Anti-Neuroinflammatory Effects of Compounds Isolated from Quercus acuta Thunb. Fruits via NF-κB Signaling Inhibition in BV2 Microglia
Abstract
1. Introduction
2. Results
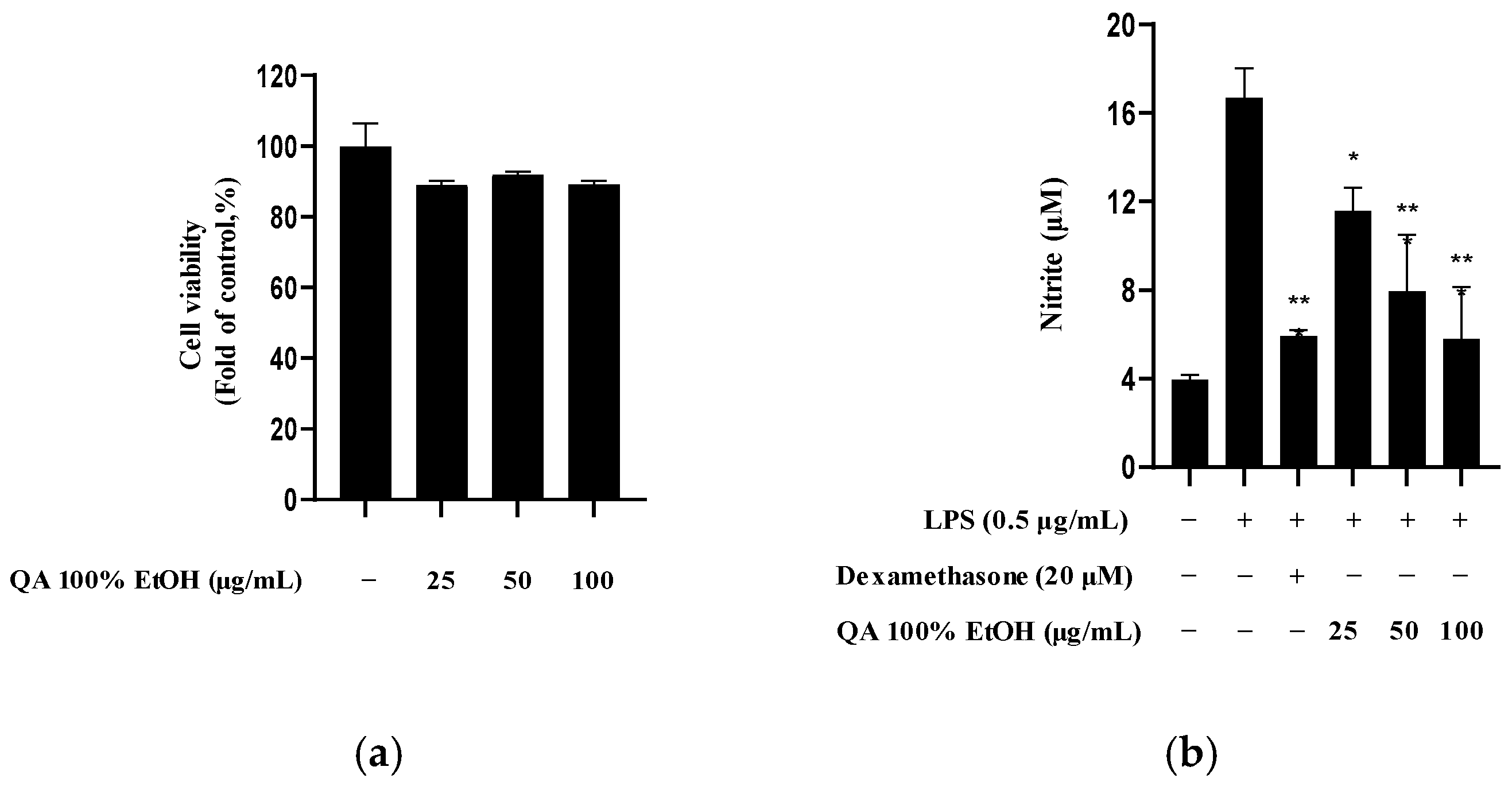
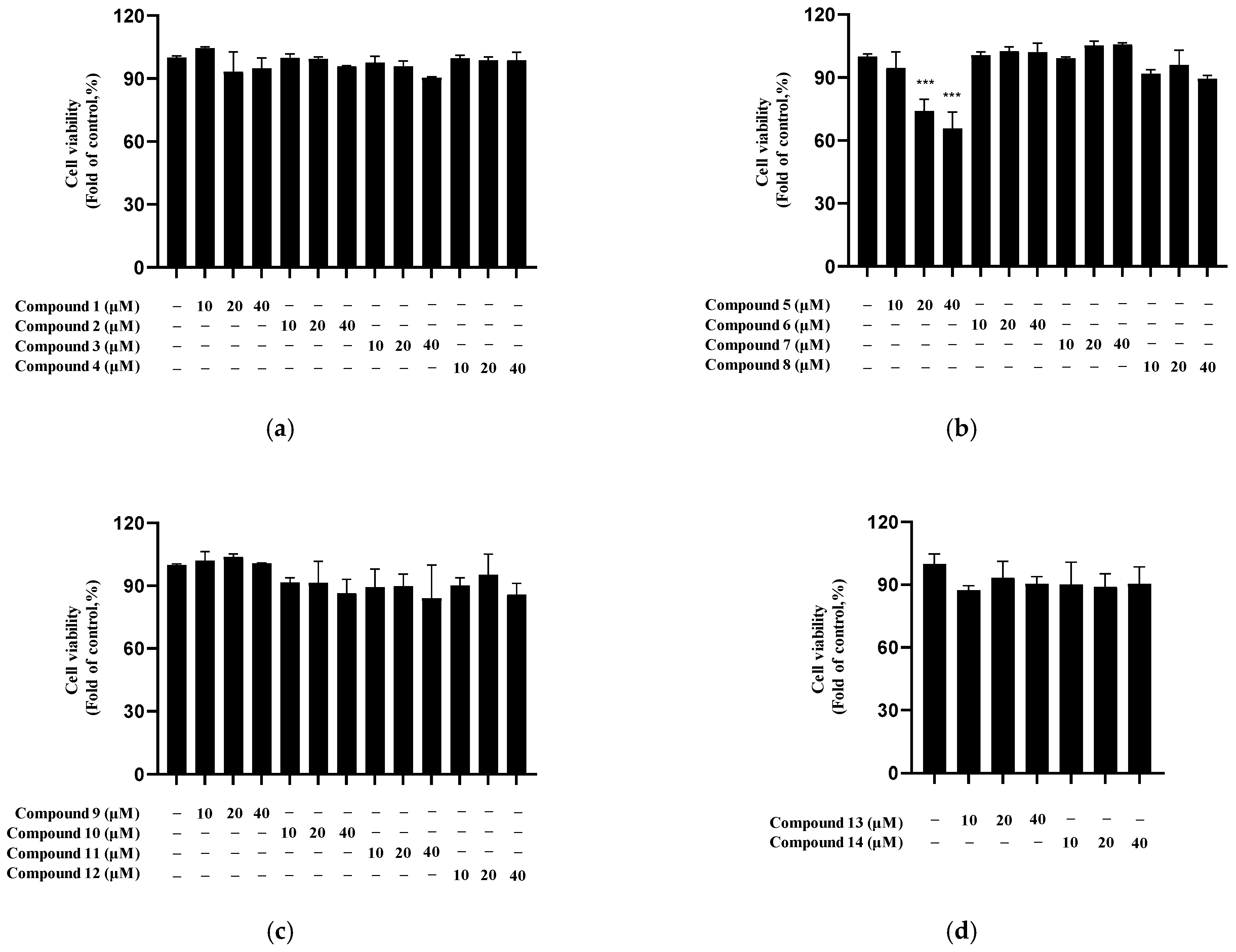
2.1. Effects of Q. acuta Fruit Ethanol Extract on Cytotoxicity and Nitrite Production
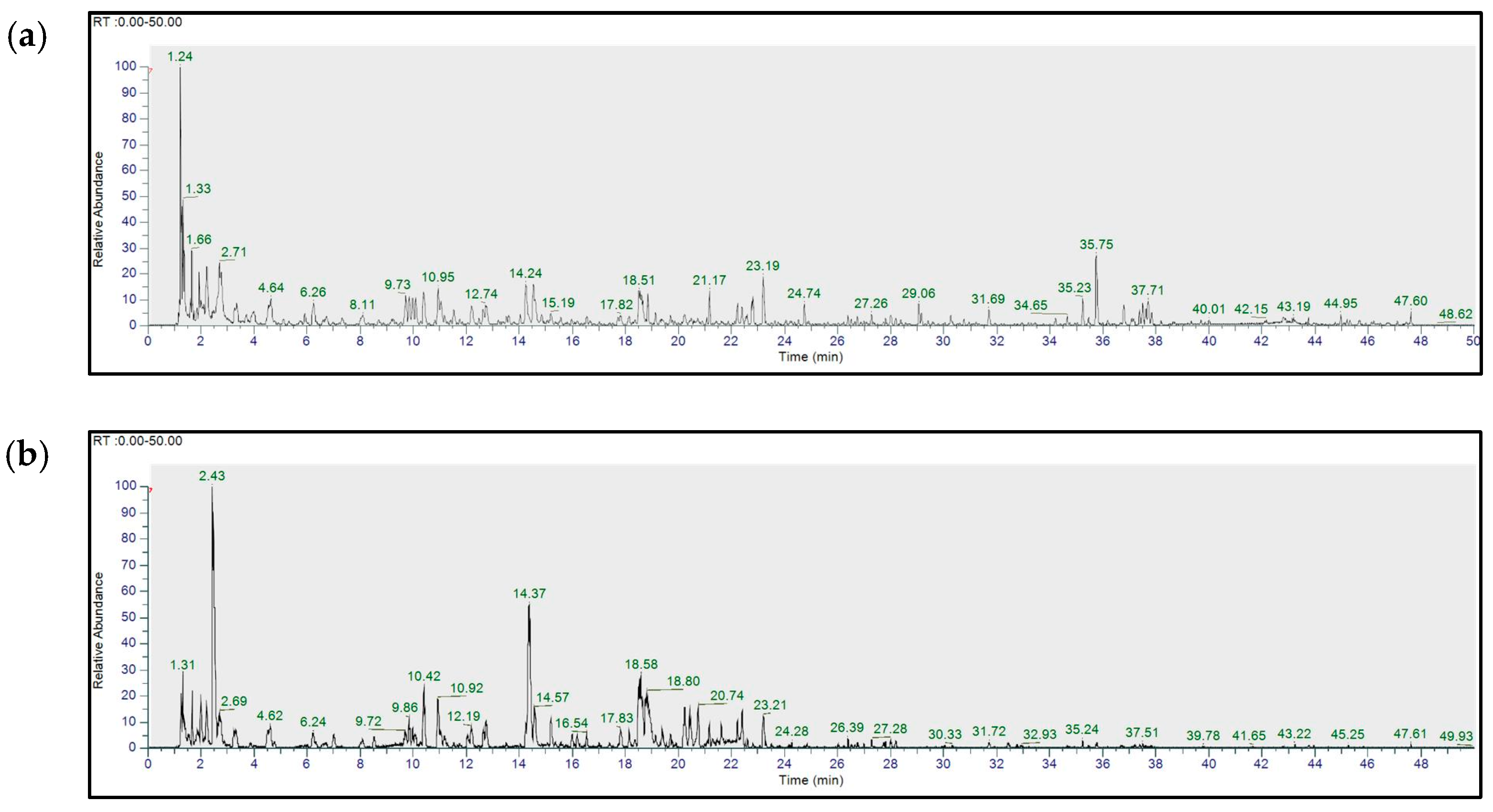
2.2. UHPLC-HR-MS/MS-Based Metabolite Profiling of Q. acuta Fruit Ethanol Extract
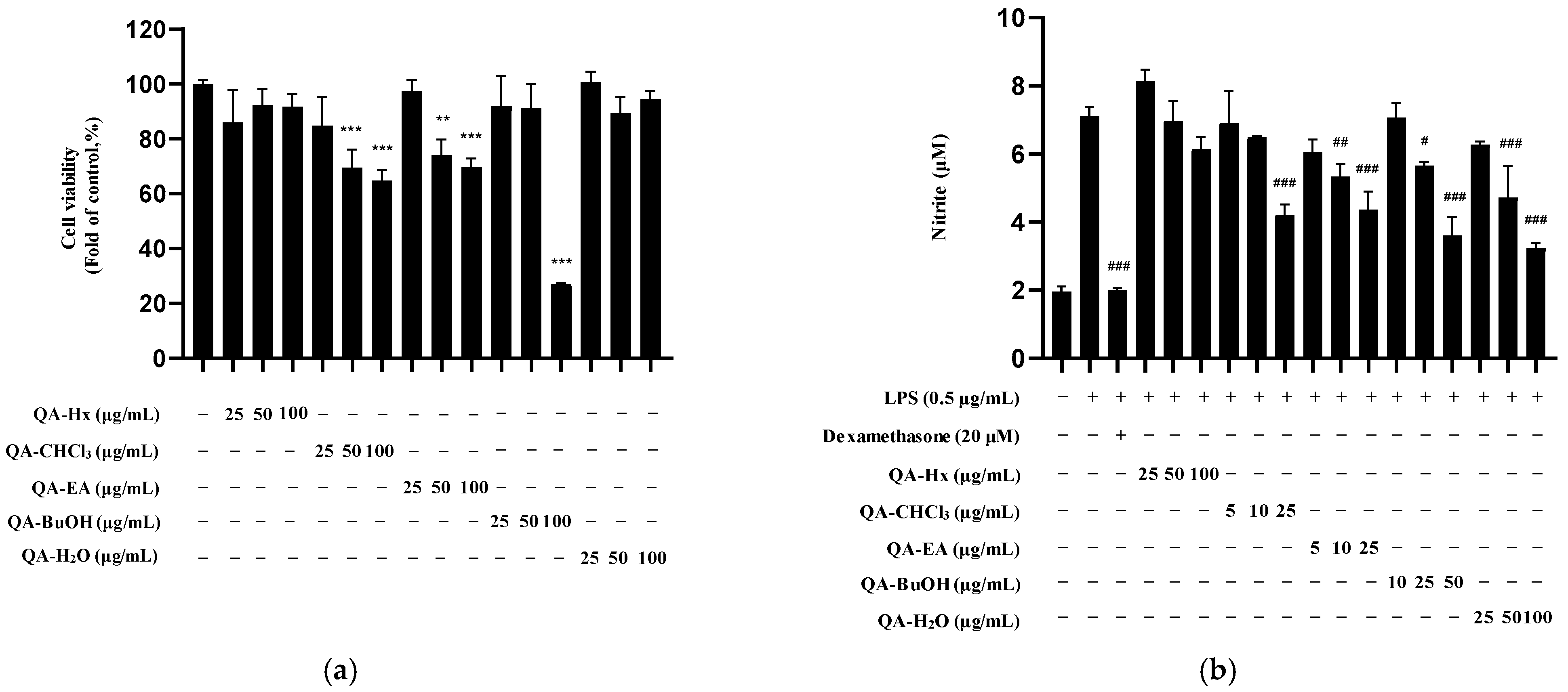
2.3. Bioactivity of Q. acuta Fruit EtOH Extract Fractions
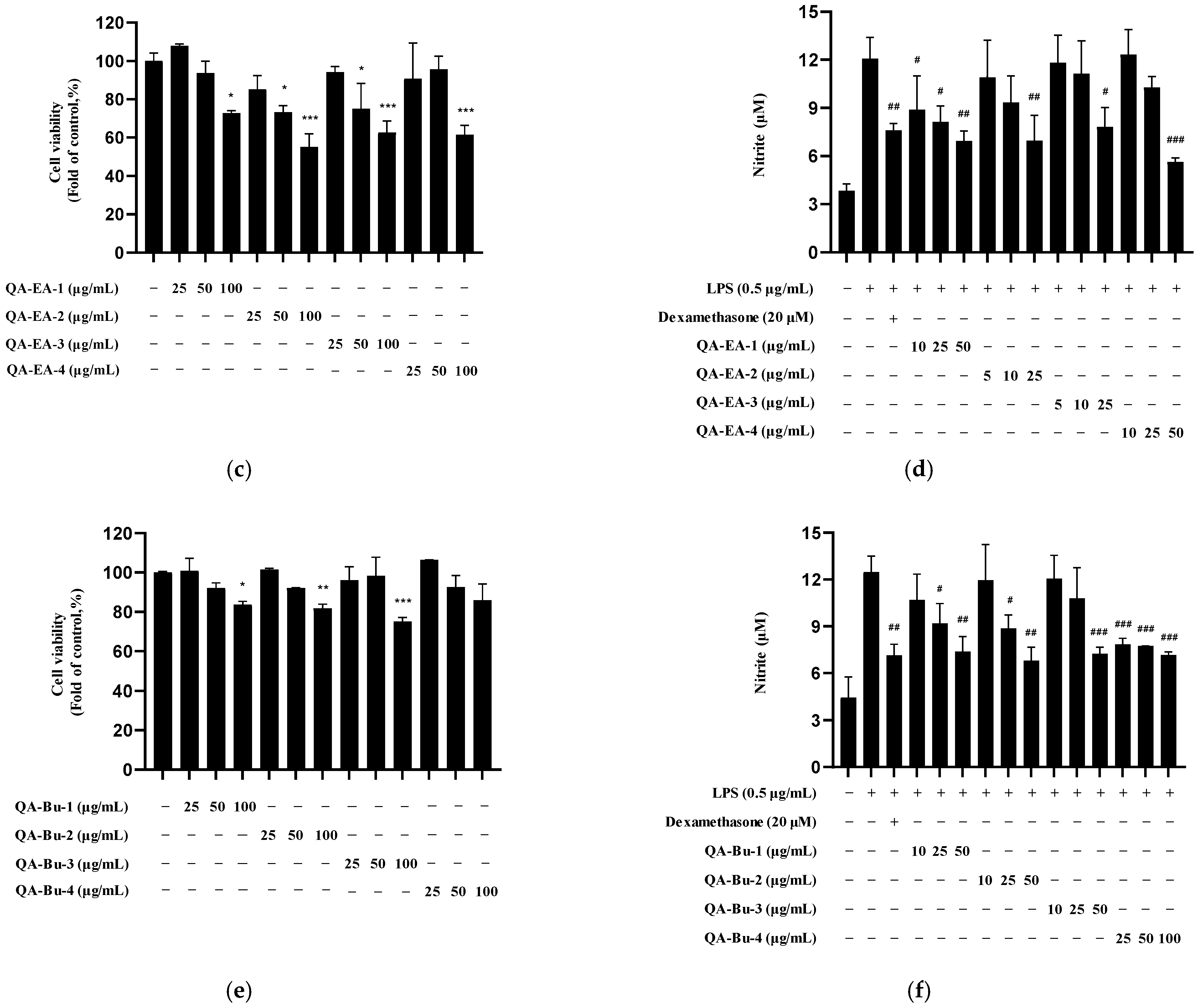
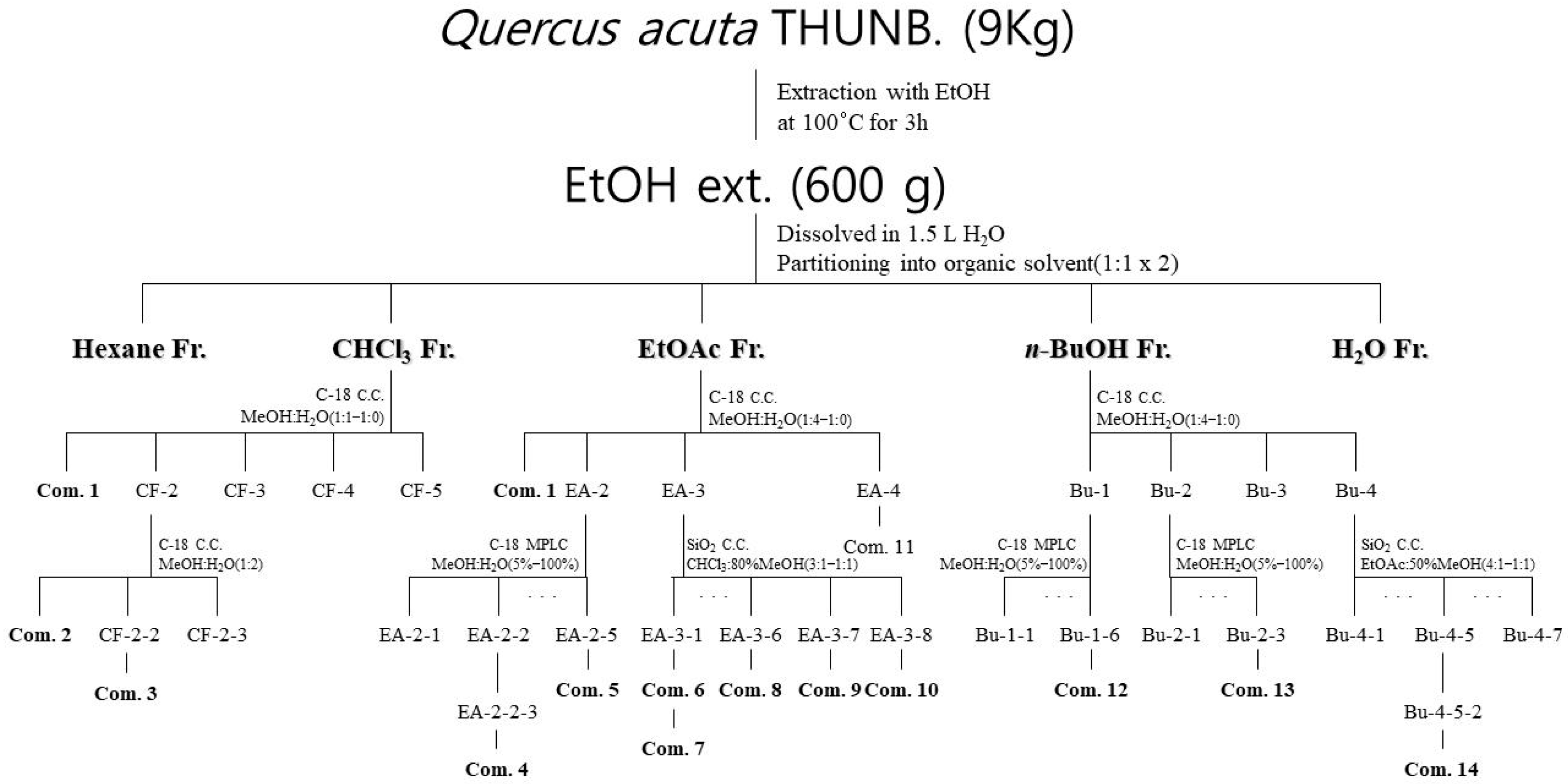
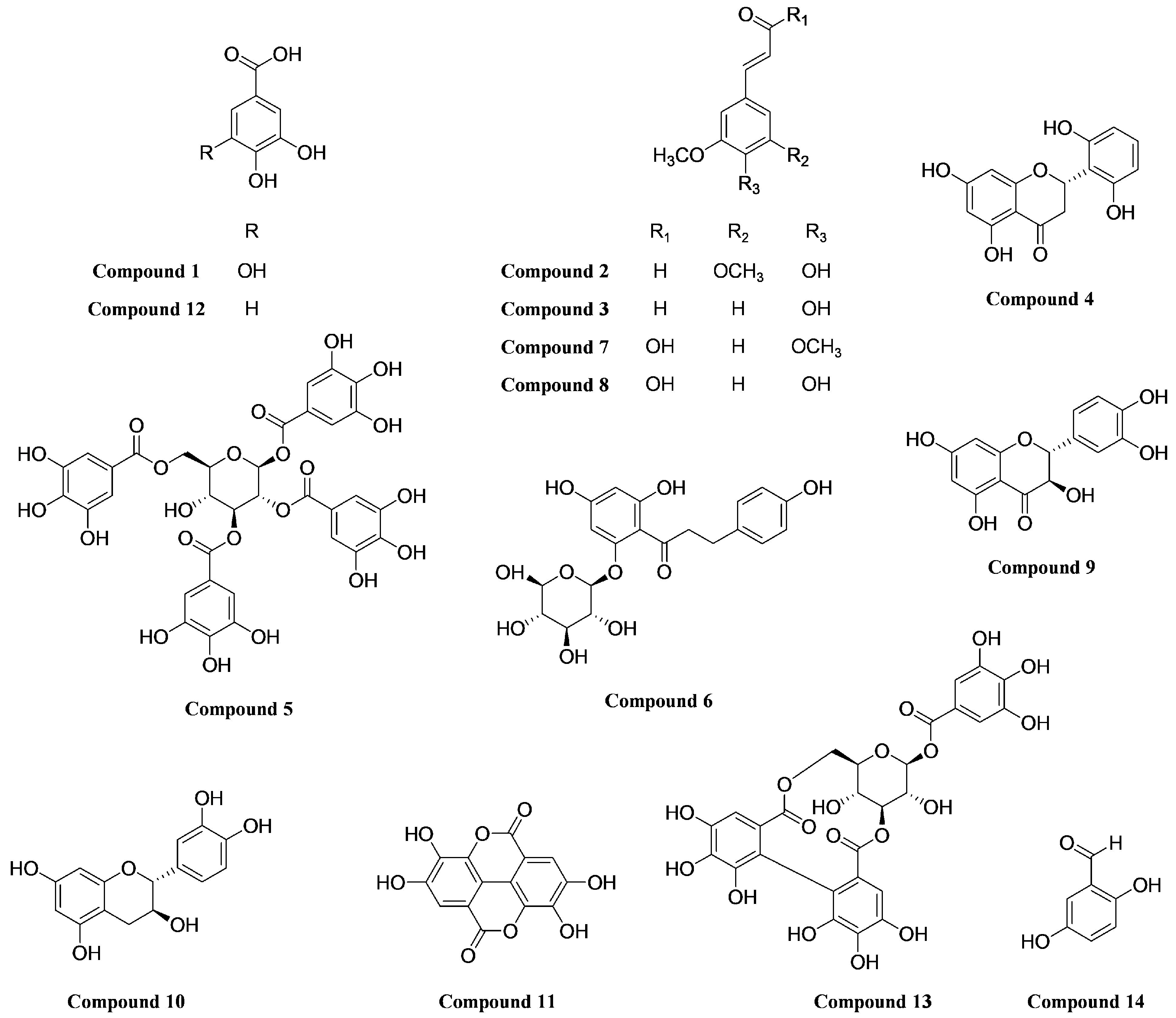
2.4. Isolation and Structural Identification of Compounds
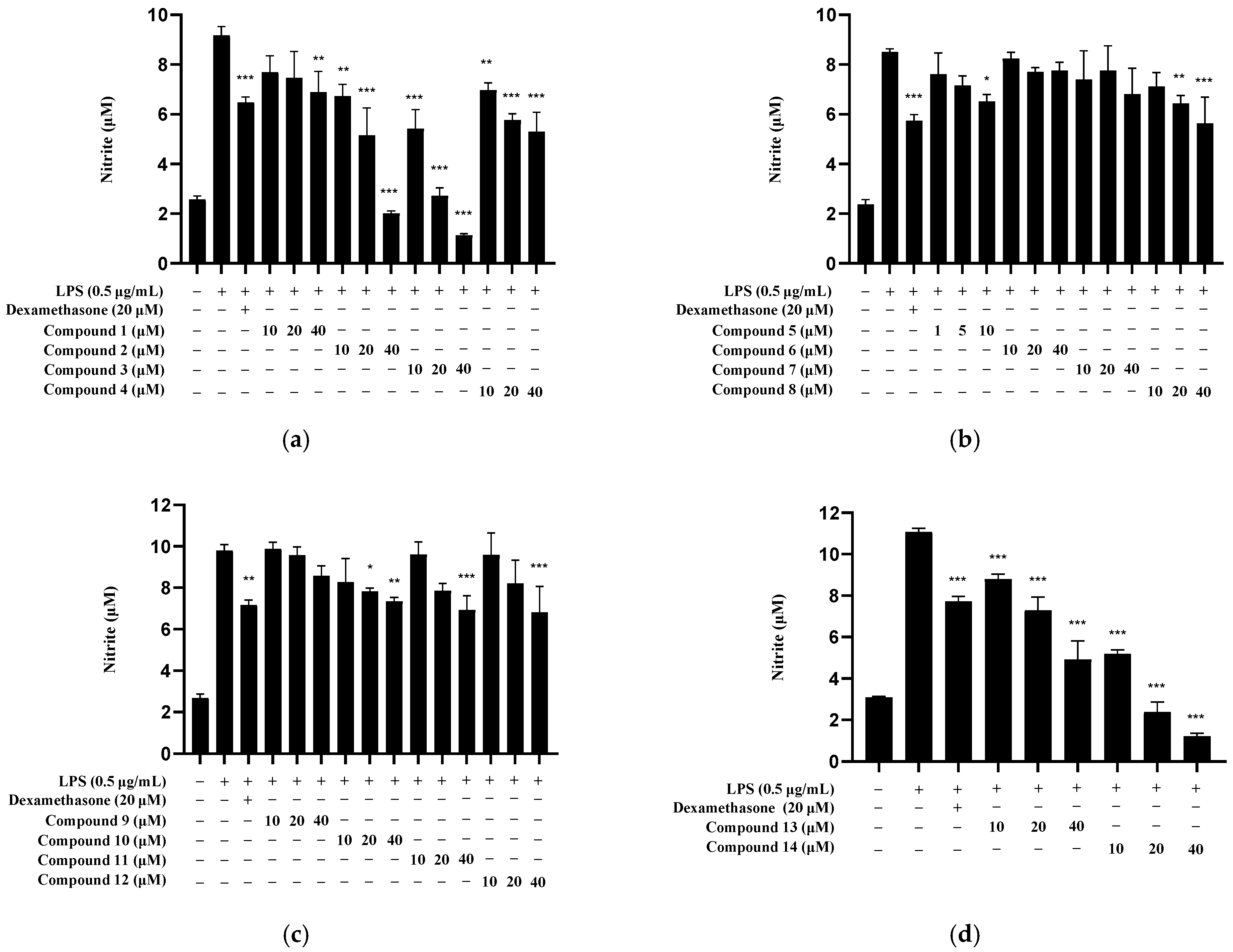
2.5. Nitrite Inhibition by Isolated Compounds
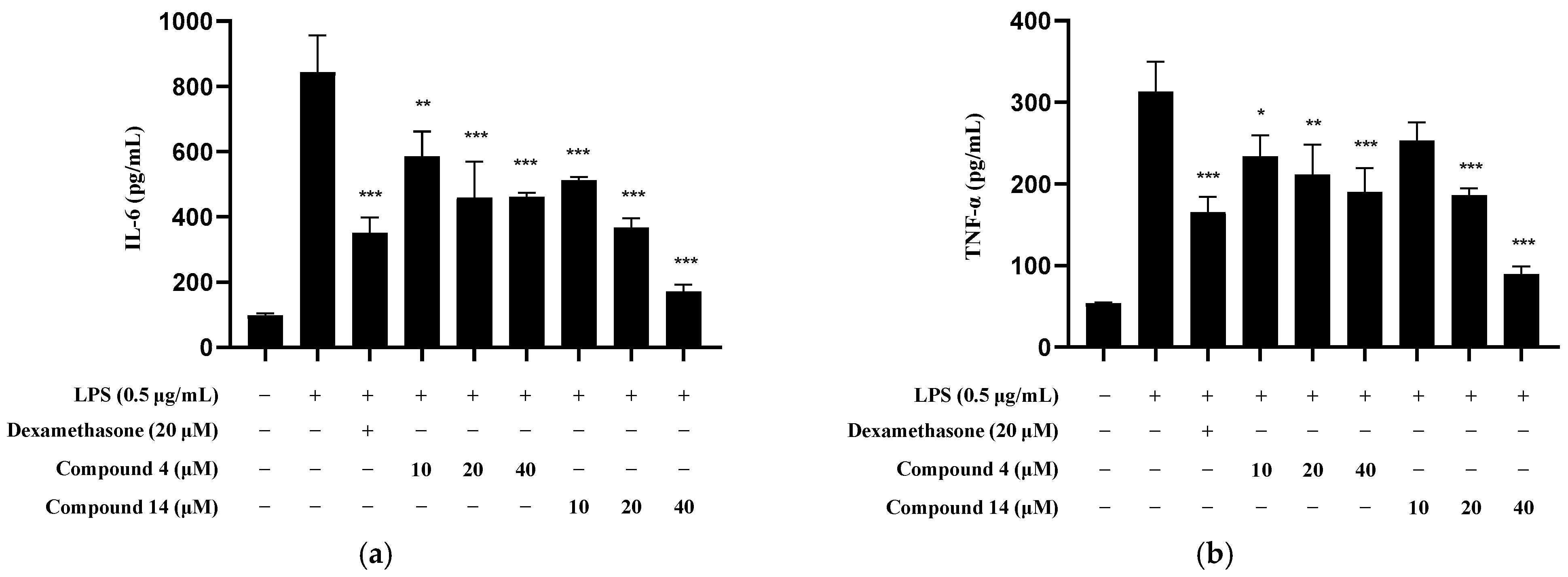
2.6. Inhibitory Effect of Compounds 4 and 16 on Inflammatory Cytokine Production
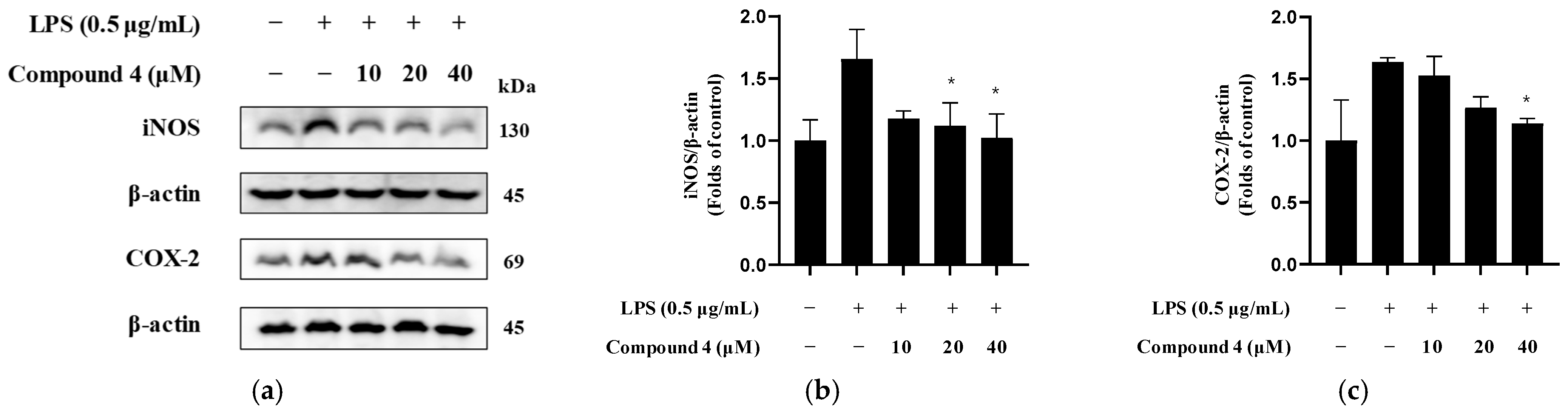
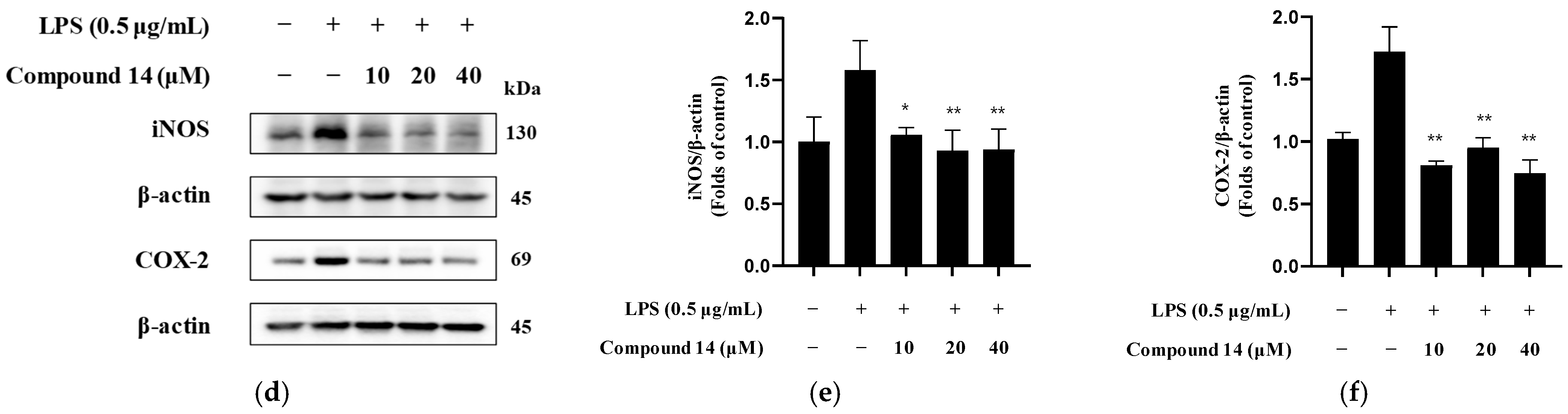
2.7. Inhibition of iNOS and COX-2 Expression
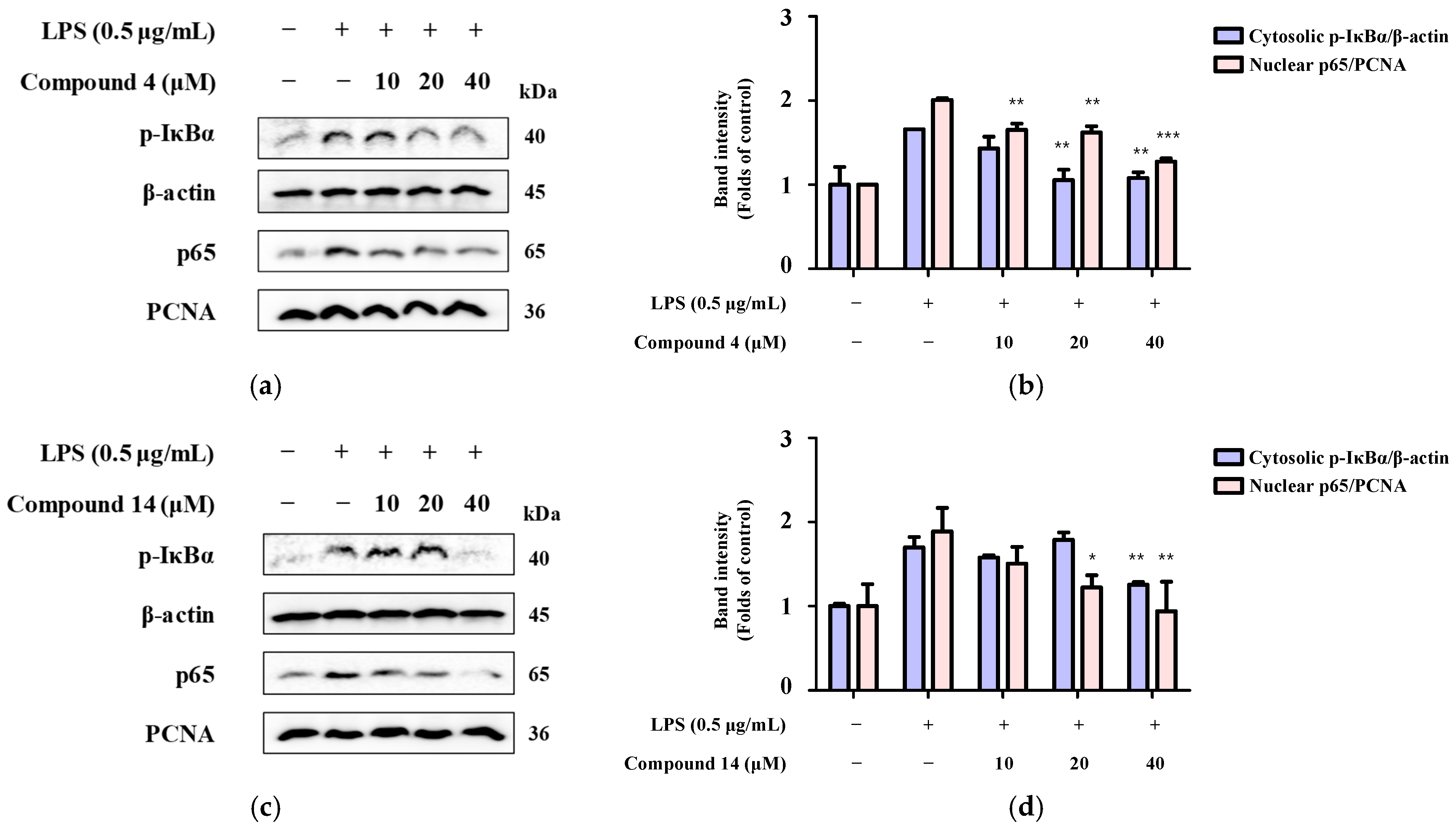
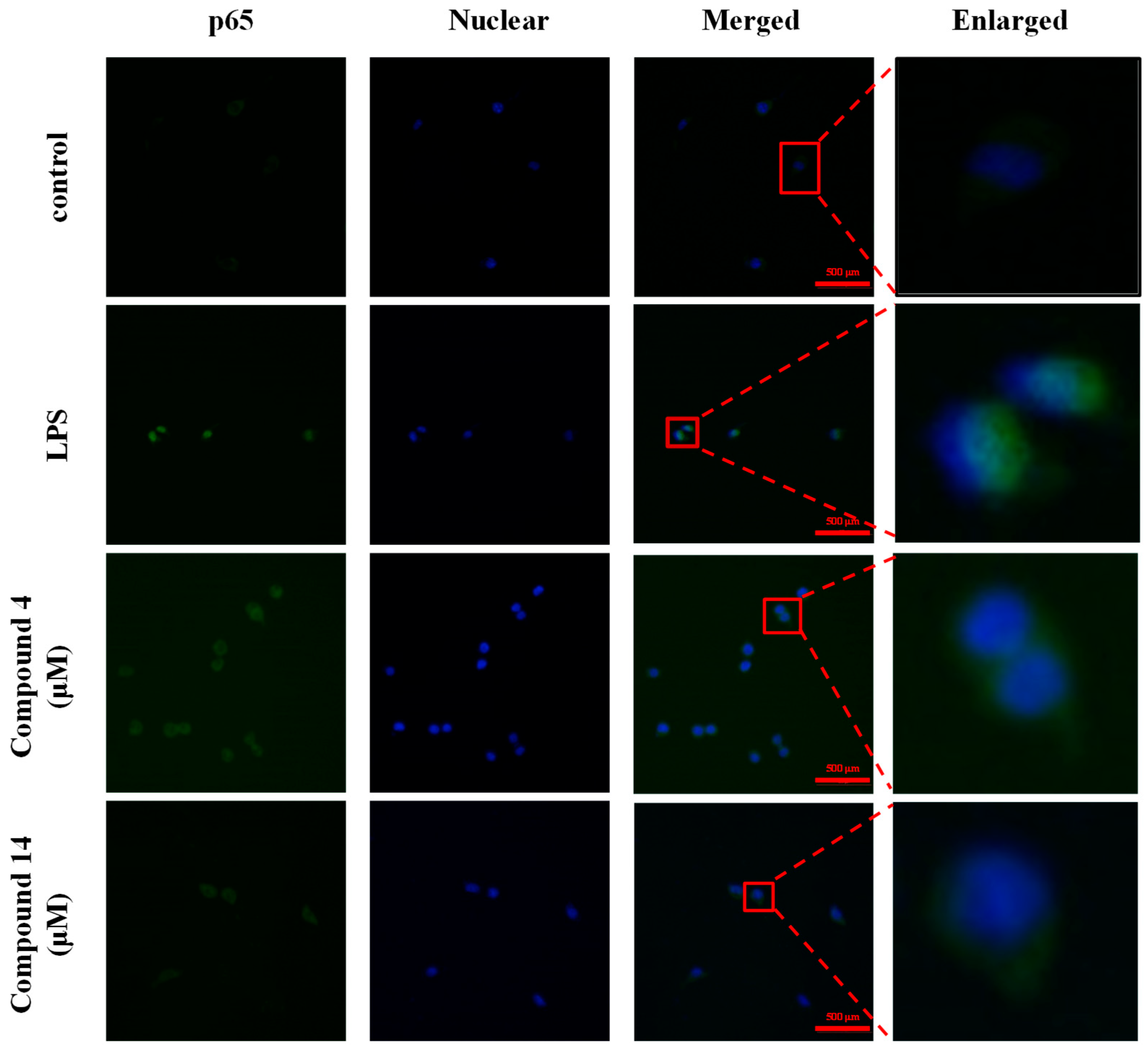
2.8. Inhibition of NF-κB Activation
3. Discussion
4. Materials and Methods
4.1. Chemicals, Solvents, and Instruments
4.2. Plant Materials
4.3. Cell Culture and Reagents
4.4. Metabolite Profiling Analysis
4.5. Compound Extraction and Isolation
4.6. Measurement of Nitrite Production
4.7. IL-6 and TNF-α Analysis
4.8. Western Blot Analysis
4.9. NF-κB Localization and Immunofluorescence
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Bu | n-butanol |
| CC | Column chromatography |
| CF | Chloroform |
| COX-2 | Cyclooxygenase-2 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMSO | Dimethyl sulfoxide |
| DW | Deionized H2O |
| EA | Ethyl acetate |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Fetal bovine serum |
| H-ESI | Heated electrospray ionization |
| HPLC | High-Performance Liquid Chromatography |
| HRP | horseradish peroxidase |
| Hx | n-hexane |
| iNOS | Inducible nitric oxide synthase |
| IL-6 | Interleukin-6 |
| LPS | Lipopolysaccharide |
| MPLC | Medium-pressure liquid chromatography |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| Q. acuta | Quercus acuta Thunb. |
| RPMI-1640 | Roswell Park Memorial Institute 1640 medium |
| RP-18 | Reversed-phase C18 |
| SiO2 | Silica gel |
| TIC | Total ion current (chromatogram) |
| TNF-α | Tumor necrosis factor-alpha |
| HR-MS/MS | High resolution tandem mass spectrometry |
References
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Qin, J.; Ma, Z.; Chen, X.; Shu, S. Microglia activation in central nervous system disorders: A review of recent mechanistic investigations and development efforts. Front. Neurol. 2023, 14, 1103416. [Google Scholar] [CrossRef] [PubMed]
- Henn, A.; Lund, S.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The suitability of BV2 cells for microglia studies. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef]
