Electrochemical Simulation of 25B-NBOMe Phase I Metabolism and Metabolite Profiling by HPLC-QTOF-MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Experimental Conditions of 25B-NBOMe Electrochemical Conversion
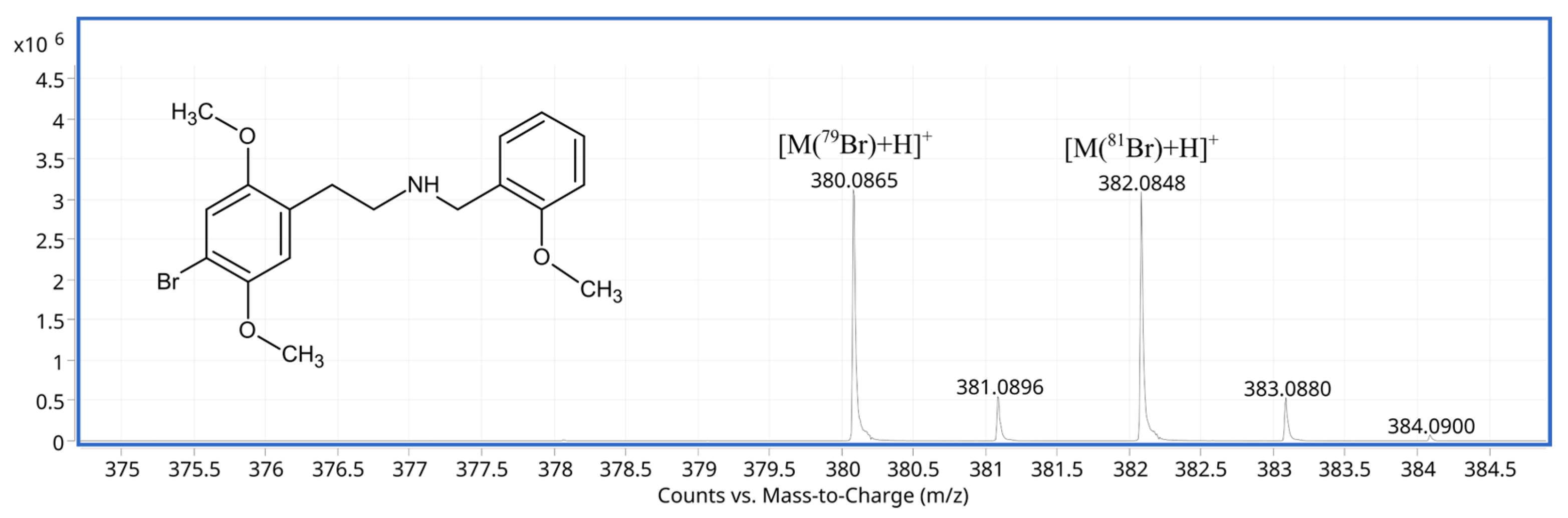
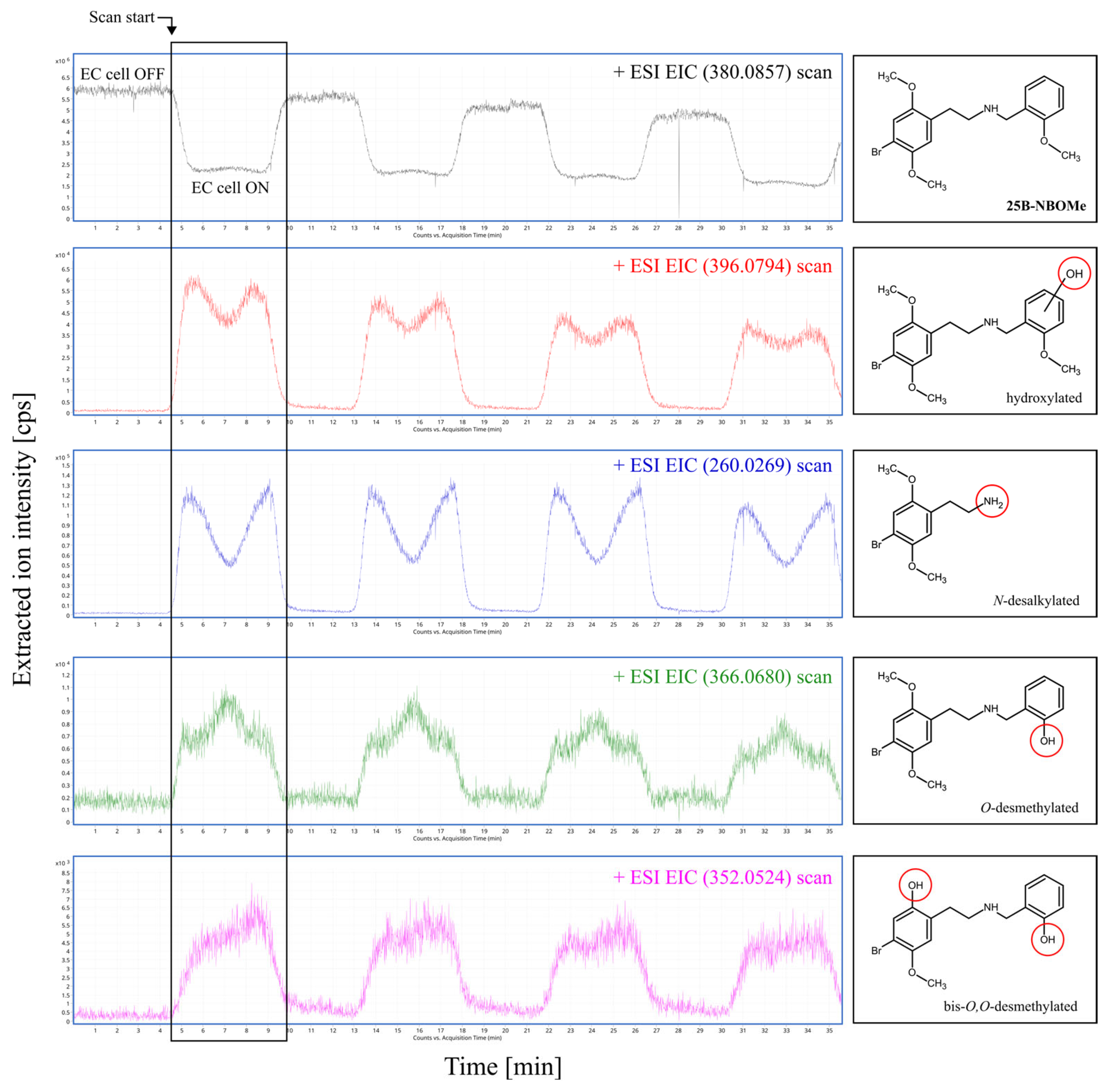
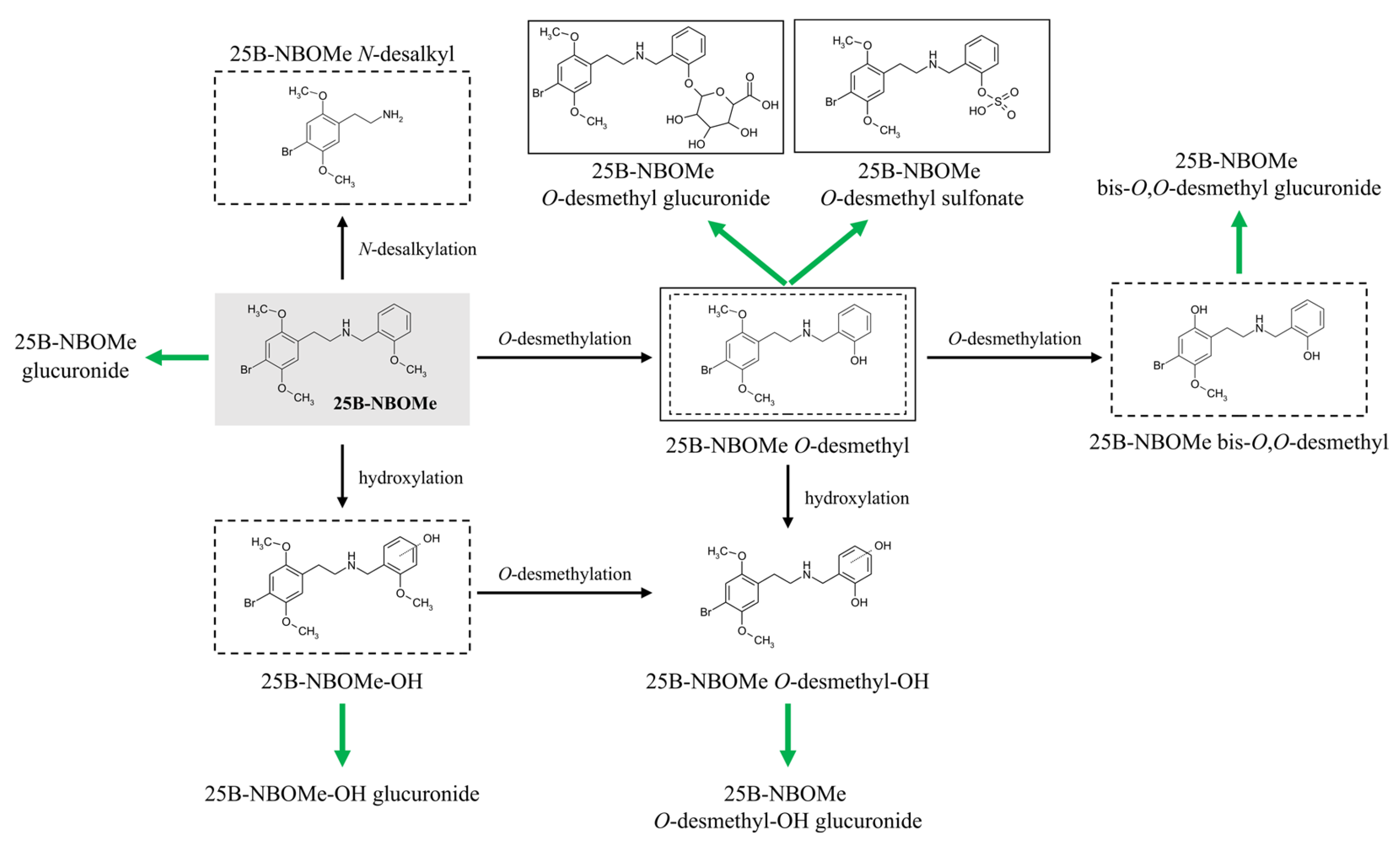
2.2. Results of Electrochemical Simulation of Phase I Metabolism of 25B-NBOMe
2.3. Detection and Tentative Identification of 25B-NBOMe Metabolites in Biological Samples of Severe Intoxications
3. Materials and Methods
3.1. Chemicals and Biological Materials
3.2. Untargeted Analysis
3.2.1. Electrochemical Simulation of Phase I Metabolism of 25B-NBOMe
3.2.2. Screening of 25B-NBOMe Metabolites Present in Human Biological Materials
3.3. General Instrumentation
3.3.1. Mimicking of Phase I Metabolism-EC Conditions
3.3.2. Qualitative Analysis-HPLC-(ESI)-Q-TOF-MS Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations: Office on Drugs and Crime (UNODC). Online World Drug Report 2023—Latest Data and Trend Analysis. 2023. Available online: www.unodc.org/unodc/en/data-and-analysis/wdr-2023-online-segment.html (accessed on 20 September 2025).
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report 2025: Trends and Developments. 2025. Available online: https://www.euda.europa.eu/publications/european-drug-report/2025_en (accessed on 20 September 2025).
