Nanomaterials for Photocatalytic Inactivation and Eradication of Candida spp. Biofilms in Healthcare Environment: A Novel Approach in Modern Clinical Practice
Abstract
1. Biofilm Candida spp. as a Healthcare Problem
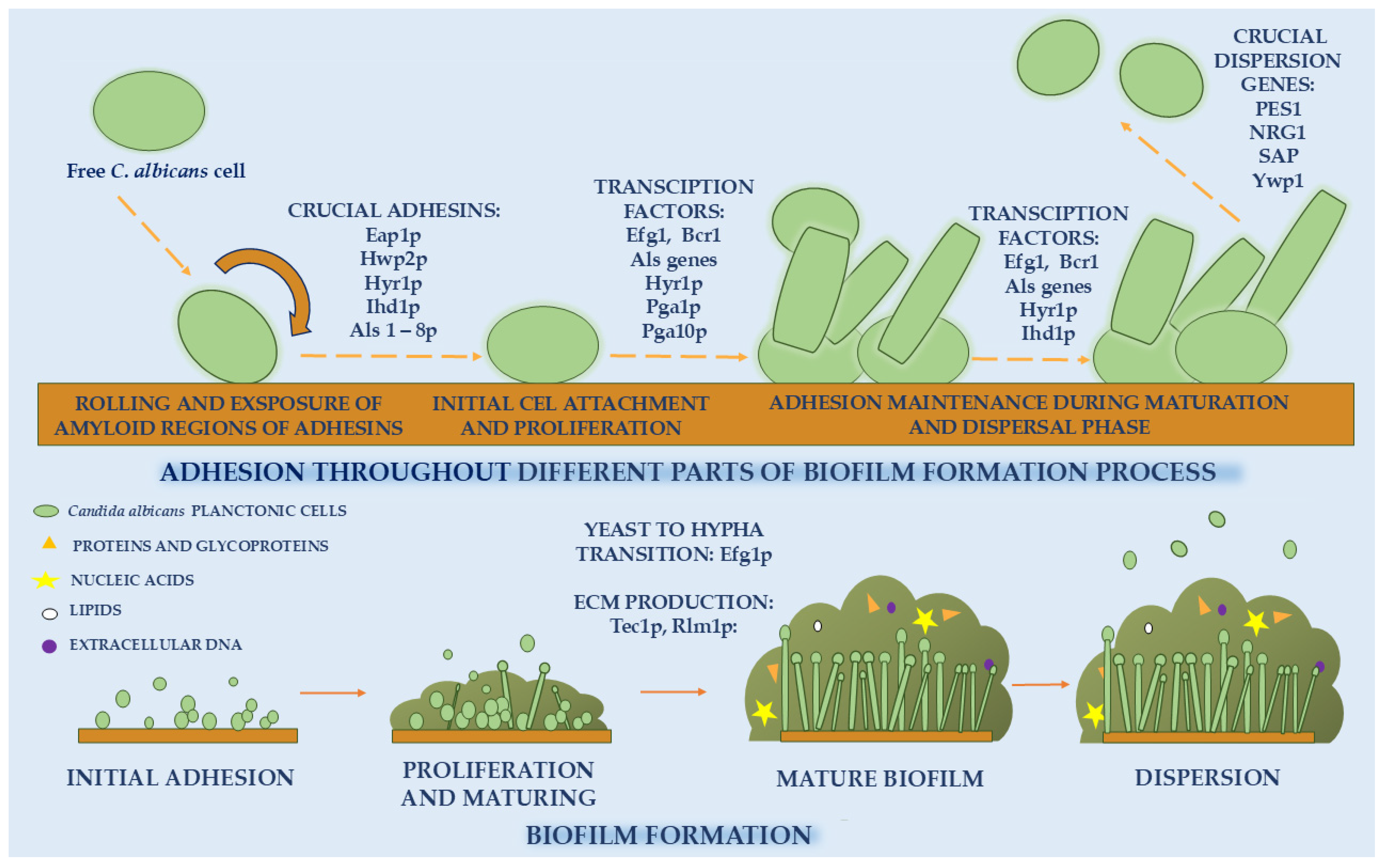
2. Biofilm Formation by Candida spp.
2.1. Biofilm Formation Problem
2.2. Adhesion
2.3. Proliferation and Maturing
2.4. Dispersal Phase
3. Candida spp. Biofilm Resistance Factors
3.1. Extracellular Matrix
3.2. Efflux Pumps
3.3. Quorum Sensing
3.4. Persister Cells
3.5. Goliath Cells
4. Nanomaterials Against Candida spp. Biofilms
| Nanomaterial Type | Advantages | Limitations | Applied in Photocatalysis Against Biofilm Candida spp. —Subsection | References |
