Ball-Milling-Assisted Coating and Magnetic Properties of Fluorescent Biodegradable Powders for Fingerprint Detection
Abstract
1. Introduction
2. Results and Discussion
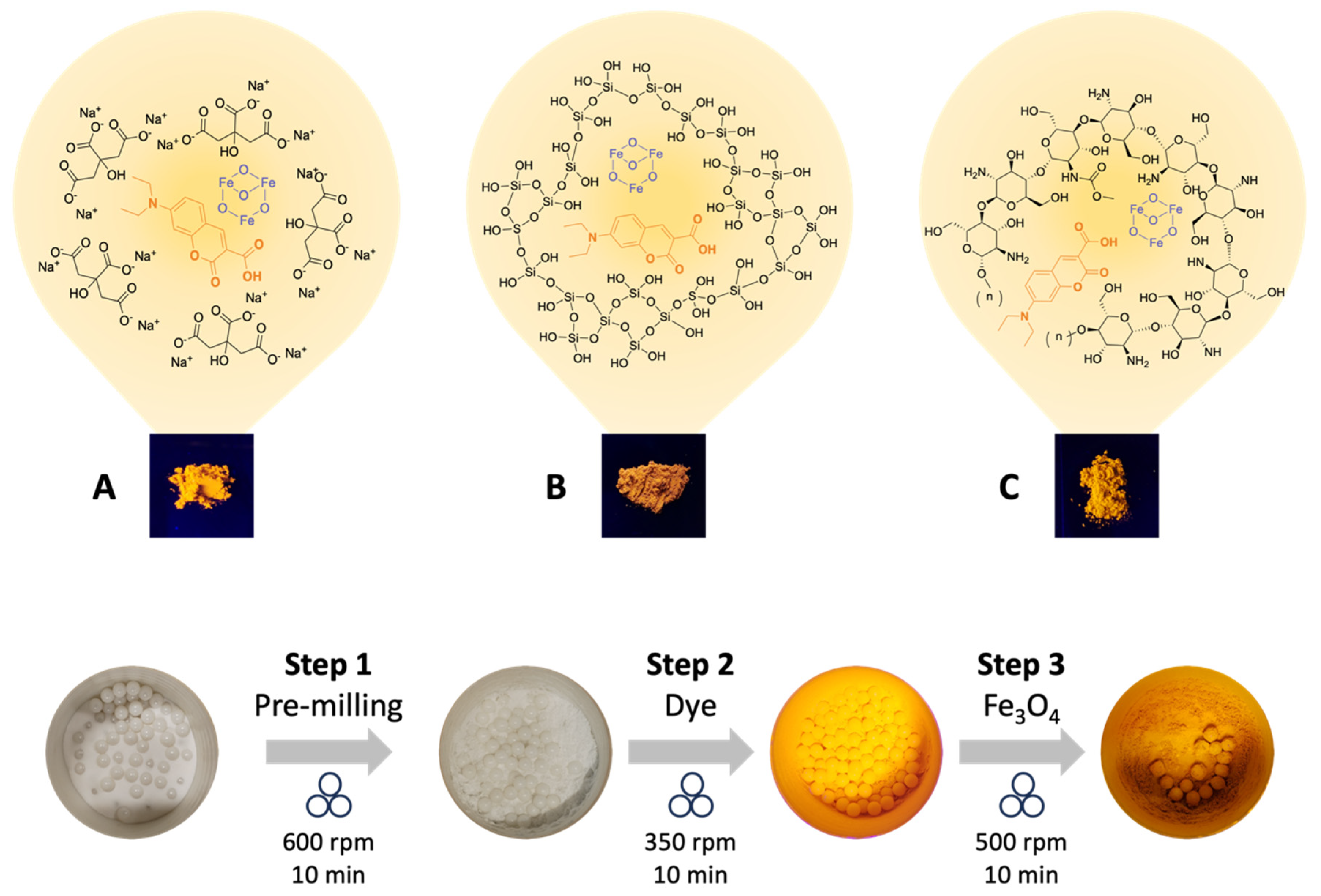
2.1. Ball-Milling-Assisted Coating of Fluorescent Magnetic Powders
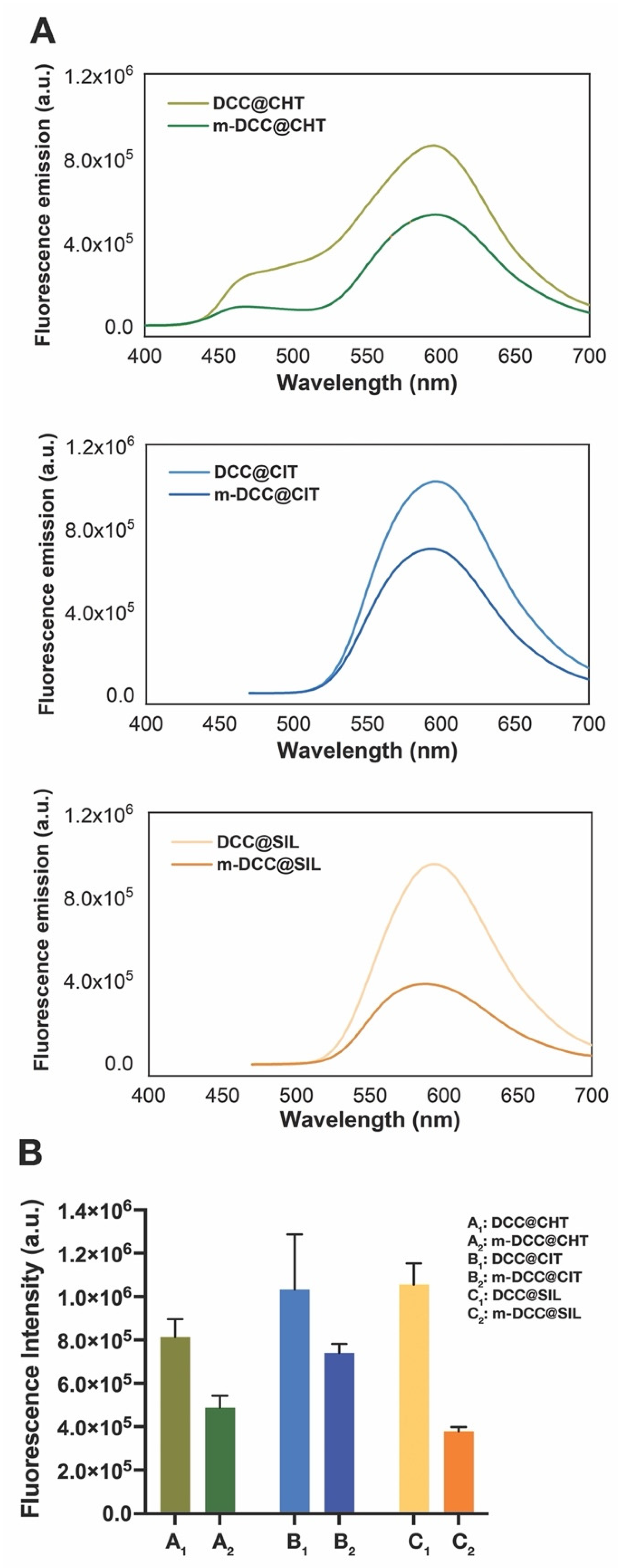
2.2. Optical Properties of Fluorescent Magnetic Powders
2.3. Magnetic Properties of Fluorescent Magnetic Powders
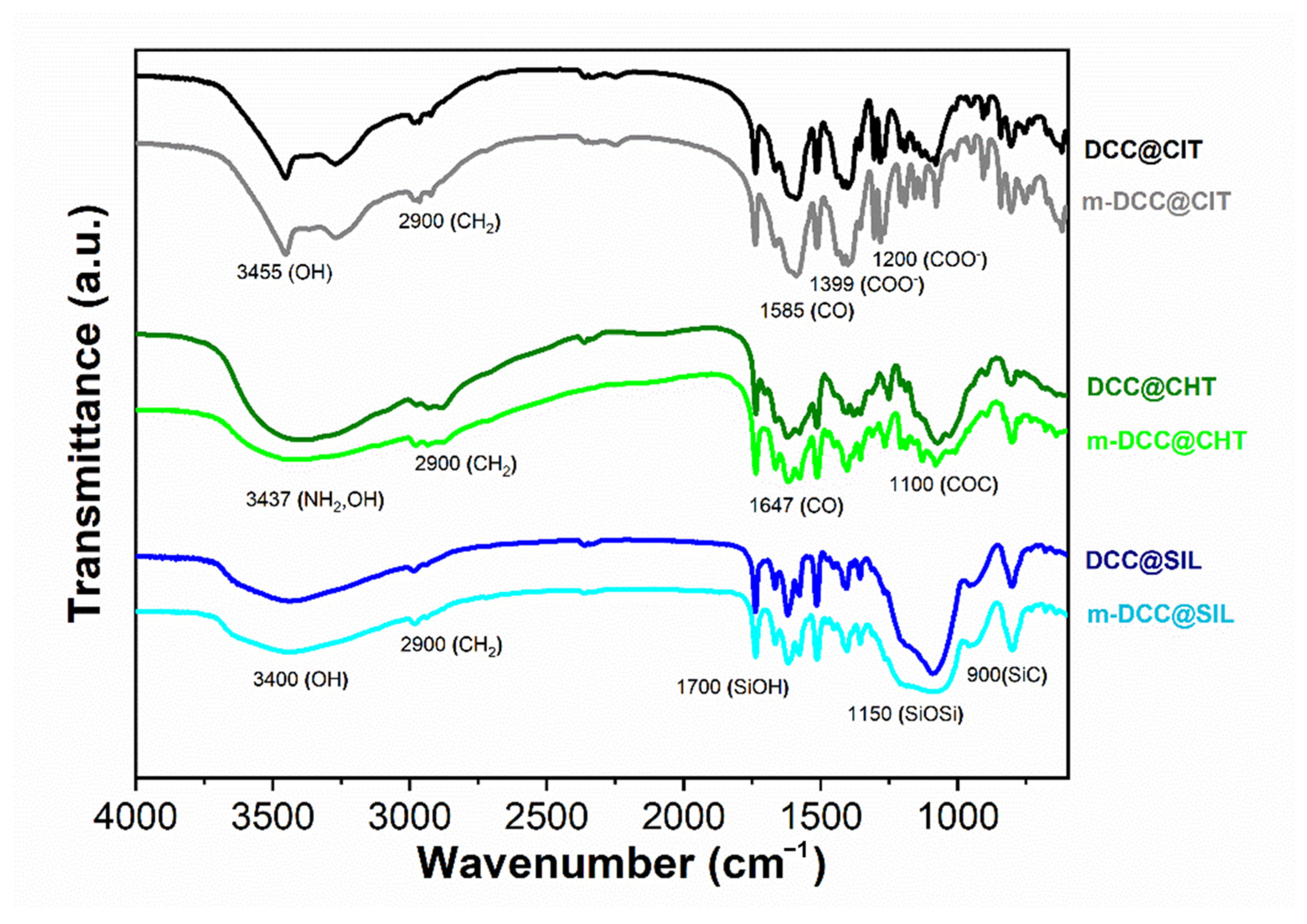
2.4. Infrared Spectra of Fluorescent Magnetic Powders
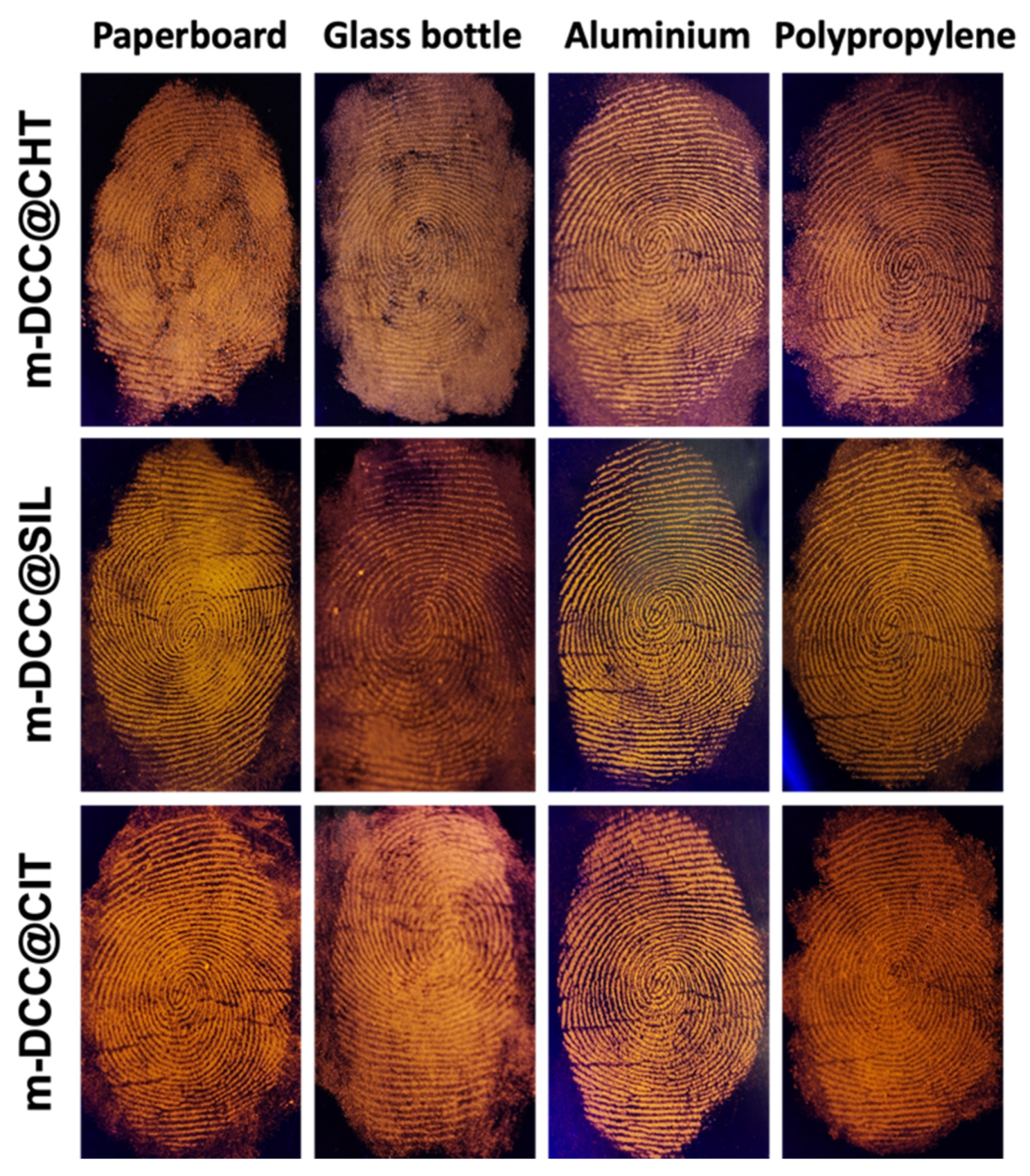
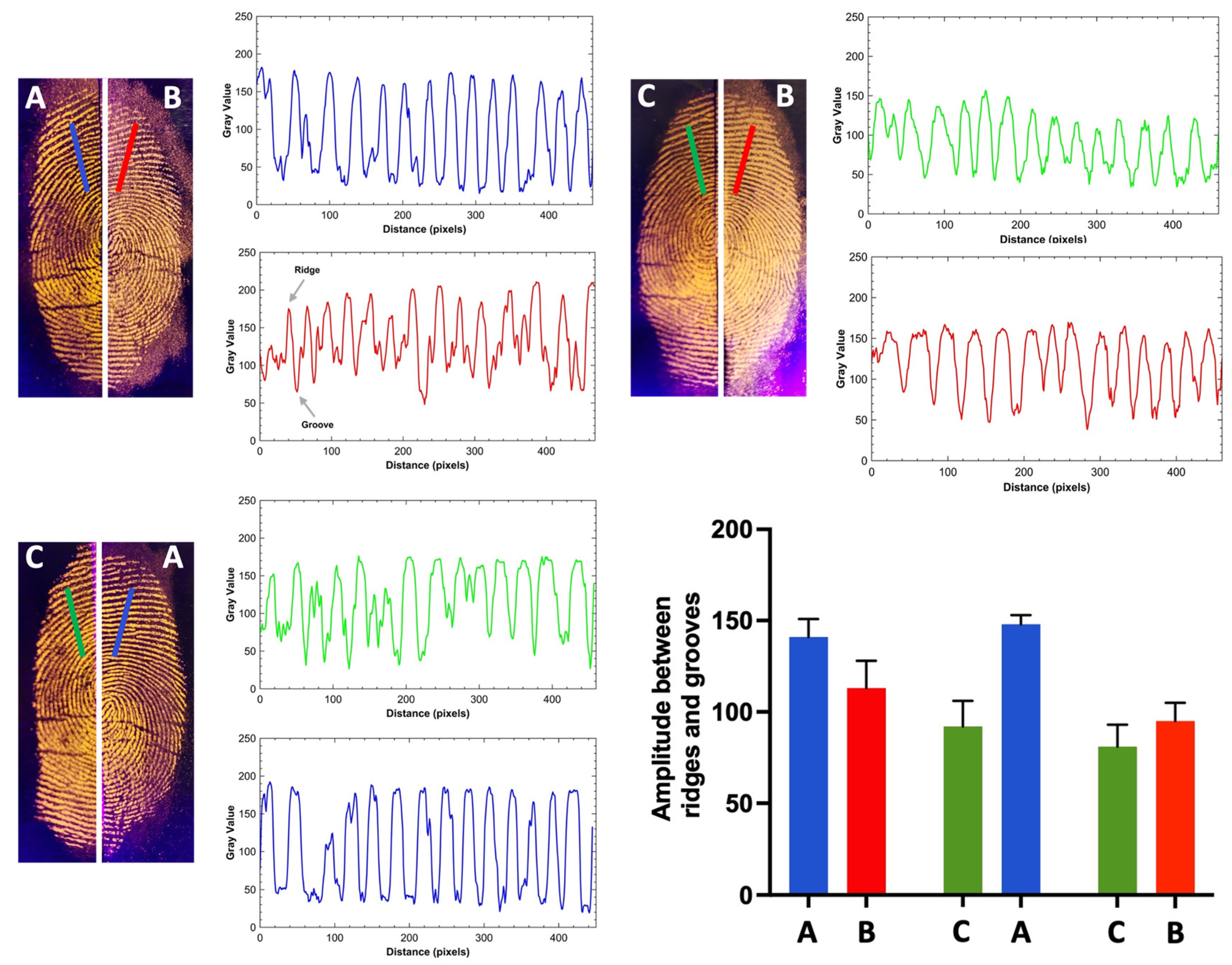
2.5. Detection of Fingerprint on Different Type of Surfaces
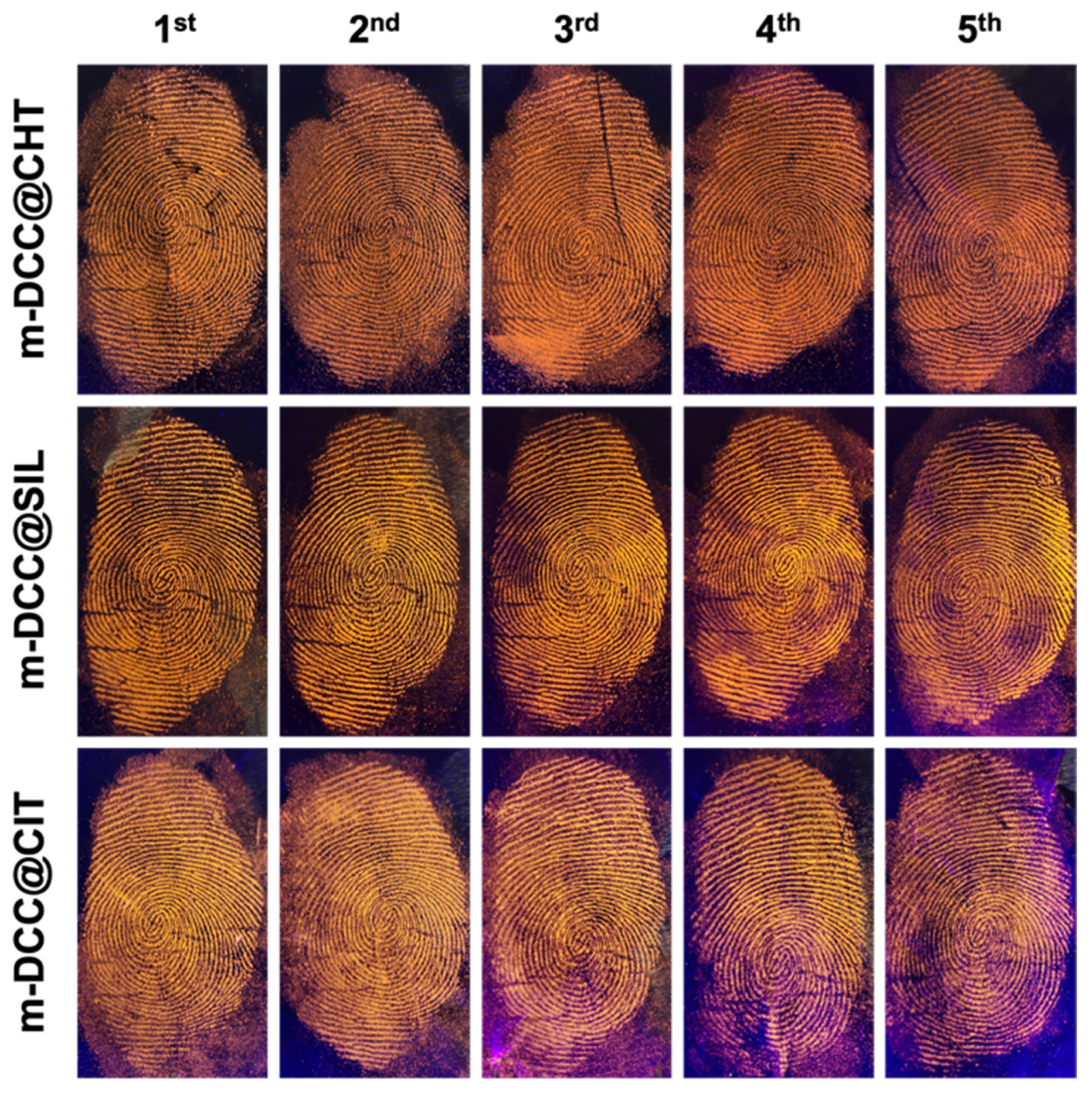
2.6. Sensibility Studies by Sequential Deposition
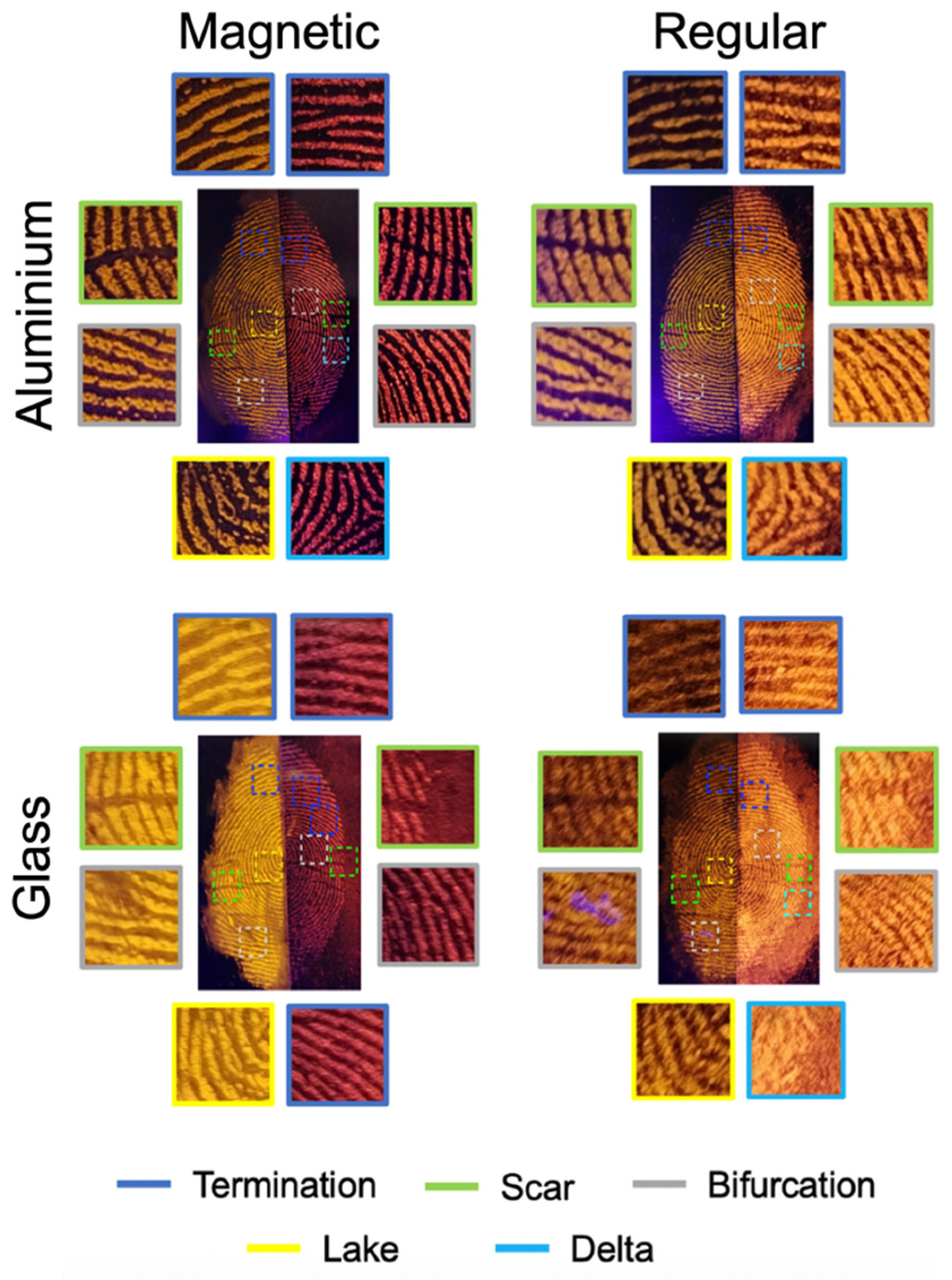
2.7. Comparison Between Regular and Magnetic Fingerprint Developers
2.8. Comparison Between Proposed and Commercial Fingerprint Developers
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Pre-Treatment and Preparation of Fluorescent Magnetic Powders
3.4. Fingerprint Samples
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lian, J.; Meng, F.; Wang, W.; Zhang, Z. Recent trends in fluorescent organic materials for latent fingerprint imaging. Front. Chem. 2020, 8, 594864. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, E.; Pillay, K. Nanomaterials for latent fingerprint detection: A review. J. Mater. Res. Technol. 2021, 12, 1856–1885. [Google Scholar] [CrossRef]
