Magnetic Nanoparticles for Rhodamine B Depletion in Wastewater—Theoretical and Experimental Approach
Abstract
1. Introduction
2. Results and Discussion
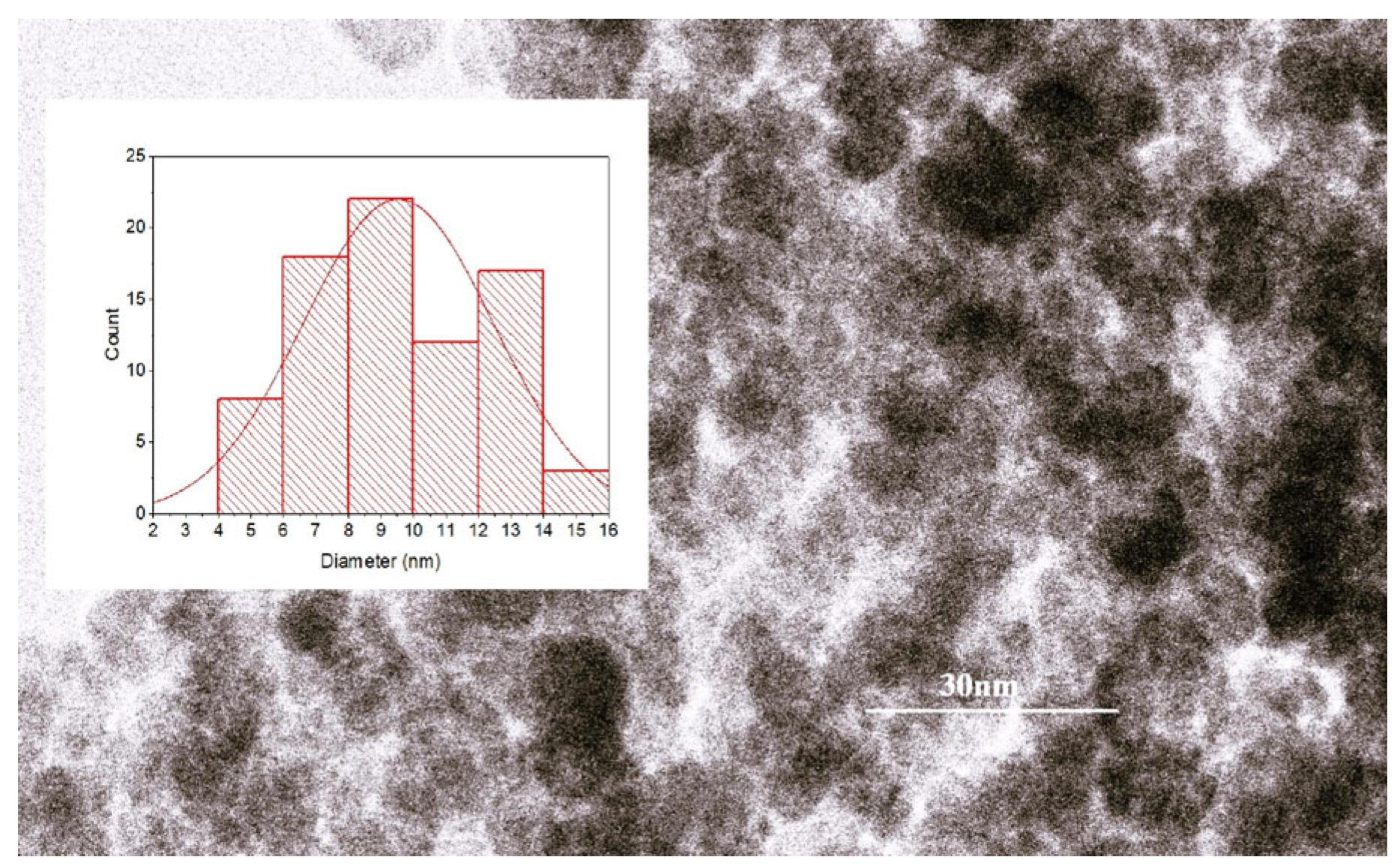
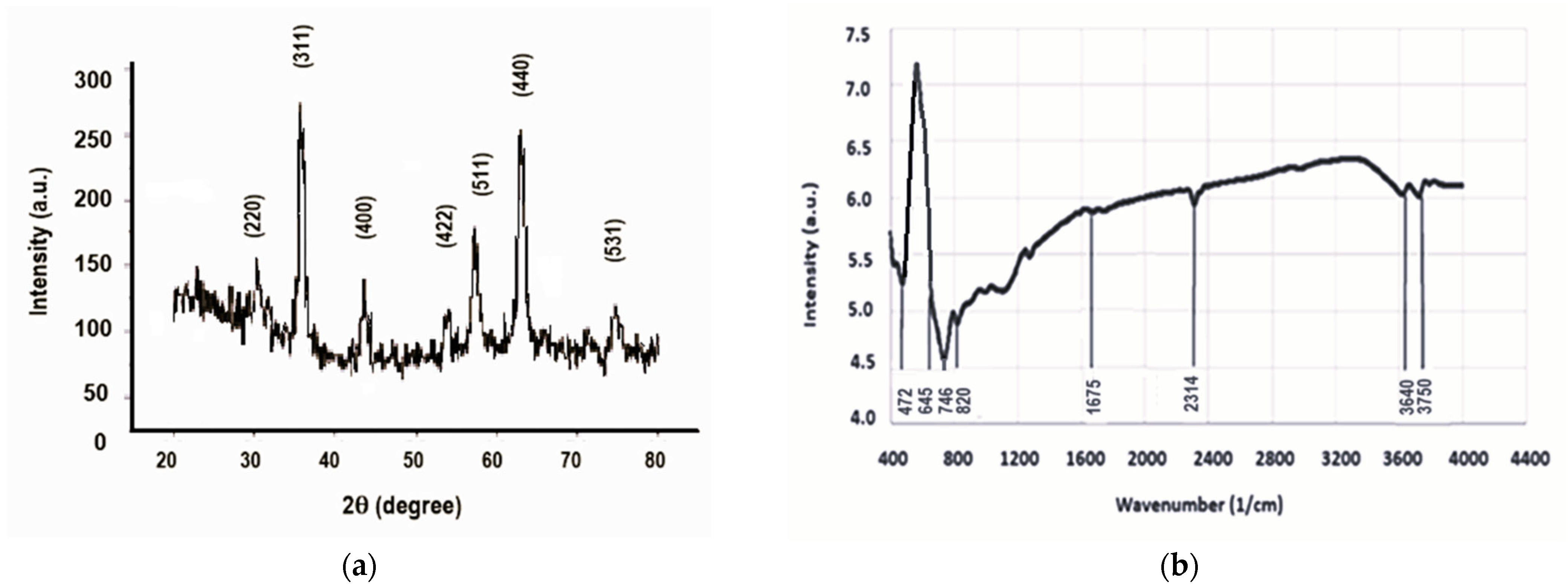
2.1. Magnetic Nanoparticles Characterization
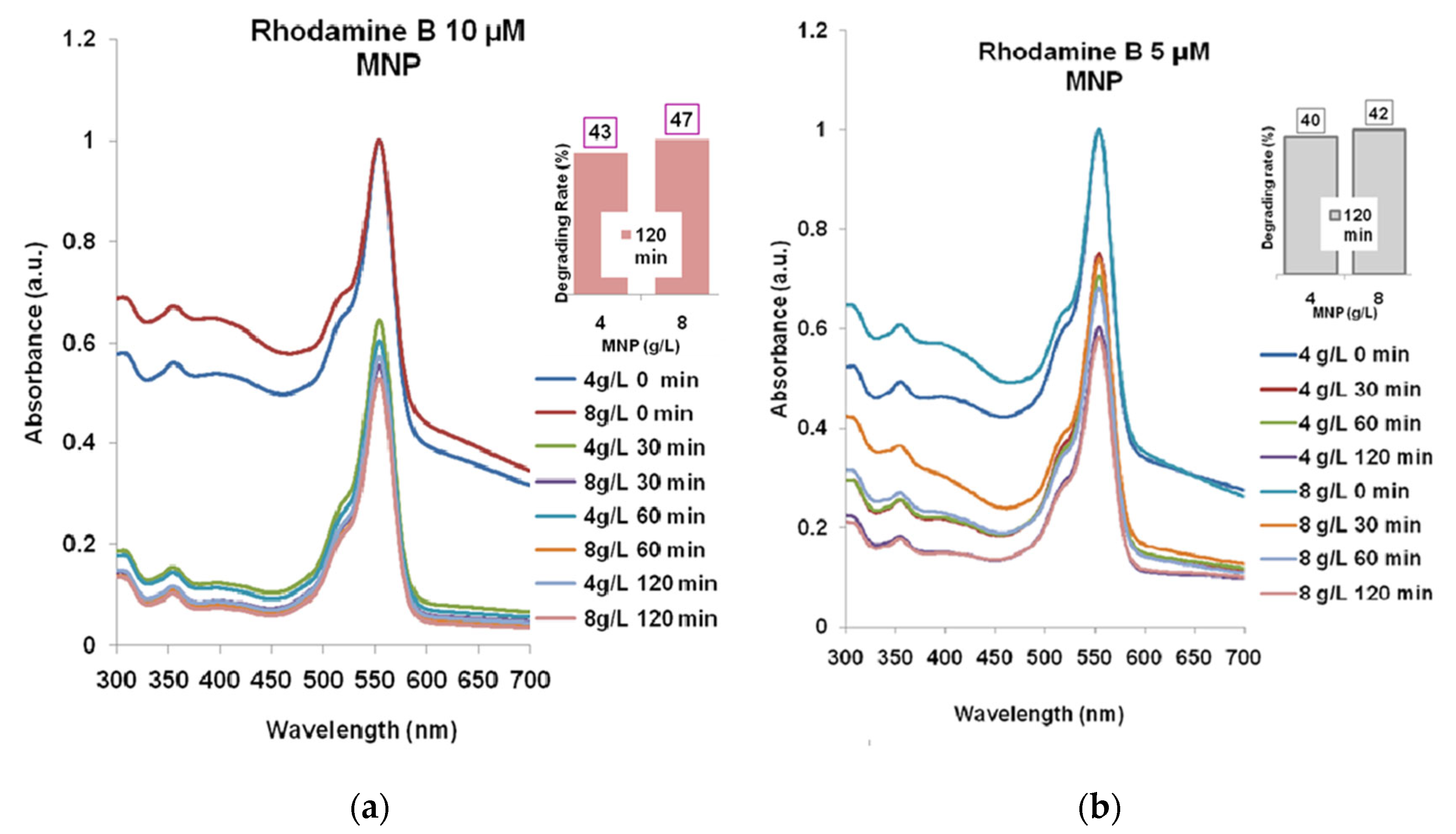
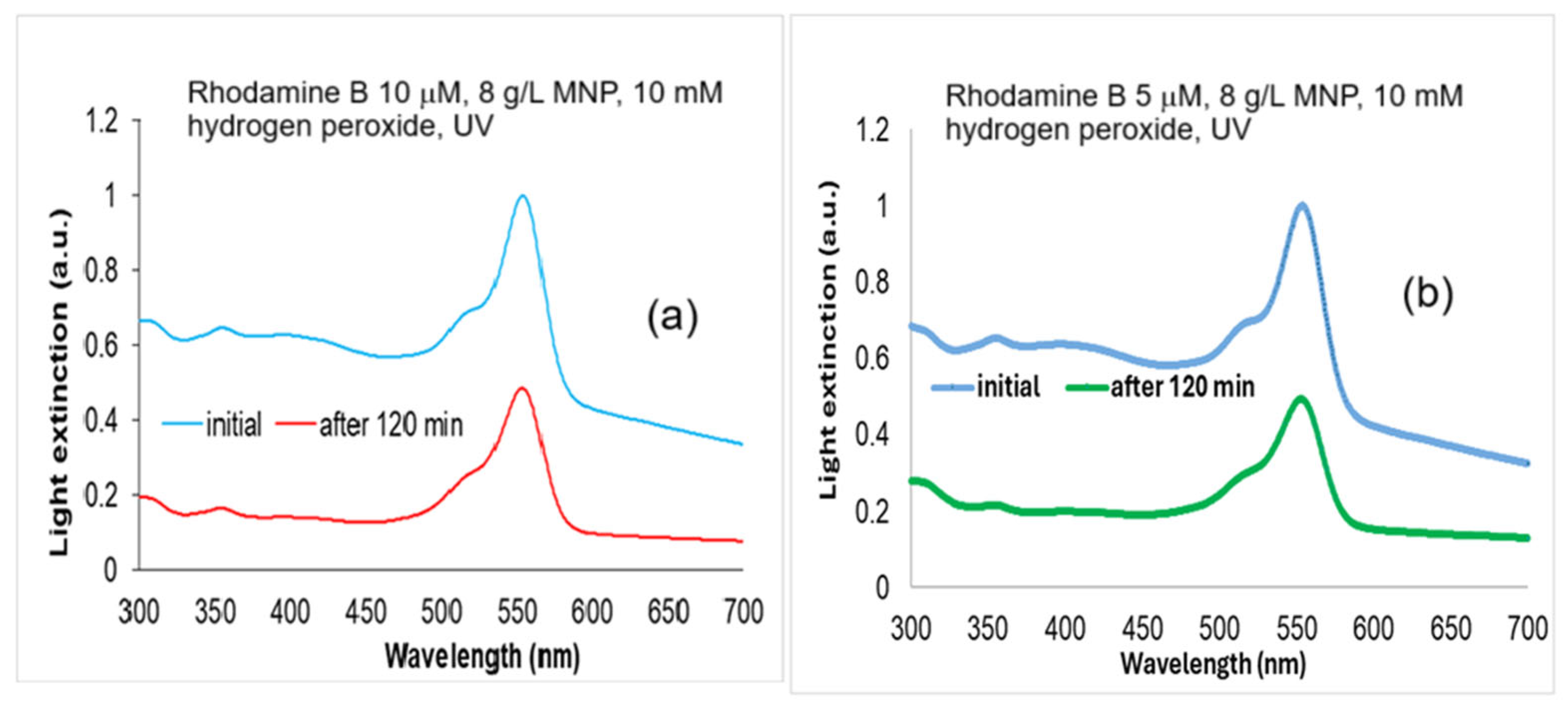
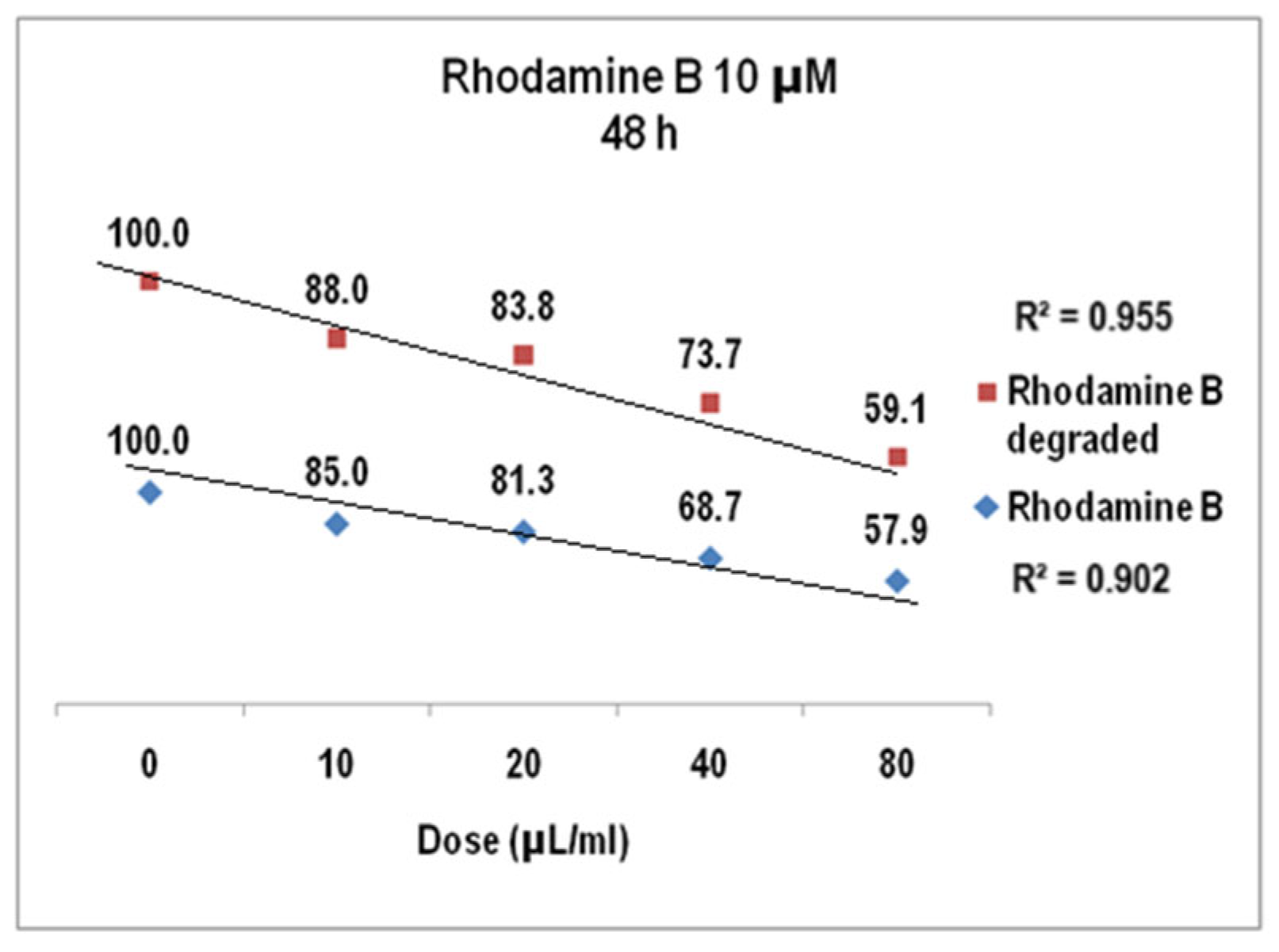
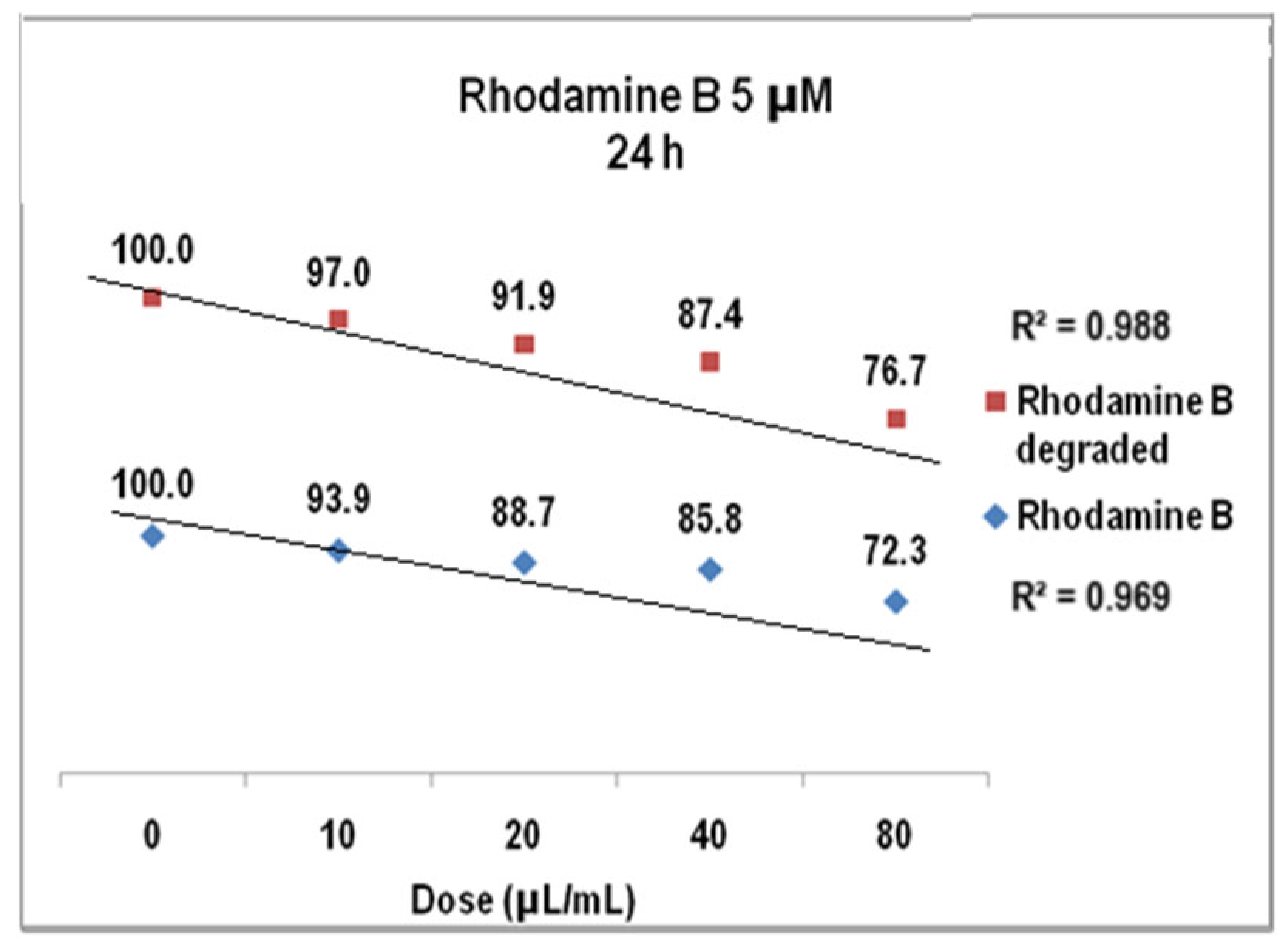
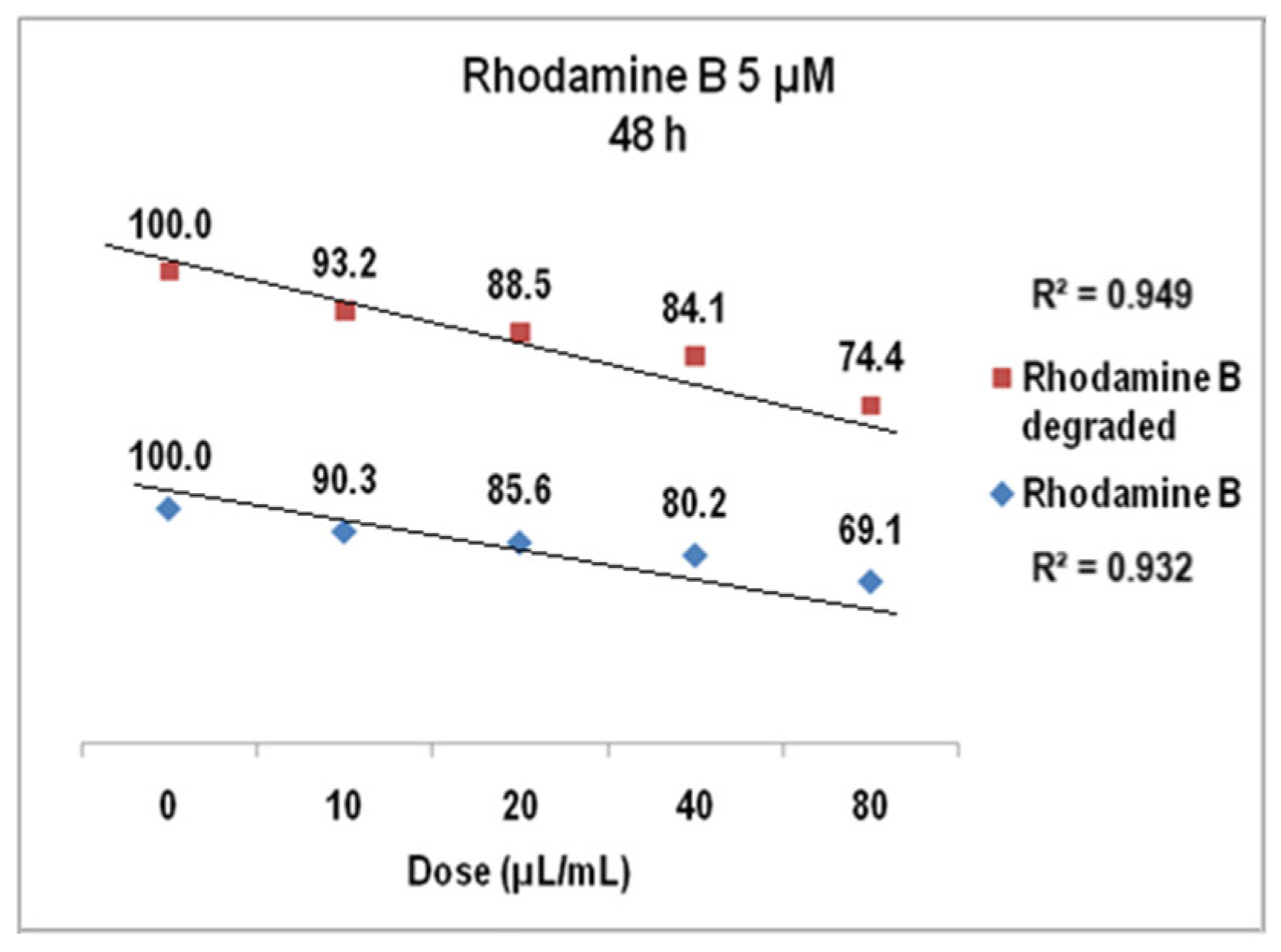
2.2. Rhodamine B Solutions Photodegradation
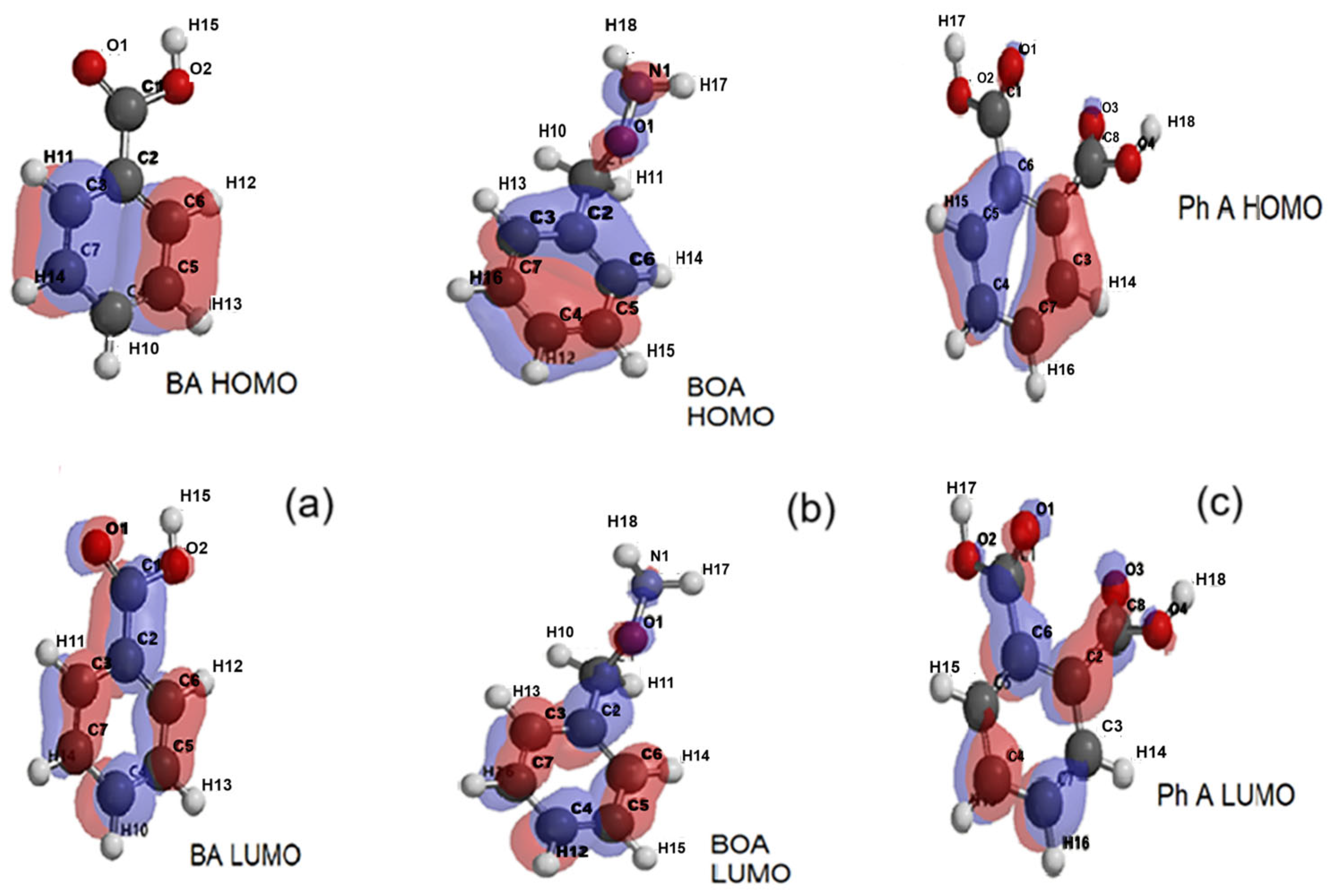
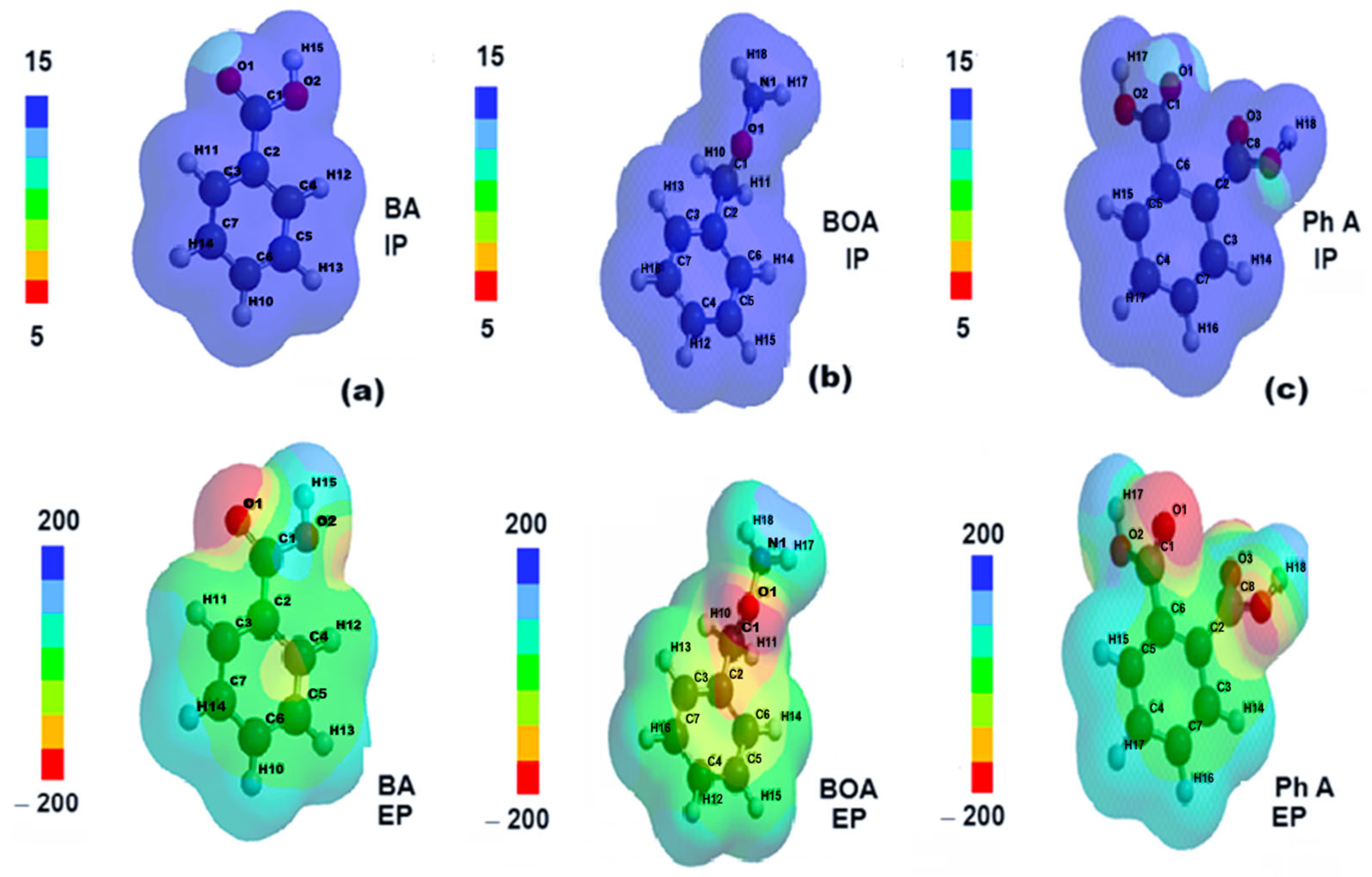
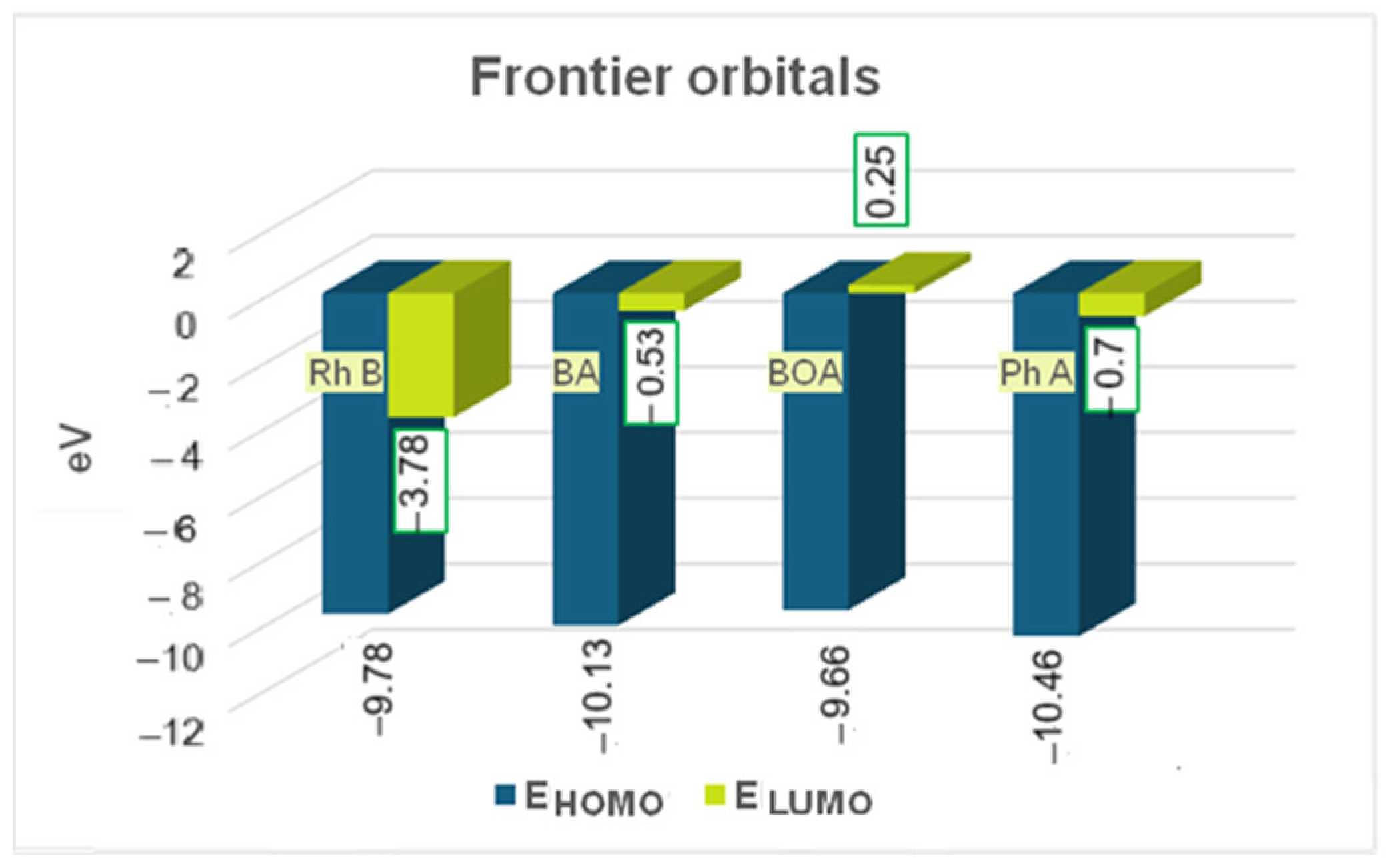
2.3. Quantum Chemical Parameters of Rhodamine B and Its Degradation Products
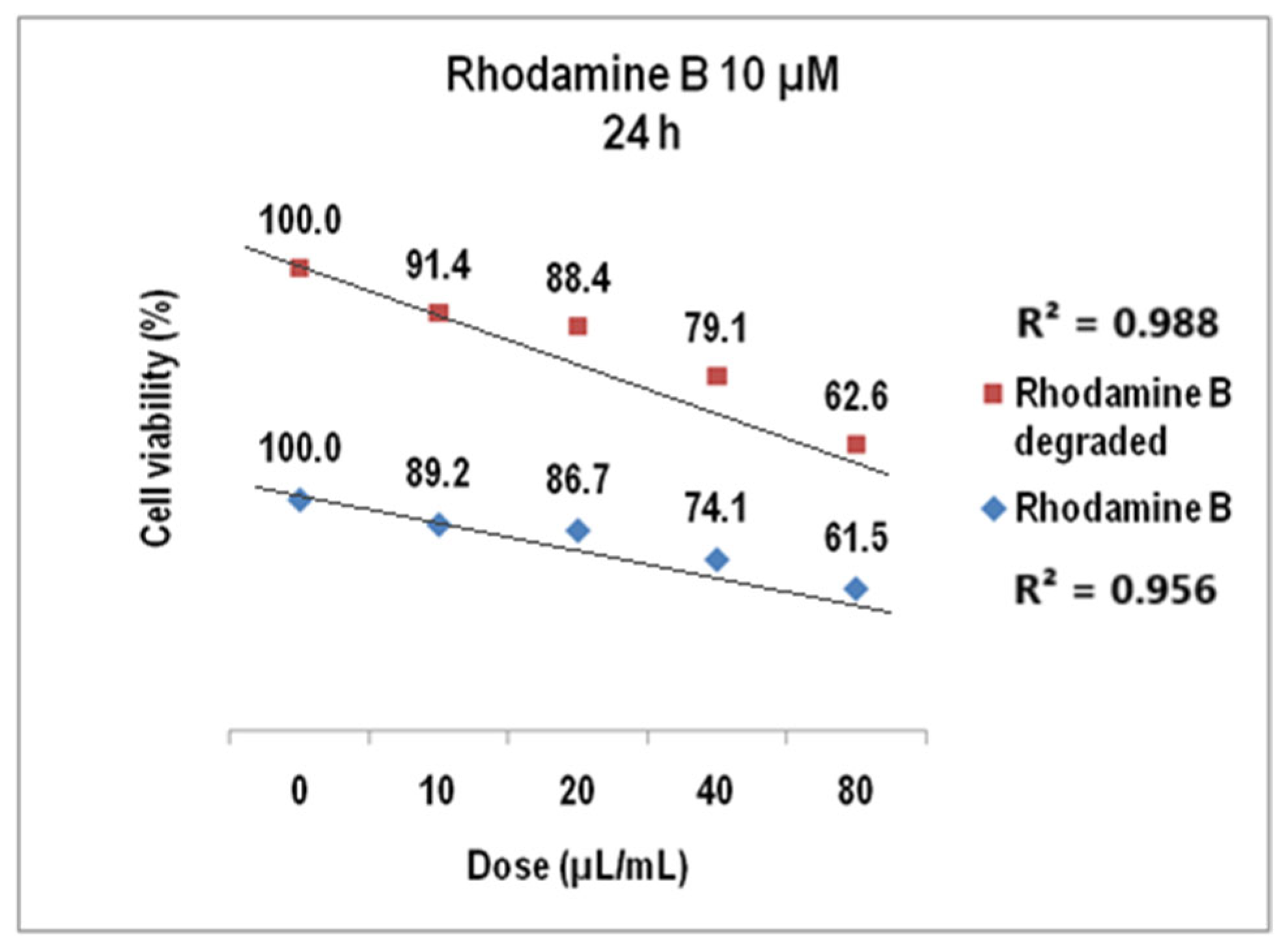
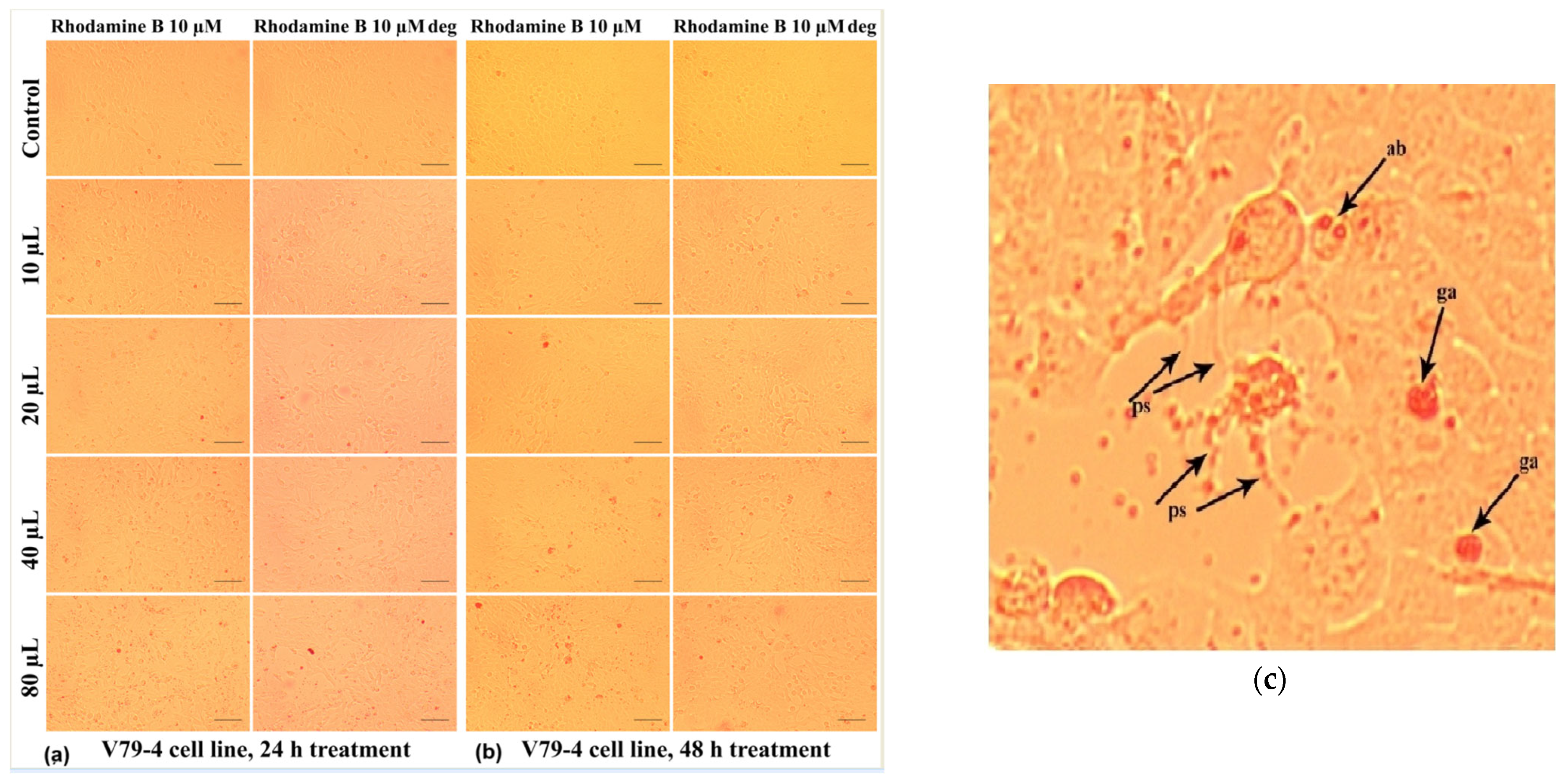
2.4. Cytotoxicity Test Results
| Magnetic Nanoparticles | Cell Viability | Reference | ||
|---|---|---|---|---|
| Uncoated magnetite | 0.01 to 0.40 mg/mL | HeLa cancer cells | Fluorescence microscopy | Li et al. (2012) [55] |
| Uncoated magnetite | 0.01 to 0.5 mg/mL | Neural PC12 cells | XTT test | Marcus et al. (2016) [56] |
| Carboxymethyl dextran-magnetite | 0.3–1.5 mg/mL | Caco-2 and MCF-7 human cancer cells. | Spectrofluorimetric assay | Rodríguez-Luccioni et al. (2011) [57] |
| DMSA coated magnetite | 0.05–0.4 mg/mL | MCF-7 breast cancer cells | MTT test | Calero et al. (2015) [59] |
| Polymer coated magnetite | <200 µg/mL | V79 hamster lung fibroblasts | MTT test | Zavisova et al. (2015) [60] |
| Biological Material | Rhodamine B Effect | Reference |
|---|---|---|
| Human fibroblasts isolated from the lips (KD cells) | Decreased cell proliferation, inhibition of collagen synthesis | Kaji et al. (1991) [34] Kaji et al. (1992) [66] |
| Cerebellum and brainstem tissue of Rattus norvegicus | Cell apoptosis | Sulistina et al. (2020) [68] |
| H. verticillata cells | Inhibitory effects on photosystem II | Sharma et al. (2022) [26] |
| Uterine cervix of Wistar albino rats | Lipid peroxidation | Safitri et al. (2015) [67] |
| Zebrafish | Reproductive toxicity | Priya et al. (2024) [23] |
| Wistar rats | Ovarian toxicity, decreased follicles number | Maryanti et al. (2014) [25] |
3. Materials and Methods
3.1. Methods of Magnetic Nanoparticles Investigation
3.2. Rhodamine B Samples
3.2.1. Rhodamine B Wastewater Models
3.2.2. Rhodamine B Solution Irradiation
3.3. Theoretical Reactivity Modeling
3.4. Biological Material
3.5. Experimental
3.5.1. Rhodamine B Photodegradation
3.5.2. Cell Culture and Treatment
3.5.3. Microscopic Study
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Q.; Hao, L. Adsorption Technologies in Wastewater Treatment Processes. Water 2025, 17, 2335. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Mishra, S.; Sundaram, B. A review of the photocatalysis process used for wastewater treatment. Mater. Today Proc. 2024, 102, 393–409. [Google Scholar] [CrossRef]
