Clinoptilolite-Based Adsorbents for Paracetamol Removal
Abstract
1. Introduction
- To prepare and characterize natural clinoptilolite, its proton-exchanged form, and an HDTMA-modified organo-zeolite;
- To evaluate how these modification strategies influence the structural and surface properties of clinoptilolite;
- To investigate the adsorption of paracetamol from aqueous solutions onto the three zeolite forms;
- To determine the effect of surface chemistry on adsorption performance;
- To critically assess the practical limitations of clinoptilolite-based materials as adsorbents for weakly polar pharmaceuticals.
2. Results and Discussion
2.1. Materials Characterization
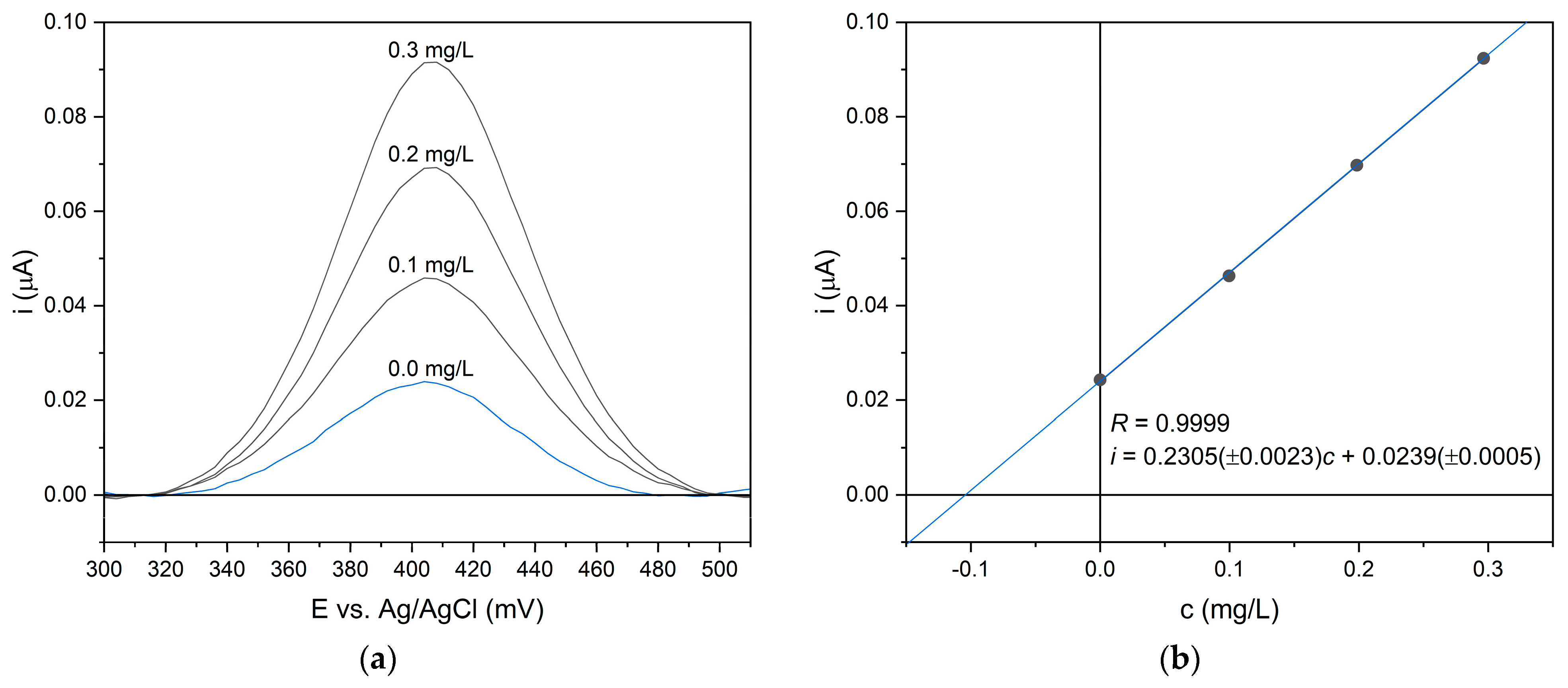
2.2. Adsorption Studies Determined by Voltammetric Analysis
3. Materials and Methods
3.1. Starting Materials
3.2. Characterization Methods
3.3. Adsorption Experiments
3.4. Differential Pulse Voltammetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGCE | activated glassy carbon electrodes |
| AOP | advanced oxidation process |
| CPE | carbon paste electrodes |
| DPV | differential pulse voltammetry |
| FT-IR | Fourier-transform infrared spectroscopy |
| GC | gas chromatography |
| GCE | glassy carbon electrodes |
| HDTMA | hexadecyltrimethylammonium |
| HPLC | high-performance liquid chromatography |
| PCT | Paracetamol |
| rGO | reduced graphene oxide |
| SEM | scanning electron microscope |
| SWV | square wave voltammetry |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
References
- Bosch, M.E.; Sánchez, A.J.R.; Rojas, F.S.; Ojeda, C.B. Determination of paracetamol: Historical evolution. J. Pharm. Biomed. Anal. 2006, 42, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N. Paracetamol—A contaminant of high concern: Existence in environment and adverse effect. PDRAJ 2022, 5, 000128. [Google Scholar] [CrossRef]
