Magnetite-Doped Activated Carbon Beads and Powder Derived from Chitosan for Adsorption of Emerging Contaminants in Drinkable Water
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Synthesis Procedures
2.2.1. Synthesis of Oleic Acid Coated Fe3O4 Nanoparticles
2.2.2. Synthesis of NaOH Impregnated Hydrogel Beads of Pure CS, Fe-Doped CS and CS/GO
2.2.3. Activation of CS-Based Beads
2.3. Characterization of the AC-Based Beads
2.4. Adsorption Experiment for Mixture of ECs Removal
2.5. Kinetic and Isotherm Modeling
3. Results and Discussion
3.1. Characterization of the Magnetic Nanoparticles
3.1.1. XRD Pattern of the Nanoparticles and Carbon Beads
3.1.2. SEM Images of the Nanoparticles
3.2. Characterization of Synthesized Carbon Beads
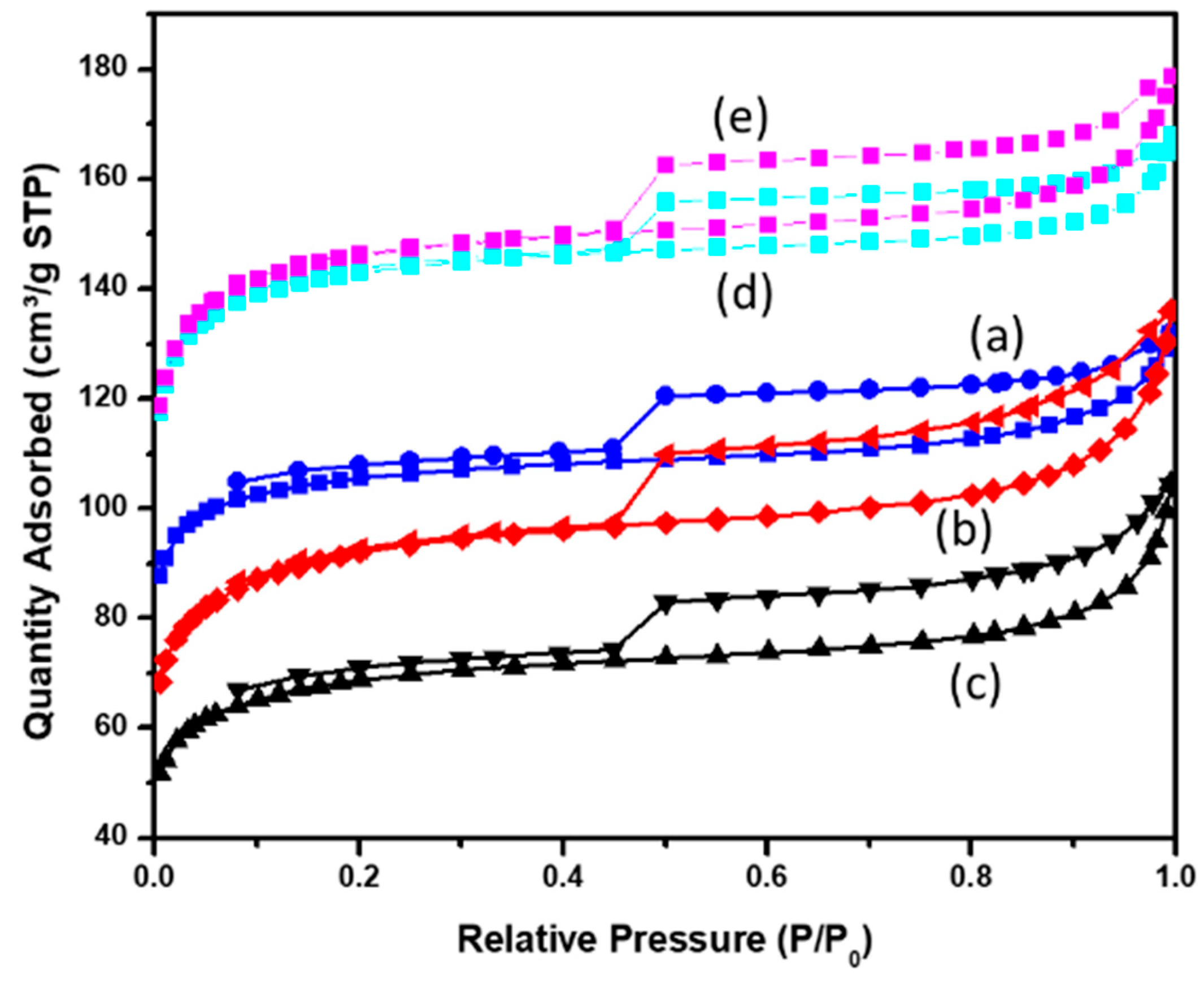
3.2.1. Surface Area and Porosity Characterization of Synthesized Samples by Gas Adsorption–Desorption
3.2.2. Morphological and Compositional Analysis of Samples: SEM-EDS and TEM
3.2.3. Chemical Characterization of Samples by XPS
3.3. Adsorption of ECs onto Carbon Adsorbents
3.3.1. Kinetics
3.3.2. MECs Adsorption Isotherms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ladan, M.T.; Okukpon, I.; Maduekwe, N.C. Realising Sustainable Access to Water and Sanitation in Africa: Role of Critical Institutions. In SDGs in Africa and the Middle East Region; Springer: Cham, Switzerland, 2024; pp. 1309–1332. [Google Scholar] [CrossRef]
- Berihun, G.; Abebe, M.; Hassen, S.; Gizeyatu, A.; Berhanu, L.; Teshome, D.; Walle, Z.; Desye, B.; Sewunet, B.; Keleb, A. Drinking Water Contamination Potential and Associated Factors among Households with Under-Five Children in Rural Areas of Dessie Zuria District, Northeast Ethiopia. Front. Public Health 2023, 11, 1199314. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Bhattarai, T.N.; Acharya, G.; Timalsina, H.; Marks, S.J.; Uprety, S.; Paudel, S.R. Water, Sanitation, and Hygiene of Nepal: Status, Challenges, and Opportunities. ACS EST Water 2023, 3, 1429–1453. [Google Scholar] [CrossRef]
- Norvivor, F.A.; Peprah, E.K.; Kyeremeh, E.A.; Konutse, O.W.; Armah, N.A.; Yirenkyi, M.B. Assessment of Improved Water and Sanitation Facilities in the Volta Region; Evidence from Ghana Demographic Health Survey 2022 Report. Sciety 2025. [Google Scholar] [CrossRef]
- Rosa, L.; Sangiorgio, M. Global Water Gaps under Future Warming Levels. Nat. Commun. 2025, 16, 1–11. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, A.Z.N.; Zisan, Z.; Rahman, M.M. Changes in Global Domestic Water Use Due to Handwashing for Preventing COVID-19: An Assessment. Water 2023, 15, 1219. [Google Scholar] [CrossRef]