- Stansley, B.; Post, J.; Hensley, K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J. Neuroinflamm. 2012, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S. Innate immunity and neuroinflammation in the CNS: The role of microglia in Toll-like receptor-mediated neuronal injury. Glia 2010, 58, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Ko, W.; Baek, J.S.; Liu, Z.; Dong, L.; Kim, N.; Lee, H.; Yoon, C.S.; Kim, N.Y.; Kim, S.C.; Lee, D.S. Anti-Inflammatory Activity of 1,6,7-Trihydroxy-2-(1,1-dimethyl-2-propenyl)-3-methoxyxanthone Isolated from Cudrania tricuspidata via NF-kappaB, MAPK, and HO-1 Signaling Pathways in Lipopolysaccharide-Stimulated RAW 264.7 and BV2 Cells. Molecules 2023, 28, 7299. [Google Scholar] [CrossRef]
- Lee, H.; Liu, Z.; Dong, L.; Lee, D.Y.; Yoon, D.; Oh, H.; Kim, Y.C.; An, R.B.; Lee, D.S. Anti-Neuroinflammatory and Neuroprotective Effect of Intermedin B Isolated from the Curcuma longa L. via NF-kappaB and ROS Inhibition in BV2 Microglia and HT22 Hippocampal Cells. Int. J. Mol. Sci. 2023, 24, 7390. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lee, H.; Dong, L.; Cheong, S.H.; Lee, D.S. Fatsia japonica extract exerts antioxidant and anti-neuroinflammatory effects on neuronal cells and a zebrafish model. J. Ethnopharmacol. 2024, 324, 117813. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.H. Estimating carbon storage and CO2 absorption by developing allometric equations for Quercus acuta in South Korea. For. Sci. Technol. 2017, 13, 55–60. [Google Scholar] [CrossRef]
- Kim, J.K.; Yang, H.J.; Go, Y. Quercus acuta Thunb. Suppresses LPS-Induced Neuroinflammation in BV2 Microglial Cells via Regulating MAPK/NF-kappaB and Nrf2/HO-1 Pathway. Antioxidants 2022, 11, 1851. [Google Scholar] [CrossRef]
- Kim, B.; Kim, Y.S.; Hwang, Y.H.; Yang, H.J.; Li, W.; Kwon, E.B.; Kim, T.I.; Go, Y.; Choi, J.G. Quercus acuta Thunb. (Fagaceae) and Its Component, Isoquercitrin, Inhibit HSV-1 Replication by Suppressing Virus-Induced ROS Production and NF-kappaB Activation. Antioxidants 2021, 10, 1638. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, D.H.; Bae, M.S.; Song, S.H.; Seo, H.J.; Han, D.G.; Oh, D.S.; Jung, S.T.; Cho, Y.C.; Park, K.M.; et al. Analysis of the Active Constituents and Evaluation of the Biological Effects of Quercus acuta Thunb. (Fagaceae) Extracts. Molecules 2018, 23, 1772. [Google Scholar] [CrossRef]
- Yoon, I.S.; Park, D.H.; Bae, M.S.; Oh, D.S.; Kwon, N.H.; Kim, J.E.; Choi, C.Y.; Cho, S.S. In Vitro and In Vivo Studies on Quercus acuta Thunb. (Fagaceae) Extract: Active Constituents, Serum Uric Acid Suppression, and Xanthine Oxidase Inhibitory Activity. Evid. Based Complement. Alternat. Med. 2017, 2017, 4097195. [Google Scholar] [CrossRef]
- Hong, J.A.; Bae, D.; Oh, K.N.; Oh, D.R.; Kim, Y.; Kim, Y.; Im, S.J.; Choi, E.J.; Lee, S.G.; Kim, M.; et al. Protective effects of Quercus acuta Thunb. fruit extract against UVB-induced photoaging through ERK/AP-1 signaling modulation in human keratinocytes. BMC Complement. Med. Ther. 2022, 22, 6. [Google Scholar] [CrossRef]
- Ferrario, G.; Baron, G.; Gado, F.; Della, V.L.; Bombardelli, E.; Carini, M.; D’Amato, A.; Aldini, G.; Altomare, A. Polyphenols from Thinned Young Apples: HPLC-HRMS Profile and Evaluation of Their Anti-Oxidant and Anti-Inflammatory Activities by Proteomic Studies. Antioxidants 2022, 11, 1577. [Google Scholar] [CrossRef]