- Vieira, Y.; Spode, J.E.; Dotto, G.L.; Georgin, J.; Franco, D.S.P.; dos Reis, G.S.; Lima, E.C. Paracetamol environmental remediation and ecotoxicology: A review. Environ. Chem. Lett. 2024, 22, 2343–2373. [Google Scholar] [CrossRef]
- Jóźwiak-Bebenista, M.; Nowak, J.Z. Paracetamol: Mechanism of action, applications and safety concern. Acta Pol. Pharm. 2014, 71, 11–23. [Google Scholar] [PubMed]
- Bertolini, A.; Ferrari, A.; Ottani, A.; Guerzoni, S.; Tacchi, R.; Leone, S. Paracetamol: New vistas of an old drug. CNS Drug Rev. 2006, 12, 250–275. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Wu, Z.-L.; Lin, S.-K. Electrochemical detection of paracetamol using zeolitic imidazolate framework and graphene oxide derived zinc/nitrogen-doped carbon. Sens. Actuators. B Chem. 2024, 409, 135600. [Google Scholar] [CrossRef]
- Skwarczynska-Wojsa, A.; Puszkarewicz, A. Removal of acetaminophen from aqueous solutions in an adsorption process. Materials 2024, 17, 431. [Google Scholar] [CrossRef]
- Bauer, A.Z.; Swan, S.H.; Kriebel, D.; Liew, Z.; Taylor, H.S.; Bornehag, C.-G.; Andrade, A.M.; Olsen, J.; Jensen, R.H.; Mitchell, R.T.; et al. Paracetamol use during pregnancy—A call for precautionary action. Nat. Rev. Endocrinol. 2021, 17, 757–766. [Google Scholar] [CrossRef]
- Khmiri, Y.; Attia, A.; Jallouli, N.; Chabanon, E.; Charcosset, C.; Mahouche-Chergui, S.; Dammak, L.; Algieri, C.; Chakraborty, S.; Amar, R.B. Synthesis of a cost-effective ZnO/zeolite photocatalyst for paracetamol removal. Emergent Mater. 2025. [Google Scholar] [CrossRef]
- Montaseri, H.; Forbes, P.B.C. Analytical techniques for the determination of acetaminophen: A review. TrAC Trends Anal. Chem. 2018, 108, 122–134. [Google Scholar] [CrossRef]
- Al-howri, B.M.; Azha, S.F.; Shamsudin, M.S.; Hamid, N.A.; Alsobaai, A.M.; Ismail, S. Paracetamol in diverse water sources: Health hazards and treatment efficacy emphasizing adsorption techniques—A review. Int. J. Environ. Sci. Technol. 2024, 21, 9743–9762. [Google Scholar] [CrossRef]