|---|---|---|---|---|
| Metallic nanoparticles |
|
| 5.2, 5.3, 5.6 | [38] |
| Metal oxide nanoparticles |
|
| 5.1, 5.2, 5.3, 5.6 | [39,40] |
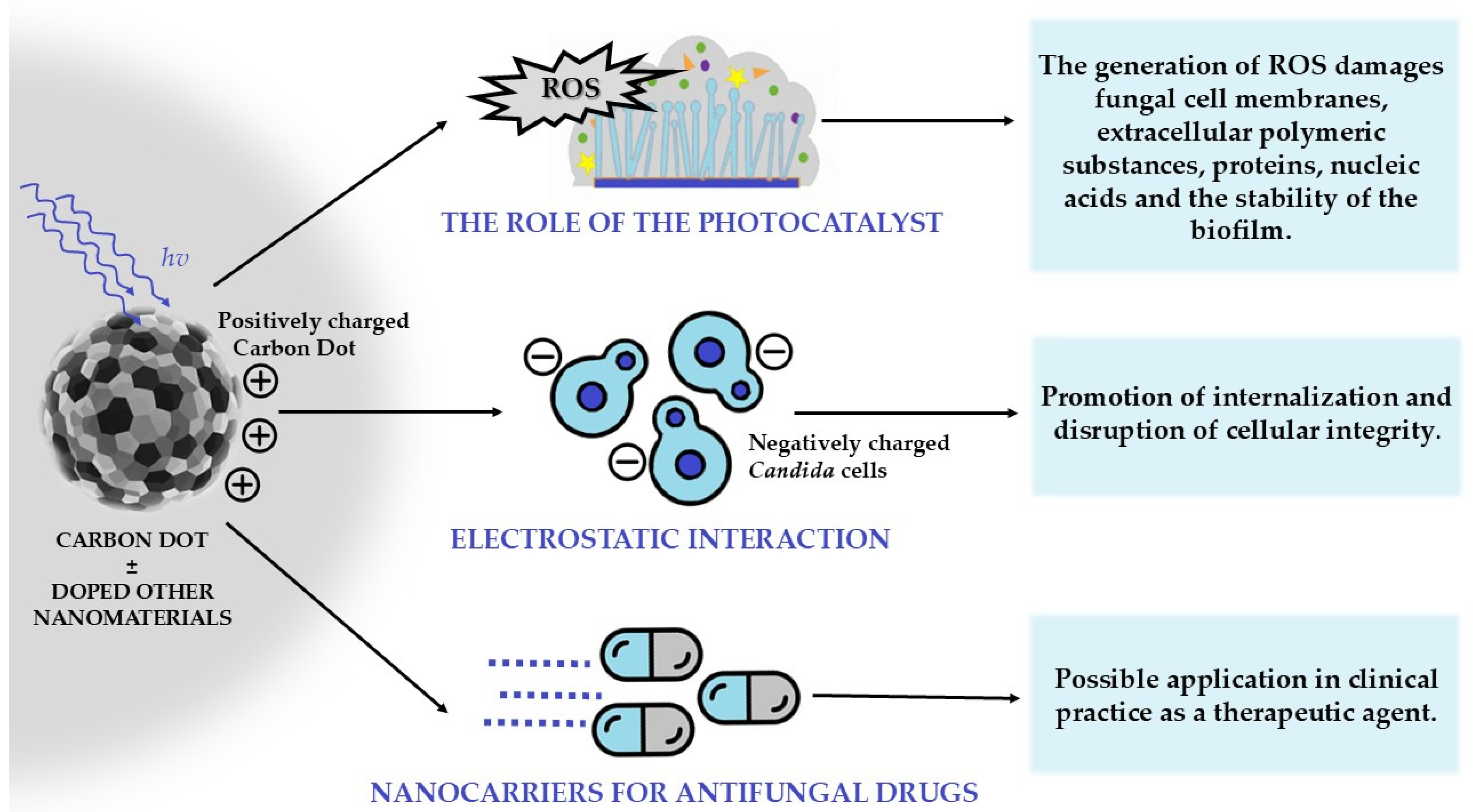
| Carbon dots |
|
| 5.5 | [41,42,43] |
| Chitosan-based nanoparticles |
|
| Not yet, only possible as a photocatalyst carrier. | [44,45,46,47] |
| Liposomes |
|
| Not yet, only possible as a photocatalyst carrier. | [48,49,50,51] |
| Solid lipid nanoparticles (SLNs) |
|
| Not yet, only possible as a photocatalyst carrier. | [52] |
| Nanoenzymes |
|
| 5.4 | [53,54,55,56] |
5. Photocatalysis Against Candida spp. Biofilms
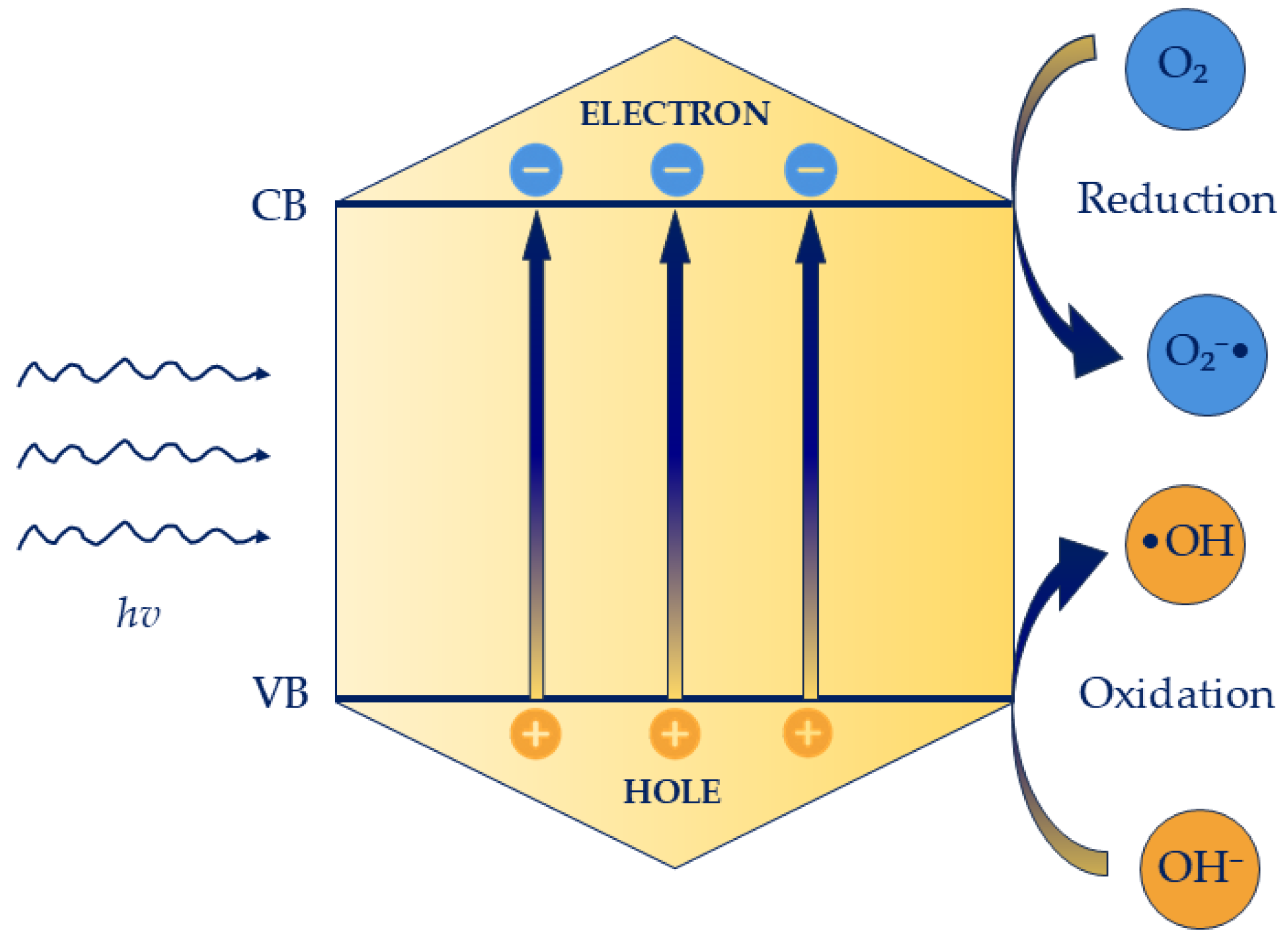
5.1. Photocatalysis
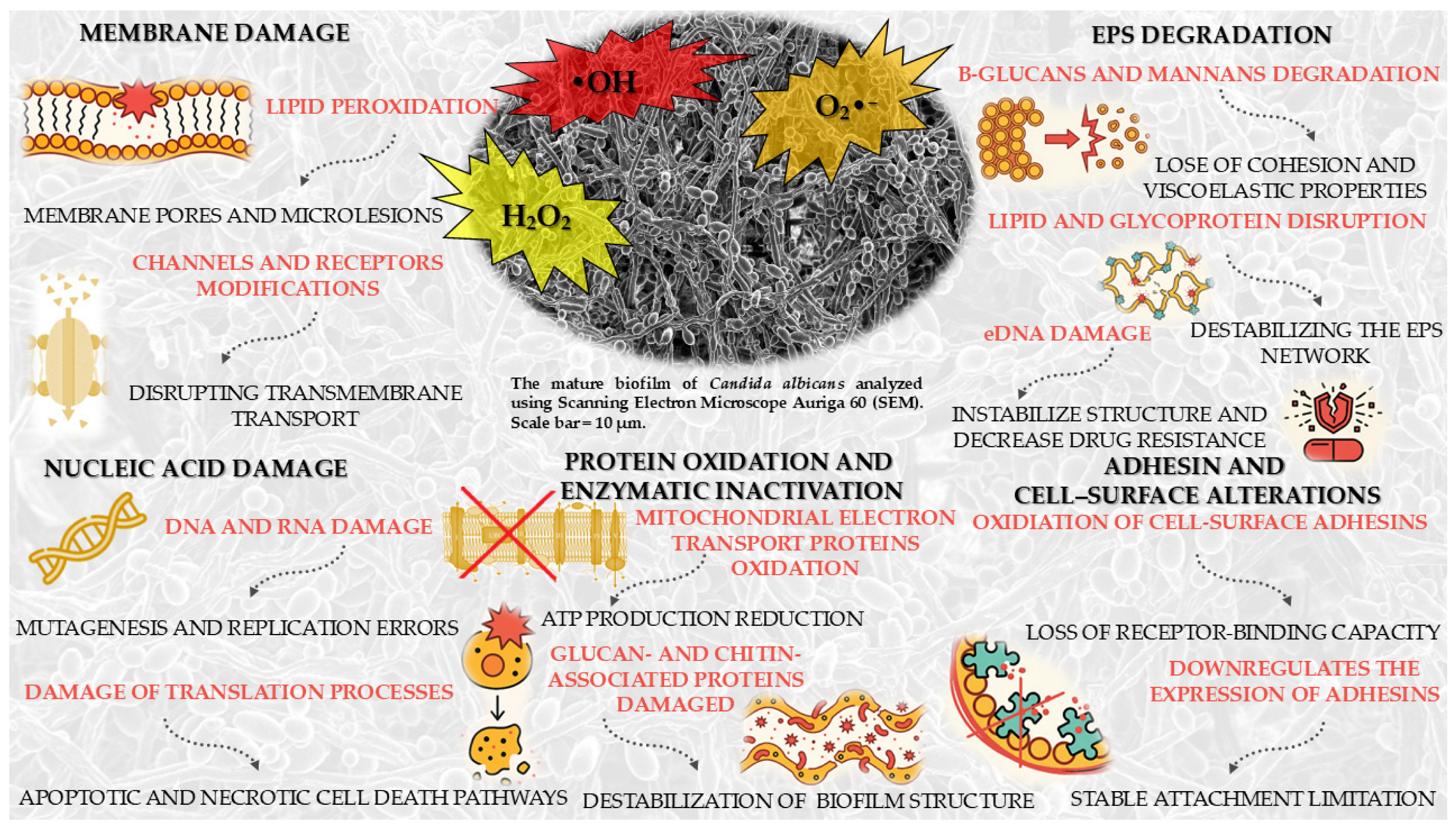
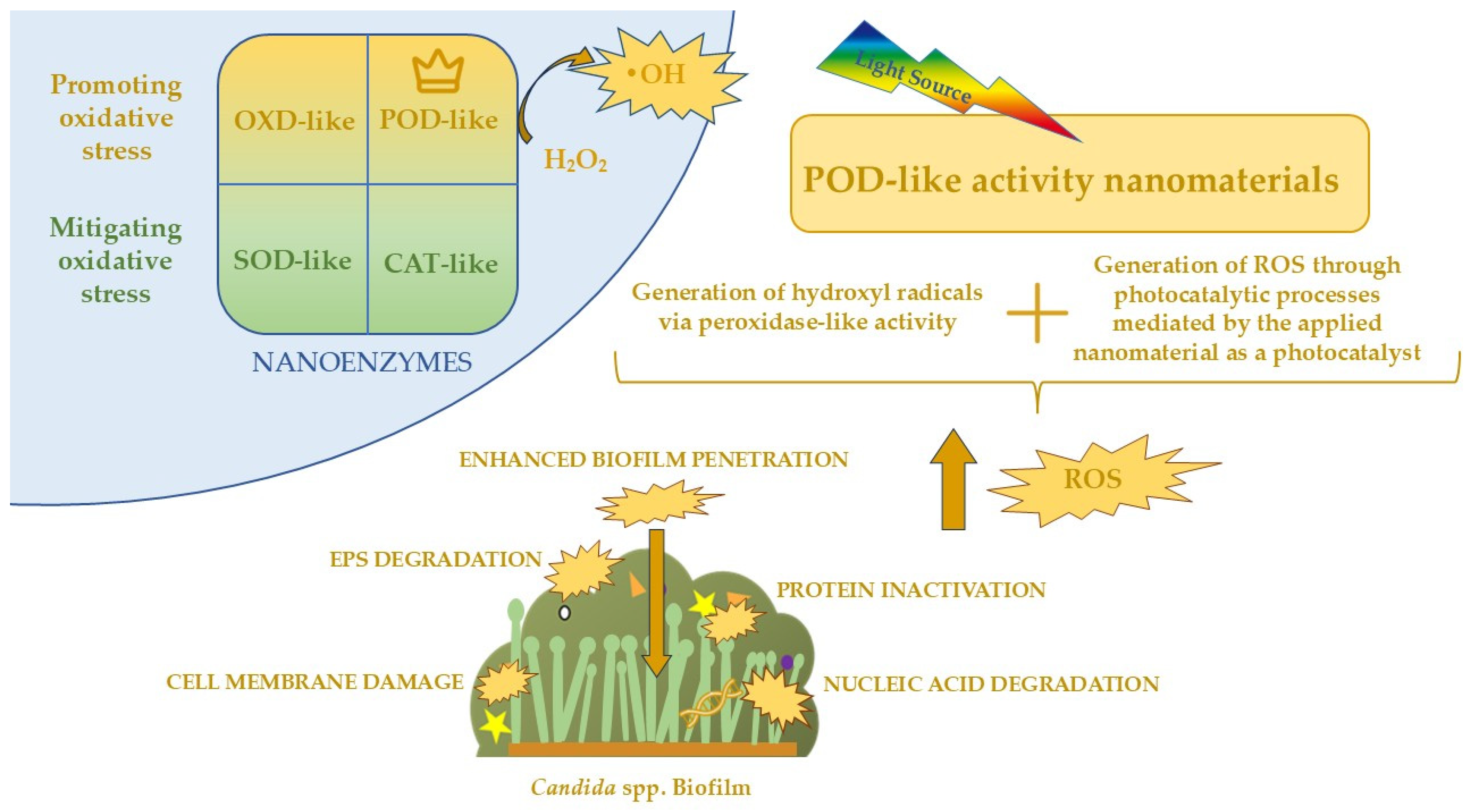
5.2. Mechanism of Photocatalysis Against Candida spp. Biofilm
5.3. Candida spp. Response Mechanisms to Oxidative Stress Induced by ROS
6. Methods of Photocatalytic Inactivation and Eradication of Candida spp. Biofilms
6.1. Titanium Dioxide: The Foundation of Nanomaterials Research Against Candida Biofilms
6.2. Transition Metal Oxide Nanoparticles: Structural Modifications and Doping Approaches
6.3. Zinc Oxide Nanostructures and Hybrid Systems in Combating Candida Biofilms
6.4. Nanozymes as a Novel Antifungal Concept
6.5. Carbon-Based Nanomaterials: Photocatalytic Properties and Anti-Candida Biofilm Applications
6.6. Modified Composites and Medical Biomaterials in the Control of Candida spp.
7. Materials and Methods
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Dorzi, H.M.; Sakkijha, H.; Khan, R.; Aldabbagh, T.; Toledo, A.; Ntinika, P.; Al Johani, S.M.; Arabi, Y.M. Invasive Candidiasis in Critically Ill Patients: A Prospective Cohort Study in Two Tertiary Care Centers. J. Intensive Care Med. 2020, 35, 542–553. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Nosanchuk, J.D. Fungal diseases as neglected pathogens: A wake-up call to public health officials. PLoS Negl. Trop. Dis. 2020, 14, e0007964. [Google Scholar] [CrossRef]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef]
- Cornely, O.A.; Sprute, R.; Bassetti, M.; Chen, S.C.; Groll, A.H.; Kurzai, O.; Lass-Flörl, C.; Ostrosky-Zeichner, L.; Rautemaa-Richardson, R.; Revathi, G.; et al. Global guideline for the diagnosis and management of candidiasis: An initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2025, 25, e280–e293. [Google Scholar] [CrossRef]
- Cristina, M.L.; Spagnolo, A.M.; Sartini, M.; Carbone, A.; Oliva, M.; Schinca, E.; Boni, S.; Pontali, E. An overview on Candida auris in healthcare settings. J. Fungi 2023, 9, 913. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Barrero-García, I.; León-Moya, C. Fungal infections in immunocompromised critically ill patients. J. Intensive Med. 2024, 4, 299–306. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Malinovská, Z.; Čonková, E.; Váczi, P. Biofilm formation in medically important Candida species. J. Fungi 2023, 9, 955. [Google Scholar] [CrossRef] [PubMed]
- Mikziński, P.; Kraus, K.; Widelski, J.; Paluch, E. Modern microbiological methods to detect biofilm formation in orthopedy and suggestions for antibiotic therapy, with particular emphasis on prosthetic joint infection (PJI). Microorganisms 2024, 12, 1198. [Google Scholar] [CrossRef] [PubMed]
- de Barros, P.P.; Rossoni, R.D.; de Souza, C.M.; Scorzoni, L.; Fenley, J.C.; Junqueira, J.C. Candida biofilms: An update on developmental mechanisms and therapeutic challenges. Mycopathologia 2020, 185, 415–424. [Google Scholar] [CrossRef]
- de Groot, P.W.; Bader, O.; de Boer, A.D.; Weig, M.; Chauhan, N. Adhesins in human fungal pathogens: Glue with plenty of stick. Eukaryot. Cell 2013, 12, 470–481. [Google Scholar] [CrossRef]
- McCall, A.D.; Pathirana, R.U.; Prabhakar, A.; Cullen, P.J.; Edgerton, M. Candida albicans Biofilm Development Is Governed by Cooperative Attachment and Adhesion Maintenance Proteins. npj Biofilms Microbiomes 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Granger, B.L. Accessibility and Contribution to Glucan Masking of Natural and Genetically Tagged Versions of Yeast Wall Protein 1 of Candida albicans. PLoS ONE 2018, 13, e0191194. [Google Scholar] [CrossRef] [PubMed]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans Biofilms and Polymicrobial Interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef]
- Kumari, A.; Tripathi, A.H.; Gautam, P.; Gahtori, R.; Pande, A.; Singh, Y.; Madan, T.; Upadhyay, S.K. Adhesins in the Virulence of Opportunistic Fungal Pathogens of Humans. Mycology 2021, 12, 296–324. [Google Scholar] [CrossRef]
- de Souza, C.M.; Dos Santos, M.M.; Furlaneto-Maia, L.; Furlaneto, M.C. Adhesion and Biofilm Formation by the Opportunistic Pathogen Candida tropicalis: What Do We Know? Can. J. Microbiol. 2023, 69, 207–218. [Google Scholar] [CrossRef]
