Potential Molecular Biomarkers for Predicting and Monitoring Complications in Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Biomarkers Help Monitor Responses to Medications
3. Emerging Theories of the Pathophysiology of T2DM
- Subtype 1—Severe autoimmune diabetes mellitus.
- Subtype 2—Severe insulin-deficient diabetes mellitus.
- Subtype 3—Severe insulin-resistant diabetes mellitus.
- Subtype 4—Mild obesity-related diabetes.
- Subtype 5—Mild age-related diabetes.
4. Potential Biomarkers of Adverse Drug Reaction for Oral Antidiabetic Medications
4.1. Mechanisms of Action, Benefits, and Risks of Oral Antihyperglycemics
4.1.1. Metformin
Mechanism by Which Metformin May Cause Side Effects
4.1.2. Insulin-Releasing Medications
Sulfonylureas
Meglitinide
Cardiovascular Risks and Mechanistic Insights into Insulin Secretagogues
4.1.3. Thiazolidinedione
Understanding the Off-Target Effects of Thiazolidinedione
4.1.4. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
DPP-4 Inhibitor: Insights into Preventing the Adverse Effects
4.1.5. Glucagon-like Peptide-1 (GLP-1) Agonist
Insights into Potential Mechanisms of GLP-1R Agonist Side Effects
4.1.6. Sodium–Glucose Co-Transporter-2 Inhibitors
5. Emerging Biomarkers in T2DM
Markers of Chronic or Low-Grade Inflammation
6. Vascular Complications Associated with T2DM
6.1. Microvascular Complications
6.1.1. Retinopathy
Potential Biomarker Options for Retinopathy
- Proangiogenic agents
- Proinflammatory agents
- Metabolite- and lipid-derived biomarkers
- Thickness changes in the outer plexiform layer may correlate with renal-related diseases such as diabetes
6.1.2. Nephropathy
Potential Biomarker Options for Nephropathy
- Dysregulated miRNA in diabetic kidney disease
- Growth Factors
- Biomarkers of oxidative stress and inflammation
- Hepatic and cardiac biomarkers
6.1.3. Neuropathy
Potential Biomarker Options in Neuropathies
- Neuroinflammatory mediators
- Hyperglycemia-induced molecules affecting metabolic and hemodynamic pathways
6.2. Macrovascular Complications
6.2.1. Coronary Artery Disease
Potential Biomarkers in Coronary Arterial Disease
- Hormones as biomarkers
- Oxidative stress
- Metabolic messengers
- Indicators of cell damage
6.2.2. Cerebrovascular Disease
Potential Biomarkers of Cerebrovascular Disease
- Biochemical indicators
- Neovasculogenesis
6.2.3. Peripheral Artery Disease
Potential Biomarker Options for Peripheral Arterial Disease
- Blood-based factors
- Inflammatory mediators
- Cell-derived molecules
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACCORD | Action to Control Cardiovascular Risk in Diabetes |
| ADA | American Diabetes Association |
| CV | Cardiovascular |
| CNS | Central nervous system |
| CAD | Coronary artery disease |
| DCCT | Diabetes Control and Complications Trial |
| DPP-4 | Dipeptidyl peptidase-4 |
| FDA | Food and Drug Administration |
| GLP-1 | Glucagon-like peptide-1 |
| GIP | Glucose-dependent insulinotropic polypeptide |
| HbA1c | Plasma glycosylated hemoglobin A1c |
| HHS | Hyperosmolar hyperglycemic state |
| IDF | International Diabetes Federation |
| SGLT2 | Sodium–glucose co-transporter-2 |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| UKPDS | United Kingdom Prospective Diabetes Study |
| WHO | World Health Organization |
References
- The International Diabetes Federation (IDF). Diabetes Facts and Figures. 2025. Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 30 July 2025).
- Markandu, K.; Dekhne, A.; Islam, H.; Islam, R.; Maddineni, K.; Potluri, G.; Parisapogu, A.; Teja Chinthapalli, M.; Dineshbhai Desai, H. 1420-P: Global Burden and Trend of Type 2 DM in 38 OECD Countries from 1990–2019—A Benchmarking Systematic Analysis. Diabetes 2024, 73 (Suppl. 1), 1420-P. [Google Scholar] [CrossRef]
- Koya, D.; Araki, S.; Haneda, M. Therapeutic management of diabetic kidney disease. J. Diabetes Investig. 2011, 2, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Rajbhandari, J.; Fernandez, C.J.; Agarwal, M.; Yeap, B.X.Y.; Pappachan, J.M. Diabetic heart disease: A clinical update. World J. Diabetes 2021, 12, 383–406. [Google Scholar] [CrossRef]
- Mota, R.I.; Morgan, S.E.; Bahnson, E.M. Diabetic vasculopathy: Macro and microvascular injury. Curr. Pathobiol. Rep. 2020, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Galiero, R.; Caturano, A.; Vetrano, E.; Beccia, D.; Brin, C.; Alfano, M.; Di Salvo, J.; Epifani, R.; Piacevole, A.; Tagliaferri, G.; et al. Peripheral Neuropathy in Diabetes Mellitus: Pathogenetic Mechanisms and Diagnostic Options. Int. J. Mol. Sci. 2023, 24, 3554. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Diabete. 2024. Available online: https://www.who.int/en/news-room/fact-sheets/detail/diabetes (accessed on 10 June 2025).
- Umemura, T.; Kawamura, T. Effect of diabetes on stroke symptoms and mortality: Lessons from a recent large population-based cohort study. J. Diabetes Investig. 2014, 5, 14–16. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, W.; Liu, J.; Xie, M.; Liu, Q.; Li, S. Vascular complications of diabetes: A narrative review. Medicine 2023, 102, e35285. [Google Scholar] [CrossRef]
- Pan, S.; Worker, C.J.; Feng Earley, Y. The hypothalamus as a key regulator of glucose homeostasis: Emerging roles of the brain renin-angiotensin system. Am. J. Physiol. Cell Physiol. 2023, 325, C141–C154. [Google Scholar] [CrossRef]
- Benoit, S.R.; Zhang, Y.; Geiss, L.S.; Gregg, E.W.; Albright, A. Trends in Diabetic Ketoacidosis Hospitalizations and In-Hospital Mortality-United States, 2000–2014. MMWR Morb. Mortal. Wkly Rep. 2018, 67, 362–365. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; Umpierrez, G.E.; Miles, J.M.; Fisher, J.N. Hyperglycemic Crises in Adult Patients With Diabetes. Diabetes Care 2009, 32, 1335–1343. [Google Scholar] [CrossRef]
- Scott, A.R.; Joint British Diabetes Societies. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet. Med. 2015, 32, 714–724. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Davis, G.M.; ElSayed, N.A.; Fadini, G.P.; Galindo, R.J.; Hirsch, I.B.; Klonoff, D.C.; McCoy, R.G.; Misra, S.; Gabbay, R.A.; et al. Hyperglycaemic crises in adults with diabetes: A consensus report. Diabetologia 2024, 67, 1455–1479. [Google Scholar] [CrossRef]
- Wei, W.T.; Lin, S.M.; Hsu, J.Y.; Wu, Y.Y.; Loh, C.H.; Huang, H.K.; Liu, P.P. Association between Hyperosmolar Hyperglycemic State and Venous Thromboembolism in Diabetes Patients: A Nationwide Analysis in Taiwan. J. Pers. Med. 2022, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Arabshomali, A.; Bazzazzadehgan, S.; Mahdi, F.; Shariat-Madar, Z. Potential Benefits of Antioxidant Phytochemicals in Type 2 Diabetes. Molecules 2023, 28, 7209. [Google Scholar] [CrossRef] [PubMed]
- Karslioglu French, E.; Donihi, A.C.; Korytkowski, M.T. Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome: Review of acute decompensated diabetes in adult patients. BMJ 2019, 365, l1114. [Google Scholar] [CrossRef] [PubMed]
- Kitabchi, A.E.; Umpierrez, G.E.; Murphy, M.B.; Kreisberg, R.A. Hyperglycemic Crises in Adult Patients With Diabetes: A consensus statement from the American Diabetes Association. Diabetes Care 2006, 29, 2739–2748. [Google Scholar] [CrossRef]
- Shariff, S.Z.; Bejaimal, S.A.; Sontrop, J.M.; Iansavichus, A.V.; Haynes, R.B.; Weir, M.A.; Garg, A.X. Retrieving clinical evidence: A comparison of PubMed and Google Scholar for quick clinical searches. J. Med. Internet Res. 2013, 15, e164. [Google Scholar] [CrossRef]
- Morshed, T.; Hayden, S. Google Versus PubMed: Comparison of Google and PubMed’s Search Tools for Answering Clinical Questions in the Emergency Department. Ann. Emerg. Med. 2020, 75, 408–415. [Google Scholar] [CrossRef]
- Laakso, M. Biomarkers for type 2 diabetes. Mol. Metab. 2019, 27, S139–S146. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Pham, M.; Maloney, E.; Rizzo, N.O.; Morton, G.J.; Wisse, B.E.; Kirk, E.A.; Chait, A.; Schwartz, M.W. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arter. Thromb. Vasc. Biol. 2008, 28, 1982–1988. [Google Scholar] [CrossRef]
- Suzuki, K.; Hatzikotoulas, K.; Southam, L.; Taylor, H.J.; Yin, X.; Lorenz, K.M.; Mandla, R.; Huerta-Chagoya, A.; Melloni, G.E.M.; Kanoni, S.; et al. Genetic drivers of heterogeneity in type 2 diabetes pathophysiology. Nature 2024, 627, 347–357. [Google Scholar] [CrossRef]
- Bazzazzadehgan, S.; Shariat-Madar, Z.; Mahdi, F. Distinct Roles of Common Genetic Variants and Their Contributions to Diabetes: MODY and Uncontrolled T2DM. Biomolecules 2025, 15, 414. [Google Scholar] [CrossRef]
- Rosen, E.D.; Kaestner, K.H.; Natarajan, R.; Patti, M.E.; Sallari, R.; Sander, M.; Susztak, K. Epigenetics and Epigenomics: Implications for Diabetes and Obesity. Diabetes 2018, 67, 1923–1931. [Google Scholar] [CrossRef]
- Burini, R.; McLellan, K.C.P.; Sloan, L.; Manda, R.M. Epigenetics of Glucose Metabolism and the Basis for T2DM Interventions. In Type 2 Diabetes; Masuo, K., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Sanches, J.M.; Zhao, L.N.; Salehi, A.; Wollheim, C.B.; Kaldis, P. Pathophysiology of type 2 diabetes and the impact of altered metabolic interorgan crosstalk. Febs. J. 2023, 290, 620–648. [Google Scholar] [CrossRef]
- Berbudi, A.; Khairani, S.; Tjahjadi, A.I. Interplay Between Insulin Resistance and Immune Dysregulation in Type 2 Diabetes Mellitus: Implications for Therapeutic Interventions. Immunotargets Ther. 2025, 14, 359–382. [Google Scholar] [CrossRef]
- Roh, E.; Song, D.K.; Kim, M.S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016, 48, e216. [Google Scholar] [CrossRef] [PubMed]
- Gerozissis, K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur. J. Pharmacol. 2008, 585, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Ahrițculesei, R.-V.; Boldeanu, L.; Dijmărescu, A.L.; Assani, M.-Z.; Boldeanu, M.V.; Siloși, I.; Vere, C.C. Neurotransmitter Alterations in Prediabetes and Type 2 Diabetes Mellitus: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 7847. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lv, B.; Zhi, L.; Shao, Y.; Liu, X.; Mitteregger, M.; Chakaroun, R.; Tremaroli, V.; Hazen, S.L.; Wang, R.; et al. Microbiome–metabolome dynamics associated with impaired glucose control and responses to lifestyle changes. Nat. Med. 2025, 31, 2222–2231. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, Y.; Ke, L.; Yu, Y. Elevated circulating free fatty acids levels causing pancreatic islet cell dysfunction through oxidative stress. J. Endocrinol. Investig. 2010, 33, 388–394. [Google Scholar] [CrossRef]
- Ivovic, A.; Yung, J.H.M.; Oprescu, A.I.; Vlavcheski, F.; Mori, Y.; Rahman, S.M.N.; Ye, W.; Eversley, J.A.; Wheeler, M.B.; Woo, M.; et al. β-Cell Insulin Resistance Plays a Causal Role in Fat-Induced β-Cell Dysfunction In Vitro and In Vivo. Endocrinology 2024, 165, bqae044. [Google Scholar] [CrossRef]
- Ji, X.; Yin, H.; Gu, T.; Xu, H.; Fang, D.; Wang, K.; Sun, H.; Tian, S.; Wu, T.; Nie, Y.; et al. Excessive free fatty acid sensing in pituitary lactotrophs elicits steatotic liver disease by decreasing prolactin levels. Cell Rep. 2024, 43, 114465. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.H.; Genest, J.; Gotto, A.M.; Kastelein, J.J.P.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Hepworth, M.R.; O’Sullivan, T.E. Regulation of systemic metabolism by tissue-resident immune cell circuits. Immunity 2023, 56, 1168–1186. [Google Scholar] [CrossRef]
- World Health Organization (WHO)/IDF Consultation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia; WHO: Geneva, Switzerland, 2006; ISBN 9241594934. Available online: https://www.who.int/publications/i/item/definition-and-diagnosis-of-diabetes-mellitus-and-intermediate-hyperglycaemia (accessed on 21 July 2025).
