Comprehensive Chemical Composition Evaluation of Ziziphus jujuba var. spinosa Germplasm Resources and Selection of Elite Cultivars for Seed, Pulp, and Leaf Utilization
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of Chemical Composition in ZS Tissues
2.2. Quantification of Major Chemical Composition Across ZS Germplasms
2.2.1. Validation of the Quantitative Analytical Method
2.2.2. Content of Major Chemical Composition in Seeds
2.2.3. Content of Major Chemical Composition in Pulp
2.2.4. Content of Major Chemical Composition in Leaves
2.3. Quality Evaluation of ZS Germplasm
2.3.1. Quality Evaluation of Seed-Use ZS Germplasm Based on Major Seed Chemical Composition
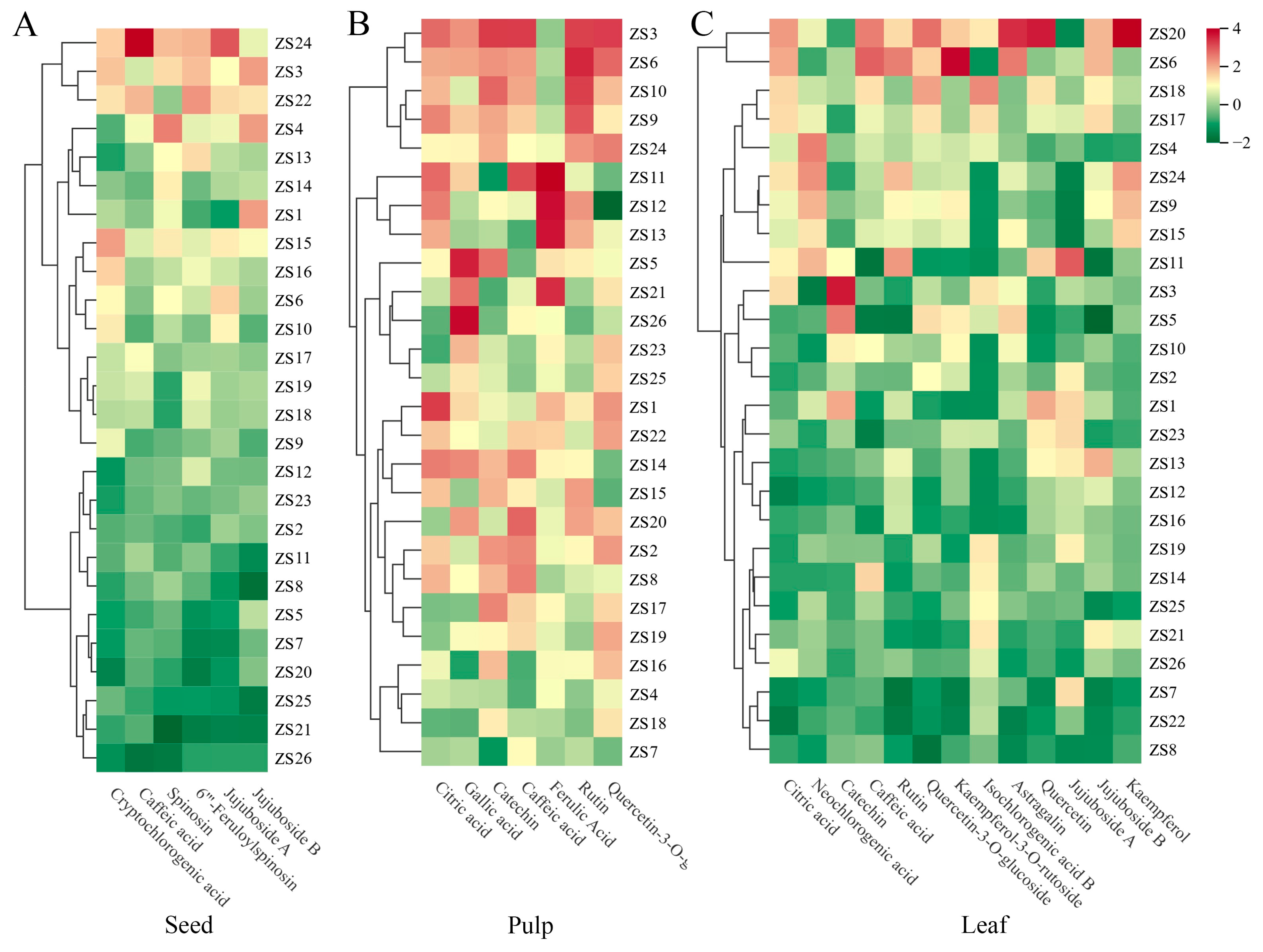
Hierarchical Cluster Analysis (HCA)
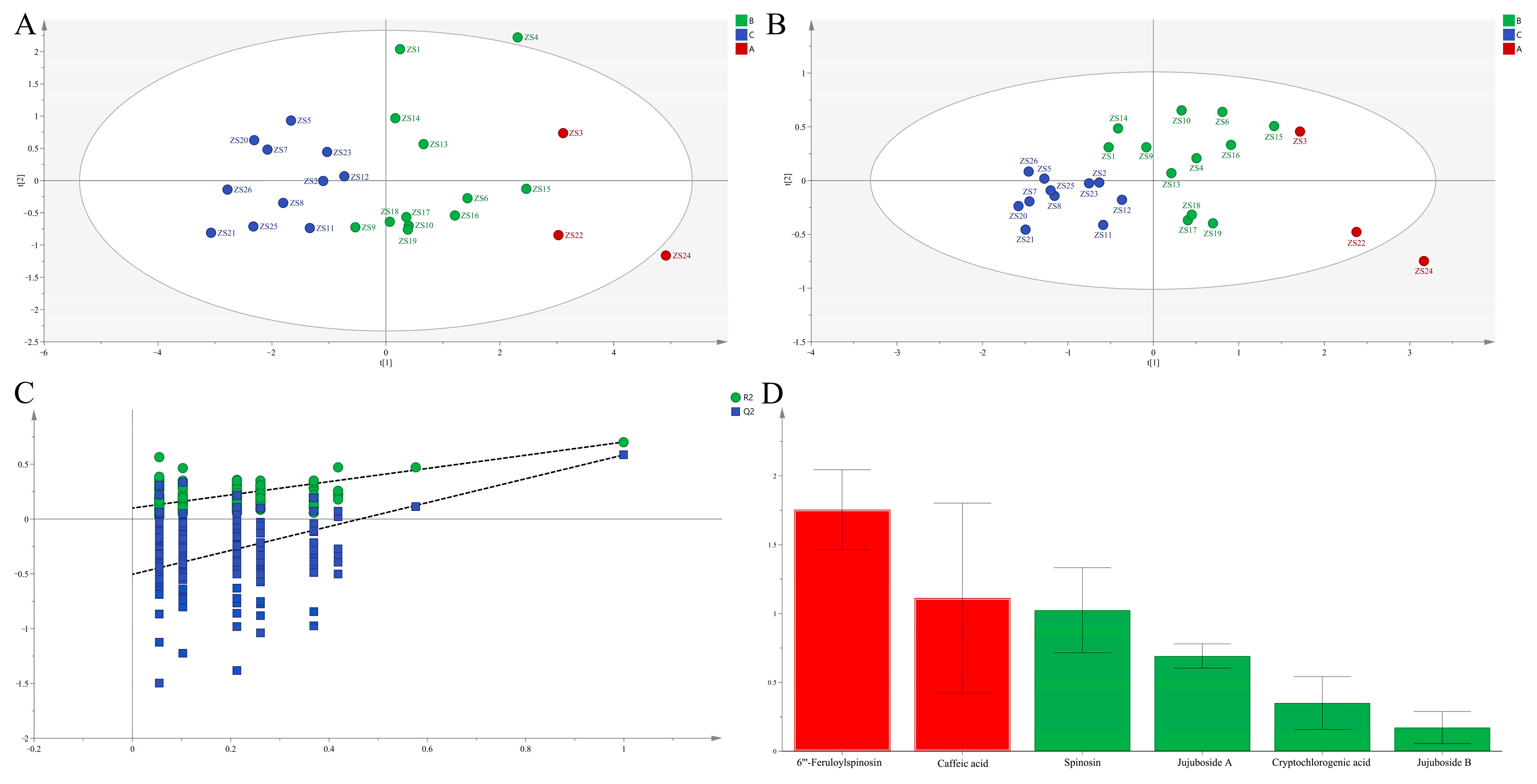
Principal Component Analysis (PCA)
Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
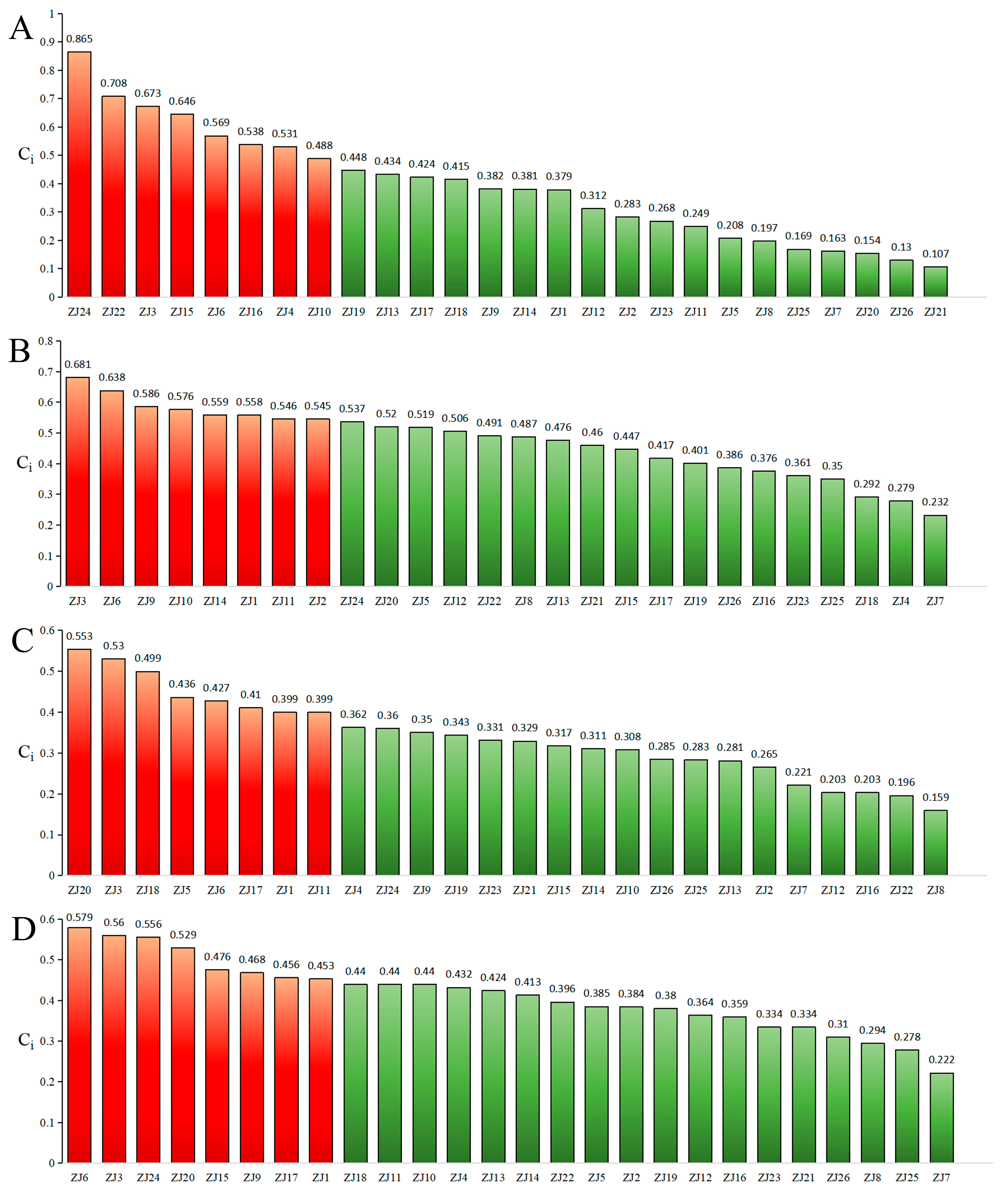
Entropy-Weighted TOPSIS Analysis (EWT)
2.3.2. Quality Evaluation of Pulp-Use ZS Germplasm Based on Major Chemical Composition in Pulp
HCA
PCA
OPLS-DA
EWT
2.3.3. Quality Evaluation of Leaf-Use ZS Germplasm Based on Major Chemical Composition in Leaves
HCA
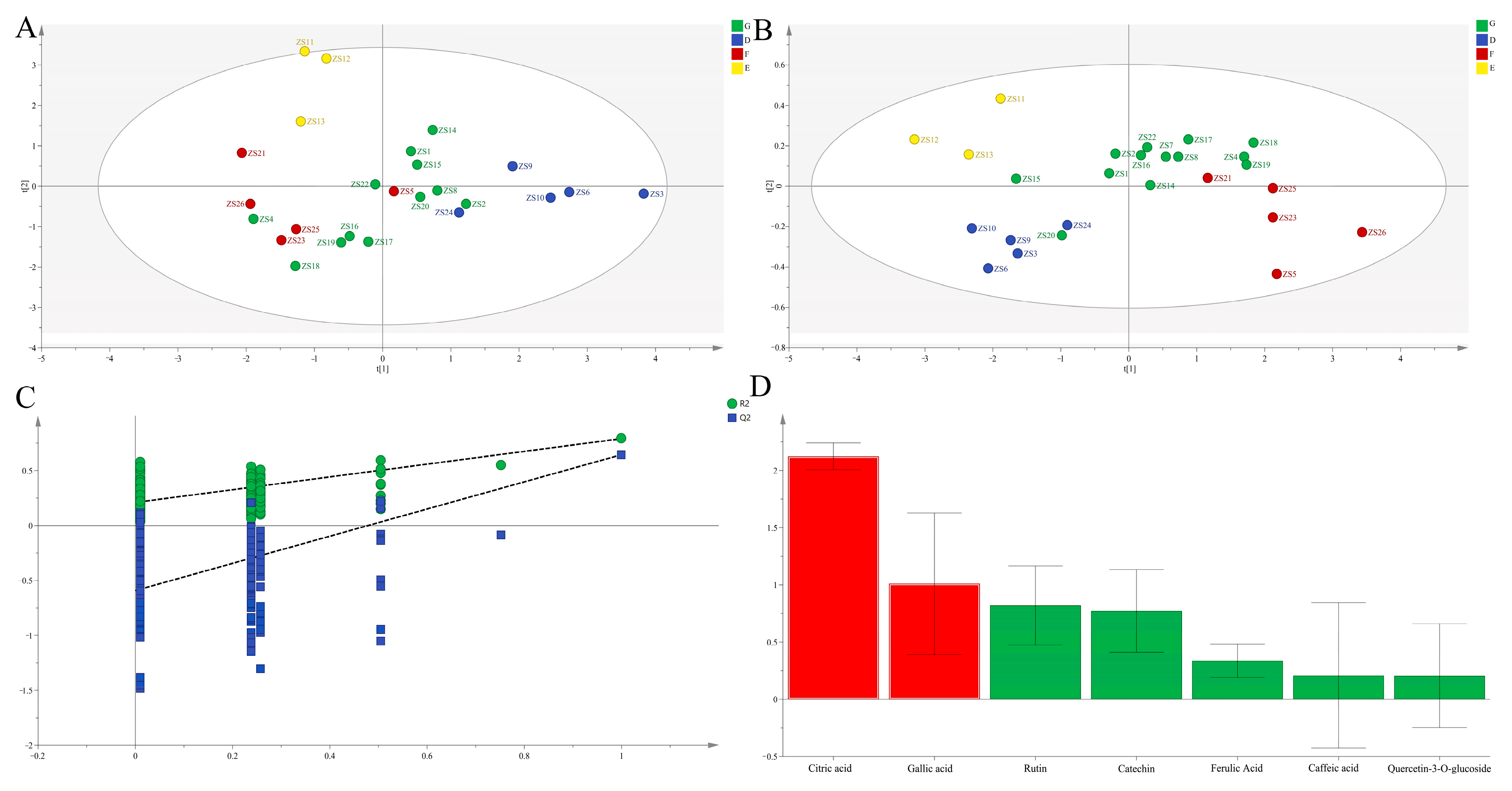
PCA
OPLS-DA
EWT
2.3.4. Comprehensive Quality Evaluation of ZS Germplasm Integrating Major Chemical Composition from Seeds, Pulp, and Leaves
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Plant Material
4.1.2. Chemicals and Reagents
4.2. Methods
4.2.1. Preparation of Sample Solutions
4.2.2. Preparation of Standard Solutions
4.2.3. UPLC-Q-Exactive-Orbitrap-MS/MS Conditions for Qualitative Analysis
4.2.4. HPLC-ELSD Conditions for Quantitative Analysis
4.2.5. Validation of the Quantitative Method
4.2.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Part | NO. | Compounds | Regression Equation | R2 | Linear Range (mg/mL) | Precision (RSD%; n = 6) | Repeatability (RSD%; n = 6) | Stability (RSD%; n = 6) | Recovery | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | RSD% | |||||||||
| Seed | 1 | Cryptochlorogenic acid | y = 181.7x − 8.2576 | 0.999 | 4.80 × 10−5–9.60 × 10−4 | 0.63 | 0.29 | 2.93 | 98.48 | 0.63 |
| 2 | Caffeic acid | y = 331.74x + 16.201 | 0.999 | 1.20 × 10−3–2.40 × 10−2 | 0.57 | 0.52 | 2.82 | 98.01 | 0.01 | |
| 3 | Spinosin | y = 169.99x + 9.557 | 1.000 | 9.90 × 10−4–1.98 × 10−2 | 0.22 | 0.07 | 0.97 | 99.28 | 1.08 | |
| 4 | 6‴-Feruloylspinosin | y = 142.31x + 7.0431 | 0.999 | 1.09 × 10−3–2.18 × 10−2 | 0.31 | 0.03 | 0.98 | 99.14 | 0.85 | |
| 5 | Jujuboside A | y = 20.72x − 44.533 | 0.999 | 9.60 × 10−4–1.92 × 10−2 | 0.37 | 0.21 | 2.99 | 98.51 | 0.32 | |
| 6 | Jujuboside B | y = 16.945x − 37.072 | 0.999 | 9.50 × 10−4–1.90 × 10−2 | 0.44 | 0.32 | 2.64 | 99.20 | 1.05 | |
| Pulp | 7 | Citric acid | y = 259.6x + 95.312 | 0.999 | 1.48 × 10−3–4.44 × 10−2 | 1.82 | 2.91 | 1.45 | 99.55 | 2.05 |
| 8 | Gallic acid | y = 194.22x − 4.8952 | 0.999 | 1.41 × 10−5–1.41 × 10−3 | 0.35 | 2.64 | 2.08 | 100.79 | 0.93 | |
| 9 | Catechin | y = 85.116x + 10.774 | 0.999 | 1.34 × 10−5–1.34 × 10−3 | 0.43 | 2.34 | 2.03 | 100.11 | 0.50 | |
| 2 | Caffeic acid | y = 484.79x + 11.078 | 0.999 | 1.49 × 10−6–2.97 × 10−4 | 0.38 | 2.78 | 2.36 | 102.12 | 0.38 | |
| 10 | Ferulic Acid | y = 439.01x + 12.786 | 0.999 | 1.54 × 10−6–3.07 × 10−4 | 0.57 | 1.42 | 1.44 | 100.75 | 0.97 | |
| 11 | Rutin | y = 91.964x + 13.828 | 0.999 | 1.34 × 10−5–1.34 × 10−3 | 0.47 | 1.96 | 2.44 | 99.76 | 1.35 | |
| 12 | Quercetin-3-O-glucoside | y = 129x + 6.4427 | 0.999 | 7.29 × 10−6–7.29 × 10−4 | 0.42 | 2.95 | 2.78 | 102.32 | 2.06 | |
| Leaf | 7 | Citric acid | y = 22.597x − 28.666 | 0.999 | 2.59 × 10−4–2.59 × 10−3 | 1.98 | 2.98 | 2.92 | 101.40 | 2.97 |
| 13 | Neochlorogenic acid | y = 16.42x + 3.6476 | 0.999 | 5.58 × 10−6–1.12 × 10−4 | 0.89 | 2.97 | 2.03 | 101.10 | 2.55 | |
| 9 | Catechin | y = 68.325x + 2.3997 | 0.999 | 1.97 × 10−5–7.88 × 10−4 | 0.34 | 2.89 | 2.90 | 102.12 | 0.66 | |
| 2 | Caffeic acid | y = 62.608x + 8.4197 | 0.999 | 9.85 × 10−6–3.94 × 10−4 | 0.54 | 2.99 | 2.59 | 100.50 | 2.85 | |
| 11 | Rutin | y = 1371.3x + 1479 | 0.999 | 5.50 × 10−3–5.50 × 10−2 | 0.43 | 2.92 | 2.73 | 99.86 | 2.64 | |
| 12 | Quercetin-3-O-glucoside | y = 92.852x + 9.7727 | 0.999 | 2.04 × 10−4–2.04 × 10−3 | 0.46 | 2.34 | 2.31 | 100.14 | 2.71 | |
| 14 | Kaempferol-3-O-rutoside | y = 108.22x + 13.042 | 0.999 | 5.65 × 10−5–2.26 × 10−3 | 0.15 | 2.99 | 2.74 | 102.98 | 0.80 | |
| 15 | Isochlorogenic acid B | y = 1898.4x + 29.204 | 1.000 | 1.08 × 10−6–1.08 × 10−2 | 2.95 | 2.32 | 2.72 | 97.50 | 1.02 | |
| 16 | Astragalin | y = 35.406x + 2.4954 | 0.999 | 1.09 × 10−5–4.36 × 10−4 | 2.94 | 2.82 | 2.86 | 101.46 | 2.66 | |
| 17 | Quercetin | y = 100.09x − 122.13 | 0.999 | 1.12 × 10−4–1.68 × 10−3 | 2.96 | 2.71 | 2.72 | 101.74 | 0.47 | |
| 5 | Jujuboside A | y = 46.259x − 55.494 | 0.999 | 1.18 × 10−4–2.35 × 10−3 | 2.21 | 2.94 | 2.09 | 100.73 | 1.90 | |
| 6 | Jujuboside B | y = 29.055x − 28.964 | 0.999 | 1.07 × 10−4–2.13 × 10−3 | 1.41 | 2.74 | 2.51 | 100.76 | 2.01 | |
| 18 | Kaempferol | y = 102.2x − 81.167 | 0.999 | 1.03 × 10−4–4.12 × 10−3 | 2.21 | 2.69 | 2.45 | 102.65 | 0.83 | |
| No. | Cryptochlorogenic Acid | Caffeic Acid | Spinosin | 6‴-Feruloyl-Spinosyn | Jujuboside A | Jujuboside B | Total Content |
|---|---|---|---|---|---|---|---|
| ZS1 | 0.1062 | 0.7112 | 1.2886 | 2.3730 | 0.3141 | 0.1938 | 4.9869 |
| ZS2 | 0.0727 | 0.6286 | 0.7910 | 2.2690 | 0.4826 | 0.1521 | 4.3960 |
| ZS3 | 0.1646 | 0.9589 | 1.4800 | 5.0200 | 0.6344 | 0.1938 | 8.4517 |
| ZS4 | 0.0725 | 1.0770 | 1.8418 | 3.9023 | 0.6084 | 0.1942 | 7.6962 |
| ZS5 | 0.0587 | 0.5209 | 0.8595 | 1.8880 | 0.3145 | 0.1618 | 3.8034 |
| ZS6 | 0.1395 | 0.6972 | 1.3440 | 3.6170 | 0.7288 | 0.1564 | 6.6829 |
| ZS7 | 0.0565 | 0.6188 | 0.8110 | 1.6747 | 0.2615 | 0.1494 | 3.5719 |
| ZS8 | 0.0623 | 0.6414 | 1.0281 | 2.5806 | 0.2980 | 0.1250 | 4.7354 |
| ZS9 | 0.1281 | 0.5376 | 0.8448 | 2.8698 | 0.4869 | 0.1448 | 5.0120 |
| ZS10 | 0.1466 | 0.5632 | 1.1062 | 2.8243 | 0.6565 | 0.1462 | 5.4430 |
| ZS11 | 0.0741 | 0.8066 | 0.7994 | 2.9534 | 0.3466 | 0.1328 | 5.1129 |
| ZS12 | 0.0537 | 0.6482 | 0.9176 | 3.7911 | 0.4211 | 0.1499 | 5.9816 |
| ZS13 | 0.0602 | 0.7359 | 1.3451 | 4.6438 | 0.5297 | 0.1584 | 7.4731 |
| ZS14 | 0.0909 | 0.6125 | 1.4105 | 2.7143 | 0.5073 | 0.1621 | 5.4976 |
| ZS15 | 0.1818 | 0.9821 | 1.4245 | 3.8416 | 0.6744 | 0.1721 | 7.2765 |
| ZS16 | 0.1577 | 0.7805 | 1.1325 | 4.0440 | 0.5537 | 0.1585 | 6.8269 |
| ZS17 | 0.1129 | 1.0943 | 0.9379 | 3.2048 | 0.4883 | 0.1541 | 5.9923 |
