A Carboxyl-Modified Polyaniline Cathode for High-Performance Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
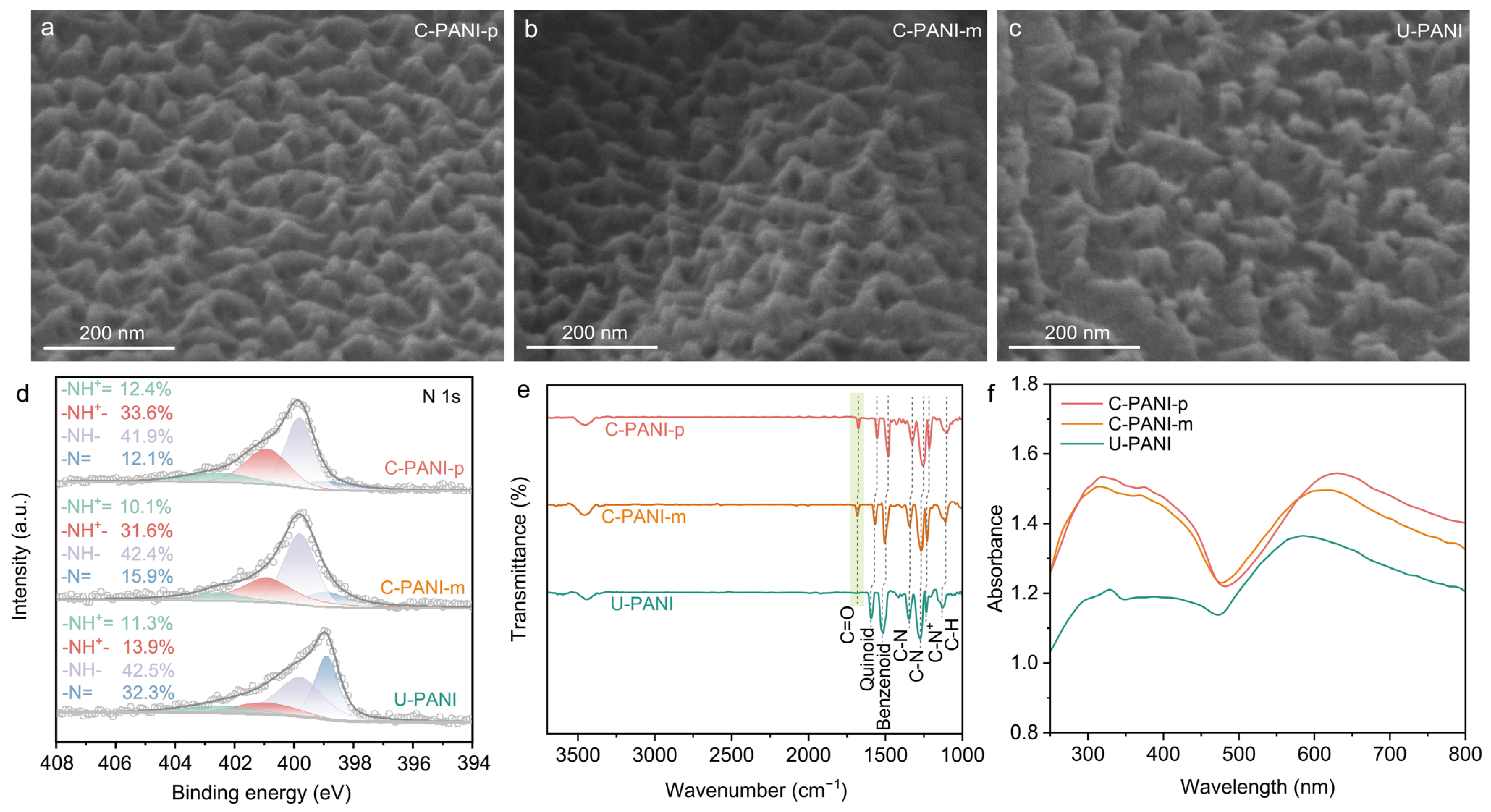
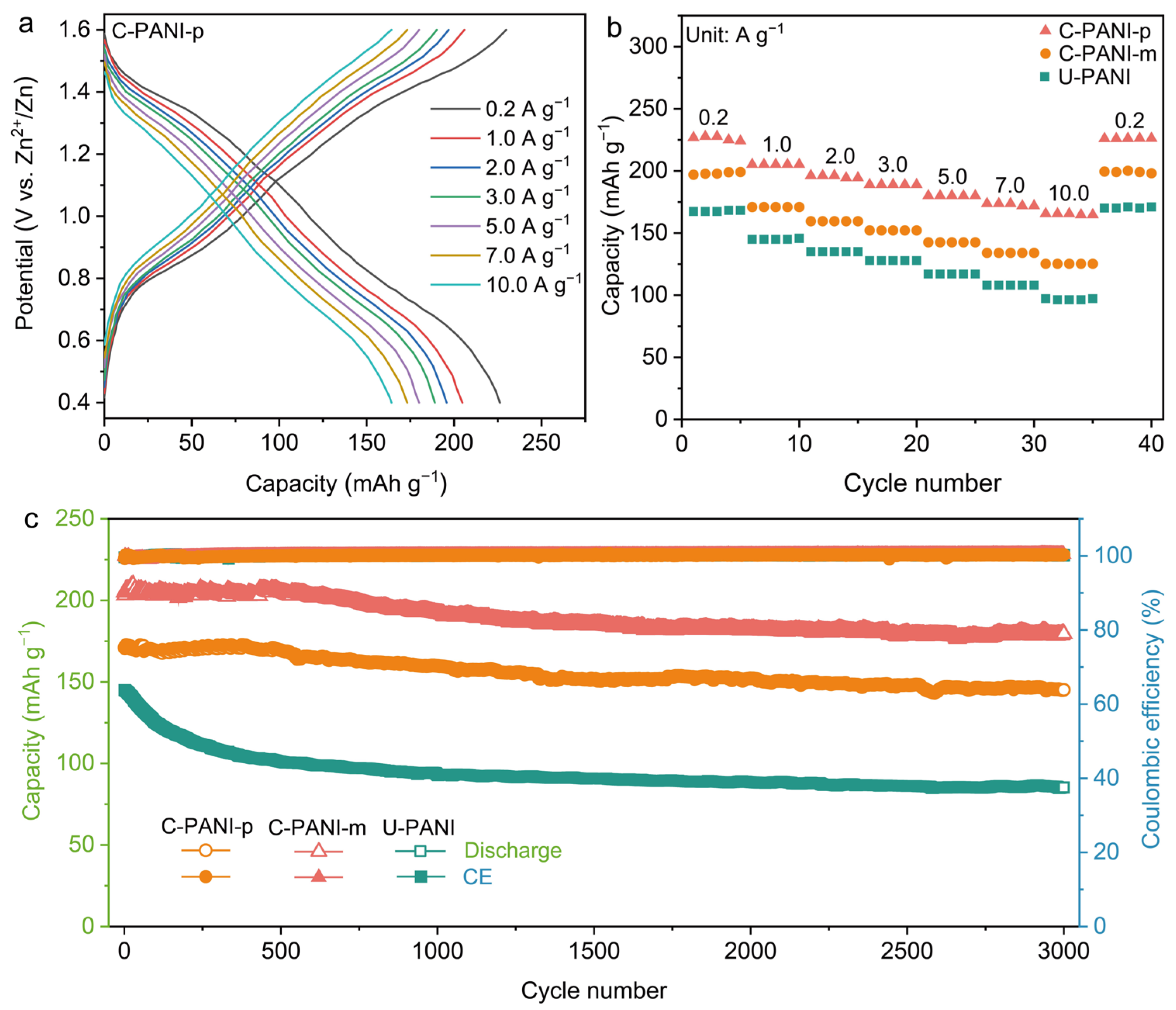
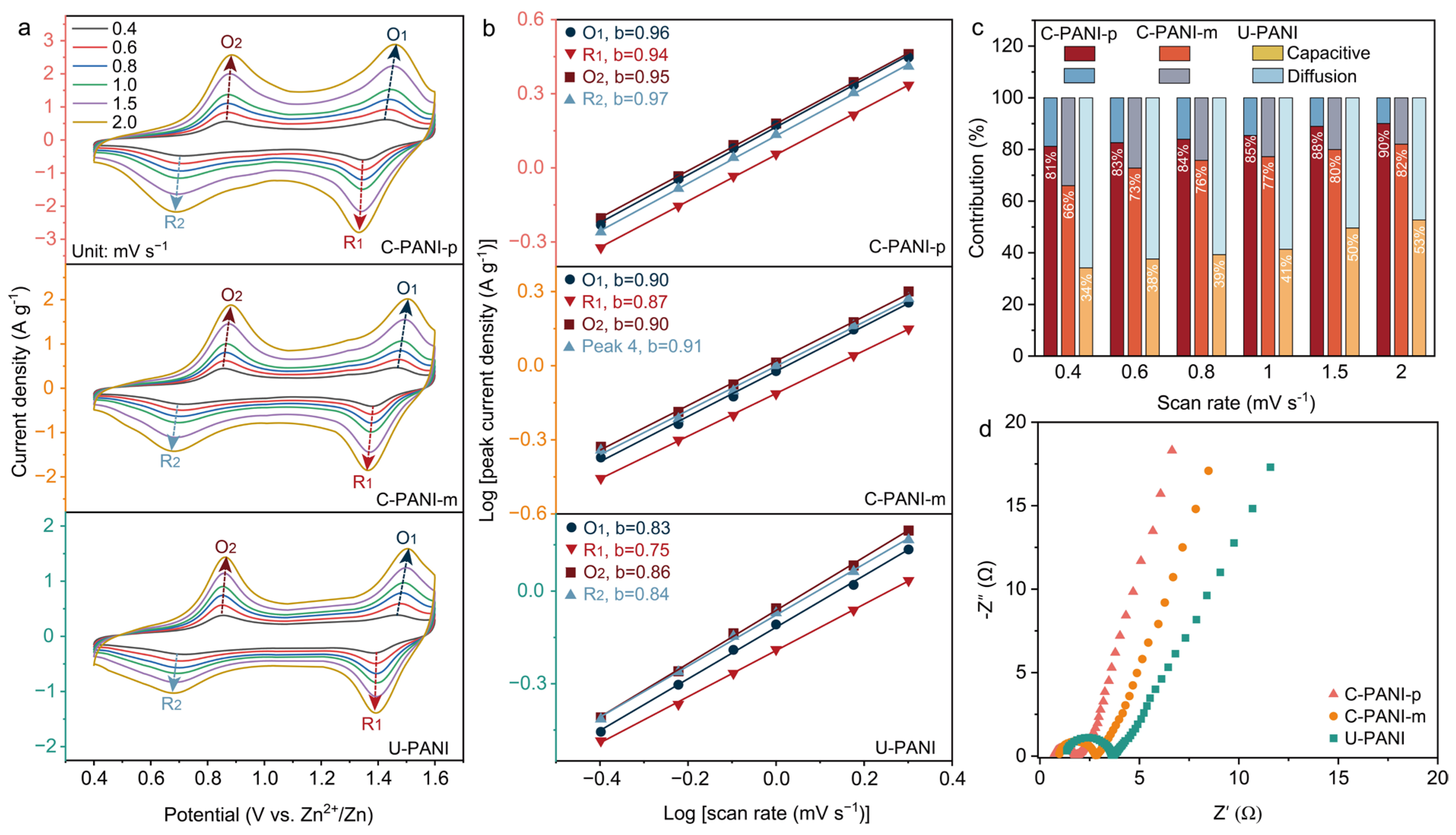
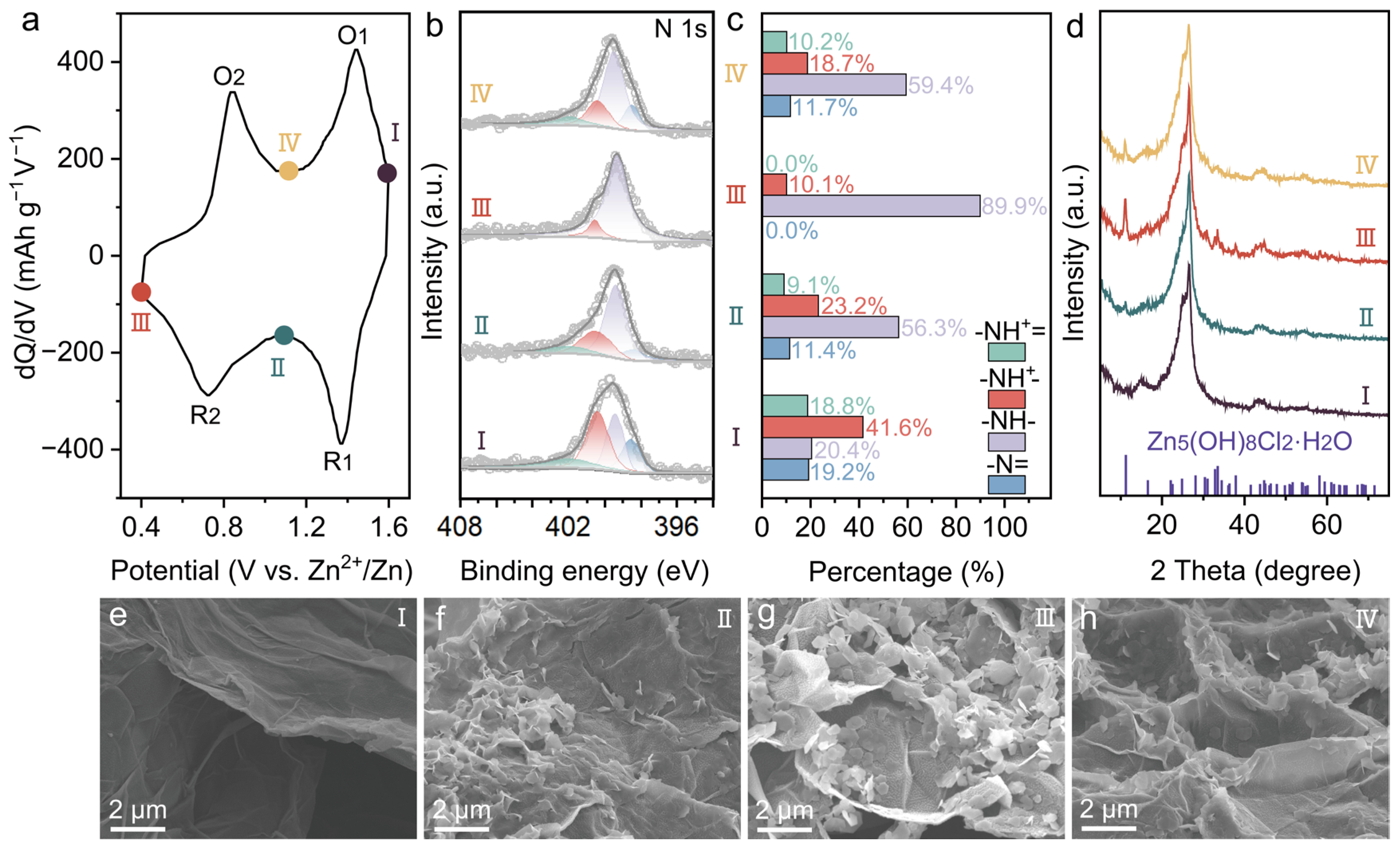
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Electrochemical Exfoliation of Graphite Foil (EG)
3.3. Electrochemical Deposition of Polyaniline (PANI)
3.4. Fabrication of the Carbon Film Coated Zn Anode
3.5. Materials Characterization
3.6. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, Y.; Qin, Y.; Hao, J.; Zhang, H.; Li, C.M. Advances and Perspectives of Ion-Intercalated Vanadium Oxide Cathodes for High-Performance Aqueous Zinc Ion Battery. Adv. Funct. Mater. 2024, 34, 2310393. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Yue, Y.; Yang, X.; Yang, C.; Niu, J.; Zeng, Z.; Kidkhunthod, P.; Wannapaiboon, S.; Zhang, X.; et al. Unveiling the X-Ray Absorption Chemistry of H3.78V6O13 Cathode for Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2307270. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, W.; Yu, G.; He, Z.; Tang, W.; Hu, P.; Yang, W.; Zhu, J.; Su, Q.; An, Q.; et al. Bilayered Vanadium Oxides Pillared by Strontium Ions and Water Molecules as Stable Cathodes for Rechargeable Zn-Metal Batteries. Small 2024, 20, 2404893. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, J.; Huang, C.; Liu, R.; Xia, Z.; Lu, S.; Wang, L.; Zhang, L.; Chen, L. Anchored VN Quantum Dots Boosting High Capacity and Cycle Durability of Na3V2(PO4)3@NC Cathode for Aqueous Zinc-Ion Battery and Organic Sodium-Ion Battery. Small 2024, 20, 2402927. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, T.; Huang, K. NaCa0.6V6O16·3H2O as an Ultra-Stable Cathode for Zn-Ion Batteries: The Roles of Pre-Inserted Dual-Cations and Structural Water in V3O8 Layer. Adv. Energy Mater. 2019, 9, 1901968. [Google Scholar] [CrossRef]