- Fatin, N.; Said, N.; Anuar, S.N.; Zakaria, Y.; Rajan, R.; Shukri, N.M.; Fakhuruddin, N.; Hassan, N. Recycling potential of natural waste products in the development of fingerprint powders for forensic application. Malays. J. Med. Health Sci. 2021, 17, 196–204. [Google Scholar]
- Kim, E.J.; Lee, D.E.; Park, S.W.; Seo, K.S.; Choi, S.W. A pilot study of a new fingerprint powder application method for the reduction of health risk. Anal. Sci. Technol. 2019, 32, 196–209. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Yao, H.; Ye, Y.; Zhou, J. Development of latent fingerprints by degradable highly-adhering powder—A long-term strategy for the fading of fingerprint residues. Dye. Pigment. 2023, 219, 111597. [Google Scholar] [CrossRef]
- Hejjaji, E.M.A.; Smith, A.M.; Morris, G.A. The potential of chitosan-tripolyphosphate microparticles in the visualization of latent fingermarks. Food Hydrocoll. 2017, 71, 290–298. [Google Scholar] [CrossRef]
- Barros, H.L.; Bonifacio, V.D.B. Mechanosynthesis of fluorescent magnetic alumina for latent fingerprint detection. J. Colloid Interface Sci. 2025, 685, 685–695. [Google Scholar] [CrossRef]
- Yadav, N.; Mudgal, D.; Mishra, A.; Shukla, S.; Malik, T.; Mishra, V. Harnessing fluorescent carbon quantum dots from natural resources for advancing sweat latent fingerprint recognition with machine learning algorithms for enhanced human identification. PLoS ONE 2024, 19, e0296270. [Google Scholar] [CrossRef]
