Interfacial Electronic Coupling in Si@SiC@EG Core–Shell Architectures Enables High-Capacity and Long-Life Lithium-Ion Batteries
Abstract
1. Introduction
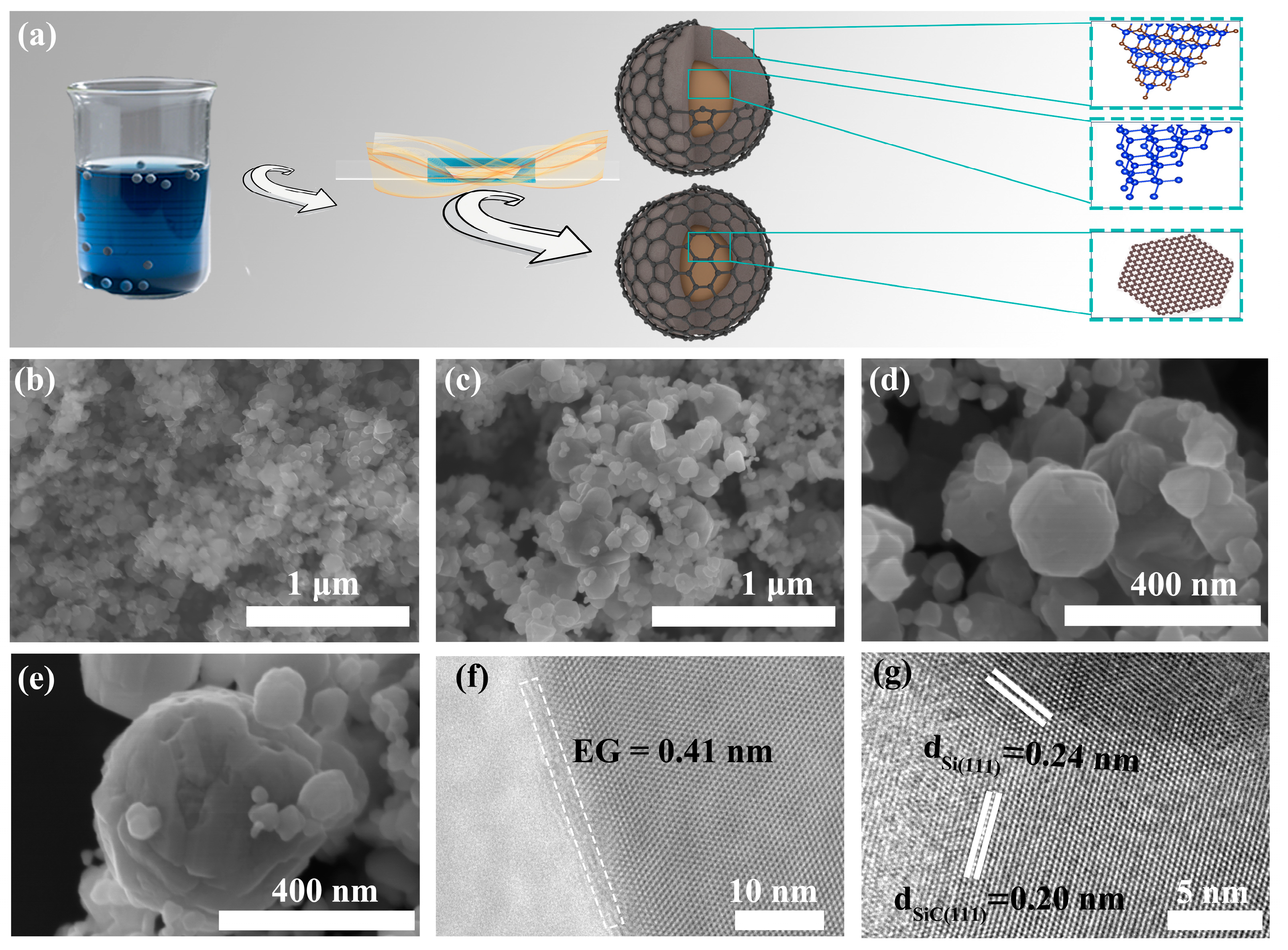
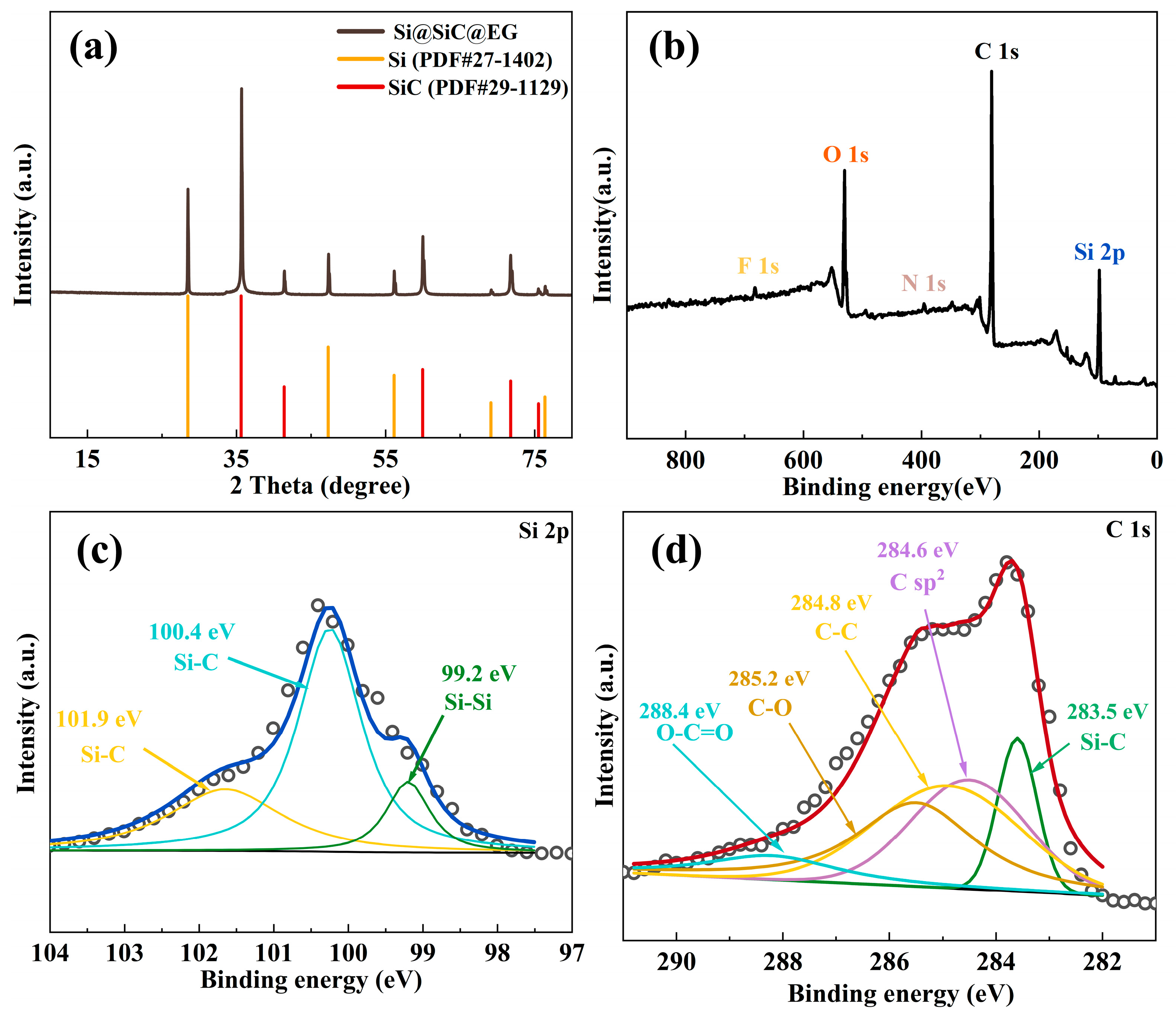
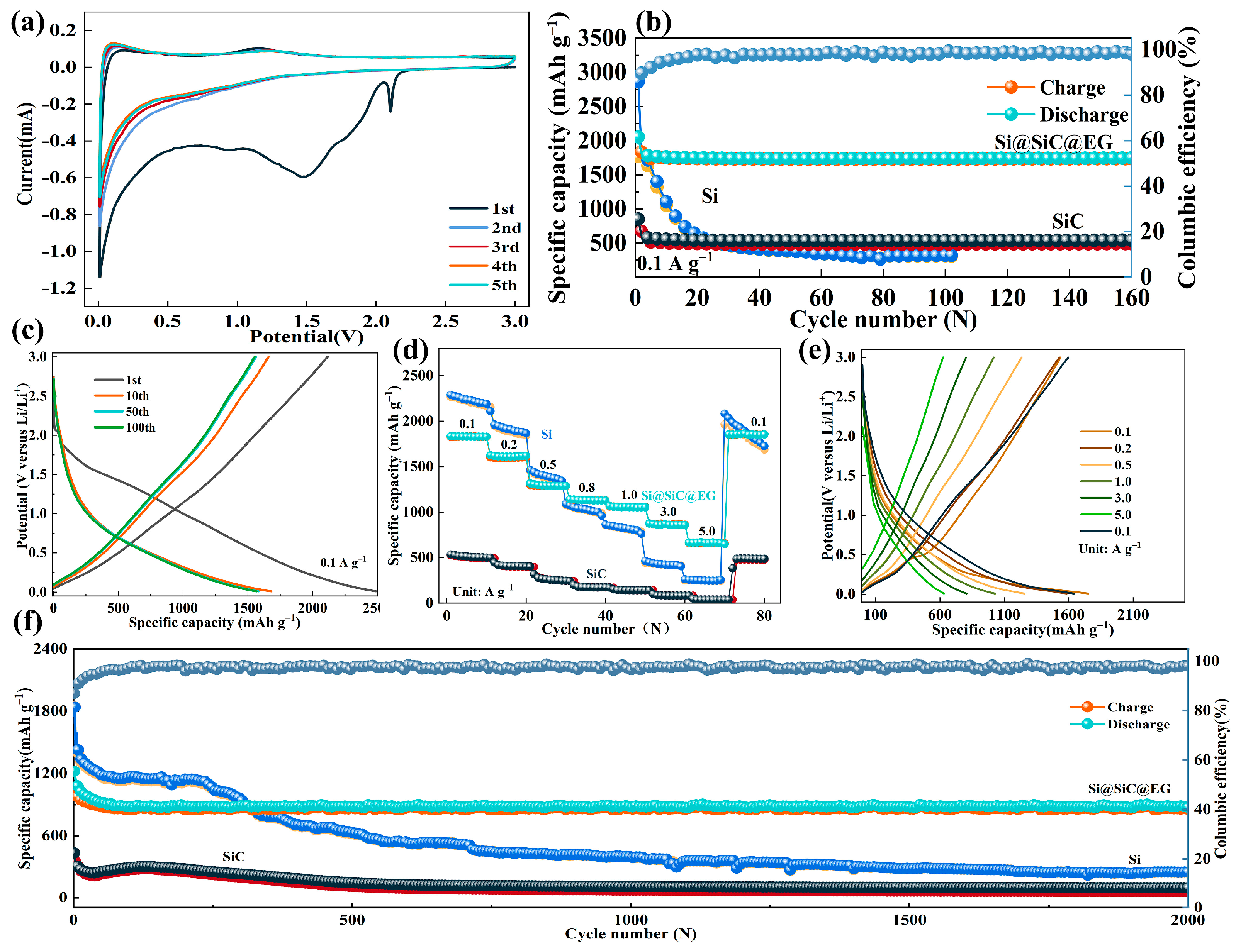
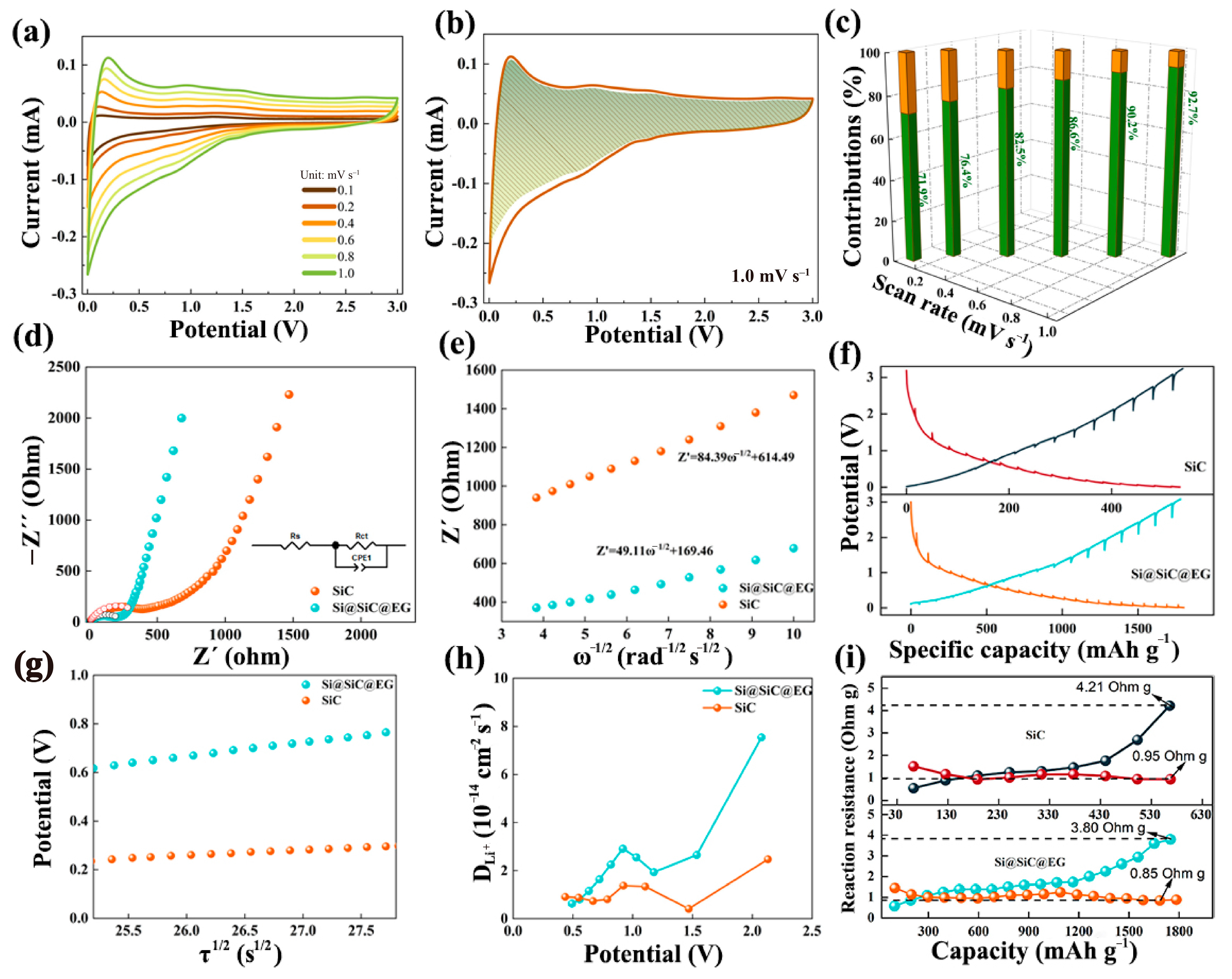
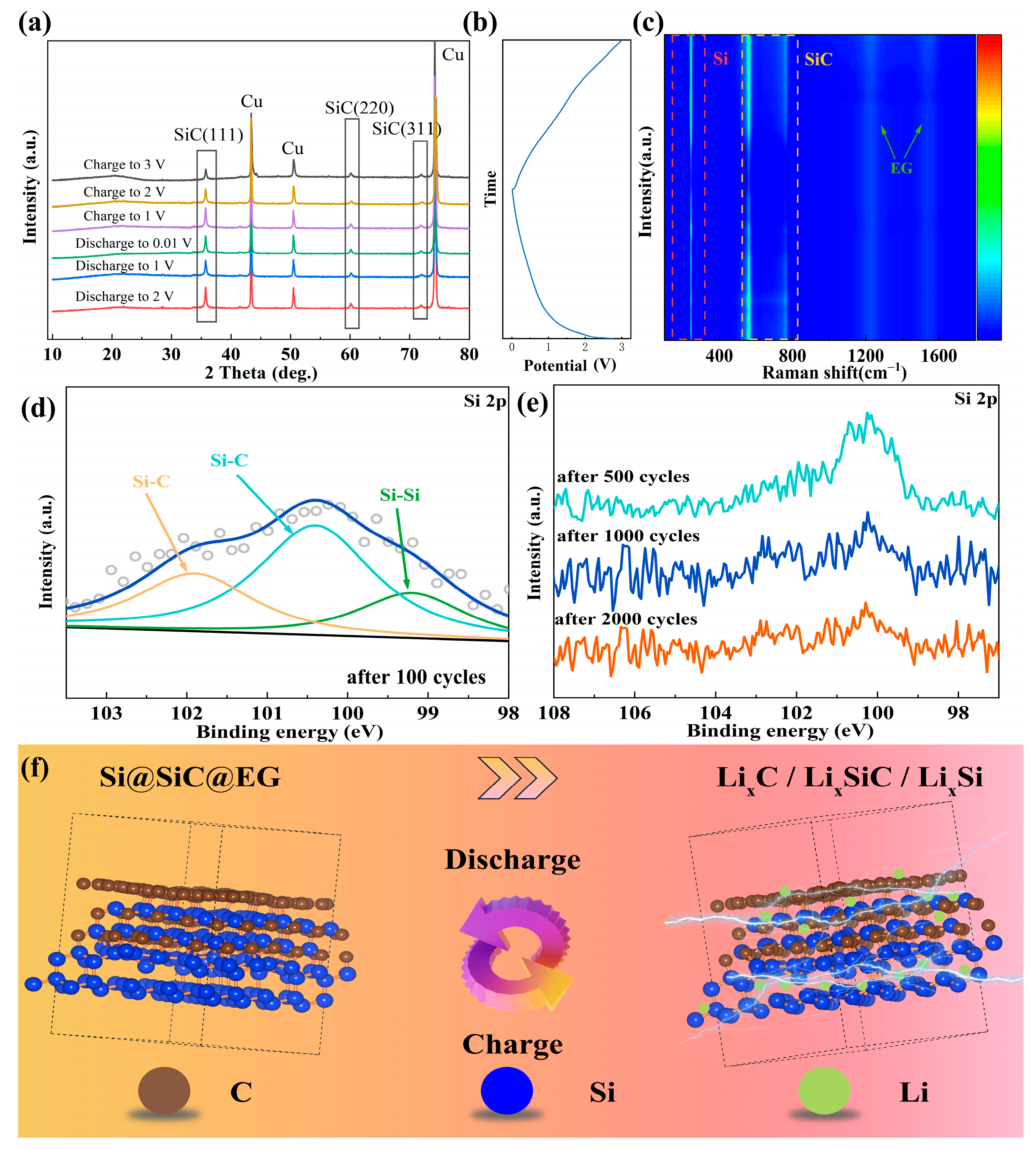
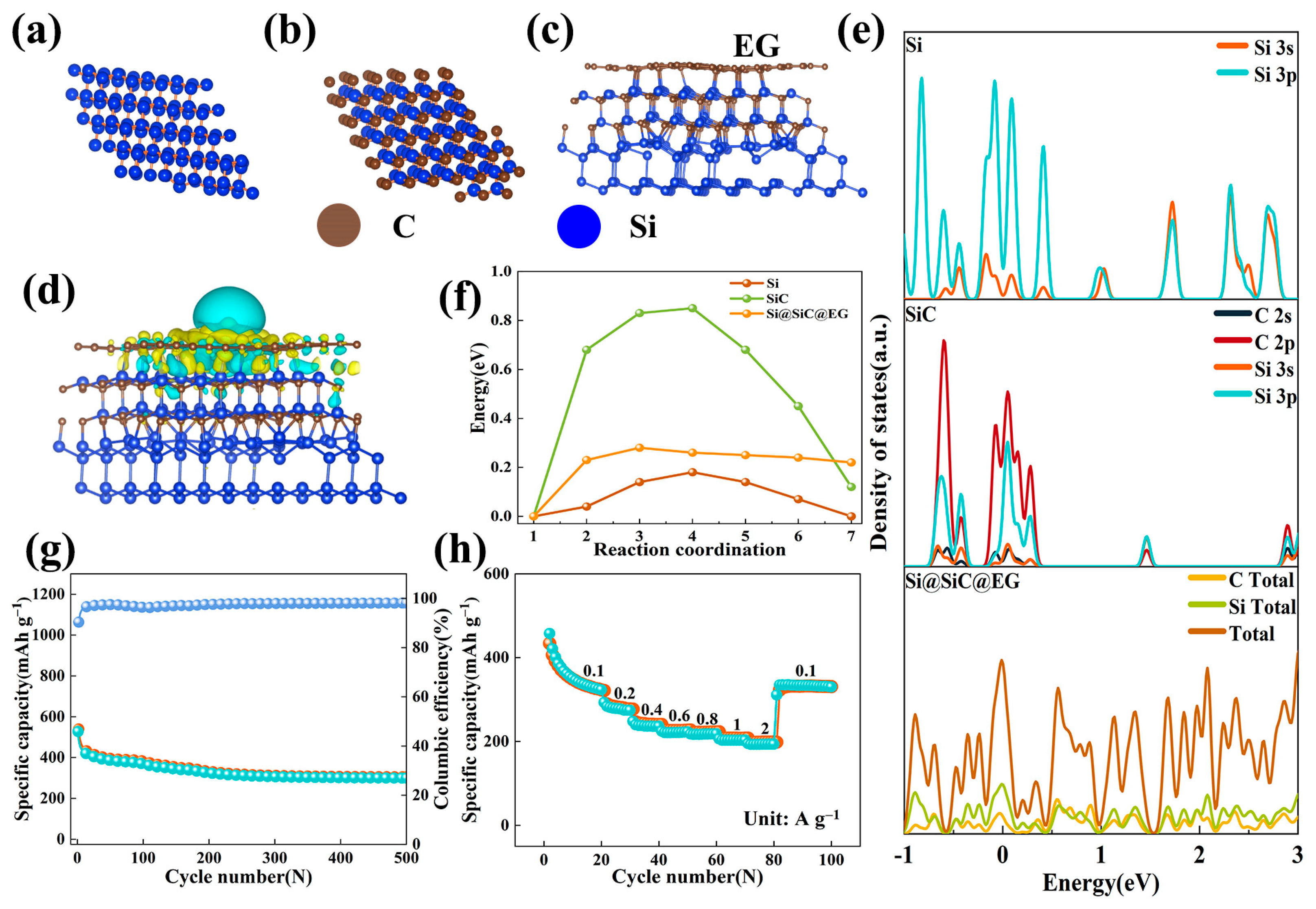
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Yin, J.; Sun, M.; Wang, W.; Chen, C.; Altunkaya, M.; Emis, A.-H.; Han, Y.; Schwingenschlögl, U.; Alshareef, H.N. Direct pyrolysis of supermolecules: An ultrahigh edge-nitrogen doping strategy of carbon anodes for potassium-ion batteries. Adv. Mater. 2020, 32, 2000732. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.; Ming, J.; Li, M.; Xie, L.; He, X.; Wang, J.; Liang, S.; Wu, Y. An exploration of new energy storage system: High energy density, high safety, and fast charging lithium ion battery. Adv. Funct. Mater. 2019, 29, 1805978. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Z.; Chen, Z.; Wan, L.; Shi, K.; Zeng, X.; Li, J.; Liu, Q. Interfacial process engineering of a co-grinding agent for recycling spent lithium-ion batteries. Green Chem. 2023, 25, 9795–9804. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Wang, S.; Ma, J.-X.; Das, P.; Zheng, S.-H.; Wu, Z.-S. Recent status and future perspectives of 2D MXene for micro-supercapacitors and micro-batteries. Energy Storage Mater. 2022, 51, 500–526. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, M.; Yin, J.; Lu, K.; Schwingenschlögl, U.; Qiu, X.; Alshareef, H.N. Accordion-like carbon with high nitrogen doping for fast and stable K ion storage. Adv. Energy Mater. 2021, 11, 2101928. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, M.; Yin, J.; Wang, W.; Huang, G.; Qiu, X.; Schwingenschlögl, U.; Alshareef, H.N. Rational design of carbon anodes by catalytic pyrolysis of graphitic carbon nitride for efficient storage of Na and K mobile ions. Nano Energy 2021, 87, 106184. [Google Scholar] [CrossRef]