| ZS18 | 0.1069 | 0.8839 | 0.7133 | 3.7788 | 0.4744 | 0.1578 | 6.1151 |
| ZS19 | 0.1139 | 0.9753 | 0.7203 | 3.9951 | 0.4863 | 0.1590 | 6.4499 |
| ZS20 | 0.0394 | 0.5856 | 0.7227 | 1.4580 | 0.2964 | 0.1523 | 3.2544 |
| ZS21 | 0.0639 | 0.5653 | 0.3413 | 1.5143 | 0.2396 | 0.1308 | 2.8552 |
| ZS22 | 0.1498 | 1.4030 | 0.9837 | 5.5312 | 0.7107 | 0.1792 | 8.9576 |
| ZS23 | 0.0592 | 0.6142 | 0.9316 | 2.6552 | 0.4238 | 0.1551 | 4.8391 |
| ZS24 | 0.1591 | 2.1300 | 1.6016 | 5.1558 | 0.9615 | 0.1695 | 10.1775 |
| ZS25 | 0.0801 | 0.4998 | 0.6658 | 2.0807 | 0.2968 | 0.1283 | 3.7515 |
| ZS26 | 0.0497 | 0.1842 | 0.4621 | 2.2039 | 0.3340 | 0.1410 | 3.3749 |
| CV(%) | 43.38 | 46.62 | 35.32 | 36.00 | 36.45 | 11.98 |
| No. | Citric Acid | Gallic Acid | Catechin | Caffeic Acid | Ferulic Acid | Rutin | Quercetin-3-O-Glucoside | Total Content |
|---|---|---|---|---|---|---|---|---|
| ZS1 | 20.4395 | 0.1283 | 0.0878 | 0.0069 | 0.0042 | 0.1586 | 0.0125 | 20.8378 |
| ZS2 | 12.6134 | 0.0907 | 0.1239 | 0.0146 | 0.0023 | 0.1455 | 0.0124 | 13.0028 |
| ZS3 | 18.0934 | 0.1619 | 0.1485 | 0.0181 | 0.0006 | 0.3071 | 0.0148 | 18.7444 |
| ZS4 | 7.2853 | 0.0814 | 0.0738 | 0.0018 | 0.0025 | 0.0533 | 0.0091 | 7.5072 |
| ZS5 | 10.0898 | 0.2129 | 0.1340 | 0.0030 | 0.0032 | 0.1538 | 0.0093 | 10.606 |
| ZS6 | 14.6059 | 0.1527 | 0.1240 | 0.0136 | 0.0011 | 0.3270 | 0.0137 | 15.238 |
| ZS7 | 5.2575 | 0.0778 | 0.0406 | 0.0089 | 0.0007 | 0.0855 | 0.0063 | 5.4773 |
| ZS8 | 13.9532 | 0.1101 | 0.1133 | 0.0150 | 0.0009 | 0.1079 | 0.0090 | 14.3094 |
| ZS9 | 16.7025 | 0.1355 | 0.1187 | 0.0111 | 0.0014 | 0.2884 | 0.0101 | 17.2677 |
| ZS10 | 13.7535 | 0.0947 | 0.1371 | 0.0131 | 0.0007 | 0.3081 | 0.0114 | 14.3186 |
| ZS11 | 18.1177 | 0.1323 | 0.0413 | 0.0175 | 0.0081 | 0.1208 | 0.0062 | 18.4439 |
| ZS12 | 17.0808 | 0.0799 | 0.0936 | 0.0078 | 0.0076 | 0.2349 | 0.0025 | 17.5071 |
| ZS13 | 14.4683 | 0.0714 | 0.0739 | 0.0017 | 0.0075 | 0.2126 | 0.0091 | 14.8445 |
| ZS14 | 17.0504 | 0.1662 | 0.1129 | 0.0148 | 0.0028 | 0.1450 | 0.0063 | 17.4984 |
| ZS15 | 13.0702 | 0.0674 | 0.1149 | 0.0095 | 0.0018 | 0.2273 | 0.0059 | 13.497 |
| ZS16 | 8.8191 | 0.0326 | 0.1123 | 0.0017 | 0.0024 | 0.1333 | 0.0114 | 9.1128 |
| ZS17 | 3.4939 | 0.0597 | 0.1281 | 0.0109 | 0.0027 | 0.0834 | 0.0107 | 3.7894 |
| ZS18 | 2.4508 | 0.0464 | 0.0983 | 0.0056 | 0.0011 | 0.0428 | 0.0103 | 2.6553 |
| ZS19 | 4.1632 | 0.1063 | 0.0947 | 0.0106 | 0.0021 | 0.0596 | 0.0120 | 4.4485 |
| ZS20 | 4.9013 | 0.1578 | 0.0803 | 0.0163 | 0.0022 | 0.2230 | 0.0112 | 5.3921 |
| ZS21 | 6.9057 | 0.1775 | 0.0510 | 0.0077 | 0.0070 | 0.0678 | 0.0103 | 7.227 |
| ZS22 | 12.9828 | 0.1075 | 0.0840 | 0.0112 | 0.0036 | 0.1039 | 0.0122 | 13.3052 |
| ZS23 | 1.4692 | 0.1441 | 0.0815 | 0.0041 | 0.0028 | 0.0831 | 0.0112 | 1.796 |
| ZS24 | 10.1719 | 0.1142 | 0.1165 | 0.0087 | 0.0023 | 0.2351 | 0.0131 | 10.6618 |
| ZS25 | 6.5215 | 0.1217 | 0.0835 | 0.0038 | 0.0023 | 0.0653 | 0.0108 | 6.8089 |
| ZS26 | 2.2088 | 0.2219 | 0.0586 | 0.0090 | 0.0025 | 0.0280 | 0.0081 | 2.5369 |
| CV(%) | 53.70 | 41.11 | 30.44 | 52.41 | 74.87 | 58.92 | 28.00 |
| No. | Citric Acid | Neochlorogenic Acid | Catechin | Caffeic Acid | Rutin | Quercetin-3-O-Glucoside | Kaempfer-ol-3-O-Rutoside | Isochlorogenic Acid B | Astragalin | Querce-Tin | Jujuboside A | Jujuboside B | Kaempferol | Total Content |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZS1 | 0.5709 | 0.0275 | 0.0897 | 0.0308 | 11.1865 | 0.3571 | 0.0921 | 0.0261 | 0.0693 | 0.2753 | 0.6068 | 0.3064 | 0.3229 | 13.9614 |
| ZS2 | 0.5089 | 0.0157 | 0.0424 | 0.0457 | 7.5060 | 0.6052 | 0.3063 | 0.0386 | 0.0610 | 0.1344 | 0.5541 | 0.2446 | 0.3106 | 10.3735 |
| ZS3 | 0.9195 | 0.0046 | 0.1344 | 0.0489 | 5.7306 | 0.5200 | 0.2046 | 2.2140 | 0.0849 | 0.1150 | 0.4081 | 0.2854 | 0.4080 | 11.078 |
| ZS4 | 0.7810 | 0.0478 | 0.0300 | 0.0709 | 10.0390 | 0.5569 | 0.2977 | 1.7245 | 0.0544 | 0.1227 | 0.3168 | 0.2025 | 0.2656 | 14.5098 |
| ZS5 | 0.5405 | 0.0155 | 0.1039 | 0.0157 | 3.0968 | 0.6526 | 0.3868 | 1.5529 | 0.1065 | 0.0967 | 0.2268 | 0.1099 | 0.4777 | 7.3823 |
| ZS6 | 1.0364 | 0.0133 | 0.0379 | 0.1302 | 18.8823 | 0.6733 | 0.7043 | 0.0416 | 0.1379 | 0.1540 | 0.4177 | 0.4441 | 0.4645 | 23.1375 |
| ZS7 | 0.4347 | 0.0107 | 0.0150 | 0.0429 | 2.8404 | 0.3405 | 0.0598 | 1.2509 | 0.0439 | 0.0910 | 0.5921 | 0.1577 | 0.2105 | 6.0901 |
| ZS8 | 0.5221 | 0.0106 | 0.0248 | 0.0526 | 5.4711 | 0.2564 | 0.1500 | 0.7973 | 0.0143 | 0.1135 | 0.1440 | 0.1711 | 0.3087 | 8.0365 |
| ZS9 | 0.8177 | 0.0415 | 0.0281 | 0.0620 | 13.3891 | 0.5901 | 0.3803 | 0.1045 | 0.0529 | 0.1316 | 0.1063 | 0.3690 | 0.9751 | 17.0482 |
| ZS10 | 0.5645 | 0.0099 | 0.0659 | 0.0830 | 9.6662 | 0.4719 | 0.3710 | 0.0332 | 0.0844 | 0.1036 | 0.2724 | 0.2821 | 0.3268 | 12.3349 |
| ZS11 | 0.8775 | 0.0410 | 0.0605 | 0.0126 | 17.7091 | 0.3422 | 0.1191 | 0.0306 | 0.0461 | 0.2499 | 0.8472 | 0.1333 | 0.4671 | 20.9362 |
| ZS12 | 0.4080 | 0.0106 | 0.0088 | 0.0391 | 11.0067 | 0.3394 | 0.2339 | 0.0024 | 0.0312 | 0.1687 | 0.4293 | 0.3416 | 0.4284 | 13.4481 |
| ZS13 | 0.5045 | 0.0136 | 0.0176 | 0.0520 | 12.2706 | 0.3904 | 0.2340 | 0.0119 | 0.0373 | 0.2217 | 0.5657 | 0.4504 | 0.5333 | 15.303 |
| ZS14 | 0.5120 | 0.0122 | 0.0102 | 0.0955 | 5.3304 | 0.4209 | 0.1608 | 2.0755 | 0.0403 | 0.1677 | 0.2860 | 0.2943 | 0.3945 | 9.8003 |
| ZS15 | 0.7865 | 0.0354 | 0.0129 | 0.0732 | 12.2471 | 0.5399 | 0.3339 | 0.0844 | 0.0909 | 0.1405 | 0.1028 | 0.2935 | 0.9070 | 15.648 |
| ZS16 | 0.5200 | 0.0141 | 0.0252 | 0.0266 | 11.1758 | 0.3514 | 0.1402 | 0.0164 | 0.0214 | 0.1681 | 0.4261 | 0.2741 | 0.3892 | 13.5486 |
| ZS17 | 0.9237 | 0.0296 | 0.0089 | 0.0769 | 10.3866 | 0.6467 | 0.2408 | 2.2678 | 0.0505 | 0.2046 | 0.4654 | 0.4048 | 0.4513 | 16.1576 |
| ZS18 | 0.9343 | 0.0261 | 0.0382 | 0.0915 | 8.7731 | 0.7476 | 0.2424 | 3.1403 | 0.0495 | 0.2369 | 0.3470 | 0.3817 | 0.5601 | 15.5687 |
| ZS19 | 0.5011 | 0.0211 | 0.0270 | 0.0514 | 5.8081 | 0.5051 | 0.1189 | 2.1670 | 0.0380 | 0.1515 | 0.5541 | 0.2828 | 0.3797 | 10.6058 |
| ZS20 | 1.0655 | 0.0296 | 0.0109 | 0.1216 | 14.7244 | 0.8187 | 0.4353 | 2.1744 | 0.1644 | 0.3637 | 0.1360 | 0.4444 | 1.5576 | 22.0465 |
| ZS21 | 0.6110 | 0.0220 | 0.0176 | 0.0498 | 5.3191 | 0.3270 | 0.1322 | 2.1690 | 0.0293 | 0.1277 | 0.2187 | 0.3844 | 0.6718 | 10.0796 |
| ZS22 | 0.3820 | 0.0133 | 0.0187 | 0.0363 | 3.1398 | 0.3311 | 0.0652 | 1.3516 | 0.0104 | 0.1034 | 0.3316 | 0.1694 | 0.2585 | 6.2113 |
| ZS23 | 0.6581 | 0.0120 | 0.0358 | 0.0180 | 7.8348 | 0.4324 | 0.3036 | 1.4796 | 0.0425 | 0.2302 | 0.6041 | 0.2018 | 0.2860 | 12.1389 |