- Zarnowski, R.; Westler, W.M.; Lacmbouh, G.A.; Marita, J.M.; Bothe, J.R.; Bernhardt, J.; Lounes-Hadj Sahraoui, A.; Fontaine, J.; Sanchez, H.; Hatfield, R.D.; et al. Novel Entries in a Fungal Biofilm Matrix Encyclopedia. mBio 2014, 5, e01333-14. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; dos Santos Fontenelle, R.O.; de Brito, E.H.S.; de Morais, S.M. Biofilm of Candida albicans: Formation, regulation and resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef]
- Atriwal, T.; Azeem, K.; Husain, F.M.; Hussain, A.; Khan, M.N.; Alajmi, M.F.; Abid, M. Mechanistic Understanding of Candida albicans Biofilm Formation and Approaches for Its Inhibition. Front. Microbiol. 2021, 12, 638609. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef]
- Wall, G.; Montelongo-Jauregui, D.; Vidal Bonifacio, B.; Lopez-Ribot, J.L.; Uppuluri, P. Candida albicans biofilm growth and dispersal: Contributions to pathogenesis. Curr. Opin. Microbiol. 2019, 52, 1–6. [Google Scholar] [CrossRef]
- Uppuluri, P.; Acosta Zaldívar, M.; Anderson, M.Z.; Dunn, M.J.; Berman, J.; Lopez Ribot, J.L.; Köhler, J.R. Candida albicans Dispersed Cells Are Developmentally Distinct from Biofilm and Planktonic Cells. mBio 2018, 9, e01338-18. [Google Scholar] [CrossRef]
- Granger, B.L. Insight into the Antiadhesive Effect of Yeast Wall Protein 1 of Candida albicans. Eukaryot. Cell 2012, 11, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Nobile, C.J. Antifungal drug-resistance mechanisms in Candida biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef] [PubMed]
- Engle, K.; Kumar, G. Tackling multi-drug resistant fungi by efflux pump inhibitors. Biochem. Pharmacol. 2024, 226, 116400. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Tripathi, M.; Gupta, M.K.; Tilak, R. Overexpression of efflux pump transporter genes and mutations in ERG11 pave the way to fluconazole resistance in Candida tropicalis: A study from a North India region. J. Glob. Antimicrob. Resist. 2020, 22, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef]
- Kovács, R.; Majoros, L. Fungal Quorum-Sensing Molecules: A Review of Their Antifungal Effect against Candida Biofilms. J. Fungi 2020, 6, 99. [Google Scholar] [CrossRef]
- Galdiero, E.; de Alteriis, E.; De Natale, A.; D’Alterio, A.; Siciliano, A.; Guida, M.; Lombardi, L.; Falanga, A.; Galdiero, S. Eradication of Candida albicans persister cell biofilm by the membranotropic peptide gH625. Sci. Rep. 2020, 10, 5780. [Google Scholar] [CrossRef]
- Malavia, D.; Lehtovirta-Morley, L.E.; Alamir, O.; Weiß, E.; Gow, N.A.R.; Hube, B.; Wilson, D. Zinc Limitation Induces a Hyper-Adherent Goliath Phenotype in Candida albicans. Front. Microbiol. 2017, 8, 2238. [Google Scholar] [CrossRef]
- Kalinina, I.; Wilson, D. Candida albicans Goliath Cells Pioneer Biofilm Formation. mBio 2025, 16, e0342524. [Google Scholar] [CrossRef]
- Lai, H.; Huang, R.; Weng, X.; Huang, B.; Yao, J.; Pian, Y. Classification and applications of nanomaterials in vitro diagnosis. Heliyon 2024, 10, e32314. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Pan, L.; Zhang, H.; Xie, L.; Wang, X.; Shou, J.; Qi, Y.; Yan, X. Recent developments on using nanomaterials to combat Candida albicans. Front. Chem. 2021, 9, 813973. [Google Scholar] [CrossRef]
- Giammarino, A.; Verdolini, L.; Simonetti, G.; Angiolella, L. Fungal biofilm: An overview of the latest nano-strategies. Antibiotics 2025, 14, 718. [Google Scholar] [CrossRef]
- Alanís-Ríos, S.A.; Sánchez-Domínguez, C.N.; Villanueva-Lozano, H.; Álvarez Villalobos, N.A.; Treviño-Rangel, R.J. Nanomaterials and Candida auris: A systematic review of emerging strategies. APMIS 2025, 133, e70044. [Google Scholar] [CrossRef]
- Fayed, B. Nanoparticles in the battle against Candida auris biofilms: Current advances and future prospects. Drug Deliv. Transl. Res. 2025, 15, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Carmo, P.H.F.D.; Garcia, M.T.; Figueiredo-Godoi, L.M.A.; Lage, A.C.P.; Silva, N.S.D.; Junqueira, J.C. Metal nanoparticles to combat Candida albicans infections: An update. Microorganisms 2023, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, A.R.; Sriyutha, P.M.; Das, A.; Priya, A.; Sushmitha, T.J.; Pandian, S.K.; Toleti, S.R. Impediment to growth and yeast-to-hyphae transition in Candida albicans by copper oxide nanoparticles. Biofouling 2020, 36, 56–72. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Ghaemi, E.; Koohsar, F. Influence of ZnO nanoparticles on Candida albicans isolates biofilm formed on the urinary catheter. Iran. J. Microbiol. 2018, 10, 424–432. [Google Scholar]