- He, F.; Du, Y.; Pan, Z.; Zeng, H.; Luo, H.; Wang, J.; Sun, Y.; Li, M. The composition of phenolic compounds in Chinese olive (Canarium album L.) cultivars and their contribution to the anti-inflammatory properties of the cultivars. Front. Nutr. 2024, 11, 1334077. [Google Scholar] [CrossRef]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of Apple Pomace Flour Obtained Industrially by Dehydration as a Source of Biomolecules with Antioxidant, Antidiabetic and Antiobesity Effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Sun, J.; Chen, P.; Lin, L.Z.; Harnly, J.M. A non-targeted approach to chemical discrimination between green tea dietary supplements and green tea leaves by HPLC/MS. J. AOAC Int. 2011, 94, 487–497. [Google Scholar] [CrossRef]
- Chang, J.B.; Lane, M.E.; Yang, M.; Heinrich, M. Disentangling the Complexity of a Hexa-Herbal Chinese Medicine Used for Inflammatory Skin Conditions-Predicting the Active Components by Combining LC-MS-Based Metabolite Profiles and in vitro Pharmacology. Front. Pharmacol. 2018, 9, 1091. [Google Scholar] [CrossRef]
- Gómez, J.; Simirgiotis, M.J.; Lima, B.; Gamarra-Luques, C.; Bórquez, J.; Caballero, D.; Feresin, G.E.; Tapia, A. UHPLC-Q/Orbitrap/MS/MS Fingerprinting, Free Radical Scavenging, and Antimicrobial Activity of Tessaria absinthiodes (Hook. & Arn.) DC. (Asteraceae) Lyophilized Decoction from Argentina and Chile. Antioxidants 2019, 8, 593. [Google Scholar] [PubMed]
- Burico, M.; Fodaroni, G.; Flamini, E.; Ascani, N.; Proietti, G.; Tamimi, S.; Quintiero, C.M.; Massa, L.; Gianni, M.; Mattoli, L. Metabolomic Fingerprint of Hamamelis virginiana L. Gallotannins by Suspect Screening Analysis with UHPLC-qToF and Their Semiquantitative Evaluation. J. Mass Spectrom. 2022, 57, e4878. [Google Scholar] [CrossRef]
- Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M.; Fernández de Simón, B.; Hernández, T.; Estrella, I. Phenolic Compounds in Chestnut (Castanea sativa Mill.) Heartwood. Effect of Toasting at Cooperage. J. Agric. Food Chem. 2010, 58, 9631–9640. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.A.; Khan, K.; Iqbal, Z.; Masood, T.; Hemeg, H.A.; Rauf, A. Metabolic and Pharmacological Profiling of Penicillium claviforme by a Combination of Experimental and Bioinformatic Approaches. Ann. Med. 2022, 54, 2102–2114. [Google Scholar] [CrossRef]
- Nagy, K.; Redeuil, K.; Williamson, G.; Rezzi, S.; Dionisi, F.; Longet, K.; Destaillats, F.; Renouf, M. First Identification of Dimethoxycinnamic Acids in Human Plasma after Coffee Intake by Liquid Chromatography–Mass Spectrometry. J. Chromatogr. A 2011, 1218, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Mirali, M.; Purves, R.W.; Stonehouse, R.; Song, R.; Bett, K.; Vandenberg, A. Genetics and Biochemistry of Zero-Tannin Lentils. PLoS ONE 2016, 11, e0164624. [Google Scholar] [CrossRef]
- Slighoua, M.; Mahdi, I.; Moussaid, F.Z.; Al Kamaly, O.; Amrati, F.E.-Z.; Conte, R.; Drioiche, A.; Saleh, A.; Housseini, A.I.; Bari, A.; et al. LC-MS/MS and GC/MS Profiling of Petroselinum sativum Hoffm. and Its Topical Application on Burn Wound Healing and Related Analgesic Potential in Rats. Metabolites 2023, 13, 260. [Google Scholar] [CrossRef]
- Zhang, Q.-F.; Guo, Y.-X.; Zheng, G.; Wang, W.-J. Chemical Constituents Comparison between Rhizoma Smilacis Glabrae and Rhizoma Smilacis Chinae by HPLC-DAD-MS/MS. Nat. Prod. Res. 2013, 27, 277–281. [Google Scholar] [CrossRef]