| ZS24 | 0.9091 | 0.0458 | 0.0094 | 0.0655 | 16.1573 | 0.5281 | 0.3047 | 0.0699 | 0.0793 | 0.1554 | 0.1221 | 0.3515 | 1.0696 | 19.8677 |
| ZS25 | 0.4940 | 0.0239 | 0.0107 | 0.0611 | 6.1704 | 0.3545 | 0.2018 | 1.9754 | 0.0509 | 0.1355 | 0.3014 | 0.1707 | 0.2315 | 10.1818 |
| ZS26 | 0.8259 | 0.0216 | 0.0088 | 0.0500 | 9.0060 | 0.3979 | 0.1736 | 1.6415 | 0.0248 | 0.1255 | 0.1866 | 0.2935 | 0.4068 | 13.1625 |
| CV(%) | 30.81 | 55.73 | 93.65 | 51.23 | 46.99 | 30.71 | 57.13 | 92.25 | 62.53 | 39.21 | 52.26 | 35.05 | 61.45 | 13.9614 |
References
- Hua, Y.; Xu, X.; Guo, S.; Xie, H.; Yan, H.; Ma, X.; Niu, Y.; Duan, J. Wild Jujube (Ziziphus jujuba var. spinosa): A Review of Its Phytonutrients, Health Benefits, Metabolism, and Applications. J. Agric. Food Chem. 2022, 70, 7871–7886. [Google Scholar] [CrossRef]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2025; p. 394. [Google Scholar]
- Zhang, Y.; Zhang, H.; Meng, X.; Qu, T.; Li, N.; Wang, D.; Zhang, Y.; Chen, J. Mechanism of Polygalae Radix-Ziziphi spinosae Semen drug Pair in the Treatment of Anxiety and Insomnia Based on Network Pharmacology and Cell Validation Experiment. Nat. Prod. Res. Dev. 2025, 37, 1942–1952. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, H.; Wen, C.; Zhao, C.; Tian, Y.; Liu, Y.; Yang, J. Mechanism of jujuboside A on improving cognitive function of mice with Alzheimer’s disease based on transcriptomic. Chin. Tradit. Herb. Drugs 2023, 54, 8094–8104. [Google Scholar]
- Wei, H.; Wang, X.; Wang, J.; Ren, S.; Mur, L.A.J.; Lu, D. Flavonoids from sour jujube leaves: Ultrasound-assisted extraction, UPLC-QQQ-MS/MS quantification, and ameliorative effect on DSS-induced ulcerative colitis in mice. Ultrason. Sonochem 2025, 114, 107279. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, W.; Li, X.; Gao, X.; Fu, K.; Zhang, J. Phenotypic Diversity and Nutrient Composition Analysis of Ziziphus jujuba var. spinosa Germplasm Resources. Seed 2024, 43, 120–126. [Google Scholar]
- Shang, Z.; Ye, Z.; Li, M.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Dynamics of microbial communities, flavor, and physicochemical properties of pickled chayote during an industrial-scale natural fermentation: Correlation between microorganisms and metabolites. Food Chem. 2022, 377, 132004. [Google Scholar] [CrossRef]
- Dong, J.; Liu, X.; Li, C.; Qi, X.; Zhu, Y.; Wang, Y.; Shan, H. Effect of jujube leaves alcohol extract on M1 and M2 polarization of mouse macrophages. Nat. Prod. Res. Dev. 2020, 32, 961–967. [Google Scholar]
- Tang, J.; Li, T.; Niu, Z.; Du, H.; Pei, X.; Du, C. Chemical constituents from the leaves of Ziziphus jujuba var. spinosa and their anti-inflammatory activities. Chin. Tradit. Pat. Med. 2024, 46, 4029–4035. [Google Scholar]
- Cheng, G.; Bai, Y.; Zhao, Y.; Tao, J.; Liu, Y.; Tu, G.; Ma, L.; Liao, N.; Xu, X. Flavonoids from Ziziphus jujuba Mill. var. spinosa. Tetrahedron 2000, 56, 8915–8920. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Ikebata, A.; Wakao, S.; Murakami, N.; Matsuda, H.; Yamahara, J. Bioactive saponins and glycosides. X. On the constituents of zizyphi spinosi semen, the seeds of Zizyphus jujuba Mill. var. spinosa Hu (1): Structures and histamine release-inhibitory effect of jujubosides A1 and C and acetyljujuboside B. Chem. Pharm. Bull. 1997, 45, 1186–1192. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.; Tang, Y.; Qiang, Y.; Zhao, J.; Qian, D.; Su, S.; Shang, E. Simultaneous qualitative and quantitative analysis of triterpenic acids, saponins and flavonoids in the leaves of two Ziziphus species by HPLC–PDA–MS/ELSD. J. Pharm. Biomed. Anal. 2011, 56, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yuan, M.; Liu, H.; Wang, L.; Zhao, X. Analysis of Metabolites and Metabolic Pathways of Three Chinese Jujube Cultivar. Metabolites 2023, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Li, D.; Wang, Y.; Sui, C.; Cao, Y.; Liang, Q. Study on the contents of cAMP and cGMP in different cultivars, growing periods and organs in Chinese jujube. Acta Hortic. Sin. 2009, 36, 1134–1139. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Nie, X.; Li, X.; Ye, Y. Research Progress on Active Ingredients and Applications of Choerospondias axillaris. Food Sci. Technol. 2024, 49, 82–87. [Google Scholar]
- Wang, F.; Wang, Z.; Luo, M.; Tang, H.; Liu, R.; Tang, X. Identification Study of Ziziphi spinosae Semen and Its Adulterants Based on Characteristic Chromatogram and Content Determination. J. Chin. Med. Mater. 2025, 9, 2273–2278. [Google Scholar]
- Chen, S.; Ye, X.; Zhang, J.; Wang, Y.; Huang, S.; Wen, N.; Yang, H.; Yang, P.; Zuo, Z.; Liu, Y. Research Progress of Zaoren Anshen Priscription and Its Predictive Analysis of Quality Markers. Chin. Pharm. J. 2025, 60, 1898–1907. [Google Scholar]
- Chen, W.; Zheng, S.; Cao, Y.; Tian, Y.; Zhang, C.; Wang, B.; Feng, H.; Tang, H.; Zhang, Q. Phytochemical components of Qin medicine Ziziphi spinosae Semen by GC-MS and UHPLC-TOF-MS. Cent. South. Pharm. 2025, 23, 901–907. [Google Scholar]
- Yang, R.; Li, Z.; Dong, L.; Heng, Y.; Song, L.; Guo, L.; Pei, X.; Yan, Y.; Du, C. Analysis of Components in Ziziphi spinosae Semen Before and After Processing Based on Targeted and Untargeted Metabolomics. Foods 2025, 14, 3771. [Google Scholar] [CrossRef]
- Gao, Q.; Dong, J.; Cui, R.; Muraki, I.; Yamagishi, K.; Sawada, N.; Iso, H.; Tsugane, S. Consumption of flavonoid-rich fruits, flavonoids from fruits and stroke risk: A prospective cohort study. Br. J. Nutr. 2021, 126, 1717–1724. [Google Scholar] [CrossRef]
- Cai, H.; You, S.; Xu, Z.; Li, Z.; Guo, J.; Ren, Z.; Fu, C. Novel extraction methods and potential applications of polyphenols in fruit waste: A review. J. Food Meas. Charact. 2021, 15, 3250–3261. [Google Scholar] [CrossRef]
- Song, H.; Xia, J.; Ji, G.; Ren, Z.; Zhang, X.; Liu, F.; Chen, L. Determination of 9 Amino Acid Endogenous Substances in Morning Urine of Depression Patients by LC-MS/MS. China Pharm. 2020, 31, 91–98. [Google Scholar]
- Sheng, J.; Zhong, H.; Zhou, Q.; Sun, L.; Jiang, H.; Zhang, S.; Guo, F.; Hou, L.; Zhang, C.; Dong, S. Analysis on chemical constituents of fruit of Chaenomeles speciosa by UPLC-Q-Exactive Orbitrap-Ms. Chin. Tradit. Herb. Drugs 2018, 49, 4773–4779. [Google Scholar]
- Chen, J.; Wu, H.; Liu, R.; Zhang, C.; Sun, Y. Study on the Chemical Constituents in Wuling Capsules by UPLC-QE-Orbitrap-MS. J. Chin. Med. Mater. 2022, 45, 639–646. [Google Scholar]