- Santos, I.C.; Maia, D.; Dinis-Oliveira, R.J.; Barbosa, D.J. New Psychoactive Substances: Health and Legal Challenges. Psychoactives 2024, 3, 285–302. [Google Scholar] [CrossRef]
- Lawn, W.; Barratt, M.; Williams, M.; Horne, A.; Winstock, A. The NBOMe hallucinogenic drug series: Patterns of use, characteristics of users and self-reported effects in a large international sample. J. Psychopharmacol. 2014, 28, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Al-Imam, A.; AbdulMajeed, B.A. NBOMe Compounds: Systematic Review and Data Crunching of the Surface Web. Glob. J. Health Sci. 2017, 9, 126. [Google Scholar] [CrossRef][Green Version]
- JZawilska, B.; Kacela, M.; Adamowicz, P. NBOMes–Highly Potent and Toxic Alternatives of LSD. Front. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Wolfrum, K.M.; Reed, J.F.; Kim, S.O.; Johnson, R.A.; Janowsky, A. Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors. Biochem. Pharmacol. 2018, 158, 27–34. [Google Scholar] [CrossRef]
- Potts, A.J.; Thomas, S.H.L.; Hill, S.L. Pharmacology and toxicology of N-Benzyl-phenylethylamines (25X-NBOMe) hallucinogens. In Novel Psychoactive Substances; Elsevier: Amsterdam, The Netherlands, 2022; pp. 279–300. [Google Scholar] [CrossRef]
- Wood, D.M.; Sedefov, R.; Cunningham, A.; Dargan, P.I. Prevalence of use and acute toxicity associated with the use of NBOMe drugs. Clin. Toxicol. 2015, 53, 85–92. [Google Scholar] [CrossRef]
- Halberstadt, A.L. Pharmacology and Toxicology of N-Benzylphenethylamine (“NBOMe”) Hallucinogens. In Neuropharmacology of New Psychoactive Substances (NPS); Baumann, M.H., Glennon, R.A., Wiley, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 283–311. [Google Scholar] [CrossRef]
- Lipow, M.; Kaleem, S.; Espiridion, E. NBOMe Toxicity and Fatalities: A Review of the Literature. Transform. Med. 2022, 1, 12–18. [Google Scholar] [CrossRef]
- Caspar, A.T.; Helfer, A.G.; Michely, J.A.; Auwärter, V.; Brandt, S.D.; Meyer, M.R.; Maurer, H.H. Studies on the metabolism and toxicological detection of the new psychoactive designer drug 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe) in human and rat urine using GC-MS, LC-MSn, and LC-HR-MS/MS. Anal. Bioanal. Chem. 2015, 407, 6697–6719. [Google Scholar] [CrossRef]
- Wohlfarth, A.; Roman, M.; Andersson, M.; Kugelberg, F.C.; Diao, X.; Carlier, J.; Eriksson, C.; Wu, X.; Konradsson, P.; Josefsson, M.; et al. 25C-NBOMe and 25I-NBOMe metabolite studies in human hepatocytes, in vivo mouse and human urine with high-resolution mass spectrometry. Drug Test. Anal. 2017, 9, 680–698. [Google Scholar] [CrossRef]
- Matey, J.M.; Zapata, F.; Menéndez-Quintanal, L.M.; Montalvo, G.; García-Ruiz, C. Identification of new psychoactive substances and their metabolites using non-targeted detection with high-resolution mass spectrometry through diagnosing fragment ions/neutral loss analysis. Talanta 2023, 265, 124816. [Google Scholar] [CrossRef] [PubMed]
- Faber, H.; Vogel, M.; Karst, U. Electrochemistry/mass spectrometry as a tool in metabolism studies—A review. Anal. Chim. Acta 2014, 834, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Portychová, L.; Schug, K.A. Instrumentation and applications of electrochemistry coupled to mass spectrometry for studying xenobiotic metabolism: A review. Anal. Chim. Acta 2017, 993, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Temgoua, R.C.T.; Bussy, U.; Alvarez-Dorta, D.; Galland, N.; Njanja, E.; Hémez, J.; Thobie-Gautier, C.; Tonlé, I.K.; Boujtita, M. Electrochemistry-coupled to liquid chromatography-mass spectrometry-density functional theory as a new tool to mimic the environmental degradation of selected phenylurea herbicides. Environ. Sci. Process. Impacts 2021, 23, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Bugrim, A.; Nikolskaya, T.; Nikolsky, Y. Early prediction of drug metabolism and toxicity: Systems biology approach and modeling. Drug Discov. Today 2004, 9, 127–135. [Google Scholar] [CrossRef]
