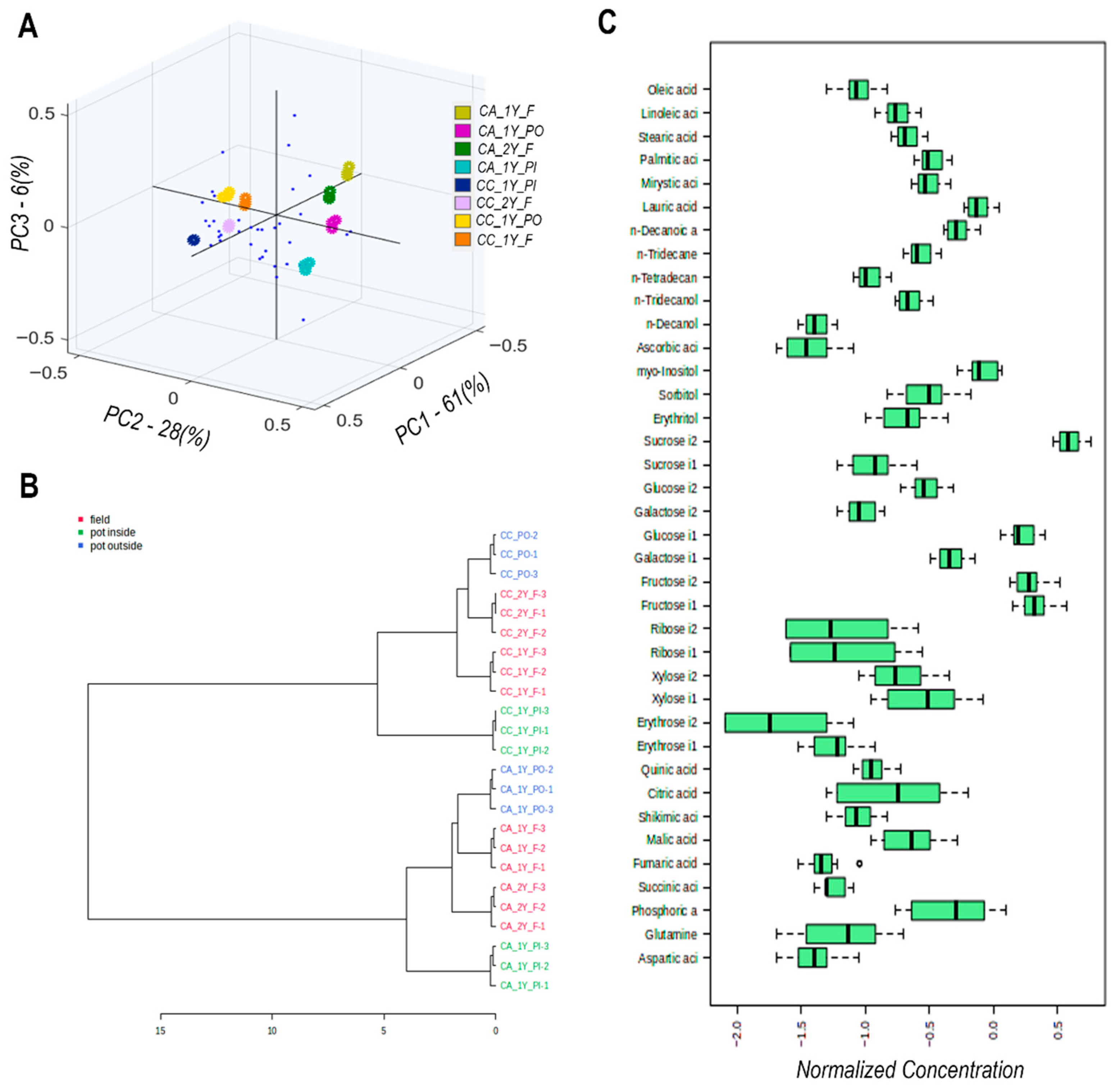
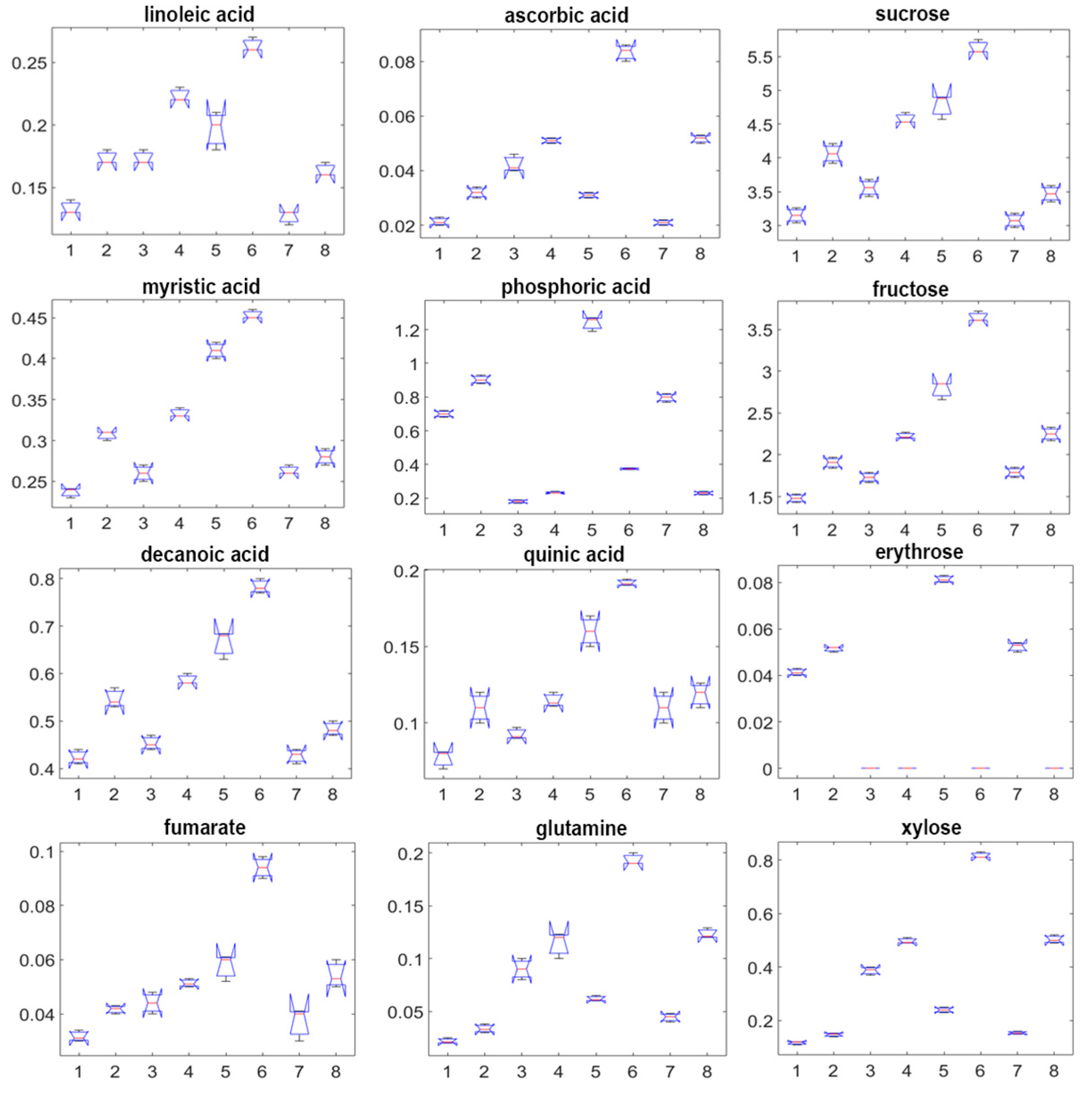
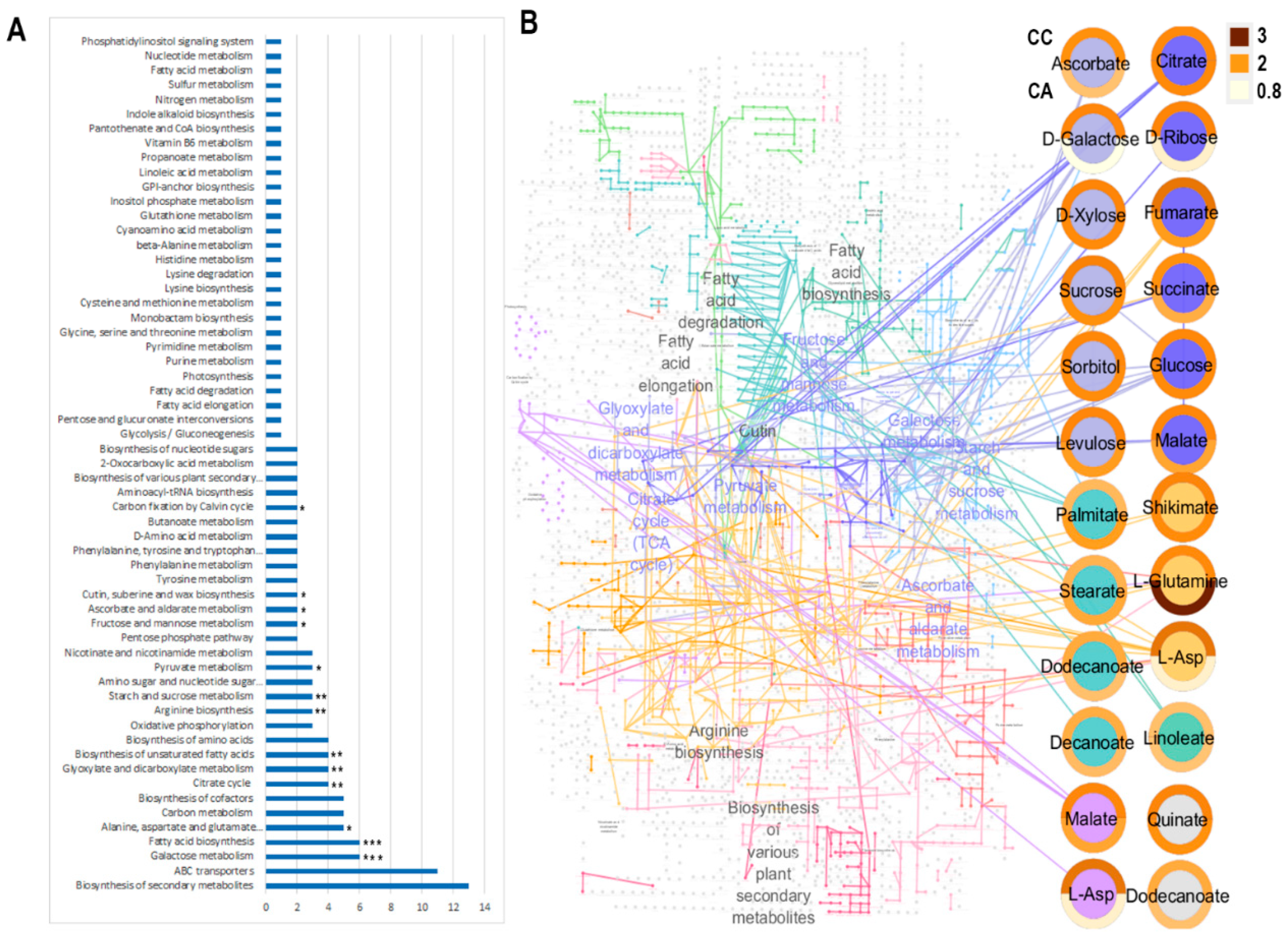
| Cv. | Cult. Sist. | Onset of Vegetative Growth | Emergence of the Floral Stem | Flowering | Entry into Dormancy | |
|---|---|---|---|---|---|---|
| Start | End | |||||
| Cv. ‘Alba’ | field | 3 March 2023 | 1 May 2023 | 26 May 2023 | 5 June 2023 | 18 July 2023 |
| 19 February 2024 | 23 April 2024 | 12 May 2024 | 27 May 2024 | 9 July 2024 | ||
| pots out | 26 March 2023 | 12 May 2023 | 2 June 2023 | 12 June 2023 | 24 July 2023 | |
| pots in | 18 December 2022 | - | - | - | - | |
| Cv. ‘Caerulea’ | field | 7 March 2023 | 17 April 2023 | 3 May 2023 | 18 May 2023 | 20 June 2023 |
| 19 February 2024 | 9 April 2024 | 19 April 2024 | 2 May 2024 | 6 June 2024 | ||
| pots out | 23 March 2023 | 4 May 2023 | 13 May 2023 | 28 May 2023 | 30 June 2023 | |
| pots in | 12 December 2022 | - | - | - | - | |