- Buamard, N.; Singh, A.; Zhang, B.; Hong, H.; Singh, P.; Benjakul, S. Ethanolic Extract of Duea Ching Fruit: Extraction, Characterization and Its Effect on the Properties and Storage Stability of Sardine Surimi Gel. Foods 2023, 12, 1635. [Google Scholar] [CrossRef]
- Fanzone, M.; González-Manzano, S.; Pérez-Alonso, J.; Escribano-Bailón, M.T.; Jofré, V.; Assof, M.; Santos-Buelga, C. Evaluation of Dihydroquercetin-3-O-Glucoside from Malbec Grapes as Copigment of Malvidin-3-O-Glucoside. Food Chem. 2015, 175, 166–173. [Google Scholar] [CrossRef]
- Plirat, W.; Chaniad, P.; Phuwajaroanpong, A.; Septama, A.W.; Punsawad, C. Phytochemical, Antimalarial, and Acute Oral Toxicity Properties of Selected Crude Extracts of Prabchompoothaweep Remedy in Plasmodium berghei-Infected Mice. Trop. Med. Infect. Dis. 2022, 7, 395. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hsu, C.C.; Huang, H.J.; Chang, C.J.; Sun, S.H.; Lin, A.M. Gallic Acid Attenuated LPS-Induced Neuroinflammation: Protein Aggregation and Necroptosis. Mol. Neurobiol. 2020, 57, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Olajide, O.A.; Kumar, A.; Velagapudi, R.; Okorji, U.P.; Fiebich, B.L. Punicalagin inhibits neuroinflammation in LPS-activated rat primary microglia. Mol. Nutr. Food Res. 2014, 58, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Yuan, T.; Yang, Z.; Zhang, Q.; Wang, W.W.; Lin, L.B.; Zhu, M.Q.; Gao, J.M. Ulmoidol, an unusual nortriterpenoid from Eucommia ulmoides Oliv. leaves prevents neuroinflammation by targeting the PU.1 transcriptional signaling pathway. Bioorg. Chem. 2021, 116, 105345. [Google Scholar] [CrossRef]
- Park, J.M.; Park, J.E.; Park, J.S.; Leem, Y.H.; Kim, D.Y.; Hyun, J.W.; Kim, H.S. Anti-Inflammatory and Antioxidant Mechanisms of Coniferaldehyde in Lipopolysaccharide-Induced Neuroinflammation: Involvement of AMPK/Nrf2 and TAK1/MAPK/NF-κB Signaling Pathways. Eur. J. Pharmacol. 2024, 979, 176850. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.; Li, X.; Sun, X.; Wang, Z.; Wang, H.; Nie, R.; Yu, W.; Zhou, Y. Coniferyl Aldehyde Inhibits the Inflammatory Effects of Leptomeningeal Cells by Suppressing the JAK2 Signaling. BioMed Res. Int. 2020, 2020, 4616308. [Google Scholar] [CrossRef]
- Kamdi, S.P.; Raval, A.; Nakhate, K.T. Phloridzin attenuates lipopolysaccharide-induced cognitive impairment via antioxidant, anti-inflammatory and neuromodulatory activities. Cytokine 2021, 139, 155408. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J.; Wang, Z.; Guo, P.; Liu, A.; Du, G. Identification of Multi-Target Anti-AD Chemical Constituents from Traditional Chinese Medicine Formulae by Integrating Virtual Screening and In Vitro Validation. Front. Pharmacol. 2021, 12, 709607. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Cheng, X.; Lin, L.; Wang, Q.; Zhan, R.; Wu, Q.; Liu, S. Protective Effect of Ferulic Acid on Lipopolysaccharide-Induced BV2 Microglia Inflammation via AMPK/mTOR Signaling Pathway. Molecules 2023, 28, 3482. [Google Scholar] [CrossRef]
- Zhang, D.; Jing, B.; Chen, Z.-N.; Li, X.; Shi, H.-M.; Zheng, Y.C.; Chang, S.Q.; Gao, L.; Zhao, G.P. Ferulic Acid Alleviates Sciatica by Inhibiting Neuroinflammation and Promoting Nerve Repair via the TLR4/NF-κB Pathway. CNS Neurosci. Ther. 2023, 29, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Nie, Y.; Huang, C.; Zhu, G.; Zhang, X.; Hu, C.; Li, Z.; Gao, Y.; Ma, Z. Ferulic Acid Produces Neuroprotection against Radiation-Induced Neuroinflammation by Affecting NLRP3 Inflammasome Activation. Int. J. Radiat. Biol. 2022, 98, 1442–1451. [Google Scholar] [CrossRef]