- American Diabetes Association. Standards of Medical Care for Patients With Diabetes Mellitus. Diabetes Care 1989, 12, 365–368. [Google Scholar] [CrossRef]
- Huang, E.S.; Liu, J.Y.; Moffet, H.H.; John, P.M.; Karter, A.J. Glycemic Control, Complications, and Death in Older Diabetic Patients: The Diabetes and Aging Study. Diabetes Care 2011, 34, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Bilal, A. Understanding Diabetes Overtreatment in Older Adults: Are We at an Intersection? Diabetes Care 2024, 48, 47–49. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Chen, Z.-Z.; Gerszten, R.E. Metabolomics and Proteomics in Type 2 Diabetes. Circ. Res. 2020, 126, 1613–1627. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, B.; Mathiesen, E.R.; Deckert, T. Effect of two years of strict metabolic control on progression of incipient nephropathy in insulin-dependent diabetes. Lancet 1986, 2, 1300–1304. [Google Scholar] [CrossRef]
- Reichard, P.; Berglund, B.; Britz, A.; Cars, I.; Nilsson, B.Y.; Rosenqvist, U. Intensified conventional insulin treatment retards the microvascular complications of insulin-dependent diabetes mellitus (IDDM): The Stockholm Diabetes Intervention Study (SDIS) after 5 years. J. Intern. Med. 1991, 230, 101–108. [Google Scholar] [CrossRef]
- Brinchmann-Hansen, O.; Dahl-Jørgensen, K.; Sandvik, L.; Hanssen, K.F. Blood glucose concentrations and progression of diabetic retinopathy: The seven year results of the Oslo study. BMJ 1992, 304, 19–22. [Google Scholar] [CrossRef]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Briggs Early, K.; Bruemmer, D.; Echouffo-Tcheugui, J.B.; Ekhlaspour, L.; Garg, R.; et al. 13. Older Adults: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48 (Suppl. 1), S266–S282. [Google Scholar] [CrossRef] [PubMed]
- Mukonda, E.; van der Westhuizen, D.J.; Dave, J.A.; Cleary, S.; Hannan, L.; Rusch, J.A.; Lesosky, M. Understanding the relationship between the frequency of HbA1c monitoring, HbA1c changes over time, and the achievement of targets: A retrospective cohort study. BMC Endocr. Disord. 2025, 25, 3. [Google Scholar] [CrossRef]
- Nunes, J.P.L.; DeMarco, J.P. A 7.0–7.7% value for glycated haemoglobin is better than a <7% value as an appropriate target for patient-centered drug treatment of type 2 diabetes mellitus. Ann. Transl. Med. 2019, 7 (Suppl. 3), S122. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; Grimm, R.H., Jr.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef]
- Buse, J.B.; Bigger, J.T.; Byington, R.P.; Cooper, L.S.; Cushman, W.C.; Friedewald, W.T.; Genuth, S.; Gerstein, H.C.; Ginsberg, H.N.; Goff, D.C., Jr.; et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: Design and methods. Am. J. Cardiol. 2007, 99, 21i–33i. [Google Scholar] [CrossRef]
- Stimson, R.H.; Dover, A.R.; Forbes, S.; Strachan, M.W.J.; McKnight, J.A.; Gibb, F.W. HbA1c Is Disproportionately Higher in Women and Older People With Type 1 Diabetes Compared With Flash Glucose Monitoring Metrics of Glycemic Control. J. Diabetes Sci. Technol. 2022, 16, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Aceves, B.; Ezekiel-Herrera, D.; Marino, M.; Datta, R.; Lucas, J.; Giebultowicz, S.; Heintzman, J. Disparities in HbA1c testing between aging US Latino and non-Latino white primary care patients. Prev. Med. Rep. 2022, 26, 101739. [Google Scholar] [CrossRef]
- Zhou, B.; Sheffer, K.E.; Bennett, J.E.; Gregg, E.W.; Danaei, G.; Singleton, R.K.; Shaw, J.E.; Mishra, A.; Lhoste, V.P.F.; Carrillo-Larco, R.M.; et al. Global variation in diabetes diagnosis and prevalence based on fasting glucose and hemoglobin A1c. Nat. Med. 2023, 29, 2885–2901. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, V.A.; Cruz Filho, R.A.; de Oliveira, T.S.; Moscavitch, S.D.; Kang, H.C.; Miranda Chagas, S.V.; Costa, D.M.; Vianna Araújo, D.; Garcia Rosa, M.L. Racial differences in HbA1c: A cross-sectional analysis of a Brazilian public primary care population. Prim. Care Diabetes 2013, 7, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Gorst, C.; Kwok, C.S.; Aslam, S.; Buchan, I.; Kontopantelis, E.; Myint, P.K.; Heatlie, G.; Loke, Y.; Rutter, M.K.; Mamas, M.A. Long-term Glycemic Variability and Risk of Adverse Outcomes: A Systematic Review and Meta-analysis. Diabetes Care 2015, 38, 2354–2369. [Google Scholar] [CrossRef]
- Teoh, H.; Home, P.; Leiter, L.A. Should A1C targets be individualized for all people with diabetes? Arguments for and against. Diabetes Care 2011, 34 (Suppl. 2), S191–S196. [Google Scholar] [CrossRef]
- Anderson, J.J.; Welsh, P.; Ho, F.K.; Ferguson, L.D.; Welsh, C.E.; Pellicori, P.; Cleland, J.G.F.; Forbes, J.; Iliodromiti, S.; Boyle, J.; et al. Ethnic differences in prevalence of actionable HbA1c levels in UK Biobank: Implications for screening. BMJ Open Diabetes Res. Care 2021, 9, e002176. [Google Scholar] [CrossRef]
- Luo, M.; Tan, K.H.X.; Tan, C.S.; Lim, W.Y.; Tai, E.S.; Venkataraman, K. Longitudinal trends in HbA(1c) patterns and association with outcomes: A systematic review. Diabetes Metab. Res. Rev. 2018, 34, e3015. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yang, L.; Zhao, D. The relationship between HbA1c control pattern and atherosclerosis progression of diabetes: A prospective study of Chinese population. Diabetol. Metab. Syndr. 2024, 16, 127. [Google Scholar] [CrossRef]
- Colayco, D.C.; Niu, F.; McCombs, J.S.; Cheetham, T.C. A1C and Cardiovascular Outcomes in Type 2 Diabetes: A nested case-control study. Diabetes Care 2010, 34, 77–83. [Google Scholar] [CrossRef]
- Ahmed, Y.; Mohamed Abuelass, F.K.; Hamd Abdelwahab, S.B.; Mukhtar, M.; Ahmed, Y.; Elfahal, M.; Mohmed Elhussein, N.S. Determinants of Poor Glycemic Control Among Type 2 Diabetes Patients: A Systematic Review. Cureus 2025, 17, e82464. [Google Scholar] [CrossRef]
- Nesti, L.; Natali, A. Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 657–669. [Google Scholar] [CrossRef]
- Griffin, S.J.; Leaver, J.K.; Irving, G.J. Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017, 60, 1620–1629. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Greyber, E.; Pasternak, G.A.; Salpeter, E.E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: Systematic review and meta-analysis. Arch. Intern. Med. 2003, 163, 2594–2602. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, B.; Wu, A.; Shin, J.I.; Sang, Y.; Alexander, G.C.; Secora, A.; Inker, L.A.; Coresh, J.; Chang, A.R.; Grams, M.E. Association of Metformin Use With Risk of Lactic Acidosis Across the Range of Kidney Function: A Community-Based Cohort Study. JAMA Intern. Med. 2018, 178, 903–910. [Google Scholar] [CrossRef]
- Verma, S.; Bhanot, S.; McNeill, J.H. Metformin decreases plasma insulin levels and systolic blood pressure in spontaneously hypertensive rats. Am. J. Physiol. 1994, 267 Pt 2, H1250–H1253. [Google Scholar] [CrossRef] [PubMed]
- Quast, D.R.; Xie, C.; Bound, M.J.; Grivell, J.; Hatzinikolas, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Nauck, M.A.; Meier, J.J.; et al. Effects of Metformin on Postprandial Blood Pressure, Heart Rate, Gastric Emptying, GLP-1, and Prevalence of Postprandial Hypotension in Type 2 Diabetes: A Double-Blind Placebo-Controlled Crossover Study. Diabetes 2025, 74, 611–618. [Google Scholar] [CrossRef]
- Huang, K.-H.; Huang, S.-W.; Yang, Y.; Gau, S.-Y.; Tsai, T.-H.; Chang, Y.-L.; Lee, C.-Y. Dose dependent relationship of metformin use and diabetic peripheral neuropathy risk in patients with type 2 diabetes mellitus. Sci. Rep. 2025, 15, 12040. [Google Scholar] [CrossRef]
- Shurrab, N.T.; Arafa, E.-S.A. Metformin: A review of its therapeutic efficacy and adverse effects. Obes. Med. 2020, 17, 100186. [Google Scholar] [CrossRef]
- European Medicine Agency Science Medicines Health. Use of Metformin to Treat Diabetes Now Expanded to Patients with Moderately Reduced Kidney Function. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/metformin-metformin-containing-medicines#topics (accessed on 29 July 2025).
- Chan, J.C.N.; Yang, A.; Chu, N.; Chow, E. Current type 2 diabetes guidelines: Individualized treatment and how to make the most of metformin. Diabetes Obes. Metab. 2024, 26 (Suppl. 3), 55–74. [Google Scholar] [CrossRef]
- Kozyraki, R.; Cases, O. Vitamin B12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef]
- Sayedali, E.; Yalin, A.E.; Yalin, S. Association between metformin and vitamin B12 deficiency in patients with type 2 diabetes. World J. Diabetes 2023, 14, 585–593. [Google Scholar] [CrossRef]
- Muralidharan, J.; Romould, G.G.; Kashyap, S.; Pasanna, R.; Sivadas, A.; Sachdev, H.S.; Kurpad, A.V.; Devi, S. Effect of calcium supplementation on reversing metformin-based inhibition of vitamin B12 bioavailability in healthy adults using a [13C] cyanocobalamin tracer—A pilot study. Clin. Nutr. ESPEN 2024, 62, 76–80. [Google Scholar] [CrossRef]
- Pellanda, P.; Ghosh, T.S.; O’Toole, P.W. Understanding the impact of age-related changes in the gut microbiome on chronic diseases and the prospect of elderly-specific dietary interventions. Curr. Opin. Biotechnol. 2021, 70, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Saboo, B.; Khader, J.; Modi, K.D.; Jindal, S.; Wangnoo, S.K.; Amarnath, S. Position of Sulfonylureas in the Current ERA: Review of National and International Guidelines. Clin. Med. Insights Endocrinol. Diabetes 2022, 15, 11795514221074663. [Google Scholar] [CrossRef] [PubMed]
- Bahardoust, M.; Mehrabi, Y.; Hadaegh, F.; Khalili, D.; Delpisheh, A. Impact of duration of treatments with metformin and sulfonylureas, individually or in combination, on diabetic retinopathy among newly diagnosed type 2 diabetic patients: A pooled cohort’s analysis. Int. J. Retin. Vitr. 2025, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Genuth, S. Should Sulfonylureas Remain an Acceptable First-Line Add-on to Metformin Therapy in Patients With Type 2 Diabetes? No, It’s Time to Move On! Diabetes Care 2014, 38, 170–175. [Google Scholar] [CrossRef]
- Lebovitz, H.E.; Feinglos, M.N. Mechanism of action of the second-generation sulfonylurea glipizide. Am. J. Med. 1983, 75, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Aschner, P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res. Clin. Pr. 2017, 132, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Kolaczynski, W.M.; Hankins, M.; Ong, S.H.; Richter, H.; Clemens, A.; Toussi, M. Microvascular Outcomes in Patients with Type 2 Diabetes Treated with Vildagliptin vs. Sulfonylurea: A Retrospective Study Using German Electronic Medical Records. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2016, 7, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, L.; Suissa, S. Sulfonylureas and the Risks of Cardiovascular Events and Death: A Methodological Meta-Regression Analysis of the Observational Studies. Diabetes Care 2017, 40, 706–714. [Google Scholar] [CrossRef]
- Douros, A.; Dell’Aniello, S.; Yu, O.H.Y.; Filion, K.B.; Azoulay, L.; Suissa, S. Sulfonylureas as second line drugs in type 2 diabetes and the risk of cardiovascular and hypoglycaemic events: Population based cohort study. BMJ 2018, 362, k2693. [Google Scholar] [CrossRef]
- Lee, T.T.L.; Hui, J.M.H.; Lee, Y.H.A.; Satti, D.I.; Shum, Y.K.L.; Kiu, P.T.H.; Wai, A.K.C.; Liu, T.; Wong, W.T.; Chan, J.S.K.; et al. Sulfonylurea Is Associated With Higher Risks of Ventricular Arrhythmia or Sudden Cardiac Death Compared With Metformin: A Population-Based Cohort Study. J. Am. Heart Assoc. 2022, 11, e026289. [Google Scholar] [CrossRef]
- Gerich, J.E. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch. Intern. Med. 2003, 163, 1306–1316. [Google Scholar] [CrossRef]
- Schmitz, O.; Lund, S.; Andersen, P.H.; Jønler, M.; Pørksen, N. Optimizing Insulin Secretagogue Therapy in Patients With Type 2 Diabetes: A randomized double-blind study with repaglinide. Diabetes Care 2002, 25, 342–346. [Google Scholar] [CrossRef]
- Philip, J.; Fernandez, C.J. Efficacy and Cardiovascular Safety of Meglitinides. Curr. Drug Saf. 2021, 16, 207–216. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Pharmacologic therapy for type 2 diabetes mellitus. Ann. Intern. Med. 1999, 131, 281–303. [Google Scholar] [CrossRef]