| Growth/Cultivar/T | SumActT (°C·d) | IAOe_Total | Total P (mm) | Days_Stress | Deficit (mm) | Irrigation (mm) |
|---|---|---|---|---|---|---|
| Field/Alba/T1 | 6.53 ± 9.96 | 18.76 ± 21.6 | 25.24 ± 26.4 | 3.6 ± 6.2 | 8.36 ± 9.3 | 0 ± 0 |
| Field/Alba/T2 | 180.27 ± 170.1 | 185.89 ± 151.6 | 80.9 ± 69.7 | 16.6 ± 14.8 | 196.6 ± 155.4 | 21 ± 1 |
| Field/Caerulea/T1 | 6.53 ± 9.96 | 18.76 ± 21.6 | 25.24 ± 26.4 | 3.6 ± 6.2 | 8.36 ± 9.3 | 0 ± 0 |
| Field/Caerulea/T2 | 180.27 ± 170.1 | 185.89 ± 151.6 | 80.9 ± 69.7 | 16.6 ± 14.8 | 196.6 ± 155.4 | 21 ± 1 |
| Pot/Alba/T1 | 6.53 ± 9.96 | 18.14 ± 20.0 | 25.24 ± 26.4 | 4.4 ± 7.7 | 8.36 ± 9.3 | 0 ± 0 |
| Pot/Alba/T2 | 180.27 ± 170.1 | 159.38 ± 58.5 | 80.9 ± 69.7 | 17.0 ± 14.4 | 196.6 ± 155.4 | 31 ± 1 |
| Pot/Caerulea/T1 | 6.53 ± 9.96 | 18.14 ± 20.0 | 25.24 ± 26.4 | 4.4 ± 7.7 | 8.36 ± 9.3 | 0 ± 0 |
