In Vivo Behavior of Biomimetic Nanoparticles: Strategies for Clearance Avoidance, Targeting, and Functional Delivery
Abstract
1. Introduction
2. Fabrication Methods
2.1. Cell Lysis
2.2. Membrane Extraction
2.3. Inorganic Particle Coating
3. Pharmacokinetics Models
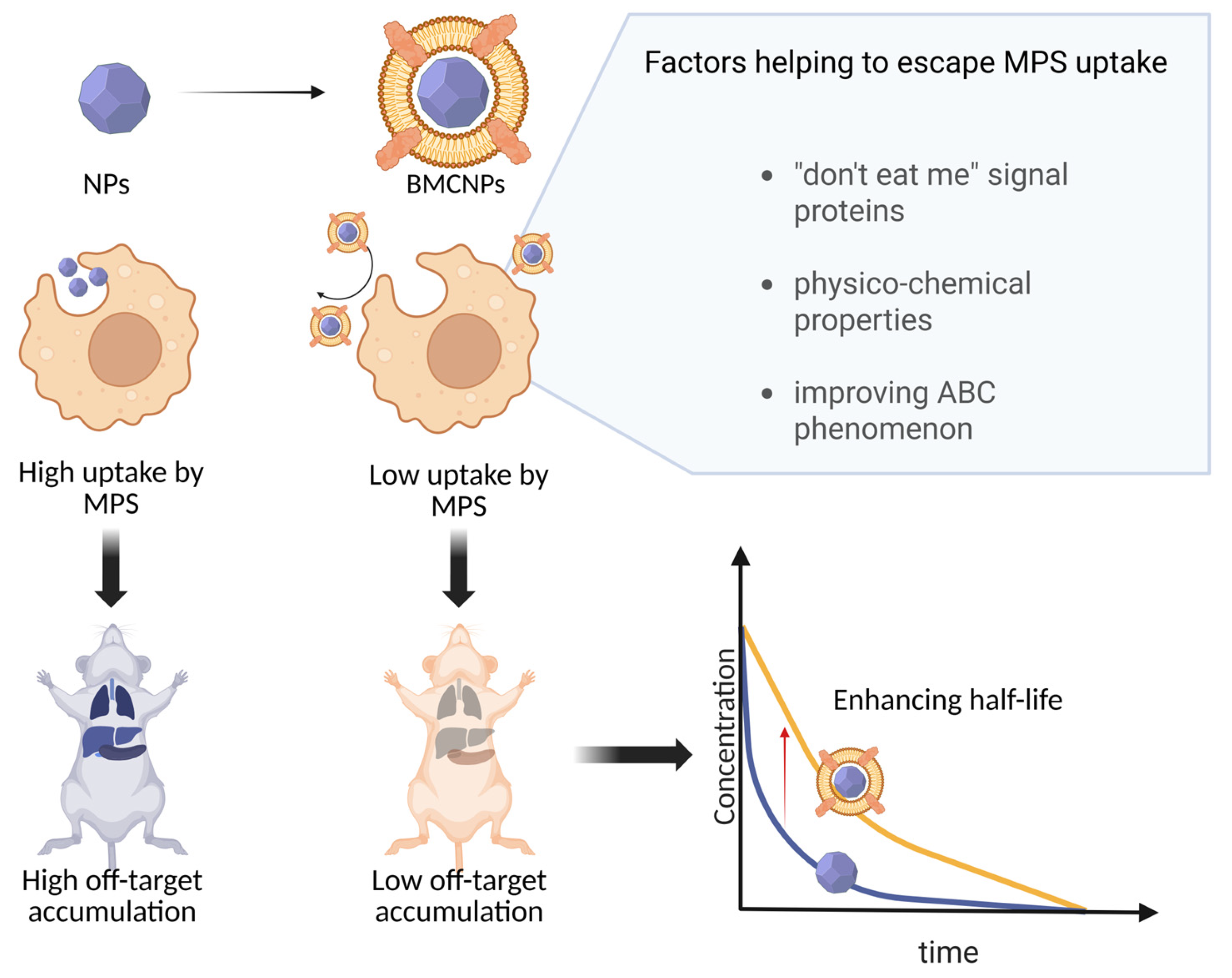
4. Factors Affecting MPS Uptake and Its Role in BMCNP Clearance
4.1. Functional Significance of Membrane Molecular Composition
4.2. Roles of Physicochemical Parameters
4.3. ABC Phenomenon
4.4. Determination of QTTP
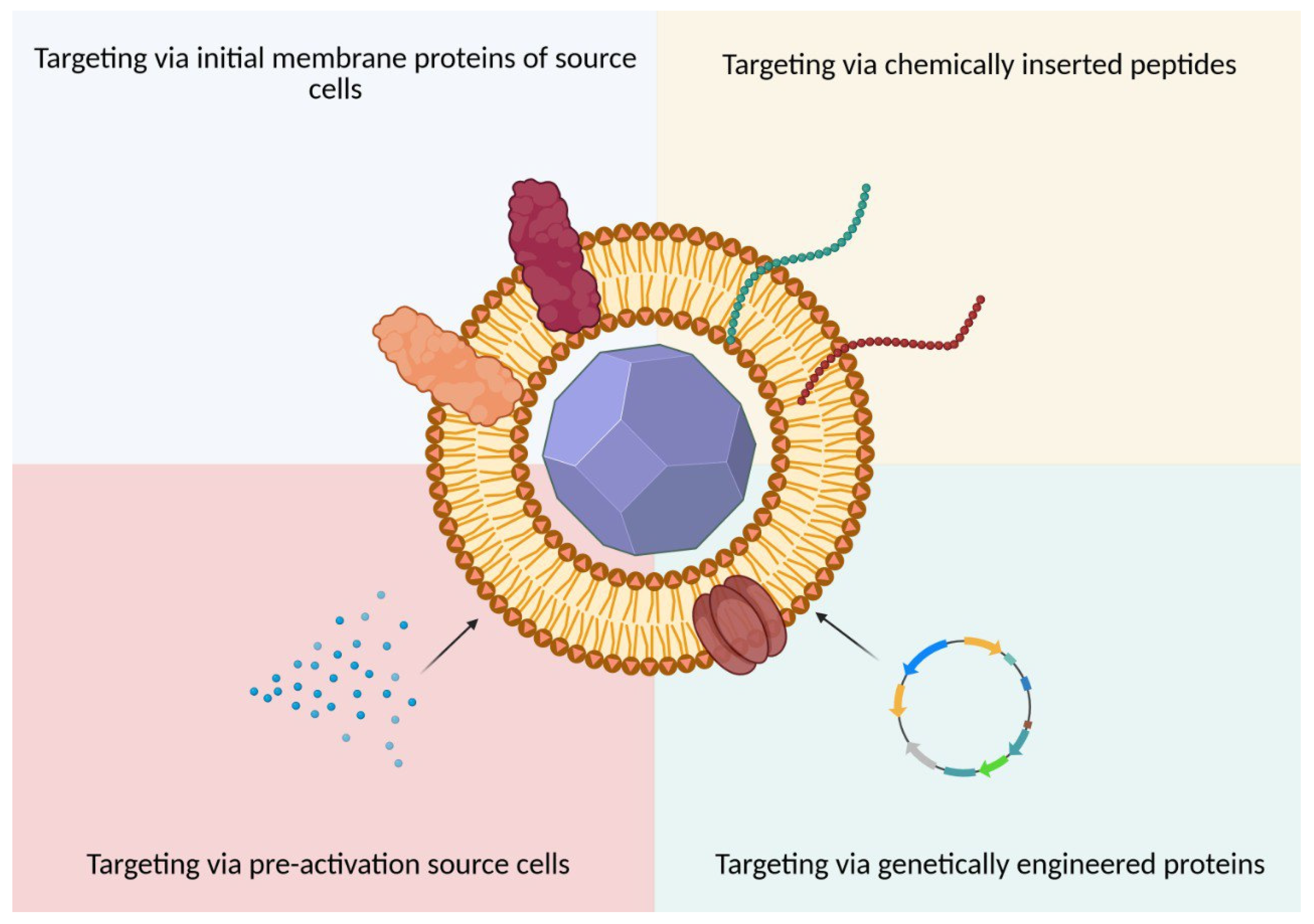
5. Targeted Delivery
5.1. Targeting by Initial Membrane Characteristics
5.2. Targeting via Exogenous Exposure to BMCNPs
5.2.1. Chemical and Physical Modifications
5.2.2. Genetic Modifications
5.2.3. Pre-Activation of Source Cells
5.3. Penetration of Biological Barriers
5.3.1. Endolysosomal Compartment
- Proton sponge effect (e.g., PAMAM, PEG-PCL-PEI, PPTS);
- Osmotic lysis (e.g., PMPC-b-PDPAEMA, DOPA, DOTAP, CaP);
- Swelling-induced escape (e.g., PDEAEMA, PAEMA, PEGDMA, mPEG);
- Pore formation (e.g., LPEI polyplexes, PEI, gp41 peptide);
- Membrane disruption (e.g., DSPE-PCB, ultrasound-responsive polymersomes, temperature-sensitive bubble liposomes);
- Membrane fusion and photochemical internalization.