- Bi, S.; Zhang, Y.; Deng, S.; Tie, Z.; Niu, Z. Proton-Assisted Aqueous Manganese-Ion Battery Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202200809. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; Wang, T.; Wu, J.; Xu, X.; Li, P.; Ji, X.; Hou, H.; Qu, X.; Jiao, L.; et al. Unraveling the “Gap-Filling” Mechanism of Multiple Charge Carriers in Aqueous Zn-MoS2 Batteries. Angew. Chem. Int. Ed. 2024, 63, e202320075. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Shi, Y.; Song, G.; Pei, C.; Yan, Y.; Chen, Z.; Pang, H. Bimetallic Design Based on the Nucleation Rate of Prussian Blue Analogues for Enhanced Aqueous Zinc Ion Batteries with Glycerol-Water Hybrid Electrolytes. Angew. Chem. Int. Ed. 2025, 64, e202425391. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, J.; Wang, Y.; Chi, Z.; Lv, L.; Wang, H.; Luo, W.; Diao, Q.; Xie, H.; Cai, X. Heterointerface Built Up by Ultrathin Amorphous MoO3 Conformal Coating Enables V5O12·6H2O with Superior Properties for Aqueous Zinc Batteries. Nano Lett. 2025, 25, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.; Chandra Tripathy, B.; Sanjay, K.; Subbaiah, T.; Minakshi, M. Electrolytic Manganese Dioxide (EMD): A Perspective on Worldwide Production, Reserves and its Role in Electrochemistry. RSC Adv. 2015, 5, 58255–58283. [Google Scholar] [CrossRef]
- Minakshi, M. Lithium Intercalation into Amorphous FePO4 Cathode in Aqueous Solutions. Electrochim. Acta 2010, 55, 9174–9178. [Google Scholar] [CrossRef]