- Yenealem, D.; Admassie, S.; Pilli, S.R.; Motana, S.; Tibebe, D. Detection and separation of paracetamol and p-aminophenol using activated glassy carbon electrodes. Electrocatalysis 2025, 16, 548–557. [Google Scholar] [CrossRef]
- Weheabby, S.; Wu, Z.; Al-Hamry, A.; Pašti, I.A.; Anurag, A.; Dentel, D.; Tegenkamp, C.; Kanoun, O. Paracetamol detection in environmental and pharmaceutical samples using multi-walled carbon nanotubes decorated with silver nanoparticles. Microchem. J. 2023, 193, 109192. [Google Scholar] [CrossRef]
- Nguyen, T.-K.-T.; Nguyen, T.-B.; Chen, C.-W.; Chen, W.-H.; Chen, L.; Hsieh, S.; Dong, C.-D. Kumquat peel-derived biochar to support zeolitic imidazole framework-67 (ZIF-67) for enhancing peracetic acid activation to remove acetaminophen from aqueous solution. Environ. Pollut. 2024, 350, 123970. [Google Scholar] [CrossRef]
- Kryuchkova, M.; Batasheva, S.; Akhatova, F.; Babaev, V.; Buzyurova, D.; Vikulina, A.; Volodkin, D.; Fakhrullin, R.; Rozhina, E. Pharmaceuticals removal by adsorption with montmorillonite nanoclay. Int. J. Mol. Sci. 2021, 22, 9670. [Google Scholar] [CrossRef] [PubMed]
- Parus, A.; Gaj, M.; Karbowska, B.; Zembrzuska, J. Investigation of acetaminophen adsorption with a biosorbent as a purification method of aqueous solution. Chem. Ecol. 2020, 36, 705–725. [Google Scholar] [CrossRef]
- Grela, A.; Kuc, J.; Bajda, T. A review on the application of zeolites and mesoporous silica materials in the removal of non-steroidal anti-inflammatory drugs and antibiotics from water. Materials 2021, 14, 4994. [Google Scholar] [CrossRef]
- Al-rimawi, F.; Daana, M.; Khamis, M.; Karaman, R.; Khoury, H.; Qurie, M. Removal of selected pharmaceuticals from aqueous solutions using natural Jordanian zeolite. Arab. J. Sci. Eng. 2019, 44, 209–215. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Arboleda-Echavarría, J.; Echavarría-Isaza, A.; Torres-Palma, R.A. Removal and elimination of pharmaceuticals in water using zeolites in diverse adsorption processes and catalytic advanced oxidation technologies—A critical review. Environ. Sci. Pollut. Res. 2024, 31, 63427–63457. [Google Scholar] [CrossRef]