- Hu, Z.; Xuan, L.; Wu, T.; Jiang, N.; Liu, X.; Chang, J.; Wang, T.; Han, N.; Tian, X. Taxifolin attenuates neuroinflammation and microglial pyroptosis via the PI3K/Akt signaling pathway after spinal cord injury. Int. Immunopharmacol. 2023, 114, 109616. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Llorens Folgado, S.; Rajabpour-Sanati, A.; Khazdair, M.R.; Samarghandian, S. Green tea catechins inhibit microglial activation which prevents the development of neurological disorders. Neural Regen. Res. 2020, 15, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Dornelles, G.L.; de Oliveira, J.S.; de Almeida, E.J.R.; Mello, C.B.E.; Rodrigues, B.R.E.; da Silva, C.B.; Petry, L.D.S.; Pillat, M.M.; Palma, T.V.; de Andrade, C.M. Ellagic Acid Inhibits Neuroinflammation and Cognitive Impairment Induced by Lipopolysaccharides. Neurochem. Res. 2020, 45, 2456–2473. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, M.; Jeon, C.J.; Goh, G.; Kim, M. Neuroinflammatory suppression with protocatechuic acid attenuates Alzheimer’s disease phenotypes in 5 FAD transgenic mice. Biomed. Pharmacother. 2025, 192, 118598. [Google Scholar] [CrossRef]
- Guan, S.; Miao, F.; Wang, D.; Hu, J.; Wang, H. Corilagin Attenuates Morphine-Induced BV2 Microglial Activation and Inflammation via Regulating TLR2-Mediated Endoplasmic Reticulum Stress. J. Toxicol. Sci. 2023, 48, 387–398. [Google Scholar] [CrossRef]
- Kim, H.; Ralph, J.; Lu, F.; Ralph, S.A.; Boudet, A.M.; MacKay, J.J.; Sederoff, R.R.; Ito, T.; Kawai, S.; Ohashi, H.; et al. NMR analysis of lignins in CAD-deficient plants. Part 1. Incorporation of hydroxycinnamaldehydes and hydroxybenzaldehydes into lignins. Org. Biomol. Chem. 2003, 1, 268–281. [Google Scholar] [CrossRef]
- Jiang, W.J.; Ishiuchi, K.; Furukawa, M.; Takamiya, T.; Kitanaka, S.; Iijima, H. Stereospecific inhibition of nitric oxide production in macrophage cells by flavanonols: Synthesis and the structure-activity relationship. Bioorg. Med. Chem. 2015, 23, 6922–6929. [Google Scholar] [CrossRef]
- Sudjaroen, Y.; Hull, W.E.; Erben, G.; Würtele, G.; Changbumrung, S.; Ulrich, C.M.; Owen, R.W. Isolation and characterization of ellagitannins as the major polyphenolic components of Longan (Dimocarpus longan Lour) seeds. Phytochemistry 2012, 77, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Hashida, K.; Tabata, M.; Kuroda, K.; Otsuka, Y.; Kubo, S.; Makino, R.; Kubojima, Y.; Tonosaki, M.; Ohara, S. Phenolic extractives in the trunk of Toxicodendron vernicifluum: Chemical characteristics, contents and radial distribution. J. Wood Sci. 2014, 60, 160–168. [Google Scholar] [CrossRef]
- Choi, H.-R.; Nam, K.-M.; Lee, H.-S.; Yang, S.-H.; Kim, Y.-S.; Lee, J.; Date, A.; Toyama, K.; Park, K.-C. Phlorizin, an Active Ingredient of Eleutherococcus senticosus, Increases Proliferative Potential of Keratinocytes with Inhibition of MiR135b and Increased Expression of Type IV Collagen. Oxid. Med. Cell. Longev. 2016, 2016, 3859721. [Google Scholar] [CrossRef]