- Sun, M.; Yan, Y.; Schwingenschlögl, U. Beryllene: A promising anode material for Na- and K-ion batteries with ultrafast charge/discharge and high specific capacity. J. Phys. Chem. Lett. 2020, 11, 9051–9056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yin, J.; Jian, W.; Wu, Y.; Chen, L.; Sun, M.; Schwingenschlögl, U.; Qiu, X.; Alshareef, H.N. Supermolecule-mediated defect engineering of porous carbons for zinc-ion hybrid capacitors. Nano Energy 2022, 103, 107827. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, M.; Yin, J.; Abou-Hamad, E.; Schwingenschlögl, U.; Costa, P.M.F.J.; Alshareef, H.N. A cyclized polyacrylonitrile anode for alkali metal ion batteries. Angew. Chem. Int. Ed. 2021, 133, 1375–1383. [Google Scholar] [CrossRef]
- Li, Z.; Gao, K.; Han, Y.; Ding, S.; Cui, Y.; Hu, M.; Zhao, J.; Zhang, M.; Meng, A.; Yun, J.; et al. Atomic insights of electronic states engineering of GaN nanowires by Cu cation substitution for highly efficient lithium ion battery. J. Energy Chem. 2022, 67, 46–54. [Google Scholar] [CrossRef]
- Gao, K.; Miao, Z.; Han, Y.; Li, D.; Sun, W.; Zhang, M.; Meng, A.; Sun, C.; Li, Z. One-step method synthesis of cobalt-doped GeZn1.7ON1.8 particle for enhanced lithium-ion storage performance. Electrochim. Acta 2023, 442, 141876. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, L.; Mi, N.; He, J.; Xu, J. Silicon@carbon composite with bioinspired root-nodule nanostructures as anode for high-performance lithium-ion batteries. Molecules 2025, 30, 4157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, M.; Du, Q.; Zhai, G.; He, H. Simple and safe synthesis of yolk-shell-structured silicon/carbon composites with enhanced electrochemical properties. Molecules 2024, 29, 1301. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-P.; Fan, Y.-B.; Zhu, J.; Li, S.; Kuang, M.; Wang, Y.-Q. Research progress and standardization construction investigation of silicon carbon anode materials for lithium-ion batteries. Adv. Ceram. 2024, 45, 408–416. [Google Scholar]
- Huang, L.-P.; Zhu, J.-T.; Liu, J.-X.; Wu, H.-Z.; Zhang, G.-J. Emerging high-entropy strategy: A booster to the development of cathode materials for power batteries. J. Adv. Ceram. 2024, 13, 1093–1118. [Google Scholar] [CrossRef]
- Zhou, X.-C.; Zhang, Z.; Jiang, X.-Y.; Tan, S.-C.; Pan, Z.-D.; Rao, X.-Y.; Wu, Y.-T.; Wang, Z.-L.; Liu, X.; Gu, J.; et al. Porous hexagonal Mn5O8 nanosheets as fast-charging anode materials for lithium-ion batteries. J. Adv. Ceram. 2024, 13, 1635–1642. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, J.; Liu, M.; Liu, Z.; Cheng, X.; Chang, J.; Yang, X.; Li, B.; Liu, B.; Liu, R. Enhanced strength and toughness of SiC/C composite ceramics via SiC@graphene core–shell nanoparticles. J. Am. Ceram. Soc. 2025, 108, e20151. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Cheng, S.; Zhou, W.-J.; Jia, J.; Yang, L.-F.; Yao, M.-H.; Wang, M.-K.; Zhou, J.; Wu, P.; Liu, M. Construction and performance characterization of α-Fe2O3/rGO composite for long-cycling-life supercapacitor anode. ACS Sustainable Chem. Eng. 2017, 5, 5067–5074. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Ji, X.; Cheng, S.; Chern, Z.-Y.; Jia, J.; Yang, L.-F.; Luo, H.-W.; Yu, J.-Y.; Peng, X.-W.; Wang, J.-H.; et al. Fast energy storage in two-dimensional MoO2 enabled by uniform oriented tunnels. ACS Nano 2019, 13, 9091–9099. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, K.; Li, Y.; Li, Z.; Wu, C.; Pan, J.H. Engineering nanostructure, interface, and prelithiation of advanced silicon-based lithium-ion battery anodes. Energy Mater. Adv. 2025, 6, 0175. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, G.; Li, J.; Wu, J.; Gu, B.-L.; Duan, W. Lithium intercalation induced decoupling of epitaxial graphene on SiC(0001): Electronic property and dynamic process. J. Phys. Chem. C 2011, 115, 23992–23997. [Google Scholar] [CrossRef]
- Wu, P.; Guo, C.; Han, J.; Yu, K.; Dong, X.; Yue, G.; Yue, H.; Guan, Y.; Liu, A. Fabrication of double core–shell Si-based anode materials with nanostructure for lithium-ion battery. RSC Adv. 2018, 8, 9094–9102. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.-J.; Gu, H.; Fan, H.; Yang, G.; Ignaszak, A.; Tang, X.; Liu, D.; Zhang, J. Interfacial coupled design of epitaxial graphene@SiC Schottky junction with built-in electric field for high-performance anodes of lithium ion batteries. Nano Energy 2020, 77, 105092. [Google Scholar] [CrossRef]
- Wu, W.-J.; Zou, Y.; Li, C.-H.; Li, Y.-W.; Wang, Z.-Y.; Chang, N.; Shi, Y.-S. Effect of impregnated phenolic resin on the properties of Si–SiC ceramic matrix composites fabricated by SLS-RMI. Ceram. Int. 2023, 49, 1624–1635. [Google Scholar] [CrossRef]
- Ding, S.; Zhu, S.; Zeng, Y.; Jiang, D. Effect of Y2O3 addition on the properties of reaction-bonded porous SiC ceramics. Ceram. Int. 2006, 32, 461–466. [Google Scholar] [CrossRef]
- Cao, J.; Lu, J.; Jiang, L.; Wang, Z. Sinterability, microstructure and compressive strength of porous glass-ceramics from metallurgical silicon slag and iste glass. Ceram. Int. 2016, 42, 10079–10084. [Google Scholar] [CrossRef]