- Black, C.; Donnelly, P.; McIntyre, L.; Royle, P.L.; Shepherd, J.P.; Thomas, S. Meglitinide analogues for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2007, 2007, Cd004654. [Google Scholar] [CrossRef]
- Jovanovic, L.; Dailey, G., 3rd; Huang, W.C.; Strange, P.; Goldstein, B.J. Repaglinide in type 2 diabetes: A 24-week, fixed-dose efficacy and safety study. J. Clin. Pharmacol. 2000, 40, 49–57. [Google Scholar] [CrossRef]
- Schramm, T.K.; Gislason, G.H.; Vaag, A.; Rasmussen, J.N.; Folke, F.; Hansen, M.L.; Fosbøl, E.L.; Køber, L.; Norgaard, M.L.; Madsen, M.; et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: A nationwide study. Eur. Heart J. 2011, 32, 1900–1908. [Google Scholar] [CrossRef]
- Papa, G.; Fedele, V.; Rizzo, M.R.; Fioravanti, M.; Leotta, C.; Solerte, S.B.; Purrello, F.; Paolisso, G. Safety of Type 2 Diabetes Treatment With Repaglinide Compared With Glibenclamide in Elderly People: A randomized, open-label, two-period, cross-over trial. Diabetes Care 2006, 29, 1918–1920. [Google Scholar] [CrossRef]
- Huang, H.-K.; Yeh, J.-I. Comparison of mortality and cardiovascular event risk associated with various insulin secretagogues: A nationwide real-world analysis. Diabetes Res. Clin. Pract. 2019, 152, 103–110. [Google Scholar] [CrossRef]
- Huang, Y.; Abdelmoneim, A.S.; Light, P.; Qiu, W.; Simpson, S.H. Comparative cardiovascular safety of insulin secretagogues following hospitalization for ischemic heart disease among type 2 diabetes patients: A cohort study. J. Diabetes Its Complicat. 2015, 29, 196–202. [Google Scholar] [CrossRef]
- Davies, M.J.; Lawrence, I.G. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction): Theory and practice. Diabetes Obes. Metab. 2002, 4, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Chutkow, W.A.; Simon, M.C.; Le Beau, M.M.; Burant, C.F. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes 1996, 45, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, S.; Kondo, C.; Yamada, M.; Matsumoto, S.; Higashiguchi, O.; Horio, Y.; Matsuzawa, Y.; Kurachi, Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J. Biol. Chem. 1996, 271, 24321–24324. [Google Scholar] [CrossRef]
- Aubert, G.; Barefield, D.Y.; Demonbreun, A.R.; Ramratnam, M.; Fallon, K.S.; Warner, J.L.; Rossi, A.E.; Hadhazy, M.; Makielski, J.C.; McNally, E.M. Deletion of Sulfonylurea Receptor 2 in the Adult Myocardium Enhances Cardiac Glucose Uptake and Is Cardioprotective. JACC Basic Transl. Sci. 2019, 4, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Domingo, A.; Tan, B.H.; Crotti, L.; Tester, D.J.; Eckhardt, L.; Cuoretti, A.; Kroboth, S.L.; Song, C.; Zhou, Q.; Kopp, D.; et al. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010, 7, 1466–1471. [Google Scholar] [CrossRef]
- de Wet, H.; Proks, P. Molecular action of sulphonylureas on KATP channels: A real partnership between drugs and nucleotides. Biochem. Soc. Trans. 2015, 43, 901–907. [Google Scholar] [CrossRef]
- Ding, D.; Wu, J.-X.; Duan, X.; Ma, S.; Lai, L.; Chen, L. Structural identification of vasodilator binding sites on the SUR2 subunit. Nat. Commun. 2022, 13, 2675. [Google Scholar] [CrossRef]
- Leonard, C.E.; Hennessy, S.; Han, X.; Siscovick, D.S.; Flory, J.H.; Deo, R. Pro- and Antiarrhythmic Actions of Sulfonylureas: Mechanistic and Clinical Evidence. Trends Endocrinol. Metab. 2017, 28, 561–586. [Google Scholar] [CrossRef]
- Nichols, C.G.; Singh, G.K.; Grange, D.K. KATP channels and cardiovascular disease: Suddenly a syndrome. Circ. Res. 2013, 112, 1059–1072. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Cecchini, A.L.; Flex, A.; Landolfi, R. Biomarkers of vascular disease in diabetes: The adipose-immune system cross talk. Intern. Emerg. Med. 2020, 15, 381–393. [Google Scholar] [CrossRef]
- Mantella, L.E.; Liblik, K.; Johri, A.M. Vascular imaging of atherosclerosis: Strengths and weaknesses. Atherosclerosis 2021, 319, 42–50. [Google Scholar] [CrossRef]
- Soccio, R.E.; Chen, E.R.; Lazar, M.A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014, 20, 573–591. [Google Scholar] [CrossRef]
- Bloomgarden, Z.T. Thiazolidinediones. Diabetes Care 2005, 28, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.E.; Han, X.; Brensinger, C.M.; Bilker, W.B.; Cardillo, S.; Flory, J.H.; Hennessy, S. Comparative risk of serious hypoglycemia with oral antidiabetic monotherapy: A retrospective cohort study. Pharmacoepidemiol. Drug Saf. 2018, 27, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.; Benny, T.; Hale, G.; Seamon, M. What is the role of pioglitazone for patients with type 2 diabetes in value-based care settings? Drugs Ther. Perspect. 2024, 40, 131–136. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Kernan, W.N.; Viscoli, C.M.; Furie, K.L.; Young, L.H.; Inzucchi, S.E.; Gorman, M.; Guarino, P.D.; Lovejoy, A.M.; Peduzzi, P.N.; Conwit, R.; et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N. Engl. J. Med. 2016, 374, 1321–1331. [Google Scholar] [CrossRef]
- Lee, R.H.; Sloane, R.; Pieper, C.; Lyles, K.W.; Adler, R.A.; Van Houtven, C.; LaFleur, J.; Colón-Emeric, C. Clinical Fractures Among Older Men With Diabetes Are Mediated by Diabetic Complications. J. Clin. Endocrinol. Metab. 2018, 103, 281–287. [Google Scholar] [CrossRef]
- Oei, L.; Zillikens, M.C.; Dehghan, A.; Buitendijk, G.H.; Castaño-Betancourt, M.C.; Estrada, K.; Stolk, L.; Oei, E.H.; van Meurs, J.B.; Janssen, J.A.; et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: The Rotterdam Study. Diabetes Care 2013, 36, 1619–1628. [Google Scholar] [CrossRef]
- Kaku, K.; Hashiramoto, M. Thiazolidinediones and bone fractures. J. Diabetes Investig. 2011, 2, 354–355. [Google Scholar] [CrossRef] [PubMed]
- Bhattoa, H.P. Laboratory aspects and clinical utility of bone turnover markers. Ejifcc 2018, 29, 117–128. [Google Scholar] [PubMed]
- Bełtowski, J.; Rachańczyk, J.; Włodarczyk, M. Thiazolidinedione-Induced Fluid Retention: Recent Insights into the Molecular Mechanisms. PPAR Res. 2013, 2013, 628628. [Google Scholar] [CrossRef]
- Daza-Arnedo, R.; Rico-Fontalvo, J.-E.; Pájaro-Galvis, N.; Leal-Martínez, V.; Abuabara-Franco, E.; Raad-Sarabia, M.; Montejo-Hernández, J.; Cardona-Blanco, M.; Cabrales-Juan, J.; Uparella-Gulfo, I.; et al. Dipeptidyl Peptidase-4 Inhibitors and Diabetic Kidney Disease: A Narrative Review. Kidney Med. 2021, 3, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014, 35, 992–1019. [Google Scholar] [CrossRef]
- Fonseca, V.A. New developments in diabetes management: Medications of the 21st century. Clin. Ther. 2014, 36, 477–484. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef]
- Deacon, C.F. Peptide degradation and the role of DPP-4 inhibitors in the treatment of type 2 diabetes. Peptides 2018, 100, 150–157. [Google Scholar] [CrossRef]
- Bayanati, M.; Ismail Mahboubi Rabbani, M.; Sirous Kabiri, S.; Mir, B.; Rezaee, E.; Tabatabai, S.A. Dipeptidyl Peptidase-4 Inhibitors: A Systematic Review of Structure-Activity Relationship Studies. Iran. J. Pharm. Res. 2024, 23, e151581. [Google Scholar] [CrossRef]
- Bohannon, N. Overview of the gliptin class (dipeptidyl peptidase-4 inhibitors) in clinical practice. Postgrad. Med. 2009, 121, 40–45. [Google Scholar] [CrossRef]
- Scheen, A.J. DPP-4 inhibitors in the management of type 2 diabetes: A critical review of head-to-head trials. Diabetes Metab. 2012, 38, 89–101. [Google Scholar] [CrossRef]
- Drakul, M.; Čolić, M. Immunomodulatory activity of dipeptidyl peptidase-4 inhibitors in immune-related diseases. Eur. J. Immunol. 2023, 53, 2250302. [Google Scholar] [CrossRef]
- Kawanami, D.; Takashi, Y.; Takahashi, H.; Motonaga, R.; Tanabe, M. Renoprotective Effects of DPP-4 Inhibitors. Antioxidants 2021, 10, 246. [Google Scholar] [CrossRef]
- Gupta, S.; Sen, U. More than just an enzyme: Dipeptidyl peptidase-4 (DPP-4) and its association with diabetic kidney remodelling. Pharmacol. Res. 2019, 147, 104391. [Google Scholar] [CrossRef]
- Huang, J.; Jia, Y.; Sun, S.; Meng, L. Adverse event profiles of dipeptidyl peptidase-4 inhibitors: Data mining of the public version of the FDA adverse event reporting system. BMC Pharmacol. Toxicol. 2020, 21, 68. [Google Scholar] [CrossRef]
- Pathak, R.; Bridgeman, M.B. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors In the Management of Diabetes. Pharm. Ther. 2010, 35, 509–513. [Google Scholar]
- Patoulias, D.I.; Boulmpou, A.; Teperikidis, E.; Katsimardou, A.; Siskos, F.; Doumas, M.; Papadopoulos, C.E.; Vassilikos, V. Cardiovascular efficacy and safety of dipeptidyl peptidase-4 inhibitors: A meta-analysis of cardiovascular outcome trials. World J. Cardiol. 2021, 13, 585–592. [Google Scholar] [CrossRef]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef]
- Mannucci, E.; Mosenzon, O.; Avogaro, A. Analyses of Results From Cardiovascular Safety Trials With DPP-4 Inhibitors: Cardiovascular Outcomes, Predefined Safety Outcomes, and Pooled Analysis and Meta-analysis. Diabetes Care 2016, 39 (Suppl. 2), S196–S204. [Google Scholar] [CrossRef]
- Berra, C.C.; Manfrini, R.; Ghelardi, R.; Bollati, P.M.; Bucciarelli, L.; Mirani, M.; Muratori, M.; Folli, F.; Group, A.S. 367-P: Cardiovascular Risk Categories in Patients with Diabetes According to 2019 ESC/EASD Guidelines in Clinical Practice: Use of a Dedicated App (AWARE). Diabetes 2021, 70 (Suppl. 1), 367-P. [Google Scholar] [CrossRef]
- Ospelt, C.; Mertens, J.C.; Jüngel, A.; Brentano, F.; Maciejewska-Rodriguez, H.; Huber, L.C.; Hemmatazad, H.; Wüest, T.; Knuth, A.; Gay, R.E.; et al. Inhibition of fibroblast activation protein and dipeptidylpeptidase 4 increases cartilage invasion by rheumatoid arthritis synovial fibroblasts. Arthritis. Rheum. 2010, 62, 1224–1235. [Google Scholar] [CrossRef]
- Sayiner, Z.A.; Okyar, B.; Kısacık, B.; Akarsu, E.; Özkaya, M.; Araz, M. DPP-4 Inhibitors Increase the Incidence of Arthritis/Arthralgia But Do Not Affect Autoimmunity. Acta. Endocrinol. 2018, 14, 473–476. [Google Scholar] [CrossRef]
- Charoenngam, N.; Rittiphairoj, T.; Ponvilawan, B.; Ungprasert, P. Use of dipeptidyl peptidase-4 inhibitors is associated with a lower risk of rheumatoid arthritis in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of cohort studies. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 249–255. [Google Scholar] [CrossRef]
- Rai, P.; Dwibedi, N.; Rowneki, M.; Helmer, D.A.; Sambamoorthi, U. Dipeptidyl Peptidase-4 Inhibitors and Joint Pain: A Retrospective Cohort Study of Older Veterans with Type 2 Diabetes Mellitus. Am. Health Drug Benefits 2019, 12, 223–231. [Google Scholar]
- Padron, S.; Rogers, E.; Demory Beckler, M.; Kesselman, M. DPP-4 inhibitor (sitagliptin)-induced seronegative rheumatoid arthritis. BMJ Case Rep. 2019, 12, 223–230. [Google Scholar] [CrossRef]
- Park, T.; Bresnahan, M.; Griggs, S.K.; Chen, J.; Cho, A.H.; Gousse, Y.; Feinglos, M. Comparative risk of musculoskeletal adverse reactions among new users of dipeptidyl peptidase-4 inhibitors: A retrospective cohort study. Explor Res. Clin. Soc. Pharm. 2021, 2, 100022. [Google Scholar] [CrossRef]
- De Vries, J.H.; Rosenstock, J. DPP-4 Inhibitor–Related Pancreatitis: Rare but Real! Diabetes Care 2017, 40, 161–163. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef]
- Wen, S.; Yuan, Y.; Li, Y.; Xu, C.; Chen, L.; Ren, Y.; Wang, C.; He, Y.; Li, X.; Gong, M.; et al. The effects of non-insulin anti-diabetic medications on the diabetic microvascular complications: A systematic review and meta-analysis of randomized clinical trials. BMC Endocr. Disord. 2025, 25, 179. [Google Scholar] [CrossRef]
- Anderer, S. GLP-1 Drugs Linked to Higher Risk of Age-Related Macular Degeneration. JAMA 2025, 334, 383. [Google Scholar] [CrossRef]
- Ko, J.; Jahromi, Y. New onset diabetic retinopathy with glucagon-like peptide-1 receptor agonists: A case report. J. Am. Pharm. Assoc. 2025, 65, 102475. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Natale, P.; Tunnicliffe, D.J.; Toyama, T.; Palmer, S.C.; Saglimbene, V.M.; Ruospo, M.; Gargano, L.; Stallone, G.; Gesualdo, L.; Strippoli, G.F. Sodium-glucose co-transporter protein 2 (SGLT2) inhibitors for people with chronic kidney disease and diabetes. Cochrane Database Syst. Rev. 2024, 5, Cd015588. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Hsu, T.-W.; Liu, J.-H.; Pan, H.-C.; Lai, C.-F.; Yang, S.-Y.; Wu, V.-C. Kidney and Cardiovascular Outcomes Among Patients With CKD Receiving GLP-1 Receptor Agonists: A Systematic Review and Meta-Analysis of Randomized Trials. Am. J. Kidney Dis. 2025, 85, 555–569.e551. [Google Scholar] [CrossRef]