- Zhou, D.; Duan, H.; Wa, Q.; Wu, Y.; Peng, X.; Sun, X. Analysis of Chemical Constituents in Aerial Parts of Anemone vitifolia Based on UHPLC-Q-Exactive Orbitrap MS. Mod. Chin. Med. 2025, 27, 1018–1030. [Google Scholar]
- Liang, H.; Li, H.; Ren, Y.; Tian, D.; Zhang, M. Mechanism of Rehmanniae Radix-Salviae Miltiorrhizae Radix in the treatment of diabetic retinopathy based on network pharmacology and experimental verification. Nat. Prod. Res. Dev. 2025, 37, 1341–1355+1340. [Google Scholar]
- Zhang, X.; Yu, X.; Sun, X.; Meng, X.; Fan, J.; Zhang, F.; Zhang, Y. Comparative study on chemical constituents of different medicinal parts of Lonicera japonica Thunb. Based on LC-MS com-bined with multivariate statistical analysis. Heliyon 2024, 10, e31722. [Google Scholar] [CrossRef]
- Liu, P.; Wang, N.; Xie, S.; Wang, L.; Liao, Z.; Lai, R.; Qin, M. Rapid Determination of Fatty Acids in Ziziphi spinosae Semen from Different Origins by Internal Extractive Electrospray Ionization Mass Spectrometry. J. Chin. Mass. Spectrom. Soc. 2025, 46, 503–510. [Google Scholar]
- Niu, Y.; Wang, S. Analysis on chemical constituents in Danggui-Shaoyao-San by LC-Q- TOF-MS and LC-IT-MSn. Chin. Tradit. Herb. Drugs 2014, 45, 1056–1062. [Google Scholar]
- Chen, Y.; Yu, M.; Dai, X.; Jia, H.; Zhang, H.; Ma, Z.; Zou, J. Characterization of major constituents and determination of protocatechuic acid in Chinese herbal medicine Cibotii Rhizoma. Chin. Tradit. Herb. Drugs 2023, 54, 2254–2261. [Google Scholar]
- Yang, L.; Yang, L.; Jia, P.; Lan, W.; Zhang, Y.; Wang, S.; Zhang, P.; Zheng, X. HPLC-Q-TOF-MS/MS-based analysis of chemical constituents in Choerospondiatis fructus. Acad. J. Nav. Med. Univ. 2016, 37, 159–166. [Google Scholar]
- Cai, W.; Wang, Y.; Jin, W.; Yang, L.; Wang, J.; Zhang, Z. Chemical constituents of Qiang medicine Primula chungensis by UPLC-Q-Exactive-Orbitrap-MS. Nat. Prod. Res. Dev. 2023, 35, 986–996. [Google Scholar]
- Wang, X.; Liu, H.; Ding, S.; Luo, J.; Zhao, C.; Yao, Z.; Li, Q.; Liu, J.; Yu, J.; Wei, X.; et al. Comparison of Chemical Components between Ziziphi spinosae Semen or Schisandrae Chinensis Fructus Monodecoction and Their Codecoction by HPLC-LTQ-Orbitrap-MSn. Mod. Chin. Med. 2021, 23, 1949–1966. [Google Scholar]
- Li, B.; Qie, L.; Wang, X.; Niu, L. Simultaneous determination of fifteen constituents in Suanzao Anshen Granules by UPLC-MS/MS. Chin. Tradit. Pat. Med. 2022, 4406, 1751–1755. [Google Scholar]
- Li, R.; Li, S.; Liu, W.; Qi, S.; Peng, Y. Analysis of chemical constituents of Agrimonia pilosa based on UHPLC-Q-Exactive Orbitrap HRMS technology. J. Shenyang Pharm. Univ. 2025, 4204, 342–357. [Google Scholar]
- Qu, T.; Li, N.; Lu, J.; Ren, H.; Cui, X.; Hu, J.; Chen, Z.; Zhang, H. Analysis on chemical constituents in Ziziphi spinosae Semen by UPLC-Q-Exactive Focus-MS/MS and network pharmacology study on anti-Alzheimer’s disease mechanism. Drug Eval. Res. 2023, 46, 2563–2579. [Google Scholar]
- Liu, J.; Wei, J.; Wu, J.; Du, C.; Yan, Y. Identification of Chemical Constituents in Suanzaoren Tang Granules by UPLC-Q-TOF-MS/MS. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 1–12. [Google Scholar]
- Ren, T.; Li, Z.; Lian, J.; Su, M.; Cheng, S.; Nie, Z.; Liu, P.; Shi, J. Determination of components of 12 species of Ziziphi spinosi Semen before and after processing by UPLC-MS. West. China J. Pharm. Sci. 2023, 38, 300–305. [Google Scholar]
- Shi, Y.; Nan, Y.; Zheng, W.; Yao, L.; Lian, H.; Chen, X.; Song, J.; Zhang, J.; Jia, D.; Wang, Q.; et al. Qualitative and semiquantitative analyses of the chemical components of the seed coat and kernel of Ziziphi spinosae Semen. Chin. J. Chromatogr. 2024, 42, 234–244. [Google Scholar] [CrossRef]
- Li, Y.; Cao, L.; Chen, Z. Comparison of chemical compositions before and after charcoal-frying of Scutellariae Radix based on HPLC fingerprint and UPLC-Q Exactive Focus MS/MS. Chin. Tradit. Herb. Drugs 2024, 55, 8425–8434. [Google Scholar]
- Sun, J.; He, S. Identification of metabolites of (+)-catechin in rat liver microsomes based on UPLC-LTQ-Orbitrap and multiple mass defect filter method. Chin. J. Hosp. Pharm. 2016, 36, 1264–1268. [Google Scholar]
- Li, Z.; Du, H.; Xie, Y.; Heng, Y.; Duan, H.; Pei, X.; Yan, Y.; Du, C. Prediction and analysis of Q-Markers of fried Ziziphi spinosae Semen pieces based on multivariate statistical analysis and network pharmacology. Chin. Tradit. Herb. Drugs 2021, 52, 4811–4824. [Google Scholar]
- Li, X.; Chen, Q.; Chen, C.; Zhang, T.; Xu, L.; Zheng, P.; Yang, P. Pharmacokinetic Studies of Total Flavonoids of Desmodium Styracifolium After Oral Administration of Different Doses in SD Rats by HPLC-QTRAP-MS/MS. Chin. Pharm. J. 2021, 56, 1810–1817. [Google Scholar]
- Huang, X.; Mao, Y.; Li, H.; Wang, H.; Liu, Y. Difference in Chemical Constituents of Ziziphi spinosae Semen from Different Producing Areas by UHPLC-LTQ-Qrbitrap-Ms-based Metabolomics. Mod. Chin. Med. 2021, 23, 2077–2087. [Google Scholar]
- Yan, Y.; Du, C.; Feng, H.; Qi, J.; Qin, X. Integrated strategy of UHPLC-Q Exactive Orbitrap-HRMS and HPLC-CL to study with constituents in Ziziphi spinosae Folium. Chin. Tradit. Herb. Drugs 2016, 47, 3109–3114. [Google Scholar]
- Guo, X.; Li, H.; Feng, H.; Qi, H.; Zhang, L.; Xu, W.; Wu, Y.; Wang, C.; Liang, X. Quality analysis of Ziziphi spinosae Semen extracts based on high-performance liquid chromatography quantitative fingerprint and ultra-high performance liquid chromatography-tandem mass spectrometry quantification. Chin. J. Chromatogr. 2021, 39, 989–997. [Google Scholar] [CrossRef]
- Ye, J.; Yang, M.; Yang, X.; Zhang, H.; Zan, L. Analysis of chemical constituents in Ziziphus jujuba var. spinosa folium by UPLC-QTOF-MS. Nat. Prod. Res. Dev. 2019, 31, 1183–1191. [Google Scholar]
- Liu, J.; Long, K.; Ma, Z.; Du, X.; Zhang, H. Analysis of chemical constituents of Yifei jiedu Granules by UHPLC-Q Exactive Focus MS/MS. Chin. Pharm. J. 2023, 58, 1940–1954. [Google Scholar]
- Cai, Z.; Hu, Y.; Liu, W.; Wang, S.; Kong, X.; Yang, Y.; Qian, M.; Cao, L.; Wang, Z. Analysis of chemical components of Yin-Qiao-Qing-Re Tablets by UPLC-Q-TOF-MS/MS and GC-MS. J. Nanjing Univ. Tradit. Chin. Med. 2025, 1198–1212. [Google Scholar] [CrossRef]
- Ma, Z.; Sang, S.; Duo, J. Content Determination of 6 Kinds of Triterpene Acid in Tibetan Medicine Rubus biflorus by Pre-column Derivatization HPLC/FLD APCI/MS. China Pharm. 2019, 30, 2243–2247. [Google Scholar]