- Sturabotti, E.; Camilli, A.; Georgian Moldoveanu, V.; Bonincontro, G.; Simonetti, G.; Valletta, A.; Serangeli, I.; Miranda, E.; Amato, F.; Giacomo Marrani, A.; et al. Targeting the antifungal activity of carbon dots against Candida albicans biofilm formation by tailoring their surface functional groups. Chemistry 2024, 30, e202303631. [Google Scholar] [CrossRef]
- Shaikh, A.F.; Tamboli, M.S.; Patil, R.H.; Bhan, A.; Ambekar, J.D.; Kale, B.B. Bioinspired carbon quantum dots: An antibiofilm agents. J. Nanosci. Nanotechnol. 2019, 19, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Bao, Y.-W.; Wu, F.-G. Carbon dots for sensing and killing microorganisms. C 2019, 5, 33. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Fu, J.; Zhang, W.; Shu, G.; Lin, J.; Li, H.; Xu, F.; Tang, H.; Peng, G.; et al. Nanocarriers for combating biofilms: Advantages and challenges. J. Appl. Microbiol. 2022, 133, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.C.; Gupta, P.; Saini, S.; Mohiyuddin, S.; Pruthi, V.; Prasad, R. Plausible mechanistic insights in biofilm eradication potential against Candida spp. using in situ-synthesized tyrosol-functionalized chitosan gold nanoparticles as a versatile antifouling coating on implant surfaces. ACS Omega 2022, 7, 8350–8363. [Google Scholar] [CrossRef] [PubMed]
- Gondim, B.L.C.; Castellano, L.R.C.; de Castro, R.D.; Machado, G.; Carlo, H.L.; Valença, A.M.G.; de Carvalho, F.G. Effect of chitosan nanoparticles on the inhibition of Candida spp. biofilm on denture base surface. Arch. Oral Biol. 2018, 94, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ikono, R.; Vibriani, A.; Wibowo, I.; Saputro, K.E.; Muliawan, W.; Bachtiar, B.M.; Mardliyati, E.; Bachtiar, E.W.; Rochman, N.T.; Kagami, H.; et al. Nanochitosan antimicrobial activity against Streptococcus mutans and Candida albicans dual-species biofilms. BMC Res. Notes 2019, 12, 383. [Google Scholar] [CrossRef]
- Jaromin, A.; Zarnowski, R.; Markowski, A.; Zagórska, A.; Johnson, C.J.; Etezadi, H.; Kihara, S.; Mota-Santiago, P.; Nett, J.E.; Boyd, B.J.; et al. Liposomal formulation of a new antifungal hybrid compound provides protection against Candida auris in the ex vivo skin colonization model. Antimicrob. Agents Chemother. 2024, 68, e0095523. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, Q.; Xiao, Y.; Wu, Y.; Ding, L.; Huang, H.; Li, Y.; Yang, J.; Meng, L. The progress and future of the treatment of Candida albicans infections based on nanotechnology. J. Nanobiotechnol. 2024, 22, 568. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, H.; Pang, S.; Ya, M.; Qiu, F.; Qin, P.; Zeng, C.; Lu, Y. Liposomes as multifunctional nano-carriers for medicinal natural products. Front. Chem. 2022, 10, 963004. [Google Scholar] [CrossRef]
- Basak, S.; Das, T.K. Liposome-based drug delivery systems: From laboratory research to industrial production—Instruments and challenges. ChemEngineering 2025, 9, 56. [Google Scholar] [CrossRef]
- Almawash, S. Solid lipid nanoparticles, an effective carrier for classical antifungal drugs. Saudi Pharm. J. 2023, 31, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Ju, T.; Chen, X.; Li, X.; Wu, L.A. Nanozymes: A promising solution for dental antibacterial applications. RSC Adv. 2024, 14, 36945–36959. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Liu, Q.; Wang, X.; Zhou, Z.; Zhao, X.; Zhou, W.; Liu, W.; Zhang, Y.; Liu, S.; Zhu, C.; et al. A probiotic nanozyme hydrogel regulates vaginal microenvironment for Candida vaginitis therapy. Sci. Adv. 2023, 9, eadg0949. [Google Scholar] [CrossRef]
- Jeyachandran, S.; Srinivasan, R.; Ramesh, T.; Parivallal, A.; Lee, J.; Sathiyamoorthi, E. Recent development and application of “nanozyme” artificial enzymes—A review. Biomimetics 2023, 8, 446. [Google Scholar] [CrossRef]
- Zhang, J.; Dou, T.; Shen, Y.; Wang, W.; Wang, L.; Wu, X.; Zhang, M.; Wang, D.; Yu, P. Application of nanozymes in problematic biofilm control: Progress, challenges and prospects. Front. Environ. Sci. Eng. 2024, 18, 136. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, G.; Wang, Y.; Yang, D. Nanorobots to treat Candida albicans infection. Research 2024, 10, 0455. [Google Scholar] [CrossRef]
- Ji, X.; Yang, H.; Liu, W.; Ma, Y.; Wu, J.; Zong, X.; Yuan, P.; Chen, X.; Yang, C.; Li, X.; et al. Multifunctional parachute-like nano-motors for enhanced skin penetration and synergistic antifungal therapy. ACS Nano 2021, 15, 14218–14228. [Google Scholar] [CrossRef]
- Silva Pontes, C.; Garcia de Carvalho, G.; Rosa Perin Leite, A.; Chorilli, M.; Palomari Spolidorio, D.M. Improving drug delivery on Candida albicans using geraniol nanoemulsion. Pharmaceutics 2023, 15, 2475. [Google Scholar] [CrossRef]