- UNESCO. World Water Assessment The United Nations World Water Development Report 2019: Leaving No One Behind; UNESCO: Paris, France, 2019; ISBN 978-92-3-100309-7. [Google Scholar]
- Mekonnen, M.M.; Hoekstra, A.Y. Sustainability: Four Billion People Facing Severe Water Scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef]
- Khilchevskyi, V.; Karamushka, V. Global Water Resources: Distribution and Demand. In Clean Water and Sanitation. Encyclopedia of the UN Sustainable Development Goals; Leal Filho, W., Azul, A.M., Brandli, L., Lange Salvia, A., Wall, T., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Sadigov, R. Rapid Growth of the World Population and Its Socioeconomic Results. Sci. World J. 2022, 2022, 8110229. [Google Scholar] [CrossRef]
- Aria, S.H.; Asadollahfardi, G. Sustainable Drinking Water Management. ACS Symp. Ser. 2025, 1502, 41–63. [Google Scholar] [CrossRef]
- Hattab, S.; Alaya, C.; Banni, M. Emerging Pollutants in Wastewater: A Challenge for Water Reuse. In Emerging Pollutants. Advances in Water Security; Zandaryaa, S., Fares, A., Eckstein, G., Eds.; Springer: Cham, Switzerland, 2025; pp. 297–313. [Google Scholar] [CrossRef]
- Maliga, I.; Purwono, S.; Harini, R.; Soetarto, A. Critical Indicators for Determining Sustainable Domestic Wastewater Management for the Achievement of SDG Goal 6. Discov. Water 2025, 5, 1–25. [Google Scholar] [CrossRef]
- Gu, J.; Liu, H.; Wang, S.; Zhang, M.; Liu, Y. An Innovative Anaerobic MBR-Reverse Osmosis-Ion Exchange Process for Energy-Efficient Reclamation of Municipal Wastewater to NEWater-like Product Water. J. Clean. Prod. 2019, 230, 1287–1293. [Google Scholar] [CrossRef]
- Tortajada, C. Contributions of Recycled Wastewater to Clean Water and Sanitation Sustainable Development Goals. NPJ Clean Water 2020, 3, 1–6. [Google Scholar] [CrossRef]
- Al-Khatib, L.A.; AlHanaktah, A.M. Wastewater Treatment Plant Upgrade and Its Interlinkages with the Sustainable Development Goals. Resources 2025, 14, 62. [Google Scholar] [CrossRef]
- Intisar, A.; Ramzan, A.; Hafeez, S.; Hussain, N.; Irfan, M.; Shakeel, N.; Gill, K.A.; Iqbal, A.; Janczarek, M.; Jesionowski, T. Adsorptive and Photocatalytic Degradation Potential of Porous Polymeric Materials for Removal of Pesticides, Pharmaceuticals, and Dyes-Based Emerging Contaminants from Water. Chemosphere 2023, 336, 139203. [Google Scholar] [CrossRef] [PubMed]