| Pot/Caerulea/T2 | 180.27 ± 170.1 | 159.38 ± 58.5 | 80.9 ± 69.7 | 17.0 ± 14.4 | 196.6 ± 155.4 | 31 ± 1 |
| Parameters | Granulometric Analysis | Micro Elements, Accessible Form (ppm) | |||
|---|---|---|---|---|---|
| pH | 7.77 | Coarse sand 2.0–0.2% | 1.3 | Zn | 8.60 |
| N% | 0.15 | Fine sand 0.2–0.002% | 39.7 | Cu | 13.87 |
| P (ppm) | 268 | Dust 0.02–0.002% | 26.3 | Mn | 31.56 |
| K (ppm) | 230 | Colloidal clay < 0.002% | 32.7 | Fe | 10.49 |
| Hummus% | 3.83 | Physical clay < 0.01% | 44.1 | ||
| Organic matter% | 4.86 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozarchevici, A.-Ş.; Badjakov, I.; Mladenov, P.; Dincheva, I.; Cioroiu, B.-I.; Draghia, L. Comparative Analysis of Growth Traits and Metabolic Profiles in Camassia Cultivars ‘Alba’ and ‘Caerulea’ Under Varying Cultivation Conditions. Molecules 2025, 30, 4520. https://doi.org/10.3390/molecules30234520