- Pirvu, F.; Covaliu-Mierlă, C.I.; Catrina, G.A. Removal of acetaminophen drug from wastewater by Fe3O4 and ZSM-5 Materials. Nanomaterials 2023, 13, 1745. [Google Scholar] [CrossRef]
- Fu, M.; He, M.; Heijman, B.; van der Hoek, J.P. Ozone-based regeneration of granular zeolites loaded with acetaminophen. Sep. Purif. Technol. 2021, 256, 117616. [Google Scholar] [CrossRef]
- Araújo, A.; Soares, O.S.G.P.; Orge, C.A.; Gonçalves, A.G.; Rombi, E.; Cutrufello, M.G.; Fonseca, A.M.; Pereira, M.F.R.; Neves, I.C. Metal-zeolite catalysts for the removal of pharmaceutical pollutants in water by catalytic ozonation. J. Environ. Chem. Eng. 2021, 9, 106458. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, D.; Chen, Y.; He, J.; Li, Q. Mechanistic study of highly effective phosphate removal from aqueous solutions over a new lanthanum carbonate fabricated carbon nanotube film. J. Environ. Manag. 2024, 359, 120938. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.H.H.; Mustafa, F.S.; Karim, M.A.H.; Hama, S. Pharmaceutical pollution in the aquatic environment: Advanced oxidation processes as efficient treatment approaches: A review. Mater. Adv. 2025, 6, 3433–3454. [Google Scholar] [CrossRef]
- Onyekachukwu, E.; Nesbitt, H.; Tretsiakova-McNally, S.; Coleman, H. Low-cost adsorbents for the removal of pharmaceuticals from surface waters. Water 2025, 17, 2619. [Google Scholar] [CrossRef]
- Hastuti, B.; Lutfiah, S.; Hadi, S.; Utomo, S.B.; Kamari, A. Development of chitosan/alginate/montmorillonite hydrogel microcomposite as adsorbent for paracetamol removal from waters. Pure Appl. Chem. 2025, 97, 911–927. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Botero-Coy, A.M.; Arboleda-Echavarría, J.; Echavarría-Isaza, A.; Hernández, F.; Torres-Palma, R.A. Simultaneous elimination of multiple pharmaceuticals in real wastewater effluent using a commercial zeolite and peroxymonosulfate. J. Clean. Prod. 2025, 508, 145550. [Google Scholar] [CrossRef]