- Silori, R.; Zang, J.; Raval, N.P.; Giri, B.S.; Mahlknecht, J.; Mora, A.; Dueñas-Moreno, J.; Tauseef, S.M.; Kumar, M. Adsorptive Removal of Ciprofloxacin and Sulfamethoxazole from Aqueous Matrices Using Sawdust and Plastic Waste-Derived Biochar: A Sustainable Fight against Antibiotic Resistance. Bioresour. Technol. 2023, 387, 129537. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Malule, H.; Quiñones-Murillo, D.H.; Manotas-Duque, D. Emerging Contaminants as Global Environmental Hazards. A Bibliometric Analysis. Emerg. Contam. 2020, 6, 179–193. [Google Scholar] [CrossRef]
- Egbuna, C.; Amadi, C.N.; Patrick-Iwuanyanwu, K.C.; Ezzat, S.M.; Awuchi, C.G.; Ugonwa, P.O.; Orisakwe, O.E. Emerging Pollutants in Nigeria: A Systematic Review. Environ. Toxicol. Pharmacol. 2021, 85, 103638. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Ptacek, C.J.; Groza, L.G.; Staples, R.; Blowes, D.W. Occurrence and Distribution of Emerging Contaminants in Mine-Impacted Lake Water and Potential Use as Co-Tracers of Anthropogenic Activity in the Subarctic Region, Northwest Territories, Canada. Environ. Res. 2022, 207, 112034. [Google Scholar] [CrossRef]
- Suzuki, T.; Hidaka, T.; Kumagai, Y.; Yamamoto, M. Environmental Pollutants and the Immune Response. Nat. Immunol. 2020, 21, 1486–1495. [Google Scholar] [CrossRef]
- Nwokediegwu, Z.Q.S.; Daraojimba, O.H.; Oliha, J.S.; Obaigbena, A.; Dada, M.A.; Majemite, M.T. Review of Emerging Contaminants in Water: USA and African Perspectives. Int. J. Sci. Res. Arch. 2024, 11, 350–360. [Google Scholar] [CrossRef]
- Wiest, L.; Gosset, A.; Fildier, A.; Libert, C.; Hervé, M.; Sibeud, E.; Giroud, B.; Vulliet, E.; Bastide, T.; Polomé, P.; et al. Occurrence and removal of emerging pollutants in urban sewage treatment plants using LC-QToF-MS suspect screening and quantification. Sci. Total Environ. 2021, 774, 145779. [Google Scholar] [CrossRef]
- Čelić, M.; Farré, M.; de Alda, M.L.; Perez, S.; Barceló, D.; Petrovic, M. Environmental Analysis: Emerging Pollutants. In Liquid Chromatography (Third Edition): Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 549–578. ISBN 9780323999694. [Google Scholar]
- Kumar, M.; Sridharan, S.; Sawarkar, A.D.; Shakeel, A.; Anerao, P.; Mannina, G.; Sharma, P.; Pandey, A. Current Research Trends on Emerging Contaminants Pharmaceutical and Personal Care Products (PPCPs): A Comprehensive Review. Sci. Total Environ. 2023, 859, 160031. [Google Scholar] [CrossRef]
- Hassan, R.; Zahoor, I. Strategies and Technologies for Emerging Contaminants. Int. J. Chem. Biochem. Sci. (IJCBS) 2024, 25, 2024. [Google Scholar]
- Diniz, V.; Cunha, D.G.F.; Rath, S. Adsorption of Recalcitrant Contaminants of Emerging Concern onto Activated Carbon: A Laboratory and Pilot-Scale Study. J. Environ. Manag. 2023, 325, 116489. [Google Scholar] [CrossRef] [PubMed]
- WHO Potable Reuse: Guidance for Producing Safe Drinking-Water; WHO: Geneva, Switzerland, 2017.