- Yan, Y.; Chu, Y.; Duan, H.; Wang, H.; Qin, X.; Du, C. Study on the tissue distribution of eight effective components of Ziziphi spinosae Semen aqueous extract by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Acta Pharm. Sin. 2023, 58, 740–749. [Google Scholar] [CrossRef]
- Kang, Q.; Li, Z.; Fan, S.; Rong, R.; Jiang, H.; Jiang, X.; Zhang, J.; Gong, L. Qualitative Analysis on Perilla frutescens Leaves and Stalks by UPLC-Q-Exactive-Orbitrap-MS. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 156–162. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Xie, J.; Wang, Q.; Qi, W. Protective Effect of 6’’’-Feruloylspinosin on Aβ1-42-Induced SH-SY5Y Cells Injury. Sci. Technol. Food Ind. 2022, 43, 373–380. [Google Scholar]
- Guo, S.; Duan, J.; Li, Y.; Wang, Y.; Yan, H.; Qian, D.; Tang, Y.; Su, S. Comparison of the Bioactive Components in Two Seeds of Ziziphus Species by Different Analytical Approaches Combined with Chemometrics. Front. Pharmacol. 2017, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Bian, T.; Chen, J.; Liang, H.; Xu, M.; Wei, X.; Fu, K.; Li, X. Research progress on Ziziphi spinosae Semen and their developmentand application in prevention and treatment of insomnia. Chin. Tradit. Herb. Drugs 2025, 56, 6823–6832. [Google Scholar]
- Turan, E.; Gules, O.; Kilimci, F.S.; Kara, M.E.; Dilek, O.G.; Sabanci, S.S.; Tatar, M. The mixture of liquid foam soap, ethanol and citric acid as a new fixative–preservative solution in veterinary anatomy. Ann. Anat.-Anat. Anz. 2017, 209, 11–17. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, F.; Ramírez-Rodrigues, M.M. Influence of time-temperature in the antioxidant activity, anthocyanin and polyphenols profile, and color of Ardisia compressa K. extracts, with the addition of sucrose or citric acid. Food Chem. 2024, 440, 138181. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Q.; Sui, Y.; Wang, Y.; Qiu, X. Rutin protects endothelial dysfunction by disturbing Nox4 and ROS-sensitive NLRP3 inflammasome. Biomed. Pharmacother. 2017, 86, 32–40. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Zhang, X.; Fang, Q.; Xu, Y.; Wang, H. Rutin alleviates Pb-induced oxidative stress, inflammation and cell death via activating Nrf2/ARE system in SH-SY5Y cells. NeuroToxicology 2024, 104, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Niu, Z.; Mei, D.; Zhang, B. Mechanisms and Protective Effect of lsochlorogenic Acid B on Liver Injury in Mice. Her. Med. 2020, 39, 895–899. [Google Scholar]




| No. | tR (min) | MS1 1 (m/z) | Molecular Formula | Error ppm | Source | MS2 2 (m/z) | Identification | Type | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seed | Pulp | Leaf | |||||||||
| 1 | 0.32 | 118.0863 [M+H]+ | C5H11NO2 | 0.25 | + | + | + | 72.0443, 59.0732 [M+H−COOCH3]+, 58.0662 | Valine | Amino Acids | [22] |
| 2 | 0.45 | 117.0192 [M−H]− | C4H6O4 | −1.37 | + | + | + | 99.9256, 73.0294 [M−H−CO2]− | Succinic acid | Organic Acids | [23] |
| 3 | 0.72 | 179.0558 [M−H]− | C6H12O6 | −1.74 | + | + | + | 161.0452 [M−H−H2O]−, 89.0243 | D-Mannitol | Carbohydrate | [24] |
| 4 | 0.79 | 138.0550 [M+H]+ | C7H7NO2 | 0.14 | + | − | − | 94.0653 [M−H−C2H4O]− | Trigonelline | Alkaloids | [24] |
| 5 | 0.80 | 341.1088 [M−H]− | C12H22O11 | −0.38 | + | − | − | 179.0559, 119.0348, 89.0243 | Trehalose | Carbohydrate | [24] |
| 6 | 0.91 | 104.1071 [M+H]+ | C5H13NO | 0.86 | + | + | − | 60.0808 [M−H−C2H4O]−, 58.0661 | Choline hydroxide | Alkaloids | [25] |
| 7 | 1.24 | 118.0863 [M+H]+ | C5H11NO2 | 0.08 | + | + | + | 59.0731 [M+H−COOCH3]+ | Betaine | Alkaloids | [26] |
| 8 | 1.28 | 132.1020 [M+H]+ | C6H13NO2 | 0.38 | + | + | + | 86.0965 [M+H−HCOOH]+, 69.0610 [M+H−HCOOH-NH3]+ | Isoleucine | Amino Acids | [27] |
| 9 | 1.32 | 148.0605 [M+H]+ | C5H9NO4 | 0.27 | + | − | − | 130.0499 [M+H−H2O]+, 102.0549, 84.0444 | L-Glutamic acid | Amino Acids | [24] |
| 10 | 1.36 | 279.2308 [M−H]− | C18H32O2 | −7.70 | + | − | − | 261.3580 [M−H−H2O]−, 259.0010, 243.0622 [M−H−2H2O]− | Linoleic acid | Fatty Acids | [28] |
| 11 | 1.42 | 243.0621 [M−H]− | C9H12N2O6 | −0.70 | + | + | + | 200.0562, 110.0246, 82.0294 | Uridine | Nucleotides | [24] |
| 12 | 1.69 | 128.0352 [M−H]− | C5H7NO3 | −0.70 | + | − | + | 82.0297 | L-Pyroglutamic acid | - | [25] |
| 13 | 1.71 | 191.0558 [M−H]− | C7H12O6 | −1.83 | + | − | + | 173.0088, 85.0294 | Quinic acid | Organic Acids | [27] |
| 14 | 1.73 | 205.0353 [M−H]− | C7H10O7 | −0.59 | − | + | − | 143.0349 [M−H−CH3C(OH)2]−, 87.0085 [M−H−CH3C(OH)2−C3H3O]− | Methylcitrate | Organic Acids | [29] |
| 15 | 1.84 | 219.0511 [M−H]− | C8H12O7 | 0.23 | − | + | − | 111.0092 [M−H−CH3COOCH2CH3−H2O]−, 87.0088 | Triethyl citrate | Organic Acids | [29] |
| 16 | 1.90 | 175.1190 [M+H]+ | C6H14N4O2 | 0.06 | + | − | − | 130.0978 [M+H−OCOH]+, 116.0705 [M+H−COOCH3]+, 70.0651, 60.0556 | Arginine | Amino Acids | [27] |
| 17 | 1.93 | 132.0301 [M−H]− | C4H7NO4 | −1.06 | + | − | − | 115.0035 [M−H−OH]−, 71.0138 | L-Aspartic acid | Amino Acids | [23] |
| 18 | 1.93 | 132.1020 [M+H]+ | C6H13NO2 | 0.38 | + | + | + | 86.0965, 69.0700 | Leucine | Amino Acids | [23] |
| 19 | 2.10 | 153.0192 [M−H]− | C7H6O4 | −0.59 | + | − | − | 109.0297 [M−H−CO2]−, 108.9152, 91.5104 [M−H−CO2−H2O]−, 81.0345 | Protocatechuic acid | Phenolic Acids | [30] |
| 20 | 2.12 | 127.0390 [M+H]+ | C6H6O3 | −0.08 | + | − | + | 109.0284 [M+H−H2O]+, 81.0334 [M+H−H2O−CO]+, 59.6169 | 5-Hydroxymethylfurfural | Organic Acids | [31] |
| 21 | 2.18 | 115.0036 [M−H]− | C4H4O4 | −1.04 | − | + | + | 71.0137 | Fumaric acid | Organic Acids | [32] |
| 22 | 2.23 | 166.0863 [M+H]+ | C9H11NO2 | 0.18 | + | + | + | 149.0597 [M+H−OH]+, 120.0808, 103.0544 | L-Phenylalanine | Amino Acids | [24] |
| 23 | 2.31 | 191.0194 [M−H]− | C6H8O7 | −1.88 | + | + | + | 130.9985 [M−H−CH3C(OH)2]−, 110.6740 | Citric acid | Organic Acids | [33] |
| 24 | 2.31 | 133.0141 [M−H]− | C4H6O5 | −1.20 | + | + | + | 115.0035 [M−H−H2O]−, 73.0001 [M−H−H2O−CH2CO]−, 71.0137 [M−H−H2O−CO2]− | Malic acid | Organic Acids | [31] |
| 25 | 2.32 | 166.0864 [M+H]+ | C9H11NO2 | 0.60 | + | + | + | 149.0595 [M+H−NH3]+, 120.0808 [M+H−HCOOH]+, 103.0538 [M+H−HCOOH−NH3]+ | Phenylalanine | Amino Acids | [27] |
| 26 | 2.32 | 166.1221 [M+H]+ | C10H15NO | −3.25 | + | − | − | 166.0864, 121.0843 [M+H−OCOH]+, 103.0538 | Hordenine | Alkaloids | [25] |
| 27 | 2.34 | 136.0616 [M+H]+ | C5H5N5 | −1.25 | + | + | + | 119.9505 [M+H−OH]+, 109.9771, 91.9666 | Adenine | Nucleotides | [24] |
| 28 | 2.35 | 169.0140 [M−H]− | C7H6O5 | −1.66 | + | + | + | 168.8887, 124.6000 [M−H−COOH]− | Gallic acid | Phenolic Acids | [34] |
| 29 | 2.39 | 111.0088 [M−H]− | C5H4O3 | 0.09 | + | + | + | 67.0189 | 2-Furoic acid | Organic Acids | [25] |