- Merovci, A.; Mari, A.; Solis-Herrera, C.; Xiong, J.; Daniele, G.; Chavez-Velazquez, A.; Tripathy, D.; Urban McCarthy, S.; Abdul-Ghani, M.; DeFronzo, R.A. Dapagliflozin lowers plasma glucose concentration and improves β-cell function. J. Clin. Endocrinol. Metab. 2015, 100, 1927–1932. [Google Scholar] [CrossRef]
- Packer, M. Mechanisms of enhanced renal and hepatic erythropoietin synthesis by sodium-glucose cotransporter 2 inhibitors. Eur. Heart J. 2023, 44, 5027–5035. [Google Scholar] [CrossRef]
- Hess, D.A.; Terenzi, D.C.; Trac, J.Z.; Quan, A.; Mason, T.; Al-Omran, M.; Bhatt, D.L.; Dhingra, N.; Rotstein, O.D.; Leiter, L.A.; et al. SGLT2 Inhibition with Empagliflozin Increases Circulating Provascular Progenitor Cells in People with Type 2 Diabetes Mellitus. Cell Metab. 2019, 30, 609–613. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Rodgers, G.P. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study: Continuing to Build on 40 Years of Diabetes Research. Diabetes Care 2024, 47, 1518–1521. [Google Scholar] [CrossRef]
- Skyler, J.S. Effects of Glycemic Control on Diabetes Complications and on the Prevention of Diabetes. Clin. Diabetes 2004, 22, 162–166. [Google Scholar] [CrossRef]
- Patel, S.B.; Belalcazar, L.M.; Afreen, S.; Balderas, R.; Hegele, R.A.; Karpe, F.; Ponte-Negretti, C.I.; Rajpal, A. American Association of Clinical Endocrinology Consensus Statement: Algorithm for Management of Adults with Dyslipidemia—2025 Update. Endocr. Pract. 2025, 31, 1207–1238. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Eliasson, B.; Svensson, A.-M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjörnsdottir, S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Early, K.B.; Bruemmer, D.; Das, S.R.; Echouffo-Tcheugui, J.B.; et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48 (Suppl. 1), S207–S238. [Google Scholar] [CrossRef]
- Patel, S.B.; Wyne, K.L.; Afreen, S.; Belalcazar, L.M.; Bird, M.D.; Coles, S.; Marrs, J.C.; Peng, C.C.; Pulipati, V.P.; Sultan, S.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline on Pharmacologic Management of Adults With Dyslipidemia. Endocr. Pr. 2025, 31, 236–262. [Google Scholar] [CrossRef]
- Li, J.; Hou, D.; Li, J.; Li, R.; Sun, M. Association between the atherogenic index of plasma and the systemic immuno-inflammatory index using NHANES data from 2005 to 2018. Sci. Rep. 2025, 15, 11245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xiao, M.; Zhang, H.; Tan, Q.; Ji, J.; Cheng, Y.; Lu, F. Relationship between plasma atherogenic index and incidence of cardiovascular diseases in Chinese middle-aged and elderly people. Sci. Rep. 2025, 15, 8775. [Google Scholar] [CrossRef]
- Jeong, S.H.; Vorachitti, M.; Fuentes, F. A Case of Euglycemic Diabetic Ketoacidosis (DKA), Influenza, and a Dipeptidyl Peptidase-4 (DPP-4) Inhibitor. Cureus 2023, 15, e39012. [Google Scholar] [CrossRef]
- Lenka, J.; Sharma, N. The Domino Effect: Euglycemic Diabetic Ketoacidosis From Acute Pancreatitis After Sitagliptin Use In Type 1 Diabetes Mellitus. CHEST 2019, 155, 106A. [Google Scholar] [CrossRef]
- Chow, E.; Clement, S.; Garg, R. Euglycemic diabetic ketoacidosis in the era of SGLT-2 inhibitors. BMJ Open Diabetes Res. Care 2023, 11, e003666. [Google Scholar] [CrossRef]
- Turchin, A.; Petito, L.C.; Hegermiller, E.; Carnahan, R.; DeVries, A.; Goel, S.; Lansang, M.C.; McDonnell, M.E.; Nair, V.; Priest, E.; et al. Cardiovascular Events in Individuals Treated With Sulfonylureas or Dipeptidyl Peptidase 4 Inhibitors. JAMA Netw. Open 2025, 8, e2523067. [Google Scholar] [CrossRef]
- Sato, Y.; Yamada, T.; Taniguchi, R.; Nagai, K.; Makiyama, T.; Okada, H.; Kataoka, K.; Ito, H.; Matsumori, A.; Sasayama, S.; et al. Persistently increased serum concentrations of cardiac troponin t in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation 2001, 103, 369–374. [Google Scholar] [CrossRef]
- Shomanova, Z.; Ohnewein, B.; Schernthaner, C.; Höfer, K.; Pogoda, C.A.; Frommeyer, G.; Wernly, B.; Brandt, M.C.; Dieplinger, A.M.; Reinecke, H.; et al. Classic and Novel Biomarkers as Potential Predictors of Ventricular Arrhythmias and Sudden Cardiac Death. J. Clin. Med. 2020, 9, 578. [Google Scholar] [CrossRef]
- Leonard, C.E.; Brensinger, C.M.; Aquilante, C.L.; Bilker, W.B.; Boudreau, D.M.; Deo, R.; Flory, J.H.; Gagne, J.J.; Mangaali, M.J.; Hennessy, S. Comparative Safety of Sulfonylureas and the Risk of Sudden Cardiac Arrest and Ventricular Arrhythmia. Diabetes Care 2018, 41, 713–722. [Google Scholar] [CrossRef]
- Ziyadeh, N.; McAfee, A.T.; Koro, C.; Landon, J.; Arnold Chan, K. The thiazolidinediones rosiglitazone and pioglitazone and the risk of coronary heart disease: A retrospective cohort study using a US health insurance database. Clin. Ther. 2009, 31, 2665–2677. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Berezin, A.E. Diabetes mellitus related biomarker: The predictive role of growth-differentiation factor-15. Diabetes Metab Syndr. 2016, 10 (Suppl. 1), S154–S157. [Google Scholar] [CrossRef]
- Weir, D.L.; McAlister, F.A.; Senthilselvan, A.; Minhas-Sandhu, J.K.; Eurich, D.T. Sitagliptin use in patients with diabetes and heart failure: A population-based retrospective cohort study. JACC Heart Fail 2014, 2, 573–582. [Google Scholar] [CrossRef]
- Wang, K.-L.; Liu, C.-J.; Chao, T.-F.; Huang, C.-M.; Wu, C.-H.; Chen, S.-J.; Yeh, C.-M.; Chen, T.-J.; Lin, S.-J.; Chiang, C.-E. Sitagliptin and the risk of hospitalization for heart failure: A population-based study. Int. J. Cardiol. 2014, 177, 86–90. [Google Scholar] [CrossRef]
- Muanda, F.T.; Weir, M.A.; Bathini, L.; Clemens, K.K.; Perkovic, V.; Sood, M.M.; McArthur, E.; Sontrop, J.M.; Kim, R.B.; Garg, A.X. Higher-Dose Sitagliptin and the Risk of Congestive Heart Failure in Older Adults with CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 1728–1739. [Google Scholar] [CrossRef]
- Samad, M.; Malempati, S.; Restini, C.B.A. Natriuretic Peptides as Biomarkers: Narrative Review and Considerations in Cardiovascular and Respiratory Dysfunctions. Yale J. Biol. Med. 2023, 96, 137–149. [Google Scholar] [CrossRef]
- Morgenstern, J.; Groener, J.B.; Jende, J.M.E.; Kurz, F.T.; Strom, A.; Göpfert, J.; Kender, Z.; Le Marois, M.; Brune, M.; Kuner, R.; et al. Neuron-specific biomarkers predict hypo- and hyperalgesia in individuals with diabetic peripheral neuropathy. Diabetologia 2021, 64, 2843–2855. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Xie, M.; Yan, L.; Chen, J.; Wang, H. NSE, a Potential Biomarker, Is Closely Connected to Diabetic Peripheral Neuropathy. Diabetes Care 2013, 36, 3405–3410. [Google Scholar] [CrossRef]
- Dhanapalaratnam, R.; Issar, T.; Wang, L.L.; Tran, D.; Poynten, A.M.; Milner, K.-L.; Kwai, N.C.G.; Krishnan, A.V. Effect of Metformin on Peripheral Nerve Morphology in Type 2 Diabetes: A Cross-Sectional Observational Study. Diabetes 2024, 73, 1875–1882. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Iskander, C.; Wang, C.; Xiong, L.Y.; Shah, B.R.; Edwards, J.D.; Kapral, M.K.; Herrmann, N.; Lanctôt, K.L.; Masellis, M.; et al. Association of sulfonylureas with the risk of dementia: A population-based cohort study. J. Am. Geriatr. Soc. 2023, 71, 3059–3070. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells 2022, 11, 3362. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Sabaner, M.C.; Akdogan, M.; Doğan, M.; Oral, A.Y.; Duman, R.; Koca, T.; Bozkurt, E. Inflammatory cytokines, oxidative and antioxidative stress levels in patients with diabetic macular edema and hyperreflective spots. Eur. J. Ophthalmol. 2021, 31, 2535–2545. [Google Scholar] [CrossRef]
- Viggiano, P.; Vujosevic, S.; Palumbo, F.; Grassi, M.O.; Boscia, G.; Borrelli, E.; Reibaldi, M.; Sborgia, L.; Molfetta, T.; Evangelista, F.; et al. Optical coherence tomography biomarkers indicating visual enhancement in diabetic macular edema resolved through anti-VEGF therapy: OCT biomarkers in resolved DME. Photodiagnosis Photodyn. Ther. 2024, 46, 104042. [Google Scholar] [CrossRef]
- Song, J.; Chen, S.; Liu, X.; Duan, H.; Kong, J.; Li, Z. Relationship between C-Reactive Protein Level and Diabetic Retinopathy: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0144406. [Google Scholar] [CrossRef]
- Mazzeo, A.; Beltramo, E.; Lopatina, T.; Gai, C.; Trento, M.; Porta, M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp. Eye Res. 2018, 176, 69–77. [Google Scholar] [CrossRef]
- Wu, P.C.; Wu, V.C.; Lin, C.J.; Pan, C.F.; Chen, C.Y.; Huang, T.M.; Wu, C.H.; Chen, L.; Wu, C.J. Meglitinides increase the risk of hypoglycemia in diabetic patients with advanced chronic kidney disease: A nationwide, population-based study. Oncotarget 2017, 8, 78086–78095. [Google Scholar] [CrossRef]
- Chow, L.S.; Chen, H.; Miller, M.E.; Marcovina, S.M.; Seaquist, E.R. Biomarkers related to severe hypoglycaemia and lack of good glycaemic control in ACCORD. Diabetologia 2015, 58, 1160–1166. [Google Scholar] [CrossRef]
- Nauck, M.A.; Feldmann, A.; Meier, J.J.; Nauck, M.; Kapitza, C. 342-P: Effects of Sitagliptin on Hypoglycemic Episodes and Counterregulation in Type 2 Diabetic Patients Tightly Titrated with Insulin Glargine. Diabetes 2021, 70 (Suppl. 1), db21–db342. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Knapen, L.M.; de Jong, R.G.; Driessen, J.H.; Keulemans, Y.C.; van Erp, N.P.; De Bruin, M.L.; Leufkens, H.G.; Croes, S.; de Vries, F. Use of incretin agents and risk of acute and chronic pancreatitis: A population-based cohort study. Diabetes Obes. Metab. 2017, 19, 401–411. [Google Scholar] [CrossRef]
- Basnayake, C.; Ratnam, D. Blood tests for acute pancreatitis. Aust. Prescr. 2015, 38, 128–130. [Google Scholar] [CrossRef]
- Nelson, M.; Bhandari, N.; Wener, J. Sitagliptin-induced pancreatitis—A longer road than expected. Clin. Case Rep. 2014, 2, 149–152. [Google Scholar] [CrossRef]
- Alkayali, T.; Ricardo, J.; Busari, K.; Saad, I. Sitagliptin-induced Pancreatitis: Chronic Use Would Not Spare You the Complication. Cureus 2020, 12, e7389. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Kumar, V.; Das, R.; Sharma, V.; Mehta, D.K. Biomarkers of Hepatic Toxicity: An Overview. Curr. Ther. Res. Clin. Exp. 2024, 100, 100737. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Ramos, R.; Guadarrama-López, A.L.; Martínez-Carrillo, B.E.; Benítez-Arciniega, A.D. Vitamins and type 2 diabetes mellitus. Endocr. Metab. Immune Disord Drug Targets 2015, 15, 54–63. [Google Scholar] [CrossRef]
- Hannibal, L.; Lysne, V.; Bjørke-Monsen, A.L.; Behringer, S.; Grünert, S.C.; Spiekerkoetter, U.; Jacobsen, D.W.; Blom, H.J. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front. Mol. Biosci. 2016, 3, 27. [Google Scholar] [CrossRef]
- Gorgojo-Martínez, J.J.; Mezquita-Raya, P.; Carretero-Gómez, J.; Castro, A.; Cebrián-Cuenca, A.; de Torres-Sánchez, A.; García-de-Lucas, M.D.; Núñez, J.; Obaya, J.C.; Soler, M.J.; et al. Clinical Recommendations to Manage Gastrointestinal Adverse Events in Patients Treated with Glp-1 Receptor Agonists: A Multidisciplinary Expert Consensus. J. Clin. Med. 2022, 12, 145. [Google Scholar] [CrossRef]
- Pasricha, P.J.; Grover, M.; Yates, K.P.; Abell, T.L.; Koch, K.L.; McCallum, R.W.; Sarosiek, I.; Bernard, C.E.; Kuo, B.; Bulat, R.; et al. Progress in Gastroparesis—A Narrative Review of the Work of the Gastroparesis Clinical Research Consortium. Clin. Gastroenterol. Hepatol. 2022, 20, 2684–2695.e2683. [Google Scholar] [CrossRef]
- He, L.; Wang, J.; Ping, F.; Yang, N.; Huang, J.; Li, Y.; Xu, L.; Li, W.; Zhang, H. Association of Glucagon-Like Peptide-1 Receptor Agonist Use With Risk of Gallbladder and Biliary Diseases: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2022, 182, 513–519. [Google Scholar] [CrossRef]
- Gong, J.; Feng, Y.; Mei, Y.; Han, S.; Sun, X.; Niu, P.; Tian, J.; Yan, Q.; Li, H.; Zhu, W. Plasma metabolomics and proteomics reveal novel molecular insights and biomarker panel for cholelithiasis. J. Pharm. Biomed. Anal. 2024, 238, 115806. [Google Scholar] [CrossRef] [PubMed]