- Spencer, W.; Ibana, D.; Singh, P.; Nikoloski, A.N. Sustainable Production of Activated Carbon from Waste Wood Using Goethite Iron Ore. Sustainability 2025, 17, 681. [Google Scholar] [CrossRef]
- Hossain, Z.; Chowdhury, M.B.I.; Hossain, Z.; Chowdhury, M.B.I. Biobased Activated Carbon and Its Application. In Biomass Based Products; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Merin Rose, K.E.; Soumya, M.; Mohanan, M.; Maria, H.J.; Thomas, S. Bulk Carbon Materials for the Environment: Synthesis Routes and Properties. In Carbon: Bulk-to-Nano Forms for Detection and Remediation of Environmental Contaminants; Springer: Cham, Switzerland, 2025; pp. 57–93. [Google Scholar] [CrossRef]
- Sellaoui, L.; Gómez-Avilés, A.; Dhaouadi, F.; Bedia, J.; Bonilla-Petriciolet, A.; Rtimi, S.; Belver, C. Adsorption of Emerging Pollutants on Lignin-Based Activated Carbon: Analysis of Adsorption Mechanism via Characterization, Kinetics and Equilibrium Studies. Chem. Eng. J. 2023, 452, 139399. [Google Scholar] [CrossRef]
- Al-sareji, O.J.; Meiczinger, M.; Somogyi, V.; Al-Juboori, R.A.; Grmasha, R.A.; Stenger-Kovács, C.; Jakab, M.; Hashim, K.S. Removal of Emerging Pollutants from Water Using Enzyme-Immobilized Activated Carbon from Coconut Shell. J. Environ. Chem. Eng. 2023, 11, 109803. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water. C—J. Carbon Res. 2018, 4, 63. [Google Scholar] [CrossRef]
- Aytar, E.C.; Basılı, T.; Durmaz, A.; Aydın, B.; Seyfeli, R.C.; Kahyaoğlu, İ.M.; Karakuş, S. Zero-Waste Production of Copper Nanoparticles and Activated Carbon from Erigeron canadensis L.: A Sustainable Approach for Environmental and Health Applications. ChemistrySelect 2025, 10, e202405073. [Google Scholar] [CrossRef]
- Montoya-Bautista, C.V.; Mohamed, B.A.; Li, L.Y. Sludge-Based Activated Carbon from Two Municipal Sewage Sludge Precursors for Improved Secondary Wastewater-Treatment Discharge-Effluent. J. Environ. Chem. Eng. 2022, 10, 108704. [Google Scholar] [CrossRef]
- Zhao, M.; Ji, D.; Wu, G. Sludge-Based Activated Carbon Experiment Design, Char Properties, and Evaluation of Methyl Orange Adsorption. Biomass Convers. Biorefinery 2025, 15, 8585–8596. [Google Scholar] [CrossRef]
- Belo, C.R.; Cansado, I.P.d.P.; Mourão, P.A.M. Synthetic Polymers Blend Used in the Production of High Activated Carbon for Pesticides Removals from Liquid Phase. Environ. Technol. 2017, 38, 285–296. [Google Scholar] [CrossRef]
- Wu, H.Y.; Chen, S.S.; Liao, W.; Wang, W.; Jang, M.F.; Chen, W.H.; Ahamad, T.; Alshehri, S.M.; Hou, C.H.; Lin, K.S.; et al. Assessment of Agricultural Waste-Derived Activated Carbon in Multiple Applications. Environ. Res. 2020, 191, 110176. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, M.S.; Azha, S.F.; Sellaoui, L.; Badawi, M.; Bonilla-Petriciolet, A.; Ismail, S. Performance and Interactions of Diclofenac Adsorption Using Alginate/Carbon-Based Films: Experimental Investigation and Statistical Physics Modelling. Chem. Eng. J. 2022, 428, 131929. [Google Scholar] [CrossRef]