- Agarwalla, S.V.; Ellepola, K.; Silikas, N.; Castro Neto, A.H.; Seneviratne, C.J.; Rosa, V. Persistent inhibition of Candida albicans biofilm and hyphae growth on titanium by graphene nanocoating. Dent. Mater. 2021, 37, 370–377. [Google Scholar] [CrossRef]
- Hasanin, M.S.; El Saied, H.; Morsy, F.A.; Hassan Abdel Latif Rokbaa, H. Green nanocoating-based polysaccharides decorated with ZnONPs doped Egyptian kaolinite for antimicrobial coating paper. Sci. Rep. 2023, 13, 11461. [Google Scholar] [CrossRef]
- Fu, Y.; Chi, J.; Wu, Y.; Li, J.; Tan, M.; Li, C.; Du, H.; Hao, D.; Zhu, H.; Wang, Q.; et al. Synergistic Electric Fields Induced by Unilateral Doping Modulation for Enhanced Organic Pollutant Degradation and Sterilization. Appl. Surf. Sci. 2025, 692, 162711. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A. Nanomaterials as photocatalysts—Synthesis and their potential applications. Materials 2022, 16, 193. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, W.; Zhang, X.; Song, Z.; Tong, T. An Overview of Stimuli-Responsive Intelligent Antibacterial Nanomaterials. Pharmaceutics 2023, 15, 2113. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Fang, R.H.; Zhang, L. Engineering of stimuli-responsive self-assembled bio-mimetic nanoparticles. Adv. Drug Deliv. Rev. 2021, 179, 114006. [Google Scholar] [CrossRef]
- Fatima, M.; Almalki, W.H.; Khan, T.; Sahebkar, A.; Kesharwani, P. Harnessing the power of stimuli-responsive nanoparticles as an effective therapeutic drug delivery system. Adv. Mater. 2024, 36, 2312939. [Google Scholar] [CrossRef]
- Lozano-Rosas, R.; Ruíz-Osorio, J.J.; Ramos-García, R.; Silva-González, R.; Spezzia-Mazzocco, T.; Robles-Águila, M.J. Photoexcitation of Ag-doped TiO2 nanoparticles with visible light for antimicrobial photodynamic therapy against Candida albicans. J. Nanopart. Res. 2025, 27, 236. [Google Scholar] [CrossRef]
- Tang, N.; Yuan, S.; Luo, Y.; Wang, A.J.; Sun, K.; Liu, N.N.; Tao, K. Nanoparticle-based photodynamic inhibition of Candida albicans biofilms with interfering quorum sensing. ACS Omega 2023, 8, 4357–4368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against Staphylococcus aureus and Pseudomonas putida. J. Hazard. Mater. 2017, 340, 160–170. [Google Scholar] [CrossRef]
- Tzeng, J.-H.; Weng, C.-H.; Chang, C.-J.; Yen, L.-T.; de Luna, M.D.G.; Huang, J.-W.; Lin, Y.-T. N-Schorl TiO2 nanocomposite for visible-light photocatalysis deactivation of yeast exemplified by Candida albicans. Chem. Eng. J. 2022, 435, 134294. [Google Scholar] [CrossRef]
- Mikziński, P.; Kraus, K.; Seredyński, R.; Widelski, J.; Paluch, E. Photocatalysis and photodynamic therapy in diabetic foot ulcers (DFUs) care: A novel approach to infection control and tissue regeneration. Molecules 2025, 30, 2323. [Google Scholar] [CrossRef]
- Shen, B.; Du, H.; Liu, A.; Li, N.; Chen, C.; Shen, L.; Hui, Y.; Huo, R.; Zhang, Z.; Wang, Q. Interfacial electric field steering S-scheme charge transfer in MIL-88A(Fe)/polydopamine heterojunctions: Dual-redox pathways for efficient pollutant mineralization. J. Clean. Prod. 2025, 523, 146458. [Google Scholar] [CrossRef]
- Sinar Mashuri, S.I.; Ibrahim, M.L.; Kasim, M.F.; Mastuli, M.S.; Rashid, U.; Abdullah, A.H.; Islam, A.; Asikin Mijan, N.; Tan, Y.H.; Mansir, N.; et al. Photocatalysis for organic wastewater treatment: From the basis to current challenges for society. Catalysts 2020, 10, 1260. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.C.; El Nemr, A. Principles of photocatalysts and their different applications: A review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef] [PubMed]
- Mohamadpour, F.; Amani, A.M. Photocatalytic systems: Reactions, mechanism, and applications. RSC Adv. 2024, 14, 20609–20645. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; López-Duarte, I.; Charalambidis, G.; Ladomenou, K.; Ince, M.; Coutsolelos, A.G. Porphyrins and phthalocyanines as biomimetic tools for photocatalytic H2 production and CO2 reduction. Chem. Soc. Rev. 2022, 51, 6965–7045. [Google Scholar] [CrossRef]
- Nasir, A.; Khalid, S.; Yasin, T.; Mazare, A. A review on the progress and future of TiO2/graphene photocatalysts. Energies 2022, 15, 6248. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Zolotukhina, A. Heterogeneous dendrimer-based catalysts. Polymers 2022, 14, 981. [Google Scholar] [CrossRef]
- Trentin, G.; Bitencourt, T.A.; Guedes, A.; Pessoni, A.M.; Brauer, V.S.; Pereira, A.K.; Costa, J.H.; Fill, T.P.; Almeida, F. Mass spectrometry analysis reveals lipids induced by oxidative stress in Candida albicans extracellular vesicles. Microorganisms 2023, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Swenson, K.A.; Min, K.; Konopka, J.B. Candida albicans pathways that protect against organic peroxides and lipid peroxidation. PLoS Genet. 2024, 20, e1011455. [Google Scholar] [CrossRef] [PubMed]