- Htike, Z.Z.; Zaccardi, F.; Papamargaritis, D.; Webb, D.R.; Khunti, K.; Davies, M.J. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes. Metab. 2017, 19, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, K.; Stefanska, A.; Krintus, M.; Sypniewska, G. Discordance between lipoprotein (a) and LDL-cholesterol levels in cardiovascular risk assessment in apparently healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1429–1436. [Google Scholar] [CrossRef]
- Briand, F.; Mayoux, E.; Brousseau, E.; Burr, N.; Urbain, I.; Costard, C.; Mark, M.; Sulpice, T. Empagliflozin, via Switching Metabolism Toward Lipid Utilization, Moderately Increases LDL Cholesterol Levels Through Reduced LDL Catabolism. Diabetes 2016, 65, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; Szarek, M.; Jukema, J.W.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Fazio, S.; Garon, G.; Goodman, S.G.; Harrington, R.A.; et al. Relation of Low-Density Lipoprotein Cholesterol, High-Sensitivity C-Reactive Protein, and Lipoprotein(a) Each to Future Cardiovascular Events and Death After Acute Coronary Syndrome on High-Intensity Statin Therapy: An Analysis of the Placebo Arm of Odyssey Outcomes. Circulation 2025, 151, 1047–1050. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; McCarty, M.F. Is interleukin-6 the link between low LDL cholesterol and increased non-cardiovascular mortality in the elderly? Open Heart 2018, 5, e000789. [Google Scholar] [CrossRef]
- Antoniadou, C.; Gavriilidis, E.; Ritis, K.; Tsilingiris, D. Anemia in diabetes mellitus: Pathogenetic aspects and the value of early erythropoietin therapy. Metab. Open 2025, 25, 100344. [Google Scholar] [CrossRef]
- Northrop-Clewes, C.A.; Thurnham, D.I. Biomarkers for the differentiation of anemia and their clinical usefulness. J. Blood Med. 2013, 4, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; Burrack, N.; Heymann, A.; Grossman, A.; Neuman, T.; Abuhasira, R. Sodium-Glucose Cotransporter 2 Inhibitors, Erythrocytosis, and Thrombosis in Adults With Type 2 Diabetes. JAMA Netw. Open. 2025, 8, e2517086. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Hsieh, T.Y.J.; Sun, A.Y.-E.; Rastogi, N.; Wei, J.C.-C.; Lee, C.-H. Effect of SGLT2 Inhibitors on Erythrocytosis and Arterial Thrombosis Risk in Patients with Type 2 Diabetes Mellitus: A Real-World Multi-Center Cohort Study across the United States. Blood 2024, 144 (Suppl. 1), 5214. [Google Scholar] [CrossRef]
- Gullaksen, S.; Vernstrøm, L.; Sørensen, S.S.; Ringgaard, S.; Laustsen, C.; Funck, K.L.; Poulsen, P.L.; Laugesen, E. Separate and combined effects of semaglutide and empagliflozin on kidney oxygenation and perfusion in people with type 2 diabetes: A randomised trial. Diabetologia 2023, 66, 813–825. [Google Scholar] [CrossRef]
- Vainchenker, W.; Constantinescu, S.N. A unique activating mutation in JAK2 (V617F) is at the origin of polycythemia vera and allows a new classification of myeloproliferative diseases. Hematol. Am. Soc. Hematol. Educ. Program 2005, 2005, 195–200. [Google Scholar] [CrossRef]
- Schumacher, K.A.; Gosmanov, A.R. Hemochromatosis Gene Mutation in Persons Developing Erythrocytosis on Combined Testosterone and SGLT-2 Inhibitor Therapy. J. Investig. Med. High Impact. Case Rep. 2022, 10, 23247096221111774. [Google Scholar] [CrossRef]
- Aoun, M.; Jadoul, M.; Anders, H.-J. Erythrocytosis and CKD: A Review. Am. J. Kidney Dis. 2024, 84, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, J.; Hajirawala, M.; Jalali, J.; Wild, L. The First Report Of Angioedema And Anaphylaxis With Temporal Association To Semaglutide. Ann. Allergy Asthma Immunol. 2022, 129, S85. [Google Scholar] [CrossRef]
- Hofman, Z.L.M.; de Maat, S.; Suffritti, C.; Zanichelli, A.; van Doorn, C.; Sebastian, S.A.E.; Veszeli, N.; Csuka, D.; Renné, T.; Pasterkamp, G.; et al. Cleaved kininogen as a biomarker for bradykinin release in hereditary angioedema. J. Allergy Clin. Immunol. 2017, 140, 1700–1703.e1708. [Google Scholar] [CrossRef]
- Betschel, S.; Badiou, J.; Binkley, K.; Borici-Mazi, R.; Hébert, J.; Kanani, A.; Keith, P.; Lacuesta, G.; Waserman, S.; Yang, B.; et al. The International/Canadian Hereditary Angioedema Guideline. Allergy Asthma. Clin. Immunol. 2019, 15, 72. [Google Scholar] [CrossRef]
- Sharma, N.R.; Sharma, B.; Lamichhane, S.; Pokhrel, M.; Shrestha, P. A Rare Case Report of Sitagliptin-Induced Angioedema. Cureus 2022, 14, e30077. [Google Scholar] [CrossRef]
- Pradhan, R.; Montastruc, F.; Rousseau, V.; Patorno, E.; Azoulay, L. Exendin-based glucagon-like peptide-1 receptor agonists and anaphylactic reactions: A pharmacovigilance analysis. Lancet Diabetes Endocrinol. 2020, 8, 13–14. [Google Scholar] [CrossRef]
- Beck, S.C.; Wilding, T.; Buka, R.J.; Baretto, R.L.; Huissoon, A.P.; Krishna, M.T. Biomarkers in Human Anaphylaxis: A Critical Appraisal of Current Evidence and Perspectives. Front. Immunol. 2019, 10, 494. [Google Scholar] [CrossRef]
- Anthony, M.S.; Aroda, V.R.; Parlett, L.E.; Djebarri, L.; Berreghis, S.; Calingaert, B.; Beachler, D.C.; Crowe, C.L.; Johannes, C.B.; Juhaeri, J.; et al. Risk of Anaphylaxis Among New Users of GLP-1 Receptor Agonists: A Cohort Study. Diabetes Care 2024, 47, 712–719. [Google Scholar] [CrossRef]
- Saini, K.; Sharma, S.; Khan, Y. DPP-4 inhibitors for treating T2DM-hype or hope? an analysis based on the current literature. Front. Mol. Biosci. 2023, 10, 1130625. [Google Scholar] [CrossRef]
- Zaresharifi, S.; Niroomand, M.; Borran, S.; Dadkhahfar, S. Dermatological side effects of dipeptidyl Peptidase-4 inhibitors in diabetes management: A comprehensive review. Clin. Diabetes Endocrinol. 2024, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Prescribing Information. JANUVIA (Sitagliptin); Merck: Rahway, NJ, USA, 2006. [Google Scholar]
- Williams-Herman, D.; Round, E.; Swern, A.S.; Musser, B.; Davies, M.J.; Stein, P.P.; Kaufman, K.D.; Amatruda, J.M. Safety and tolerability of sitagliptin in patients with type 2 diabetes: A pooled analysis. BMC Endocr. Disord. 2008, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Morel, E.; Alvarez, L.; Cabanas, R.; Fiandor, A.; Diaz, R.; Escamochero, S.; Prior, N.; Blanca, M.; Bellón, T. Expression of α-defensin 1–3 in T cells from severe cutaneous drug-induced hypersensitivity reactions. Allergy 2011, 66, 360–367. [Google Scholar] [CrossRef]
- Kinoshita, M.; Ogawa, Y.; Hama, N.; Ujiie, I.; Hasegawa, A.; Nakajima, S.; Nomura, T.; Adachi, J.; Sato, T.; Koizumi, S.; et al. Neutrophils initiate and exacerbate Stevens-Johnson syndrome and toxic epidermal necrolysis. Sci. Transl. Med. 2021, 13, eaax2398. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Abe, R. Stevens–Johnson syndrome and toxic epidermal necrolysis: Updates in pathophysiology and management. Chin. Med. J. 2024, 137, 2294–2307. [Google Scholar] [CrossRef]
- Kocaaga, A.; Kocaaga, M. A review of the immunogenetics of Stevens-Johnson syndrome and toxic epidermal necrolysis. Glob. Med. Genet. 2025, 12, 100054. [Google Scholar] [CrossRef]
- Vujčić, S.; Kotur-Stevuljević, J.; Vekić, J.; Perović-Blagojević, I.; Stefanović, T.; Ilić-Mijailović, S.; Koprivica Uzelac, B.; Bosić, S.; Antonić, T.; Guzonjić, A.; et al. Oxidative Stress and Inflammatory Biomarkers in Patients with Diabetic Foot. Medicina 2022, 58, 1866. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.E. ‘Ammonia, lactate and blood gases—A user’s guide’. Paediatr. Child. Health 2019, 29, 142–145. [Google Scholar] [CrossRef]
- De Fronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Yang, H.; Dai, H.; Chen, X.; Huang, J.; Miao, F.; Lv, J.; Zhang, J. Risk of medication-induced lactic acidosis and hyperlactatemia: A pharmacovigilance study of the United States Food and Drug Administration’s Adverse Event Reporting System database. Front. Pharmacol. 2025, 16, 1555955. [Google Scholar] [CrossRef]
- See, K.C. Metformin-associated lactic acidosis: A mini review of pathophysiology, diagnosis and management in critically ill patients. World J. Diabetes 2024, 15, 1178–1186. [Google Scholar] [CrossRef]
- Smeijer, J.D.; Kohan, D.E.; de Zeeuw, D.; Heerspink, H.J.L. Diuretic medication and change in fluid retention biomarkers during treatment with the endothelin receptor antagonist atrasentan. Diabetes Obes. Metab. 2023, 25, 2419–2422. [Google Scholar] [CrossRef]
- Singer, E.; Markó, L.; Paragas, N.; Barasch, J.; Dragun, D.; Müller, D.N.; Budde, K.; Schmidt-Ott, K.M. Neutrophil gelatinase-associated lipocalin: Pathophysiology and clinical applications. Acta Physiol. 2013, 207, 663–672. [Google Scholar] [CrossRef]
- Moledina, D.G.; Wilson, F.P.; Pober, J.S.; Perazella, M.A.; Singh, N.; Luciano, R.L.; Obeid, W.; Lin, H.; Kuperman, M.; Moeckel, G.W.; et al. Urine TNF-α and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight. 2019, 4, e127456. [Google Scholar] [CrossRef]
- Ostermann, M.; Legrand, M.; Meersch, M.; Srisawat, N.; Zarbock, A.; Kellum, J.A. Biomarkers in acute kidney injury. Ann. Intensive Care 2024, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.J.; Grigoryev, D.N.; Linfert, D.; Jang, H.R.; Watkins, T.; Cheadle, C.; Racusen, L.; Rabb, H. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am. J. Physiol. Ren. Physiol. 2010, 298, F1472–F1483. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Bright, R.; Truong, V.-K.; Li, J.; Juneja, R.; Vasilev, K. Key biomarkers in type 2 diabetes patients: A systematic review. Diabetes Obes. Metab. 2025, 27, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8, S3. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Singh, B.; Goyal, A.; Patel, B.C. C-Reactive Protein: Clinical Relevance and Interpretation. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Rees, R.F.; Gewurz, H.; Siegel, J.N.; Coon, J.; Potempa, L.A. Expression of a C-reactive protein neoantigen (neo-CRP) in inflamed rabbit liver and muscle. Clin. Immunol. Immunopathol. 1988, 48, 95–107. [Google Scholar] [CrossRef]
- Khreiss, T.; József, L.; Potempa, L.A.; Filep, J.G. Opposing Effects of C-Reactive Protein Isoforms on Shear-Induced Neutrophil-Platelet Adhesion and Neutrophil Aggregation in Whole Blood. Circulation 2004, 110, 2713–2720. [Google Scholar] [CrossRef]
- Eisenhardt, S.U.; Habersberger, J.; Murphy, A.; Chen, Y.-C.; Woollard, K.J.; Bassler, N.; Qian, H.; von zur Muhlen, C.; Hagemeyer, C.E.; Ahrens, I.; et al. Dissociation of Pentameric to Monomeric C-Reactive Protein on Activated Platelets Localizes Inflammation to Atherosclerotic Plaques. Circ. Res. 2009, 105, 128–137. [Google Scholar] [CrossRef]
- Ji, S.-R.; Ma, L.; Bai, C.-J.; Shi, J.-M.; Li, H.-Y.; Potempa, L.A.; Filep, J.G.; Zhao, J.; Wu, Y. Monomeric C-reactive protein activates endothelial cells via interaction with lipid raft microdomains. FASEB J. 2009, 23, 1806–1816. [Google Scholar] [CrossRef]
- Ménégaut, L.; Laubriet, A.; Crespy, V.; Leleu, D.; Pilot, T.; Van Dongen, K.; de Barros, J.P.; Gautier, T.; Petit, J.M.; Thomas, C.; et al. Inflammation and oxidative stress markers in type 2 diabetes patients with Advanced Carotid atherosclerosis. Cardiovasc Diabetol 2023, 22, 248. [Google Scholar] [CrossRef] [PubMed]
- Shaheer, A.K.; Tharayil, J.K.; Krishna, P.W. A Comparative Study of High Sensitivity C-Reactive Protein and Metabolic Variables in Type 2 Diabetes Mellitus with and without Nephropathy. J. Clin. Diagn. Res. 2017, 11, Bc01–Bc04. [Google Scholar] [CrossRef]
- Ghiasi Hafezi, S.; Sahranavard, T.; Kooshki, A.; Hosseini, M.; Mansoori, A.; Fakhrian, E.A.; Rezaeifard, H.; Ghamsary, M.; Esmaily, H.; Ghayour-Mobarhan, M. Predicting high sensitivity C-reactive protein levels and their associations in a large population using decision tree and linear regression. Sci. Rep. 2024, 14, 30298. [Google Scholar] [CrossRef] [PubMed]
- Bowker, N.; Shah, R.L.; Sharp, S.J.; Luan, J.a.; Stewart, I.D.; Wheeler, E.; Ferreira, M.A.R.; Baras, A.; Wareham, N.J.; Langenberg, C.; et al. Meta-analysis investigating the role of interleukin-6 mediated inflammation in type 2 diabetes. eBioMedicine 2020, 61, 103062. [Google Scholar] [CrossRef]