- Minakshi, M.; Kandhasamy, S. Utilizing Active Multiple Dopants (Co and Ni) in Olivine LiMnPO4. Curr. Opin. Solid State Mater. Sci. 2012, 16, 163–167. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, C.; Li, X.; Peng, L.; Ding, W.; Guo, X.; Hou, W. Systematic Modification of MoO3-Based Cathode by the Intercalation Engineering for High-Performance Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2022, 33, 2210010. [Google Scholar] [CrossRef]
- Fang, K.; Zhang, H.; Chen, P.; Zhang, H.Y.; Wei, Z.; Ding, L.; Ye, X.A.; Liu, J.; Liu, Y.L.; Wang, G.G.; et al. Synergistic Structure Engineering and Electrochemical Activation Modulating Vanadium Oxide Cathode toward Superior Zinc-Ion Storage. Chem. Eng. J. 2024, 496, 153736. [Google Scholar] [CrossRef]
- Han, X.; Zhao, R.; Yu, L.; Wang, L.; Zhang, X.; Zhang, A.; Yang, J.; Hu, Z.; Lv, M.; Miao, T.; et al. Dynamic Protective Multi-Layers for MnO2 Cathodes: Ion Sorting and Structural Protection for Superior Zinc-Ion Battery Cycling Performance. Adv. Mater. 2025, 18, e13548. [Google Scholar] [CrossRef] [PubMed]
- Dallaev, R. Conductive Polymer Thin Films for Energy Storage and Conversion: Supercapacitors, Batteries, and Solar Cells. Polymers 2025, 17, 2346. [Google Scholar] [CrossRef]
- Hoang, T.K.A.; Doan, T.N.L.; Sun, K.E.K.; Chen, P. Corrosion Chemistry and Protection of Zinc & Zinc Alloys by Polymer-Containing Materials for Potential Use in Rechargeable Aqueous Batteries. RSC Adv. 2015, 5, 41677–41691. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.h.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.W.; O’Neal, J.; Shao, L.; Lutkenhaus, J.L. Charge Storage in Polymer Acid-Doped Polyaniline-Based Layer-by-Layer Electrodes. ACS Appl. Mater. Interfaces 2013, 5, 10127–10136. [Google Scholar] [CrossRef]