- Linares, C.E.; Giacomelli, S.R.; Altenhofen, D.; Alves, S.H.; Morsch, V.M.; Schetinger, M.R. Fluconazole and amphotericin-B resistance are associated with increased catalase and superoxide dismutase activity in Candida albicans and Candida dubliniensis. Rev. Soc. Bras. Med. Trop. 2013, 46, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Grijalva-Castillo, M.C.; Saénz-Hernández, R.J.; Cobos-Márquez, A.A.; Herrera-Ojeda, F.A.; Díaz-Chávez, F.E.; Acosta-Galindo, I.R.; Leyva-Porras, C.; Castillo-González, A.R.; Favila-Pérez, M.A.; Quiñonez-Flores, C.M.; et al. Synergistic Disinfection of Photocatalytic Nanomaterials Exposed to UVC, Electricity and Magnetic Fields Against Candida albicans. Coatings 2025, 15, 968. [Google Scholar] [CrossRef]
- Thabet, S.; Simonet, F.; Lemaire, M.; Guillard, C.; Cotton, P. Impact of photocatalysis on fungal cells: Depiction of cellular and molecular effects on Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2014, 80, 7527–7535. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.d.S.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Cheng, J.; Sun, A.Y.; Wang, L.; Mirchevska, G.; Calderone, R.; Li, D. Respiratory stress in mitochondrial electron transport chain complex mutants of Candida albicans activates Snf1 kinase response. Fungal Genet. Biol. 2018, 111, 73–84. [Google Scholar] [CrossRef]
- Zubair, M.; Husain, F.M.; Al-Amri, M.; Hasan, I.; Hassan, I.; Albalawi, T.; Fatima, F.; Khan, A.; Arshad, M.; Alam, P. In vitro inhibition of biofilm and virulence factor production in azole-resistant strains of Candida albicans isolated from diabetic foot by Artemisia vulgaris-stabilized tin (IV) oxide nanoparticles. Front. Cell. Infect. Microbiol. 2024, 13, 1322778. [Google Scholar] [CrossRef]
- Lara, H.H.; Ixtepan-Turrent, L.; Jose Yacaman, M.; Lopez-Ribot, J. Inhibition of Candida auris biofilm formation on medical and environmental surfaces by silver nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 21183–21191. [Google Scholar] [CrossRef]
- Smolarz, M.; Zawrotniak, M.; Satala, D.; Rapala-Kozik, M. Extracellular nucleic acids present in the Candida albicans biofilm trigger the release of neutrophil extracellular traps. Front. Cell. Infect. Microbiol. 2021, 11, 681030. [Google Scholar] [CrossRef]
- Nguyen, K.N.; Sao, L.; Kyllo, K.; Hernandez, D.; Salomon, S.; Shah, K.; Oh, D.; Kao, K.C. Antibiofilm activity of PDMS/TiO2 against Candida glabrata through inhibited hydrophobic recovery. ACS Omega 2024, 9, 42593–42601. [Google Scholar] [CrossRef]
- Arribas, V.; Gil, C.; Molero, G. Deciphering the oxidative stress response in Candida albicans. Fungal Biol. Rev. 2025, 52, 100427. [Google Scholar] [CrossRef]
- Arribas, V.; Monteoliva, L.; Hernáez, M.L.; Gil, C.; Molero, G. Unravelling the role of Candida albicans Prn1 in the oxidative stress response through a proteomics approach. Antioxidants 2024, 13, 527. [Google Scholar] [CrossRef]
- Briones-Martin-Del-Campo, M.; Orta-Zavalza, E.; Juarez-Cepeda, J.; Gutierrez-Escobedo, G.; Cañas-Villamar, I.; Castaño, I.; De Las Peñas, A. The oxidative stress response of the opportunistic fungal pathogen Candida glabrata. Rev. Iberoam. Micol. 2014, 31, 67–71. [Google Scholar] [CrossRef]
- Haque, F.; Blanchard, A.; Laipply, B.; Dong, X. Visible-light-activated TiO2-based photocatalysts for the inactivation of pathogenic bacteria. Catalysts 2024, 14, 855. [Google Scholar] [CrossRef]
- Helmy, E.T.; Abouellef, E.M.; Soliman, U.A.; Pan, J.H. Novel Green Synthesis of S-Doped TiO2 Nanoparticles Using Malva parviflora Plant Extract and Their Photocatalytic, Antimicrobial and Antioxidant Activities under Sunlight Illumination. Chemosphere 2021, 271, 129524. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, J.-H.; Weng, C.-H.; Yen, L.-T.; Gaybullaev, G.; Chang, C.-J.; de Luna, M.D.G.; Lin, Y.-T. Inactivation of pathogens by visible light photocatalysis with nitrogen-doped TiO2 and tourmaline-nitrogen co-doped TiO2. Sep. Purif. Technol. 2021, 274, 118979. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.K.; Kanojia, R.; Attri, P. Transition Metal Oxides and Their Composites for Photocatalytic Dye Degradation. J. Compos. Sci. 2021, 5, 82. [Google Scholar] [CrossRef]
- Batool, M.; Khurshid, S.; Qureshi, Z.; Hassan, A.; Siddique, M.B.A.; Naveed, S.; Siddique, S.A. Study of biogenically fabricated transition metal oxides nanoparticles on oral cavity infectious microbial strains. Inorg. Nano-Met. Chem. 2021, 51, 856–866. [Google Scholar] [CrossRef]