- Lee, C.C.; Adler, A.I.; Sandhu, M.S.; Sharp, S.J.; Forouhi, N.G.; Erqou, S.; Luben, R.; Bingham, S.; Khaw, K.T.; Wareham, N.J. Association of C-reactive protein with type 2 diabetes: Prospective analysis and meta-analysis. Diabetologia 2009, 52, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Anness, A.R.; Baldo, A.; Webb, D.R.; Khalil, A.; Robinson, T.G.; Mousa, H.A. Effect of metformin on biomarkers of placental- mediated disease: A systematic review and meta-analysis. Placenta 2021, 107, 51–58. [Google Scholar] [CrossRef]
- Gu, Y.; Hu, K.; Huang, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Xia, Y.; Bao, X.; Shi, H.; et al. White blood cells count as an indicator to identify whether obesity leads to increased risk of type 2 diabetes. Diabetes Res. Clin. Pr. 2018, 141, 140–147. [Google Scholar] [CrossRef]
- Bonfigli, A.R.; Spazzafumo, L.; Prattichizzo, F.; Bonafè, M.; Mensà, E.; Micolucci, L.; Giuliani, A.; Fabbietti, P.; Testa, R.; Boemi, M.; et al. Leukocyte telomere length and mortality risk in patients with type 2 diabetes. Oncotarget 2016, 7, 50835–50844. [Google Scholar] [CrossRef]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The Good and the Bad: Monocytes’ and Macrophages’ Diverse Functions in Inflammation. Cells 2022, 11, 50835–50844. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus-An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef]
- Wang, D.; Chen, F.; Han, Z.; Yin, Z.; Ge, X.; Lei, P. Relationship Between Amyloid-β Deposition and Blood–Brain Barrier Dysfunction in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 695479. [Google Scholar] [CrossRef]
- Trim, W.V.; Lynch, L. Immune and non-immune functions of adipose tissue leukocytes. Nat. Rev. Immunol. 2022, 22, 371–386. [Google Scholar] [CrossRef]
- Chapman, N.M.; Chi, H. Metabolic adaptation of lymphocytes in immunity and disease. Immunity 2022, 55, 14–30. [Google Scholar] [CrossRef]
- Reimold, A.M. TNFalpha as therapeutic target: New drugs, more applications. Curr. Drug Targets Inflamm. Allergy 2002, 1, 377–392. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef]
- McGrath, E.R.; Beiser, A.S.; O’Donnell, A.; Himali, J.J.; Pase, M.P.; Satizabal, C.L.; Seshadri, S. Determining Vascular Risk Factors for Dementia and Dementia Risk Prediction Across Mid- to Later Life: The Framingham Heart Study. Neurology 2022, 99, e142–e153. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.A.; Bersano, A.; Manso-Calderón, R.; Jia, J.P.; Schmidt, H.; Middleton, L.; Nacmias, B.; Siddiqi, S.; Adams, H.H. Genetics of vascular dementia—Review from the ICVD working group. BMC Med. 2017, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.; Matu, J.; Tang, E.Y.H.; Gregory, S.; Anderson, E.; Fairley, A.; Townsend, R.; Stevenson, E.; Stephan, B.C.M.; Siervo, M.; et al. Foods, dietary patterns, and risk of vascular dementia: A systematic review. Nutr. Metab. 2024, 21, 105. [Google Scholar] [CrossRef]
- Saeed, A.; Lopez, O.; Cohen, A.; Reis, S.E. Cardiovascular Disease and Alzheimer’s Disease: The Heart—Brain Axis. J. Am. Heart Assoc. 2023, 12, e030780. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lelieveldt, T.; Sturkenboom, M.; Biessels, G.J.; Ahmadizar, F. Evaluating the Causal Association Between Type 2 Diabetes and Alzheimer’s Disease: A Two-Sample Mendelian Randomization Study. Biomedicines 2025, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qian, H.; Feng, L.; Li, M. Causal Association Between Type 2 Diabetes Mellitus and Alzheimer’s Disease: A Two-Sample Mendelian Randomization Study. J. Alzheimers. Dis. Rep. 2024, 8, 945–957. [Google Scholar] [CrossRef]
- Bender, E.C.; Tareq, H.S.; Suggs, L.J. Inflammation: A matter of immune cell life and death. npj Biomed. Innov. 2025, 2, 7. [Google Scholar] [CrossRef]
- Tan, E.-K.; Chao, Y.-X.; West, A.; Chan, L.-L.; Poewe, W.; Jankovic, J. Parkinson disease and the immune system—Associations, mechanisms and therapeutics. Nat. Rev. Neurol. 2020, 16, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Zhang, W.; McDonald, D. Diabetic retinopathy: Mechanism, diagnosis, prevention, and treatment. Biomed Res. Int. 2015, 2015, 854593. [Google Scholar] [CrossRef]
- Mapanga, R.F.; Essop, M.F. Damaging effects of hyperglycemia on cardiovascular function: Spotlight on glucose metabolic pathways. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H153–H173. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Li, Z.; Han, J.; Xia, P.; Shen, Y.; Ma, J.; Liu, X.; Zhang, J.; Yu, P. Advances in the study of RNA-binding proteins in diabetic complications. Mol. Metab. 2022, 62, 101515. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Kropp, M.; Golubnitschaja, O.; Mazurakova, A.; Koklesova, L.; Sargheini, N.; Vo, T.K.S.; de Clerck, E.; Polivka, J., Jr.; Potuznik, P.; Polivka, J.; et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications-risks and mitigation. Epma J. 2023, 14, 21–42. [Google Scholar] [CrossRef]
- Jeng-Miller, K.W.; Baumal, C.R. Chapter 5—Genetics of Diabetic Retinopathy. In Current Management of Diabetic Retinopathy; Baumal, C.R., Duker, J.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–40. [Google Scholar]
- Gupta, S.; Thool, A.R. A Narrative Review of Retinopathy in Diabetic Patients. Cureus 2024, 16, e52308. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Takeda, N.; Hara, H.; Ishii, S.; Numata, G.; Tokiwa, H.; Katoh, M.; Maemura, S.; Suzuki, T.; Takiguchi, H.; et al. PGC-1α–mediated angiogenesis prevents pulmonary hypertension in mice. JCI Insight 2023, 8, e162632. [Google Scholar] [CrossRef]
- Cunningham, K.F.; Beeson, G.C.; Beeson, C.C.; Baicu, C.F.; Zile, M.R.; McDermott, P.J. Estrogen-Related Receptor α (ERRα) is required for adaptive increases in PGC-1 isoform expression during electrically stimulated contraction of adult cardiomyocytes in sustained hypoxic conditions. Int. J. Cardiol. 2015, 187, 393–400. [Google Scholar] [CrossRef]
- Shoag, J.; Arany, Z. Regulation of Hypoxia-Inducible Genes by PGC-1α. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 662–666. [Google Scholar] [CrossRef]
- Willy, P.J.; Murray, I.R.; Qian, J.; Busch, B.B.; Stevens, W.C.; Martin, R.; Mohan, R.; Zhou, S.; Ordentlich, P.; Wei, P.; et al. Regulation of PPARγ coactivator 1α (PGC-1α) signaling by an estrogen-related receptor α (ERRα) ligand. Proc. Natl. Acad. Sci. USA 2004, 101, 8912–8917. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Nawaz, M.I.; Ahmad, A.; Siddiquei, M.M.; Allegaert, E.; Gikandi, P.W.; De Hertogh, G.; Opdenakker, G. A key role of the PGC-1alpha/ERR-alpha pathway in regulation of angiogenic factors in proliferative diabetic retinopathy. Front. Endocrinol. 2025, 16, 1615103. [Google Scholar] [CrossRef]
- Jonas, J.B.; Neumaier, M. Erythropoietin levels in aqueous humour in eyes with exudative age-related macular degeneration and diabetic retinopathy. Clin. Exp. Ophthalmol. 2007, 35, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, A.A.R.; Jayashree, K.; Senthilkumar, G.P.; Thomas, S.E.; Babu, K.R. An Assessment of Serum Irisin and Intercellular Adhesion Molecule-1 as Potential Indicators of Retinopathy in Type 2 Diabetes Mellitus. Niger. Postgrad. Med. J. 2025, 32, 240–246. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, N.; Zhang, Y.; Gu, X.; Li, H.; Yu, X. Postprandial C-Peptide to Glucose Ratio as a Promising Systemic Marker of Diabetic Retinopathy in Type 2 Diabetes. Transl. Vis. Sci. Technol. 2025, 14, 27. [Google Scholar] [CrossRef]
- Wei, Z.M.; Zhao, Y.; Ding, R.R.; Zeng, Y.S.; Zeng, Z.; He, Z.T.; Hao, J.; Hu, J.J.; Yu, J.G.; You, C.Y. Combination of red blood cell distribution width and platelet-to-lymphocyte ratio for predicting severity of diabetic retinopathy. Int. J. Ophthalmol. 2025, 18, 1506–1514. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Y.; Jiang, F.; Zhang, K.; Meng, X.; Zheng, Y.; He, M. Unveiling biomarkers via plasma metabolome profiling for diabetic macrovascular and microvascular complications. Cardiovasc. Diabetol. 2025, 24, 341. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Benedikter, B.J.; Mokhtar, S.B.A.; van der Heide, F.C.T.; Kumaramanickavel, G.; van Greevenbroek, M.M.J.; Webers, C.A.B.; Berendschot, T. Plasma Sphingomyelins as Biomarkers for Diabetic Retinal Neurodegeneration: The Maastricht Study. Ophthalmol. Sci. 2025, 5, 100870. [Google Scholar] [CrossRef]
- Hao, Y.; She, X.; Huang, G.; Chu, X.; Zhao, S.; Lv, Z.; Tao, J.; Zhang, Y. Metabolomic insights into vitreous humor with therapy outcome in type 2 diabetic retinopathy. BMC Ophthalmol. 2025, 25, 460. [Google Scholar] [CrossRef]
- Zooravar, D.; Shojaei, S.; Mousavi, A.; Soltani, P.; Amiri, B.S.; Radkhah, H. Novel Lipid Biomarkers and Microvascular Complications in Patients with Diabetes Mellitus: A Systematic Review and Meta-analysis. Clin. Med. Insights Endocrinol Diabetes 2025, 18, 11795514251365301. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Yang, Q.; Hong, Y.; Tan, R.; Ibrahim, F.N.I.; Lim, C.; Choo, J.; Sabanayagam, C.; Coffman, T.M.; Wong, T.Y.; et al. Retinal Neuronal Changes and Kidney Dysfunction in Diabetes Mellitus. Clin. Exp. Ophthalmol. 2025, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Conserva, F.; Gesualdo, L.; Papale, M. A Systems Biology Overview on Human Diabetic Nephropathy: From Genetic Susceptibility to Post-Transcriptional and Post-Translational Modifications. J. Diabetes Res. 2016, 2016, 7934504. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.P. Diabetic Nephropathy and End-Stage Renal Disease. In Complete Nurse’s Guide to Diabetes Care; Childs, B.P., Cypress, M., Spollett, G.R., Eds.; American Diabetes Association: Arlington, VA, USA, 2017. [Google Scholar]
- Agarwal, R. Pathogenesis of Diabetic Nephropathy. Compendia 2021, 2021, 2–7. [Google Scholar] [CrossRef]
- Natesan, V.; Kim, S.J. Diabetic Nephropathy—A Review of Risk Factors, Progression, Mechanism, and Dietary Management. Biomol. Ther. 2021, 29, 365–372. [Google Scholar] [CrossRef]
- Hostetter, T.H.; Rennke, H.G.; Brenner, B.M. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am. J. Med. 1982, 72, 375–380. [Google Scholar] [CrossRef]
- Wu, T.; Ding, L.; Andoh, V.; Zhang, J.; Chen, L. The Mechanism of Hyperglycemia-Induced Renal Cell Injury in Diabetic Nephropathy Disease: An Update. Life 2023, 13, 539. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhang, Y.; Fang, X.; Zhang, Y.; Miao, R.; Yao, Y.; Guan, H.; Tian, J. Discovering diabetes complications-related microRNAs: Meta-analyses and pathway modeling approach. BMC Med. Genom. 2025, 18, 86. [Google Scholar] [CrossRef]
- Abdel-Tawab, M.S.; Mohamed, M.G.; Doudar, N.A.; Rateb, E.E.; Reyad, H.R.; Elazeem, N.A.A. Circulating hsa-miR-221 as a possible diagnostic and prognostic biomarker of diabetic nephropathy. Mol. Biol. Rep. 2023, 50, 9793–9803. [Google Scholar] [CrossRef] [PubMed]
- Abdelgayed, G.; Hosni, A.; Abdel-Moneim, A.; Malik, A.; Zaky, M.Y.; Hasona, N.A. Integrated analysis of long non-coding RNA megacluster, microRNA-132 and microRNA-133a and their implications for cardiovascular risk and kidney failure progression in diabetic patients. Exp. Ther. Med. 2025, 29, 35. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Liu, Y.; Sattar, N.; Yavin, Y.; Pollock, C.A.; Butler, J.; Jardine, M.; Heerspink, H.J.L.; Masson, S.; Breyer, M.; et al. Vascular endothelial growth factors and risk of cardio-renal events: Results from the CREDENCE trial. Am. Heart J. 2024, 271, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kralisch, S.; Fasshauer, M. Adipocyte fatty acid binding protein: A novel adipokine involved in the pathogenesis of metabolic and vascular disease? Diabetologia 2013, 56, 10–21. [Google Scholar] [CrossRef]
- Xie, T.; Leung, P.S. Fibroblast growth factor 21: A regulator of metabolic disease and health span. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E292–E302. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Quan, X.; Qin, X.; Zhou, Y.; Liu, Z.; Chao, Z.; Jia, C.; Qin, H.; Zhang, H. Pigment epithelium-derived factor and its role in microvascular-related diseases. Biochimie 2022, 200, 153–171. [Google Scholar] [CrossRef]
- Cheung, C.Y.Y.; Lee, C.-H.; Tang, C.S.; Xu, A.; Au, K.-W.; Fong, C.H.Y.; Ng, K.K.K.; Kwok, K.H.M.; Chow, W.-S.; Woo, Y.-C.; et al. Genetic Regulation of Pigment Epithelium-Derived Factor (PEDF): An Exome-Chip Association Analysis in Chinese Subjects With Type 2 Diabetes. Diabetes 2018, 68, 198–206. [Google Scholar] [CrossRef]