5.3.2. Blood–Brain Barrier
6. Therapeutic Efficiency
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMCNPs | Biomimetic cell membrane-coated nanoparticles |
| NPs | Nanoparticles |
| PLGA | Poly(lactic-co-glycolic acid) |
| RBC | Red blood cells |
| UC | Ultracentrifugation |
| DGU | Density gradient ultracentrifugation |
| FNC | Flash NanoComplexation |
| Half-time | t1/2 |
| MM-coated NPs | Macrophage membrane-coated nanoparticles |
| SM-coated NPs | Stem cell-coated nanoparticles |
| CM-coated NPs | Cancer cell-coated nanoparticles |
| CNS | Central nervous system |
| PS | Phosphatidylserine |
| ABC | Accelerated blood clearance |
| ROS | Reactive oxygen species |
| CMAs | Critical material attributes |
| QTPP | Quality Target Product Profile |
| PEG | Polyethylene glycol |
| EPR | Enhanced permeability and retention |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| ICAM-1 | Intercellular adhesion molecule 1 |
| TME | Tumor microenvironment |
| NM-coated NPs | Neutrophile membrane-coated NPs |
| LPS | Lipopolysaccharide |
| PM-coated NPs | Platelet-coated nanoparticles |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| CAR | Chimeric antigen receptors |
| GBM | Glioblastoma |
| TLR | Toll-like receptors |
| BBB | Blood–brain barrier |
| ApoE | Apolipoprotein E |
| LDLRs | Low-density lipoprotein receptors |
| Ang | Angiopep-2 |
| Lex | Lexiscan |
| SDF-1 | Stromal cell-derived factor-1 |
| PDT | Photodynamic therapy |
| DCs | Dendritic cells |
| TAAs | Tumor-Associated Antigens |
| MHC | Major histocompatibility complex |
| CTLs | Cytotoxic T lymphocytes |
| PAMPs | Pathogen-associated molecular patterns |
| TAMs | Tumor-associated macrophages |
References
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Beh, C.Y.; Prajnamitra, R.P.; Chen, L.-L.; Hsieh, P.C.-H. Advances in Biomimetic Nanoparticles for Targeted Cancer Therapy and Diagnosis. Molecules 2021, 26, 5052. [Google Scholar] [CrossRef]
- Li, H.; Jin, K.; Luo, M.; Wang, X.; Zhu, X.; Liu, X.; Jiang, T.; Zhang, Q.; Wang, S.; Pang, Z. Size Dependency of Circulation and Biodistribution of Biomimetic Nanoparticles: Red Blood Cell Membrane-Coated Nanoparticles. Cells 2019, 8, 881. [Google Scholar] [CrossRef]
- Tikhonov, A.; Kachanov, A.; Yudaeva, A.; Danilik, O.; Ponomareva, N.; Karandashov, I.; Kostyusheva, A.; Zamyatnin, A.A.; Parodi, A.; Chulanov, V.; et al. Biomimetic Nanoparticles for Basic Drug Delivery. Pharmaceutics 2024, 16, 1306. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Dai, L.; He, Q.; Li, J. Macrophage Cell Membrane Camouflaged Mesoporous Silica Nanocapsules for In Vivo Cancer Therapy. Adv. Healthc. Mater. 2015, 4, 1645–1652. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Correia, I.J.; Moreira, A.F. Red Blood Cell Membrane-Camouflaged Gold-Core Silica Shell Nanorods for Cancer Drug Delivery and Photothermal Therapy. Int. J. Pharm. 2024, 655, 124007. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, B.; Shen, S.; Tuo, Y.; Luo, Z.; Hu, Y.; Pang, Z.; Jiang, X. Tumor Microenvironment Modulation by Cyclopamine Improved Photothermal Therapy of Biomimetic Gold Nanorods for Pancreatic Ductal Adenocarcinomas. ACS Appl. Mater. Interfaces 2017, 9, 31497–31508. [Google Scholar] [CrossRef]
- Pang, L.; Qin, J.; Han, L.; Zhao, W.; Liang, J.; Xie, Z.; Yang, P.; Wang, J. Exploiting Macrophages as Targeted Carrier to Guide Nanoparticles into Glioma. Oncotarget 2016, 7, 37081–37091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Du, Y.; Wang, S.; Chen, B. A Review of Biomimetic Nanoparticle Drug Delivery Systems Based on Cell Membranes. Drug Des. Devel. Ther. 2020, 14, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pan, D.; Chen, S.; Martikainen, M.-V.; Kårlund, A.; Ke, J.; Pulkkinen, H.; Ruhanen, H.; Roponen, M.; Käkelä, R.; et al. Systematic Design of Cell Membrane Coating to Improve Tumor Targeting of Nanoparticles. Nat. Commun. 2022, 13, 6181. [Google Scholar] [CrossRef]
- Liu, H.; Su, Y.-Y.; Jiang, X.-C.; Gao, J.-Q. Cell Membrane-Coated Nanoparticles: A Novel Multifunctional Biomimetic Drug Delivery System. Drug Deliv. Transl. Res. 2023, 13, 716–737. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Na, Y.; Yin, S.; Yan, C.; Gu, J.; Zhang, N.; Geng, F. Cell Membrane Biomimetic Nanoparticles with Potential in Treatment of Alzheimer’s Disease. Molecules 2023, 28, 2336. [Google Scholar] [CrossRef] [PubMed]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M. Immune Cell Membrane—Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small 2021, 17, 2006484. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red Blood Cell Membrane-Camouflaged Nanoparticles: A Novel Drug Delivery System for Antitumor Application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Choi, B.; Park, W.; Park, S.-B.; Rhim, W.-K.; Han, D.K. Recent Trends in Cell Membrane-Cloaked Nanoparticles for Therapeutic Applications. Methods 2020, 177, 2–14. [Google Scholar] [CrossRef]
- Arduino, I.; Di Fonte, R.; Tiboni, M.; Porcelli, L.; Serratì, S.; Fondaj, D.; Rafaschieri, T.; Cutrignelli, A.; Guida, G.; Casettari, L.; et al. Microfluidic Development and Biological Evaluation of Targeted Therapy-Loaded Biomimetic Nano System to Improve the Metastatic Melanoma Treatment. Int. J. Pharm. 2024, 650, 123697. [Google Scholar] [CrossRef]
- Liu, L.; Bai, X.; Martikainen, M.-V.; Kårlund, A.; Roponen, M.; Xu, W.; Hu, G.; Tasciotti, E.; Lehto, V.-P. Cell Membrane Coating Integrity Affects the Internalization Mechanism of Biomimetic Nanoparticles. Nat. Commun. 2021, 12, 5726. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yang, C.; Zhang, F.; Li, M.; Tu, Z.; Mu, L.; Dawulieti, J.; Lao, Y.; Xiao, Z.; Yan, H.; et al. A Versatile and Robust Platform for the Scalable Manufacture of Biomimetic Nanovaccines. Adv. Sci. 2021, 8, 2002020. [Google Scholar] [CrossRef]
- Kutumova, E.; Akberdin, I.; Kiselev, I.; Sharipov, R.; Kolpakov, F. Modular Representation of Physiologically Based Pharmacokinetic Models: Nanoparticle Delivery to Solid Tumors in Mice as an Example. Mathematics 2022, 10, 1176. [Google Scholar] [CrossRef]
- Talevi, A.; Bellera, C.L. Two-Compartment Pharmacokinetic Model. In The ADME Encyclopedia; Springer International Publishing: Cham, Switzerland, 2022; pp. 1167–1174. [Google Scholar]
- Ben-Akiva, E.; Meyer, R.A.; Yu, H.; Smith, J.T.; Pardoll, D.M.; Green, J.J. Biomimetic Anisotropic Polymeric Nanoparticles Coated with Red Blood Cell Membranes for Enhanced Circulation and Toxin Removal. Sci. Adv. 2020, 6, eaay9035. [Google Scholar] [CrossRef]
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte—Membrane—Enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy. Adv. Mater. 2017, 29, 1701429. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, M.; Chen, J.; Liu, Y.; Chen, Y.; Fan, G.; Liu, Y.; Cheng, J.; Wang, Z.; Wang, S.; et al. Cancer-Erythrocyte Hybrid Membrane-Camouflaged Magnetic Nanoparticles with Enhanced Photothermal-Immunotherapy for Ovarian Cancer. ACS Nano 2021, 15, 19756–19770. [Google Scholar] [CrossRef]