- Kim, J.; Bayram, C.; Park, H.; Cheng, C.-W.; Dimitrakopoulos, C.; Ott, J.A.; Reuter, K.B.; Bedell, S.W.; Sadana, D.K. Principle of direct van der Waals epitaxy of single-crystalline films on epitaxial graphene. Nat. Commun. 2014, 5, 4836. [Google Scholar] [CrossRef]
- Luo, T.; Chen, X.; Wang, P.; Li, C.; Cao, B.; Zeng, H. Laser irradiation-induced SiC@graphene sub-microspheres: A bioinspired core–shell structure for enhanced tribology properties. Adv. Mater. Interfaces 2018, 5, 1700839. [Google Scholar] [CrossRef]
- Krasovskii, P.V.; Lebedev, A.M. Evidence for heterogeneous oxidation on the surface of a plasma SiC nanopowder based on synchrotron-excited XPS. Ceram. Int. 2023, 49, 40995–41000. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Ge, B.; Shi, Z.; Jin, Z. Fabrication of Si2N2O ceramic with silicon kerf waste as raw material. Ceram. Int. 2018, 44, 5581–5586. [Google Scholar] [CrossRef]
- Queraltó, A.; del Pino, A.P.; Logofatu, C.; Datcu, A.; Amade, R.; Bertran-Serra, E.; György, E. Reduced graphene oxide/iron oxide nanohybrid flexible electrodes grown by laser-based technique for energy storage applications. Ceram. Int. 2018, 44, 20409–20416. [Google Scholar] [CrossRef]
- Gao, J.; Yu, J.; Zhou, L.; Muhammad, J.; Dong, X.; Wang, Y.; Yu, H.; Quan, X.; Li, S.; Jung, Y. Interface evolution in the platelet-like SiC@C and SiC@SiO2 monocrystal nanocapsules. Nano Res. 2017, 10, 2644–2656. [Google Scholar] [CrossRef]
- Yuan, D.; Adekoya, D.; Dou, Y.; Tian, Y.; Chen, H.; Wu, Z.; Qin, J.; Yu, L.; Zhang, J.; Liu, X.; et al. Cation-vacancy induced Li+ intercalation pseudocapacitance at atomically thin heterointerface for high capacity and high power lithium-ion batteries. J. Energy Chem. 2021, 62, 281–288. [Google Scholar] [CrossRef]
- Hasa, I.; Haregewoin, A.M.; Zhang, L.; Tsai, W.-Y.; Guo, J.; Veith, G.M.; Ross, P.N.; Kostecki, R. Electrochemical reactivity and passivation of silicon thin-film electrodes in organic carbonate electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 40879–40890. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Y.; Lu, Y.-Q.; Li, J.-T.; Zanna, S.; Seyeux, A.; Huang, L.; Sun, S.-G.; Marcus, P.; Światowska, J. Influence of carbonate solvents on solid electrolyte interphase composition over Si electrodes monitored by in situ and ex situ spectroscopies. ACS Omega 2021, 6, 27335–27350. [Google Scholar] [CrossRef]