- Saeed, A.; Qasim, M. An efficient total synthesis of trilepisiumic acid. Nat. Prod. Res. 2014, 28, 1121–1126. [Google Scholar] [CrossRef]
- Sajjadi, S.E.; Shokoohinia, Y.; Moayedi, N.-S. Isolation and Identification of Ferulic Acid from Aerial Parts of Kelussia odoratissima Mozaff. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.J.; Park, H.J.; Park, J.H.; Cho, J.G.; Kang, J.H.; Jeong, T.S.; Kang, H.C.; Lee, D.Y.; Kim, H.S.; Byun, S.Y.; et al. Flavonoids from Machilus japonica Stems and Their Inhibitory Effects on LDL Oxidation. Int. J. Mol. Sci. 2014, 15, 16418–16429. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, C.F.; Shen, S.M.; Wang, G.L.; Liu, P.; Liu, Z.M.; Wang, Y.Y.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Antioxidant Phenolic Compounds from Pu-erh Tea. Molecules 2012, 17, 14037–14045. [Google Scholar] [CrossRef]
- Orabi, M.A.A.; Orabi, E.A.; Al Awadh, A.A.; Alshahrani, M.M.; Abdel-Wahab, B.A.; Sakagami, H.; Hatano, T. New Megastigmane and Polyphenolic Components of Henna Leaves and Their Tumor-Specific Cytotoxicity on Human Oral Squamous Carcinoma Cell Lines. Antioxidants 2023, 12, 1951. [Google Scholar] [CrossRef]
- Teethaisong, Y.; Win, H.H.; Thummavongsa, T.; Eumkeb, G.; Dunkhunthod, B.; Posoongnoen, S.; Santhi, M.; Gorantla, J.N.; Ketudat-Cairns, M.; Ketudat-Cairns, J.R. Identification of Protocatechuic Acid as an Anti-Acne Component in Extracts of Black Rice Bran. Sci. Rep. 2025, 15, 21151. [Google Scholar] [CrossRef]
- Adesina, S.K.; Idowu, O.; Ogundaini, A.O.; Oladimeji, H.; Olugbade, T.A.; Onawunmi, G.O.; Pais, M. Antimicrobial Constituents of the Leaves of Acalypha wilkesiana and Acalypha hispida. Phytother. Res. 2000, 14, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Iqbal, E.Y.; Siddiqui, P.J.A.; Sattar, M.A.; Faizi, S. Nematicidal Potential of Ceriops tagal Extracts and Authentic Compounds against Cyst Nematode Heterodera zeae. J. Agric. Food Chem. 2025, 73, 17550–17564. [Google Scholar] [CrossRef]
- Liu, Z.; Yoon, C.S.; Lee, H.; Kim, E.; Yim, J.H.; Kim, T.K.; Oh, H.; Lee, D.S. The neuroprotective and anti-neuroinflammatory effects of ramalin synthetic derivatives in BV2 and HT22 cells. Biochem. Pharmacol. 2025, 231, 116654. [Google Scholar] [CrossRef]
- She, Y.; Shao, C.Y.; Liu, Y.F.; Huang, Y.; Yang, J.; Wan, H.T. Catalpol reduced LPS induced BV2 immunoreactivity through NF-κB/NLRP3 pathways: An in Vitro and in silico study. Front. Pharmacol. 2024, 15, 1415445. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, D.; Yin, H.; Li, H.; Du, J.; Bao, H. Ganoderic acid A attenuates LPS-induced neuroinflammation in BV2 microglia by activating farnesoid X receptor. Neurochem. Res. 2021, 46, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Vinh, L.B.; Tuan, N.Q.; Lee, H.; Kim, E.; Kim, Y.C.; Sohn, J.H.; Yim, J.H.; Lee, H.J.; Lee, D.S.; et al. Macrosphelides from Antarctic fungus Pseudogymnoascus sp. (strain SF-7351) and their neuroprotective effects on BV2 and HT22 cells. Chem. Biol. Interact. 2023, 385, 110718. [Google Scholar] [CrossRef] [PubMed]
| R.T. (min) | M.F. | M.W. | Adduct | MS2 Ion Fragments (m/z) | I.D. | M.A.S. | Class | I.D. ref. |
|---|---|---|---|---|---|---|---|---|
| 2.01 | C13H16O10 | 332.1 | [M − H]− | 125.023, 169.011 | Gallic acid hexoside | 1 | Phenol | [20] |