| 30 | 2.41 | 277.2172 [M−H]− | C18H30O2 | −0.29 | − | − | + | 233.1538 [M−H−CO2]− | α-Linolenic acid | Fatty Acids | [33] |
| 31 | 2.51 | 153.0191 [M−H]− | C7H6O4 | −1.70 | + | − | − | 109.0291 [M−H−C2H4O]− | Epythricine | Phenolic Acids | [25] |
| 32 | 2.54 | 145.0496 [M+H]+ | C6H8O4 | 0.14 | + | + | + | 127.0389 [M+H−OH]+, 99.0440, 71.0491 | 5-hydroxy-4-methoxy-5,6-dihydro-2H-pyran-2-One | - | [24] |
| 33 | 2.63 | 268.1040 [M+H]+ | C10H13N5O4 | 0.00 | + | − | + | 136.0618 | Adenosine | Nucleotides | [25] |
| 34 | 2.93 | 116.0706 [M+H]+ | C5H9NO2 | 0.26 | + | + | + | 70.0651 | DL-Proline | Amino Acids | [24] |
| 35 | 2.98 | 137.0243 [M−H]− | C7H6O3 | −1.17 | + | + | + | 93.9087 [M−H−CO2]−, 65.0393 [M−H−CO2−CH2CH2]− | p-Hydroxybenzoic acid | Phenolic Acids | [31] |
| 36 | 3.14 | 167.0344 [M−H]− | C8H8O4 | −3.05 | + | + | + | 152.0120 [M−H−CH3]− | Vanillic acid | Phenolic Acids | [35] |
| 37 | 3.23 | 341.1096 [M−H]− | C12H22O11 | 2.02 | + | − | − | 179.0563, 89.0244 | Sucrose | Carbohydrate | [27] |
| 38 | 3.47 | 368.0988 [M−H]− | C16H19NO9 | 0.24 | + | − | − | 176.0714 [M−H−Glc−CH2O]−, 158.0598 [M−H−Glc−CH2O−H2O]−, 144.0453 [M−H−Glc−CH2O−H2O−CH2]− | 3R-N-glc-3-hydroxy indoleacetic acid | Amino Acids | [36] |
| 39 | 3.87 | 188.0708 [M+H]+ | C11H9NO2 | 1.12 | + | + | + | 170.0597, 146.0600, 118.0655 | Trans-3-Indoleacrylic acid | - | [25] |
| 40 | 3.88 | 153.0191 [M−H]− | C7H6O4 | −1.31 | + | − | − | 135.9736 [M−H−H2O]−, 109.0296 [M−H−CO2]− | Protocatechuic acid | Phenolic Acids | [27] |
| 41 | 4.26 | 370.1133 [M+H]+ | C16H19NO9 | −1.35 | − | − | + | 212.0710, 208.0606 [M+H−Glc]+, 190.0500 [M+H−Glc−H2O]+, 188.0708, 146.0603 [M+H−Glc−H2O−CO2]+ | 3R-1-N-β-D-glucopyranosyl-3-hydroxy-indole-3-acetic acid | Amino Acids | [37] |
| 42 | 4.44 | 116.0706 [M+H]+ | C5H9NO2 | 0.00 | + | − | − | 70.0652 [M+H−CH2CNH]+, 58.6513 [M+H−CH2CNH−H2O]+ | Proline | Amino Acids | [32] |
| 43 | 4.51 | 179.0350 [M−H]− | C9H8O4 | 0.22 | + | + | + | 178.9775, 134.9880 [M−H−CO2]− | Caffeic acid | Phenolic Acids | [38] |
| 44 | 4.57 | 137.0243 [M−H]− | C7H6O3 | −0.73 | + | + | − | 93.0345 [M−H−CO2]− | 4-Hydroxybenzoic acid | Phenolic Acids | [27] |
| 45 | 4.79 | 342.1704 [M]+ | C20H24NO4 | −0.35 | + | + | + | 297.1124 [M−OCOH]+, 265.0879 [M−C6H6]+, 58.0652 | Magnoflorine | Alkaloids | [39] |
| 46 | 4.98 | 137.0243 [M−H]− | C7H6O3 | −0.78 | + | + | − | 108.0216 [M−H-CHO]−, 92.9199 [M−H−COOH]− | Protocatechualdehyde | Phenolic Acids | [31] |
| 47 | 5.11 | 352.1038 [M−H]− | C16H19NO8 | 0.03 | + | + | + | 188.0714 [M−H−Glc]−, 146.0609 [M−H−Glc−CH2CNH]− | N-glc-indoleacetic acid | Amino Acids | [36] |
| 48 | 5.33 | 181.0503 [M−H]− | C9H10O4 | −1.71 | + | + | + | 166.0267 [M−H−CH3]−, 151.0400 [M−H−CH2O]− | Syringic acid | Phenolic Acids | [40] |
| 49 | 5.53 | 314.1752 [M]+ | C19H24NO3 | −1.21 | + | − | − | 314.2743, 269.1175 [M−OCOH]+, 175.0759 [M−OCOH−C6H5O]+, 127.0917 [M−OCOH-C6H5O−NO−H2O]+, 107.0491 | Magnocurarine | Alkaloids | [39] |
| 50 | 5.77 | 447.1892 [M]+ | C23H29NO8 | −0.16 | − | − | + | 174.9562 [M−Glc−NO−C6H7]+ | 6-glc-Coclaurine | - | [33] |
| 51 | 5.98 | 353.0879 [M−H]− | C16H18O9 | 0.20 | + | + | + | 352.1035, 191.0560 [M−H−CA]−, 173.0093 [M−H−CA−H2O]−, 161.0243 [M−H−QA]−, 79.0560 | Chlorogenic acid | Phenolic Acids | [39] |
| 52 | 6.05 | 289.0719 [M−H]− | C15H14O6 | 0.38 | + | + | + | 271.0603 [M−H−H2O]−, 245.0817 [M−H−CO2]−, 231.0302 [M−H−COOCH3]−, 221.0813 [M−H−CO2−H2O]−, 205.0504, 203.0714, 187.0400 [M−H−COOCH3−CO2]−, 161.0603 [M−H−H2O−C6H6O2]−, 125.0242, 109.0295 [M−H−H2O−Glc]−, 97.0293 | (+)-Catechin/(−)-Epicatechin | Flavonoids | [41] |
| 53 | 6.10 | 353.0874 [M−H]− | C16H18O9 | −1.25 | + | − | + | 335.1316 [M−H−H2O]− | Cryptochlorogenic acid | Phenolic Acids | [39] |
| 54 | 6.10 | 353.0874 [M−H]− | C16H18O9 | −1.25 | − | − | + | 352.2861, 335.1330 [M−H−H2O]−, 135.5516 [M−H−CO2]− | Neochlorogenic acid | Phenolic Acids | [39] |
| 55 | 6.15 | 344.1852 [M]+ | C20H26NO4 | −2.99 | − | + | + | 299.1273 [M−COOH]+, 175.0749, 137.0599 [M−COOH−Glc]+ | Tembetarin | Alkaloids | [39] |
| 56 | 6.45 | 127.0390 [M+H]+ | C6H6O3 | 0.31 | + | + | + | 99.0441 [M+H−CO]+, 69.0334 | Phloroglucinol | Phenolic Acids | [27] |
| 57 | 6.49 | 286.1438 [M+H]+ | C17H19NO3 | 0.00 | + | + | + | 269.1173 [M+H−NH3]+, 237.0910 [M+H−H2O]+, 209.0952 [M+H−H2O−CO]+, 175.0753 [M+H−NH3−C6H6O]+, 107.0491 | Coclaurine | Alkaloids | [42] |
| 58 | 6.82 | 463.0881 [M−H]− | C21H20O12 | −0.26 | + | + | + | 301.0290 [M−H−Glc]−, 271.0321 [M−H−Glc−H2O]− | Quercetin-3-O-glucoside | Flavonoids | [38] |
| 59 | 7.47 | 282.1128 [M + H]+ | C17H15NO3 | 1.17 | + | − | − | 282.2211, 188.0713 [M+H−H2O−NO−OCOH]+ | Juzirine | Alkaloids | [42] |
| 60 | 7.55 | 220.0615 [M−H]− | C11H11NO4 | −0.36 | − | + | − | 176.0718 [M−H−C2H4O]−, 69.0813 | 6-Hydroxy indole lactic acid | Amino Acids | [31] |
| 61 | 8.25 | 433.1143 [M−H]− | C21H22O10 | 0.55 | − | − | + | 271.0618, 119.0349 | Prunin | Flavonoids | [32] |
| 62 | 8.25 | 193.0505 [M−H]− | C10H10O4 | −0.78 | − | + | + | 192.9289, 133.1016 [M−H−COOCH3]− | Ferulic Acid | Phenolic Acids | [34] |
| 63 | 8.45 | 465.1013 [M+H]+ | C21H20O12 | −3.2 | − | + | + | 303.0497 [M+H−Glc]+, 283.0301 [M+H−Glc−H2O]+, 85.0283 | Hyperin | Flavonoids | [31] |
| 64 | 8.52 | 449.1092 [M−H]− | C21H22O11 | 0.51 | − | + | − | 287.0562 [M−H−Glu]−, 269.0455 [M−H−Glu−H2O]−, 259.0614 [M−H−Glu−H2O−CH2O]−, 125.0247 | Hovetrichoside C | Flavonoids | [37] |
| 65 | 8.82 | 449.1083 [M+H]+ | C21H20O11 | 1.07 | − | + | + | 303.0516 [M+H−Rha]+, 287.0549 [M+H−Glc]+, 85.0283, 71.0495 | Quercetin-3-O-rjamnoside | Flavonoids | [31] |
| 66 | 9.43 | 447.0926 [M−H]− | C21H20O11 | −1.61 | − | − | + | 446.1139, 283.0261 [M−H−Glu]−, 145.0291 | Astragalin | Flavonoids | [38] |
| 67 | 9.56 | 268.1332 [M+H]+ | C17H17NO2 | −0.07 | + | + | + | 251.1066 [M+H−H2O]+, 236.0833 [M+H−CH3OH]+, 219.0804, 191.0869 [M+H−H2O−CH3COOH]+ | Caaverine | Alkaloids | [37] |
| 68 | 10.03 | 193.0495 [M+H]+ | C10H8O4 | −0.10 | − | + | − | 178.0264, 133.0285 [M+H−CH3COOH]+ | Scopoletin | - | [31] |
| 69 | 10.37 | 593.1508 [M−H]− | C27H30O15 | −0.72 | + | − | − | 559.1457, 533.1267 [M−H−CH3COOH]−, 503.1179, 473.1088 [M−H−2CH3COOH]−, 457.1128, 395.0818, 383.0748, 379.0808, 353.0658, 283.0651 | Vicenin II | Flavonoids | [43] |
| 70 | 10.44 | 515.1217 [M−H]− | C25H24O12 | 4.27 | − | − | + | 515.2872, 353.0975 [M−H−Glu]− | Isochlorogenic acid B | - | [34] |
| 71 | 10.83 | 137.0242 [M−H]− | C7H6O3 | −1.31 | + | + | − | 109.0295, 93.0347 [M−H−CO2]− | Salicylic acid | Phenolic Acids | [40] |