- Bajda, T.; Grela, A.; Pamuła, J.; Kuc, J.; Klimek, A.; Matusik, J.; Franus, W.; Alagarsamy, S.K.K.; Danek, T.; Gara, P. Using zeolite materials to remove pharmaceuticals from water. Materials 2024, 17, 3848. [Google Scholar] [CrossRef]
- Mallah, M.A.; Sherazi, S.T.H.; Bhanger, M.I.; Mahesar, S.A.; Bajeer, M.A. A Rapid Fourier-transform infrared (FTIR) spectroscopic method for direct quantification of paracetamol content in solid pharmaceutical formulations. Spectrochim. Acta A 2015, 141, 64–70. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Perveen, S.; Hussain, S.G.; Khan, A.A.; Ejaz, S.M.W.; Rizvi, S.M.A. Design of a facile, green and efficient graphene oxide-based electrochemical sensor for analysis of acetaminophen drug. Chem. Pap. 2023, 77, 2275–2294. [Google Scholar] [CrossRef]
- Eskezia Ayalew, M.; Yenealem Ayitegeb, D. Differential pulse voltammetric determination of paracetamol using activated glassy carbon electrode. Am. J. Phys. Chem. 2021, 10, 16. [Google Scholar] [CrossRef]
- Engin, C.; Yilmaz, S.; Saglikoglu, G.; Yagmur, S.; Sadikoglu, M. Electroanalytical investigation of paracetamol on glassy carbon electrode by voltammetry. Int. J. Electrochem. Sci. 2015, 10, 1916–1925. [Google Scholar] [CrossRef]
- Chitravathi, S.; Munichandraiah, N. Voltammetric Determination of Paracetamol, Tramadol and caffeine using poly(nile blue) modified glassy carbon electrode. J. Electroanal. Chem. 2016, 764, 93–103. [Google Scholar] [CrossRef]
- Delolo, F.G.; Rodrigues, C.; Silva, M.M.d.; Dinelli, L.R.; Delling, F.N.; Zukerman-Schpector, J.; Batista, A.A. A new electrochemical sensor containing a film of chitosan-supported ruthenium: Detection and quantification of sildenafil citrate and acetaminophen. J. Braz. Chem. Soc. 2014, 25, 550–559. [Google Scholar] [CrossRef]