- Jacob, M.M.; Ponnuchamy, M.; Kapoor, A.; Sivaraman, P. Adsorptive Decontamination of Organophosphate Pesticide Chlorpyrifos from Aqueous Systems Using Bagasse-Derived Biochar Alginate Beads: Thermodynamic, Equilibrium, and Kinetic Studies. Chem. Eng. Res. Des. 2022, 186, 241–251. [Google Scholar] [CrossRef]
- Farghal, H.H.; Nebsen, M.; El-Sayed, M.M.H. Exploitation of Expired Cellulose Biopolymers as Hydrochars for Capturing Emerging Contaminants from Water. RSC Adv. 2023, 13, 19757–19769. [Google Scholar] [CrossRef]
- Kumar, A.; Patra, C.; Rajendran, H.K.; Narayanasamy, S. Activated Carbon-Chitosan Based Adsorbent for the Efficient Removal of the Emerging Contaminant Diclofenac: Synthesis, Characterization and Phytotoxicity Studies. Chemosphere 2022, 307, 135806. [Google Scholar] [CrossRef]
- Ayouch, I.; Aboulhrouz, S.; Jioui, I.; Dânoun, K.; Oumam, M.; Zahouily, M. Wastewater Treatment: Use of Biopolymers as a Sustainable Approach. In Handbook of Sustainable Industrial Wastewater Treatment; CRC Press: London, UK, 2025. [Google Scholar]
- Krishna Rao, K.S.V.; Sudha Vani, T.J.; Hemalatha, D.; Rao, K.M.; Reddy, G.V.; Naidu, B.V.K. Chitosan-Based Ecofriendly Nanocomposites: Recent Advances for Environmental Remediation Applications. In Carbohydrate Polymer Nanotechnologies. Smart Nanomaterials Technology; Krishna Rao, K.S.V., Suresh Reddy, K.V.N., Alle, M., Eds.; Springer: Singapore, 2025. [Google Scholar] [CrossRef]
- Marrakchi, F.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous-Activated Carbon Prepared from Chitosan Flakes via Single-Step Sodium Hydroxide Activation for the Adsorption of Methylene Blue. Int. J. Biol. Macromol. 2017, 98, 233–239. [Google Scholar] [CrossRef]
- Guy, F.; Runtti, H.; Duclaux, L.; Ondarts, M.; Reinert, L.; Outin, J.; Gonze, E.; Bonnamy, S.; Soneda, Y. Synthesis and Characterization of Cu Doped Activated Carbon Beads from Chitosan. Microporous Mesoporous Mater. 2021, 322, 111147. [Google Scholar] [CrossRef]
- Mitra, A.K.; Nayak, S. Sustainable Nanomaterials in Wastewater Remediation. In Nanomaterials in Wastewater Research. Advances in Wastewater Research; Agarwal, N., Shah, M.P., Solanki, V.S., Singh, N., Eds.; Springer: Singapore, 2025. [Google Scholar] [CrossRef]
- Varghese, A.M.; Kuppireddy, S.; Tsatsos, S.; Kyriakou, G.; Alamoodi, N.; Karanikolos, G.N. Tailored Metal-Doped Activated Carbon Adsorbents Exhibiting High-Capacity, Selective, and Reversible Hydrogen Storage at Room Temperature. Ind. Eng. Chem. Res. 2025, 64, 16845–16861. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y. Preparation and Characterization of Activated Carbon (AC) Doped Iron Oxide (Fe2O3) Nanoparticles. Mater. Sci. 2025, XX, 2025. [Google Scholar] [CrossRef]