| 2.46 | C7H6O5 | 170.0 | [M + H]+ | 81.034, 107.013, 127.039, 153.018 | Gallic acid | 1 | Phenol | [20,21] |
| 3.70 | C20H20O13 | 126.0 | [M + H]+ | 53.039, 81.034, 109.029 | Pyrogallol | 0.97 | Phenol | [23] |
| 4.00 | C13H16O10 | 468.1 | [M + H]+ | 111.044, 125.024, 153.018, 299.076 | Ginnalin A | 1 | Phenol | [24] |
| 4.56 | C20H20O14 | 484.1 | [M − H]− | 85.028, 95.012, 113.023, 125.023, 169.013, 313.057 | Hamamelitannin | 1 | Tannin | [25] |
| 4.74 | C7H6O4 | 154.0 | [M − H]− | 81.033, 108.020, 109.028 | Protocatechuic acid | 0.81 | Phenol | [19,21] |
| 6.96 | C7H6O3 | 138.0 | [M − H]− | 81.033, 108.020, 109.028, 136.015 | Protocatechuic aldehyde | 0.77 | Phenol | [26] |
| 9.20 | C30H26O12 | 578.1 | [M − H]− | 109.028, 125.023, 161.024, 289.072, 339.086, 407.077, 425.089 | Procyanidin B1 | 1 | Flavonoid | [20,25] |
| 9.68 | C7H6O3 | 138.0 | [M + H]+ | 55.019, 65.039, 69.034, 111.044 | 2,5-Dihydroxybenzaldehyde | 1 | Phenol | [32] |
| 9.68 | C15H14O6 | 290.1 | [M − H]− | 57.033, 97.028, 109.028, 125.023, 151.039, 203.070, 245.082 | Catechin | 1 | Flavonoid | [22,23] |
| 11.12 | C27H22O18 | 634.1 | [M − H]− | 123.008, 125.023, 169.013, 173.024, 185.024, 275.021, 300.999 | Corilagin | 0.98 | Tannin | [20] |
| 12.19 | C27H24O18 | 636.1 | [M − H]− | 65.853, 75.418, 85.028, 95.012, 125.023, 169.013, 313.057, 465.067 | 1,3,6-tri-O-galloyl glucose | 0.98 | Tannin | [22,25] |
| 13.28 | C15H14O6 | 290.1 | [M − H]− | 109.028, 123.044, 151.039, 179.034, 203.071, 245.082, 289.072 | Epicatechin | 0.97 | Flavonoid | [20,25] |
| 13.37 | C16H18O8 | 338.1 | [M + H]+ | - | 1-Coumaroylquinic acid | 1 | Phenol | [19] |
| 13.76 | C10H8O5 | 208.0 | [M + H]+ | 107.049, 135.044, 153.055, 163.039, 194.021 | Fraxetin | 0.99 | Coumarin | [27] |
| 14.53 | C15H12O7 | 304.1 | [M + H]+ | 123.044, 149.023, 153.018, 195.029, 231.065, 259.060, 287.055 | (2R,3R)-2-(2,6-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one | 0.99 | Flavonoid | [23] |
| 15.51 | C21H22O12 | 466.1 | [M − H]− | 107.012, 151.003, 151.039, 178.997, 285.041, 303.003, 339.073 | (2R,3R)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-2,3-dihydrochromen-4-one | 1 | Flavonoid | [33] |
| 16.65 | C10H8O4 | 192.0 | [M + H]+ | 133.029, 149.060, 150.031, 178.026 | Scopoletin | 0.98 | Coumarin | [26] |
| 16.90 | C10H10O4 | 194.1 | [M − H]− | - | Ferulic acid | 0.91 | Phenol | [20] |
| 17.91 | C15H12O7 | 304.1 | [M + H]+ | 123.044, 149.023, 153.018, 231.065, 259.060 | Taxifolin | 1 | Flavonoid | [21] |
| 18.12 | C11H10O5 | 222.1 | [M + H]+ | 107.050, 134.036, 162.031, 190.026, 208.037 | Fraxidin | 1 | Coumarin | [31] |
| 18.78 | C14H6O8 | 302.0 | [M + H]+ | 173.024, 201.018, 229.013, 257.008, 285.003 | Ellagic acid | 1 | Tannin | [20,21] |
| 18.86 | C25H32O13 | 540.2 | [M − H]− | 59.012, 89.025, 101.025, 123.008, 163.111 | Oleuropein | 1 | Phenol | [30] |
| 19.23 | C22H18O10 | 442.1 | [M − H]− | - | Epicatechin gallate | 1 | Flavonoid | [22] |
| 19.38 | C15H12O8 | 320.1 | [M − H]− | 102.288, 133.029, 145.028, 173.024, 189.019 | Dihydromyricetin | 1 | Flavonoid | [29] |
| 19.41 | C10H10O3 | 178.1 | [M + H]+ | 55.019, 119.049, 133.065, 147.044, 161.060 | Coniferaldehyde | 1 | Phenol | [26] |
| 20.18 | C11H12O4 | 208.1 | [M + H]+ | - | Sinapoyl aldehyde | 1 | Phenol | [26] |