- Wang, F.; Xiang, Y.; Zhang, Y.; Zhou, X.; Zhang, J.; He, C.; Zhang, H.; Xiong, Z.; Zhou, P.; Zhou, H.; et al. A comprehensive review on oxygen vacancies modified catalysts: Synthesis, characterization, and crucial role in catalytic ozonation. Chin. Chem. Lett. 2025, 36, 111315. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Akram, S.; Lal, B.; Hassan, S.U.; Ashraf, R.; Kezembayeva, G.; Hosseini-Bandegharaei, A. Advanced photocatalysis as a viable and sustainable wastewater treatment process: A comprehensive review. Environ. Res. 2024, 253, 118947. [Google Scholar] [CrossRef]
- Wei, R.; Wang, P.; Zhang, G.; Wang, N.; Zheng, T. Microwave-responsive catalysts for wastewater treatment: A review. Chem. Eng. J. 2020, 382, 122781. [Google Scholar] [CrossRef]
- Liu, N.; Tian, M.; Zhang, Y.; Yang, J.; Wang, Z.; Dai, W.; Quan, G.; Lei, J.; Zhang, X.; Tang, L. Three-dimensional MIL-88A(Fe)-derived α-Fe2O3 and graphene composite for efficient photo-Fenton-like degradation of ciprofloxacin. Chin. Chem. Lett. 2025, 36, 111063. [Google Scholar] [CrossRef]
- Zhang, N.; He, C.; Jing, Y.; Qian, Y.; Obuchi, M.; Toyoshima, R.; Kondoh, H.; Oka, K.; Wu, B.; Li, L.; et al. Enhanced nitrous oxide decomposition on zirconium-supported rhodium catalysts by iridium augmentation. Environ. Sci. Technol. 2025, 59, 1598–1607. [Google Scholar] [CrossRef]
- Wlodarczyk, A.; Gorgon, S.; Radon, A.; Bajdak-Rusinek, K. Magnetite nanoparticles in magnetic hyperthermia and cancer therapies: Challenges and perspectives. Nanomaterials 2022, 12, 1807. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; Garcia-Martin, M.L. Magnetic nanoparticles as MRI contrast agents. Top. Curr. Chem. 2020, 378, 49–91. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Eltoukhy, A.A.; Love, K.T.; Langer, R.; Anderson, D.G. Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett. 2013, 13, 1059–1064. [Google Scholar] [CrossRef]
- Kennaz, H.; Harat, A.; Guellati, O.; Momodu, D.Y.; Barzegar, F.; Dangbegnon, J.K.; Manyala, N.; Guerioune, M. Synthesis and electrochemical investigation of spinel cobalt ferrite magnetic nanoparticles for supercapacitor application. J. Solid State Electrochem. 2018, 22, 835–847. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, F.; Li, Q.; Xia, Q.; Li, Z.; Li, X.; Ye, W.; Li, S.; Ge, C. Reversible control of magnetization in Fe3O4 nanoparticles by a supercapacitor. J. Phys. Condens. Matter 2020, 32, 334001. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, I.; Geiser, A.; Somborn-Schulz, A. Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Barjasteh-Askari, F.; Davoudi, M.; Dolatabadi, M.; Ahmadzadeh, S. Iron-modified activated carbon derived from agrowaste for enhanced dye removal from aqueous solutions. Heliyon 2021, 7, e07191. [Google Scholar] [CrossRef]