- Miao, Y.; Yang, Y.; Guo, L.; Chen, M.; Zhou, X.; Zhao, Y.; Nie, D.; Gan, Y.; Zhang, X. Cell Membrane-Camouflaged Nanocarriers with Biomimetic Deformability of Erythrocytes for Ultralong Circulation and Enhanced Cancer Therapy. ACS Nano 2022, 16, 6527–6540. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, Y.; Guo, R.; Yao, X.; Sung, S.; Pang, Z.; Yang, W. Erythrocyte-Cancer Hybrid Membrane-Camouflaged Melanin Nanoparticles for Enhancing Photothermal Therapy Efficacy in Tumors. Biomaterials 2019, 192, 292–308. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Q.; Wu, T.; He, Z.; Li, Y.; Li, Z.; Hou, X.; He, Y.; Ruan, S.; Wang, Z.; et al. A Novel Multi-Functionalized Multicellular Nanodelivery System for Non-Small Cell Lung Cancer Photochemotherapy. J. Nanobiotechnol. 2021, 19, 245. [Google Scholar] [CrossRef]
- Huang, S.; Song, C.; Miao, J.; Zhu, X.; Jia, Y.; Liu, Y.; Fu, D.; Li, B.; Miao, M.; Duan, S.; et al. Red Blood Cell Membrane-Coated Functionalized Au Nanocage as a Biomimetic Platform for Improved MicroRNA Delivery in Hepatocellular Carcinoma. Int. J. Pharm. 2023, 642, 123044. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.-B.; Lee, S.M.Y.; Wang, R. Treatment of Atherosclerosis by Macrophage-Biomimetic Nanoparticles via Targeted Pharmacotherapy and Sequestration of Proinflammatory Cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Xiang, Y.; Liu, S.; Zhang, Y.; Wan, J.; Ci, Z.; Cui, M.; Shen, L.; Li, N.; Guan, Y. Macrophage Membrane Modified Baicalin Liposomes Improve Brain Targeting for Alleviating Cerebral Ischemia Reperfusion Injury. Nanomed. Nanotechnol. Biol. Med. 2022, 43, 102547. [Google Scholar] [CrossRef]
- Kang, T.; Zhu, Q.; Wei, D.; Feng, J.; Yao, J.; Jiang, T.; Song, Q.; Wei, X.; Chen, H.; Gao, X.; et al. Nanoparticles Coated with Neutrophil Membranes Can Effectively Treat Cancer Metastasis. ACS Nano 2017, 11, 1397–1411. [Google Scholar] [CrossRef]
- Yao, Q.; Yang, G.; Wang, H.; Liu, J.; Zheng, J.; Lv, B.; Yang, M.; Yang, Y.; Gao, C.; Guo, Y. Aging Erythrocyte Membranes as Biomimetic Nanometer Carriers of Liver-Targeting Chromium Poisoning Treatment. Drug Deliv. 2021, 28, 1455–1465. [Google Scholar] [CrossRef]
- Yuan, P.; Chen, X.; Li, X.; Zong, X.; Yang, C.; Li, Y.; Xue, W.; Dai, J. Effect of Cell Membrane—cloaked Nanoparticle Elasticity on Nano—Bio Interaction. Small Methods 2023, 7, 2201548. [Google Scholar] [CrossRef]
- Parodi, A.; Quattrocchi, N.; van de Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.V.; et al. Synthetic Nanoparticles Functionalized with Biomimetic Leukocyte Membranes Possess Cell-like Functions. Nat. Nanotechnol. 2013, 8, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Debnath, G.; Vasu, B.; Gorla, R.S.R. Current State-of-the-Art in Multi-Scale Modeling in Nano-Cancer Drug Delivery: Role of AI and Machine Learning. Cancer Nanotechnol. 2025, 16, 45. [Google Scholar] [CrossRef]
- Rout, S.; Mallick, R.; Kumar Sahu, S. Exploring the Significance of Feature Analysis in AI/ML Modeling. In Proceedings of the 2023 OITS International Conference on Information Technology (OCIT), Raipur, India, 13 December 2023; pp. 580–585. [Google Scholar]
- Chou, W.-C.; Chen, Q.; Yuan, L.; Cheng, Y.-H.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E.; Lin, Z. An Artificial Intelligence-Assisted Physiologically-Based Pharmacokinetic Model to Predict Nanoparticle Delivery to Tumors in Mice. J. Control. Release 2023, 361, 53–63. [Google Scholar] [CrossRef]
- Lin, Z.; Chou, W.-C.; Cheng, Y.-H.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E. Predicting Nanoparticle Delivery to Tumors Using Machine Learning and Artificial Intelligence Approaches. Int. J. Nanomed. 2022, 17, 1365–1379. [Google Scholar] [CrossRef]
- Mi, K.; Chou, W.-C.; Chen, Q.; Yuan, L.; Kamineni, V.N.; Kuchimanchi, Y.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E.; Lin, Z. Predicting Tissue Distribution and Tumor Delivery of Nanoparticles in Mice Using Machine Learning Models. J. Control. Release 2024, 374, 219–229. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Jin, K.; Zhang, B.; Peng, S.; Nayak, A.K.; Pang, Z. Recent Advances in Erythrocyte Membrane-Camouflaged Nanoparticles for the Delivery of Anti-Cancer Therapeutics. Expert Opin. Drug Deliv. 2022, 19, 965–984. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Shevchenko, K.G.; Deyev, S.M. Rediscovery of Mononuclear Phagocyte System Blockade for Nanoparticle Drug Delivery. Nat. Commun. 2024, 15, 4366. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.A.; Liu, F.; Jarrett, T.R.; Fletcher, N.L.; Thurecht, K.J. Nanoparticle Based Medicines: Approaches for Evading and Manipulating the Mononuclear Phagocyte System and Potential for Clinical Translation. Biomater. Sci. 2022, 10, 3029–3053. [Google Scholar] [CrossRef]
- Lu, J.; Gao, X.; Wang, S.; He, Y.; Ma, X.; Zhang, T.; Liu, X. Advanced Strategies to Evade the Mononuclear Phagocyte System Clearance of Nanomaterials. Exploration 2023, 3, 20220045. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A. The Mononuclear Phagocyte System. Curr. Opin. Immunol. 2006, 18, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, A.; Fenaroli, F.; Monopoli, M.P.; Åberg, C.; Dawson, K.A.; Salvati, A. Effects of the Presence or Absence of a Protein Corona on Silica Nanoparticle Uptake and Impact on Cells. ACS Nano 2012, 6, 5845–5857. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, X.; Zhang, W.; Zhang, K.; Pan, L.; Zhu, M.; Qin, H.; Zou, C.; Wang, W.; Zhang, C.; et al. Biomimetic Macrophage Membrane-Camouflaged Nanoparticles Induce Ferroptosis by Promoting Mitochondrial Damage in Glioblastoma. ACS Nano 2023, 17, 23746–23760. [Google Scholar] [CrossRef]
- Lai, J.; Deng, G.; Sun, Z.; Peng, X.; Li, J.; Gong, P.; Zhang, P.; Cai, L. Scaffolds Biomimicking Macrophages for a Glioblastoma NIR-Ib Imaging Guided Photothermal Therapeutic Strategy by Crossing Blood-Brain Barrier. Biomaterials 2019, 211, 48–56. [Google Scholar] [CrossRef]
- Li, Y.-S.; Wu, H.-H.; Jiang, X.-C.; Zhang, T.-Y.; Zhou, Y.; Huang, L.-L.; Zhi, P.; Tabata, Y.; Gao, J.-Q. Active Stealth and Self-Positioning Biomimetic Vehicles Achieved Effective Antitumor Therapy. J. Control. Release 2021, 335, 515–526. [Google Scholar] [CrossRef]
- Bose, R.J.; Kim, B.J.; Arai, Y.; Han, I.; Moon, J.J.; Paulmurugan, R.; Park, H.; Lee, S.-H. Bioengineered Stem Cell Membrane Functionalized Nanocarriers for Therapeutic Targeting of Severe Hindlimb Ischemia. Biomaterials 2018, 185, 360–370. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, C.; Wu, Z.; Zhang, W.; Wang, L.; Yu, J.; Li, L.; Zhang, J.; Duan, Y. Engineered PD-1/TIGIT Dual-Activating Cell-Membrane Nanoparticles with Dexamethasone Act Synergistically to Shape the Effector T Cell/Treg Balance and Alleviate Systemic Lupus Erythematosus. Biomaterials 2022, 285, 121517. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Jiang, J.; Chen, F.; Fang, X. Doxorubicin Delivered Using Nanoparticles Camouflaged with Mesenchymal Stem Cell Membranes to Treat Colon Cancer. Int. J. Nanomed. 2020, 15, 2873–2884. [Google Scholar] [CrossRef]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth Coating of Nanoparticles in Drug-Delivery Systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef]