- Gan, C.; Zhang, C.; Wen, W.; Liu, Y.; Chen, J.; Xie, Q.; Luo, X. Enhancing delithiation reversibility of Li15Si4 alloy of silicon nanoparticles-carbon/graphite anode materials for stable-cycling lithium ion batteries by restricting the silicon particle size. ACS Appl. Mater. Interfaces 2019, 11, 35809–35819. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, X.; Cao, Z.; Wang, Y.; Wang, Y.; Lv, L.; Huang, W.; Huang, Y.; Zheng, H. Unveiling the mechanisms into Li-trapping induced (ir)reversible capacity loss for silicon anode. Energy Storage Mater. 2023, 55, 660–668. [Google Scholar] [CrossRef]
- Yang, Y.; Ni, C.; Gao, M.; Wang, J.; Liu, Y.; Pan, H. Dispersion-strengthened microparticle silicon composite with high anti-pulverization capability for Li-ion batteries. Energy Storage Mater. 2018, 14, 279–288. [Google Scholar] [CrossRef]
- Wang, X.; Fan, F.; Wang, J.; Wang, H.; Tao, S.; Yang, A.; Liu, Y.; Beng Chew, H.; Mao, S.X.; Zhu, T.; et al. High damage tolerance of electrochemically lithiated silicon. Nat. Commun. 2015, 6, 8417. [Google Scholar] [CrossRef]
- He, Y.; Jiang, L.; Chen, T.; Xu, Y.; Jia, H.; Yi, R.; Xue, D.; Song, M.; Genc, A.; Bouchet-Marquis, C.; et al. Progressive growth of the solid–electrolyte interphase towards the Si anode interior causes capacity fading. Nat. Nanotech. 2021, 16, 1113–1120. [Google Scholar] [CrossRef]
- Yue, X.; Fan, J.; Xiang, Q. Internal electric field on steering charge migration: Modulations, determinations and energy-related applications. Adv. Funct. Mater. 2022, 32, 2110258. [Google Scholar] [CrossRef]
- Galvez-Aranda, D.E.; Verma, A.; Hankins, K.; Seminario, J.M.; Mukherjee, P.P.; Balbuena, P.B. Chemical and mechanical degradation and mitigation strategies for Si anodes. J. Power Sources 2019, 419, 208–218. [Google Scholar] [CrossRef]
- Chen, H.; Hua, Y.; Luo, N.; He, X.; Li, Y.; Zhang, Y.; Chen, W.; Huang, S. Lithiation abilities of SiC bulks and surfaces: A first-principles study. J. Phys. Chem. C 2020, 124, 7031–7038. [Google Scholar] [CrossRef]
- Chen, G.; Yan, L.; Luo, H.; Guo, S. Nanoscale engineering of heterostructured anode materials for boosting lithium-ion storage. Adv. Mater. 2016, 28, 7580–7602. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, M.; Yao, L.; Yang, W.; Li, Y. Controlled growth lateral/vertical heterostructure interface for lithium storage. Adv. Mater. 2024, 36, 2402961. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, T.; Li, Y.; Yang, J.; Huang, B.; Ou, S.; Meng, C.; Zhang, S.; Tong, Y. Consecutive chemical bonds reconstructing surface structure of silicon anode for high-performance lithium-ion battery. Energy Storage Mater. 2021, 39, 354–364. [Google Scholar] [CrossRef]