- Alizadeh Fard, M.; Vosoogh, A.; Barkdoll, B.; Aminzadeh, B. Using Polymer Coated Nanoparticles for Adsorption of Micropollutants from Water. Colloids Surf. A Physicochem. Eng. Asp. 2017, 531, 189–197. [Google Scholar] [CrossRef]
- Lourens, A.; Falch, A.; Malgas-Enus, R. Magnetite Immobilized Metal Nanoparticles in the Treatment and Removal of Pollutants from Wastewater: A Review. J. Mater. Sci. 2023, 58, 2951–2970. [Google Scholar] [CrossRef]
- Alizadeh Fard, M.; Barkdoll, B. Magnetic Activated Carbon as a Sustainable Solution for Removal of Micropollutants from Water. Int. J. Environ. Sci. Technol. 2019, 16, 1625–1636. [Google Scholar] [CrossRef]
- Hashemzadeh, F.; Ariannezhad, M.; Derakhshandeh, S.H. Sustainable Removal of Tetracycline and Paracetamol from Water Using Magnetic Activated Carbon Derived from Pine Fruit Waste. Sci. Rep. 2024, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.L.; de Oliveira, H.L.; Serpa, J.A.S.; Torres, J.A.; Nogueira, F.G.E.; de Freitas, V.A.A.; Borges, K.B.; Silva, M.C. Nanomagnets Based on Activated Carbon/Magnetite Nanocomposite for Determination of Endocrine Disruptors in Environmental Water Samples. Microchem. J. 2021, 168, 106366. [Google Scholar] [CrossRef]
- Xu, Q.; Lai, D.; Xing, Z.; Liu, X.; Wang, Y. Strengthened Removal of Emerging Contaminants over S/Fe Codoped Activated Carbon Fabricated by a Mild One-Step Thermal Transformation Scheme. Chemosphere 2023, 310, 136897. [Google Scholar] [CrossRef]
- Rios, R.D.F.; Binatti, I.; Ardisson, J.D.; Moura, F.C.C. Compounds Based on Iron Mining Tailing Dams and Activated Carbon from Macauba Palm for Removal of Emerging Contaminants and Phosphate from Aqueous Systems. Environ. Sci. Pollut. Res. 2023, 30, 60212–60224. [Google Scholar] [CrossRef]
- Raval, N.P.; Kumar, M. Geogenic Arsenic Removal through Core–Shell Based Functionalized Nanoparticles: Groundwater in-Situ Treatment Perspective in the Post–COVID Anthropocene. J. Hazard. Mater. 2021, 402, 123466. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Low, M.J.D. Kinetics of Chemisorption of Gases on Solids. Chem. Rev. 2002, 60, 267–312. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Vermeulen, T. Theory for Irreversible and Constant-Pattern Solid Diffusion. Ind. Eng. Chem. 2002, 45, 1664–1670. [Google Scholar] [CrossRef]
- Poulin, S.; França, R.; Moreau-Bélanger, L.; Sacher, E. Confirmation of X-Ray Photoelectron Spectroscopy Peak Attributions of Nanoparticulate Iron Oxides, Using Symmetric Peak Component Line Shapes. J. Phys. Chem. C 2010, 114, 10711–10718. [Google Scholar] [CrossRef]