- Dolatabadi, M.; Ehrampoush, M.H.; Pournamdari, M.; Ebrahimi, A.A.; Fallahzadeh, H.; Ahmadzadeh, S. Catalytic electrodes’ characterization study serving polluted water treatment: Environmental healthcare and ecological risk assessment. J. Environ. Sci. Health B 2023, 58, 594–602. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Garud, H.B.; Patil, A.H.; Patil, G.D.; Patil, C.R.; Dongale, T.D.; Patil, P.S. Recent advancements in silica nanoparticle-based technologies for removal of dyes from water. Colloid Interface Sci. Commun. 2019, 30, 100181. [Google Scholar] [CrossRef]
- Narayani, H.; Jose, M.; Srira, K.; Shukla, S. Hydrothermal synthesized magnetically separable mesostructured H2Ti3O7/γ-Fe2O3 nanocomposite for organic dye removal via adsorption and its regeneration/reuse through synergistic non-radiation driven H2O2 activation. Environ. Sci. Pollut. Res. 2017, 25, 20304–20319. [Google Scholar] [CrossRef]
- Peres, E.C.; Slaviero, J.C.; Cunha, A.M.; Hosseini-Bandegharaei, A.; Dotto, G.L. Microwave synthesis of silica nanoparticles and its application for methylene blue adsorption. J. Environ. Chem. Eng. 2018, 6, 649–659. [Google Scholar] [CrossRef]
- Xiao, Y.; Deng, Y.; Huan, W.; Li, J.; Zhang, J.; Xing, M. Hollow-structured Fe2O3/Au/SiO2 nanorods with enhanced and recyclable photo-Fenton oxidation for the remediation of organic pollutants. Mater. Today Chem. 2019, 11, 86–93. [Google Scholar] [CrossRef]
- Ghasemi, A.; Es’haghi, Z.; Jamali, M.R. Removal of Sudan dyes from environmental waters and food samples with amine functionalized magnetic silica nanoparticles as solid-phase extraction adsorbent. Water Environ. J. 2018, 32, 630–636. [Google Scholar] [CrossRef]
- Priya, P.S.; Nandhini, P.P.; Vaishnavi, S.; Pavithra, V.; Almutairi, M.H.; Almutairi, B.O.; Arokiyaraj, S.; Pachaiappan, R.; Arockiaraj, J. Rhodamine B, an organic environmental pollutant, induces reproductive toxicity in parental and teratogenicity in F1 generation in vivo. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 280, 109898. [Google Scholar] [CrossRef] [PubMed]
- Honeychurch, K.C. Voltammetric behaviour of Rhodamine B at a screen-printed carbon electrode and its trace determination in environmental water samples. Sensors 2022, 22, 4631. [Google Scholar] [CrossRef] [PubMed]
- Maryanti, S.A.; Suciati, S.; Wahyuni, E.S.; Santoso, S.; Wiyasa, W.A. Rhodamine B triggers ovarian toxicity through oxidative stress. Cukurova Med. J. 2014, 39, 451–457. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Bhatt, U.; Soni, V. Toxic effects of Rhodamine B on antioxidant system and photosynthesis of Hydrilla verticillata. J. Hazard. Mater. Lett. 2022, 3, 100069. [Google Scholar] [CrossRef]