- Yuan, C.; Li, M.; Wang, M.; Zhang, L. Cationic dye–diatomite composites: Novel dusting powders for developing latent fingerprints. Dye. Pigment. 2018, 153, 18–25. [Google Scholar] [CrossRef]
- Huang, W.; Li, X.; Wang, H.; Xu, X.; Liu, H.; Wang, G. Synthesis of amphiphilic silica nanoparticles for latent fingerprint detection. Anal. Lett. 2015, 48, 1524–1535. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, K.; Sharma, A.; Singh, P. Magical fluorescent powder and ink for instantaneous imaging of latent fingerprints (level 1–3) and anti-counterfeiting labels. Results Chem. 2024, 7, 101310. [Google Scholar] [CrossRef]
- Jain, R.; Sunil, D.; Bhagavath, P. Organic fluorophores in developing latent fingerprints: An up-to-date review. J. Coat. Technol. Res. 2024, 22, 117–147. [Google Scholar] [CrossRef]
- Yu, T.; Yang, S.; Zhao, Y.; Zhang, H.; Han, X.; Fan, D.; Qiu, Y.; Chen, L. Synthesis, crystal structures and fluorescence properties of 3-(2-pyridyl)coumarin derivatives. J. Photochem. Photobiol. A Chem. 2010, 214, 92–99. [Google Scholar] [CrossRef]
- Hua, C.J.; Niu, W.J.; Li, Y.J. Optical property investigations of coumarin and indene diketone structure dyes: Experiment and calculation. Results Chem. 2022, 4, 100257. [Google Scholar] [CrossRef]
- Chen, G.F.; Zhang, L.Y.; Li, H.Y.; Chen, B.H. Synthesis of coumarin derivatives containing 2-aminothiazole moiety and their recognition of metal ions. Res. Chem. Intermed. 2015, 41, 4273–4281. [Google Scholar] [CrossRef]