- Gautam, S.; Das, D.K.; Kaur, J.; Kumar, A.; Ubaidullah, M.; Hasan, M.; Yadav, K.K.; Gupta, R.K. Transition metal-based nanoparticles as potential antimicrobial agents: Recent advancements, mechanistic, challenges, and future prospects. Discover. Nano 2023, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Lin, I.H.; Krisnawati, D.I.; Khaerunnisa, S.; Khafid, M.; Widodo; Hsiao, Y.C.; Kuo, T.R. Antibacterial pathways in transition metal-based nanocomposites: A mechanistic overview. Int. J. Nanomed. 2022, 17, 6821–6842. [Google Scholar] [CrossRef]
- Jeba, R.; Radhika, S.; Padma, C.M.; Ascar Davix, X. Structural, optical, thermal, magnetic properties of zirconia nano-rods and their photocatalytic and antimicrobial properties. J. Water Environ. Nanotechnol. 2021, 6, 252–264. [Google Scholar]
- Abbas, S.; Uzair, B.; Sajjad, S.; Leghari, S.A.K.; Noor, S.; Niazi, M.B.K.; Farooq, I.; Iqbal, H. Dual-functional green facile CuO/MgO nanosheets composite as an efficient antimicrobial agent and photocatalyst. Arab. J. Sci. Eng. 2022, 47, 5895–5909. [Google Scholar] [CrossRef]
- El-Khodary, S.A.; Menazea, A.A.; Abdelhamid, S.A.; Khalaf, M. Tuning the optical, electrical, anti-microbial, and swelling activity of nanowires manganese dioxide-loaded chitosan matrix. Int. J. Biol. Macromol. 2025, 311, 143745. [Google Scholar] [CrossRef]
- Mp, N.; Rao, B.M. Anodized CuO nanoflakes for the antibacterial and antifungal applications. Heliyon 2025, 11, e42304. [Google Scholar] [CrossRef]
- Nasr, R.A.; El-Sayed, A.F.; El Komy, G.M.; El-Bassyouni, G.T.; Mousa, S.M. Modification of photocatalytic activity and antibacterial properties of Mn2O3 by Zn ions doping. Sci. Rep. 2025, 15, 14325. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Kumar, S.S.; Manikandan, M.; Saravanan, M. Photocatalytic properties and antimicrobial efficacy of Fe-doped CuO nanoparticles against the pathogenic bacteria and fungi. Microb. Pathog. 2018, 122, 84–89. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X. Nanomaterial ZnO Synthesis and Its Photocatalytic Applications: A Review. Nanomaterials 2025, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.S.H.; Karthikeyan, C.; Kumar, V.S.; Kumaresan, S.; Sasikumar, S. Effect of Mg2+, Ca2+, Sr2+ and Ba2+ Metal Ions on the Antifungal Activity of ZnO Nanoparticles Tested against Candida albicans. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Saqib Saif, M.; Zafar, A.; Waqas, M.; Hassan, S.G.; Haq, A.U.; Tariq, T.; Batool, S.; Dilshad, M.; Hasan, M.; Shu, X. Phyto-Reflexive Zinc Oxide Nano-Flowers Synthesis: An Advanced Photocatalytic Degradation and Infectious Therapy. J. Mater. Res. Technol. 2021, 13, 2375–2391. [Google Scholar] [CrossRef]
- Arzate-Quintana, C.; Leyva-Porras, C.; Favila-Pérez, M.; Castillo, A.; Quiñonez-Flores, C.; Faudoa, A. Biofilm Integrity and Cytomorphology of Candida albicans after Exposure to UV-Light on ZnO Thin Films: SEM Analysis. Microsc. Microanal. 2021, 27, 1896–1898. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choe, Y.E.; Shin, S.J.; Park, J.H.; Dashnyam, K.; Kim, H.S.; Jun, S.K.; Knowles, J.C.; Kim, H.W.; Lee, J.H.; et al. Photocatalytic Effect-Assisted Antimicrobial Activities of Acrylic Resin Incorporating Zinc Oxide Nanoflakes. Biomater. Adv. 2022, 139, 213025. [Google Scholar] [CrossRef]
- Song, Y.; Chang, M.; Dong, H.; Li, N.; Zeng, G.; Wang, Y.; Yang, D. Nanozymes: An emerging arsenal for the treatment of Candida albicans infection. Fundam. Res. 2025, 5, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zou, S.; Wang, Q.; Chen, L.; Yan, X.; Gao, L. Catalytic defense against fungal pathogens using nanozymes. Nanotechnol. Rev. 2021, 10, 1277–1292. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Ouyang, D.; Ye, K.; Chen, Y.; Li, Q.; Xia, Q.; Wu, X.; Yang, Y. Copper- and iodine-doped nanozymes with simulated enzyme activity and efficient antifungal activity against Candida albicans. Biochem. Eng. J. 2023, 191, 108791. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Yuan, T.; Zhu, J.; Yang, Y. Influence of the iodine content of nitrogen- and iodine-doped carbon dots as a peroxidase mimetic nanozyme exhibiting antifungal activity against Candida albicans. Biochem. Eng. J. 2021, 175, 108139. [Google Scholar] [CrossRef]
- Oh, M.J.; Yoon, S.; Babeer, A.; Liu, Y.; Ren, Z.; Xiang, Z.; Miao, Y.; Cormode, D.P.; Chen, C.; Steager, E.; et al. Nanozyme-based robotics approach for targeting fungal infection. Adv. Mater. 2024, 36, e2300320. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dizaj, S.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, X.; Shi, L.; Yang, Y. Deep eutectic solvents-derived carbon dots for detection of mercury (II), photocatalytic antifungal activity and fluorescent labeling for Candida albicans. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 220, 117080. [Google Scholar] [CrossRef]