- Wang, H.; Feng, X.; Bo, X.; Zhou, M.; Guo, L. Nickel-Based Metal-Organic Framework/Crosslinked Tubular Poly(3,4-ethylenedioxythiophene) Composite as an Electrocatalyst for the Detection of Gallic Acid and Tinidazole. ChemElectroChem 2020, 7, 4031–4037. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, L.; Zhang, W.; Dai, Z.; Wei, W.; Luo, S.; Chen, X.; Chen, W.; Rao, F.; Wang, L.; et al. Conjugated System of PEDOT:PSS-Induced Self-Doped PANI for Flexible Zinc-Ion Batteries with Enhanced Capacity and Cyclability. ACS Appl. Mater. Interfaces 2019, 11, 30943–30952. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Zheng, R.; Pan, J.; Niu, J.; Zou, X.; Jia, C. A Flexible Electrochromic Rechargeable Zn-Ion Battery Based on Actiniae-Like Self-Doped Polyaniline Cathode. J. Mater. Chem. A 2020, 8, 12799–12809. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, S.; Petkov, P.S.; Zhang, P.; Qi, H.; Nguyen, N.N.; Zhang, W.; Yoon, J.; Li, P.; Brumme, T.; et al. Two-Dimensional Polyaniline Crystal with Metallic out-of-plane Conductivity. Nature 2025, 638, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shi, H.Y.; Lin, Z.; Yang, X.; Li, C.; Lin, L.; Song, Y.; Guo, D.; Liu, X.X.; Sun, X. The Controlled Quinone Introduction and Conformation Modification of Polyaniline Cathode Materials for Rechargeable Aqueous Zinc-Polymer Batteries. Chem. Eng. J. 2021, 419, 129659. [Google Scholar] [CrossRef]
- Amaya, T.; Abe, Y.; Hirao, T. Deprotonation-Induced Efficient Delocalization of Polaron in Self-Doped Poly(anilinephosphonic acid). Macromolecules 2014, 47, 8115–8118. [Google Scholar] [CrossRef]