- Sbriscia, M.; Caccese, S.; Marchegiani, F.; Recchioni, R.; Matacchione, G.; Giordani, C.; Francini, E.; Salvioli, S.; Conte, M.; Landolfo, M.; et al. A scoring model integrating CXCL9, GDF15, FGF21, and NfL, predicts long-term mortality in type 2 diabetes: A retrospective study. Cardiovasc. Diabetol. 2025, 24, 270. [Google Scholar] [CrossRef]
- van Heck, J.I.P.; Ajie, M.; Joosten, L.A.B.; Tack, C.J.; Stienstra, R. Circulating inflammatory proteins are elevated in type 1 and type 2 diabetes and associated to complications. Diabetes Obes. Metab. 2025, 27, 719–728. [Google Scholar] [CrossRef]
- AlMajed, H.T.; Abu-Farha, M.; Alshawaf, E.; Devarajan, S.; Alsairafi, Z.; Elhelaly, A.; Cherian, P.; Al-Khairi, I.; Ali, H.; Jose, R.M.; et al. Increased Levels of Circulating IGFBP4 and ANGPTL8 with a Prospective Role in Diabetic Nephropathy. Int. J. Mol. Sci. 2023, 24, 4244. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, C.G.; Ma, L.J. Role of GDF-15 in diabetic nephropathy: Mechanisms, diagnosis, and therapeutic potential. Int. Urol. Nephrol. 2025, 57, 169–175. [Google Scholar] [CrossRef]
- Hou, Q.; Huang, K.; Liang, Y.; Yu, W.; Long, L.; Wang, K.; Yi, B. Associations between serum JAML, nesfatin-1, and 25(OH)D and the risk of diabetic kidney disease in patients with type 2 diabetes. Sci. Rep. 2025, 15, 27438. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; van Goor, H.; Bakker, S.J.L.; Hillebrands, J.L.; Bilo, H.J.G.; Dullaart, R.P.F.; van Dijk, P.R. Serum peroxiredoxin-4, a biomarker of oxidative stress, is associated with the development of nephropathy in patients with type 2 diabetes (Zodiac-65). Free Radic. Biol. Med. 2024, 212, 186–190. [Google Scholar] [CrossRef]
- Ghareghani, O.; Ghareghani, S.; Takhshid, M.A. Diagnostic values of ischemia modified albumin in diabetes-related complications: A narrative review. J. Diabetes Metab. Disord. 2023, 22, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Spoto, B.; Politi, C.; Pizzini, P.; Parlongo, R.M.; Testa, A.; Mobrici, M.; Tripepi, G.L.; Mallamaci, F.; Zoccali, C. 8-hydroxy-2′-deoxyguanosine, a biomarker of oxidative DNA injury, in diabetic kidney disease. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103722. [Google Scholar] [CrossRef]
- Roointan, A.; Shafieizadegan, S.; Ghaeidamini, M.; Gheisari, Y.; Hudkins, K.L.; Gholaminejad, A. The potential of cardiac biomarkers, NT-ProBNP and troponin T, in predicting the progression of nephropathy in diabetic patients: A meta-analysis of prospective cohort studies. Diabetes Res. Clin. Pr. 2023, 204, 110900. [Google Scholar] [CrossRef] [PubMed]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.K.; Inglis, J.T.; Madden, K.M.; Boyd, L.A. Brain and Body: A Review of Central Nervous System Contributions to Movement Impairments in Diabetes. Diabetes 2019, 69, 3–11. [Google Scholar] [CrossRef]
- Erus, G.; Battapady, H.; Zhang, T.; Lovato, J.; Miller, M.E.; Williamson, J.D.; Launer, L.J.; Bryan, R.N.; Davatzikos, C. Spatial patterns of structural brain changes in type 2 diabetic patients and their longitudinal progression with intensive control of blood glucose. Diabetes Care 2015, 38, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Kawamura, N.; Dyck, P.J.; Dyck, P.J.B.; Kihara, M.; Low, P.A. Spectrum of diabetic neuropathies. Diabetol. Int. 2020, 11, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Lee, M.K. Autonomic dysfunction, diabetes and metabolic syndrome. J. Diabetes Investig. 2021, 12, 2108–2111. [Google Scholar] [CrossRef]
- Serhiyenko, V.A.; Serhiyenko, A.A. Cardiac autonomic neuropathy: Risk factors, diagnosis and treatment. World J. Diabetes 2018, 9, 1–24. [Google Scholar] [CrossRef]
- Itani, M.; Gylfadottir, S.S.; Krøigård, T.; Kristensen, A.G.; Christensen, D.H.; Karlsson, P.; Möller, S.; Andersen, H.; Tankisi, H.; Nielsen, J.S.; et al. Small and large fiber sensory polyneuropathy in type 2 diabetes: Influence of diagnostic criteria on neuropathy subtypes. J. Peripher. Nerv. Syst. 2021, 26, 55–65. [Google Scholar] [CrossRef]
- Li, Y.; Wu, W.; Xue, L.; Zhao, T.; Lu, Y.; Qiao, X.; Ding, H. Plantar Tissue Characteristics in People With Diabetes With and Without Peripheral Neuropathy: A Novel Explanatory Model for DPN Risk Assessment. J. Diabetes 2025, 17, e70094. [Google Scholar] [CrossRef]
- Ascaso, P.; Palanca, A.; Martinez-Hervas, S.; Sanz, M.J.; Ascaso, J.F.; Piqueras, L.; Real, J.T. Peripheral blood levels of CXCL10 are a useful marker for diabetic polyneuropathy in subjects with type 2 diabetes. Int. J. Clin. Pr. 2021, 75, e14302. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, C.; Pramanik, S.; Dash, D.; Mukherjee, B.; Malik, R.A.; Mukhopadhyay, S. Circulating netrin-1 levels are reduced and related to corneal nerve fiber loss in patients with diabetic neuropathy. J. Diabetes Investig. 2024, 15, 1068–1074. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K.W. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Hviid, C.V.B.; Rasmussen, N.H.; Roikjer, J. Glial fibrillary acidic protein: A potential biomarker for small fiber neuropathy? Acta. Diabetol. 2025, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Z.; Li, Y.; Liu, S.; Hu, P.; Luo, E. Advanced glycation end products and reactive oxygen species: Uncovering the potential role of ferroptosis in diabetic complications. Mol. Med. 2024, 30, 141. [Google Scholar] [CrossRef]
- Jakhotia, S.; Sivaprasad, M.; Shalini, T.; Reddy, P.Y.; Viswanath, K.; Jakhotia, K.; Sahay, R.; Sahay, M.; Reddy, G.B. Circulating levels of Hsp27 in microvascular complications of diabetes: Prospects as a biomarker of diabetic nephropathy. J. Diabetes Complicat. 2018, 32, 221–225. [Google Scholar] [CrossRef]
- Matuschik, L.; Riabov, V.; Schmuttermaier, C.; Sevastyanova, T.; Weiss, C.; Klüter, H.; Kzhyshkowska, J. Hyperglycemia Induces Inflammatory Response of Human Macrophages to CD163-Mediated Scavenging of Hemoglobin-Haptoglobin Complexes. Int. J. Mol. Sci. 2022, 23, 1385. [Google Scholar] [CrossRef]
- Le Jeune, S.; Sadoudi, S.; Charue, D.; Abid, S.; Guigner, J.M.; Helley, D.; Bihan, H.; Baudry, C.; Lelong, H.; Mirault, T.; et al. Low grade intravascular hemolysis associates with peripheral nerve injury in type 2 diabetes. PLoS ONE 2022, 17, e0275337. [Google Scholar] [CrossRef]
- Shao, C.; Wang, J.; Tian, J.; Tang, Y.-d. Coronary Artery Disease: From Mechanism to Clinical Practice. In Coronary Artery Disease: Therapeutics and Drug Discovery; Wang, M., Ed.; Springer: Singapore, 2020; pp. 1–36. [Google Scholar]
- Dziopa, K.; Chaturvedi, N.; Asselbergs, F.W.; Schmidt, A.F. Identifying and ranking non-traditional risk factors for cardiovascular disease prediction in people with type 2 diabetes. Commun Med. 2025, 5, 77. [Google Scholar] [CrossRef]
- Morales-Villegas, E. Dyslipidemia, Hypertension and Diabetes Metaflammation. A Unique Mechanism for 3 Risk Factors. Curr. Hypertens Rev. 2014, 9, 278–296. [Google Scholar] [CrossRef]
- Jacoby, R.M.; Nesto, R.W. Acute myocardial infarction in the diabetic patient: Pathophysiology, clinical course and prognosis. J. Am. Coll. Cardiol. 1992, 20, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Patoulias, D.; Vlachakis, P.K.; Antoniadis, A.P.; Fragakis, N. Diabetes-Driven Atherosclerosis: Updated Mechanistic Insights and Novel Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 2196. [Google Scholar] [CrossRef] [PubMed]
- John, J.E.; Claggett, B.; Skali, H.; Solomon, S.D.; Cunningham, J.W.; Matsushita, K.; Konety, S.H.; Kitzman, D.W.; Mosley, T.H.; Clark, D.; et al. Coronary Artery Disease and Heart Failure With Preserved Ejection Fraction: The ARIC Study. J. Am. Heart Assoc. 2022, 11, e021660. [Google Scholar] [CrossRef]
- Braunwald, E.; Morrow, D.A. Unstable Angina. Circulation 2013, 127, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.A. Chapter 15—Chronic Stable Angina. In Cardiology Secrets, 5th ed.; Levine, G.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–142. [Google Scholar]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease. The Framingham study. Jama 1979, 241, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, Y.; Ning, Y.; Peng, X.; Zhao, H.; Feng, J.; Lin, L.; Ruan, C.; Chen, S.; Tian, J.; et al. Modifiable Factors and 10-Year and Lifetime Risk of Cardiovascular Disease in Adults With New-Onset Diabetes: The Kailuan Cohort Study. J. Am. Heart Assoc. 2025, 14, e041223. [Google Scholar] [CrossRef]
- Jasaszwili, M.; Billert, M.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin as A Fat-Burning Hormone with Multiple Functions-Review of a Decade of Research. Molecules 2020, 25, 549. [Google Scholar] [CrossRef]
- Mushala, B.A.S.; Scott, I. Adropin: A hepatokine modulator of vascular function and cardiac fuel metabolism. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H238–H244. [Google Scholar] [CrossRef]
- Berezina, T.A.; Berezin, O.O.; Novikov, E.V.; Berezin, A.E. The Association of Adropin with Asymptomatic Coronary Calcification in Patients in Early Stages of Chronic Kidney Disease. Int. J. Mol. Sci. 2025, 26, 7816. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, C.G.; Guo, Y.L.; Wu, N.Q.; Dong, Q.; Xu, R.X.; Wu, Y.J.; Qian, J.; Li, J.J. Prognostic Value of Plasma Endothelin-1 in Predicting Worse Outcomes in Patients with Prediabetes and Diabetes and Stable Coronary Artery Diseases. Diabetes Metab. J. 2024, 48, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Boieriu, A.M.; Luca, C.D.; Neculoiu, C.D.; Bisoc, A.; Tint, D. Endothelial Dysfunction and Oxidative Stress in Patients with Severe Coronary Artery Disease: Does Diabetes Play a Contributing Role? Medicina 2025, 61, 135. [Google Scholar] [CrossRef]
- Hwang, D.; Park, S.-H.; Koo, B.-K. Ischemia With Nonobstructive Coronary Artery Disease. JACC Asia 2023, 3, 169–184. [Google Scholar] [CrossRef]
- Ferrone, M.; Ciccarelli, M.; Varzideh, F.; Kansakar, U.; Guerra, G.; Cerasuolo, F.A.; Buonaiuto, A.; Fiordelisi, A.; Venga, E.; Esposito, M.; et al. Endothelial microRNAs in INOCA patients with diabetes mellitus. Cardiovasc. Diabetol. 2024, 23, 268. [Google Scholar] [CrossRef]
- Zuo, Y.; NaveenKumar, S.K.; Navaz, S.; Liang, W.; Sugur, K.; Kmetova, K.; Ayers, C.R.; Kluge, L.; Chong, E.; Shah, A.M.; et al. Epidemiological and Translational Study of Calprotectin and Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2025, 10, 718–727. [Google Scholar] [CrossRef]
- Carnazzo, V.; Redi, S.; Basile, V.; Natali, P.; Gulli, F.; Equitani, F.; Marino, M.; Basile, U. Calprotectin: Two sides of the same coin. Rheumatology 2024, 63, 26–33. [Google Scholar] [CrossRef]
- Gigante, B.; Chen, Q.; Bjorkbacka, H.; Bjornson, E.; Brinck, J.; Chorell, E.; Djekic, D.; Edsfeldt, A.; Engstrom, G.; Eriksson, J.W.; et al. Lipoproteins and lipoprotein lipid composition are associated with stages of dysglycemia and subclinical coronary atherosclerosis. Int. J. Cardiol. 2025, 419, 132698. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, W.; Bourron, O.; Materne, C.; Galier, S.; Phan, F.; Tan-Chen, S.; Guillas, I.; Hartemann, A.; Salem, J.E.; Redheuil, A.; et al. Inverse relationship between circulating sphingosine-1-phosphate and precursor species and coronary artery calcification score in type 2 diabetes. Cardiovasc. Diabetol. 2025, 24, 85. [Google Scholar] [CrossRef]
- Xu, L.; Yan, X.; Tang, Z.; Feng, B. Association between circulating oxidized OxLDL/LDL-C ratio and the severity of coronary atherosclerosis, along with other emerging biomarkers of cardiovascular disease in patients with type 2 diabetes. Diabetes Res. Clin. Pr. 2022, 191, 110040. [Google Scholar] [CrossRef]
- Hultgårdh-Nilsson, A.; Borén, J.; Chakravarti, S. The small leucine-rich repeat proteoglycans in tissue repair and atherosclerosis. J. Intern. Med. 2015, 278, 447–461. [Google Scholar] [CrossRef]