- Vandchali, N.R.; Moadab, F.; Taghizadeh, E.; Tajbakhsh, A.; Gheibihayat, S.M. CD47 Functionalization of Nanoparticles as a Poly(Ethylene Glycol) Alternative: A Novel Approach to Improve Drug Delivery. Curr. Drug Targets 2021, 22, 1750–1759. [Google Scholar] [CrossRef]
- Kaur, S.; Isenberg, J.S.; Roberts, D.D. CD47 (Cluster of Differentiation 47). Atlas Genet. Cytogenet. Oncol. Haematol. 2021, 25, 83–102. [Google Scholar] [PubMed]
- Yang, H.; Shao, R.; Huang, H.; Wang, X.; Rong, Z.; Lin, Y. Engineering Macrophages to Phagocytose Cancer Cells by Blocking the CD47/SIRPɑ Axis. Cancer Med. 2019, 8, 4245–4253. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal “Self” Peptides That Inhibit Phagocytic Clearance and Enhance Delivery of Nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Fang, R.H.; Luk, B.T.; Chen, K.N.H.; Carpenter, C.; Gao, W.; Zhang, K.; Zhang, L. ‘Marker-of-Self’ Functionalization of Nanoscale Particles through a Top-down Cellular Membrane Coating Approach. Nanoscale 2013, 5, 2664. [Google Scholar] [CrossRef]
- Li, Y.; Che, J.; Chang, L.; Guo, M.; Bao, X.; Mu, D.; Sun, X.; Zhang, X.; Lu, W.; Xie, J. CD47—and Integrin α 4/ β 1—Comodified—Macrophage—Membrane—Coated Nanoparticles Enable Delivery of Colchicine to Atherosclerotic Plaque. Adv. Healthc. Mater. 2022, 11, 2101788. [Google Scholar] [CrossRef]
- Dehaini, D.; Wei, X.; Fang, R.H.; Masson, S.; Angsantikul, P.; Luk, B.T.; Zhang, Y.; Ying, M.; Jiang, Y.; Kroll, A.V.; et al. Erythrocyte–Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization. Adv. Mater. 2017, 29, 1606209. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Meng, Q.; Bu, L.; Cai, B.; Huang, Q.; Sun, Z.; Zhang, W.; Li, A.; Guo, S.; Liu, W.; et al. Erythrocyte Membrane-Coated Upconversion Nanoparticles with Minimal Protein Adsorption for Enhanced Tumor Imaging. ACS Appl. Mater. Interfaces 2017, 9, 2159–2168. [Google Scholar] [CrossRef]
- Meng, Q.; Cheng, Y.; Huang, Q.; Zan, M.; Xie, W.; Sun, Y.; Li, R.; Wei, X.; Guo, S.; Zhao, X.; et al. Biomimetic Immunomagnetic Nanoparticles with Minimal Nonspecific Biomolecule Adsorption for Enhanced Isolation of Circulating Tumor Cells. ACS Appl. Mater. Interfaces 2019, 11, 28732–28739. [Google Scholar] [CrossRef]
- Tang, J.C.; Lee, C.-H.; Lu, T.; Vankayala, R.; Hanley, T.; Azubuogu, C.; Li, J.; Nair, M.G.; Jia, W.; Anvari, B. Membrane Cholesterol Enrichment of Red Blood Cell-Derived Microparticles Results in Prolonged Circulation. ACS Appl. Bio Mater. 2022, 5, 650–660. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Z.; Gao, N.; Pi, Z.; Du, X.; Ren, J.; Qu, X. Self-Protecting Biomimetic Nanozyme for Selective and Synergistic Clearance of Peripheral Amyloid-β in an Alzheimer’s Disease Model. J. Am. Chem. Soc. 2020, 142, 21702–21711. [Google Scholar] [CrossRef] [PubMed]
- Haripriyaa, M.; Suthindhiran, K. Pharmacokinetics of Nanoparticles: Current Knowledge, Future Directions and Its Implications in Drug Delivery. Futur. J. Pharm. Sci. 2023, 9, 113. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Wibowo, D.; Yang, G.; Middelberg, A.P.J.; Gao, H.; Zhao, C.-X. Nanoparticle Elasticity Regulates Phagocytosis and Cancer Cell Uptake. Sci. Adv. 2020, 6, eaaz4316. [Google Scholar] [CrossRef]
- Kong, S.M.; Costa, D.F.; Jagielska, A.; Van Vliet, K.J.; Hammond, P.T. Stiffness of Targeted Layer-by-Layer Nanoparticles Impacts Elimination Half-Life, Tumor Accumulation, and Tumor Penetration. Proc. Natl. Acad. Sci. USA 2021, 118, e2104826118. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Impact of Particle Elasticity on Particle-Based Drug Delivery Systems. Adv. Drug Deliv. Rev. 2017, 108, 51–67. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.-X. Role of Nanoparticle Mechanical Properties in Cancer Drug Delivery. ACS Nano 2019, 13, 7410–7424. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Wu, Z.; Yi, X.; Hui, Y.; Yang, G.; Liu, Y.; Tengjisi; Wang, H.; Brooks, A.; Wang, H.; et al. Nanoparticle Elasticity Regulates the Formation of Cell Membrane-Coated Nanoparticles and Their Nano-Bio Interactions. Proc. Natl. Acad. Sci. USA 2023, 120, e2214757120. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Y.; Chen, Y.; Zhang, C.; Deng, H.; Lu, J.; Chen, W. Emerging Strategies against Accelerated Blood Clearance Phenomenon of Nanocarrier Drug Delivery Systems. J. Nanobiotechnol. 2025, 23, 138. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.; Xu, J.; Cai, B.; Yu, G.; Yu, X.; He, Z.; Huang, Q.; Li, A.; Guo, S.; et al. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small 2015, 11, 6225–6236. [Google Scholar] [CrossRef]
- Guo, K.; Liu, Y.; Tang, L.; Shubhra, Q.T.H. Homotypic Biomimetic Coating Synergizes Chemo-Photothermal Combination Therapy to Treat Breast Cancer Overcoming Drug Resistance. Chem. Eng. J. 2022, 428, 131120. [Google Scholar] [CrossRef]
- Malhotra, S.; Dumoga, S.; Singh, N. Red Blood Cells Membrane—derived Nanoparticles: Applications and Key Challenges in Their Clinical Translation. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1776. [Google Scholar] [CrossRef]
- Martinez, J.O.; Molinaro, R.; Hartman, K.A.; Boada, C.; Sukhovershin, R.; De Rosa, E.; Kuri, D.; Zhang, S.; Evangelopoulos, M.; Carter, A.M.; et al. Biomimetic Nanoparticles with Enhanced Affinity towards Activated Endothelium as Versatile Tools for Theranostic Drug Delivery. Theranostics 2018, 8, 1131–1145. [Google Scholar] [CrossRef]
- Ortega-Gómez, A.; Perretti, M.; Soehnlein, O. Resolution of Inflammation: An Integrated View. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Yusuf-Makagiansar, H.; Anderson, M.E.; Yakovleva, T.V.; Murray, J.S.; Siahaan, T.J. Inhibition of LFA—1/ICAM—1 and VLA—4/VCAM—1 as a Therapeutic Approach to Inflammation and Autoimmune Diseases. Med. Res. Rev. 2002, 22, 146–167. [Google Scholar] [CrossRef]
- Cuff, C.A.; Kothapalli, D.; Azonobi, I.; Chun, S.; Zhang, Y.; Belkin, R.; Yeh, C.; Secreto, A.; Assoian, R.K.; Rader, D.J.; et al. The Adhesion Receptor CD44 Promotes Atherosclerosis by Mediating Inflammatory Cell Recruitment and Vascular Cell Activation. J. Clin. Invest. 2001, 108, 1031–1040. [Google Scholar] [CrossRef]
- Ley, K. The Role of Selectins in Inflammation and Disease. Trends Mol. Med. 2003, 9, 263–268. [Google Scholar] [CrossRef]
- Chen, L.; Fu, C.; Zhang, Q.; He, C.; Zhang, F.; Wei, Q. The Role of CD44 in Pathological Angiogenesis. FASEB J. 2020, 34, 13125–13139. [Google Scholar] [CrossRef] [PubMed]
- Langston, W.; Chidlow, J.H.; Booth, B.A.; Barlow, S.C.; Lefer, D.J.; Patel, R.P.; Kevil, C.G. Regulation of Endothelial Glutathione by ICAM-1 Governs VEGF-A-Mediated ENOS Activity and Angiogenesis. Free Radic. Biol. Med. 2007, 42, 720–729. [Google Scholar] [CrossRef]
- Ding, Y.-B. Association of VCAM-1 Overexpression with Oncogenesis, Tumor Angiogenesis and Metastasis of Gastric Carcinoma. World J. Gastroenterol. 2003, 9, 1409. [Google Scholar] [CrossRef]