- Wilson, D.; Langell, M.A. XPS Analysis of Oleylamine/Oleic Acid Capped Fe3O4 Nanoparticles as a Function of Temperature. Appl. Surf. Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]







| Molecule Name | Bisphenol A (BPA) | Carbofuran (CBF) | Carbamazepine (CBZ) | Diclofenac (DCF) | Dimethoate (DMA) | Imidacloprid (ICP) |
|---|---|---|---|---|---|---|
| Molecular formula | C15H16O2 | C12H15NO3 | C15H12N2O | C14H11Cl2NO2 | C5H12NO3PS2 | C9H10ClN5O2 |
| Structure |  |  |  |  |  |  |
| Polarizability (Å3) | 25.4 | 23.3 | 25 | 27.9 | 21.4 | 23.2 |
| Water solubility (mg.L−1) | 120–300 | 351 | 17.7 | 2.37 | 39,000 | 510 |
| Log Kow | 3.64 | 2.32 | 2.45 | 4.51 | 0.70 | 0.57 |
| pKa | 9.6 | 11.95 | 13.90 | 4.15 | 4.20 | 1.56/11.12 |
| Usage | Polycarbonate precursor | N-methyl carbamate insecticide and nematicide | Analgesic, anti-epileptic | Analgesic, anti-inflammatory | Organophosphorus insecticide | Neonicotinoid insecticide |
| Sample Name | CS Concentration in Acetic Acid Solution (Mass. %) | IR of NaOH (%) * | Gelification Time (h) | Fe3O4 Addition (Mass. % of Solid) | GO Addition (Mass. %) | Pyrolysis T (°C) |
|---|---|---|---|---|---|---|
| C-Cs-T # | 5 | ~5–20 | 1–6 | - | - | 600–1000 |
| C-CsF/1% | 5 | ~9 | 2 | 1 | - | 700 |
| C-CsF/2% | 5 | ~9 | 2 | 2 | - | 700 |
| C-CsF/5% | 5 | ~9 | 2 | 5 | - | 700 |
| C-CsG | 2.5 | ~9 | 2 | - | ~8 | 700 |
| AC Beads Type | SBET §a (m2.g−1) | Vmicro §b (cm3.g−1) | Vmeso §c (cm3.g−1) | Vtotal §d (cm3.g−1) |
|---|---|---|---|---|
| C-Cs-1000 | 666 | 0.24 | 0.73 | 0.97 |
| C-Cs-900 | 684 | 0.26 | 0.45 | 0.71 |
| C-Cs-800 | 776 | 0.33 | 0.20 | 0.53 |
| C-Cs-700 | 561 | 0.22 | 0.04 | 0.26 |
| C-CsF/1% | 415 | 0.16 | 0.04 | 0.20 |
| C-CsF/2% | 346 | 0.14 | 0.07 | 0.21 |
| C-CsF/5% | 260 | 0.10 | 0.06 | 0.16 |
| C-CsG | 572 | 0.23 | 0.05 | 0.28 |
| AC Name | Fe Total (at. %) | Fe3+/Fe2+ | Fe3+ Oct. | Fe3+ Tet. | Fe3+ Oct./ Fe3+ Tet. | O Lattice | O/Fe |
|---|---|---|---|---|---|---|---|
| C-CsF/1% | 5.93 | 1.65 | 2.51 | 1.18 | 2.13 | 7.97 | 1.34 |
| C-CsF/2% | 8.20 | 1.10 | 3.52 | 1.72 | 2.04 | 9.99 | 1.22 |
| C-CsF/5% | 15.56 | 0.83 | 5.34 | 1.73 | 3.08 | 9.01 | 0.58 |
| EC Name | qe(exp) (µg.g−1) | Pseudo-First-Order Rate Parameters | Pseudo-Second-Order Rate Parameters | Elovitch Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe(cal) (µg.g−1) | K1 (min−1) | R2 | qe(cal) (µg.g−1) | K2 (g.µg−1.min−1) | R2 | α (g.µg−1.min−1) | β (µg.g−1) | R2 | ||
| BPA | 327 | 298 | 0.024 | 0.958 | 336 | 1 × 10−4 | 0.987 | 1.5 × 105 | 3.28 | 0.977 |
| CBF | 369 | 330 | 0.386 | 0.946 | 342 | 2.1 × 10−3 | 0.959 | 1.7 × 1013 | 6.32 | 0.921 |
| CBZ | 449 | 419 | 0.033 | 0.891 | 455 | 1 × 10−4 | 0.948 | 1.7 × 108 | 4.27 | 0.992 |
| DCF | 371 | 334 | 0.022 | 0.897 | 373 | 1 × 10−4 | 0.946 | 1.0 × 106 | 3.57 | 0.996 |