- Kirankaya, A.; Tugrul, S.; Ozcan, S.; Ince, O.; Donmez, E.; Atici, A.; Hancioglu, E.; Okuyan, E.; Sahin, I. Correlation between the serum lumican level and the severity of coronary artery disease. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Hershenson, R.; Nardi-Agmon, I.; Leshem-Lev, D.; Kornowski, R.; Eisen, A. The effect of empagliflozin on circulating endothelial progenitor cells in patients with diabetes and stable coronary artery disease. Cardiovasc. Diabetol. 2024, 23, 386. [Google Scholar] [CrossRef] [PubMed]
- Taheri, H.; Chiti, H.; Reshadmanesh, T.; Gohari, S.; Jalilvand, A.; Arsang-Jang, S.; Ismail-Beigi, F.; Ghanbari, S.; Dadashi, M.; Asgari, A.; et al. Empagliflozin improves high-sensitive cardiac troponin-I and high-density lipoprotein cholesterol in patients with type 2 diabetes mellitus and coronary artery disease: A post-hoc analysis of EMPA-CARD Trial. J. Diabetes Metab. Disord. 2023, 22, 1723–1730. [Google Scholar] [CrossRef]
- Jin, Q.; Ma, R.C.W. Metabolomics in Diabetes and Diabetic Complications: Insights from Epidemiological Studies. Cells 2021, 10, 2832. [Google Scholar] [CrossRef] [PubMed]
- Muriach, M.; Flores-Bellver, M.; Romero, F.J.; Barcia, J.M. Diabetes and the brain: Oxidative stress, inflammation, and autophagy. Oxid Med. Cell Longev. 2014, 2014, 102158. [Google Scholar] [CrossRef]
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New insights into the mechanisms of diabetic complications: Role of lipids and lipid metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.A.; Lingaraju, A.; Schoenfelt, K.Q.; Zhou, G.; Cui, C.; Jacobs-El, H.; Babenko, I.; Hoofnagle, A.; Czyz, D.; Shuman, H.; et al. Obesity and Insulin Resistance Promote Atherosclerosis through an IFNγ—Regulated Macrophage Protein Network. Cell Rep. 2018, 23, 3021–3030. [Google Scholar] [CrossRef]
- Ding, P.F.; Zhang, H.S.; Wang, J.; Gao, Y.Y.; Mao, J.N.; Hang, C.H.; Li, W. Insulin resistance in ischemic stroke: Mechanisms and therapeutic approaches. Front. Endocrinol. 2022, 13, 1092431. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.-Q.; Yang, S.; Dong, M.-H.; Chen, L.; Lu, Y.-L.; Zhang, L.-Y.; Zhang, L.; Chu, Y.-H.; Xu, L.-L.; et al. The foam cell-derived exosomes exacerbate ischemic white matter injury via transmitting metabolic defects to microglia. Cell Metab. 2025, 37, 1636–1654.e1610. [Google Scholar] [CrossRef]
- Edgar, L.; Akbar, N.; Braithwaite, A.T.; Krausgruber, T.; Gallart-Ayala, H.; Bailey, J.; Corbin, A.L.; Khoyratty, T.E.; Chai, J.T.; Alkhalil, M.; et al. Hyperglycemia Induces Trained Immunity in Macrophages and Their Precursors and Promotes Atherosclerosis. Circulation 2021, 144, 961–982. [Google Scholar] [CrossRef]
- Mavridis, A.; Viktorisson, A.; Eliasson, B.; von Euler, M.; Sunnerhagen, K.S. Risk of Ischemic and Hemorrhagic Stroke in Individuals With Type 1 and Type 2 Diabetes: A Nationwide Cohort Study in Sweden. Neurology 2025, 104, e213480. [Google Scholar] [CrossRef]
- Dogan, Z.; Senyigit, A.; Durmus, S.; Duvarci, C.; Gelisgen, R.; Uzun, H.; Tabak, O. The relationship between oxLDL, sLOX-1, PCSK9 and carotid intima-media thickness in patients with prediabetes and type 2 diabetes. Sci. Rep. 2025, 15, 4554. [Google Scholar] [CrossRef]
- Chang, D.; Sharma, L.; Dela Cruz, C.S. Chitotriosidase: A marker and modulator of lung disease. Eur. Respir. Rev. 2020, 29, 190143. [Google Scholar] [CrossRef] [PubMed]
- Artieda, M.; Cenarro, A.; Gañán, A.; Jericó, I.; Gonzalvo, C.; Casado, J.M.; Vitoria, I.; Puzo, J.; Pocoví, M.; Civeira, F. Serum Chitotriosidase Activity Is Increased in Subjects With Atherosclerosis Disease. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1645–1652. [Google Scholar] [CrossRef]
- Cerqueira, A.; Quelhas-Santos, J.; Ferreira, I.; Sampaio, S.; Relvas, M.; Marques, N.; Dias, C.C.; Pestana, M. Circulating Renalase as Predictor of Renal and Cardiovascular Outcomes in Pre-Dialysis CKD Patients: A 5-Year Prospective Cohort Study. Life 2021, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef]
- Pan, H.C.; Chou, K.M.; Lee, C.C.; Yang, N.I.; Sun, C.Y. Circulating Klotho levels can predict long-term macrovascular outcomes in type 2 diabetic patients. Atherosclerosis 2018, 276, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Liao, Y.F.; Zeng, T.S.; Li, Y.M.; Yu, F.; Li, H.Q. Number and function of circulating endothelial progenitor cell in diabetics with different vascular complications. Zhonghua Yi Xue Za Zhi 2009, 89, 1234–1239. [Google Scholar] [PubMed]
- Zhang, X.; Xiao, W.; Zhang, Q.; Xia, D.; Gao, P.; Su, J.; Yang, H.; Gao, X.; Ni, W.; Lei, Y.; et al. Progression in Moyamoya Disease: Clinical Features, Neuroimaging Evaluation, and Treatment. Curr. Neuropharmacol. 2022, 20, 292–308. [Google Scholar] [CrossRef]
- Bao, X.Y.; Fan, Y.N.; Liu, Y.; Wang, Q.N.; Zhang, Y.; Zhu, B.; Liu, B.; Duan, L. Circulating endothelial progenitor cells and endothelial cells in moyamoya disease. Brain Behav. 2018, 8, e01035. [Google Scholar] [CrossRef]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Thiruvoipati, T.; Kielhorn, C.E.; Armstrong, E.J. Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World J. Diabetes 2015, 6, 961–969. [Google Scholar] [CrossRef]
- Abu-Jableh, W.; Younis, A.; Hjazeen, A.; Mashaqbeh, E.; Al-Sharif, E. Asymptomatic Peripheral Arterial Disease Among Jordanian Patients With Diabetic Foot Ulcer. Cureus 2024, 16, e73722. [Google Scholar] [CrossRef]
- Smolderen, K.G.; Ujueta, F.; Buckley Behan, D.; Vlaeyen, J.W.S.; Jackson, E.A.; Peters, M.; Whipple, M.; Phillips, K.; Chung, J.; Mena-Hurtado, C.; et al. Understanding the Pain Experience and Treatment Considerations Along the Spectrum of Peripheral Artery Disease: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Qual. Outcomes 2025, 18, e000135. [Google Scholar] [CrossRef] [PubMed]
- Sangeeta, S.; Siripuram, C.; Konka, S.; Vaithilingam, K.; Periasamy, P.; Velu, R.K.; Kandimalla, R. Biomarkers of Inflammation, Oxidative Stress, and Endothelial Dysfunction in Early Detection of Diabetic Foot Ulcers. Cureus 2025, 17, e82174. [Google Scholar] [CrossRef]
- Jude, E.B. Intermittent claudication in the patient with diabetes. Br. J. Diabetes Vasc. Dis. 2004, 4, 238–242. [Google Scholar] [CrossRef]
- Nadeau, M.; Rosas-Arellano, M.P.; Gurr, K.R.; Bailey, S.I.; Taylor, D.C.; Grewal, R.; Lawlor, D.K.; Bailey, C.S. The reliability of differentiating neurogenic claudication from vascular claudication based on symptomatic presentation. Can. J. Surg. 2013, 56, 372–377. [Google Scholar] [CrossRef]
- Khan, Z.; Zeb, S.; Ashraf, R.; Ali, A.; Aleem, F.; Omair, F. The Relationship Between Plasma Fibrinogen Levels and the Severity of Diabetic Foot Ulcers in Diabetic Patients. Cureus 2025, 17, e81118. [Google Scholar] [CrossRef] [PubMed]
- Yachmaneni, A., Jr.; Jajoo, S.; Mahakalkar, C.; Kshirsagar, S.; Dhole, S. A Comprehensive Review of the Vascular Consequences of Diabetes in the Lower Extremities: Current Approaches to Management and Evaluation of Clinical Outcomes. Cureus 2023, 15, e47525. [Google Scholar] [CrossRef]
- Alsararatee, H.H.; Langley, J.C.S.; Thorburn, M.; Burton-Gow, H.; Whitby, S.; Powell, S. Assessment of the diabetic foot in inpatients. Br. J. Nurs. 2025, 34, S12–S23. [Google Scholar] [CrossRef]
- Wilson, A.M.; Kimura, E.; Harada, R.K.; Nair, N.; Narasimhan, B.; Meng, X.Y.; Zhang, F.; Beck, K.R.; Olin, J.W.; Fung, E.T.; et al. Beta2-microglobulin as a biomarker in peripheral arterial disease: Proteomic profiling and clinical studies. Circulation 2007, 116, 1396–1403. [Google Scholar] [CrossRef]
- Al-Kafaji, G.; Al-Mahroos, G.; Abdulla Al-Muhtaresh, H.; Sabry, M.A.; Abdul Razzak, R.; Salem, A.H. Circulating endothelium-enriched microRNA-126 as a potential biomarker for coronary artery disease in type 2 diabetes mellitus patients. Biomarkers 2017, 22, 268–278. [Google Scholar] [CrossRef]
- Méndez-Valdés, G.; Gómez-Hevia, F.; Lillo-Moya, J.; González-Fernández, T.; Abelli, J.; Cereceda-Cornejo, A.; Bragato, M.C.; Saso, L.; Rodrigo, R. Endostatin and Cancer Therapy: A Novel Potential Alternative to Anti-VEGF Monoclonal Antibodies. Biomedicines 2023, 11, 718. [Google Scholar] [CrossRef]
- Golledge, J.; Clancy, P.; Hankey, G.J.; Yeap, B.B.; Norman, P.E. Serum endostatin concentrations are higher in men with symptoms of intermittent claudication. Dis. Markers 2014, 2014, 298239. [Google Scholar] [CrossRef]
- Naka, K.K.; Papathanassiou, K.; Bechlioulis, A.; Pappas, K.; Tigas, S.; Makriyiannis, D.; Antoniou, S.; Kazakos, N.; Margeli, A.; Papassotiriou, I.; et al. Association of vascular indices with novel circulating biomarkers as prognostic factors for cardiovascular complications in patients with type 2 diabetes mellitus. Clin. Biochem. 2018, 53, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Funahashi, T.; Shimomura, I. Adiponectin as a routine clinical biomarker. Best. Pr. Res. Clin. Endocrinol. Metab. 2014, 28, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Samman Tahhan, A.; Hayek, S.S.; Sandesara, P.; Hajjari, J.; Hammadah, M.; O’Neal, W.T.; Kelli, H.M.; Alkhoder, A.; Ghasemzadeh, N.; Ko, Y.A.; et al. Circulating soluble urokinase plasminogen activator receptor levels and peripheral arterial disease outcomes. Atherosclerosis 2017, 264, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shaikh, F.; Younes, H.; Abuhalimeh, B.; Zamzam, A.; Abdin, R.; Qadura, M. The Prognostic Potential of Insulin-like Growth Factor-Binding Protein 1 for Cardiovascular Complications in Peripheral Artery Disease. J. Cardiovasc. Dev. Dis. 2025, 12, 253. [Google Scholar] [CrossRef]
- Furuhashi, M. Fatty Acid-Binding Protein 4 in Cardiovascular and Metabolic Diseases. J. Atheroscler. Thromb. 2019, 26, 216–232. [Google Scholar] [CrossRef]
- Aragones, G.; Ferre, R.; Lazaro, I.; Cabre, A.; Plana, N.; Merino, J.; Heras, M.; Girona, J.; Masana, L. Fatty acid-binding protein 4 is associated with endothelial dysfunction in patients with type 2 diabetes. Atherosclerosis 2010, 213, 329–331. [Google Scholar] [CrossRef]
- Li, B.; Shaikh, F.; Younes, H.; Abuhalimeh, B.; Zamzam, A.; Abdin, R.; Qadura, M. Matrix Metalloproteinases 7 and 10 Are Prognostic Biomarkers for Systemic Cardiovascular Risk in Individuals with Peripheral Artery Disease. Biomolecules 2025, 15, 853. [Google Scholar] [CrossRef]
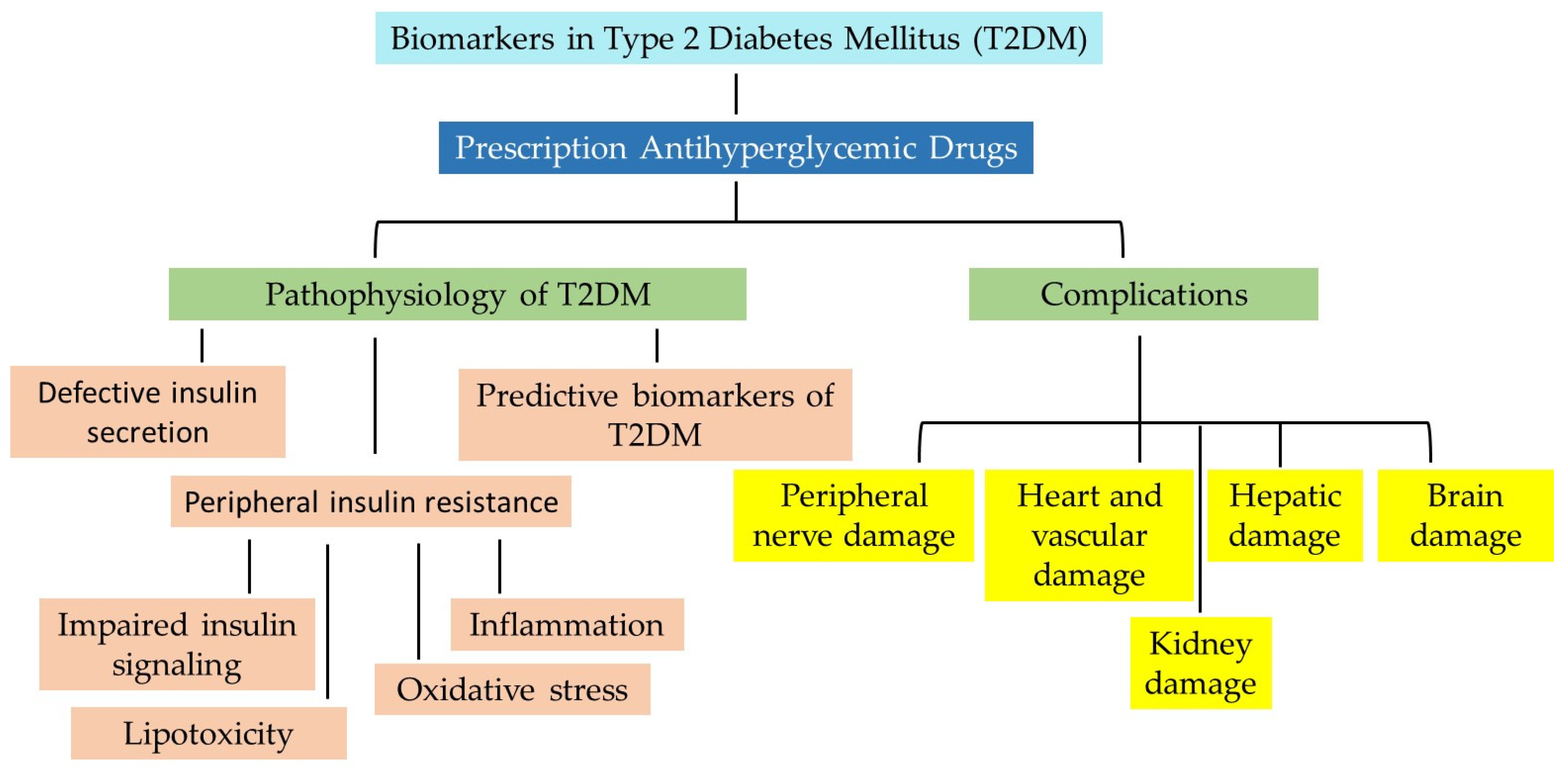
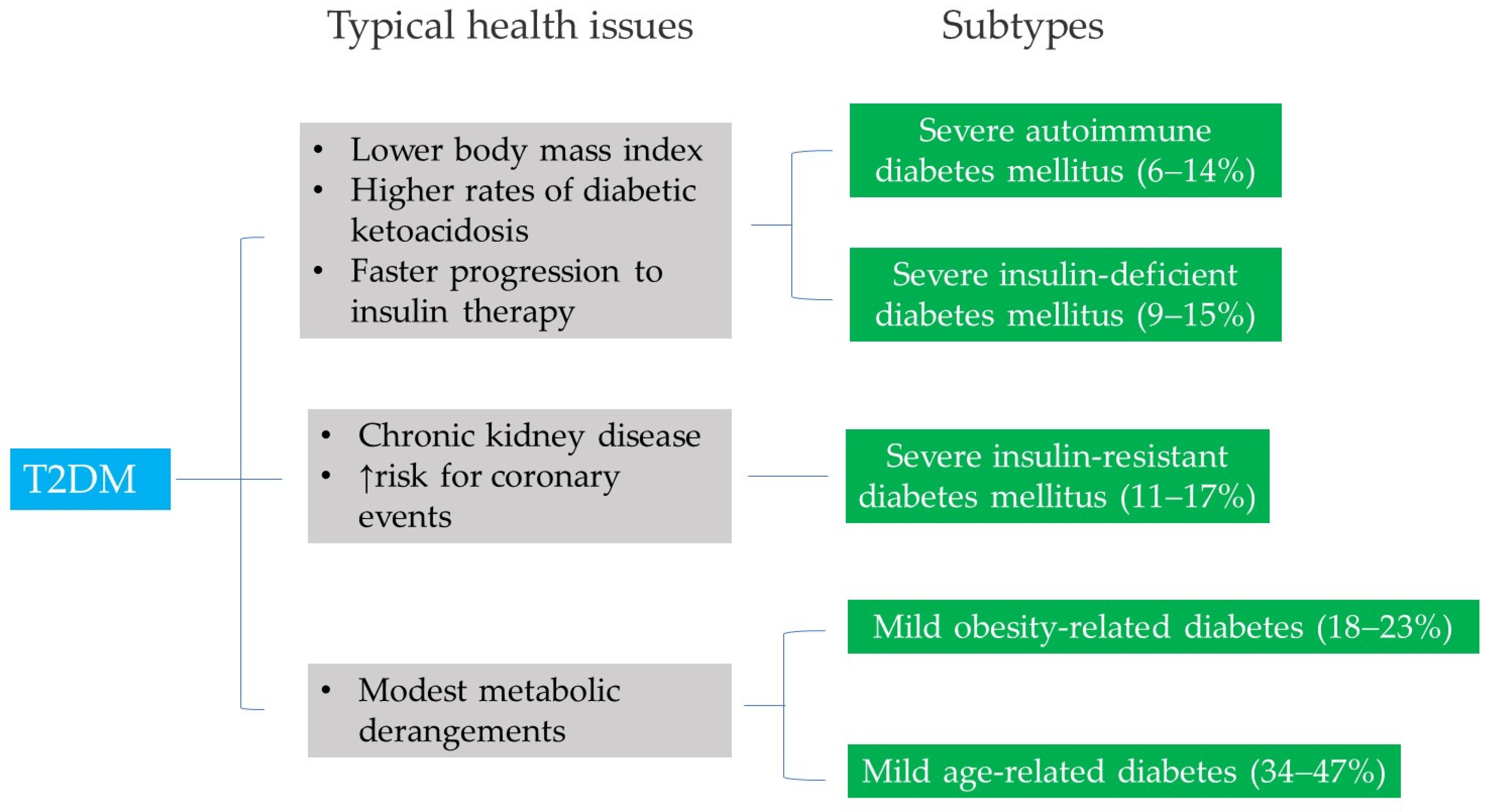
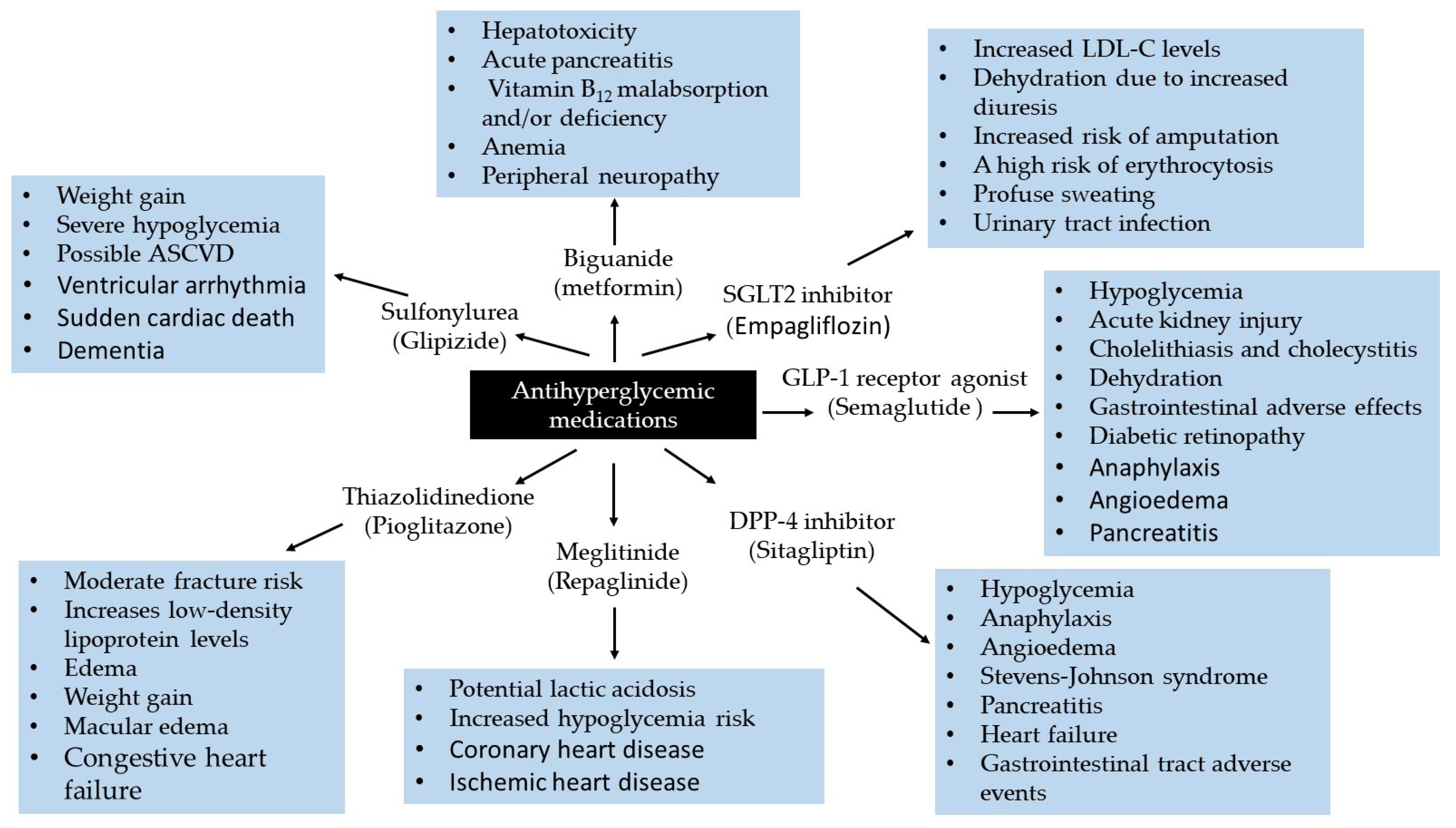
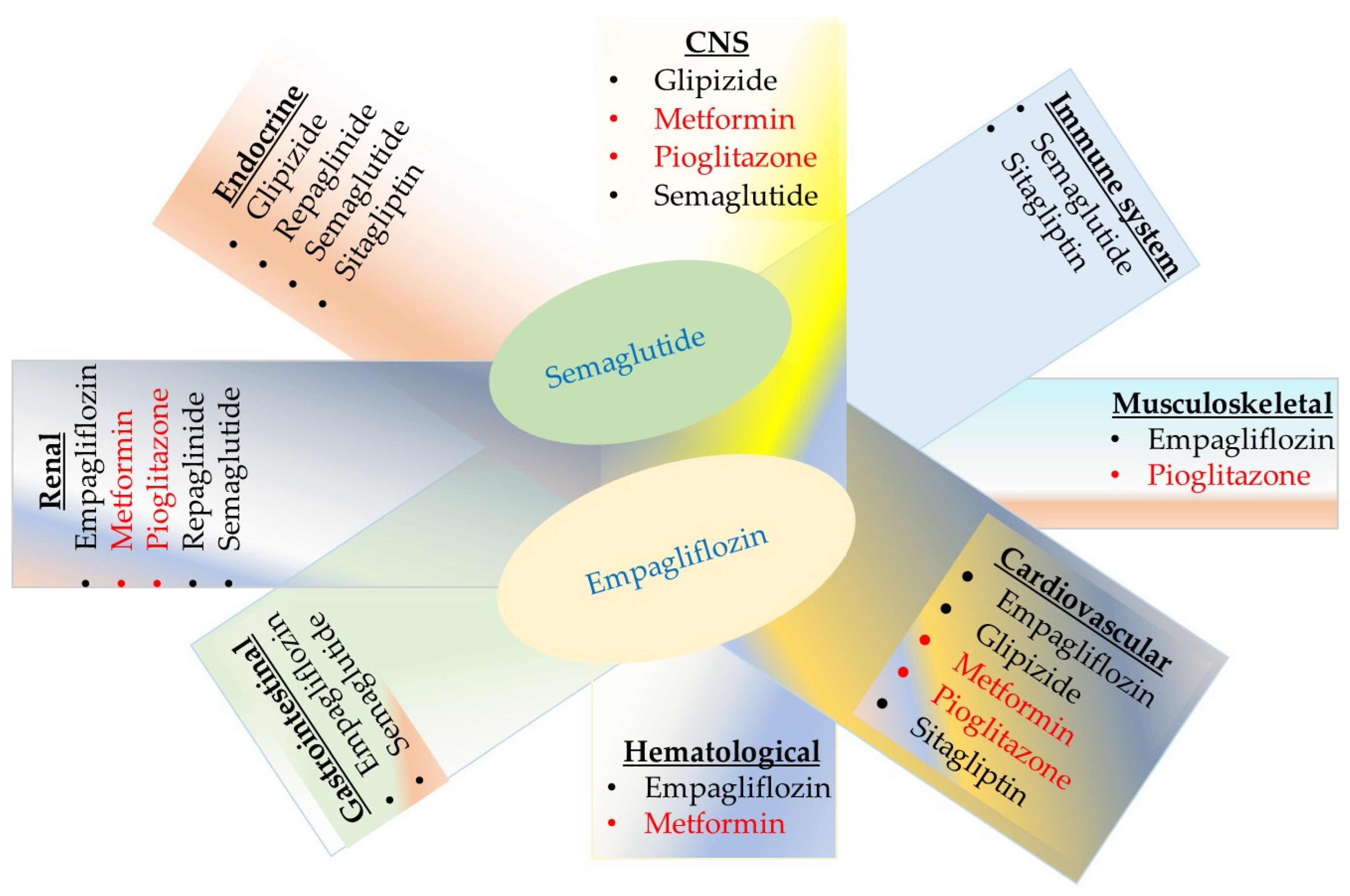
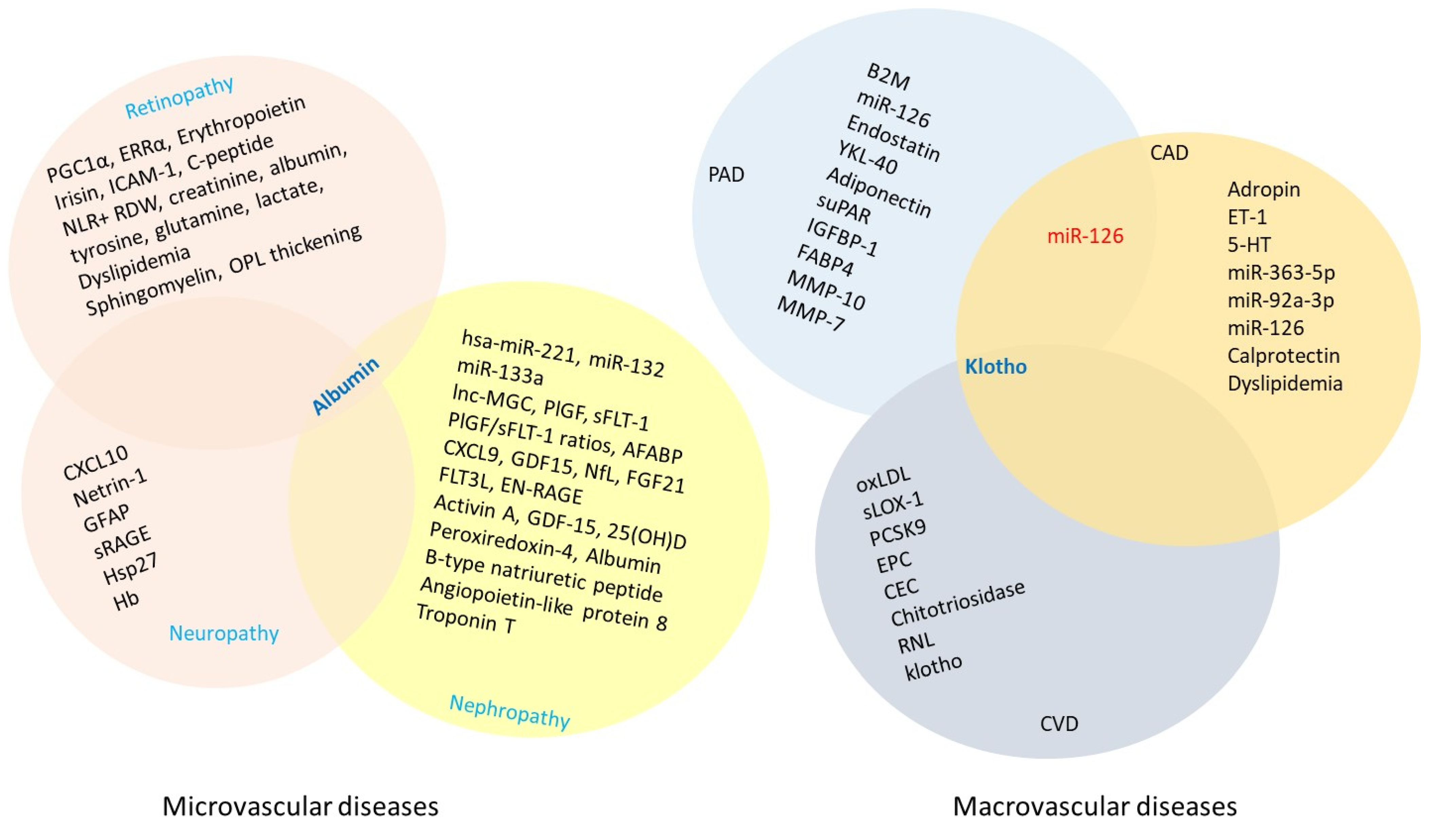
| System (Node) | Serious Side Effects | Glucose-Lowering Medication * | Biomarker Test | Ref. |
|---|---|---|---|---|
| Cardiovascular |
|
Glipizide Pioglitazone Sitagliptin † |
| [171,172,173] [174,175,176,177] [111,112] [178,179,180,181,182,183] [123,184] |
| CNS |
| Metformin Glipizide Pioglitazone Semaglutide |
No blood-based biomarkers are available
| [185,186,187] [188] [189,190,191] [192] [193,194] |
| Endocrine |
|
|
| [115,195,196,197] [198,199,200,201,202] |
| Gastrointestinal |
|
Semaglutide Empagliflozin |
| [203] [204,205] [206,207] [208,209] [210] [211,212,213,214] |
| Hematological |
| Metformin Empagliflozin |
| [215,216] [217,218,219,220,221,222] |
| Immune system |
|
|
| [223,224,225,226] [227,228,229,230,231] [232,233,234,235,236,237] |
| Musculoskeletal |
| Pioglitazone Empagliflozin † |
| [121,122] [238,239] |
| Renal |
|
Pioglitazone Semaglutide
|
Urinary neutrophil gelatinase-associated lipocalin test, kidney injury molecule-1 | [240,241,242,243,244] [123,245,246] [247,248] [246,249] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shariat-Madar, Z.; Mahdi, F. Potential Molecular Biomarkers for Predicting and Monitoring Complications in Type 2 Diabetes Mellitus. Molecules 2025, 30, 4448. https://doi.org/10.3390/molecules30224448
Shariat-Madar Z, Mahdi F. Potential Molecular Biomarkers for Predicting and Monitoring Complications in Type 2 Diabetes Mellitus. Molecules. 2025; 30(22):4448. https://doi.org/10.3390/molecules30224448
Chicago/Turabian StyleShariat-Madar, Zia, and Fakhri Mahdi. 2025. "Potential Molecular Biomarkers for Predicting and Monitoring Complications in Type 2 Diabetes Mellitus" Molecules 30, no. 22: 4448. https://doi.org/10.3390/molecules30224448
APA StyleShariat-Madar, Z., & Mahdi, F. (2025). Potential Molecular Biomarkers for Predicting and Monitoring Complications in Type 2 Diabetes Mellitus. Molecules, 30(22), 4448. https://doi.org/10.3390/molecules30224448