| 21.17 | C21H24O10 | 436.1 | [M + H]+ | 81.034, 109.029, 125.022, 143.034, 171.029 | Phloretin-2′-O-glucoside | 1 | Phenol | [19] |
| 22.55 | C11H12O4 | 208.1 | [M + H]+ | 133.065, 148.052, 163.075, 191.071 | Dimethylcaffeic acid | 1 | Phenol | [28] |
| 22.76 | C19H22O5 | 330.1 | [M + H]+ | 137.060, 151.075, 189.091, 227.107, 255.102, 285.112, 287.128, 313.144, 331.202 | Tetrahydrosappanone A Trimethyl Ether | 0.99 | Phenol | [34] |
| 23.82 | C15H10O7 | 302.0 | [M − H]− | - | Quercetin | 0.99 | Flavonoid | [19,20] |
| Compound | Name | IC50 ± S.D. |
|---|---|---|
| Compound 1 | Gallic acid | - |
| Compound 2 | Sinapaldehyde | 13.68 ± 2.38 |
| Compound 3 | Coniferaldehyde | 9.96 ± 3.03 |
| Compound 4 | 3,5,7,2′,6′-pentahydroxyflavanone | 22.55 ± 0.77 |
| Compound 5 | 1,2,3,6-Tetrakis-O-galloyl-beta-D-glucose | - |
| Compound 6 | Phlorizin | - |
| Compound 7 | 3,4-Dimethoxycinnamic acid | - |
| Compound 8 | Ferulic acid | - |
| Compound 9 | Taxifolin | - |
| Compound 10 | Catechin | - |
| Compound 11 | Ellagic acid | - |
| Compound 12 | Protocatechuic acid | - |
| Compound 13 | Corilagin | 19.60 ± 1.53 |
| Compound 14 | 2,5-Dihydroxybenzaldehyde | 7.41 ± 1.40 |
| Compound Name (I.D. Number) | Neuro-Inflammation Activity Determination Model | Ref. |
|---|---|---|
| Gallic acid (1) | BV2 microglial cells, rat primary microglia | [35,36] |
| Sinapaldehyde (2) | BV2 microglial cells | [37] |
| Coniferaldehyde (3) | BV2 microglial cells, leptomeningeal cells | [38,39] |
| 3,5,7,2′,6′-Pentahydroxyflavanone (4) | - | - |
| 1,2,3,6-Tetrakis-O-galloyl-β-D-glucose (5) | - | - |
| Phlorizin (6) | Mice | [40] |
| 3,4-Dimethoxycinnamic acid (7) | BV2 microglial cells | [41] |
| Ferulic acid (8) | BV2 microglial cells, rats, mice | [42,43,44] |
| Taxifolin (9) | BV2 microglial cells | [45] |
| Catechin (10) | BV2 microglial cells | [46] |
| Ellagic acid (11) | Rats | [47] |
| Protocatechuic acid (12) | Mice | [48] |
| Corilagin (13) | BV2 microglial cells | [49] |
| 2,5-Dihydroxybenzaldehyde (14) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Jin, Y.; Hong, J.-A.; Bai, C.; Lee, G.; Woo, S.; Yoon, C.-S.; Bae, D.; Lee, D.-S. Anti-Neuroinflammatory Effects of Compounds Isolated from Quercus acuta Thunb. Fruits via NF-κB Signaling Inhibition in BV2 Microglia. Molecules 2025, 30, 4514. https://doi.org/10.3390/molecules30234514
Lee H, Jin Y, Hong J-A, Bai C, Lee G, Woo S, Yoon C-S, Bae D, Lee D-S. Anti-Neuroinflammatory Effects of Compounds Isolated from Quercus acuta Thunb. Fruits via NF-κB Signaling Inhibition in BV2 Microglia. Molecules. 2025; 30(23):4514. https://doi.org/10.3390/molecules30234514
Chicago/Turabian StyleLee, Hwan, Yezhi Jin, Ji-Ae Hong, Chenyang Bai, Gyoyoung Lee, Suhyeon Woo, Chi-Su Yoon, Donghyuck Bae, and Dong-Sung Lee. 2025. "Anti-Neuroinflammatory Effects of Compounds Isolated from Quercus acuta Thunb. Fruits via NF-κB Signaling Inhibition in BV2 Microglia" Molecules 30, no. 23: 4514. https://doi.org/10.3390/molecules30234514
APA StyleLee, H., Jin, Y., Hong, J.-A., Bai, C., Lee, G., Woo, S., Yoon, C.-S., Bae, D., & Lee, D.-S. (2025). Anti-Neuroinflammatory Effects of Compounds Isolated from Quercus acuta Thunb. Fruits via NF-κB Signaling Inhibition in BV2 Microglia. Molecules, 30(23), 4514. https://doi.org/10.3390/molecules30234514