- Grifasi, N.; Ziantoni, B.; Fino, D.; Piumetti, M. Fundamental properties and sustainable applications of the natural zeolite clinoptilolite. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Bahmanzadegan, F.; Ghaemi, A. Modification and functionalization of zeolites to improve the efficiency of CO2 adsorption: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100564. [Google Scholar] [CrossRef]
- Akyalcin, S.; Akyalcin, L.; Ertugrul, E. Modification of natural clinoptilolite zeolite to enhance its hydrogen adsorption capacity. Res. Chem. Intermed. 2024, 50, 1455–1473. [Google Scholar] [CrossRef]
- Li, Z.; Bowman, R.S. Sorption of perchloroethylene by surfactant-modified zeolite as controlled by surfactant loading. Environ. Sci. Technol. 1998, 32, 2278–2282. [Google Scholar] [CrossRef]
- Dávila-Estrada, M.; Ramírez-García, J.J.; Solache-Ríos, M.J.; Gallegos-Pérez, J.L. Kinetic and equilibrium sorption studies of ceftriaxone and paracetamol by surfactant-modified zeolite. Water Air Soil Pollut. 2018, 229, 123. [Google Scholar] [CrossRef]
- Mozgawa, W.; Król, M.; Bajda, T. IR spectra in the studies of anion sorption on natural sorbents. J. Mol. Struct. 2011, 993, 109–114. [Google Scholar] [CrossRef]
- Verma, Y.; Sharma, G.; Kumar, A.; Wang, T.; Dhiman, P.; Tessema Mola, G. Zeolites and their composites as novel remediation agent for antibiotics: A review. Environ. Eng. Res. 2025, 30, 240062. [Google Scholar] [CrossRef]
- Porada, R.; Wenninger, N.; Bernhart, C.; Fendrych, K.; Kochana, J.; Baś, B.; Kalcher, K.; Ortner, A. Targeted modification of the carbon paste electrode by natural zeolite and graphene oxide for the enhanced analysis of paracetamol. Microchem. J. 2023, 187, 108455. [Google Scholar] [CrossRef]
- Smiljanić, D.; Daković, A.; Obradović, M.; Ožegović, M.; Izzo, F.; Germinario, C.; de Gennaro, B. Application of surfactant modified natural zeolites for the removal of salicylic acid—A contaminant of emerging concern. Materials 2021, 14, 7728. [Google Scholar] [CrossRef]