- Chen, G.F.; Jia, H.M.; Zhang, L.Y.; Hu, J.; Chen, B.H.; Song, Y.L.; Li, J.T.; Bai, G.Y. A highly selective fluorescent sensor for Fe3+ ion based on coumarin derivatives. Res. Chem. Intermed. 2013, 39, 4081–4090. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, P.; Zhao, Y.; Zhang, H.; Meng, J.; Fan, D. Synthesis, characterization and high-efficiency blue electroluminescence based on coumarin derivatives of 7-diethylamino-coumarin-3-carboxamide. Org. Electron. 2009, 10, 653–660. [Google Scholar] [CrossRef]
- Xue, L.; Feng, Y.; Song, Y.; Wang, R.; Liu, D.; Du, J.; Yang, Q.; Li, Y. A highly selective and sensitive ratiometric fluorescent probe for hypochlorite and its application. Chem. Res. Chin. Univ. 2018, 34, 536–540. [Google Scholar] [CrossRef]
- Secci, D.; Carradori, S.; Bolasco, A.; Chimenti, P.; Yáñez, M.; Ortuso, F.; Alcaro, S. Synthesis and selective human monoamine oxidase inhibition of 3-carbonyl, 3-acyl, and 3-carboxyhydrazido coumarin derivatives. Eur. J. Med. Chem. 2011, 46, 4846–4852. [Google Scholar] [CrossRef]
- Corrie, J.E.T. Thiol-reactive fluorescent probes for protein labelling. J. Chem. Soc. Perkin Trans. 1994, 1, 2975–2982. [Google Scholar] [CrossRef]
- Shen, S.L.; Zhang, X.F.; Bai, S.Y.; Miao, J.Y.; Zhao, B.X. A novel ratiometric pH probe for extreme acidity based on FRET and PET. RSC Adv. 2015, 5, 13341–13346. [Google Scholar] [CrossRef]