- Kukita, K.; Sakaguchi, M.; Inoue, H.; Imamura, Y.; Shin, Y. Type IV Collagen Expression Is Regulated by Notch3-Mediated Notch Signaling during Angiogenesis. Biochem. Biophys. Res. Commun. 2025, 749, 151351. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the Site of Inflammation: The Leukocyte Adhesion Cascade Updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Newham, P.; Craig, S.E.; Seddon, G.N.; Schofield, N.R.; Rees, A.; Edwards, R.M.; Jones, E.Y.; Humphries, M.J. A4 Integrin Binding Interfaces on VCAM-1 and MAdCAM-1. J. Biol. Chem. 1997, 272, 19429–19440. [Google Scholar] [CrossRef]
- Marlin, S.D.; Springer, T.A. Purified Intercellular Adhesion Molecule-1 (ICAM-1) Is a Ligand for Lymphocyte Function-Associated Antigen 1 (LFA-1). Cell 1987, 51, 813–819. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer Platelet—Mimicking Nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Blanco, P.; Nigrovic, P.A. Platelets: Active Players in the Pathogenesis of Arthritis and SLE. Nat. Rev. Rheumatol. 2012, 8, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Su, M.; Guo, E.; Zhou, Y.; Yang, X.; Li, S.; Ye, Y. Tissue-Resident Macrophage Membrane-Coated Nanomedicine for Targeted Tumor Therapy. ACS Nano 2025, 19, 26296–26319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, X.; Li, J.; Wang, H.; Xu, X.; Wu, Y.; Wang, J.; Wang, S.; Li, Y.; Zhang, Z. M2 Macrophage Microvesicle-Inspired Nanovehicles Improve Accessibility to Cancer Cells and Cancer Stem Cells in Tumors. J. Nanobiotechnol. 2021, 19, 397. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Dai, L.; Li, J.; He, Q. Macrophage Cell Membrane Camouflaged Au Nanoshells for in Vivo Prolonged Circulation Life and Enhanced Cancer Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 9610–9618. [Google Scholar] [CrossRef]
- Nai, J.; Zhang, J.; Li, J.; Li, H.; Yang, Y.; Yang, M.; Wang, Y.; Gong, W.; Li, Z.; Li, L.; et al. Macrophage Membrane- and CRGD-Functionalized Thermosensitive Liposomes Combined with CPP to Realize Precise SiRNA Delivery into Tumor Cells. Mol. Ther. Nucleic Acids 2022, 27, 349–362. [Google Scholar] [CrossRef]
- Yue, Y.; Li, F.; Li, Y.; Wang, Y.; Guo, X.; Cheng, Z.; Li, N.; Ma, X.; Nie, G.; Zhao, X. Biomimetic Nanoparticles Carrying a Repolarization Agent of Tumor-Associated Macrophages for Remodeling of the Inflammatory Microenvironment Following Photothermal Therapy. ACS Nano 2021, 15, 15166–15179. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Duan, S.; Li, L.; Zhao, G.; Wei, L.; Zhang, B.; Ma, Y.; Wu, M.X.; Mao, Y.; Lu, M. Bio—Responsive Sliver Peroxide—Nanocarrier Serves as Broad—Spectrum Metallo—β—lactamase Inhibitor for Combating Severe Pneumonia. Adv. Mater. 2024, 36, e2310532. [Google Scholar] [CrossRef]
- Phatale, V.; Famta, P.; Srinivasarao, D.A.; Vambhurkar, G.; Jain, N.; Pandey, G.; Kolipaka, T.; Khairnar, P.; Shah, S.; Singh, S.B.; et al. Neutrophil Membrane-Based Nanotherapeutics: Propitious Paradigm Shift in the Management of Cancer. Life Sci. 2023, 331, 122021. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Wu, W.; Gao, F.; Li, R.; Song, W.; Zhuang, Z.; Liu, C.; Zhang, X. Artificial Super Neutrophils for Inflammation Targeting and HClO Generation against Tumors and Infections. Adv. Mater. 2019, 31, 1901179. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, Y.; Zhang, Q.; Hu, C.; Li, J.; Feng, J.; Hu, C.; Su, Y.; Lou, J.; Long, L.; et al. Neutrophil Nanodecoys Inhibit Tumor Metastasis by Blocking the Interaction between Tumor Cells and Neutrophils. ACS Nano 2024, 18, 7363–7378. [Google Scholar] [CrossRef]
- Liang, J.; Piao, Y.; Holmes, L.; Fuller, G.N.; Henry, V.; Tiao, N.; de Groot, J.F. Neutrophils Promote the Malignant Glioma Phenotype through S100A4. Clin. Cancer Res. 2014, 20, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil Membrane-Coated Nanoparticles Inhibit Synovial Inflammation and Alleviate Joint Damage in Inflammatory Arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef]
- Ouyang, S.; Lu, P.; Li, J.; Jin, H.; Wu, W.; Luo, R.; Wang, B.; Huang, X.; Lian, X.; Huang, G. Inhaled Tea Polyphenol-Loaded Nanoparticles Coated with Platelet Membranes Largely Attenuate Asthmatic Inflammation. Respir. Res. 2024, 25, 311. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xie, W.; Zan, H.-M.; Liu, Z.; Wang, G.; Wang, Y.; Liu, W.; Dong, W. Platelet Membrane-Coated Nanoparticles for Targeted Drug Delivery and Local Chemo-Photothermal Therapy of Orthotopic Hepatocellular Carcinoma. J. Mater. Chem. B 2020, 8, 4648–4659. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, X.; Han, S.; Wang, T.; Zhao, J.; He, Y.; Chen, S.; Deng, S.; Wang, C.; Wang, J. Irradiation Pretreatment Enhances the Therapeutic Efficacy of Platelet-Membrane-Camouflaged Antitumor Nanoparticles. J. Nanobiotechnol. 2020, 18, 101. [Google Scholar] [CrossRef]
- Liu, H.; Cai, G.; Yuan, S.; Zhou, X.; Gui, R.; Huang, R. Platelet Membrane-Camouflaged Silver Metal–Organic Framework Biomimetic Nanoparticles for the Treatment of Triple-Negative Breast Cancer. Mol. Pharm. 2024, 21, 3577–3590. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, Q.; Li, J.; Zhao, Q.; Huang, X.; Dong, H.; Liu, H.; Gui, R.; Nie, X. Platelet-Membrane-Camouflaged Zirconia Nanoparticles Inhibit the Invasion and Metastasis of Hela Cells. Front. Chem. 2020, 8, 377. [Google Scholar] [CrossRef]
- Dai, J.; Wu, M.; Xu, Y.; Yao, H.; Lou, X.; Hong, Y.; Zhou, J.; Xia, F.; Wang, S. Platelet Membrane Camouflaged AIEgen—mediated Photodynamic Therapy Improves the Effectiveness of anti—PD—L1 Immunotherapy in Large—burden Tumors. Bioeng. Transl. Med. 2023, 8, e10417. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Nie, L.; Shavandi, A.; Yunusov, K.E.; Aharodnikau, U.E.; Solomevich, S.O.; Sun, Y.; Jiang, G. Platelet Membrane-Camouflaged Copper Doped CaO 2 Biomimetic Nanomedicines for Breast Cancer Combination Treatment. ACS Biomater. Sci. Eng. 2024, 10, 7492–7506. [Google Scholar] [CrossRef]
- Song, B.; Na, Y.-G.; Kim, B.J.; Jin, M.; Song, Y.H.; Kim, D.-E.; Hwang, S.; Baek, J.-S.; Lee, H.-K.; Cho, C.-W. Platelet Membrane-Coated Poly (Lactic-Co-Glycolic Acid) Nanoparticles as a Targeting Drug Delivery System for Multidrug-Resistant Breast Cancer. Int. J. Nanomed. 2025, 20, 8529–8545. [Google Scholar] [CrossRef]
- Lindemann, S.; Krämer, B.; Seizer, P.; Gawaz, M. Platelets, Inflammation and Atherosclerosis. J. Thromb. Haemost. 2007, 5, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, T.; Xu, M.; Wang, S.; Wu, A.; Zhang, M.; Zhou, Y.L.; Shi, J. Platelet Membrane-Camouflaged Nanoparticles Carry MicroRNA Inhibitor against Myocardial Ischaemia—reperfusion Injury. J. Nanobiotechnol. 2022, 20, 434. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, X.; Wang, T.; Xu, M.; Huang, Z.; Yu, R.; Jiang, Y.; Zhou, Y.; Shi, J. Platelet-Membrane-Encapsulated Carvedilol with Improved Targeting Ability for Relieving Myocardial Ischemia–Reperfusion Injury. Membranes 2022, 12, 605. [Google Scholar] [CrossRef]