| 72 | 11.36 | 1091.5641 [M−H]− | C53H88O23 | −0.25 | − | − | + | 1073.5580 [M−H−H2O]−, 929.5077 [M−H−Glu]− | 3-O-β-D-glucopyranosyl-3β, 20S, 23S, 30-tetrahydroxydammar-24-en-16-on-3-O-β-D-Glucopyranosyl-(1→3)-[α-Lrhamnopyranosyl-(1→2)]-α-Larabinopyranoside | Flavonoids | [44] |
| 73 | 12.45 | 919.2495 [M−H]− | C42H48O23 | −2.01 | + | − | − | 757.1979 [M−H−Glc]−, 427.1057 | 6‴-(4‴-O-Glc)-Vanilloyspinosin | Flavonoids | [44] |
| 74 | 12.52 | 595.1611 [M+H]+ | C27H30O15 | −7.85 | + | − | − | 433.1130 [M+H−Glu]+, 415.1009 [M+H−Glu−H2O]+, 367.0828, 337.0708, 313.0707, 283.0651 | Isovitexin-2″-O-β-D-glucopyranoside | Flavonoids | [33] |
| 75 | 12.61 | 595.1635 [M+H]+ | C27H30O15 | −3.85 | + | − | − | 433.1130 [M+H−Glu]+, 415.1022 [M+H−Glu−H2O]+, 397.0919 [M+H−Glu−2H2O]+, 379.0805 [M+H−Glu−3H2O]+, 367.0814, 337.0709, 313.0706, 283.0599 | Meloside A | Flavonoids | [39] |
| 76 | 12.61 | 595.1635 [M+H]+ | C27H30O15 | −3.85 | + | + | + | 287.0567 [M+H−Rha−Glc]+, 285.0402, 284.0322, 85.0286 | Kaempferol-3-O-rutinoside | Flavonoids | [45] |
| 77 | 12.73 | 609.1816 [M+H]+ | C28H32O15 | 0.41 | + | − | − | 447.1283 [M+H−Glu]+, 429.1180 [M+H−Glu−H2O]+, 411.1071 [M+H−Glu−2H2O]+, 393.0971, 351.0866 [M+H−Glu−H2O−C2H6O3]+, 327.0863 [M+H−Glu−C4H8O4]+, 297.0757 [M+H−Glu−C5H8O5]+, 285.0748 [M+H−2−Glu]+, 85.0286 | Isospinosin | Flavonoids | [39] |
| 78 | 12.77 | 433.1147 [M+H]+ | C21H20O10 | 4.16 | + | − | − | 415.1037 [M+H−H2O]+, 397.0919 [M+H−2H2O]+, 379.0822 [M+H−3H2O]+, 367.0814, 337.0712 [M+H−2H2O−CH3COOH]+, 313.0708 [M+H−2CH3COOH]+, 283.0613 | Vitexin | Flavonoids | [37] |
| 79 | 12.82 | 725.1926 [M−H]− | C32H38O19 | −1.13 | + | + | + | 593.1478 [M−H−Glu]−, 575.1392 [M-H−Glu−H2O]−, 285.0404, 284.0328 | Camellianin B | Flavonoids | [37] |
| 80 | 12.83 | 282.1489 [M+H]+ | C18H19NO2 | 0.04 | + | + | − | 282.1489, 266.1258, 265.1223 [M+H−H2O]+, 250.0991 [M+H−CH3OH]+, 234.1039, 191.1497 | N-nornuciferine | Alkaloids | [37] |
| 81 | 12.83 | 609.1820 [M+H]+ | C28H32O15 | 0.92 | + | − | − | 489.1374, 447.1283 [M+H−Glu]+, 429.1184 [M+H−Glu−H2O]+, 411.1076 [M+H−Glu−2H2O]+, 393.0975, 381.0971, 351.0868 [M+H−Glu−H2O−C2H6O3]+, 327.0866 [M+H−Glu−C4H8O4]+, 297.0766 [M+H−Glu−C5H8O5]+, 285.0757 [M+H−2−Glu]+ | Spinosin | Flavonoids | [37] |
| 82 | 12.87 | 433.1137 [M+H]+ | C21H20O10 | 1.89 | + | − | − | 415.1037 [M+H−H2O]+, 397.0919 [M+H−2H2O]+, 379.0839 [M+H−3H2O]+, 367.0814, 337.0705 [M+H−2H2O−CH3COOH]+, 313.0714 [M+H−2CH3COOH]+, 283.0610 | Isovitexin | Flavonoids | [37] |
| 83 | 12.89 | 447.1284 [M+H]+ | C22H22O10 | −0.40 | + | + | − | 429.1183 [M+H−H2O]+, 411.1060 [M+H−2H2O]+, 393.0972 [M+H−3H2O]+, 351.0856, 327.0875 [M+H−2CH3COOH]+, 297.0762 [M+H−2CH3COOH−CO]+, 281.0668 [M+H−2CH3COOH−CO−CH3]+ | Swertisin | Flavonoids | [37] |
| 84 | 12.90 | 607.1665 [M−H]− | C28H32O15 | −0.56 | + | − | − | 299.0244 [M−H−Rha−Glc]− | Diosmin | Flavonoids | [27] |
| 85 | 13.03 | 609.1821 [M+H]+ | C28H32O15 | 1.12 | + | − | − | 489.1410 [M+H−2CH3COOH]+, 447.1286 [M+H−Glu]+, 429.1185 [M+H−Glu−H2O]+, 327.0861 | Genistein-7-O-glucuronide | Flavonoids | [33] |
| 86 | 13.09 | 609.1468 [M−H]− | C27H30O16 | 1.13 | + | + | + | 301.0349 [M−H−Glc−Rha]−, 178.9975 | Rutin | Flavonoids | [45] |
| 87 | 13.11 | 485.3113 [M+H]+ | C27H40N4O4 | −2.02 | + | − | + | 115.1312, 114.1278 | Mucronine J | Alkaloids | [42] |
| 88 | 13.23 | 795.4533 [M−H]− | C42H68O14 | −0.39 | − | + | + | 749.4515 [M−H−COOH]− | Jujubasaponin VI | Terpenoids | [44] |
| 89 | 13.24 | 759.2126 [M+H]+ | C36H38O18 | −0.66 | + | + | + | 639.1703 [M+H−2CH3COOH]+, 429.1180, 381.0974, | 6‴-vanilloylspinosin | Flavonoids | [33] |
| 90 | 13.26 | 771.2130 [M+H]+ | C37H38O18 | −0.10 | + | − | + | 651.1766 [M+H−2CH3COOH]+, 609.1599 [M+H−Glu]+, 337.0719, 145.0282 | Isovitexin-2″-O-(6-feruloyl)-glucopyranoside | Flavonoids | [33] |
| 91 | 13.28 | 729.2051 [M+H]+ | C35H36O17 | 3.51 | + | − | − | 609.1555 [M+H−2CH3COOH]+, 447.1273, 429.1181 | 6‴-O-p-Hydroxybenzoylspinosin | Flavonoids | [33] |
| 92 | 13.28 | 944.2814 [M+H]+ | C44H49NO22 | −0.49 | + | − | − | 824.2395 [M+H−CH3COOH]+, 327.0864, 297.0758 | 6‴-O-(3-Glc-indole-acetyl)spinosin | Alkaloids | [39] |
| 93 | 13.28 | 945.2865 [M+H]+ | C44H50NO22 | −3.41 | + | − | − | 824.2395 [M+H−Glu]+, 429.1182, 285.0759 | 6‴-O-(3-Indoleglucoside-Acetyl)spinosin | Alkaloids | [33] |
| 94 | 13.30 | 741.1977 [M+H]+ | C36H36O17 | −6.57 | + | − | − | 433.11353 [M+H−Glu]+, 337.07095, 313.07071, 147.04408 | Isovitexin-2′’-O-(6-p-Coumaroyl)Glucopyranoside | Flavonoids | [42] |
| 95 | 13.36 | 815.2410 [M+H]+ | C39H42O19 | 2.05 | + | − | − | 695.2031, 609.1816 [M+H−sinapoyl]+, 447.1293, 429.1179 [M+H−sinapoyl−Glc−H2O]+, 411.1063 [M+H−siapoyl−Glc−2H2O]+, 393.0981 [M+H−sinapoyl−Glc−3H2O]+, 351.0850, 327.0864, 297.0758, 207.0652 | 6‴-Sinapoylspinosin | Flavonoids | [39] |
| 96 | 13.42 | 755.2183 [M+H]+ | C37H38O17 | 0.20 | + | − | − | 635.1754 [M+H−2CH3COOH]+, 447.1288, 327.0865 | 6‴-p-Coumaroylspinosin | Flavonoids | [44] |
| 97 | 13.42 | 785.2286 [M+H]+ | C38H40O18 | −0.13 | + | − | − | 665.1857 [M+H−2CH3COOH]+, 605.1653, 489.1408 | 6‴-Feruloylspinosin | Flavonoids | [36] |
| 98 | 13.44 | 873.3129 [M+H]+ | C43H52O19 | −5.29 | + | − | − | 855.3099 [M+H−H2O]+, 735.2687 [M+H−H2O−2CH3COOH]+, 447.1285 | 6‴-Dihydrophaseoylspinosin | Flavonoids | [36] |
| 99 | 13.51 | 785.2272 [M+H]+ | C38H40O18 | −2.00 | + | − | − | 665.1873 [M+H−2CH3COOH]+, 447.1285, 177.0548 | 6‴-Feruloylisospinosin | Flavonoids | [36] |
| 100 | 13.78 | 869.2864 [M−H]− | C43H50O19 | −1.05 | + | − | − | 839.2766 [M−H−CH2O]−, 607.1705, 427.1036 | 6‴-(-)-Phaseoylspinosin | Flavonoids | [33] |
| 101 | 13.95 | 961.2761 [M−H]− | C48H50O21 | −1.10 | + | − | − | 943.2663 [M−H−H2O]−, 931.2672 [M−H−2CH3]− | 6″-p-coumaroyl-6‴-sinapoylspinosin | Flavonoids | [33] |
| 102 | 14.00 | 535.3284 [M+H]+ | C31H42N4O4 | 1.03 | + | − | − | 149.1153, 148.1122 | Sanjoinine A | Alkaloids | [42] |
| 103 | 14.00 | 1121.3337 [M+H]+ | C54H58NO25 | −2.96 | + | + | + | 1102.3140 [M+H−H2O]+, 940.2723 [M+H−Glc]+, 327.0863 | 6″-O-(3-glc-indole-acetyl)-6‴-feruloylspinosin | Alkaloids | [46] |
| 104 | 14.10 | 632.3815 [M+H]+ | C36H49N5O5 | 1.41 | + | − | + | 344.1968, 289.1912, 148.1121 | Amphibin D | Alkaloids | [37] |
| 105 | 14.54 | 301.0354 [M−H]− | C15H10O7 | −0.03 | + | + | + | 301.2016 [M−H]−, 300.0259, 273.0399 [M−H−CO]−, 257.1294 [M−H−CO−H2O]−, 178.0036 | Quercetin | Flavonoids | [38] |