- Barros, H.L.; Tavares, L.; Stefani, V. Dye-doped starch microparticles as a novel fluorescent agent for the visualization of latent fingermarks on porous and non-porous substrates. Forensic Chem. 2020, 20, 100264. [Google Scholar] [CrossRef]
- Pu, Z.H.; He, J.; Liu, X.; Wang, J.; Bai, Q.H.; Wang, C.H.; Xiao, X. Dual-mode visual imaging of latent fingerprints based on organic fluorescent probe with enhanced TICT emission. Sens. Actuators B Chem. 2025, 423, 136874. [Google Scholar] [CrossRef]
- Brini, L.; Bennour, I.; Toncelli, A.; Maalej, R.; Abdelhedi, M. Eu-doped pyrochlore crystal nano-powders as fluorescent solids for fingerprint visualization and anti-counterfeiting applications. Materials 2022, 15, 2423. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Yu, A.; Zhu, Y.; Yang, M.; Mao, C. Fluorescent nanomaterials for the development of latent fingerprints in forensic sciences. Adv. Funct. Mater. 2017, 27, 1606243. [Google Scholar] [CrossRef]
- Ding, L.; Peng, D.; Wang, R.; Li, Q. A user-secure and highly selective enhancement of latent fingerprints by magnetic composite powder based on carbon dot fluorescence. J. Alloys Compd. 2021, 856, 158160. [Google Scholar] [CrossRef]
- Pan, P.; Zhang, T.; Yu, B.; Ma, R.; Yue, Q.; Alghamdi, A.A.; Deng, Y. A facile construction of bifunctional core–shell magnetic fluorescent Fe3O4@YVO4:Eu3+ microspheres for latent fingerprint detection. J. Colloid Interface Sci. 2022, 605, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakash, G.; Mahadevan, S.; Jayakumar, T.; Kalyanasundaram, P.; Philip, J.; Raj, B. Effect of initial pH and temperature of iron salt solutions on formation of magnetite nanoparticles. Mater. Chem. Phys. 2007, 103, 168–175. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room temperature co-precipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef]
- Knobel, M.; Nunes, W.C.; Socolovsky, L.M.; De Biasi, E.; Vargas, J.M.; Denardin, J.C. Superparamagnetism and other magnetic features in granular materials: A review on ideal and real systems. J. Nanosci. Nanotechnol. 2008, 8, 2836–2857. [Google Scholar] [CrossRef]
- Arévalo-Cid, P.; Isasi, J.; Martín-Hernández, F. Comparative study of core–shell nanostructures based on amino-functionalized Fe3O4@SiO2 and CoFe2O4@SiO2 nanocomposites. J. Alloys Compd. 2018, 766, 609–618. [Google Scholar] [CrossRef]
- Daou, T.J.; Pourroy, G.; Bégin-Colin, S.; Grenèche, J.M.; Ulhaq-Bouillet, C.; Legaré, P.; Bernhardt, P.; Leuvrey, C.; Rogez, G. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem. Mater. 2006, 18, 4399–4404. [Google Scholar] [CrossRef]
- Digigow, R.G.; Dechézelles, J.F.; Dietsch, H.; Geissbühler, I.; Vanhecke, D.; Geers, C.; Hirt, A.M.; Rothen-Rutishauser, B.; Petri-Fink, A. Preparation and characterization of functional silica hybrid magnetic nanoparticles. J. Magn. Magn. Mater. 2014, 362, 72–79. [Google Scholar] [CrossRef]