- He, H.; Chai, K.; Wu, T.; Qiu, Z.; Wang, S.; Hong, J. Adsorption of Rhodamine B from simulated wastewater onto kaolin-bentonite composites. Materials 2022, 15, 4058. [Google Scholar] [CrossRef]
- Al-Kahtani, A.A. Photocatalytic degradation of Rhodamine B dye in wastewater using gelatin/CuS/PVA nanocomposites under solar light irradiation. J. Biomater. Nanobiotechnol. 2017, 8, 66–82. [Google Scholar] [CrossRef]
- Deveci, İ.; Mercimek, B. Performance of SiO2/Ag core/shell particles in sonocatalytic degradation of Rhodamine B. Ultrason. Sonochem. 2019, 51, 197–205. [Google Scholar] [CrossRef]
- Flores, A.; Nesprias, K.; Vitale, P.; Tasca, J.; Lavat, A.; Eyler, N.; Canizo, A. Heterogeneous photocatalytic discoloration/degradation of Rhodamine B with H2O2 and spinel copper ferrite magnetic nanoparticles. Aust. J. Chem. 2014, 67, 609–614. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Morad, N.; Tan, K.A.; Mathiyarasi, R. Removal of Rhodamine B dye using activated carbon prepared from palm kernel shell and coated with iron oxide nanoparticles. Sep. Sci. Technol. 2012, 47, 742–752. [Google Scholar] [CrossRef]
- Peng, L.; Qin, P.; Lei, M.; Zeng, Q.; Song, H.; Yang, J.; Shao, J.; Liao, B.; Gu, J. Modifying Fe3O4 nanoparticles with humic acid for removal of Rhodamine B in water. J. Hazard. Mater. 2012, 209–210, 193–198. [Google Scholar] [CrossRef]
- Cao, H.L.; Yang, C.; Qian, H.L.; Yan, X.P. Urea-linked covalent organic framework functionalized polytetrafluoroethylene film for selective and rapid thin film microextraction of rhodamine B. J. Chromatogr. A 2022, 1673, 463133. [Google Scholar] [CrossRef]
- Kaji, T.; Kawashima, T.; Yamamoto, C.; Sakamoto, M. Rhodamine B inhibition of glycosaminoglycan production by cultured human lip fibroblasts. Toxicol. Appl. Pharmacol. 1991, 111, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Cheng, L.; Zhang, Y.; Wang, Q.; Wu, Q.; Xue, Y.; Meng, X. Efficiency and mechanisms of rhodamine B degradation in Fenton-like systems based on zero-valent iron. RSC Adv. 2020, 10, 28509–28515. [Google Scholar] [CrossRef] [PubMed]
- Kusic, H.; Koprivanac, N.; Srsan, L. Azo dye degradation using Fenton-type processes assisted by UV irradiation: A kinetic study. J. Photochem. Photobiol. A Chem. 2006, 181, 195–202. [Google Scholar] [CrossRef]
- He, H.; Zhong, Y.; Liang, X.; Tan, W.; Zhu, J.; Wang, C.Y. Natural magnetite: An efficient catalyst for the degradation of organic contaminant. Sci. Rep. 2015, 5, 10139. [Google Scholar] [CrossRef]
- Maldonado, K.L.L.; De La Presa, P.; De La Rubia, M.A.; Crespo, P.; De Frutos, J.; Hernando, A.; Aquino, J.A.M.; Galindo, J.T.E. Effects of grain boundary width and crystallite size on conductivity and magnetic properties of magnetite nanoparticles. J. Nanoparticle Res. 2014, 16, 2482. [Google Scholar] [CrossRef]