| DMA | 359 | 295 | 0.091 | 0.780 | 400 | 1 × 10−4 | 0.794 | 1.2 × 109 | 4.77 | 0.977 |
| ICP | 552 | 487 | 0.186 | 0.912 | 525 | 5 × 10−4 | 0.964 | 4.3 × 1012 | 5.69 | 0.979 |
| EC Name | qe(exp) (µg.g−1) | Parameters of Diffusion Model of Weber and Morris | Parameters of Diffusion-Controlled Adsorption of Vermeulen | ||||
|---|---|---|---|---|---|---|---|
| Kd1 (µg.g−1. min−1/2) | C (µg.g−1) | R2 | Kd2 (min−1) | qe(cal) (µg.g−1) | R2 | ||
| BPA | 327 | 15.22 | 48.4 | 0.924 | 0.0081 | 322 | 0.989 |
| CBF | 369 | 16.07 | 163.5 | 0.557 | 0.2382 | 331 | 0.948 |
| CBZ | 449 | 20.73 | 113.1 | 0.882 | 0.0139 | 440 | 0.972 |
| DCF | 371 | 16.08 | 70.4 | 0.940 | 0.0082 | 361 | 0.975 |
| DMA | 359 | 19.16 | 85.8 | 0.860 | 0.022 | 331 | 0.887 |
| ICP | 552 | 27.46 | 208.3 | 0.691 | 0.064 | 509 | 0.942 |
| qmax (µg.g−1) | KL (L.µg−1) | ΔHads (kJ.mol−1) | R2 | KH (µg.g−1.mol−1) | |
|---|---|---|---|---|---|
| BPA | 10,009 | 0.051 | −0.9 | 0.9776 | 739 |
| CBF | 2700 | 0.049 | −0.2 | 0.9266 | 142 |
| CBZ | 2010 | 0.301 | −0.2 | 0.9076 | 642 |
| DCF | 2467 | 0.310 | −0.2 | 0.9493 | 763 |
| DMA | 1900 | 0.008 | −4.2 | 0.9604 | 71 |
| ICP | 10,000 | 0.047 | −1.2 | 0.9603 | 767 |
| KF (L1/n.g−1.μg1−1/n) | n | R2 | |
|---|---|---|---|
| BPA | 706 | 1.1 | 0.9780 |
| CBF | 269 | 1.86 | 0.9542 |
| CBZ | 633 | 2.99 | 0.9699 |
| DCF | 655 | 2.44 | 0.9817 |
| DMA | 9.91 | 1.63 | 0.8766 |
| ICP | 544 | 0.86 | 0.9362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raval, N.P.; Reinert, L.; Duclaux, L.; Cottin, N.; Yoshizawa, N.; Nicolle, J.; Chandran, A.; Muller, F. Magnetite-Doped Activated Carbon Beads and Powder Derived from Chitosan for Adsorption of Emerging Contaminants in Drinkable Water. Molecules 2025, 30, 4443. https://doi.org/10.3390/molecules30224443
Raval NP, Reinert L, Duclaux L, Cottin N, Yoshizawa N, Nicolle J, Chandran A, Muller F. Magnetite-Doped Activated Carbon Beads and Powder Derived from Chitosan for Adsorption of Emerging Contaminants in Drinkable Water. Molecules. 2025; 30(22):4443. https://doi.org/10.3390/molecules30224443
Chicago/Turabian StyleRaval, Nirav P., Laurence Reinert, Laurent Duclaux, Nathalie Cottin, Noriko Yoshizawa, Jimmy Nicolle, Anandu Chandran, and Fabrice Muller. 2025. "Magnetite-Doped Activated Carbon Beads and Powder Derived from Chitosan for Adsorption of Emerging Contaminants in Drinkable Water" Molecules 30, no. 22: 4443. https://doi.org/10.3390/molecules30224443
APA StyleRaval, N. P., Reinert, L., Duclaux, L., Cottin, N., Yoshizawa, N., Nicolle, J., Chandran, A., & Muller, F. (2025). Magnetite-Doped Activated Carbon Beads and Powder Derived from Chitosan for Adsorption of Emerging Contaminants in Drinkable Water. Molecules, 30(22), 4443. https://doi.org/10.3390/molecules30224443