- Sun, C.; Xu, X.; Gui, C.; Chen, F.; Wang, Y.; Chen, S.; Shao, M.; Wang, J. High-quality epitaxial N doped graphene on SiC with tunable interfacial interactions via electron/ion bridges for stable lithium-ion storage. Nano-Micro Lett. 2023, 15, 202. [Google Scholar] [CrossRef]
- Zhao, E.; Gao, K.; Luo, X.; Li, L.; Zhao, J.; Li, H. Heterostructure VO2@VS2 tailored by one-step hydrothermal synthesis for stable and highly efficient Zn-ion storage. Mater. Futures 2024, 3, 045101. [Google Scholar] [CrossRef]
- Qin, T.; Zhao, X.; Sui, Y.; Wang, D.; Chen, W.; Zhang, Y.; Luo, S.; Pan, W.; Guo, Z.; Leung, D.Y.C. Heterointerfaces: Unlocking superior capacity and rapid mass transfer dynamics in energy storage electrodes. Adv. Mater. 2024, 36, 2402644. [Google Scholar] [CrossRef] [PubMed]
- Fafure, A.V.; Bem, D.B.; Kahuthu, S.W.; Adediran, A.A.; Bodunrin, M.O.; Fabuyide, A.A.; Ajanaku, C. Advances in silicon carbon composites anodes derived from agro istes for applications in lithium-ion battery: A review. Heliyon 2024, 10, 386. [Google Scholar] [CrossRef]
- Sarkar, L.; Pal, S.; Das, S.; Sultana, F.; Banerjee, A.; Show, B.; Nandi, U. Enhanced cycling stability of ZnO-doped NiCo2O4 electrodes for acidic solid-state symmetric supercapacitors. Sustain. Energy Fuels 2025, 9, 5327–5341. [Google Scholar] [CrossRef]
- Bueno, P.R.; Leite, E.R. Nanostructured Li ion insertion electrodes. 1. Discussion on fast transport and short path for ion diffusion. J. Phys. Chem. B 2003, 107, 8868–8877. [Google Scholar] [CrossRef]
- Deiss, E. Spurious chemical diffusion coefficients of Li+ in electrode materials evaluated with GITT. Electrochim. Acta 2005, 50, 2927–2932. [Google Scholar] [CrossRef]
- Kidalov, V.V.; Kukushkin, S.A.; Osipov, A.V.; Redkov, A.V.; Grashchenko, A.S.; Soshnikov, I.P.; Boiko, M.E.; Sharkov, M.D.; Dyadenchuk, A.F. Properties of SiC films obtained by the method of substitution of atoms on porous silicon. ECS J. Solid State Sci. 2018, 7, P158. [Google Scholar] [CrossRef]