- Asha, S.; Vidyavathi, M. Role of Human Liver Microsomes in In Vitro Metabolism of Drugs—A Review. Appl. Biochem. Biotechnol. 2010, 160, 1699–1722. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, G.; Ding, X.; Lu, C. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm. Sin. B 2012, 2, 549–561. [Google Scholar] [CrossRef]
- Brandon, E.F.A.; Raap, C.D.; Meijerman, I.; Beijnen, J.H.; Schellens, J.H.M. An update on in vitro test methods in human hepatic drug biotransformation research: Pros and cons. Toxicol. Appl. Pharmacol. 2003, 189, 233–246. [Google Scholar] [CrossRef]
- Baumann, A.; Karst, U. Online electrochemistry/mass spectrometry in drug metabolism studies: Principles and applications. Expert Opin. Drug Metab. Toxicol. 2010, 6, 715–731. [Google Scholar] [CrossRef]
- Faber, H.; Lutze, H.; Lareo, P.L.; Frensemeier, L.; Vogel, M.; Schmidt, T.C.; Karst, U. Liquid chromatography/mass spectrometry to study oxidative degradation of environmentally relevant pharmaceuticals by electrochemistry and ozonation. J. Chromatogr. A 2014, 1343, 152–159. [Google Scholar] [CrossRef]
- Nikzad, N.; Rafiee, M. Electrochemical study of drug metabolism. Curr. Opin. Electrochem. 2024, 44, 101446. [Google Scholar] [CrossRef]
- Electrochemistry and LC–MS for Metabolite Generation and Identification: Tools, Technologies and Trends. Available online: https://www.chromatographyonline.com/view/electrochemistry-and-lc-ms-metabolite-generation-and-identification-tools-technologies-and-trends-1 (accessed on 4 October 2025).
- Orhan, H.; Vermeulen, N.P.E. Conventional and Novel Approaches in Generating and Characterization of Reactive Intermediates from Drugs/Drug Candidates. Curr. Drug Metab. 2011, 12, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.; Lohmann, W.; Schubert, B.; Oberacher, H.; Karst, U. Metabolic studies of tetrazepam based on electrochemical simulation in comparison to in vivo and in vitro methods. J. Chromatogr. A 2009, 1216, 3192–3198. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Nigjeh, E.; Bischoff, R.; Bruins, A.P.; Permentier, H.P. Electrochemistry in the Mimicry of Oxidative Drug Metabolism by Cytochrome P450s. Curr. Drug Metab. 2011, 12, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.; Melles, D.; Jacquoilleot, S.; Sanderson, P.; Zazzeroni, R.; Karst, U. Combination of Electrochemistry and Nuclear Magnetic Resonance Spectroscopy for Metabolism Studies. Anal. Chem. 2012, 84, 8777–8782. [Google Scholar] [CrossRef]
- Bussy, U.; Giraudeau, P.; Tea, I.; Boujtita, M. Understanding the degradation of electrochemically-generated reactive drug metabolites by quantitative NMR. Talanta 2013, 116, 554–558. [Google Scholar] [CrossRef]
- Herl, T.; Matysik, F.-M. Recent developments in electrochemistry–mass spectrometry. ChemElectroChem 2020, 7, 2498–2512. [Google Scholar] [CrossRef]
- Temgoua, R.C.T.; Tonlé, I.K.; Boujtita, M.M. Electrochemistry coupled with mass spectrometry for the prediction of the environmental fate and elucidation of the degradation mechanisms of pesticides: Current status and future prospects. Environ. Sci. Process. Impacts 2023, 25, 340–350. [Google Scholar] [CrossRef]