Ozarchevici A-Ş, Badjakov I, Mladenov P, Dincheva I, Cioroiu B-I, Draghia L. Comparative Analysis of Growth Traits and Metabolic Profiles in Camassia Cultivars ‘Alba’ and ‘Caerulea’ Under Varying Cultivation Conditions. Molecules. 2025; 30(23):4520. https://doi.org/10.3390/molecules30234520
Chicago/Turabian StyleOzarchevici, Alina-Ştefana, Ilian Badjakov, Petko Mladenov, Ivayla Dincheva, Bogdan-Ionel Cioroiu, and Lucia Draghia. 2025. "Comparative Analysis of Growth Traits and Metabolic Profiles in Camassia Cultivars ‘Alba’ and ‘Caerulea’ Under Varying Cultivation Conditions" Molecules 30, no. 23: 4520. https://doi.org/10.3390/molecules30234520
APA StyleOzarchevici, A.-Ş., Badjakov, I., Mladenov, P., Dincheva, I., Cioroiu, B.-I., & Draghia, L. (2025). Comparative Analysis of Growth Traits and Metabolic Profiles in Camassia Cultivars ‘Alba’ and ‘Caerulea’ Under Varying Cultivation Conditions. Molecules, 30(23), 4520. https://doi.org/10.3390/molecules30234520