- Karthickraja, D.; Karthi, S.; Kumar, G.A.; Sardar, D.K.; Dannangoda, G.C.; Martirosyan, K.S.; Girija, E.K. Fabrication of core–shell CoFe2O4@HAp nanoparticles: A novel magnetic platform for biomedical applications. New J. Chem. 2019, 43, 13584–13592. [Google Scholar] [CrossRef]
- Ristić, M.; De Grave, E.; Musić, S.; Popović, S.; Orehovec, Z. Transformation of low crystalline ferrihydrite to α-Fe2O3 in the solid state. J. Mol. Struct. 2007, 834–836, 454–460. [Google Scholar] [CrossRef]
- Safi, R.; Ghasemi, A.; Shoja-Razavi, R.; Tavousi, M. The role of pH on the particle size and magnetic consequence of cobalt ferrite. J. Magn. Magn. Mater. 2015, 396, 288–294. [Google Scholar] [CrossRef]
- Aly, S.T.; Saed, A.; Mahmoud, A.; Badr, M.; Garas, S.S.; Yahya, S.; Hamad, K.H. Preparation of magnetite nanoparticles and their application in the removal of methylene blue dye from wastewater. Sci. Rep. 2024, 14, 20100. [Google Scholar] [CrossRef]
- Łoński, S.; Łukowiec, D.; Barbusiński, K.; Babilas, R.; Szeląg, B.; Radoń, A. Flower-like magnetite nanoparticles with unfunctionalized surface as an efficient catalyst in photo-Fenton degradation of chemical dyes. Appl. Surf. Sci. 2023, 638, 158127. [Google Scholar] [CrossRef]
- Ribeiro, J.A.S.; Alves, J.F.; Salgado, B.C.B.; Oliveira, A.C.; Araújo, R.S.; Rodríguez-Castellón, E. Heterogeneous photo-Fenton degradation of azo dyes over a magnetite-based catalyst: Kinetic and thermodynamic studies. Catalysts 2024, 14, 591. [Google Scholar] [CrossRef]
- Zawadzki, P.; Deska, M. Degradation efficiency and kinetics analysis of an advanced oxidation process utilizing ozone, hydrogen peroxide and persulfate to degrade the dye Rhodamine B. Catalysts 2021, 11, 974. [Google Scholar] [CrossRef]
- Ansari, M.S.; Raees, K.; Ali Khan, M.; Rafiquee, M.Z.A.; Otero, M. Kinetic studies on the catalytic degradation of Rhodamine B by hydrogen peroxide: Effect of surfactant-coated and non-coated iron(III) oxide nanoparticles. Polymers 2020, 12, 2246. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.X.; Xu, D.D.; Zou, L.P.; Yuan, H.; Hu, X. Heterogeneous Fenton oxidation of refractory dye Rhodamine B in aqueous solution with mesoporous Fe/SBA-15. Acta Phys.-Chim. Sin. 2015, 31, 771–782. [Google Scholar] [CrossRef]
- Hua, Y.; Wang, S.; Xiao, J.; Cui, C.; Wang, C. Preparation and characterization of Fe3O4/gallic acid/graphene oxide magnetic nanocomposites as highly efficient Fenton catalysts. RSC Adv. 2017, 7, 28979–28986. [Google Scholar] [CrossRef]
- Jakimińska, A.; Pawlicki, M.; Macyk, W. Photocatalytic transformation of Rhodamine B to Rhodamine-110—The mechanism revisited. J. Photochem. Photobiol. A Chem. 2022, 433, 114176. [Google Scholar] [CrossRef]