- Göldner, V.; Fangmeyer, J.; Karst, U. Mass spectrometric methods for the analysis of electrochemical transformation products. TrAC Trends Anal. Chem. 2025, 185, 118178. [Google Scholar] [CrossRef]
- Caspar, A.T.; Brandt, S.D.; Stoever, A.E.; Meyer, M.R.; Maurer, H.H. Metabolic fate and detectability of the new psychoactive substances 2-(4-bromo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25B-NBOMe) and 2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25C-NBOMe) in human and rat urine by GC–MS, LC–MS n, and LC–HR–MS/MS approaches. J. Pharm. Biomed. Anal. 2017, 134, 158–169. [Google Scholar] [CrossRef]
- Leth-Petersen, S.; Gabel-Jensen, C.; Gillings, N.; Lehel, S.; Hansen, H.D.; Knudsen, G.M.; Kristensen, J.L. Metabolic Fate of Hallucinogenic NBOMes. Chem. Res. Toxicol. 2016, 29, 96–100. [Google Scholar] [CrossRef]
- Seo, H.; Kim, I.S.; Kim, Y.-H.; Yoo, H.H.; Hong, J. Metabolic profile determination of 25N-NBOMe in human liver microsomes by liquid chromatography-quadrupole time-of-flight mass spectrometry. Int. J. Leg. Med. 2019, 133, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Kinyua, J.; Negreira, N.; Ibáñez, M.; Bijlsma, L.; Hernández, F.; Covaci, A.; van Nuijs, A.L.N. A data-independent acquisition workflow for qualitative screening of new psychoactive substances in biological samples. Anal. Bioanal. Chem. 2015, 407, 8773–8785. [Google Scholar] [CrossRef] [PubMed]
- Wiergowski, M.; Aszyk, J.; Kaliszan, M.; Wilczewska, K.; Anand, J.S.; Kot-Wasik, A.; Jankowski, Z. Identification of novel psychoactive substances 25B-NBOMe and 4-CMC in biological material using HPLC-Q-TOF-MS and their quantification in blood using UPLC–MS/MS in case of severe intoxications. J. Chromatogr. B 2017, 1041–1042, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, P.; Raoof, H.; Kotlinska, J.H.; Stefanowicz, P.; Szewczuk, Z.; Suder, P.; Silberring, J. Electrochemical Simulation of Cocaine Metabolism—A Step toward Predictive Toxicology for Drugs of Abuse. Eur. J. Mass Spectrom. 2014, 20, 279–285. [Google Scholar] [CrossRef]
- Jerszyńska, P.; Szultka-Młyńska, M. Electrochemical simulation of psychotropic drug metabolism compared to in vivo processes using liquid chromatography and mass spectrometry. Front. Pharmacol. 2025, 16, 1637852. [Google Scholar] [CrossRef]
- Smoluch, M.; Mielczarek, P.; Reszke, E.; Hieftje, G.M.; Silberring, J. Determination of psychostimulants and their metabolites by electrochemistry linked on-line to flowing atmospheric pressure afterglow mass spectrometry. Analyst 2014, 139, 4350–4355. [Google Scholar] [CrossRef]
- Poklis, J.L.; Nanco, C.R.; Troendle, M.M.; Wolf, C.E.; Poklis, A. Determination of 4-bromo-2,5-dimethoxy-N-[(2-methoxyphenyl)methyl]-benzeneethanamine (25B-NBOMe) in serum and urine by high performance liquid chromatography with tandem mass spectrometry in a case of severe intoxication. Drug Test. Anal. 2014, 6, 764–769. [Google Scholar] [CrossRef]
- Boumrah, Y.; Humbert, L.; Phanithavong, M.; Khimeche, K.; Dahmani, A.; Allorge, D. In vitro characterization of potential CYP- and UGT-derived metabolites of the psychoactive drug 25B-NBOMe using LC-high resolution MS. Drug Test. Anal. 2016, 8, 248–256. [Google Scholar] [CrossRef]
- Johansson, T.; Weidolf, L.; Jurva, U. Mimicry of phase I drug metabolism—Novel methods for metabolite characterization and synthesis. Rapid Commun. Mass Spectrom. 2007, 21, 2323–2331. [Google Scholar] [CrossRef]