- Ji, M.; Tang, Q.; Olatunji, O.Y.; Ge, R.; Ying, Y.; Pan, J.; Yunusov, K.E.; Jiang, G. Platelet Membrane-Camouflaged Bioactive Glass Nano-Formulations for Enhanced Drug Delivery in the Treatment of Acute Arterial Thrombosis. Acta Biomater. 2025, 199, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Luo, R.; Li, J.; Zhao, H.; Ouyang, S.; Yao, Y.; Chen, D.; Ling, Z.; Zhu, W.; Chen, M.; et al. Inhaled Platelet Vesicle-Decoyed Biomimetic Nanoparticles Attenuate Inflammatory Lung Injury. Front. Pharmacol. 2022, 13, 1050224. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Cai, G.-Q.; Li, J.; Li, X.-S.; Liu, H.-T.; Shang, X.-L.; Zhou, J.-D.; Nie, X.-M.; Gui, R. Platelet Membrane-Camouflaged Silver Metal-Organic Framework Drug System against Infections Caused by Methicillin-Resistant Staphylococcus Aureus. J. Nanobiotechnol. 2021, 19, 229. [Google Scholar] [CrossRef]
- Liu, C.; Gao, J.; Cheng, Y.; Zhang, S.; Fu, C. Homologous-Adhering/Targeting Cell Membrane- and Cell-Mediated Delivery Systems: A Cancer-Catch-Cancer Strategy in Cancer Therapy. Regen. Biomater. 2025, 12, bae135. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Hu, X.; Li, Q.; Yin, M.; Song, H.; Hu, J.; Wang, L.; Fan, C.; Chen, N. Unraveling Cell-Type-Specific Targeted Delivery of Membrane-Camouflaged Nanoparticles with Plasmonic Imaging. Nano Lett. 2020, 20, 5228–5235. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, D.; Marino, A.; Tapeinos, C.; Pucci, C.; Rocchiccioli, S.; Michelucci, E.; Finamore, F.; McDonnell, L.; Scarpellini, A.; Lauciello, S.; et al. Homotypic Targeting and Drug Delivery in Glioblastoma Cells through Cell Membrane-Coated Boron Nitride Nanotubes. Mater. Des. 2020, 192, 108742. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.; Li, F.; Wang, S.; Zhao, J.; Wang, J.; Liu, J.; Lyu, C.; Ye, P.; Tan, H.; et al. Engineered Biomimetic Nanoparticles Achieve Targeted Delivery and Efficient Metabolism-Based Synergistic Therapy against Glioblastoma. Nat. Commun. 2022, 13, 4214. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Bu, L.; Cai, B.; Xu, J.; Li, A.; Zhang, W.; Sun, Z.; Guo, S.; Liu, W.; Wang, T.; et al. Cancer Cell Membrane—Coated Upconversion Nanoprobes for Highly Specific Tumor Imaging. Adv. Mater. 2016, 28, 3460–3466. [Google Scholar] [CrossRef]
- Harris, J.C.; Scully, M.A.; Day, E.S. Cancer Cell Membrane-Coated Nanoparticles for Cancer Management. Cancers 2019, 11, 1836. [Google Scholar] [CrossRef]
- Prajapati, A.; Rangra, S.; Patil, R.; Desai, N.; Jyothi, V.G.S.S.; Salave, S.; Amate, P.; Benival, D.; Kommineni, N. Receptor-Targeted Nanomedicine for Cancer Therapy. Receptors 2024, 3, 323–361. [Google Scholar] [CrossRef]
- Marshall, S.K.; Angsantikul, P.; Pang, Z.; Nasongkla, N.; Hussen, R.S.D.; Thamphiwatana, S.D. Biomimetic Targeted Theranostic Nanoparticles for Breast Cancer Treatment. Molecules 2022, 27, 6473. [Google Scholar] [CrossRef]
- Wen, Q.; Zhang, Y.; Muluh, T.A.; Xiong, K.; Wang, B.; Lu, Y.; Wu, Z.; Liu, Y.; Shi, H.; Xiao, S.; et al. Erythrocyte Membrane-Camouflaged Gefitinib/Albumin Nanoparticles for Tumor Imaging and Targeted Therapy against Lung Cancer. Int. J. Biol. Macromol. 2021, 193, 228–237. [Google Scholar] [CrossRef]
- Daniyal, M.; Jian, Y.; Xiao, F.; Sheng, W.; Fan, J.; Xiao, C.; Wang, Z.; Liu, B.; Peng, C.; Yuhui, Q.; et al. Development of A Nanodrug-Delivery System Camouflaged by Erythrocyte Membranes for The Chemo/Phototherapy of Cancer. Nanomedicine 2020, 15, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, X.; Ouyang, Y.; Chu, L.; Xu, M.; Wang, K.; Tong, X. Engineering of Neutrophil Membrane Camouflaging Nanoparticles Realizes Targeted Drug Delivery for Amplified Antitumor Therapy. Int. J. Nanomed. 2021, 16, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, M.; Wu, L.; Song, Y.; Yu, S.; Wan, Y.; Cheng, W.; Yang, B.; Mou, X.; Yu, H.; et al. Peptide-Anchored Neutrophil Membrane-Coated Biomimetic Nanodrug for Targeted Treatment of Rheumatoid Arthritis. J. Nanobiotechnol. 2023, 21, 13. [Google Scholar] [CrossRef]
- Curley, N.; Levy, D.; Do, M.A.; Brown, A.; Stickney, Z.; Marriott, G.; Lu, B. Sequential Deletion of CD63 Identifies Topologically Distinct Scaffolds for Surface Engineering of Exosomes in Living Human Cells. Nanoscale 2020, 12, 12014–12026. [Google Scholar] [CrossRef]
- Wei, G.; Xiao, T.; Xi, Y.; Ju, R. A Macrophage-like Biomimetic Nanoparticle with High-Efficiency Biofilm Disruption and Innate Immunity Activation for Implant-Related Infection Therapy. Mater. Today Bio 2025, 31, 101575. [Google Scholar] [CrossRef]
- Kostyusheva, A.; Brezgin, S.; Ponomareva, N.; Frolova, A.; Lunin, A.; Bayurova, E.; Tikhonov, A.; Slatinskaya, O.; Demina, P.; Kachanov, A.; et al. Biologics-Based Technologies for Highly Efficient and Targeted RNA Delivery. Mol. Ther. 2025, 33, 168–183. [Google Scholar] [CrossRef]
- Choi, H.; Yim, H.; Park, C.; Ahn, S.-H.; Ahn, Y.; Lee, A.; Yang, H.; Choi, C. Targeted Delivery of Exosomes Armed with Anti-Cancer Therapeutics. Membranes 2022, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Geng, X.; Wu, Y.; Dai, Y.; Zeng, J.; Wang, Z.; Fang, H.; Sun, Y.; Chen, X. Engineered Macrophage Membrane—Coated Nanoparticles with Enhanced CCR2 Expression Promote Spinal Cord Injury Repair by Suppressing Neuroinflammation and Neuronal Death. Small 2024, 20, 2305659. [Google Scholar] [CrossRef]
- Chang, Y.; Cai, X.; Syahirah, R.; Yao, Y.; Xu, Y.; Jin, G.; Bhute, V.J.; Torregrosa-Allen, S.; Elzey, B.D.; Won, Y.-Y.; et al. CAR-Neutrophil Mediated Delivery of Tumor-Microenvironment Responsive Nanodrugs for Glioblastoma Chemo-Immunotherapy. Nat. Commun. 2023, 14, 2266. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Q.; Huang, J.; Zhao, X.; Shao, S.; Zhang, F.; Yao, Z.; Ping, Y.; Liang, T. Engineered a Dual-Targeting Biomimetic Nanomedicine for Pancreatic Cancer Chemoimmunotherapy. J. Nanobiotechnol. 2022, 20, 85. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Zhang, L.; Miron, R.J.; Liang, J.; Shi, M.; Mo, W.; Zheng, S.; Zhao, Y.; Zhang, Y. Pretreated Macrophage—Membrane—Coated Gold Nanocages for Precise Drug Delivery for Treatment of Bacterial Infections. Adv. Mater. 2018, 30, e1804023. [Google Scholar] [CrossRef]
- Cao, H.; Gao, Y.; Jia, H.; Zhang, L.; Liu, J.; Mu, G.; Gui, H.; Wang, Y.; Yang, C.; Liu, J. Macrophage-Membrane-Camouflaged Nonviral Gene Vectors for the Treatment of Multidrug-Resistant Bacterial Sepsis. Nano Lett. 2022, 22, 7882–7891. [Google Scholar] [CrossRef]
- Qiu, C.; Xia, F.; Zhang, J.; Shi, Q.; Meng, Y.; Wang, C.; Pang, H.; Gu, L.; Xu, C.; Guo, Q.; et al. Advanced Strategies for Overcoming Endosomal/Lysosomal Barrier in Nanodrug Delivery. Research 2023, 6, 148. [Google Scholar] [CrossRef]