- Nakashima, S.; Harima, H. Raman investigation of SiC polytypes. Phys. Stat. Sol. (a) 1997, 162, 39–64. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, S.; Zhang, Q.; Xin, Y.; Dong, S.; Liu, H.; Li, J.; Wang, C.; Lu, C.; Yang, W.; et al. Unshackling the reversible capacity of SiOx/graphite-based full cells via selective LiF-induced lithiation. Sci. China Mater. 2022, 65, 2335–2342. [Google Scholar] [CrossRef]
- Cai, Z.; Kuru, Y.; Han, J.W.; Chen, Y.; Yildiz, B. Surface electronic structure transitions at high temperature on perovskite oxides: The case of strained La0.8Sr0.2CoO3 thin films. J. Am. Chem. Soc. 2011, 133, 17696–17704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Song, Z.; Chen, Z.; Li, X.; Yuan, R.; Zhang, D.; Li, A.; Chen, X.; Yao, X.; et al. Tuning diffusion preferences in silicon-based anodes for enhanced rapid and homogeneous lithiation. ACS Nano 2025, 19, 31246–31257. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- He, T.; Feng, J.; Ru, J.; Feng, Y.; Lian, R.; Yang, J. Constructing heterointerface of metal atomic layer and amorphous anode material for high-capacity and fast lithium storage. ACS Nano 2018, 13, 830–838. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Zhang, X.; Guo, G.; Hu, J.; Dong, A.; Yang, D. Bowl-like 3C-SiC nanoshells encapsulated in hollow graphitic carbon spheres for high-rate lithium-ion batteries. Chem. Mater. 2016, 28, 1179–1186. [Google Scholar] [CrossRef]
- Chang, W.-S.; Park, C.-M.; Kim, J.-H.; Kim, Y.-U.; Jeong, G.; Sohn, H.-J. Quartz (SiO2): A new energy storage anode material for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6895–6899. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; He, R.; Luo, W.; Meng, J.; Yu, Q.; Zhao, D.; Zhou, L.; Mai, L. Yolk@shell SiOx/C microspheres with semi-graphitic carbon coating on the exterior and interior surfaces for durable lithium storage. Energy Storage Mater. 2019, 19, 299–305. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, Y.; Qian, J.; Ai, X.; Yang, H. Antimony-coated SiC nanoparticles as stable and high-capacity anode materials for Li-ion batteries. J. Phys. Chem. C 2010, 114, 15196–15201. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, J.-K.; Yin, Y.-X.; Guo, Y.-G. Facile synthesis of blocky SiOx/C with graphite-like structure for high-performance lithium-ion battery anodes. Adv. Funct. Mater. 2018, 28, 1705235. [Google Scholar] [CrossRef]