- Gelman, F.; Dybala-Defratyka, A. Bromine isotope effects: Predictions and measurements. Chemosphere 2020, 246, 125746. [Google Scholar] [CrossRef]
- Almazroo, O.A.; Miah, M.K. Venkataramanan, Drug metabolism in the liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]





| Parameter | Value/Characteristic | |
|---|---|---|
| EC setting | Flow rate | 10 µL/min |
| Working electrode potential | 0–2500 mV (10 mV steps) | |
| EC operating mode | Scan, continuous | |
| EC temperature | Room, not controlled |
| Measured m/z a | Theoretical m/z b | Relative Mass Accuracy [ppm] | Ion Formula | Modification | Metabolic Reaction (Phase I) |
|---|---|---|---|---|---|
| 380.0857 | 380.08558 | 0.32 | [C18H23BrNO3]+ | [M+H]+ | (parent compound) |
| 378.0699 | 378.06993 | −0.08 | [C18H21BrNO3]+ | [M-2H+H]+ | dehydrogenation |
| 396.0794 | 396.08050 | −2.78 | [C18H23BrNO4]+ | [M+O+H]+ | hydroxylation |
| 394.0649 | 394.06485 | 0.13 | [C18H21BrNO4]+ | [M+O-2H+H]+ | hydroxylation-dehydrogenation |
| 260.0269 | 260.02807 | −4.50 | [C10H15BrNO2]+ | [M-C8H8O+H]+ | N-desalkylation |
| 258.0124 | 258.01242 | −0.08 | [C10H13BrNO2]+ | [M-C8H8O-2H+H]+ | N-desalkylation-dehydrogenation |
| 366.0680 | 366.06993 | −5.27 | [C17H21BrNO3]+ | [M-CH2+H]+ | O-desmethylation |
| 352.0524 | 352.05428 | −5.34 | [C16H19BrNO3]+ | [M-2CH2+H]+ | bis-O,O-desmethylation |
| 408.0808 | 408.08050 | 0.74 | [C19H23BrNO4]+ | [M+CO+H]+ | carbonylation (formylation) |
| 440.0710 | 440.07033 | 1.52 | [C19H23BrNO6]+ | [M+CO+2O+H]+ | carbonylation (formylation)-bis-hydroxylation |
| Retention Time [min] | Measured m/z a | Theoretical m/z b | Relative Mass Accuracy [ppm] | Ion Formula | Modification | Metabolic Reaction |
|---|---|---|---|---|---|---|
| 9.45 | 380.0876 | 380.08558 | 5.31 | [C18H23BrNO3]+ | [M+H]+ | (parent compound) |
| 8.80 | 366.0710 | 366.06993 | 2.92 | [C17H21BrNO3]+ | [M-CH2+H]+ | O-desmethylation/phase I |
| 8.72 | 446.0281 | 446.02675 | 3.03 | [C17H21BrNO6S]+ | [M-CH2+SO3+H]+ | O-desmethylation and sulfation/phase II |
| 7.91 | 542.1024 | 542.10202 | 0.70 | [C23H29BrNO9]+ | [M-CH2+C6H8O6+H]+ | O-desmethylation and glucuronidation/phase II |
| m/z of Parent Ion | m/z of Product Ions | CE [V] |
|---|---|---|
| 366 | 243, 201, 179, 121, 55 | 24.6 |
| 446 | 229, 121, 93, 91, 55 | 27.8 |
| 542 | 366, 275, 184, 121, 91 | 31.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kot-Wasik, A.; Potęga, A.; Aszyk-Woźniak, J.; Garwolińska, D.; Wiergowski, M.; Wasik, A. Electrochemical Simulation of 25B-NBOMe Phase I Metabolism and Metabolite Profiling by HPLC-QTOF-MS. Molecules 2025, 30, 4450. https://doi.org/10.3390/molecules30224450
Kot-Wasik A, Potęga A, Aszyk-Woźniak J, Garwolińska D, Wiergowski M, Wasik A. Electrochemical Simulation of 25B-NBOMe Phase I Metabolism and Metabolite Profiling by HPLC-QTOF-MS. Molecules. 2025; 30(22):4450. https://doi.org/10.3390/molecules30224450
Chicago/Turabian StyleKot-Wasik, Agata, Agnieszka Potęga, Justyna Aszyk-Woźniak, Dorota Garwolińska, Marek Wiergowski, and Andrzej Wasik. 2025. "Electrochemical Simulation of 25B-NBOMe Phase I Metabolism and Metabolite Profiling by HPLC-QTOF-MS" Molecules 30, no. 22: 4450. https://doi.org/10.3390/molecules30224450
APA StyleKot-Wasik, A., Potęga, A., Aszyk-Woźniak, J., Garwolińska, D., Wiergowski, M., & Wasik, A. (2025). Electrochemical Simulation of 25B-NBOMe Phase I Metabolism and Metabolite Profiling by HPLC-QTOF-MS. Molecules, 30(22), 4450. https://doi.org/10.3390/molecules30224450