- Kumar, M.S.; Vijayaraghavan, G.V.; Rajesh, K.; Krishnan, S. Influence of different solvents on the growth, thermal and dielectric properties of phthalic acid single crystals. Mater. Res. Innov. 2020, 24, 422–432. [Google Scholar] [CrossRef]
- Delgado, J.C.; Selsby, R.G. Density functional theory calculations on rhodamine B and pinacyanol chloride: Optimized ground state, dipole moment, vertical ionization potential, adiabatic electron affinity and lowest excited triplet state. Photochem. Photobiol. 2013, 89, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.G.; Liao, W.; Yu, Z.X. A frontier molecular orbital theory approach to understanding the Mayr equation and to quantifying nucleophilicity and electrophilicity by using HOMO and LUMO energies. Asian J. Org. Chem. 2012, 1, 336–345. [Google Scholar] [CrossRef]
- Malik, B.A.; Mir, J.M. Synthesis, characterization and DFT aspects of some oxovanadium(IV) and manganese(II) complexes involving dehydroacetic acid and β-diketones. J. Coord. Chem. 2018, 71, 104–119. [Google Scholar] [CrossRef]
- Pegu, D.; Deb, J.; Van Alsenoy, C.; Sarkar, U. Theoretical investigation of electronic, vibrational, and nonlinear optical properties of 4-fluoro-4-hydroxybenzophenone. Spectrosc. Lett. 2017, 50, 232–243. [Google Scholar] [CrossRef]
- Li, L.; Mak, K.Y.; Shi, J.; Koon, H.K.; Leung, C.H.; Wong, C.M.; Leung, C.W.; Mak, C.S.K.; Chan, N.M.M.; Zhong, W.; et al. Comparative in vitro cytotoxicity study on uncoated magnetic nanoparticles: Effects on cell viability, cell morphology, and cellular uptake. J. Nanosci. Nanotechnol. 2012, 12, 9010–9017. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.; Karni, M.; Baranes, K.; Levy, I.; Alon, N.; Margel, S.; Shefi, O. Iron oxide nanoparticles for neuronal cell applications: Uptake study and magnetic manipulations. J. Nanobiotechnol. 2016, 14, 37. [Google Scholar] [CrossRef]
- Rodríguez-Luccioni, H.L.; Latorre-Esteves, M.; Méndez-Vega, J.; Soto, O.; Rodríguez, A.R.; Rinaldi, C.; Torres-Lugo, M. Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles. Int. J. Nanomed. 2011, 6, 373–380. [Google Scholar] [CrossRef]
- Rafieepour, A.; Azari, M.R.; Peirovi, H.; Khodagholi, F.; Jaktaji, J.P.; Mehrabi, Y.; Naserzadeh, P.; Mohammadian, Y. Investigation of the effect of magnetite iron oxide particle size on cytotoxicity in A549 cell line. Toxicol. Ind. Health 2019, 35, 703–713. [Google Scholar] [CrossRef]
- Calero, M.; Chiappi, M.; Lazaro-Carrillo, A.; Rodríguez, M.J.; Chichón, F.J.; Crosbie-Staunton, K.; Prina-Mello, A.; Volkov, Y.; Villanueva, A.; Carrascosa, J.L. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J. Nanobiotechnology 2015, 13, 16. [Google Scholar] [CrossRef]
- Zavisova, V.; Koneracka, M.; Kovac, J.; Kubovcikova, M.; Antal, I.; Kopcansky, P.; Bednarikova, M.; Muckova, M. The cytotoxicity of iron oxide nanoparticles with different modifications evaluated in vitro. J. Magn. Magn. Mater. 2015, 380, 85–89. [Google Scholar] [CrossRef]