- Shahbazi, N.; Zare-Dorabei, R. A facile colorimetric and spectrophotometric method for sensitive determination of metformin in human serum based on citrate-capped gold nanoparticles: Central composite design optimization. ACS Omega 2019, 4, 17519–17526. [Google Scholar] [CrossRef] [PubMed]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głab, M.; Kedzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical investigations of chitosan-based hydrogels containing Aloe vera designed for biomedical use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, S.; Zhu, Y.; Huang, Z.; Yang, B.; Yang, W.; Wu, M.; Wu, Q.; Yang, J. Facile fabrication of Janus magnetic microcapsules via double in situ miniemulsion polymerization. Polym. Chem. 2013, 4, 1459–1466. [Google Scholar] [CrossRef]
- Barros, H.L.; Stefani, V. Synthesis and photophysical behavior of fluorescent benzazole dyes and fluorescent microparticles: Their use as fingerprint developers. J. Photochem. Photobiol. A Chem. 2021, 420, 113494. [Google Scholar] [CrossRef]
- Barros, H.L.; Stefani, V. Micro-structured fluorescent powders for detecting latent fingerprints on different types of surfaces. J. Photochem. Photobiol. A Chem. 2019, 368, 137–146. [Google Scholar] [CrossRef]
- Ramezanalizadeh, H. On the role of mechanical milling on structural and morphological features of nano-sized Al3Mg2 powder. Adv. Mater. Process. Technol. 2022, 8, 122–129. [Google Scholar] [CrossRef]
- International Fingerprint Research Group (IFRG), Guidelines for the Assessment of Fingermark Detection Techniques. 2014. Available online: https://www.theiai.org/docs/JFI-2014-02-174.pdf (accessed on 7 November 2025).