- Doczekalska, B.; Kuśmierek, K.; Świątkowski, A. The adsorptive removal of paracetamol as a model pollutant from an aqueous environment using activated carbons made from selected nutshells as agricultural waste. Processes 2025, 13, 2198. [Google Scholar] [CrossRef]
- Iancu, V.-I.; Chiriac, L.-F.; Paun, I.; Dinu, C.; Pirvu, F.; Cojocaru, V.; Tenea, A.G.; Cimpean, I.A. Pharmaceutical contaminants occurrence and ecological risk assessment along the Romanian Black Sea coast. Toxics 2025, 13, 498. [Google Scholar] [CrossRef]
- Baerlocher, C.h.; Brouwer, D.; Marler, B.; McCusker, L.B. Database of Zeolite Structures. Available online: https://www.iza-structure.org/databases/ (accessed on 28 October 2025).
- Barczyk, K.; Mozgawa, W.; Król, M. Studies of anions sorption on natural zeolites. Spectrochim. Acta A 2014, 133, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Haro, N.K.; Dávila, I.V.J.; Nunes, K.G.P.; Espina de Franco, M.A.; Marcilio, N.R.; Féris, L.A. Kinetic, equilibrium and thermodynamic studies of the adsorption of paracetamol in activated carbon in batch model and fixed-bed column. Appl. Water Sci. 2021, 11, 38. [Google Scholar] [CrossRef]
- Aydin, S.; Celik Karakaya, M.; Karakaya, N.; Aydin, M.E. Effective removal of selected pharmaceuticals from sewerage treatment plant effluent using natural clay (Na-montmorillonite). Appl. Water Sci. 2023, 13, 129. [Google Scholar] [CrossRef] [PubMed]
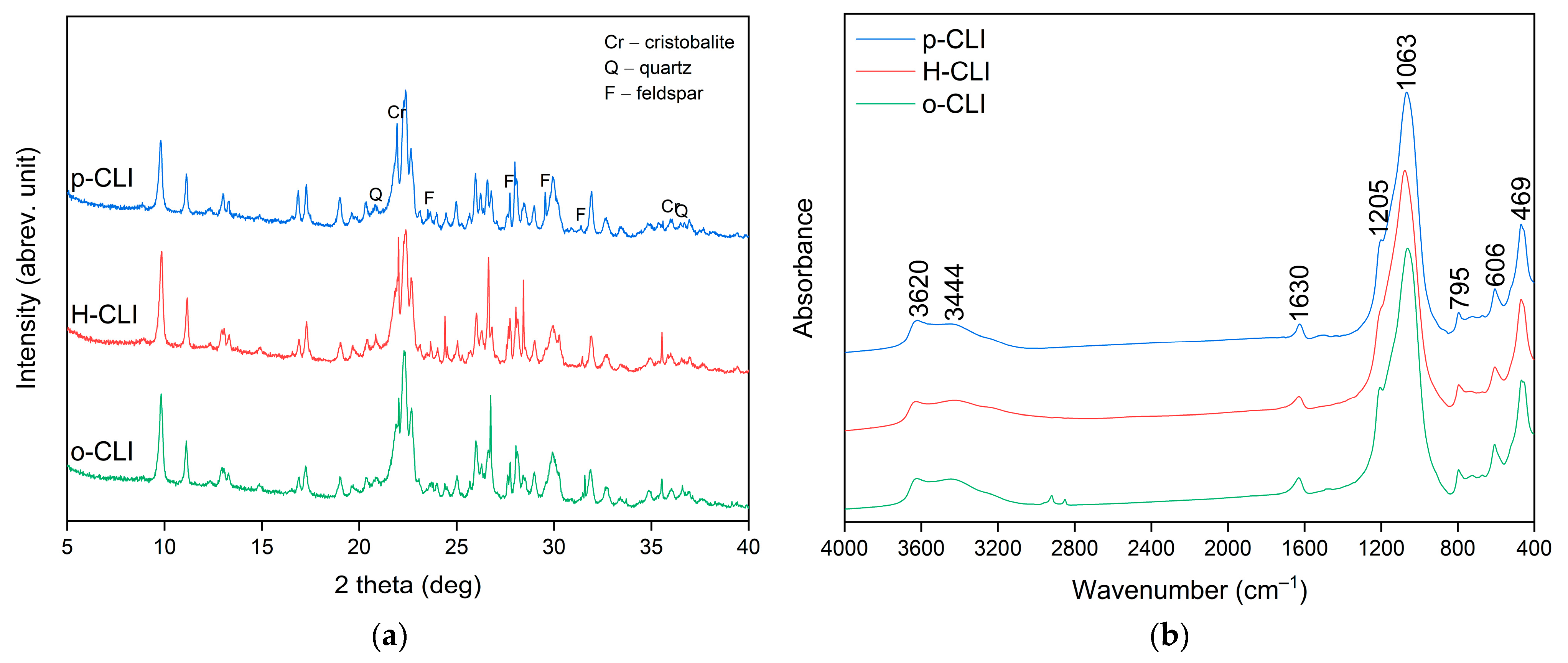

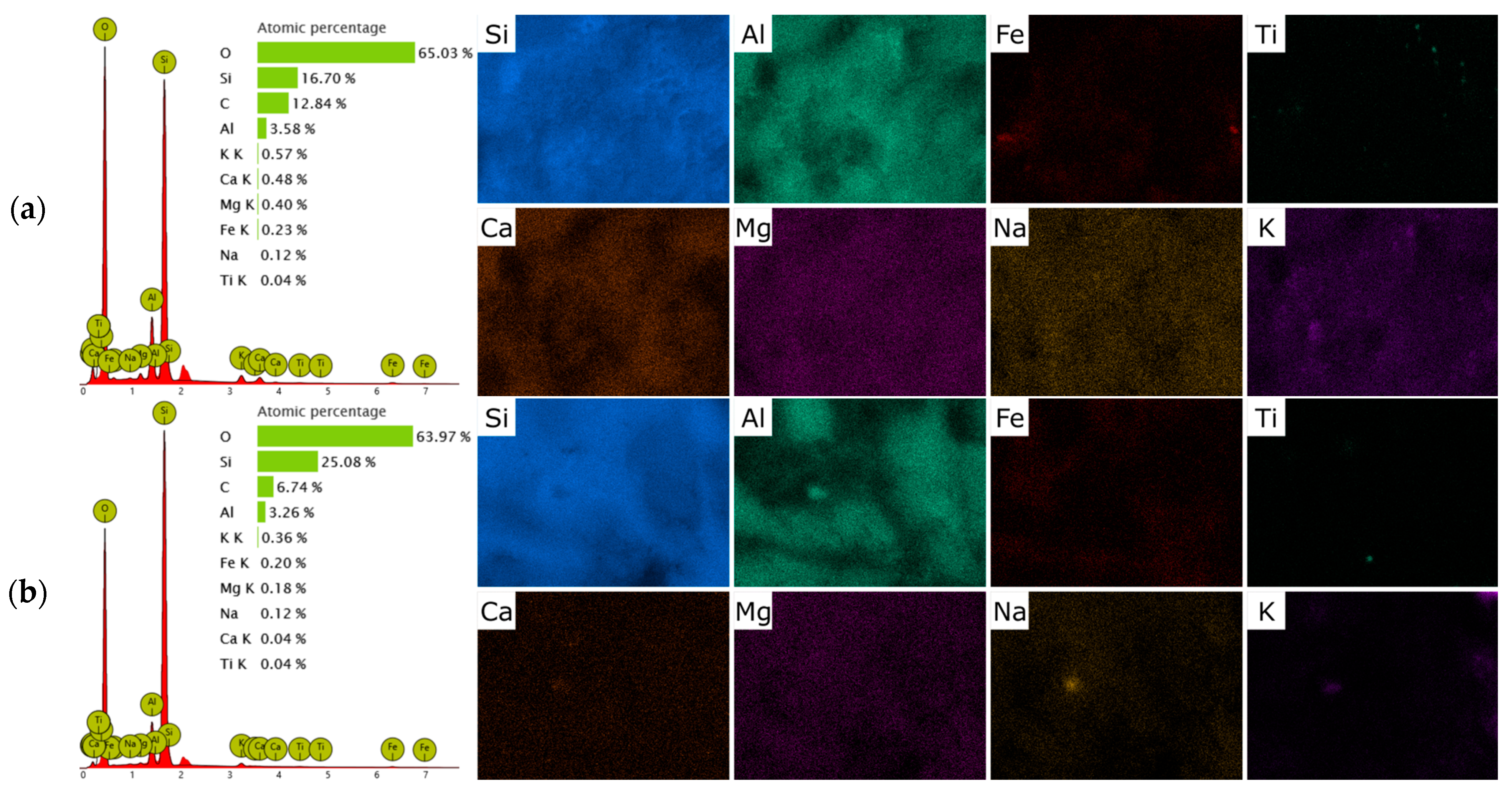
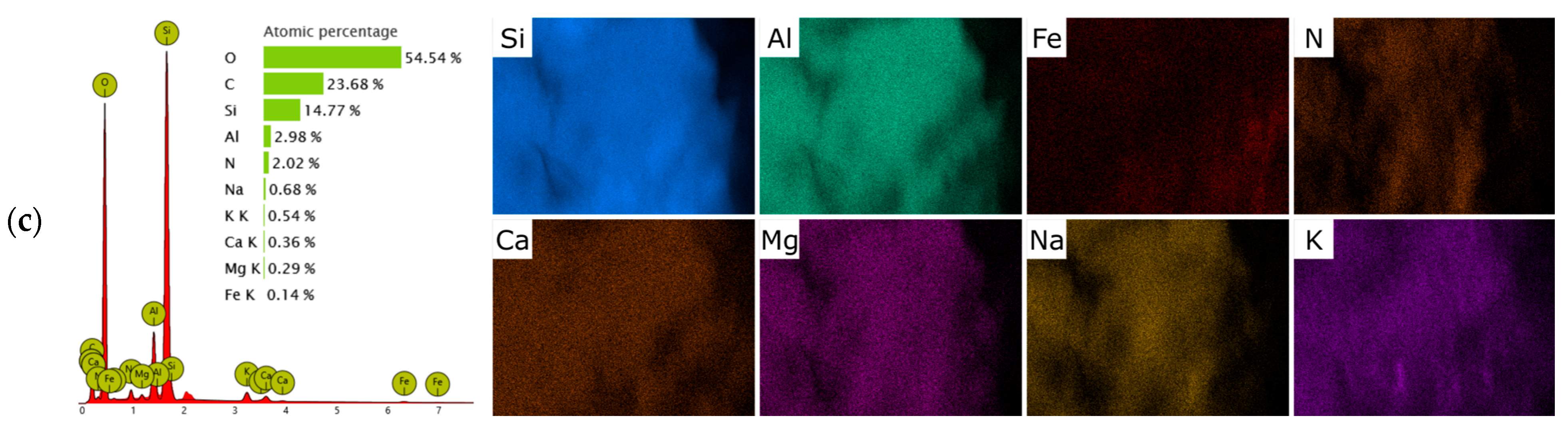

| Oxides | SiO2 | TiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | Others |
|---|---|---|---|---|---|---|---|---|---|
| Ratio by weight, (%) | 75.46 | 0.33 | 11.89 | 2.34 | 4.45 | 0.54 | 3.87 | 0.83 | 0.29 |
| Name | C0, mg/L | R * | Ce, mg/L | R * | qe, mg/g |
|---|---|---|---|---|---|
| p-CLI | 0.57 ± 0.07 | 0.9990 | 0.52 ± 0.01 | 0.9999 | 0.013 ± 0.001 |
| 1.05 ± 0.13 | 0.9987 | 0.92 ± 0.01 | 0.9997 | 0.013 ± 0.001 | |