- Gunjal, A. Magnetic nanoparticles and their interaction with living organisms. Nano Mater. Nano Drugs 2023, 3, 61. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Kafayati, M.E.; Raheb, J.; Torabi Angazi, M.; Alizadeh, S.; Bardania, H. The effect of magnetic Fe3O4 nanoparticles on the growth of genetically manipulated bacterium, Pseudomonas aeruginosa (PTSOX4). Iran. J. Biotechnol. 2013, 11, 41–46. [Google Scholar] [CrossRef]
- Stavilă, C.; Herea, D.D.; Zară, M.C.; Stoian, G.; Minuti, A.E.; Labușcă, L.; Grigoraș, M.; Chiriac, H.; Lupu, N.; Petrovici, A.; et al. Enhancement of chemotherapy effects by non-lethal magneto-mechanical actuation of gold-coated magnetic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2024, 60, 102766. [Google Scholar] [CrossRef]
- Binder, R.L.; Volpenhein, M.E. An evaluation of the effects of culture medium osmolality and pH on metabolic cooperation between Chinese hamster V79 cells. Carcinogenesis 1987, 8, 1257–1261. [Google Scholar] [CrossRef]
- Kaji, T.; Kawashima, T.; Yamamoto, C.; Sakamoto, M. Rhodamine B inhibits collagen synthesis by human lip fibroblasts in culture. Toxicol. Lett. 1992, 61, 81–87. [Google Scholar] [CrossRef]
- Safitri, Y.A.; Indrawa, I.W.A.; Winarsih, S. Rhodamine B induces oxidative stress and cervical epithelial cell proliferation in the uterus. Toxicol. Rep. 2015, 2, 1434–1436. [Google Scholar] [CrossRef]
- Sulistina, D.R.; Martini, S. The effect of rhodamine B on the cerebellum and brain stem tissue of Rattus norvegicus. J. Public Health Res. 2020, 9, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.M.; Datta, A.R. Acid tolerance of enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 1995, 61, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Kabakaş, H.Ö.; Kürkçü, M.S.; Taşdelen, K.A.O.; Çöl, B. The cytotoxic effect of benzoic acid on ten different cancer cell lines. Eskişehir Tech. Univ. J. Sci. Technol. A-Appl. Sci. Eng. 2024, 25, 66–77. [Google Scholar] [CrossRef]
- Bang, D.Y.; Lee, I.; Lee, B.M. Toxicological characterization of phthalic acid. Toxicol. Res. 2011, 27, 191–203. [Google Scholar] [CrossRef]
- Koyama, R.; Mizuta, R. Acrolein scavengers cysteamine and N-benzylhydroxylamine reduce mouse liver damage after acetaminophen overdose. J. Vet. Med. Sci. 2016, 78, 1903–1905. [Google Scholar] [CrossRef]
- Miret-Casals, L.; Baelo, A.; Julián, E.; Astola, J.; Lobo-Ruiz, A.; Albericio, F.; Torrents, E. Hydroxylamine derivatives as a new paradigm in the search of antibacterial agents. ACS Omega 2018, 3, 17057–17069. [Google Scholar] [CrossRef]