- Cong, S.; Zhou, S.; You, J.; Wang, L.; Wang, X. Directionally designed double Z-scheme heterojunction: BiOBr and carbon dots-reduced TiO2−x for targeted photocarriers separation in photocatalytic sterilization. Small 2025, 21, e05905. [Google Scholar] [CrossRef]
- Darbari, S.; Abdi, Y.; Haghighi, F.; Mohajerzadeh, S.; Haghighi, N. Investigating the antifungal activity of TiO2 nanoparticles deposited on branched carbon nanotube arrays. J. Phys. D Appl. Phys. 2011, 44, 245401. [Google Scholar] [CrossRef]
- Ballo, M.K.S.; Rtimi, S.; Kiwi, J.; Pulgarin, C.; Entenza, J.M.; Bizzini, A. Fungicidal Activity of Copper-Sputtered Flexible Surfaces under Dark and Actinic Light against Azole-Resistant Candida albicans and Candida glabrata. J. Photochem. Photobiol. B Biol. 2017, 174, 229–234. [Google Scholar] [CrossRef]
- Faudoa-Arzate, A.; Camarillo-Cisneros, J.; Castillo-González, A.R.; Favila-Pérez, M.A.; Sáenz-Hernández, R.J.; Realyvazquez-Guevara, P.R.; Arzate-Quintana, C. Disinfection Mechanism of the Photocatalytic Activity of SnO2 Thin Films Against Candida albicans, Proposed from Experimental and Simulated Perspectives. Can. J. Microbiol. 2021, 67, 667–676. [Google Scholar] [CrossRef]
- Ariani, N.; Vissink, A.; van Oort, R.P.; Kusdhany, L.; Djais, A.; Rahardjo, T.B.; van der Mei, H.C.; Krom, B.P. Microbial Biofilms on Facial Prostheses. Biofouling 2012, 28, 583–591. [Google Scholar] [CrossRef]
- Widodo, T.T.; Siswomiharjo, W.; Sunarintyas, S.; Yulianto, D.K. Effect of Method and Concentration of Titanium Dioxide Addition on Anti-Biofilm Ability in Extraoral Maxillofacial Prosthetic Fungus. Int. J. Adv. Med. 2022, 10, 1–9. [Google Scholar] [CrossRef]
- Shafie, S.; Shimy, A.; Elsheredy, A.; Moustafa, M. Antifungal Effect of Photocatalytic Nano-Titanium Dioxide Incorporated in Silicone Elastomer. Alexandria Dent. J. 2019, 44, 52–60. [Google Scholar] [CrossRef]
- Nett, J.E.; Marchillo, K.; Spiegel, C.A.; Andes, D.R. Development and Validation of an In Vivo Candida albicans Biofilm Denture Model. Infect. Immun. 2010, 78, 3650–3659. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Liu, X.; Chen, H.; Huang, Y.; Li, A.; Guo, L. Study on Mechanical Properties, Optical Properties, Cytotoxicity of TiO2-HAP Nanoparticles-Modified PMMA and Photodynamically Assisted Antibacterial Activity Against Candida albicans in Vitro. Int. J. Nanomed. 2025, 20, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Alhasani, A.H.; Al-Akwa, A.A.Y.; Al-Shamahy, H.A.W.; Al-deen, H.M.S.; Al-labani, M.A. Biofilm Formation and Antifungal Susceptibility of Candida Isolates from Oral Cavity after the Introduction of Fixed Orthodontic Appliances. Univ. J. Pharm. Res. 2020, 5, 21–27. [Google Scholar] [CrossRef]
- Grzegocka, K.; Krzyściak, P.; Hille-Padalis, A.; Loster, J.E.; Talaga-Ćwiertnia, K.; Loster, B.W. Candida Prevalence and Oral Hygiene Due to Orthodontic Therapy with Conventional Brackets. BMC Oral Health 2020, 20, 277. [Google Scholar] [CrossRef]
- Cao, S.; Liu, B.; Fan, L.; Yue, Z.; Liu, B.; Cao, B. Highly Antibacterial Activity of N-Doped TiO2 Thin Films Coated on Stainless Steel Brackets under Visible Light Irradiation. Appl. Surf. Sci. 2014, 309, 119–127. [Google Scholar] [CrossRef]
- Özyıldız, F.; Güden, M.; Uzel, A.; Karaboz, I.; Akil, O.; Bulut, H. Antimicrobial Activity of TiO2-Coated Orthodontic Ceramic Brackets against Streptococcus mutans and Candida albicans. Biotechnol. Bioprocess Eng. 2010, 15, 680–685. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraus, K.; Mikziński, P.; Widelski, J.; Paluch, E. Nanomaterials for Photocatalytic Inactivation and Eradication of Candida spp. Biofilms in Healthcare Environment: A Novel Approach in Modern Clinical Practice. Molecules 2025, 30, 4500. https://doi.org/10.3390/molecules30234500
Kraus K, Mikziński P, Widelski J, Paluch E. Nanomaterials for Photocatalytic Inactivation and Eradication of Candida spp. Biofilms in Healthcare Environment: A Novel Approach in Modern Clinical Practice. Molecules. 2025; 30(23):4500. https://doi.org/10.3390/molecules30234500
Chicago/Turabian StyleKraus, Karolina, Paweł Mikziński, Jarosław Widelski, and Emil Paluch. 2025. "Nanomaterials for Photocatalytic Inactivation and Eradication of Candida spp. Biofilms in Healthcare Environment: A Novel Approach in Modern Clinical Practice" Molecules 30, no. 23: 4500. https://doi.org/10.3390/molecules30234500
APA StyleKraus, K., Mikziński, P., Widelski, J., & Paluch, E. (2025). Nanomaterials for Photocatalytic Inactivation and Eradication of Candida spp. Biofilms in Healthcare Environment: A Novel Approach in Modern Clinical Practice. Molecules, 30(23), 4500. https://doi.org/10.3390/molecules30234500