- McLaren, C.; Lennard, C.; Stoilovic, M. Methylamine pretreatment of dry latent fingermarks on polyethylene for enhanced detection by cyanoacrylate fuming. J. Forensic Identif. 2010, 60, 199–222. [Google Scholar]
- Berthelot, T.; Laïn, G.L.; Latxague, L.; Déleris, G. Synthesis of novel fluorogenic L-Fmoc lysine derivatives as potential tools for imaging cells. J. Fluoresc. 2004, 14, 145–152. [Google Scholar] [CrossRef] [PubMed]
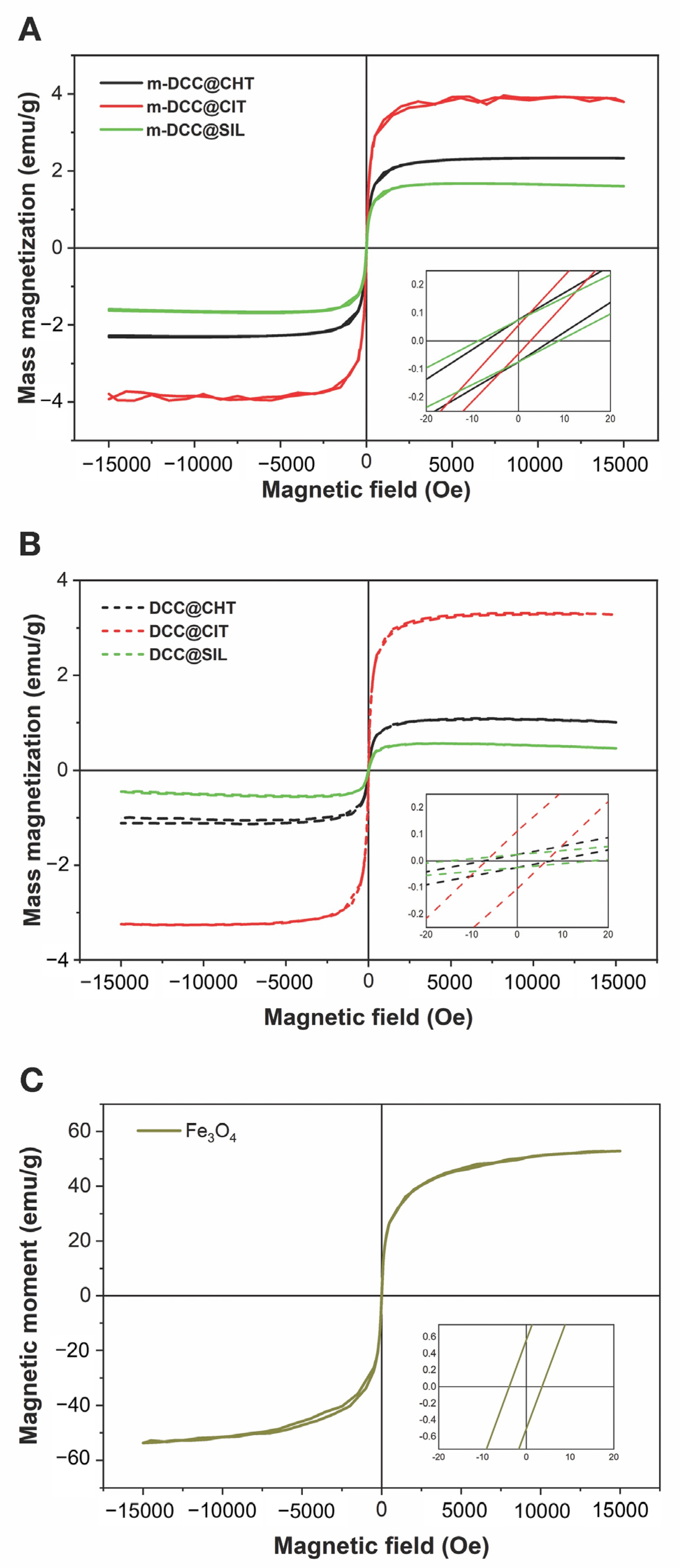

| Compounds | Ms (emu/g) | Mr (Oe) | Hc (emu/g) | Mr/Ms |
|---|---|---|---|---|
| Fe3O4 | 52.9 | 0.56 | 3.53 | 0.01 |
| m-CHT | 3.8 | 0.05 | 2.54 | 0.01 |
| m-CIT | 3.4 | 0.05 | 6.99 | 0.02 |
| m-SIL | 1.6 | 0.06 | 8.73 | 0.04 |
| m-DCC@CHT | 3.3 | 0.1 | 4.60 | 0.03 |
| m-DCC@CIT | 1.2 | 0.02 | 9.05 | 0.02 |
| m-DCC@SIL | 0.5 | 0.02 | 17.33 | 0.05 |
| Method | Developer | Surface Type | |
|---|---|---|---|
| Aluminum Foil | Glass | ||
| Proposed (half left) | m-DCC@CIT | 0 | +1 |
| Commercial (half left) | Red Magnetic | 0 | −1 |
| Proposed (half left) | DCC@CIT | 0 | 0 |
| Commercial (half left) | Orange Flu | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, H.L.; Martinho, N.; Cardoso, S.; Bonifácio, V.D.B. Ball-Milling-Assisted Coating and Magnetic Properties of Fluorescent Biodegradable Powders for Fingerprint Detection. Molecules 2025, 30, 4481. https://doi.org/10.3390/molecules30224481
Barros HL, Martinho N, Cardoso S, Bonifácio VDB. Ball-Milling-Assisted Coating and Magnetic Properties of Fluorescent Biodegradable Powders for Fingerprint Detection. Molecules. 2025; 30(22):4481. https://doi.org/10.3390/molecules30224481
Chicago/Turabian StyleBarros, Hélio L., Nuno Martinho, Susana Cardoso, and Vasco D. B. Bonifácio. 2025. "Ball-Milling-Assisted Coating and Magnetic Properties of Fluorescent Biodegradable Powders for Fingerprint Detection" Molecules 30, no. 22: 4481. https://doi.org/10.3390/molecules30224481
APA StyleBarros, H. L., Martinho, N., Cardoso, S., & Bonifácio, V. D. B. (2025). Ball-Milling-Assisted Coating and Magnetic Properties of Fluorescent Biodegradable Powders for Fingerprint Detection. Molecules, 30(22), 4481. https://doi.org/10.3390/molecules30224481