- Yan, H.; Mu, X.; Song, Y.; Qin, Z.; Guo, D.; Sun, X.; Liu, X.X. Protonating Imine Sites of Polyaniline for Aqueous Zinc Batteries. Chem. Commun. 2022, 58, 1693–1696. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, J.; Yu, P.; Li, M.; Huang, L.; Hu, Z.; Wang, Y.; Song, Z. Toward Full Utilization and Stable Cycling of Polyaniline Cathode for Nonaqueous Rechargeable Batteries. Adv. Energy Mater. 2023, 13, 2301520. [Google Scholar] [CrossRef]
- Jiménez, P.; Levillain, E.; Alévêque, O.; Guyomard, D.; Lestriez, B.; Gaubicher, J. Lithiumn-Doped Polyaniline as a High-Performance Electroactive Material for Rechargeable Batteries. Angew. Chem. Int. Ed. 2017, 56, 1553–1556. [Google Scholar] [CrossRef]
- Romanova, J.; Madjarova, G.; Tadjer, A.; Gospodinova, N. Solvent Polarity and Dopant Effect on the Electronic Structure of the Emeraldine Salt. Inter. J. Quantum Chem. 2010, 11, 435–443. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Wang, X.; Bi, S.; Niu, Z.; Chen, J. An Aqueous Rechargeable Zinc-Organic Battery with Hybrid Mechanism. Adv. Funct. Mater. 2018, 28, 201804975. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Epstein, A.J. Polyanilines: A Vovel Class of Conducting Polymers. Faraday Discuss. Chem. Soc. 1989, 88, 317–332. [Google Scholar] [CrossRef]
- Hong, Y.; Jia, K.; Zhang, Y.; Li, Z.; Jia, J.; Chen, J.; Liang, Q.; Sun, H.; Gao, Q.; Zhou, D.; et al. Energetic and Durable All-Polymer Aqueous Battery for Sustainable, Flexible Power. Nat. Commun. 2024, 15, 9539. [Google Scholar] [CrossRef]
- Vujković, M.J.; Etinski, M.; Vasić, B.; Kuzmanović, B.; Bajuk-Bogdanović, D.; Dominko, R.; Mentus, S. Polyaniline as a Charge Storage Material in an Aqueous Aluminum-Based Electrolyte: Can Aluminum Ions Play the Role of Protons? J. Power Sources 2021, 482, 228937. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, J.; Emwas, A.H.; Mohammed, O.F.; Alshareef, H.N. An Aqueous Mg2+-Based Dual-Ion Battery with High Power Density. Adv. Funct. Mater. 2021, 31, 2107523. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Z.; Zhang, W.; Jiang, Y.; Peng, J.; Wu, D.; Chen, B.; Wei, W.; Chen, X.; Liu, Z.; et al. Sulfonic-Group-Grafted Ti3C2Tx MXene: A Silver Bullet to Settle the Instability of Polyaniline toward High-Performance Zn-Ion Batteries. ACS Nano 2021, 15, 9065–9075. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Yang, L.S.; Huang, W.S.; Humphrey, B.D. Polyaniline: Electrochemistry and Application to Rechargeable Batteries. Synthetic Met. 1987, 18, 393–398. [Google Scholar] [CrossRef]
- Huang, W.S.; Humphrey, B.D.; MacDiarmid, A.G. Polyaniline, a Novel Conducting Polymer. Morphology and Chemistry of its Oxidation and Reduction in Aqueous Electrolytes. J. Chem. Soc. Faraday Trans. 1 1986, 82, 2385–2400. [Google Scholar] [CrossRef]
- Shi, H.Y.; Ye, Y.J.; Liu, K.; Song, Y.; Sun, X. A Long-Cycle-Life Self-Doped Polyaniline Cathode for Rechargeable Aqueous Zinc Batteries. Angew. Chem. Int. Ed. 2018, 57, 16359–16363. [Google Scholar] [CrossRef]
- Dong, H.; Wang, L.; Zhang, F.; Li, H.; Zhao, X.; Wei, W.; Kang, Y.; Yan, C.; Sang, Y.; Liu, H.; et al. Green Proton Reservoirs of PANI for Disposable High-Performance Zinc-Ion Batteries. Nano Energy 2024, 128, 109768. [Google Scholar] [CrossRef]
- Yue, J.; Wang, Z.H.; Cromack, K.R.; Epstein, A.J.; MacDiarmid, A.G. Effect of Sulfonic Acid Group on Polyaniline Backbone. J. Am. Chem. Soc. 2002, 113, 2665–2671. [Google Scholar] [CrossRef]