- Ponomareva, N.; Brezgin, S.; Karandashov, I.; Kostyusheva, A.; Demina, P.; Slatinskaya, O.; Bayurova, E.; Silachev, D.; Pokrovsky, V.S.; Gegechkori, V.; et al. Swelling, Rupture and Endosomal Escape of Biological Nanoparticles Per Se and Those Fused with Liposomes in Acidic Environment. Pharmaceutics 2024, 16, 667. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood–Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Blood-Brain Barrier Active Efflux Transporters: ATP-Binding Cassette Gene Family. NeuroRX 2005, 2, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Gong, L.; Xu, Q.; Wang, J.; Yang, Y.; Zhang, S.; Dong, J.; Lin, K.; Liang, Z.; Sun, Y.; et al. Revolutionizing Neurocare: Biomimetic Nanodelivery Via Cell Membranes. Adv. Mater. 2024, 36, 2402445. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.S.; Wasan, K.M. Potential Role of the Low-Density Lipoprotein Receptor Family as Mediators of Cellular Drug Uptake. Adv. Drug Deliv. Rev. 2004, 56, 1315–1334. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, J.; Meng, F.; Zhong, Z. Apolipoprotein E Peptide-Directed Chimeric Polymersomes Mediate an Ultrahigh-Efficiency Targeted Protein Therapy for Glioblastoma. ACS Nano 2018, 12, 11070–11079. [Google Scholar] [CrossRef]
- He, W.; Li, X.; Morsch, M.; Ismail, M.; Liu, Y.; Rehman, F.U.; Zhang, D.; Wang, Y.; Zheng, M.; Chung, R.; et al. Brain-Targeted Codelivery of Bcl-2/Bcl-Xl and Mcl-1 Inhibitors by Biomimetic Nanoparticles for Orthotopic Glioblastoma Therapy. ACS Nano 2022, 16, 6293–6308. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; An, Y.; Sun, Y.; Li, J.; Zheng, M.; Zou, Y.; Shi, B. Non-Invasive PTEN MRNA Brain Delivery Effectively Mitigates Growth of Orthotopic Glioblastoma. Nano Today 2023, 49, 101790. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Liu, W.; Yang, J.; Sun, C.; Sun, R.; Li, H.; Shen, S.; Luo, Y.; Ye, X.; et al. Regulating the Surface Poly(Ethylene Glycol) Density of Polymeric Nanoparticles and Evaluating Its Role in Drug Delivery in Vivo. Biomaterials 2015, 69, 1–11. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Y.; Yang, Z.; Zhang, D.; Lu, Y.; Zheng, M.; Xue, X.; Geng, J.; Chung, R.; Shi, B. Effective and Targeted Human Orthotopic Glioblastoma Xenograft Therapy via a Multifunctional Biomimetic Nanomedicine. Adv. Mater. 2018, 30, e1803717. [Google Scholar] [CrossRef]
- Cencioni, C.; Melchionna, R.; Straino, S.; Romani, M.; Cappuzzello, C.; Annese, V.; Wu, J.C.; Pompilio, G.; Santoni, A.; Gaetano, C.; et al. Ex Vivo Acidic Preconditioning Enhances Bone Marrow Ckit+ Cell Therapeutic Potential via Increased CXCR4 Expression. Eur. Heart J. 2013, 34, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zang, G.; Liu, B.; Qin, X.; Zhang, Y.; Chen, Y.; Zhang, H.; Wu, W.; Wang, G. Bioengineering CXCR4-Overexpressing Cell Membrane Functionalized ROS-Responsive Nanotherapeutics for Targeting Cerebral Ischemia-Reperfusion Injury. Theranostics 2021, 11, 8043–8056. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Bowman, R.L.; Klemm, F.; Akkari, L.; Pyonteck, S.M.; Sevenich, L.; Quail, D.F.; Dhara, S.; Simpson, K.; Gardner, E.E.; Iacobuzio-Donahue, C.A.; et al. Macrophage Ontogeny Underlies Differences in Tumor-Specific Education in Brain Malignancies. Cell Rep. 2016, 17, 2445–2459. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Engelhardt, B. The Anatomical and Cellular Basis of Immune Surveillance in the Central Nervous System. Nat. Rev. Immunol. 2012, 12, 623–635. [Google Scholar] [CrossRef]
- Xiao, T.; He, M.; Xu, F.; Fan, Y.; Jia, B.; Shen, M.; Wang, H.; Shi, X. Macrophage Membrane-Camouflaged Responsive Polymer Nanogels Enable Magnetic Resonance Imaging-Guided Chemotherapy/Chemodynamic Therapy of Orthotopic Glioma. ACS Nano 2021, 15, 20377–20390. [Google Scholar] [CrossRef]
- Zuo, H.; Tao, J.; Shi, H.; He, J.; Zhou, Z.; Zhang, C. Platelet-Mimicking Nanoparticles Co-Loaded with W18O49 and Metformin Alleviate Tumor Hypoxia for Enhanced Photodynamic Therapy and Photothermal Therapy. Acta Biomater. 2018, 80, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer–Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer Immunotherapy Comes of Age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Chekaoui, A.; Garofalo, M.; Gad, B.; Staniszewska, M.; Chiaro, J.; Pancer, K.; Gryciuk, A.; Cerullo, V.; Salmaso, S.; Caliceti, P.; et al. Cancer Vaccines: An Update on Recent Achievements and Prospects for Cancer Therapy. Clin. Exp. Med. 2024, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, S.; Zhang, S.; Wang, L.; Yuan, H.; Hu, F. Cell Membrane Coated-Nanoparticles for Cancer Immunotherapy. Acta Pharm. Sin. B 2022, 12, 3233–3254. [Google Scholar] [CrossRef]
- Zou, W. Immunosuppressive Networks in the Tumour Environment and Their Therapeutic Relevance. Nat. Rev. Cancer 2005, 5, 263–274. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Dendritic-Cell-Based Therapeutic Cancer Vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef]
- Gan, J.; Du, G.; He, C.; Jiang, M.; Mou, X.; Xue, J.; Sun, X. Tumor Cell Membrane Enveloped Aluminum Phosphate Nanoparticles for Enhanced Cancer Vaccination. J. Control. Release 2020, 326, 297–309. [Google Scholar] [CrossRef]
- Li, M.; Qin, M.; Song, G.; Deng, H.; Wang, D.; Wang, X.; Dai, W.; He, B.; Zhang, H.; Zhang, Q. A Biomimetic Antitumor Nanovaccine Based on Biocompatible Calcium Pyrophosphate and Tumor Cell Membrane Antigens. Asian J. Pharm. Sci. 2021, 16, 97–109. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Jiang, W.; Yang, C.; Miao, H.; Wang, Y. Nanovaccines Integrating Endogenous Antigens and Pathogenic Adjuvants Elicit Potent Antitumor Immunity. Nano Today 2020, 35, 101007. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune Modulation by Bacterial Outer Membrane Vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Zhou, J.; Chekuri, S.; Wei, X.; Kroll, A.V.; Yu, C.L.; Duan, Y.; Gao, W.; Fang, R.H.; et al. Engineered Cell—Membrane—Coated Nanoparticles Directly Present Tumor Antigens to Promote Anticancer Immunity. Adv. Mater. 2020, 32, e2001808. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, C.; Jin, Y.; Li, Y.; Zhong, C.; Ma, J.; Yang, J.; Zhang, N.; Li, Y.; Wang, C.; et al. Artificial Mini Dendritic Cells Boost T Cell–Based Immunotherapy for Ovarian Cancer. Adv. Sci. 2020, 7, 1903301. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2020, 9, 1512. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Guo, X.; Zhao, J.; Zhou, S. A Biomimetic Polymer Magnetic Nanocarrier Polarizing Tumor—Associated Macrophages for Potentiating Immunotherapy. Small 2020, 16, 2003543. [Google Scholar] [CrossRef]
- Deng, G.; Sun, Z.; Li, S.; Peng, X.; Li, W.; Zhou, L.; Ma, Y.; Gong, P.; Cai, L. Cell-Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth. ACS Nano 2018, 12, 12096–12108. [Google Scholar] [CrossRef]
- Xiao, P.; Han, X.; Huang, Y.; Yang, J.; Chen, L.; Cai, Z.; Hu, N.; Cui, W.; Huang, W. Reprogramming Macrophages via Immune Cell Mobilized Hydrogel Microspheres for Osteoarthritis Treatments. Bioact. Mater. 2024, 32, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yang, C.; Shi, K.; Liu, Y.; Hu, D.; He, X.; Yang, Y.; Chu, B.; Peng, J.; Zhou, Z.; et al. Activated Macrophage Membrane-Coated Nanoparticles Relieve Osteoarthritis-Induced Synovitis and Joint Damage. Biomaterials 2023, 295, 122036. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Zhang, Y.; Qian, J.; Wang, W.; Yu, X.; Huang, J.; Zhou, L.; Zheng, S. M2-Type Macrophage Membrane-Mediated Delivery of Carvedilol Nanocomplex for Acute Liver Failure Treatment and Remodeling Inflammatory Microenvironment. Nano Res. 2024, 17, 6362–6375. [Google Scholar] [CrossRef]