| 2.81 ± 0.09 | 0.9999 | 2.19 ± 0.03 | 0.9997 | 0.063 ± 0.003 | |
| 5.18 ± 0.13 | 1.0000 | 4.51 ± 0.14 | 0.9962 | 0.067 ± 0.014 | |
| 10.20 ± 0.11 | 0.9991 | 8.70 ± 0.28 | 0.9999 | 0.150 ± 0.035 | |
| H-CLI | 0.59 ± 0.02 | 0.9997 | 0.28 ± 0.01 | 0.9999 | 0.031 ± 0.001 |
| 1.14 ± 0.09 | 0.9989 | 0.68 ± 0.04 | 1.0000 | 0.047 ± 0.001 | |
| 2.80 ± 0.22 | 0.9985 | 1.43 ± 0.14 | 1.0000 | 0.137 ± 0.002 | |
| 5.64 ± 0.16 | 0.9999 | 4.07 ± 0.10 | 0.9999 | 0.157 ± 0.004 | |
| 14.53 ± 0.30 | 0.9999 | 8.55 ± 0.23 | 0.9999 | 0.598 ± 0.002 | |
| o-CLI | 0.59 ± 0.02 | 0.9997 | 0.47 ± 0.02 | 0.9997 | 0.013 ± 0.001 |
| 1.14 ± 0.09 | 0.9989 | 0.95 ± 0.06 | 0.9995 | 0.019 ± 0.001 | |
| 2.80 ± 0.22 | 0.9985 | 1.89 ± 0.13 | 0.9988 | 0.091 ± 0.002 | |
| 5.64 ± 0.16 | 0.9999 | 4.45 ± 0.16 | 0.9999 | 0.119 ± 0.008 | |
| 14.53 ± 0.30 | 0.9999 | 8.86 ± 0.53 | 0.9996 | 0.567 ± 0.028 |
| Name | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax, mg/g | KL, L/mg | R2 | KF, (mg/g)(L/mg)1⁄n | 1/n | R2 | |
| p-CLI | −0.18 | −0.0634 | 0.3122 | 0.0136 | 1.2830 | 0.9471 |
| H-CLI | 1.45 | 0.0541 | 0.1274 | 0.0787 | 0.8140 | 0.9250 |
| o-CLI | −0.38 | −0.0639 | 0.4147 | 0.0282 | 1.2604 | 0.9416 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, S.; Fendrych, K.; Mozgawa, W.; Król, M. Clinoptilolite-Based Adsorbents for Paracetamol Removal. Molecules 2025, 30, 4506. https://doi.org/10.3390/molecules30234506
Wójcik S, Fendrych K, Mozgawa W, Król M. Clinoptilolite-Based Adsorbents for Paracetamol Removal. Molecules. 2025; 30(23):4506. https://doi.org/10.3390/molecules30234506
Chicago/Turabian StyleWójcik, Szymon, Katarzyna Fendrych, Włodzimierz Mozgawa, and Magdalena Król. 2025. "Clinoptilolite-Based Adsorbents for Paracetamol Removal" Molecules 30, no. 23: 4506. https://doi.org/10.3390/molecules30234506
APA StyleWójcik, S., Fendrych, K., Mozgawa, W., & Król, M. (2025). Clinoptilolite-Based Adsorbents for Paracetamol Removal. Molecules, 30(23), 4506. https://doi.org/10.3390/molecules30234506