- Yue, J.; Epstein, A.J.; Macdiarmid, A.G. Sulfonic Acid Ring-Substituted Polyaniline, A Self-Doped Conducting Polymer. Mol. Cryst. Liq. Cryst. 1990, 189, 255–261. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, Q.; Qi, Y.; Xu, J.; Peng, Y.; Shu, J.; Ma, J.; Wang, Y. Towards High-Performance Aqueous Zinc Batteries via a Semi-Conductive Bipolar-Type Polymer Cathode. Angew. Chem. Int. Ed. 2022, 61, e202211107. [Google Scholar] [CrossRef]
- Zhao, L.; Jia, Y.; Wu, Y.; Gu, T.; Zhou, X.; Wang, X.; Zhong, L.; Zhan, S.; Lv, H.; Zhi, C.; et al. An Ultra-Stable 2D Linear Polymer Cathode for High-Performance Aqueous Zinc-Organic Batteries. Angew. Chem. Int. Ed. 2025, 64, e202425082. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, S.; Xu, L. Enhanced Conductivity of Polyaniline by Conjugated Crosslinking. Macromol. Rapid Commun. 2011, 32, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Ni, Y.; Li, Y.; Cheng, W.; Shao, Z.; Feng, X.; Niu, Z.; Zhao, Q.; Li, Y.A.; Li, H.; et al. A Crystalline Polymer Cathode for Sodium-Organic Batteries. Angew. Chem. Int. Ed. 2025, 64, e202501134. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, L.; Ren, X.; Lin, L.; Zhan, C.; Weng, Q.; Sun, X.; Yu, X. Asymmetric Charge Distribution of Active Centers in Small Molecule Quinone Cathode Boosts High-Energy and High-Rate Aqueous Zinc-Organic Batteries. Adv. Funct. Mater. 2024, 34, 2313241. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, P.; Hu, H.; Xia, X.; Lu, X.; Yu, S.; Tempeld, H.; Eichel, R.A.; Liao, X.; Chen, Y. Impact of Electrostatic Interaction on Non-Radiative Recombination Energy Losses in Organic Solar Cells Based on Asymmetric Acceptors. Angew. Chem. Int. Ed. 2023, 62, e202304931. [Google Scholar] [CrossRef]