- Sun, X.; Shao, C.; Zhang, F.; Li, Y.; Wu, Q.-H.; Yang, Y. SiC nanofibers as long-life lithium-ion battery anode materials. Front. Chem. 2018, 6, 166. [Google Scholar] [CrossRef]
- Suzuki, N.; Cervera, R.B.; Ohnishi, T.; Takada, K. Silicon nitride thin film electrode for lithium-ion batteries. J. Power Sources 2013, 231, 186–189. [Google Scholar] [CrossRef]
- de Guzman, R.C.; Yang, J.; Ming-Cheng Cheng, M.; Salley, S.O.; Ng, K.Y.S. High capacity silicon nitride-based composite anodes for lithium ion batteries. J. Mater. Chem. A 2014, 2, 14577–14584. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, F.; Li, X.; Luo, W.; Li, L.; Zhang, H.; Wang, L.; Wang, Y.; Jiang, W.; Liu, H.K.; et al. Engineering the distribution of carbon in silicon oxide nanospheres at the atomic level for highly stable anodes. Angew. Chem. Int. Ed. 2019, 58, 6669–6673. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, Y.-Z.; Zhang, F.; Li, Q.; Anjum, D.H.; Liang, H.; Liu, Y.; Liu, C.-s.; Alshareef, H.N.; Pang, H. A novel strategy for the synthesis of highly stable ternary SiOx composites for Li-ion-battery anodes. J. Mater. Chem. A 2019, 7, 15969–15974. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; He, S.; Sun, C.; Gao, K.; Li, H.; Zheng, Q.; Geng, L.; Wang, Y.-J.; Zhao, E.; Zhu, Y. Interfacial Electronic Coupling in Si@SiC@EG Core–Shell Architectures Enables High-Capacity and Long-Life Lithium-Ion Batteries. Molecules 2025, 30, 4517. https://doi.org/10.3390/molecules30234517
Zhao H, He S, Sun C, Gao K, Li H, Zheng Q, Geng L, Wang Y-J, Zhao E, Zhu Y. Interfacial Electronic Coupling in Si@SiC@EG Core–Shell Architectures Enables High-Capacity and Long-Life Lithium-Ion Batteries. Molecules. 2025; 30(23):4517. https://doi.org/10.3390/molecules30234517
Chicago/Turabian StyleZhao, Huangyu, Sihao He, Changlong Sun, Kesheng Gao, Honglin Li, Qiuju Zheng, Lingshan Geng, Yan-Jie Wang, Enyue Zhao, and Yuanyuan Zhu. 2025. "Interfacial Electronic Coupling in Si@SiC@EG Core–Shell Architectures Enables High-Capacity and Long-Life Lithium-Ion Batteries" Molecules 30, no. 23: 4517. https://doi.org/10.3390/molecules30234517
APA StyleZhao, H., He, S., Sun, C., Gao, K., Li, H., Zheng, Q., Geng, L., Wang, Y.-J., Zhao, E., & Zhu, Y. (2025). Interfacial Electronic Coupling in Si@SiC@EG Core–Shell Architectures Enables High-Capacity and Long-Life Lithium-Ion Batteries. Molecules, 30(23), 4517. https://doi.org/10.3390/molecules30234517