| 106 | 14.43 | 1205.5936 [M−H]− | C58H94O26 | −0.57 | + | + | + | 1055.3833 [M−H−Rha−H2O]−, 911.3578 [M−H−Rha−Glc]−, 749.2642 [M−H−Rha−2Glc]−, 603.2457 [M−H−Rha−2Glc−Xyl]−, 455.2535 | Jujuboside A | Terpenoids | [33] |
| 107 | 14.56 | 933.2449 [M−H]− | C46H46O21 | −1.02 | + | − | − | 783.2073, 663.1733, 577.1345, 235.0812 | 6″-feruloyl-6‴-vanillylspinosin | Flavonoids | [44] |
| 108 | 14.80 | 484.3199 [M−H]− | C30H45O5 | 0.97 | − | + | + | 423.3266 [M−H−CH3C(OH)2]− | Epiceanothic acid | Terpenoids | [47] |
| 109 | 14.98 | 285.0406 [M−H]− | C15H10O6 | 0.46 | − | + | + | 284.1950, 151.0036 [M−H−C4H4O4−H2O]− | Kaempferol | Flavonoids | [34] |
| 110 | 15.20 | 455.3519 [M+H]+ | C30H46O3 | −0.15 | + | + | + | 409.3472, 189.1637 | Betulonic acid | Terpenoids | [48] |
| 111 | 15.24 | 391.2837 [M+H]+ | C24H38O4 | −0.46 | + | − | − | 167.1049, 149.1322 | Bis(2-ethylhexyl) phthalate | - | [24] |
| 112 | 15.27 | 959.2600 [M−H]− | C48H48O21 | −1.58 | + | − | − | 783.2116 [M−H−Glu]− | 6″,6‴-diferuloylspinosin | Flavonoids | [44] |
| 113 | 15.49 | 385.1633 [M+H]+ | C22H24O6 | −3.27 | − | + | − | 285.0781 | Schisandrin C | - | [33] |
| 114 | 15.53 | 329.2331 [M−H]− | C18H34O5 | −0.70 | + | + | + | 229.1440, 211.1340 | 9,12,13-Trihydroxy-11-octadecenoic acid | Fatty Acids | [49] |
| 115 | 15.54 | 473.3625 [M+H]+ | C30H48O4 | −0.11 | + | + | + | 455.3545 [M+H−H20]+, 437.3425 [M+H−2H20]+, 409.3479, 391.2832 | Corosolic acid | Terpenoids | [50] |
| 116 | 15.84 | 1043.5425 [M−H]− | C52H84O21 | −0.72 | + | + | + | 911.5001 [M−H−Xyl]−, 893.4887, 749.4475 [M−H−Xyl−Glu]−, 603.3878 [M−H−Xyl−Glu−Xyl]− | Jujuboside B | Terpenoids | [51] |
| 117 | 16.18 | 254.2246 [M−H]− | C16H31O2 | −1.97 | − | + | − | 255.0879 | Hexadecanoic acid | Fatty Acids | [47] |
| 118 | 16.20 | 1085.5529 [M−H]− | C54H86O22 | −0.86 | + | − | − | 1043.5425 [M−H−CH2CO]−, 1025.5322 [M−H−CH3COOH]−, 765.4409, 749.4477 | Acetyl-jujuboside B | Terpenoids | [33] |
| 119 | 16.72 | 474.3709 [M+H]+ | C30H49O4 | 1.22 | − | + | + | 473.3645, 409.3476 [M+H−C5H5]+ | 2α-hydroxyursolic acid | Terpenoids | [47] |
| 120 | 16.94 | 501.3225 [M−H]− | C30H46O6 | 0.62 | − | + | + | 501.3222 [M−H]− | 27-Hydroxyceanothic acid | Terpenoids | [47] |
| 121 | 17.37 | 501.3218 [M−H]− | C30H46O6 | −0.76 | + | + | + | 471.3110 [M−H−CH2O]−, 439.3212 [M−H−CH3C(OH)2]− | 24-Hydroxyceanothic acid | Terpenoids | [47] |
| 122 | 17.48 | 485.3272 [M−H]− | C30H46O5 | −0.12 | − | − | + | 423.3313 | Ceanothic acid | Terpenoids | [36] |
| 123 | 17.81 | 313.2382 [M−H]− | C18H34O4 | −0.86 | + | + | − | 183.1384, 129.0920 | (±)9(10)-Dihome | Organic Acids | [24] |
| 124 | 17.93 | 313.2384 [M−H]− | C18H34O4 | −0.19 | + | + | − | 201.1133 | (±)12(14)-Dihome | Organic Acids | [24] |
| 125 | 18.05 | 320.2590 [M+H]+ | C20H33NO2 | 1.94 | + | − | − | 302.2493 [M+H−H2O]+ | Mestanolone | Alkaloids | [25] |
| 126 | 18.30 | 457.3682 [M+H]+ | C30H48O3 | 1.18 | − | − | + | 439.3756 [M+H−H2O]+, 179.1444, 135.1173 | Ursolic acid | Terpenoids | [31] |
| 127 | 18.42 | 485.3272 [M−H]− | C30H46O5 | 0.00 | + | + | + | 467.3186 [M−H−H2O]−, 423.3261, 60.9930 | Emmolic Acid | Terpenoids | [36] |
| 128 | 18.96 | 295.2277 [M−H]− | C18H32O3 | −0.71 | + | + | + | 277.2171 [M−H−H2O]−, 171.1023 | 10E,12Z-octadecadienoic acid | Fatty Acids | [24] |
| 129 | 19.01 | 281.2482 [M−H]− | C18H34O2 | −1.42 | + | + | + | 281.0876 [M−H]− | Oleic acid | Fatty Acids | [47] |
| 130 | 19.07 | 235.1694 [M+H]+ | C15H22O2 | 0.60 | + | + | + | 179.1071 | 3,5-Di-tert-butyl-4-hydroxybenzaldehyde | - | [25] |
| 131 | 19.61 | 469.3321 [M−H]− | C30H46O4 | −0.45 | + | + | + | 451.3235 [M−H−H2O]−, 423.8594 [M−H−CO−H2O]− | Zizyberanalic acid | Terpenoids | [44] |
| 132 | 20.12 | 255.2330 [M−H]− | C16H32O2 | 0.31 | + | − | + | 219.8454 [M−H−2H2O]− | Palmitic acid | Fatty Acids | [52] |
| 133 | 21.00 | 471.3479 [M−H]− | C30H48O4 | −0.23 | − | − | + | 471.3479 [M−H]− | Alphitolic acid | Terpenoids | [39] |
| 134 | 21.27 | 282.2548 [M−H]− | C18H35O2 | −5.77 | − | − | + | 283.2642 | Octadecanoic acid | Fatty Acids | [47] |
| 135 | 21.63 | 338.3418 [M+H]+ | C22H43NO | 0.09 | + | + | + | 233.2274, 163.1481, 97.1012 | Erucamide | Fatty Acids | [24] |
| 136 | 21.85 | 256.2635 [M+H]+ | C16H33NO | 0.00 | + | + | + | 88.0755 | Hexadecanamide | Fatty Acids | [24] |
| 137 | 21.90 | 455.3531 [M−H]− | C30H48O3 | 0.00 | + | + | + | 455.0169, 409.3474 [M−H−COOH]−, 407.5913, 217.1960, 191.1793, 121.1010 | Betulinic acid | Terpenoids | [39] |
| 138 | 22.20 | 282.2792 [M+H]+ | C18H35NO | 0.35 | + | + | − | 265.2527 [M+H−OH]+, 114.0914, 69.0698, 57.0698 | Oleamide | Fatty Acids | [24] |
| 139 | 22.40 | 453.3371 [M−H]− | C30H46O3 | −0.62 | + | + | + | 451.3217, 255.2330, 180.9730 | Betulonic acid | Terpenoids | [36] |
| 140 | 22.96 | 599.3210 [M−H]− | C27H53O12P | 1.32 | − | + | + | 315.0497, 283.2645, 241.0124, 152.9961 | 1-octadecanoyl-sn-glycero-3-phospho-(1′-myo-inositol) | Fatty Acids | [39] |
| 141 | 23.15 | 452.3257 [M−H]− | C30H45O3 | −8.71 | + | − | + | 409.3156 [M−H−CH3CO]− | Oleanolic acid | Terpenoids | [47] |
| 142 | 23.69 | 265.1478 [M−H]− | C12H26O4S | −0.38 | + | + | − | 96.9599 | (dodecyloxy)Sulfonic acid | - | [25] |
| 143 | 24.86 | 595.2882 [M−H]− | C27H49O12P | −1.23 | + | + | + | 595.0358, 279.2332, 241.0115 | 1-(9Z,12Z-octadecadienoyl)-glycero-3-phospho-(1′-myo-inositol) | Fatty Acids | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Zhang, Y.; Zhang, L. Comprehensive Chemical Composition Evaluation of Ziziphus jujuba var. spinosa Germplasm Resources and Selection of Elite Cultivars for Seed, Pulp, and Leaf Utilization. Molecules 2025, 30, 4470. https://doi.org/10.3390/molecules30224470
Song X, Zhang Y, Zhang L. Comprehensive Chemical Composition Evaluation of Ziziphus jujuba var. spinosa Germplasm Resources and Selection of Elite Cultivars for Seed, Pulp, and Leaf Utilization. Molecules. 2025; 30(22):4470. https://doi.org/10.3390/molecules30224470
Chicago/Turabian StyleSong, Xiaochen, Yongqing Zhang, and Longfei Zhang. 2025. "Comprehensive Chemical Composition Evaluation of Ziziphus jujuba var. spinosa Germplasm Resources and Selection of Elite Cultivars for Seed, Pulp, and Leaf Utilization" Molecules 30, no. 22: 4470. https://doi.org/10.3390/molecules30224470
APA StyleSong, X., Zhang, Y., & Zhang, L. (2025). Comprehensive Chemical Composition Evaluation of Ziziphus jujuba var. spinosa Germplasm Resources and Selection of Elite Cultivars for Seed, Pulp, and Leaf Utilization. Molecules, 30(22), 4470. https://doi.org/10.3390/molecules30224470