- Morimoto, N.; Takei, R.; Wakamura, M.; Oishi, Y.; Nakayama, M.; Suzuki, M.; Winnik, F.M. Fast and effective mitochondrial delivery of ω-Rhodamine-B-polysulfobetaine-PEG copolymers. Sci. Rep. 2018, 8, 1128. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Yuan, Z.; Mao, X. Excess of dietary benzoic acid supplementation leads to growth retardation, hematological abnormality and organ injury of piglets. Livest. Sci. 2016, 190, 94–103. [Google Scholar] [CrossRef]
- Long, F.; Ren, Y.; Ji, Y.; Li, J.; Zhang, H.; Wu, Z.; Li, H. Pollution characteristics, toxicological properties, and health risk assessment of phthalic acid esters in water, soil, and atmosphere. Atmosphere 2024, 15, 1071. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods IV: Extension of MNDO, AM1, and PM3 to more main group elements. J. Mol. Model. 2004, 10, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Freshney, R.I. Basic Principles of Cell Culture. In Culture of Cells for Tissue Engineering; Vunjak-Novakovic, G., Freshney, R.I., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 3–22. [Google Scholar]
- Bláha, P.; Koshlan, I.V.; Koshlan, N.A.; Bogdanova, Y.V.; Petrova, D.V.; Govorun, R.D.; Krasavin, E.A. Structural changes in HPRT gene of V79 cells after irradiation with heavy ions—Immediate and delayed effects. Front. Phys. 2021, 8, 584326. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Laville, N.; Aït-Aïssa, S.; Gomez, E.; Casellas, C.; Porcher, J. Effects of human pharmaceuticals on cytotoxicity, EROD activity, and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Cann, A.J. Maths from Scratch for Biologists; John Wiley & Sons: Chichester, UK, 2002; pp. 83–146. ISBN 978-0-471-49835-3. [Google Scholar]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vochița, G.; Fânaru-Balint, A.R.; Agavriloaei, A.; Gherghel, D.; Răcuciu, M.; Creangă, D. Magnetic Nanoparticles for Rhodamine B Depletion in Wastewater—Theoretical and Experimental Approach. Molecules 2025, 30, 4447. https://doi.org/10.3390/molecules30224447
Vochița G, Fânaru-Balint AR, Agavriloaei A, Gherghel D, Răcuciu M, Creangă D. Magnetic Nanoparticles for Rhodamine B Depletion in Wastewater—Theoretical and Experimental Approach. Molecules. 2025; 30(22):4447. https://doi.org/10.3390/molecules30224447
Chicago/Turabian StyleVochița, Gabriela, Andreea R. Fânaru-Balint, Anda Agavriloaei, Daniela Gherghel, Mihaela Răcuciu, and Dorina Creangă. 2025. "Magnetic Nanoparticles for Rhodamine B Depletion in Wastewater—Theoretical and Experimental Approach" Molecules 30, no. 22: 4447. https://doi.org/10.3390/molecules30224447
APA StyleVochița, G., Fânaru-Balint, A. R., Agavriloaei, A., Gherghel, D., Răcuciu, M., & Creangă, D. (2025). Magnetic Nanoparticles for Rhodamine B Depletion in Wastewater—Theoretical and Experimental Approach. Molecules, 30(22), 4447. https://doi.org/10.3390/molecules30224447