- Lin, L.; Lin, Z.; Zhu, J.; Wang, K.; Wu, W.; Qiu, T.; Sun, X. A Semi-Conductive Organic Cathode Material Enabled by Extended Conjugation for Rechargeable Aqueous Zinc Batteries. Energ. Environ. Sci. 2023, 16, 89–96. [Google Scholar] [CrossRef]
- Sun, Q.Q.; Sun, T.; Du, J.Y.; Li, K.; Xie, H.M.; Huang, G.; Zhang, X.B. A Sulfur Heterocyclic Quinone Cathode Towards High-Rate and Long-Cycle Aqueous Zn-Organic Batteries. Adv. Mater. 2023, 35, e2301088. [Google Scholar] [CrossRef]
- Ye, C.; Zhou, X.; Tang, S. An Azo Polymer with Abundant Active Sites and Extended Conjugation as a Stable Cathode for High-Performance Zinc-Organic Batteries. Angew. Chem. Int. Ed. 2025, 64, e202501743. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Y.; Lu, Y.; Li, L.; Wan, F.; Zhang, K.; Chen, J. Modulating Electrolyte Structure for Ultralow Temperature Aqueous Zinc Batteries. Nat. Commun. 2020, 11, 4463. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Tang, S.; Wang, H.; Liu, S.; Cai, X. A Carboxyl-Modified Polyaniline Cathode for High-Performance Aqueous Zinc-Ion Batteries. Molecules 2025, 30, 4498. https://doi.org/10.3390/molecules30234498
Sun Z, Tang S, Wang H, Liu S, Cai X. A Carboxyl-Modified Polyaniline Cathode for High-Performance Aqueous Zinc-Ion Batteries. Molecules. 2025; 30(23):4498. https://doi.org/10.3390/molecules30234498
Chicago/Turabian StyleSun, Zhen, Shijun Tang, Haixu Wang, Shiyu Liu, and Xiang Cai. 2025. "A Carboxyl-Modified Polyaniline Cathode for High-Performance Aqueous Zinc-Ion Batteries" Molecules 30, no. 23: 4498. https://doi.org/10.3390/molecules30234498
APA StyleSun, Z., Tang, S., Wang, H., Liu, S., & Cai, X. (2025). A Carboxyl-Modified Polyaniline Cathode for High-Performance Aqueous Zinc-Ion Batteries. Molecules, 30(23), 4498. https://doi.org/10.3390/molecules30234498