- Hu, C.; Luo, R.; Wang, Y. Heart Valves Cross-Linked with Erythrocyte Membrane Drug-Loaded Nanoparticles as a Biomimetic Strategy for Anti-Coagulation, Anti-Inflammation, Anti-Calcification, and Endothelialization. ACS Appl. Mater. Interfaces 2020, 12, 41113–41126. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Huang, K.; Ma, H.; Liang, H.; Dinh, P.; Chen, J.; Shen, D.; Allen, T.A.; Qiao, L.; Li, Z.; et al. Platelet—Inspired Nanocells for Targeted Heart Repair After Ischemia/Reperfusion Injury. Adv. Funct. Mater. 2019, 29, 1803567. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Lan, X.; Zhang, Y.; Fu, C.; Liu, L.; Cao, F.; Guo, W. Biomimetic Nanomedicines for Precise Atherosclerosis Theranostics. Acta Pharm. Sin. B 2023, 13, 4442–4460. [Google Scholar] [CrossRef]
- Wang, K.-N.; Li, Z.-Z.; Zhou, K.; Liu, B.; Rao, L.; Bu, L.-L. Cell Membrane-Coated Nanoparticles for Dental, Oral, and Craniofacial Diseases. Research 2024, 7, 478. [Google Scholar] [CrossRef]
- Yuan, S.; Hu, Q. Convergence of Nanomedicine and Neutrophils for Drug Delivery. Bioact. Mater. 2024, 35, 150–166. [Google Scholar] [CrossRef]
- Zeng, S.; Tang, Q.; Xiao, M.; Tong, X.; Yang, T.; Yin, D.; Lei, L.; Li, S. Cell Membrane-Coated Nanomaterials for Cancer Therapy. Mater. Today Bio 2023, 20, 100633. [Google Scholar] [CrossRef]
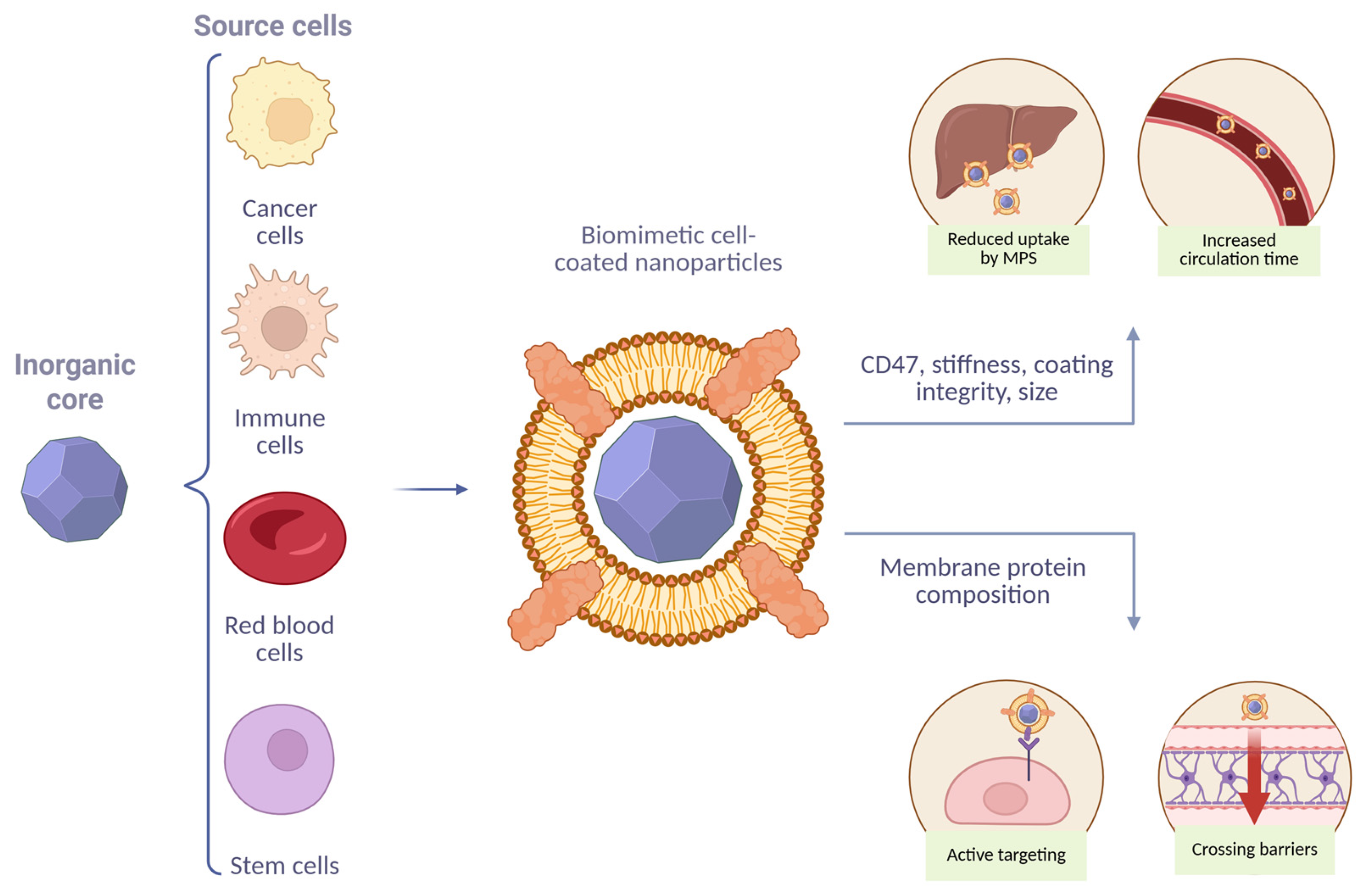
| Source Cells for Membrane Coating | Core | t1/2 (Elimination Phase) Before Coating vs. After Coating, h | Animal Model | Route of Injection | Total Clearance Cl | Volume of Distribution Vd | References |
|---|---|---|---|---|---|---|---|
| RBCs | Gold nanorods (L~50 nm) | 0.980 vs. 14.9 | Male BALB/c mice | Intravenous injection | 0.641 vs. 0.024 L/h | 0.898 vs. 0.511 L | [7] |
| PLGA NPs (d = 240 nm) | 0.41 vs. 1.08 | C57BL/6J mice | Retroorbital injection | NS | NS | [21] | |
| Perfluorocarbon encapsulated within PLGA NPs (d~400 nm) | ~7 vs. 13.93 | Female nude mice | Subcutaneous injection | NS | NS | [22] | |
| PLGA NPs (d = 80 nm) | 15.8 vs. 39.6 | Male ICR mice | Intravenous injection | NS | NS | [23] | |
| Magnetic NPs (d = 52 nm) | 2.6 vs. 8.1 | Female C57BL/6 mice with ID8 tumor | Intravenous injection | NS | NS | [24] | |
| PLGA NPs (d = 120 nm) | 28.2 vs. 51.1 | BALB/c mice | Intravenous injection | NS | NS | [25] | |
| Melanin NPs (d = 216 nm) | 4.0 vs. 11.2 | Female ICR mice | Intravenous injection | NS | NS | [26] | |
| Lipid multichambered NPs (d = 220 nm) | ~10 vs. ~25 | Tumor-bearing nude mice | Intravenous injection | ~2 vs. <1 L/h/kg | NS | [27] | |
| Au nanocages (d~150 nm) | ~8.2 vs. 29.528 | Female BALB/c mice with tumor | Subcutaneous injection | 0.021 (nmol)/(nmol/mL)·h | 0.883 (nmol)/(n mol/mL) | [28] | |
| Macrophages | Amphiphilic oxidation-sensitive chitosan oligosaccharide NPs (d = 149 nm) | 5.43 vs. 9.82 | ApoE−/− mice | Intravenous injection | NS | NS | [29] |
| Baicalin liposomes (d = 182 nm) | 4 vs. 4.7 | Rats | Intravenous injection | 0.0070 vs. 0.0032 L/min/kg | 2.29 vs. 0.62 L/kg | [30] | |
| Neutrophiles | PLGA NPs (d~75 nm) | 0.77 vs. 6.59 | Male SD rats | Intravenous injection | 3160.37 vs. 17.42 mL/h/kg | 3397.85 vs. 144.2 | [31] |
| Cancer cells | Magnetic NPs (d = 52 nm) | 2.6 vs. 4.4 | Female C57BL/6 mice with ID8 tumor | Intravenous injection | NS | NS | [24] |
| SiO2 NPs (d = 120 nm) | 9.1 vs. 23.6 | SD rats | Intravenous injection | 0.062 vs. 0.07 L/kg | 0.017 vs. 0.004 L/h/kg | [10] | |
| Melanin NPs (d = 216 nm) | 4.0 vs. 5.1 | Female ICR mice | Intravenous injection | NS | NS | [26] | |
| RBCs + cancer cells | Magnetic NPs (d = 52 nm) | 2.6 vs. 7.1 | Female C57BL/6 mice with ID8 tumor | Intravenous injection | NS | NS | [24] |
| RBCs + cancer cells (2:1) | Melanin NPs (d = 216 nm) | 4.0 vs. 10.9 | Female ICR mice | Intravenous injection | NS | NS | [26] |
| RBCs + cancer cells (1:1) | Melanin NPs (d = 216 nm) | 4.0 vs. 10.7 | Female ICR mice | Intravenous injection | NS | NS | [26] |
| CMAs | Target | Justification |
|---|---|---|
| Size | <100 nm | Optimal size for reducing non-target organ accumulation [74], maximize t1/2, and reduce uptake by MPS |
| Coating integrity | 100% | Maximize t1/2, reduce uptake by MPS, and enhance tumor accumulation [10] |
| CD47 | Presence | Maximize t1/2, reduce uptake by MPS |
| Stiffness | ~10 MPa | Maximize t1/2, reduce uptake by MPS, and enhance tumor accumulation [25] |
| Pathological Conditions | Membrane Source | BBB Penetration Mechanism | Main Effect | References |
|---|---|---|---|---|
| Ischemia–reperfusion injury | CXCR4-overexpressing primary mouse thoracic aorta endothelial cells | CXCR4/SDF-1 chemokine axis | ~3-fold increase in accumulation at ischemic region vs. control NPs | [147] |
| Glioma | RBCs | ApoE–LDLR- mediated uptake | Tumor accumulation of ApoE-BMCNP treatment reached 9.3% of the injected dose per gram of tissue, which was 1.7–2.9-fold higher than those of BMCNPs and uncoated NPs | [143] |
| Glioma | RBCs | ApoE–LDLR- mediated uptake | ApoE-BMCNP accumulation in the tumor area was enhanced 7-fold and 21-fold compared to BMCNPs and uncoated NPs, respectively | [144] |
| Glioma | RBCs | Ang-2 and Lex-mediated uptake | Ang-RBC-Lex NPs, which were 2.5-, 2.9–, 3.5-fold higher than that of RBC-Lex NPs, Ang-RBC NPs, and NPs-Lex | [146] |
| Glioma | Macrophages | Integrin-dependent macrophage penetration | ~4-fold increase in accumulation at tumor region vs. uncoated NPs | [47] |
| Glioma | Macrophages | Integrin-dependent macrophage penetration | At the same time points post-injection, BMCNPs displayed significantly higher accumulation than uncoated NPs | [152] |
| Glioma | Macrophages | Integrin-dependent macrophage penetration and Ang-2-mediated uptake | The distribution of Ang-BMCNPs in the brain was significantly higher than BMCNPs | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazareva, P.; Chulanov, V.; Kostyushev, D.; Abakumov, M. In Vivo Behavior of Biomimetic Nanoparticles: Strategies for Clearance Avoidance, Targeting, and Functional Delivery. Molecules 2025, 30, 4487. https://doi.org/10.3390/molecules30224487
Lazareva P, Chulanov V, Kostyushev D, Abakumov M. In Vivo Behavior of Biomimetic Nanoparticles: Strategies for Clearance Avoidance, Targeting, and Functional Delivery. Molecules. 2025; 30(22):4487. https://doi.org/10.3390/molecules30224487
Chicago/Turabian StyleLazareva, Polina, Vladimir Chulanov, Dmitry Kostyushev, and Maxim Abakumov. 2025. "In Vivo Behavior of Biomimetic Nanoparticles: Strategies for Clearance Avoidance, Targeting, and Functional Delivery" Molecules 30, no. 22: 4487. https://doi.org/10.3390/molecules30224487
APA StyleLazareva, P., Chulanov, V., Kostyushev, D., & Abakumov, M. (2025). In Vivo Behavior of Biomimetic Nanoparticles: Strategies for Clearance Avoidance, Targeting, and Functional Delivery. Molecules, 30(22), 4487. https://doi.org/10.3390/molecules30224487









